Abstract
The purpose of this study was to determine the minimum forward center of mass (COM) velocity required to prevent backward loss of balance in gait as function of the initial COM position. We hypothesized that these threshold values would be different from those previously published for standing because of the postural differences between gait and standing. To investigate this issue, we constructed a seven-link, nine-degree-of-freedom biomechanical model and employed dynamic optimization to estimate these threshold values under two initial postural conditions: (1) the posture at the beginning of swing phase (i.e., at toe-off), and (2) symmetric bipedal standing. In particular, for a range of possible COM positions posterior to the base of support (BOS), simulated annealing was used to search for the minimum velocity that could carry the COM into the BOS and avoid backward loss of balance. We found that the stability boundary against backward balance loss in walking had a similar overall trend as that previously published for standing. In general, the minimal COM velocity necessary to prevent a backward loss of balance in walking was greater than that in symmetric bipedal standing, and the difference could approach 30% or more when the COM started 0.5 and 1.0 foot-lengths behind the BOS. These discrepancies suggest that simpler biomechanical models, while being more efficient and easier to employ, may not always be adequate for exploring stability limits of humans.
Keywords: Forward dynamics, Optimization, Motion state, Posture, Stability
1. Introduction
Falls among older adults presents a significant medical and societal challenge. Between 25% and 35% of persons aged 65 or older report one or more falls each year. Consequently, falls are the leading cause of injury-related hospitalization and death in this older population (Holbrook, 1984; Tinetti and Williams, 1998). Injuries as a result of falls affect a broad spectrum of persons: not only frail or impaired individuals but healthy individuals as well (Morley, 2002; Rubenstein et al., 1994). Furthermore, slips that occur during daily living often result in a backward fall incident (Topper et al., 1993), and this type of fall is especially likely to cause hip fracture (Smeesters et al., 2001). Clearly, reducing the incidence of backward falls is important, and improving the stability against backward loss of balance can offer the first line of defense.
A primary objective for the control of stability is to control the relative motion between the body center of mass (COM) and its base of support (BOS). When the COM is behind the BOS and its forward velocity fails to reach above a certain threshold, the forward momentum of the COM will be insufficient to carry it into the BOS before diminishing to zero under the influence of gravity, leading to a backward balance loss. Across a range of possible COM positions posterior to the BOS, a set of corresponding COM velocity thresholds exists that would bound the region of backward loss of balance in the COM-state space. Individuals, young and old alike, can prevent unintended backward loss of balance and instability by operating above these stability limits, as in walking where forward progression of the COM is desired.
Computer simulation and optimization approaches have been applied to predict such dynamic stability limits based on general human anatomical, physiological, and environmental constraints (Pai and Patton, 1997; Pai and Iqbal, 1999). Such predictions have provided a basis for the understanding of the adaptive behavior of the motor control system when balance perturbations are introduced (Pai et al., 2003; Pavol and Pai, 2002). The existing boundaries for backward balance loss are based on an assumed symmetric bipedal standing posture (Pai and Patton, 1997; Pai and Iqbal, 1999). These results have been verified against a range of experiment results derived from symmetric standing (Pai et al., 1998, 2000, 2003; Patton et al., 1999, 2000). However, the extent to which these predictions are applicable to walking is unclear.
The primary purpose of this study was to determine the minimum forward COM velocity required to prevent backward loss of balance at toe-off in gait and during standing as function of the initial COM position. We hypothesized that because of the difference in initial posture, the threshold values derived for gait would be different from that for symmetrical standing. If the results do not support this hypothesis, the findings would imply the general applicability and robustness of the simpler models with symmetric bipedal standing, which sometimes can be determined with drastically reduced complexity and computational costs (Hof et al., 2005).
2. Methods
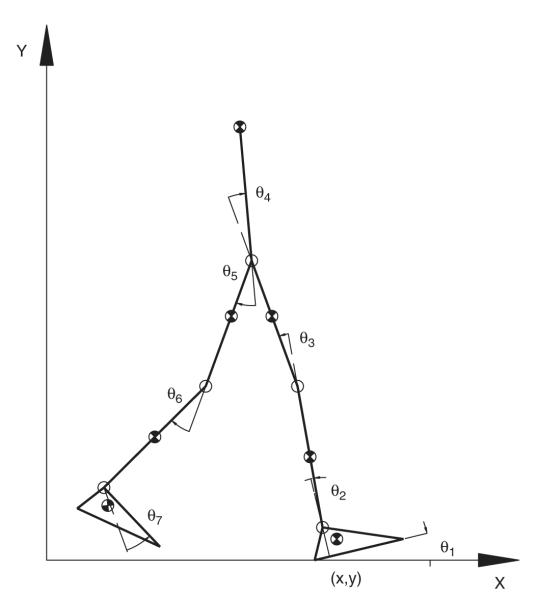
Simulations were conducted for two types of initial postural conditions: that of walking at the beginning of swing phase and that of symmetric bipedal standing. For each of these postural conditions, a series of optimization problems was solved to determine the minimum forward velocity of the COM that would allow the COM to be brought above the BOS at the end of the movement for each specified initial COM position. In order to achieve this objective, a sagittal-plane bipedal model comprised of seven rigid body segments was developed. The segments included a lumped head, arms, and trunk (HAT) segment as well as a left and a right foot, shank, and thigh. Anthropometric and inertial properties were adapted from Anderson and Pandy (1999). The position and orientation of the right foot (the base segment of the model) were defined by a planar joint with respect to the ground reference frame. The degrees of freedom corresponding to this joint were the horizontal and vertical displacements of right heel (x, y) and the angle of right foot relative to the ground θ1. The remaining segments branched out in an open chain from the right foot, all articulated with pin joints directed orthogonal to the sagittal plane (Fig. 1). Thus, a total of nine generalized coordinates, q =[x, y, θ1; θ2; θ3; θ4; θ5; θ6; θ7], were used to define the configuration of the body.
Fig. 1.
Schematic of the seven-link, nine-DOF, sagittal-plane model of the human body. The vector q = [x, y, θ1, θ2, …, θ7] represents the generalized coordinates of the model. Generalized coordinates x, y, and θ1 specify the position and orientation of the right foot, which was the base segment of the model during the stance phase, with reference to the global frame (X, Y). Joint angles θi (i = 2, 3,…,7) specify the angles of the right ankle, right knee, right hip, left hip, left knee, and left ankle, respectively. On the right side, the direction of the rotation axes is along the positive Z-axis (laterally to the right), but for the left side of the body the joint axes were in the direction of the negative Z-axis (laterally to the left). The locations of the centers of mass, shown as the half-shaded circles, were based on regression equation (McConville et al., 1980). The positive X-axis is in the direction of forward progression, and the positive Y-axis is upwards.
Each anatomical joint in the model was actuated by a single resultant joint moment with peak flexion and extension limits based on published values (Anderson and Pandy, 1999). Specifically, the resultant joint moment was computed using the following relationship:
| (1) |
where τi, ai, and Ti are the moment, activation level, and the physiological moment range of the ith joint, respectively. The superscripts E and F represent extension and flexion, or plantar flexion and dorsiflexion for the ankle, respectively. A first-order differential equation governed the rise and decay of activation level in response to a net muscle excitation (Pandy et al., 1992).
| (2) |
The rise and decay time constants, trise and tfall, were both assigned a value of 10 ms. Each muscle excitation-time history, u(t), was defined by a set of independent linearly interpolated variables or control nodes.
In walking, the joint moments in the swing leg were less variable than in the stance leg. Therefore, fewer control nodes were used to represent the excitation histories in the swing (left) leg than in the stance (right) leg. Specifically, the numbers of control nodes used were 18, 18, 18, 11, 11, and 5 (81 total) corresponding to the right ankle, right knee, right hip, left hip, left knee, and left ankle, respectively. In symmetric bipedal standing, each excitation history was approximated by 11 control nodes (66 total).
In walking, the initial configuration of model was set to correspond to body positions taken from the swing phase of gait. In standing, both feet were placed side-by-side with identical joint angles for the left and right sides of the body; both feet were constrained to remain in contact with the ground throughout the simulated movement. Contact of each foot with the ground was modeled using a set of 16 visco-elastic elements uniformly distributed beneath each foot (Anderson and Pandy, 1999). For each postural condition, the posterior starting position of the COM was systematically varied. In walking, the simulated COM initial positions normalized to foot length were −1.50, −1.25, −1.00, −0.75, −0.50, and −0.25 (negative values indicating displacements posterior to the most posterior point of the right heel with right foot in stance phase). Joint angles for positions −0.50, −0.75, and −1.00 wereobtained directly from experimental gait data (see below), in which the COM position corresponded closely to these positions at the instant of toe-off (swing initiation). Joint angles for −0.25, −1.25, and −1.50 were obtained by linear interpolation and extrapolation of the joint angles obtained from the experimental data. In standing, two initial positions of −1.00 and −0.50 were considered. Joint angles corresponding to each of these positions were obtained from Iqbal and Pai (2000). The duration for the simulations was fixed to provide adequate time for the COM to reach the BOS, ranging from 0.40 to 0.51 s depending on the initial position of the COM.
For the walking posture, the optimization performance criterion had the following terms:
| (3) |
where wi is the weight of the ith term. The subscript r and l indicate right and left, respectively. and , respectively, represent the horizontal velocity and acceleration of the COM. The symbol ⌊ ⌋ has the following behavior:
The first two terms in the cost function required the COM to lie within the BOS with the left foot forward of the right foot at termination of the simulation. The third term prevented the left foot from contacting the ground. Term four required the vertical component of the ground reaction force, Fy, to remain positive for all visco-elastic springs. Terms five and six restricted the joint angles and angular velocities to remain within physical limits determined by experimental data. Term seven was used to prevent the hyperextension of the joints by constraining the excitations. Finally, the last three terms required the COM to come to rest in static equilibrium at termination of the simulation.
For symmetric bipedal standing, the cost function had a similar form:
| (4) |
where xheel represents the position of the posterior heel, and the ground reaction force term included the forces acting on both feet. The remaining terms are the same as in Eq. (3).
The optimization problem was thus to find values for the initial joint angular velocities () and for the 81 and 66 excitation controls nodes in walking and in standing, respectively, that minimized the performance criteria expressed in Eqs. (3) and (4). Simulated annealing (Corona et al., 1987) was used to perform the optimizations. Iteration was terminated when the improvement in the cost function was less than 10−3 for 500 consecutive function evaluations. All state equations for the model (i.e., Eq. (2) for activation and the equations of motion) were integrated forward in time using a Runge–Kutta–Feldberg 5–6 variable-step integrator (Atkinson et al., 1989). Equations of motion for the dynamical linkage were derived using SD/Fast (PTC, Needham, MA).
The results of the computer simulations were evaluated in three ways. First, a sample of six COM states was taken from the backward balance loss region. It was verified that a dynamic optimization solution could not be found with any of these initial states that would lead to successful termination with the COM restored over the BOS. Second, the current simulation results were compared with those previously published in the literature. And, third, the predicted threshold for backward balance loss was checked against experimentally derived gait data to verify that it was not crossed during the course of normal walking.
In gait experiments, 39 young (age in years: 27.0±4.7) and eight older adults (75.9±7.1) were recruited. Subjects in each group performed three blocks of 10 trials each at their, respectively, preferred fast, natural, or slow speeds along a 7 m walkway. The data for the young subjects walking were obtained from a study on slipping (Bhatt et al., 2005). These 39 subjects were randomly divided into three speed groups: fast (n = 12), natural (n = 13), and slow (n = 14) where only the last (i.e., the third) block, which was always performed at the same speed at which the subsequent first slip was induced, was included here. Due to a relatively smaller sample size of older adults, the last trial of every speed block was analyzed (i.e., each subject had three representative trials, one at each speed).
3. Results
The threshold against backward balance loss in walking required normalized forward COM velocities of 0.305, 0.29, 0.259, 0.213, 0.15, and 0.075 when the COM started 1.5, 1.25, 1, 0.75, 0.5 and 0.25 foot-lengths behind the heel, respectively. The velocities required to prevent backward balance loss for walking were greater than for symmetric bipedal standing throughout the range of COM positions considered (Fig. 2: solid vs. dashed line and stars). For the two initial COM positions of −0.50 and −1.00, velocities required for walking were about the 37% higher than the corresponding standing postures (Table 1).
Fig. 2.
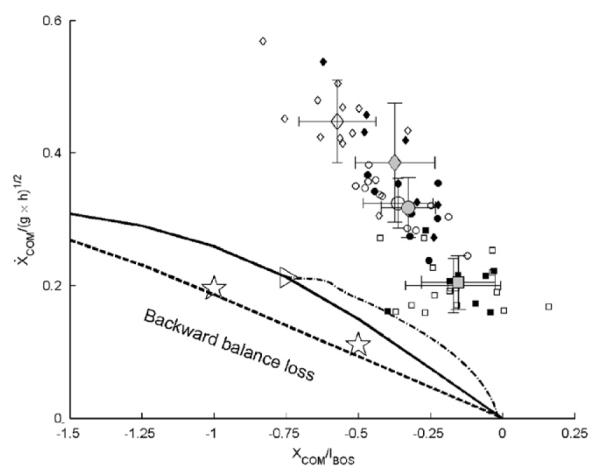
The stability boundary against backward balance loss (solid line) corresponds to initial postures at the beginning of swing phase in regular gait, interpolated based on seven individual initial center of mass (COM) positions (xCOM = 0:0, −0.25, −0.50, −0.75, −1.00, −1.25, and −1.50, normalized to foot length, lBOS, with negative values indicating distance posterior to the base of support (BOS)). All but the condition for xCOM = 0:0 were predicted by the simulation. The velocity of the COM was normalized to , where g is the acceleration due to gravity, and h the body height. To illustrate the actual result from one simulation run, the dash-dot line represents the simulated COM state trajectories for the dynamic optimization solution for walking with an initial COM position of −0.75. The triangle identifies the starting point of the COM state for this single simulation. Also included are the results (two stars) based on the present seven-link model but with two initial postures corresponding to bipedal symmetric standing (xCOM = −0:50 and −1.00), respectively. It is obvious that these states for standing are much closer to the previously published boundary for bipedal standing (dashed line, Pai and Patton, 1997) than to the boundary for walking. Finally, the experimental data from gait analysis are also included. The small open diamond, circle, and square represent the measured COM states at toe-off of the left foot obtained from 39 young subjects walking at fast (n = 12), natural (n =13), and slow (n = 14) speeds. The small closed diamond, circle, and square indicate the COM states of the older subjects (n = 8) at the three walking speeds fast, natural and slow, respectively. The large open and close markers and SD bars, respectively, indicate the corresponding mean ±1 SD values of the COM state for the young and older subjects.
Table 1.
Comparison between the initial COM horizontal velocities necessary to prevent backward balance loss for walking and for standing derived from the current study as well as the published results (in parenthesis) (Pai and Patton, 1997) at two initial COM positions
| xCOM |
|
||
|---|---|---|---|
| (a) Walking | (b) Standing | a/b (%) | |
| −0.50 | 0.150 | 0.109 (0.094*) | 138 |
| −1.00 | 0.258 | 0.190 (0.186*) | 136 |
The COM position and forward velocity are dimensionless variables expressed as a fraction of lBOS and , respectively, where lBOS represents the length of the foot, g is the gravity constant, and h is the body height.
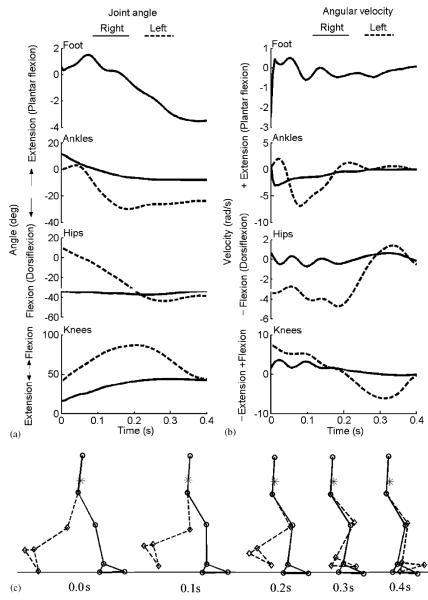
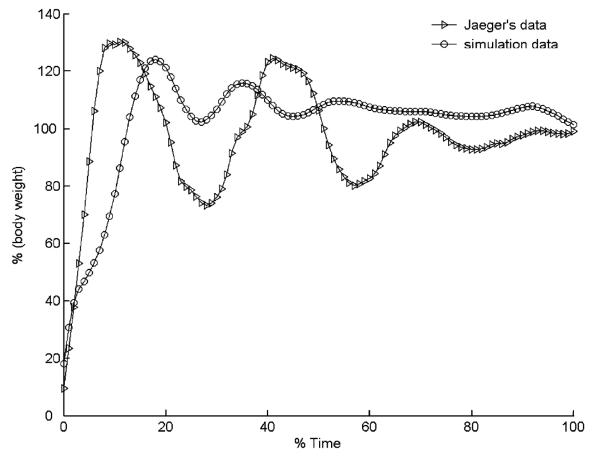
Inspection of the results for a specific simulation in walking (xCOM = −0:75, triangle and dash-dot in Fig. 2, which is representative of the simulations for the other COM positions) revealed that the optimization process resulted in a coordinated solution that enforced the various conditions expressed in the performance criterion in Eq. (3) (Fig. 3). Joint angles remained within physiological ranges, and the left foot remained above the ground throughout the simulation. At movement termination, joint angular velocities were close to zero, the left heel was anterior to the right heel, and the COM was just within the base of support. In addition, the simulated vertical ground reaction force was similar to that previously reported in a study on gait termination (Jaeger and Vantichatchavan, 1992) and was very close to body weight at movement termination (Fig. 4).
Fig. 3.
Simulated joint angular displacements (a), joint angular velocities (b), and stick-figure animation (c) for successful balance recovery starting from a normalized COM position of −0.75 during walking. The solid and dash lines are for the right and left sides, respectively. For both ankles and both hips, positive values represent extension (plantar flexion for the ankle); while for both knees, positive values indicate flexion. The number beneath each stick figure indicates the simulation time, and the asterisk represents the COM position.
Fig. 4.
Simulated vertical ground reaction force for balance recovery starting from a normalized COM position of −0.75 during walking (circles) and the experimental data from Jaeger and Vantichatchavan (1992) (triangles). The simulated and experimental data demonstrated similar oscillator behavior. At termination, the ground reaction forces stabilized near body weight.
Further confidence in the threshold for backward loss of balance comes from three sources. First, when we used initial COM states that were located inside the region of backward balance, a solution that brought the COM above the BOS was not possible (Fig. 5). Second, the results computed for symmetrical bipedal standing posture were similar to previously published results for standing posture but using a simpler inverted pendulum model (Table 1)(Pai and Patton, 1997; Pai and Iqbal, 1999). Finally, the COM states at toe-off (swing initiation) obtained from the subjects walking at different speeds were distributed well beyond the threshold that would trigger a backward loss of balance (Fig. 2). At no time during swing phase did the COM state trajectory of any subject cross the threshold computed for waling that would trigger backward balance loss.
Fig. 5.
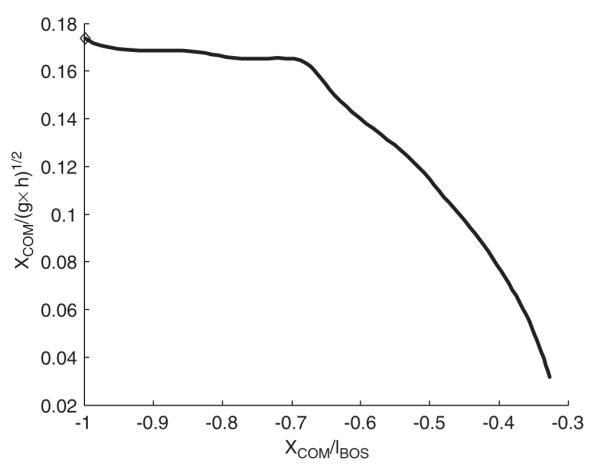
Unsuccessful balance recovery. Initial COM states were chosen to lie within the region of backward balance loss. The diamond marks the starting COM state. At the termination of the movement, the COM was still significantly behind the right heel and its horizontal velocity was nearly zero.
4. Discussion
The stability limits against backward loss of balance have been previously derived with a reductionist approach based on inverted pendulum models (Hof et al., 2005; Pai and Patton, 1997; Pai and Iqbal, 1999). They are remarkably robust and appropriate for a range of task conditions with symmetric bipedal standing postures. These task conditions include bi-manual pull (Patton et al., 1999, 2000), resisting floor perturbation (Pai et al., 2000), waist pull (Pai et al., 1998), chair-rise and a slip (Pai et al., 2003). The initial positions of the lower limbs are symmetrical and similar in all these activities. Walking, however, is characterized by a distinctly asymmetrical posture. Indeed, the present study provides evidence that the threshold for backward loss of balance for walking is different from the thresholds for activities with symmetrical foot placement.
It is generally believed that the more posterior the COM is related to the BOS, the less stable is the person. Larger step lengths at faster speeds are associated with more posteiorly located COM with respect to the base of support—the primary contributing factor for instability and backward balance loss according to this static concept. The results of the current analysis suggest, however, that a person is unlikely to be in a state of instability at toe-off during gait even when the COM is located posterior to the BOS, especially during higher walking speeds. The instability caused by large step lengths can be sufficiently offset by a correspondingly higher COM velocity (Bhatt et al., 2005). Indeed, our results have revealed that the stability at toe-off of regular gait tends to be greater (i.e., further beyond the thresholds that can trigger backward balance loss) at faster speeds regardless of the age of the individual under nonslip conditions (Fig. 2).
This misconception may be attributable to the traditional view on stability, which states that the body can keep standing still without falling as long as the vertical projection of the COM remains inside of the BOS (Borelli, 1680). Most contemporary clinical exams and balance tests are based on this centuries-old understanding of static stability (O’Sullivan, 1994). Efforts have been made to assess dynamic stability by introducing perturbations during quiet standing in a postural stress test (Wolfson et al., 1986) and in a moveable-platform setup (Nashner, 1987). It is only recently that this concept has been extended to dynamic situations with symmetric bipedal standing (Pai, 2003), and, in the present study, to walking. The thresholds derived in this study provide a quantitative basis for assessing an individual’s stability beyond that of quiet standing. The thresholds also provide information necessary to quantify the effect of adaptive training. For instance, individuals can be trained to reduce the risk of falls by adaptively improving their COM state stability (Bhatt and Pai, 2005). From this perspective, such stability limits provides the basis for setting quantifiable goals for these individuals.
In this connection, the present study lays a foundation for understanding backward balance loss during a slip, the most common reason for backward falls (Bhatt and Pai, 2005; Pai et al., 2003). The gait model, with minor modifications, together with the optimization approach used in this study, can be applied to determine the boundary for slip conditions. The difference between the boundaries for slip and nonslip conditions quantifies how much one must alter his/her COM state by increasing the forward velocity of the COM and/or anteriorly shifting the COM position in order to maintain stability even during a slip (Pai and Iqbal, 1999). The empirical evidence supports a basic premise that the central nervous system (CNS) must have learned to modify and update the internal representation of these stability limits in order to correct previous errors made during balance recovery (Pai et al., 2003). The adaptive alteration of gait would be the prerequisite for motor behavior modification and its retention intended to reduce slip-related fall incidence among older adults (Bhatt and Pai, 2005; Pai et al., 2003).
The results of this work are based on several simplifying assumptions, which may influence the simulation results and limit their applicability. Chiefly, the model permitted motion only in the sagittal plane. Although the movement of each individual limb during walking is primarily planar, the pelvis undergoes some degree of movement in both the frontal and horizontal planes. Indeed, the motion along the mediolateral direction may have an impact on stability. Similarly, neglecting the motion of the arms could also have affected the placement of the threshold for balance loss. Moreover, the simulation results are likely dependent upon the assumed strength of the model, which may be sufficiently different to account for differences between previous studies (Table 1). The impact of these limitations will be studied in the future.
Despite these limitations, however, the credibility of the results is supported by a number of sources. First, the fact that solutions were not possible when simulations were started with COM states taken from within the region of balance loss supports that the dynamic optimization solutions converged to a true stability boundary, given the current modeling assumptions, and were not sub-optimal solutions. Second, the results for standing were quite similar to previously published values (Pai and Patton, 1997). This is encouraging because those values have been verified extensively with different computational (Hof et al., 2005) or empirical methods (Pai et al., 1998, 2000, 2003; Patton et al., 1999, 2000). Finally, experimental data for normal walking both from young and older adults were also supportive of the model simulation results, as the data were clearly beyond the thresholds for initiating a backward loss of balance (Fig. 2). Taken together, it is convincing that we have been able to determine a valid range of the minimum COM state values at the instant of toe-off in walking, above which a backward loss of balance can be avoided.
In summary, the present study determined the boundary against backward loss of balance for walking, which can be substantially different from that for symmetric bipedal standing. The present study also lays a foundation for using the current model, appropriately modified, to simulate the stability boundary against backward loss of balance during slips. Together, these results can provide insights for understanding the neuromechanical adaptive mechanisms employed by the CNS and the bases for constructing effective preventive strategies aimed at reducing incidence of slip-related falls.
Acknowledgements
This work was funded by NIH 2R01-AG16727 (YCP). The authors thank N. Chandran for his initial assistance in programming and Tanvi Bhatt, Ph.D., for editing the text.
References
- Anderson FC, Pandy MG. A dynamic optimization solution for vertical jumping in three dimensions. Computational Methods in Biomechanics and Biomedical Engineering. 1999;2:201–231. doi: 10.1080/10255849908907988. [DOI] [PubMed] [Google Scholar]
- Atkinson LV, Harley PJ, Hudson JD. Numerical methods with Fortran 77: A practical introduction. Addison-Wesley; UK: 1989. [Google Scholar]
- Bhatt T, Pai Y-C. Long-term retention of gait stability improvements. Journal of Neurophysiology. 2005;94:1971–1979. doi: 10.1152/jn.00266.2005. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai Y-C. Influence of gait speed on stability: recovery from anterior slips and compensatory stepping. Gait and Posture. 2005;21:146–156. doi: 10.1016/j.gaitpost.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Borelli GA. De Motu Animalium. Springer; Berlin: 1680. p. 1989. [Google Scholar]
- Corona A, Marchesi M, Martini C, Ridella S. Minimizing multimodal functions of continuous variables with the “Simulated Annealing” algorithm. ACM Transactions on Mathematica Softward. 1987;13:262–280. [Google Scholar]
- Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. Journal of Biomechanics. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Holbrook TL. The Frequency of Occurrence, Impact and Cost of Musculoskeletal Conditions in the United States. American Academy of Orthopedic Surgeons. 1984 [Google Scholar]
- Iqbal K, Pai Y. Predicted region of stability for balance recovery: motion at the knee joint can improve termination of forward movement. Journal of Biomechanics. 2000;33:1619–1627. doi: 10.1016/s0021-9290(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Jaeger RJ, Vantichatchavan P. Ground reaction forces during termination of human gait. Journal of Biomechanics. 1992;25:1233–1236. doi: 10.1016/0021-9290(92)90080-k. [DOI] [PubMed] [Google Scholar]
- McConville JT, Clauser CE, Churchill TD, Cuzzi J, Kaleps I. Anthropometric relationships of body and body segment moments of inertia. Air Force Aerospace Medical Research Laboratory, Wright-Patterson AFB; Ohio: 1980. (AFAMRL-TR-80-119) [Google Scholar]
- Morley JE. A fall is a major event in the life of an older person. Journal of Gerontology A: Biological Science and Medical Science. 2002;57:M492–M495. doi: 10.1093/gerona/57.8.m492. [DOI] [PubMed] [Google Scholar]
- Nashner LM. A Systems Approach to Understanding and Assessing Orientation and Balance. NeuroCOm International; Clackamas, OR: 1987. [Google Scholar]
- O’Sullivan SB. Motor control assessment. In: O’Sullivan SB, Schmitz TJ, editors. Physical Rehabilitation Assessment and Treatment. third ed F. A. Davis Co.; Philadelphia: 1994. pp. 121–125. [Google Scholar]
- Pai Y-C. Movement termination and stability in standing. Exercise and Sport Science Review. 2003;31:19–25. doi: 10.1097/00003677-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Pai Y-C, Iqbal K. Simulated movement termination for balance recovery: can movement strategies be sought to maintain stability even in the presence of slipping or forced sliding? Journal of Biomechanics. 1999;32:779–786. doi: 10.1016/s0021-9290(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. Journal of Biomechanics. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Pai YC, Maki BE, Iqbal K, McIlroy WE, Perry SD. Thresholds for step initiation induced by support-surface translation: a dynamic center-of-mass model provides much better prediction than a static model. Journal of Biomechanics. 2000;33:387–392. doi: 10.1016/s0021-9290(99)00199-2. [DOI] [PubMed] [Google Scholar]
- Pai YC, Rogers MW, Patton J, Cain TD, Hanke TA. Static versus dynamic predictions of protective stepping following waist-pull perturbations in young and older adults. Journal of Biomechanics. 1998;31:1111–1118. doi: 10.1016/s0021-9290(98)00124-9. [DOI] [PubMed] [Google Scholar]
- Pai YC, Wening JD, Runtz EF, Iqbal K, Pavol MJ. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. Journal of Neurophysiology. 2003;90:755–762. doi: 10.1152/jn.01118.2002. [DOI] [PubMed] [Google Scholar]
- Pandy MG, Anderson FC, Hull DG. A parameter optimization approach for the optimal control of large-scale musculoskeletal systems. Journal of Biomechanical Engineering. 1992;114:450–460. doi: 10.1115/1.2894094. [DOI] [PubMed] [Google Scholar]
- Patton JL, Pai Y-C, Lee WA. Evaluation of a model that determines the stability limits of dynamic balance. Gait Posture. 1999;9:38–49. doi: 10.1016/s0966-6362(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Patton JL, Lee WA, Pai YC. Relative stability improves with experience in a dynamic standing task. Experimental Brain Research. 2000;135:117–126. doi: 10.1007/s002210000500. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for a potential loss of balance. Experimental Brain Research. 2002;145:528–538. doi: 10.1007/s00221-002-1143-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Annals of Internal Medicine. 1994;121:442–451. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- Smeesters C, Hayes WC, McMahon TA. Disturbance type and gait speed affect fall direction and impact location. Journal of Biomechanics. 2001;34:309–317. doi: 10.1016/s0021-9290(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. Journal of Gerontology A: Biological Science and Medical Science. 1998;53:M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- Topper AK, Maki BE, Holliday PJ. Are activity-based assessments of balance and gait in the elderly predictive of risk of falling and/or type of fall? Journal of American Geriatric Society. 1993;41:479–487. doi: 10.1111/j.1532-5415.1993.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Wolfson LI, Whipple R, Amerman P, Kleinberg A. Stressing the postural response. A quantitative method for testing balance. Journal of American Geriatric Society. 1986;34:845–850. doi: 10.1111/j.1532-5415.1986.tb07256.x. [DOI] [PubMed] [Google Scholar]