Abstract
Evolving knowledge regarding sex differences in coronary heart disease (CHD) is emerging. Given the lower burden of obstructive coronary artery disease (CAD) and preserved systolic function in women contrasted by higher rates of myocardial ischemia and near-term mortality compared to men, we propose the term ischemic heart disease (IHD) as appropriate for this discussion specific to women, rather than CAD or CHD. This paradoxical difference where women have lower rates of anatomical CAD but more symptoms, ischemia, and outcomes appear linked to coronary reactivity which includes microvascular dysfunction. Novel risk factors can improve the Framingham risk score, including inflammatory markers and reproductive hormones, as well as noninvasive imaging and functional capacity measurements. Risk for women with obstructive CAD is elevated compared to men, yet women are less likely to receive guideline-indicated therapies. In the setting of non-ST elevation acute myocardial infarction, interventional strategies are equally effective in biomarker positive women and men, while conservative management is indicated for biomarker negative women. For women with evidence of ischemia but no obstructive CAD, anti-anginal and anti-ischemic therapies can improve symptoms, endothelial function, and quality of life; however trials evaluating adverse outcomes are needed. We hypothesize that women experience more adverse outcomes compared to men because obstructive CAD remains the current focus of therapeutic strategies. Continued research is indicated to devise therapeutic regimens to improve symptom burden and reduce risk in women with IHD.
Keywords: Ischemic Heart Disease, Sex Differences, Women
Over the past several decades, an evolving knowledge regarding sex differences in coronary heart disease (CHD) has emerged. Prevalence, symptom manifestation, and pathophysiology for CHD vary between women and men. Annual CHD population statistics continue to report a higher number of deaths for women than men (455,000 versus 410,000) (1). While recent reports document declines in CHD mortality for females, reductions lag behind those realized for men (2), including mortality increases among younger women (3). The most recent Center for Disease Control data reveal that 1 in 2.6 women die from CHD contrasted to 1 in 4.6 from cancer (4). Current projections indicate a continued increase in CHD given our aging population and epidemics of obesity, diabetes, and the cardiometabolic syndrome (1,2,5-6). Notably, cardiac death remains the leading killer of women at all ages (1,7-8).
Among clinical cohorts, paradoxical sex differences are observed where women have less anatomical obstructive coronary artery disease (CAD) and relatively preserved left ventricular function yet higher rates of myocardial ischemia and mortality compared to similarly-aged males (5,9-11). Accordingly, the term ischemic heart disease (IHD) is more appropriate for a discussion specific to women; rather than CAD or CHD. Data from the NIH-NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) and related studies implicate adverse coronary reactivity (12), microvascular dysfunction (13), and plaque erosion/distal micro-embolization (14-15) as contributory to a female-specific IHD pathophysiology. Thus, knowledge beyond an anatomical description of obstructive CAD may provide important clues to IHD risk detection and treatment for women.
This review outlines our evolving knowledge of pathophysiology and mechanisms of IHD in women. We include clinical studies addressing gender-specific issues in IHD prevalence and prognosis, traditional and novel risk factors, screening and diagnostic testing, as well as therapeutic management strategies. We propose models for application of our emerging knowledge on IHD in women to clinical practice, as well as forward novel hypotheses for investigation. Finally, while it is unknown to what extent the described issues are female-specific or simply more prevalent in women, it is likely that the outlined concepts should also be applicable for men.
IHD Prevalence in Women
In addition to an absolute greater number of women dying from IHD, a higher proportion of women die of sudden cardiac death prior to hospital arrival (52%) contrasted with 42% of men (16-17). Recent data report significant declines in sudden cardiac death in men with (essentially) no change in women (18). Symptomatic women more often have persistent symptoms requiring more hospitalizations compared to men, accompanied by lower ratings of general well-being and limitations in their abilities to perform activities of daily living (19-20). Notably, these adverse outcomes are experienced by women of all ages despite a lesser extent and severity of obstructive CAD and better systolic function compared to men (11). Relatively higher CAD healthcare costs are incurred in women where resource consumption patterns are characterized by: 1) more frequent diagnoses of angina, office visits, and hospitalizations; 2) higher MI mortality; and 3) higher rates of heart failure hospitalization as compared with men (22-24).
Thus, IHD in women presents a unique and difficult challenge for clinicians due to a greater symptom burden, functional disability, greater healthcare needs, and more adverse outcomes as compared to men despite a lower prevalence and severity of anatomical CAD.
IHD Risk Factors in Women
Over 80% of midlife women have 1 or more traditional cardiac risk factors (25). Women have higher (average) blood cholesterol levels than men after their 5th decade of life (10) and exhibit mild declines in HDL-cholesterol following menopause (1,26). Obesity is prevalent in one-third of women including 7% having a body mass index (BMI) ≥40 kg/m2 with associated increased mortality (27-28). Hypertriglyceridemia is a more potent independent risk factor for women as compared to men (26, 29). Diabetic women have significantly higher IHD mortality compared with diabetic men (30-31), and an elevated 3.3-fold IHD risk compared to non-diabetic women (32). Importantly, 30-year trends reveal marked CVD mortality reduction for diabetic men but not for diabetic women (33).
IHD mortality increases with the number of traditional cardiac risk factors, with 30-year death rates (per 10,000 person-years) ranging from 1.5 to 9.1 for women with 0 to ≥2 risk factors (34). Clustering risk factors is common post-menopause, notably the combination of obesity, hypertension, and dyslipidemia (35-39); potentially related to hormonally-mediated metabolic disturbances.
Novel Risk Factors for IHD in Women
Traditional risk factors and the Framingham risk score (FRS) underestimate IHD risk in women (40-45), while novel risk markers improve risk detection (13,46,48-49). Females have, on average, higher mean C-reactive protein (CRP) measures compared with males; a sex difference apparent at the time of puberty (50). This difference in CRP is consistent with the 2- to 50-fold greater frequency of inflammatory-mediated autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosis, in women as compared to men (51) suggesting a prominent role for inflammation in IHD sex differences. Indeed, the relative risk of future IHD events increases proportionally with rising levels of high sensitivity CRP (hsCRP), acting synergistically with other risk factors to accelerate IHD risk in women (47,48,52-55). A number of inflammatory measures, including hsCRP, are related to other IHD risk markers such as the cardiometabolic syndrome, type 2-diabetes, and heart failure (53,56,57). The use of multiple biomarkers improves IHD risk assessment in women (58-60).
We and others have further demonstrated that disruption of ovulatory cycling, indicated by estrogen deficiency and hypothalamic dysfunction (61) or irregular menstrual cycling (62) in premenopausal women is associated with an increased risk of coronary atherosclerosis and adverse CVD events. Polycystic ovary syndrome (PCOS) is prevalent in 10-13% of females and is linked with a clustering of risk factors, incident type-2 diabetes mellitus (63), and adverse IHD events postmenopausally (64). The cardiometabolic syndrome is a clustering of risk factors including at least 3 of the following: insulin resistance, dyslipidemia (elevated triglycerides, low HDL cholesterol), hypertension, or abdominal obesity and is frequently associated with alterations in endogenous estrogens and androgens in women (36,62,65).
Investigation into the optimal utilization of novel risk factors for IHD risk stratification in women is needed.
Risk Assessment in Women Using Traditional Risk Factors and Scores
The FRS is used to classify patients’ 10-year risk of CAD death or MI to determine the appropriate level of therapeutic intervention for both LDL cholesterol and hypertension (66-67). Patients at highest risk should receive the most intensive therapeutic and lifestyle recommendations (i.e., secondary prevention goals). However, the FRS classifies >90% of women as low risk, with very few assigned high risk status before age 70 (41). The FRS is best used to risk stratify populations and underestimates individual patient risk, notably for women (43-45).
The Reynolds risk score is a sex-specific tool recently devised from large derivation (n=24,588) and validation (n=8,158) cohorts of women (68). This score uses the following equation: 0.0799 × age + 3.137 × natural logarithm (systolic blood pressure) + 0.180 × natural logarithm (hsCRP) + 1.382 × natural logarithm (total cholesterol) − 1.172 × natural logarithm (high-density lipoprotein cholesterol) + 0.134 × hemoglobin A1c (%) (if diabetic) + 0.818 (if current smoker) + 0.438 (if family history of premature MI). When compared to the FRS, use of the Reynold’s score resulted in risk re-classification in >40% of intermediate FRS women (68).
Several recent reports have also examined the prevalence of subclinical atherosclerosis within female FRS subsets (43-44). In a recent cross-sectional study of 2,447 consecutive, clinically-referred asymptomatic, non-diabetic women, 84% of those with significant coronary artery calcification (CAC) were classified with a low FRS (43). These data underscore the imprecision of FRS estimates in women and the prevalent, undetected burden of atherosclerosis in females.
Noninvasive Imaging of Atherosclerosis
There is a growing body of evidence on the use of atherosclerotic imaging. In women, the prevalence of an ankle brachial index ≤0.90 increases with age (ranging <5% for <60 years to 10-35% for 60-80 years) and is more prevalent in Black and Hispanic females (69-70). The hazard for death with an ankle brachial index ≤0.90 is 2.7 (95% CI=2.0-3.6) for women and 3.3 (95% CI=2.7-4.1) for men (71). Carotid intima-media thickness (cIMT) is another imaging marker that is a validated measure of risk for both women and men (72-74). A low risk cIMT is associated with a ~1% 10-year IHD risk vs. ~10% for a high risk cIMT (75); with a relatively higher risk predicted for women than men (76). CAC is another imaging measure that is highly correlated with traditional risk factors (77) but uncorrelated with hsCRP (78). CAC lags by nearly a decade in incidence for women; similar to obstructive CAD (49,79-84). From the NHLBI Multi-Ethnic Study of Atherosclerosis study (44), women with a CAC Score ≥300 had an annual IHD event rate of 2.2%; thus achieving NCEP CHD risk-equivalent status. The IHD event risk for women with a high risk CAC score and multiple risk factors is 10% higher in women than men (49,83); supporting the notion that comorbidity disproportionately accelerates risk in women.
Symptom Assessment and Prevalence of Ischemia in Women
Evaluation of women with symptoms suggestive of IHD is hampered by the definition of “typical” angina, derived from largely male populations where exertional components are more reflective of male patterns of presentation (85-86). Women report more angina despite lower rates of obstructive CAD (11,87-89). In a recent meta-analysis of 74 reports from 13,311 women and 11,511 men, angina prevalence was 11-27% higher for women <65 years of age yet similar in the elderly ≥75 years (90). Women with typical or atypical chest pain symptoms (non-exertional or prolonged discomfort unrelieved by rest) have calculated obstructive CAD probabilities substantially less than that of men (91-93) and among those undergoing coronary angiography, as much as 50% of females do not have obstructive CAD (93-94). Over half of symptomatic women without obstructive CAD continue to have signs and symptoms of ischemia, undergo repeat hospitalization and coronary angiography, with continued consumption of CAD healthcare resources often due to diagnostic and therapeutic uncertainty (20,24). Data from the Women’s Health Initiative document that women with non-specific chest pain have a 2-fold higher risk for nonfatal MI (95), while WISE data demonstrate elevated mortality in women with chest pain and no obstructive CAD (96), underscoring that prognosis in these women is not benign.
“Normal” coronary angiograms, defined as no visible obstructive CAD (luminal irregularities <50% stenosis) are also reported more frequently in women with acute coronary syndromes (ACS). In a recent large series from 600 US hospitals in 459,941 acute coronary syndrome (ACS) patients, the adjusted odds for obstructive CAD were 50% lower for women as compared to men (11). For women presenting with ACS/ST segment elevation MI (STEMI), 10-25% of women as compared to 6-10% of men have no obstructive CAD (97-100). Of the estimated 1.4 million patients discharged following an ACS each year, 600,000 are women (1). Among women, the 10-25% rate of “normal” angiography (101) translates into 60,000-150,000 women with ACS/MI having nonobstructive CAD. Specific investigation is needed to understand the paradox whereby women have less obstructive CAD and less severe MIs yet worsening clinical outcomes. The higher mortality compared to men has been attributed to advanced age, comorbidity (5,10,102,103), and underutilization of guideline care among women (104); yet the largest mortality gap is seen in younger women with a number of studies demonstrating persistent sex differences despite covariate adjustment (105-106).
Exercise ECG in Women
Clinicians often rely on exercise electrocardiography (ECG) to assess IHD risk. The exercise ECG has a lower sensitivity and specificity (≥1 mm ST segment depression ≅65%) for detection of obstructive CAD in women compared to men (107), in part due to the lower obstructive CAD prevalence (i.e., Bayesian theory). In several large female cohorts, significant exertional ST segment depression did not differ between survivors and non-survivors (108-109); although marked ST segment changes (≥2 mm horizontal or downsloping) occurring at low workloads or persisting into recovery confirm high risk status for women (110). Combining variables such as exercise duration and ST segment changes into the Duke Treadmill Score (DTS) accurately predicts IHD mortality in women (111-112). From the St. James Women Take Heart Study of 5,392 asymptomatic women, the risk of death decreased by 9% for every unit increase in the DTS; while each MET increase in exercise capacity decreased mortality by 17% (p<0.001) (111). Women undergoing exercise testing using common treadmill protocols are often incapable of performing >5 metabolic equivalents (METs) (112), a level equivalent to performing routine activities of daily living (113), elevating their risk of IHD death or MI by ~3-fold (108-110,114). Reduced functional capacity (≤7 METs) portends worsening outcome equally among lean and obese women (115). A female sex-specific nomogram of exercise capacity (in METs) has been devised and can be applied to estimate average functional abilities for women of diverse ages (116).
Noninvasive Cardiac Imaging in Women
Stress-induced changes in regional myocardial perfusion or wall motion are accurate markers of IHD risk in women (110,117-120). Although the sensitivity of echocardiographic wall motion abnormalities is diminished in the setting of an intermediate stenosis or single vessel obstructive CAD, the test’s high negative predictive value renders it useful for younger women (110). Stress-induced changes in myocardial perfusion have been extensively evaluated in women; largely employing SPECT imaging with more recent use of positron emission tomography (PET) and cardiovascular magnetic resonance (CMR) techniques (110).
The evidence is substantial that myocardial perfusion imaging effectively risk stratifies women (110,119-120). Pooled myocardial perfusion data in >7,500 women reveals a low annual IHD event rate of 0.6% in the setting of a normal study (119). Survival worsens for women with multivessel ischemia (120) or moderate-severe perfusion abnormalities yielding a 5% annual IHD mortality for women (121). Because SPECT flow is comparatively assessed across the myocardium, it can appear normal in the setting of global reductions in perfusion attributable to severe multivessel CAD but also to endothelial or microvascular dysfunction, left ventricular hypertrophy, or cardiomyopathy. Additional challenges for SPECT in women include: 1) limited spatial resolution where minor perfusion abnormalities may go undetected in smaller hearts and 2) breast tissue artifact. With regards to the latter, contemporary techniques using Tc-99m agents, prone imaging, and/or attenuation correction algorithms diminish artifact frequency (110). Thus, it is no longer appropriate to label perfusion abnormalities in the setting of nonobstructive CAD as “false positives” in women if accompanied by objective signs of ischemia, such as chest pain, electrocardiographic abnormalities, or reduced functional capacity due to the elevated IHD risk (52,106)The use of 82Rb PET has several advantages in women including quantification of absolute values of regional and global myocardial blood flow to assess microvascular disease (flow reserve) and integrated attenuation correction along with improved image quality compared to SPECT. PET has notable advantages for obese women however there is limited prognostic data with no gender-specific reports (122-123). As based on recent estimates, effective radiation dose appears slightly higher for PET when compared to single-isotope rest-stress SPECT imaging (12.6-13.5 for 82Rb PET vs. 11.3-11.4 for rest-stress Tc-99m SPECT) (124).
Stress CMR imaging uniquely allows measurement of subendocardial perfusion. In an initial report in 19 symptomatic women with abnormal stress tests and normal coronaries, subendocardial ischemia was frequently observed (125). These findings have been validated in a larger cohort reporting a strong correlation between subendocardial ischemia and abnormal coronary reactivity testing (126); although population heterogeneity has resulted in varying results (127). Investigation into the prognostic implications of CMR subendocardial ischemia with regard to IHD events and its association with future chest pain frequency and stability is needed.
Coronary computed tomographic angiography (CCTA) is a noninvasive anatomic technique with a reported high diagnostic accuracy for obstructive CAD (128-129). In a series of 51 women and 52 men, diagnostic sensitivity and specificity was similar by gender at 85% and 99% (130); although a recent larger controlled trial demonstrated a lower specificity of 90% (131). An important limitation for CCTA, and all tests of ionizing radiation exposure, is that imaging should be used cautiously in younger women due to a heightened lifetime cancer risk. CCTA is associated with effective radiation doses that average 11.3 mSv for men and 12.7 mSv for women (124). Test protocols emphasizing reductions in radiation exposure, including ECG-controlled tube current modulation, prospective gating, minimization of scan length, and optimization of tube current and voltage, should be emphasized in women. Moreover, especially for younger women, caution should be applied to use of testing that involves ionizing radiation and, in some cases, use of stress echocardiography or magnetic resonance imaging techniques may be favorable, in particular for younger women.
Importantly, women with angina and confirmatory ischemia have an elevated IHD mortality (106). In a recent report from an ambulatory population (n=56,441 women and 34,885 men), the coronary standardized mortality ratio was ~2-fold higher for females 55-74 years and increased to 12-fold higher for those aged 45-54 years (132).
In summary, abnormalities in functional capacity and noninvasive imaging are valuable IHD risk predictors in symptomatic women. Further work is needed to integrate the use of existing and novel strategies to optimize IHD risk detection in women.
Coronary Reactivity in Women
Women suffer disproportionately from a variety of generalized vascular disorders, including migraine headaches, Raynaud’s phenomenon, and autoimmune arteritis. These observations support the influence of lifelong, varying reproductive hormone levels related to ovarian cycling, pregnancy, peri-partum, and menopause are likely related to vascular function in health and disease (133). While knowledge regarding the role of coronary reactivity was historically confined to Prinzemetal’s angina, characterized by abnormal proximal epicardial coronary artery vasospasm modulated by smooth muscle dysfunction (134), it is now clear that intra-myocardial microvascular arteries (135) mediated by endothelial (136) and autonomic nervous system adrenergic pathways (137) are involved.
Microvascular Dysfunction
Recent data support a gender-specific role for coronary microvascular dysfunction in IHD pathophysiology. Autopsy data from sudden cardiac death victims suggest that women have a higher frequency of coronary plaque erosion and distal embolization compared to men (14-15,138-141). Retinal arterial narrowing, a measure of microvascular disease, is related to CVD events in women but not men (13). Additional important sex differences in the arterial remodeling/repair response to injury/atherosclerosis may prove etiologic for the development of microvascular dysfunction in women. Although the onset of atherosclerosis for women temporally lags behind that of men, evidence that the combination of smaller arterial size and more prominent positive remodeling (49,83,142) may lead to a greater role of microvascular dysfunction in IHD in women compared with men (143). Recently Han et al. (144), studied patients with obstructive CAD who underwent simultaneous intravascular ultrasound (IVUS) and coronary reactivity assessment and demonstrated that men have a greater atheroma burden and more diffuse epicardial endothelial dysfunction while women have more disease of the microcirculation. These factors may influence the higher rates of angina, ischemia, and ACS in the absence of obstructive CAD in women supporting coronary microvascular dysfunction as a prominent disorder in women compared to men (113,143).
Endothelial Dyfsunction
Endothelial function (measured centrally in the coronary or distally in the peripheral circulation) contributes to IHD pathophysiology in women. Brachial artery flow-mediated dilation, a peripheral measure of endothelial function, is impaired in hyperlipidemic, hypertensive, smokers, and diabetics (145), and exacerbated post-menopausally (146). Abnormal flow mediated dilation in a large cohort of 2,264 post-menopausal women was associated with a 1.3- to 4.4-fold increased IHD risk (p<0.0001) (147). Whether endothelial dysfunction mechanistically is a precursor to the development of hypertension, a marker for subclinical atherosclerosis, a measure of obstructive CAD severity, or related to left ventricular remodeling and diastolic dysfunction is unknown (5,148-149).
In the coronary circulation, both endothelial-dependent epicardial (endothelial dysfunction) and endothelial-independent (microvascular dysfunction) dysfunction predict adverse IHD events in patients undergoing diagnostic angiography, single vessel percutaneous coronary angioplasty (PCI), or post ACS/MI (150-153). These results are important because restoration of endothelial function is associated with improved outcome. In a study of 400 hypertensive post-menopausal women, improved endothelial function was associated with a 7.3-fold lower rate of IHD events when compared to women with no improvement (154).
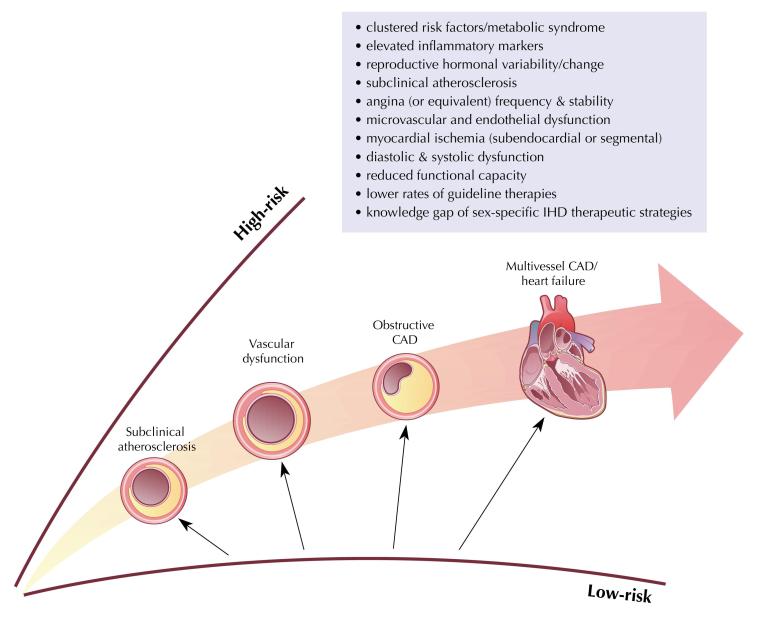
The role that coronary reactivity dysfunction plays in ischemia in women without obstructive CAD has only now been described and the relative importance of endothelial and microvascular dysfunction has been insufficiently explored. An integrated working understanding of the cascade of mechanisms and manifestations of ischemia impacting IHD risk in women is reviewed in Figure 1.
Figure 1.
Cascade of Mechanisms and Manifestations of Ischemia Impacting IHD Risk in Women
Unifying Novel Hypotheses of IHD in Women
We propose that coronary microvascular dysfunction is more prevalent in women than men due to risk factor clustering and hormonal alterations and is etiologic for the observed paradoxical frequent (atypical) symptoms, evidence of ischemia, and adverse outcomes. We propose that symptoms occurring due to coronary microvascular dysfunction which result in myocardial ischemia should be called microvascular angina. A hypothetical model of microvascular angina in women is depicted in Figure 2. This model provides a rationale for why current approaches for detection of focal obstructive coronary lesions are less effective in women with a greater prevalence of nonobstructive CAD. Abnormal coronary reactivity occurs in the setting of underlying atheroma vulnerable to clinical instability and more progressive disease states. It is for this reason that identifying nonobstructive atheroma may provide greater risk stratification in women. An overarching working model of this proposed female-specific IHD pathophysiology is depicted in Figure 3. While the relationship between microvascular dysfunction and epicardial atherosclerosis is not fully understood, a leading hypothesis is that it is a single disease process, where response to intimal injury may vary related to sex differences in vascular remodeling and vascular reactivity.
Figure 2.
Model of Microvascular Angina in Women. HTN=hypertension, PCOS=polycystic ovary syndrome.
Figure 3.
Overarching Working Model of IHD Pathophysiology in Women. Abbreviations as previous.
Prognosis in Women with IHD
A consistent pattern in the literature is a higher mortality in women compared to men with acute MI (155-157). In the Thrombolysis In Myocardial Infarction (TIMI)-II trial, significantly higher rates of death and re-infarction were observed in women compared to men at 6-weeks and 1-year, even following adjustment for age and comorbidity (158-159). The National Registry of Myocardial Infarction-2 analyzed data from 384,878 patients demonstrating that among younger patients (<50 years of age), adjusted mortality for women was more than twice that of men (105). The Primary Angioplasty in Myocardial Infarction (PAMI) trial demonstrating that primary PCI post-MI reduced the risk of intracranial bleeding resulting in comparable survival by gender; in contrast to patients treated with t-PA where in-hospital mortality from acute MI was 3.3-fold higher in women than men (160). Although absolute mortality reduction in MI patients treated with fibrinolytic therapy is similar by gender, there is a higher mortality following reperfusion with fibrinolytic therapy in women of all ages (161).
Prognosis in Women with Obstructive CAD
In women undergoing invasive coronary angiography, those with obstructive CAD have a 1.7 to 2.0-fold higher odds of in-hospital mortality as compared to nonobstructive CAD (p=0.013) (11). In-hospital mortality is highest for ACS women ranging from 22-38% for those with 1-3 vessel CAD (p<0.0001). The higher short-term mortality includes more frequent complications of reinfarction and higher procedural complications; with older age, more diabetes, and greater comorbidity contributory (5,103,113,162-163). In a recent post-infarction trial, there was a borderline increased risk of sudden cardiac arrest and resuscitated cardiac arrest occurring within the first week post-MI for women (p=0.08), suggesting a higher acute post-MI instability in women (164).
Prognosis in Women with Nonobstructive CAD
The prognosis with “normal” coronary arteries co-occurring with signs and symptoms of myocardial ischemia has historically been interpreted as benign (165-167). More recent prognostic data in patients with ACS and non-obstructive CAD does not appear to be consistent with these historical findings and notes a 2% risk of death and myocardial infarction at 30 days of follow-up (168). Notably, while a majority of these subjects were women, these datasets include men with nonobstructive CAD and comparative analyses by sex are needed. A recent investigation demonstrated that 30% of women with chest pain, “normal” angiograms, and endothelial dysfunction developed obstructive CAD during 10-year follow-up (169). A pooled analysis of women from recent, large randomized trials reveals that women with mild CAD have a worsening prognosis as compared to those with normal coronaries (170). Recently, Gulati and colleagues (96) reported 5-year CVD event rates of 16.0% for those with mild CAD (stenosis 1-49%), 7.9% for those with no coronary stenosis, and 2.4% in asymptomatic women (p≤0.002); following adjustment of cardiac risk factors. Despite these compelling findings, treatment for women with open coronary arteries remains often reassurance, sedative-hypnotic prescriptions, and/or repeated hospitalization and coronary angiography in response to refractory symptoms (97).
Given the sizeable gap in IHD prognosis between women and men, further research into sex-specific pathophysiology is needed. A model summarizing the factors known to contribute to the prognostic risk of IHD events in women with and without obstructive CAD is depicted in Figure 4.
Figure 4.
Factors Impacting Risk of IHD Events in Women. Abbreviations as previous.
Treatment of Women with IHD
Invasive Strategies for ACS in Women
For women with ACS, existing evidence-based guidelines support a stratified invasive vs. conservative strategy for high and low risk women (171). Data from a recent meta-analysis of 8 ACS trials (3,075 women and 7,075 men) compared risk reduction using an invasive compared to a conservative strategy (172). For both women and men, an invasive strategy resulted in an equivalent 19-27% relative risk reduction using a composite endpoint of death, MI, or repeat ACS. There were, however, important differences in risk reduction between biomarker-positive and -negative women. The invasive strategy was associated with a 33% lower risk of the composite endpoint in biomarker-positive women in contrast to a higher risk in biomarker-negative women, a difference that was not evident in men. Similarly, although women and men with ACS derive similar benefit from drug-eluting stents (174), women have an overall higher mortality with PCI for STEMI and non-STEMI (173).
Conservative Strategies for ACS in Women
Following fibrinolysis, the 30-day incidence of death or nonfatal MI was significantly lower in women compared to men in the enoxaparin group compared to unfractionated heparin (161), suggesting that sex differences may beneficially impact outcomes in women for specific therapies. For both women and men undergoing PCI, despite higher bleeding risk in women, the clinical benefit of glycoprotein IIb/IIIa platelet receptor blockade with abciximab for adverse events is similar (175). Overall, among patients with ACS treated with IIb/IIIa receptor blockade (not undergoing early coronary angiography), men experienced a benefit with an odds ratio (OR) of 0.81 (95% CI=0.75-0.89) compared to a suggestion of harm in women (OR=1.15, 95% CI=1.01-1.30); although high risk women with elevated troponins did derive a benefit (176). Prior studies document that women’s higher risk of bleeding is due in part to lack of dose adjustment to body size and renal function compared to men (177). A sex difference in bleeding risk was not observed when doses were adjusted for age and renal function (175). From a large international registry, women with ACS were generally treated less aggressively, including less acute heparin, angiotensin-converting enzyme inhibitors, and glycoprotein IIb/IIIa inhibitors and lower rates of discharge with aspirin, angiotensin-converting enzyme inhibitors, and statins as compared to men (104). Application of guideline-indicated therapy post-ACS is associated with abolishment of the adverse mortality gap in women (178).
Medical Therapy for IHD in Women
As noted above, one factor contributing to relatively higher IHD risk in women is less intensive use of indicated medical therapy (aspirin, beta-blocker, statin, ACE, therapeutic lifestyle counseling) (179-183); despite specific guidelines noting their benefit (6). The Cooperative Cardiovascular Project (184) showed that women received less medical treatment post-MI, including 5% less aspirin at discharge; although they were 5% more likely than men to receive ACE inhibitor, perhaps due to hypertension. A more recent registry (104) indicates that this observation has not changed with women receiving less (indicated) discharge aspirin (87.5% vs. 90.4%), beta-blockers (80.5% vs. 82.7%), and statins (55.9% vs. 69.4%) compared to men.
Treatment of Women with Obstructive CAD
Undertreatment of women has been attributed to the lower prevalence of obstructive CAD. Recent data from the Euro Heart Survey of Stable Angina reported that women with CAD less likely received coronary revascularization (OR=0.70 [95% CI=0.52-0.94], p=0.019) and were less often on lipid-lowering therapy at 1-year follow-up (76% vs. 81%, p=0.05), despite adjustment for an array of clinical factors (185). In contrast, the CRUSADE registry (104) revealed similar rates of PCI among women and men after accounting for the severity of angiographic CAD (adjusted OR=0.97, 95% CI=0.91-1.03). The GRACE study investigated women with obstructive CAD and demonstrated less use of aspirin (95% vs. 96%), beta-blockers (87% vs. 89%), and statins (75% vs. 77%) compared to men (186). The recent COURAGE trial demonstrated that women with CAD and chronic stable angina derive an equal benefit from intensive, long-term medical therapy and with no added benefit of PCI (187) (Figure 5).
Figure 5.
Relative hazard (95% confidence intervals) for death or myocardial infarction for women and men enrolled in the Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation (COURAGE). OMT+PCI=Optimal medical therapy with percutaneous coronary intervention, OMT=Optimal medical therapy. Reprinted with permission.
Thus, the weight of the evidence indicates suboptimal treatment of women with proven obstructive CAD (188), despite evidence and guidelines supporting effective risk reduction when applying acute, revascularization and/or chronic medical therapies (6,189-191).
Treatment of Women with Ischemia and Nonobstructive CAD
Much of the evidence of treatment in women with nonobstructive CAD has focused on improvement in symptoms or vascular function. Many anti-ischemic therapies have been evaluated including data that calcium antagonists reduce coronary flow reserve and fail to improve symptoms (192). Beta-blockers, however, are highly effective for improving chest pain symptoms (193). No controlled studies are available on the effects of nitrates on health status outcomes in women. Statins and ACE-inhibitors improve endothelial dysfunction (194-195) and may be of benefit in patients with nonobstructive CAD (194-196). Beneficial effects of statins on the coronary microcirculation have been documented in clinical studies (197). Combinations of drugs, specifically statins and ACE-inhibitors, may amplify these benefits (194). However, combination therapy to more fully attenuate the renin-angiotensin aldosterone system has not been explored; additional work is required to determine the translational value of this treatment. The proven benefit of exercise training in this population (198) suggests that mechanisms of adrenergic modulation play a role.
Novel therapies have been evaluated in women without obstructive CAD. Imipramine improves symptoms in patients with abnormal cardiac pain perception and normal coronary angiograms; possibly through a visceral analgesic effect. It also has anticholinergic and alpha antagonist effects demonstrated both in the coronary and peripheral circulation (199). Six-month supplementation of L-arginine improved endothelial function and symptoms in patients with nonobstructive CAD (200); although a recent post-MI trial demonstrated adverse effects of L-arginine questioning its safety (201). Menopausal hormone therapy may improve emotional well-being in postmenopausal women with angina and “normal” angiograms yet there is no symptom benefit for these patients (202).
No randomized trials comparing therapies for risk reduction and cost effectiveness in women with angina/ischemia and “normal” coronary arteries have been conducted. Future IHD research will need to specifically characterize patients as to the pathophysiological mechanism(s) of disease, with regard to the presence or absence of coronary microvascular dysfunction, in order to devise optimal clinical trials aimed at improved IHD risk and health status outcomes.
Summary
Given the relatively lower prevalence of obstructive CAD yet the notably higher prevalence of ischemia, symptom burden, and mortality relative to men, we propose use of the term IHD as more appropriate for symptomatic women in lieu of terms, CAD or CHD. Traditional risk factors contribute to accelerating risk for IHD events in women, and novel risk markers, including inflammatory markers and reproductive sex hormones, provide unique value for identifying at-risk women. More recent specific global risk scores for women, such as the Reynold’s risk score, and markers of subclinical atherosclerosis improve risk detection. Routinely available diagnostic testing can be used to accurately risk stratify women, however identification of compromised functional capacity and evidence of ischemia as markers of an adverse prognosis are particularly important. Given the frequent paradoxical findings of angina and ischemia in women without obstructive CAD, new data support the use of the term microvascular angina to reflect the occurrence of microvascular dysfunction in IHD pathophysiology in women; models linking these findings with symptoms, ischemia, and adverse outcomes should be tested. For ACS, new sex-specific guidelines indicate that conservative management is indicated for biomarker negative women however, interventional strategies are equally effective in biomarker positive women and men. Yet, the weight of evidence documents suboptimal use of evidence-based guideline therapies in women with IHD compared to men. Anti-anginal and anti-atherosclerotic strategies are effective for symptom and ischemia management in symptomatic women with evidence of ischemia and no obstructive CAD, however are infrequently used and need to be evaluated in large outcome trials. The evolving knowledge regarding sex differences in IHD appears to be at the precipice of our understanding; future investigation should identify tailored diagnostic and therapeutic strategies to optimize outcomes for women and men (203).
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, R01 HL090957-01A1, R03 AG032631-01, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey, the Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Disclosure: Leslee J. Shaw: Grant support from GE Healthcare and Bracco Diagnostics
Abbreviations
- ACE
angiotensin converting enzyme inhibitor
- ACS
acute coronary syndrome
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CHD
coronary heart disease
- hsCRP
high sensitivity C-reactive protein
- CVD
cardiovascular disease
- FRS
Framingham Risk Score
- HDL
high density lipoprotein
- IHD
ischemic heart disease
- MI
myocardial infarction
- NIH-NHLBI
National Institutes of Health-National Heart, Lung and Blood Institute
- PCI
percutaneous coronary intervention
- STEMI
ST segment myocardial infarction
- TIMI
Thrombolysis In Myocardial Infarction
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Raffaelle Bugiardini: None
C. Noel Bairey Merz: Consulting for Novartis, Karolinska Institute, Strategy Group, University of Pittsburgh, Pfizer, BSP, Kendle Internation, Inc., NHLBI; Lecture Honorarium for Northwestern University, University of California-Davis, Abbott Labs, CV Therapeutics, Boehringer Ingelheim, American College of Physicians, ProMedica, Mayo Clinic, Merck; and Stock in Boston Scientific, Medtronic, Johnson and Johnson, and Teva Pharmaceuticals.
References
- 1. [access date: August 25, 2008]; http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5106a3.htm.
- 2.Heron MP, Hoyert DL, Xu JQ, Scott C, Tejada-Vera B. Deaths: preliminary data for 2006. Natl Vital Stat Rep. 2008;56(16) [Google Scholar]
- 3.Ford ES, Capewell S. Coronary Heart Disease Mortality Among Young Adults in the U.S. From 1980 Through 2002: Concealed Leveling of Mortality Rates. J. Am. Coll. Cardiol. 50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. originally published online Nov 12, 2007. [DOI] [PubMed] [Google Scholar]
- 4. [access date: November 30, 2008]; http://circ.ahajournals.org/cgi/reprint/CIRCULATIONAHA.107.187998.
- 5.Merz CN Bairey, Shaw LJ, Reis SE. Ischemic Heart Disease in Women: Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Part II: gender-differences in presentation, diagnosis, and outcome with regard to sex-based pathophysiology of atherosclerosis, macro- and micro-vascular CAD. J Am Coll Cardiol. 2006;47(Suppl):21s–29s. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK, Expert Panel/Writing Group Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007 Mar 20;49(11):1230–50. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Smith SC, Jr, Cooper RS, Hill MN, Luepker RV. Task force #1--magnitude of the prevention problem: opportunities and challenges. 33rd Bethesda Conference. J Am Coll Cardiol. 2002;40:588–603. doi: 10.1016/s0735-1097(02)02082-x. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 9.Moriel M, Rozanski A, Klein J, Berman DS, Merz CN Bairey. Women, prognosis and coronary artery disease: the limited efficacy of exercise radionuclide ventriculography. Am J Cardiol. 1995;76:1030–5. doi: 10.1016/s0002-9149(99)80290-2. [DOI] [PubMed] [Google Scholar]
- 10.Shaw LJ, Merz Bairey CN, Reis SE, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G, for the WISE Investigators Ischemic heart disease in women: Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part I: Sex Differences in Traditional and Novel Risk Factors, Symptom Evaluation and Gender-Optimized Diagnostic Strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Shaw RE, Merz CN Bairey, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology – National Cardiovascular Data Registry (ACC-NCDR) Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 12.Von Mering GO, Arant CB, Wessel TR, McGorray SP, Merz CN Bairey, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart, Lung, and Blood Institute Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004 Feb 17;109(6):722–5. doi: 10.1161/01.CIR.0000115525.92645.16. PMID: 14970106. [DOI] [PubMed] [Google Scholar]
- 13.Wong, Klein, Sharrett, Duncan, Couper, Tielsch, Klein, Hubbard Retrinal arteriolar narrowing and risk of coronary heart disease in men and women. JAMA. 2002;287:1153. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 14.Burke AP, Farb A, Malcolm GT, Liang Y, Smialek J, Virmani R. Effects of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–16. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 15.Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. 34th Bethesda Conference: Task force #2--What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874–86. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 16. [access date: August 25, 2008]; http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5401a3.htm.
- 17.Murphy SL. Death: final data for 1998. Natl Vital Stat Rep. 2000;48:1–105. [PubMed] [Google Scholar]
- 18.Ni H, Coady S, Rosamond W, Folsom AR, Chambless L, Russell SD, Sorlie PD. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;157:46–52. doi: 10.1016/j.ahj.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson MB, Kelsey SF, Matthews K, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24:1506–14. doi: 10.1016/s0195-668x(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BD, Merz CN Bairey, Kelsey SF, Sharaf BL, Cornell CE, Handberg-Thurmond EM, Rickens C, Pakstis D. Persistent chest pain predicts cardiovascular events in women with and without obstructive coronary artery disease: Results from the NHLBI-sponsored WISE study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 21.Raine R, Hutchings A, Black N. Is publicly funded health care really distributed according to need? Health Policy. 2004;67(2):227–35. doi: 10.1016/s0168-8510(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 22. [Access date: October 14, 2008]; www.ahrq.gov/hcup/factbk3/factbk3.htm#men.
- 23.Hemingway H, Crook AM, Feder G, Banerjee S, Dawson JR, Magee P, Philpott S, Sanders J, Wood A, Timmis SD. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. New Eng J Med. 2001;344:645–54. doi: 10.1056/NEJM200103013440906. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Sharaf BL, Johnson BD, et al. The economic burden of angina in women with suspected ischemic heart disease: Results from the National Institutes of Health – National Heart, Lung, and Blood Institute – Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 25.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003 Jan 1;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 26.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–90. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 28.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, Stefanick ML, Van Horn L, Kuller L. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein level: A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–9. [PubMed] [Google Scholar]
- 30.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162:1737–45. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–31. [PubMed] [Google Scholar]
- 32.Spencer EA, Pirie KL, Stevens RJ, Beral V, Brown A, Liu B, Green J, Reeves GK, Million Women Study Collaborators Diabetes and modifiable risk factors for cardiovascular disease: the prospective Million Women Study. Eur J Epidemiol. Nov 18; doi: 10.1007/s10654-008-9298-3. [in press] [DOI] [PubMed] [Google Scholar]
- 33.Gregg EW, Gu Q, Cheng YJ, et al. Mortality trends in men and women with diabetes. Ann Intern Med. 2007;147:60520–59. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 34.Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd-Jones DM, Greenland P. Favorable Cardiovascular Risk Profile in Young Women and Long-term Risk of Cardiovascular and All-Cause Mortality. JAMA. 2004;292:1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 35.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 36.Lakka H-M, Laaksonen DE, Lakka TA, et al. The Metabolic Syndrome and Total and Cardiovascular Mortality in middle aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 37.Ford ES, Giles WH, Dietz WH. Prevalence of the Metabolic Syndrome Among US Adults - Findings From the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 38.Ramos RG, Olden K. The prevalence of metabolic syndrome among US women of childbearing age. Am J Public Health. 2008 Jun;98(6):1122–7. doi: 10.2105/AJPH.2007.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T, Cox J, Ghali WA, Grace S, Hamet P, Ho T, Kirkland S, Lambert M, Libersan D, O’Loughlin J, Paradis G, Petrovich M, Tagalakis V. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007 Mar 13;176(6):S1–44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht HS, Superko HR. Electron beam tomography and National Cholesterol Education Program guidelines in asymptomatic women. J Am Coll Cardiol. 2001;37:1506–11. doi: 10.1016/s0735-1097(01)01211-6. [DOI] [PubMed] [Google Scholar]
- 41.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003 Jun 4;41(11):1863–74. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 42.Shaw LJ, Lewis JF, Hlatky MA, et al. Women’s Ischemic Syndrome Evaluation: Current Status and Future Research Directions, Report of the National Heart Lung Blood Institute (NHLBI) Workshop, Section 5: Gender-Related Risk Factors for Ischemic Heart Disease, October 2-4, 2002. Circulation. 2004a;109:56e–58e. doi: 10.1161/01.CIR.0000116210.70548.2A. [DOI] [PubMed] [Google Scholar]
- 43.Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, Blumenthal RS. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–6. doi: 10.1016/j.atherosclerosis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007 Dec 10;167(22):2437–42. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 45.Nasir K, Michos ED, Blumenthal RS, Raggi P. Detection of high-risk young adults and women by coronary calcium and National Cholesterol Education Program Panel III guidelines. J Am Coll Cardiol. 2005 Nov 15;46(10):1931–6. doi: 10.1016/j.jacc.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 46.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Brit J Ophthalmol. 2002;86:1007–13. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 48.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145(1):21–9. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 49.Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcium. J Women Health. 2004;13(3):273–88. doi: 10.1089/154099904323016437. [DOI] [PubMed] [Google Scholar]
- 50.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–14. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 51.Bessant R, Hingorani A, Patel L, MacGregor A, Isenberg DA, Rahman A. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2004 Jul;43(7):924–9. doi: 10.1093/rheumatology/keh213. [DOI] [PubMed] [Google Scholar]
- 52.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Merz CN Bairey, Sopko G, Olson MB, Reis SE, National Heart, Lung, and Blood Institute Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004 Feb 17;109(6):726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 53.Marroquin OC, Kip KE, Kelley D, et al. The metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: A report from WISE. Circulation. 2004;1009:714–21. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–33. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 56.Kuller LH, Tracy RP. The role of inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2000;20:901–6. doi: 10.1161/01.atv.20.4.901. [DOI] [PubMed] [Google Scholar]
- 57.Tracy RP. Inflammation in cardiovascular disease: cart, horse or both--revisited. Arterioscler Thromb Vasc Biol. 2002;22:1514–5. doi: 10.1161/01.atv.0000035403.39442.db. [DOI] [PubMed] [Google Scholar]
- 58.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome on cardiovascular risk in women: A report from the Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:706–13. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 59.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 60.Arant CB, Wessel TR, Ridker PM, Olson MB, Johnson BD, Sharaf BL, Kerensky RA, Pauly DF, Handberg E, Zineh I, Sopko G, Kelsey SF, Merz CN Bairey, Pepine CJ. Multimarker Approach Predicts Adverse Cardiovascular Events In Women Evaluated for Suspected Ischemia: A Report From the NHLBI-Sponsored WISE Study. Clin Cardiol. doi: 10.1002/clc.20454. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merz CN Bairey, Johnson BD, Sharaf BL, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–9. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 62.Tannenbaum C, Barrett-Connor E, Laughlin GA, Platt RW. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: the Rancho Bernardo Study. Eur J Endocrinol. 2004;151:717–25. doi: 10.1530/eje.0.1510717. [DOI] [PubMed] [Google Scholar]
- 63.Ding EL, Song Y, Malik VS, Liu S. Sex Differences of Endogenous Sex Hormones & Risk of Type 2 Diabetes. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 64.Shaw LJ, Merz CN Bairey, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008 Apr;93(4):1276–84. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Zambon S, Zanoni S, Romanato G Giovanna, Chiara M, Noale M, Sartori L, Musacchio E, Baggio G, Crepaldi G, Manzato E. Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: The Progetto Veneto Anziani (Pro.V.A.) study. Diabetes Care. 2008 October 17; doi: 10.2337/dc08-1256. published online ahead of print. DOI: 10.2337/dc08-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. [Access date: December 8, 2008]; http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.
- 67. [Access date: December 8, 2008]; http://www.nhlbi.nih.gov/guidelines/hypertension/index.htm.
- 68.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007 Feb 14;297(6):611–9. doi: 10.1001/jama.297.6.611. 2007. [DOI] [PubMed] [Google Scholar]
- 69.Ingelsson E, Sullivan LM, Fox CS, Murabito JM, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O’Donnell CJ, Wang TJ, D’Agostino RB, Wolf PA, Vasan RS. Burden and prognostic importance of subclinical cardiovascular disease in overweight and obese individuals. Circulation. 2007 Jul 24;116(4):375–84. doi: 10.1161/CIRCULATIONAHA.107.688788. [DOI] [PubMed] [Google Scholar]
- 70.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez RH, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif J-C, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim Carotid Intima-Media Thickness Consensus (2004-2006) An Update on Behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 74.Devine J, Carlson DW, Taylor AJ. Clinical value of carotid-intima media thickness. J Nucl Cardiol. 2006;13:710–8. doi: 10.1016/j.nuclcard.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Simon A, Chironi G, Levinson J. Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. Eur Heart J. 2007;28:2967–2971. doi: 10.1093/eurheartj/ehm487. [DOI] [PubMed] [Google Scholar]
- 76.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008 Feb;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Eisenberg MJ, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ, American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Society of Atherosclerosis Imaging and Prevention. Society of Cardiovascular Computed Tomography ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007 Jan 23;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Redberg RF, Rifai N, Gee L, Ridker PM. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: implications for coronary artery disease screening. J Am Coll Cardiol. 2000 Jul;36(1):39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 79.Nasir K, Raggi P, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, Blumenthal RS. Coronary artery calcium volume scores on electron beam tomography in 12,936 asymptomatic adults. Am J Cardiol. 2004 May 1;93(9):1146–9. doi: 10.1016/j.amjcard.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 80.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001 Jun 15;87(12):1335–9. doi: 10.1016/s0002-9149(01)01548-x. [DOI] [PubMed] [Google Scholar]
- 81.Nasir K, Budoff MJ, Shaw LJ, Blumenthal RS. Value of multislice computed tomography coronary angiography in suspected coronary artery disease. J Am Coll Cardiol. 2007 May 22;49(20):2070–1. doi: 10.1016/j.jacc.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008 Mar 27;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 83.Bellasi A, Lacey C, Taylor AJ, Raggi P, Wilson PW, Budoff MJ, Vaccarino V, Shaw LJ. Comparison of prognostic usefulness of coronary artery calcium in men versus women (results from a meta- and pooled analysis estimating all-cause mortality and coronary heart disease death or myocardial infarction) Am J Cardiol. 2007;100(3):409–14. doi: 10.1016/j.amjcard.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 84.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. JACC. 2008 Jul 1;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Douglas PS, Ginsburg GS. The evaluation of chest pain in women. N Engl J Med. 1996;334:1311–5. doi: 10.1056/NEJM199605163342007. [DOI] [PubMed] [Google Scholar]
- 86.Hendrix KH, Mayhan S, Lackland DT, Egan BM. Prevalence, treatment, and control of chest pain syndromes and associated risk factors in hypertensive patients. Am J Hypertension. 2005;18(8):1026–32. doi: 10.1016/j.amjhyper.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 87.Alexander KP, Shaw LJ, Shaw LK, Delong ER, Mark DB, Peterson ED. Value of exercise treadmill testing in women. J Am Coll Cardiol. 1998;32:1657–64. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 88.Shaw LJ, Heller GV, Travin MI, Lauer M, Marwick TH, Hachamovitch R, Berman DS, Miller DD. Cost analysis of diagnostic testing for coronary artery disease in women with stable chest pain. J Nucl Cardiol. 1999;6(6):559–569. doi: 10.1016/s1071-3581(99)90091-0. [DOI] [PubMed] [Google Scholar]
- 89.O’Keefe-McCarthy S. Women’s experiences of cardiac pain: a review of the literature. Can J Cardiovasc Nurs. 2008;18(3):18–25. [PubMed] [Google Scholar]
- 90.Hemingway Circulation. 2008;117:526–1536. [Google Scholar]
- 91.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 92.Johnson BD, Kelsey SF, Merz CN Bairey. Clinical risk assessment in women: chest discomfort. Report from the WISE study. In: Shaw LJ, Redberg RF, editors. CAD in Women: Evidence-Based Diagnosis and Treatment. Humana Press; New Jersey: 2003. pp. 129–142. [Google Scholar]
- 93.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–41. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 94.Merz NB Bairey, Johnson BD, Kelsey PSF, et al. Diagnostic, prognostic, and cost assessment of coronary artery disease in women. Am J Managed Care. 2001;7:959–65. [PubMed] [Google Scholar]
- 95.Robinson JG, Wallace R, Limacher M, Ren H, Cochrane B, Wassertheil-Smoller S, Ockene JK, Blanchette PL, Ko MG. Cardiovascular risk in women with non-specific chest pain (from the Women’s Health Initiative Hormone Trials) Am J Cardiol. 2008 Sep 15;102(6):693–9. doi: 10.1016/j.amjcard.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 96.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN Bairey. Adverse Cardiovascular Outcomes In Women with Nonobstructive Coronary Artery Disease: A Report From The National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study and The St James Women Take Heart (WTH) Project. Arch Int Med. doi: 10.1001/archinternmed.2009.50. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bugiardini R, Merz CN Bairey. Angina with “normal” coronary arteries: A changing philosophy. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 98.Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 99.Hochman JS, McCabe CH, Stone PH, et al. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. J Am Coll Cardiol. 1997;30:141–148. doi: 10.1016/s0735-1097(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 100.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007 Feb 20;115(7):823–6. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 101.Panza JA. Myocardial ischemia and the pains of the heart. N Engl J Med. 2002;346:1934–5. doi: 10.1056/NEJMp020047. [DOI] [PubMed] [Google Scholar]
- 102.Humphries KH, Pu A, Gao M, Carere RG, Pilote M. Angina with “normal” coronary arteries: Sex differences in outcomes. Am Heart J. 2008;155:375–381. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Reynolds HR, Farkouh ME, Lincoff AM, Hsu A, Swahn E, Sadowski ZP, White JA, Topol EJ, Hochman JS, GUSTO V Investigators Impact of female sex on death and bleeding after fibrinolytic treatment of myocardial infarction in GUSTO V. Arch Int Med. 2007;167:2054–2060. doi: 10.1001/archinte.167.19.2054. [DOI] [PubMed] [Google Scholar]
- 104.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK. Gender disparities in diagnosis & treatment of non–ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 105.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–25. doi: 10.1056/NEJM199907223410401. PMID: 10413733. [DOI] [PubMed] [Google Scholar]
- 106.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimäki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006 Mar 22;295(12):1404–11. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 107.Kwok YS, Kim C, Grady D, et al. Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol. 1999;83:660–6. doi: 10.1016/s0002-9149(98)00963-1. [DOI] [PubMed] [Google Scholar]
- 108.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of Exercise Testing to Predict Cardiovascular and All-Cause Death in Asymptomatic Women. JAMA. 2003;290:1600–07. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 109.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women. Circulation. 2003;108:1554–9. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 110.Mieres JH, Shaw LJ, Arai A, Budoff M, Hundley G, Flamm SD, Marwick TH, Mosca L, Patel AR, Redberg RF, Taubert K, Thomas G, Wenger NK, for the Cardiovascular Imaging Committee American Heart Association – Cardiac Imaging Committee Consensus Statement: The role of cardiac imaging in the clinical evaluation of women with known or suspected coronary artery disease. Circulation. 2005;111:682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 111.Gulati M, Arnsdorf MF, Shaw LJ, Pandey DK, Thisted RA, Lauderdale DS, Wicklund RH, Al-Hani AJ, Black HR. Prognostic value of the duke treadmill score in asymptomatic women. Am J Cardiol. 2005 Aug 1;96(3):369–75. doi: 10.1016/j.amjcard.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 112.Alexander KP, Shaw LJ, Shaw LK, DeLong ER, Mark DB, Peterson ED. Diagnostic and prognostic value of the Duke treadmill score in women. J Am Coll Cardiol. 1998;32(6):1657–1664. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 113.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, Reis SE, Mankad S, Rogers WJ, Pohost GM, Arant C, Wessel T, Chaitman BR, Sopko G, Handberg E, Pepine CJ, Merz CN Bairey. The value of estimated functional capacity in estimating outcome: results from the NHLBI-sponsored women’s ischemia syndrome evaluation. J Am Coll Cardiol. 2006;47:S36–S43. doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 114.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003 Dec 17;42(12):2139–43. doi: 10.1016/j.jacc.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 115.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs BMI with CAD & CV events in women. JAMA. 2004 Sep 8;292(10):1179–87. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 116.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN Bairey, Lauer MS, Marwick TH, Pandey PK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. New Eng J Med. 2005;353:18–25. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 117.Shaw LJ, Vasey C, Sawada S, Rimmerman C, Marwick TH. Impact of gender on risk stratification by exercise and dobutamine stress echocardiography: longterm mortality in 4,234 women and 6,898 men. Eur Heart J. 2005 Mar;26(5):447–56. doi: 10.1093/eurheartj/ehi102. [DOI] [PubMed] [Google Scholar]
- 118.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007 Jan 16;49(2):227–37. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 119.Shaw LJ, Iskandrian AE. Prognostic value of stress gated SPECT in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2004;11(2):171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 120.Marwick TH, Shaw LJ, Lauer MS, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106:172–8. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 121.Berman DS, Kang X, Hayes SW, Friedman JD, Cohen I, Abidov A, Shaw LJ, Amanullah AM, Germano G, Hachamovitch R. Adenosine myocardial perfusion SPECT in women compared with men: Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41(7):1125–1133. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 122.Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, Szeto A Lok-Tin, Aung M, Davies RA, Ruddy TD, Beanlands RS. J Am Coll Cardiol. 2006 Sep 5;48(5):1029–39. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 123.Lertsburapa K, Ahlberg AW, Bateman TM, Katten D, Volker L, Cullom SJ, Heller GV. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2008 Nov-Dec;15(6):745–53. doi: 10.1007/BF03007355. [DOI] [PubMed] [Google Scholar]
- 124.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation Dose to Patients From Cardiac Diagnostic Imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 125.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. New Eng J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 126.Pilz G, Klos M, Ali E, Hoefling B, Scheck R, Bernhardt P. Angiographic correlations of patients with small vessel disease diagnosed by adenosine-stress cardiac magnetic resonance imaging. J Cardiovasc Magn Reson. 2008 Jan 31;10(1):8. doi: 10.1186/1532-429X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vermeltfoort IA, Bondarenko O, Raijmakers PG, Odekerken DA, Kuijper AF, Zwijnenburg A, van der Vis-Melsen MJ, Twisk JW, Beek AM, Teule GJ, van Rossum AC. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007 Jul;28(13):1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 128.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. New Eng J Med. 2008 Nov 27;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 129.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008 Nov 18;52(21):1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 130.Pundziute G, Schuijf JD, Jukema JW, van Werkhoven JM, Boersma E, de Roos A, van der Wall EE, Bax JJ. Gender influence on the diagnostic accuracy of 64-slice multislice computed tomography coronary angiography for detection of obstructive coronary artery disease. Heart. 2008 Jan;94(1):48–52. doi: 10.1136/hrt.2007.116715. [DOI] [PubMed] [Google Scholar]
- 131.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008 Nov 27;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 132.Sekhri N, Timmis A, Chen R, Junghans C, Walsh N, Zaman MJ, Eldridge S, Hemingway H, Feder G. Inequity of access to investigation and effect on clinical outcomes: prognostic study of coronary angiography for suspected stable angina pectoris. BMJ. 2008 May 10;336(7652):1058–61. doi: 10.1136/bmj.39534.571042.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anderson RD, Pepine CJ. Gender differences in the treatment of acute myocardial infarction: bias or biology? Circulation. 2007;115:823–6. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 134.Harding MB, Leithe ME, Mark DB, Nelson CL, Harrison JK, Hermiller JB, Davidson CJ, Pryor DB, Bashore TM. Ergonovine maleate testing during cardiac catheterization: a 10-year perspective in 3,447 patients without significant coronary artery disease or Prinzmetal’s variant angina. J Am Coll Cardiol. 1992 Jul;20(1):107–11. doi: 10.1016/0735-1097(92)90145-d. [DOI] [PubMed] [Google Scholar]
- 135.Sun H, ohri M, Shimokawa H, Usai M, Hrakami L, Takeshita A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J Am Coll Cardiol. 2002;39:847–51. doi: 10.1016/s0735-1097(02)01690-x. [DOI] [PubMed] [Google Scholar]
- 136.Hibino H, Kurachi Y. A new insight into the pathogenesis of coronary vasospasm. Circ Res. 2006;98:579–81. doi: 10.1161/01.RES.0000215571.12500.ab. [DOI] [PubMed] [Google Scholar]
- 137.Kakkar R, Ye B, Stoller DA, et al. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–9. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 138.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 139.Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001;141:S58–62. doi: 10.1067/mhj.2001.109946. [DOI] [PubMed] [Google Scholar]
- 140.Burke AP, Kolodgie F, Farb A, Virmani Gender differences in coronary plaque morphology in sudden coronary death. Circulation. 2003;108:IV–165. [Google Scholar]
- 141.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006 Apr 18;47(8 Suppl):C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 142.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/ sclerosis in the Atherosclerosis Risk in Communities (ARIC) study. Ophthalmology. 1999;106:2269–80. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 143.Reis SE, Holubkov R, Smith AJ Conrad, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 144.Han SH, Bae JH, Holmes DR, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex Differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008 Jun;29(11):1359–69. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 145.Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E, American Society of Echocardiography Society for Vascular Medicine and Biology. American society of echocardiography report. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med. 2006 Nov;11(3):201–11. doi: 10.1177/1358863x06070511. [DOI] [PubMed] [Google Scholar]
- 146.Colacurci N, Manzella D, Fornaro F, Carbonella M, Paolisso G. Endothelial function and menopause: Effects of raloxifene administration. JCEM. 2003:2135–2140. doi: 10.1210/jc.2002-021557. [DOI] [PubMed] [Google Scholar]
- 147.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. JACC. 2008 Mar 11;51(10):997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 148.Elesber AA, Redfield MM, Rihal CS, Prasad A, Lavi S, Lennon R, Mathew V, Lerman LO, Lerman A. Coronary endothelial dysfunction and hyperlipidemia are independently associated with diastolic dysfunction in humans. Am Heart J. 2007 Jun;153(6):1081–7. doi: 10.1016/j.ahj.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004 Oct 19;44(8):1636–40. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 150.Schachinger V, Britten M, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long term outcome of CHD. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 151.Lerman A, Zeiher AM. Endothelial Function: Cardiac Events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 152.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular Obstruction and the No-Reflow Phenomenon After Percutaneous Coronary Intervention. Circulation. 2008;117:3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 153.Ong P, Athanasiadis A, Hill S, Volgelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008 Aug 12;52(7):523–7. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 154.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 155.MacIntyre K, Stewart S, Capewell S, Chalmers JW, Pell JP, Boyd J, Finlayson A, Redpath A, Gilmour H, McMurray JJ. Gender and survival: a population-based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol. 2001;38:729–735. doi: 10.1016/s0735-1097(01)01465-6. [DOI] [PubMed] [Google Scholar]
- 156.Chang WC, Kaul P, Westerhout CM, Graham MM, Fu Y, Chowdhury T, Armstrong PW. Impact of sex on long-term mortality from acute myocardial infarction vs. unstable angina. Arch Intern Med. 2003;163:2476–2484. doi: 10.1001/archinte.163.20.2476. [DOI] [PubMed] [Google Scholar]
- 157.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, Simoons M, Aylward P, Van de Werf F, Califf RM. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 158.Woodfield SL, Lundergan CF, Reiner JS, Thompson MA, Rohrbeck SC, Deychak Y, Smith JO, Burton JR, McCarthy WF, Califf RM, White HD, Weaver WD, Topol EJ, Ross AM. Gender and acute myocardial infarction: is there a different response to thrombolysis? J Am Coll Cardiol. 1997;29:35–42. doi: 10.1016/s0735-1097(96)00449-4. [DOI] [PubMed] [Google Scholar]
- 159.Becker RC, Burns M, Every N, Maynard C, Frederick P, Spencer FA, Gore JM, Lambrew C. Early clinical outcomes and routine management of patients with non-ST-segment elevation myocardial infarction: a nationwide perspective. Arch Intern Med. 2001;161:601–7. doi: 10.1001/archinte.161.4.601. [DOI] [PubMed] [Google Scholar]
- 160.Grines C, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, Brodie BR, Madonna O, Eijgelshoven M, Lansky AJ, O’Neill WW, Morice MC. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341:1949–1956. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 161.Antman EM, Morrow DA, McCabe CH, Murphy SA, Ruda M, Sadowski Z, Budaj A, López-Sendón JL, Guneri S, Jiang F, White HD, Fox KA, Braunwald E, ExTRACT-TIMI 25 Investigators Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006;354:1477–88. doi: 10.1056/NEJMoa060898. [DOI] [PubMed] [Google Scholar]
- 162.Becker RC, Burns M, Every N, Maynard C, Frederick P, Spencer FA, Gore JM, Lambrew C. Early clinical outcomes and routine management of patients with non-ST-segment elevation myocardial infarction: a nationwide perspective. Arch Intern Med. 2001;161:601–7. doi: 10.1001/archinte.161.4.601. [DOI] [PubMed] [Google Scholar]
- 163.Koek HL, de Bruin A, Gast F, Gevers E, Kardaun JW, Reitsma JB, Grobbee DE, Bots ML. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am J Cardiol. 2006;98:993–999. doi: 10.1016/j.amjcard.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 164.Bonarjee VVS, Rosengren A, Snapinn SM, James MS, Dickstein K. Sex-based short- and long-term survival in patients following complicated myocardial infarction. Eur Heart J. 2006;27:2177–2183. doi: 10.1093/eurheartj/ehl160. [DOI] [PubMed] [Google Scholar]
- 165.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 166.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995;25:1013–1018. doi: 10.1016/0735-1097(94)00519-v. [DOI] [PubMed] [Google Scholar]
- 167.Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 168.Diver DJ, Bier JD, Ferreira PE, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial) Am J Cardiol. 1994;74:531–537. doi: 10.1016/0002-9149(94)90739-0. [DOI] [PubMed] [Google Scholar]
- 169.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease. A study on women with chest pain and normal angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 170.Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Int Med. 2006;166:1391–1395. doi: 10.1001/archinte.166.13.1391. [DOI] [PubMed] [Google Scholar]
- 171.Lansky AJ, Hochman JS, Ward PA, Mintz GS, Fabunmi R, Berger PB, New G, Grines CL, Pietras CG, Kern MJ, Ferrell M, Leon MB, Mehran R, White C, Mieres JH, Moses JW, Stone GW, Jacobs AK, American College of Cardiology Foundation. American Heart Association Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation. 2005 Feb 22;111(7):940–53. doi: 10.1161/01.CIR.0000155337.50423.C9. [DOI] [PubMed] [Google Scholar]
- 172.O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008 Jul 2;300(1):71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 173.Lansky AJ. Outcomes of percutaneous and surgical revascularization in women. Prog Cardiovasc Dis. 2004;46:305–319. doi: 10.1016/j.pcad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 174.Solinas E, Dangas G, Kirtane AJ, Lansky AJ, Franklin-Bond T, Boland P, Syros G, Kim YH, Gupta A, Mintz G, Fahy M, Collins M, Kodali S, Stone GW, Moses JW, Leon MB, Mehran R. Angiographic patterns of drug-eluting stent restenosis and one-year outcomes after treatment with repeated percutaneous coronary intervention. Am J Cardiol. 2008;102:311–5. doi: 10.1016/j.amjcard.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 175.Cho L, Topol EJ, Balog C, Foody JM, Booth JE, Cabot C, Kleiman NS, Tcheng JE, Califf R, Lincoff AM. Clinical benefit of glycoprotein IIb/IIIa blockade with Abciximab is independent of gender: pooled analysis from EPIC, EPILOG and EPISTENT trials. Evaluation of 7E3 for the Prevention of Ischemic Complications. Evaluation in Percutaneous Transluminal Coronary Angioplasty to Improve Long-Term Outcome with Abciximab GP IIb/IIIa blockade. Evaluation of Platelet IIb/IIIa Inhibitor for Stent. J Am Coll Cardiol. 2000;36:381–386. doi: 10.1016/s0735-1097(00)00746-4. [DOI] [PubMed] [Google Scholar]
- 176.Boersma E, Harrington RA, Moliterno DJ, White H, Théroux P, Van de Werf F, de Torbal A, Armstrong PW, Wallentin LC, Wilcox RG, Simes J, Califf RM, Topol EJ, Simoons ML. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002 Jan 19;359(9302):189–98. doi: 10.1016/S0140-6736(02)07442-1. [DOI] [PubMed] [Google Scholar]
- 177.Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED, for the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Investigators Sex Differences in Major Bleeding With Glycoprotein IIb/IIIa Inhibitors: Results From the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) Initiative. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 178.Novack V, Cutlip DE, Jotkowitz A, Lieberman N, Porath A. Reduction in sex-based mortality difference with implementation of new cardiology guidelines. Am J Med. 2008 Jul;121(7):597–603. doi: 10.1016/j.amjmed.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 179.Bowling A, Bond M, McKee D, McClay M, Banning AP, Dudley N, Elder A, Martin A, Blackman I. Equity in access to exercise tolerance testing, coronary angiography, and coronary artery bypass grafting by age, sex and clinical indications. Heart. 2001 Jun;85(6):680–6. doi: 10.1136/heart.85.6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Battleman DS, Callahan M. Gender differences in utilization of exercise treadmill testing: a claims-based analysis. J Healthc Qual. 2001 May-Jun;23(3):38–41. doi: 10.1111/j.1945-1474.2001.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 181.Stafford RS. Aspirin use is low amoung United States outpatients with coronary artery disease. Circulation. 2000 Mar 14;101(10):1097–10. doi: 10.1161/01.cir.101.10.1097. [DOI] [PubMed] [Google Scholar]
- 182.Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S, Pasternak R, Pearson TA, Redberg RF, Smith SC, Jr, Winston M, Zinberg S. Guide to Preventive Cardiology for Women.AHA/ACC Scientific Statement Consensus panel statement. Circulation. 1999 May 11;99(18):2480–4. doi: 10.1161/01.cir.99.18.2480. [DOI] [PubMed] [Google Scholar]
- 183.Rathore SS, Chen J, Wang Y, Radford MJ, Vaccarino V, Krumholz HM. Sex differences in cardiac catheterization: the role of physician gender. JAMA. 2001;286:2849–56. doi: 10.1001/jama.286.22.2849. [DOI] [PubMed] [Google Scholar]
- 184.Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, Chan L. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med. 2000;343:8–15. doi: 10.1056/NEJM200007063430102. [DOI] [PubMed] [Google Scholar]
- 185.Daly Circulation. 2006;113:490–98. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]; Daly C, Clemens F, Sendon JL Lopez, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM, Euro Heart Survey Investigators Gender differences in the management and clinical outcome of stable angina. Circulation. 2006 Jan 31;113(4):490–8. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 186.Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA, Global Registry of Acute Coronary Events investigators Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009 Jan;95(1):20–6. doi: 10.1136/hrt.2007.138537. [DOI] [PubMed] [Google Scholar]
- 187.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N Engl J Med. 2007 Apr 12;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 188.Bugiardini R, Estrada JL Navarro, Nikus K, Hall AS, Manfrini O. Gender bias in acute coronary syndrome. Curr Vasc Pharmacol. doi: 10.2174/157016110790887018. (in press) [DOI] [PubMed] [Google Scholar]
- 189.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Pearle DL, Sloan MA, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. J Am Coll Cardiol. 2008 Jan 15;51(2):210–47. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 190.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology. American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) American College of Emergency Physicians. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons. American Association of Cardiovascular and Pulmonary Rehabilitation. Society for Academic Emergency Medicine ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007 Aug 14;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 191.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O’Rourke RA, Pasternak RC, Williams SV, Gibbons RJ, Alpert JS, Antman EM, Hiratzka LF, Fuster V, Faxon DP, Gregoratos G, Jacobs AK, Smith SC, Jr, American College of Cardiology. American Heart Association Task Force on Practice Guidelines. Committee on the Management of Patients With Chronic Stable Angina ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003 Jan 7;107(1):149–58. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 192.Sutsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–143. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 193.Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–856. doi: 10.1016/s0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 194.Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme a reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–58. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 195.Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J. 2003;24:1999–2005. doi: 10.1016/s0195-668x(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 196.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 197.Manfrini O, Pizzi C, Morgagni GL, Fontana F, Bugiardini R. Effects of pravastatin on myocardial perfusion after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2004;93:1391–1393. doi: 10.1016/j.amjcard.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 198.Eriksson BE, Tyni-Lenne R, Svedenhag J, et al. Physical training in Syndrome X: physical training counteracts deconditioning and pain in Syndrome X. J Am Coll Cardiol. 2000;36:1619–25. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 199.Kelley BM, Porter JH. The role of muscarinic cholinergic receptors in the discriminative stimulus properties of clozapinein rats. Pharmacol Biochem Behav. 1997;57:707–19. doi: 10.1016/s0091-3057(96)00342-5. [DOI] [PubMed] [Google Scholar]
- 200.Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 201.Dzavík V, Cotter G, Reynolds HR, Alexander JH, Ramanathan K, Stebbins AL, Hathaway D, Farkouh ME, Ohman EM, Baran DA, Prondzinsky R, Panza JA, Cantor WJ, Vered Z, Buller CE, Kleiman NS, Webb JG, Holmes DR, Parrillo JE, Hazen SL, Gross SS, Harrington RA, Hochman JS. SHould we inhibit nitric Oxide synthase in Cardiogenic shocK 2 (SHOCK-2) investigators. Effect of nitric oxide synthase inhibition on haemodynamics and outcome of patients with persistent cardiogenic shock complicating acute myocardial infarction: a phase II dose-ranging study. Eur Heart J. 2007 May;28(9):1109–16. doi: 10.1093/eurheartj/ehm075. [DOI] [PubMed] [Google Scholar]
- 202.Adamson DL, Webb CM, Collins P. Esterified estrogens combined with methyltestosterone improve emotional well-being in postmenopausal women with chest pain and normal coronary angiograms. Menopause. 2001;8:233–238. doi: 10.1097/00042192-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 203.Berger JS, Bairey-Merz CN, Redberg RF, Douglas PS. Improving the quality of care for women with cardiovascular disease Report of a DCRI Think Tank, March 8 to 9, 2007. Am Heart J. 2008 Nov;156(5):816–825. doi: 10.1016/j.ahj.2008.06.039. [DOI] [PubMed] [Google Scholar]