Abstract
In recent years there has been increased attention in a clinical syndrome characterized by excessive sexual thoughts, sexual urges, and/or sexual behaviors that has many aspects in common with impulse control disorders. This study provides a preliminary examination of the impulsive aspects of this syndrome, Compulsive Sexual Behavior (CSB), as conceptualized by Coleman and colleagues. Sixteen male subjects, 8 CSB patients and 8 non-patient controls, completed psychometric measures of impulsivity and compulsive sexual behavior, a behavioral task designed to assess impulse control (go/no-go task), and underwent diffusion tensor imaging (DTI) procedures. The results indicated that CSB patients were significantly more impulsive; whether measured by psychometric testing or the go/no-go procedure than controls. The results also indicate that CSB patients showed significantly higher superior frontal region mean diffusivity (MD) than controls. A correlational analysis indicated significant associations between impulsivity measures and inferior frontal region fractional anisotrophy (FA) and MD, but no associations with superior frontal region measures. Similar analyses indicated a significant negative association between superior frontal lobe MD and the compulsive sexual behavior inventory. Thus, while CSB patients were more impulsive than controls, the DTI results were not consistent with impulse control disorders.
Keywords: Compulsive sexual behavior, diffusion tensor imaging, impulsivity, sexual addiction, MRI, brain structure
1. INTRODUCTION
Over the course of the last several decades, an increasing number of clinicians and researchers have become interested in a clinical syndrome involving excessive sexual thoughts, sexual urges, or sexual activity which cause distress or impairment. This phenomenon has been called Compulsive Sexual Behavior (CSB), (Quadland, 1985; Coleman, 1991), paraphilia-related disorder (Kafka, 1994), sexual impulsivity (Barth and Kinder, 1987), and sexual addiction (Carnes, 1983; Goodman, 1993). Coleman and colleagues (Coleman, et al., 2000) proposed criteria for CSB that require the presence of recurrent and intense sexually arousing fantasies, sexual urges, or behaviors over a period of at least six months that cause distress or impairment. While there are some disagreements over the nature and the etiology of compulsive sexual behavior, all of the researchers listed above agree that the syndrome includes intense, intrusive sexual urges and fantasies, along with excessive problematic sexual behavior. In this manner, CSB resembles impulse control disorders such as kleptomania, pathological gambling, and eating disorders such as bulimia nervosa and binge eating disorder.
Although there have been no brain imaging studies of CSB, it has been suggested that damage to the frontal lobes can result in disinhibition of sexual behavior, and thus, hypersexual, or CSB (Coleman, 2005). Diffusion tensor imaging (DTI) is an MRI technique that measures the self-diffusion of water in brain tissue. DTI has been used to provide quantitative information about white matter organization and integrity. The DTI data can be represented in a number of ways, including fractional anisotropy (FA), a measure of the extent to which water diffusion is directionally restricted, and mean diffusivity (MD), a measure of overall diffusivity in the tissue. Grant, et al. (2006) used DTI to examine white matter in kleptomania. These investigators found that FA was significantly lower in the inferior frontal regions of individuals with kleptomania, indicating altered white matter organization in this region of the brain, which influences executive function and inhibitory control (Hoptman, et al., 2002).
The purpose of this study is to explore white matter micro-structure with DTI in men with CSB. Given the results for kleptomania and the presence of impulsivity in CSB, we hypothesized that we would find greater disorganization of white matter on DTI in the frontal lobes of men with CSB and that this white matter disorganization would be associated with greater impulsivity in CSB patients than non-CSB controls.
2. METHODS
2.1. Subjects
Eight men who met the proposed research criteria for CSB described above were recruited from a treatment program for individuals seeking treatment for sexual problems. CSB patients all reported non-paraphilic CSB. Five of the 8 (62%) had a history of major depression, almost all (7 of 8) had a history of alcohol abuse or dependence, while 4 (50%) had a history of other substance abuse or dependence. One subject had a history of obssessive-compulsive disorder and another one subject reported current social phobia. Eight male age-matched controls were selected from a database of healthy individuals who were willing to participate in imaging research studies. The mean ages of the CSB and control groups were 44.5+/−10.6 years and 43.4+/−9.1 years respectively. Subjects ranged in age from 19 to 51 years and were not significantly different. All of the CSB participants were Caucasian and all but one of the control participants were Caucasian. Participants were most likely to have at least some college (100% of CSB group and 75% of control group) and to hold technical or professional jobs (86% of CSB group and 63% of control group). Neither the educational level or employment level variables were significantly different.
2.2. Procedures
All participants were screened to determine if they were eligible for and interested in participating in the study. Subsequently an initial evaluation was scheduled. During this appointment all participants were interviewed using the Structured Clinical Interview for DSM-IV, Patient version (SCID-P: First et al.1995) that had a section developed by our research group added to assess the symptoms of Compulsive Sexual Behavior (Raymond, et al., 1999). These interviews were used to determine if the participant met criteria for CSB and had no active major psychiatric illnesses or substance use disorder as these were conditions that would preclude participation in the study. Also, SCID results indicated no active co-morbid impulse control disorders in either CSB patients or controls.
During the initial appointment participants also completed several self-rating scales including: 1) the Compulsive Sexual Behavior Inventory (Coleman, et al., 2001; Miner, et al., 2007) a 22-item scale that assesses the severity of CSB symptoms, 2) the Barratt Impulsiveness Scale (BIS 11: Patton, et al., 1995) a 30 item scale that measures severity of impulsive traits, and 3) the Multidimensional Personality Questionnaire (Patrick, et al., 2002) a 166 item scale that assesses various personality characteristics including Constraint factor (assessing a trait that is essentially the opposite of impulsivity so that low scores on this scale indicate greater impulsivity) and Negative Emotionality factor (assessing a trait that involves difficulties with emotional regulation). A computerized go/no-go continuous performance task (Braver, et al., 2001) was also completed by all participants. The program required participants to either push or not push a button when they saw an "X" under two different conditions. During task 1 the target was presented frequently, that is respondents were instructed to push the left mouse button when they saw any letter other than an “X” (83% frequency) and inhibit pushing the button when an “X” appeared (17% frequency). This condition assesses the degree of impulsivity by computing errors of commission, when participant fails to inhibit response by pushing button in the presence of the letter X. In task two respondents push the left mouse button only when they saw an “X” (17% frequency) and the object is to remain attentive so as not to miss pushing the button when a target (the letter X) appears. This task assesses inattentiveness by computing the errors of omission, when participant fails to respond by pressing the button in the presence of the letter X.
2.2.1 Imaging Parameters
At the second appointment magnetic resonance imaging data were acquired from all participants on a research dedicated Siemens 3T Trio scanner (Erlangen, Germany). Whole brain volumetric images with T1 and proton density (PD) contrasts were obtained for use in tissue classification. T1 images were acquired with coronal orientation, using an MP-Rage sequence (TR=2530ms, TE=3.65ms, TI=1100ms, flip angle 7 degrees, 240 partitions, 1 mm isotropic voxel). PD images were acquired in the axial orientation, using a hyper-echo, turbo spin echo sequence (TR=8550ms, TE=14ms, flip angle 120 degrees, 80 contiguous slices, 1×1×2mm voxel). DTI volumes were acquired with axial orientation and aligned to the PD volume, using a double spin echo, single shot EPI acquisition with 12 diffusion gradient directions (TR=11500ms, TE=98ms, 64 contiguous 2 mm slices, 2 mm isotropic voxel, b=1000 sec/mm2, 2 averages). A dual echo field map sequence with voxel parameters common to the DTI was acquired and used to correct the DTI data for geometric distortions caused by magnetic field inhomogeneities.
2.2.2. Anatomical processing
Image data was processed using software (BET, FLIRT, FAST, FDT, FUGUE) from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/). The brain was first extracted from T1 and PD images using BET. The T1 brain was then aligned to the PD brain using FLIRT. Dual channel tissue classification was performed on the PD and aligned T1 images using FAST, producing four tissue classes (CSF, white, gray, and blood).
2.2.3. DTI processing
The raw diffusion data was first corrected for eddy current distortion and then the diffusion tensor was computed using FDT and the FA and MD maps were computed (Basser, 1995). The b=0 diffusion volume and the FA and MD volumes were corrected for the distortion caused by magnetic field inhomogeneity using the field map image and FUGUE.
Subject specific white matter masks were created on the dewarped DTI volumes by registering the partial volume estimate (PVE) white matter map from the dual channel FAST segmentation onto the distortion corrected DTI image using the inverse of the transform generated by aligning the dewarped, DTI b=0 image to the PD volume. Voxels in the DTI images were classified as white matter if the estimated white matter composition of the voxel exceeded 90% as determined by the DTI aligned PVE map.
2.2.4. Region of interest determination
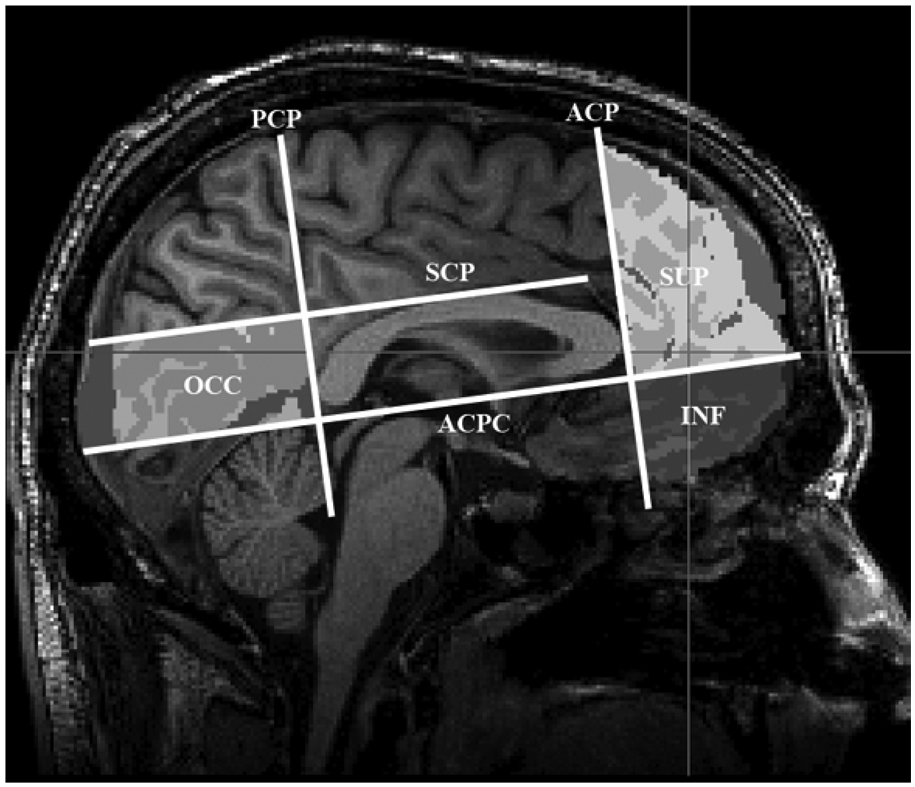
A semi-automatic process similar to that used in Wozniak, et al. (2007) was used to define regions of interest (ROIs). The T1 data were aligned to the MNI global brain using FLIRT with a 12 degree of freedom affine alignment. A trained operator determined the boundary of the ROIs for each subject by selecting four planes on the individual MNI aligned T1 image. The anterior coronal plane (ACP) was defined as the most anterior extent of the genu of the corpus callosum; the posterior coronal plane (PCP) was defined as the posterior most extent of the splenium of the corpus callosum; the AC-PC plane (ACPC) was defined to be the axial passing through the AC-PC line; the supra-callosal plane (SCP) was defined to be the axial plane above the most superior extent of the corpus callosum at the midline (see figure 1).
Figure 1.
Sagittal view: Frontal region defined as anterior to the anterior coronal place (ACP) and subdivided by the ACPC plane into the superior frontal (SUP) and inferior frontal (INF) regions.
Two regions of interest were evaluated in this analysis: the superior frontal region was defined as tissue anterior of the ACP and superior of the ACPC, and the inferior frontal region was defined as tissue anterior to the ACP and inferior to the ACPC (see figure 1). The ROIs were then projected into the DTI images using the inverse transforms of the product of the transforms that were determined from the MNI to T1, T1 to PD, and PD to dewarped DTI alignments. Mean values for white matter FA and MD in each region for every subject were determined by averaging those voxels in the white matter mask that were also in the aligned ROI.
2.3. Statistical analysis
Differences between CSB patients and controls were analyzed using Student’s t-tests calculated using SPSS Version 15 for Windows. The associations were calculated using Pearson’s Product-Moment Correlation Coefficients.
3. RESULTS
The data presented in Table 1 show that the CSB group differs from the controls on multiple measures of impulsivity. Significant CSB vs. Control differences were found for overall impulsivity, t14=−2.64, P <0.019, and Contraint, t14=2.50, P <0.026. Additionally, CSB participants showed significant higher negative emotionality, t14=−3.16, P <0.007. The CSB participants also showed significantly higher scores on the CSBI, t14= 9.57, P <0.001,
Table 1.
Mean differences between Compulsive Sexual Behavior Patients and Controls on Psychometric, Behavioral, and Neuroanatomical Measures
| CSB (n = 8) Mean (sd) |
Controls (n = 8) Mean (sd) |
P | Effect Size (d) |
|
|---|---|---|---|---|
| CSBI | 63 (9.9) | 29 (1.8) | <0.001 | 5.8 |
| Barratt Impulsivity | 61 (12.9) | 48 (5.1) | 0.019 | 1.4 |
| MPQ Constraint Factor | 38 (9.7) | 50 (10.5) | 0.026 | 1.2 |
| Negative Emotionality Factor | 50 (8.7) | 38 (6.2) | 0.007 | 1.6 |
| Go-No Go Tasks | ||||
| Task 1-Errors Target Frequent | ||||
| Commission | 8.6 (3.9) | 3.0 (3.4) | 0.008 | 1.5 |
| Omission | 11.5 (9.4) | 2.0 (3.3) | 0.018 | 1.5 |
| Task 2-Errors Target In-Frequent | ||||
| Commission | 0.75 (1.0) | 0.50 (0.8) | 0.59 | 0.3 |
| Omission | 0.12 (0.4) | 0.00 (0.0) | 0.33 | 0.6 |
| Total Errors | ||||
| Commission | 9.4 (3.9) | 3.5 (4.0) | 0.010 | 1.5 |
| Omission | 11.6 (9.3) | 2.0 (3.3) | 0.015 | 1.5 |
| Sup. Frontal FA (× 1000) | 392 (37.9) | 368 (23.4) | 0.15 | 0.8 |
| Sup. Frontal MD (µm2/s) | 781 (40.6) | 833 (34.2) | 0.02 | 1.4 |
| Inf. Frontal FA (× 1000) | 384 (33.9) | 394 (18.1) | 0.46 | 0.4 |
| Inf. Frontal MD (µm2/s) | 893 (87.6) | 869 (74.2) | 0.58 | 0.3 |
The results of a Go-No Go procedure, which is a behavioral measure of impulsivity, were that CSB participants made significantly more errors, both of commission, t14=3.09, P <0.008, and omission, t14=2.69, P <0.018, during the target frequent condition and also showed significantly more total errors over both conditions than Controls (Commission errors: t14=2.98, P<0.01; Omission errors: t14=2.76, P<0.014).
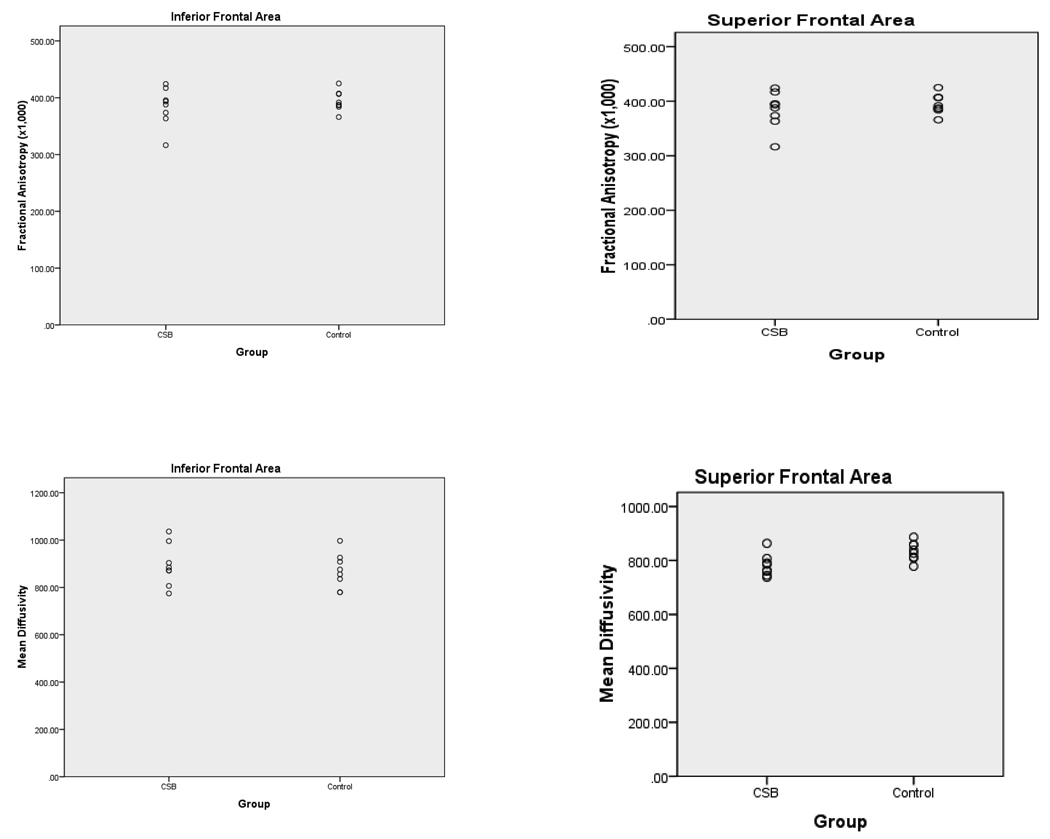
The results of the imaging studies comparing CSB participants with control participants are presented in Table 1 and Figure 2. The CSB group has significantly lower MD in the superior frontal region. While the differences between groups on FA in the superior frontal was not significant (P=0.15) the effect size of the difference (d=0.8) is medium to large (Cohen, 1988). There were no significant differences between the CSB group and control group on any measures in the inferior frontal region and the effect sizes for the differences were small.
Figure 2.
FA (× 1000) and MD by group for Inferior Frontal and Superior Frontal Regions
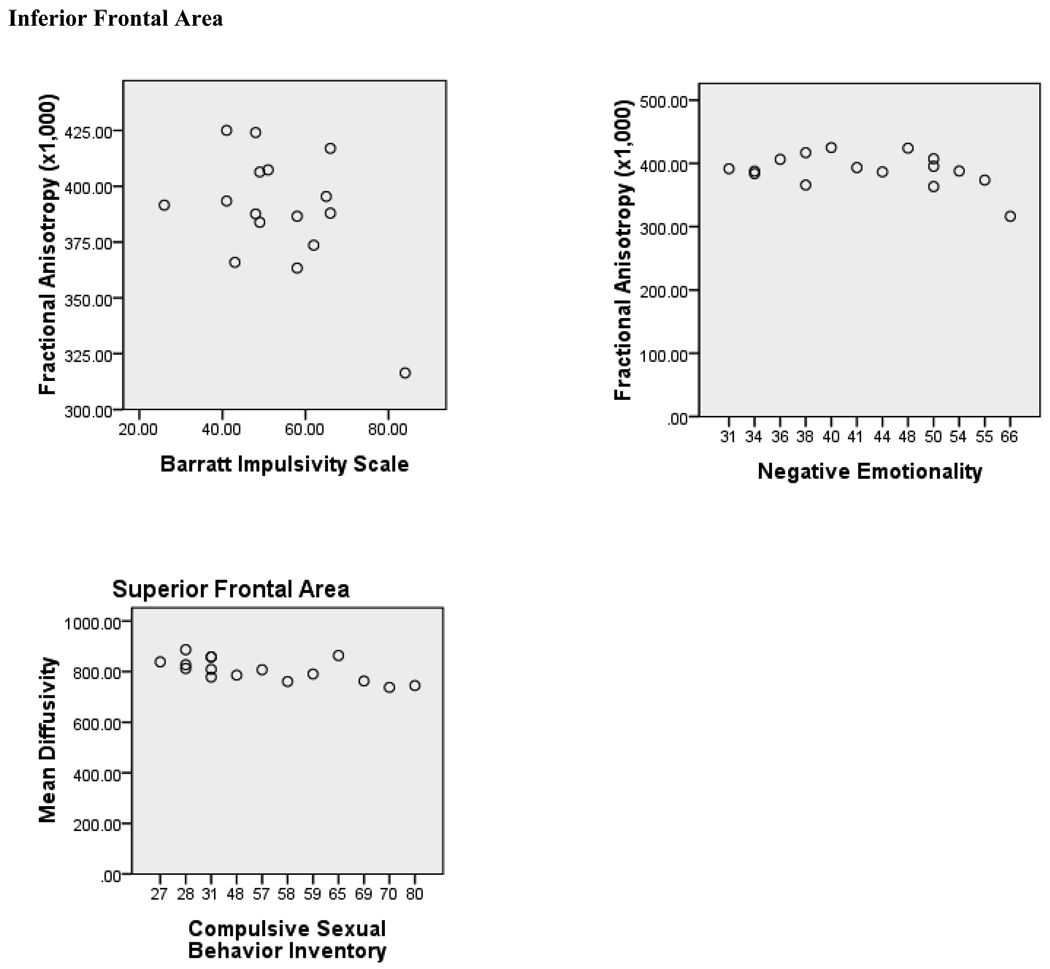
The associations of the impulsivity and emotionality measures and the imaging measures are presented in Table 2 and Figure 3. The results indicate significant, negative associations of impulsivity and negative emotionality with inferior frontal region FA. Constraint showed the opposite pattern of associations with FA, as well as trend toward a negative association with inferior frontal region MD. These measures showed no associations in the superior frontal region. The CSBI, however, showed no significant associations in the inferior frontal region, however, a significant negative association was found between CSBI score and superior frontal MD.
Table 2.
Correlations between Impulsivity and Personality Measures and Imaging Measures.
| Superior FA |
Superior MD |
Inferior FA |
Inferior MD |
|
|---|---|---|---|---|
| Barratt Impulsivity | −0.12 | −0.01 | −0.56* | 0.33 |
| MPQ | ||||
| Constraint Factor | −0.10 | 0.17 | 0 .48† | −0.49‡ |
| Negative | −0.07 | −0.04 | −0.51* | 0.17 |
| Emotionality Factor | ||||
| CSBI | 0.31 | −0.64** | 0.30 | −0.04 |
p=.059
p=.054
correlation significant at p<.05.
correlation significant at p<.01.
Figure 3.
Scatterplot of Inferior Frontal Region FA (×1000) vs. Barratt Impulsivity and Negative Imotionality and Superior Frontal Region MD vs. Compulsive Sexual Behavior.
4. DISCUSSION
The data presented in this paper are consistent with the assumption that CSB has much in common with impulse control disorders, such as kleptomania, compulsive gambling, and eating disorders. Specifically, we found that individuals who meet diagnostic criteria for compulsive sexual behavior score higher on self report measures of impulsivity, including measures of overall impulsivity and the personality factor, Constraint. However, although there was a significant difference between scores on the Barratt Impulsivity Scale between CSB patients and controls, and this effect size of this difference was substantial, our CSB patients’ scores were within the average range for a recent community sample (Spinella, 2005).
In addition to the above self-report measures, CSB patients also showed significantly more impulsivity on a behavioral task, the Go-No Go procedure. Consistent with research on attention deficit hyperactivity disorder (Dickstein, et al., 2006: Farmer and Rucklidge, 2006) and the general impulse control literature (Asahi, et al., 2004; Cheung, et al., 2004; Spinella, 2004) patients with CSB had more errors of commission on the Go-No Go procedure. However, they also showed more errors of omission than controls. In the response infrequent condition, errors of omission are a measure of inattentiveness. Our groups did not differ in errors on the response infrequent condition. The differences in errors of omission during the response frequent condition are similar to results found for obsessive-compulsive patients, where more frequent errors of omission were found in an affective Go-No Go procedure when compared to trichotillomania patients and controls (Chamberlain, et al., 2007). This would indicate that in addition to indications of impulsivity, the increased errors of commission in the CSB patients, there is also an indication of some other issue, which is indicated by the failure to respond when responses are required. It is possible that this is some form of perseveration, which may be consistent with a compulsive, in addition to impulsive, dimension of CSB.
Contrary to expectation, there were no differences between CSB patients and controls on the DTI measures, FA and MD, in the inferior frontal region. However, CSB patients did show significantly lower MD in the superior frontal region and higher FA, although the difference in FA did not reach statistical significance. These differences were of substantial size (d=0.8 for FA and 1.4 for MD). So, while our findings with respect to impulsivity are consistent with research on other impulse control disorders, our DTI white matter integrity data are not consistent with that research, which has found impulse control problems to be associated with inferior frontal white matter disorganization, that is low FA and high MD (Hoptman, et al., 2002; Grant, et al, 2006; Rüsch et al., 2007).
MD and FA are scalar measures that summarize characteristics of the diffusion tensor, which is a type of matrix and contains information describing the magnitude and direction of the water self-diffusion pattern in tissue. The diffusion pattern can be visualized as an ellipsoid with three orthogonal axes with the length of an axis representing the degree of diffusion in that axis. MD represents the overall free space available for the water to self-diffuse, thus is the average length of all the three axes. FA represents the ratio between the length of the primary axis and the other two orthogonal axes – high anisotropy would represent diffusion that is highly oriented in one direction (Wozniak & Lim, 2006). DTI measures are not absolute measures and need to be interpreted in context. To identify pathology using DTI generally requires that a comparison be made with a non pathological sample population at the same anatomical location. For example, crossing fibers results in a reduction in FA. Loss of one set of fibers in the crossing, as has been shown in stroke (Pierpaoli, et al., 2001), can result in an increase in FA in the stroke patients. Our data, showed an increase in FA and a decrease in MD in superior frontal white matter in CSB patients as compared to non-disordered comparison subjects. This could reflect altered fiber organization, possibly due to fewer crossing fibers in the superior frontal area of CSB patients and lower free space in this region, possibly due to closer packing of the tissue.
Given the differences found, we explored the DTI data further by investigating its association with our measures of impulsivity and compulsive sexual behavior. Consistent with previous research, we found substantial associations between impulsivity measures and DTI measures of decreased white matter organization in the inferior frontal cortex. However, consistent with the group differences between CSB patients and Controls and inconsistent with the results for impulse control measures, we found a substantial negative association between the CSBI and superior frontal MD. The CSBI showed no association with inferior frontal measures, and the impulsivity measures showed no association with superior frontal measures. The association of CSB with decreased MD, while inconsistent with impulsivity, is consistent with emerging data from anxiety disorders. Increased FA and decreased MD have been found in patients with panic disorder and post traumatic stress disorder (Abe, et al, 2006; Han, et al., in press). Additionally, the severity of anxiety symptoms has been found to be positively associated with FA and negatively associated with MD (Han, et al., in press). Also, our findings with respect to FA and MD are smilar to emerging DTI studies of obessive-compulsive disorder (OCD). Several DTI studies have found that OCD patients show increased FA when compared to controls in brain regions similar to the superior frontal region explored in this study (Cannistraro, et al., 2007; Yoo, et al., 2007; Menzies, et al., 2008; Nakamae, et al., 2008). Additionally, Nakamae, et al. (2008) found a higher apparent diffusion coefficient (ADC) in the left medial frontal cortex of OCD patients when compared to controls. ADC is a measure similar to MD.
Coleman (1991) discusses CSB as driven by negative affect, especially anxiety and depression. The data here appears consistent with CSB being a moderator of negative affect in that CSB patients scored higher on negative emotionality, a scale that indicates difficulties with emotional regulation (Patrick, et al., 2002), and showed DTI and Go-No Go error differences consistent with anxiety disorders. In fact, the data from this study indicates that, at least in terms of neuroantomical measures, CSB may fit more on an OCD than an impulse control spectrum.
The major limitation of this study is the sample size. Given the small samples, and the fact that we chose to conduct multiple analyses without controlling for experiment-wise error, it is possible that some of our findings are spurious. However, most of our correlation coefficients are quite substantial and the effect sizes for our group differences are also quite substantial. Thus, these preliminary analyses are promising and provide an indication that there are probably neuroanatomical and/or neurophysiological factors associated with compulsive sexual behavior. These data also indicate that CSB is likely characterized by impulsivity, but also includes other components, which may be related to the emotional reactivity and anxiety of OCD. Further studies that replicate these procedures in large, representative samples of individuals who meet diagnostic criteria for CSB and non-clinical controls are indicated. The addition of a patient comparison group with non-sexual compulsive disorder could help to parcel general compulsive features from specifically sexual compulsive features. This would further advance our understanding of this phenomenon characterized by hypersexuality. Over the years many theories have been proposed related to etiology of CSB. New neuroimaging techniques now provide us with tools to examine the neurobiological underpinnings (brain substrates, etc.) of these theories.
ACKNOWLEDGMENTS
This project was supported in part by a Grant-in-Aid of Research, Artistry and Scholarship from the University of Minnesota to Michael H. Miner, and by P41 RR008079, P30 NS057091 and M01-RR00400 National Center for Research Resources, National Institutes of Health to Kelvin O. Lim. The authors would like to thank Dr. S. Charles Schulz who provided seed funding and support for this research. We also wish to thank Dr. Eli Coleman for his counsel and support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtani T, Masuntani Y, Kato N, Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research: Neuroimaging. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Barth J, Kinder BN. The mislabeling of sexual impulsivity. Journal of Sexual and Marital Therapy. 1987;13:15–23. doi: 10.1080/00926238708403875. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(411):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulated cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depression and Anxiety. 2007;24:440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Carnes P. Out of the shadows: Understanding sexual addiction. Minneapolis, MN: CompCare; 1983. [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robiins TW, Shahkian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Mitsis EM, Halperin JM. The relationship of behavioral inhibition to executive functions in young adults. Journal of Clinical and Experimental Neurophyshcology. 2004;26:393–404. doi: 10.1080/13803390490510103. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2nd Ed. Hillsdale, N.J: Lawrence Erlbaum; 1988. [Google Scholar]
- Coleman E. Compulsive sexual behavior. New concepts and treatments. Journal of Psychology and Human Sexuality. 1991;4:37–52. [Google Scholar]
- Coleman E. Neuroanatomical and neurotransmitter dysfunction and compulsive sexual behavior. In: Hyde JS, editor. Biological substrates of human sexuality. Washington, D.C: American Psychological Association; 2005. pp. 147–169. [Google Scholar]
- Coleman E, Gratzer T, Nesvacil L, Raymond N. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: A retrospective study. Journal of Clinical Psychiatry. 2000;61:282–284. [PubMed] [Google Scholar]
- Coleman E, Miner M, Ohlerking F, Raymond N. Compulsive sexual behavior inventory: A preliminary study of reliability and validity. Journal of Sex and Marital Therapy. 2001;27:325–332. doi: 10.1080/009262301317081070. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Casellano FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Rucklidge JJ. An evaluation of the response modulation huypothesis in relation to attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2006;34:545–557. doi: 10.1007/s10802-006-9034-y. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbons M, Williams JBW. Biometrics Research Department. New York: New York State Psychiatric Institute; 1995. Structured clinical interview for the DSM-IV – patient edition (SCID-I/P, Version 2.0) [Google Scholar]
- Goodman A. Diagnosis and treatment of sexual addiction. Journal of Sex and Marital Therapy. 1993;19:225–251. doi: 10.1080/00926239308404908. [DOI] [PubMed] [Google Scholar]
- Grant JE, Correaia S, Brennan-Krohn T. White matter integrity in kleptomania: A pilot study. Psychiatry Research: Neuroimaging. 2006;147:233–237. doi: 10.1016/j.pscychresns.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager S, Chung A, Hwang J, Daniels MA, Lee YS, Lyoo IK. Altered cingulated white matter connectivity I panic disorder patients. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2007.03.002. in press. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: A preliminary study. Biological Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Kafka MP. Sertraline pharmacotherapy for paraphilias and paraphilia-related disorders: An open trial. Annals of Clinical Psychiatry. 1994;6:189–195. doi: 10.3109/10401239409149003. [DOI] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian BJ, Robbins TW, Bullmore ET. While matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. American Journal of Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Miner MH, Coleman E, Center BA, Ross M, Rosser BRS. Compulsive Sexual Behavior Inventory: Psychometric properties. Archives of Sexual Behavior. 2007;36:579–587. doi: 10.1007/s10508-006-9127-2. [DOI] [PubMed] [Google Scholar]
- Makamae T, Narumoto J, Shibata K, Matsumoto R, Kitabayashi Y, Yoshida T, Yamada K, Nishimura T, Fukui K. Alteration of fractiona anisotropy and apparent diffusion coefficient in obsessive-compulsvie disorder: A diffusion tensor imaging study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1221–1226. doi: 10.1016/j.pnpbp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsivity Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegin A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter achitecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Quadland MC. Compulsive sexual behavior: Definition of a problem and an approach to treatment. Journal of Sexual and Marital Therapy. 1985;11:121–132. doi: 10.1080/00926238508406078. [DOI] [PubMed] [Google Scholar]
- Raymond NC, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. American Journal of Psychiatry. 1999;156:786–788. doi: 10.1176/ajp.156.5.786. [DOI] [PubMed] [Google Scholar]
- Rüsch N, Weber M, Il’yasov KA, Lieb K, Ebert D, Hennig J, van Elst LT. Inferior frontal white matter microstructure and patterns of psychopathology in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. Neuroimage. 2007;35:738–747. doi: 10.1016/j.neuroimage.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Spinella M. Neurobehavioral correlates of impulsivity: Evidence of prefrontal involvement. International Journal of Neuroscience. 2004;114:95–104. doi: 10.1080/00207450490249347. [DOI] [PubMed] [Google Scholar]
- Spinella M. Normative data and a short form of the Barratt Impulsiveness Scale. International Journal of Neuroscience. 2005;117:359–368. doi: 10.1080/00207450600588881. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Krach L, Ward E, Mueller B, Muetzel R, Schnoebelen S, Kiragu A, Lim KO. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Review. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SY, Jang JH, Shin Y-W, Kim DJ, Park H-J, Moon W-J, Chung EC, Lee J-M, Kim I/Y, Kwon JS. White matter abnormalities in durg-naïve patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Act Psychiatrica Scandinavica. 2007;116:211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]