Abstract
Objectives
Determine the effect of developing a dual sensory loss (DSL) on depression over time and evaluate the impact of pre-existing single sensory loss on this effect.
Methods
Multilevel modeling was used to analyze data (N=2689) from the Health and Retirement Study.
Results
A significant increase in depression at the first report of DSL occurred, and depression increased at a significantly faster rate following DSL, in a curvilinear pattern. In addition, persons who eventually developed DSL began the study with a depression score significantly higher than persons who did not experience sensory loss. A pre-existing single sensory loss did not alter the effect of DSL on depression.
Discussion
Two sources of disparity in depression between persons with and without DSL were identified: pre-existing differences and differences that occur due to the DSL. The relationship exhibited between depression and developing a DSL is indicative of an adjustment process.
Keywords: depression, hearing loss, vision loss, dual sensory loss
Introduction
One result of the increasing lifespan of the population in the United States has been a rise in the incidence of dual sensory loss (i.e., combined vision and hearing loss) among older adults. Estimates of the prevalence of dual sensory loss (DSL) among older persons in the U.S. have ranged from 7.3% to 21%, depending on the data source, age of the sample, and the manner in which vision and hearing loss were measured (Caban, Lee, Gomez-Marin, Zhen, & Lam, 2005; Campbell, Crews, Moriarty, Zack & Blackman, 1999; Crews & Campbell, 2004; Brennan, Horowitz, & Su, 2005). Previous research has established the relationship between sensory loss and several negative outcomes. One of the strongest associations has been found between vision loss and depression, with less consistent or weaker relationships between hearing loss and depression (Brody et al., 2001; Capella-McDonnall, 2005; Kramer, Kapteyn, Kuik, & Deeg, 2002; Lee, Smith, & Kington, 1999). Research involving sensory loss and depression has documented that a substantially larger proportion of persons with visual impairment or DSL experience depression or depressive symptoms than the general population (Brody et al., 2001; Capella-McDonnall, 2005; Chou & Chi, 2004; Casten, Rovner, & Edmonds, 2002; Horowitz, Reinhardt, & Kennedy, 2005; McDonnall, 2009; Rovner, Casten, & Tasman, 2002). The reported percentages of those with visual impairment or combined visual and hearing impairments who experience depression or its symptoms have ranged from 28% to 43%, while percentages for the general population are reported to be between 8% and 16% (Blazer, 2003). This represents a major public health concern for these populations, as the experience of depression or symptoms of depression can negatively impact all areas of a person's life (Blazer, 2003).
Although a substantial amount of research related to single sensory loss has been conducted, fewer studies involving persons with DSL have been completed. The relationships documented between vision loss and negative outcomes can be expected to be the same for persons who experience hearing loss in addition to vision loss. The limited research that has been conducted in this area has confirmed that DSL is associated with depression (Crews & Campbell, 2004; Capella-McDonnall, 2005; Chou & Chi, 2004; Chia et al., 2006).
The majority of the research that has established a relationship between sensory loss and depression has been conducted cross-sectionally. It is important to study this relationship longitudinally, as results may differ from those conducted at one time point and the causal relationships can be investigated with longitudinal analysis (Cohen, Cohen, West, & Aiken, 2003). Only a small number of studies have evaluated the longitudinal relationship between sensory loss and related outcomes. Just one of these studies evaluated the effects over time of acquiring a sensory loss (Sloan, Ostermann, Brown, & Lee, 2005) and only a few have included persons with a DSL (Chou, 2008; LaForge, Spector, & Sternberg, 1992; Reuben, Mui, Damesyn, Moore, & Greendale, 1999). This longitudinal research involving persons with DSL focused on functional status and utilized small samples. Further, all but one of these longitudinal studies related to sensory loss involved analyzing data at only two time points. This allows for the control of initial differences between subjects, but does not allow for the determination of the trajectories of outcomes of interest. It also precludes the use of modern longitudinal analytic methods, such as growth curve modeling (Singer & Willet, 2003). Utilizing growth curve modeling, researchers can determine whether the development of DSL causes a change in individuals' depression and the effect of experiencing DSL on depression over time.
The one longitudinal study that evaluated the effects of acquiring a vision loss (Sloan, Ostermann, Brown, & Lee, 2005) found that depressive symptoms significantly increased, although the effect size was small. A limited number of studies have evaluated the impact of length of vision loss on psychological status. One such study found an association between shorter duration of vision loss and increased levels of emotional distress (Williams et al., 1998), while other studies provide evidence that depression in persons with vision loss persists over time (Horowitz, Reinhardt, McInerney, & Balistreri, 1994; Rovner, Zisselman, & Shmuely-Dulitzki, 1996).
Currently, research is lacking on the effects of acquiring a DSL on depression and the longitudinal relationship between DSL and depression. In addition, the effect of developing a DSL after already experiencing a single sensory loss has not been studied. It is possible that acquiring one loss first and having time to adjust to that loss would lessen the impact of experiencing DSL. This supposition particularly applies to vision loss, as the association between vision loss and depression is much stronger than between hearing loss and depression. Based on theory and prior research, the onset of DSL is expected to cause a period of increased depressive symptoms. The onset of a disability is often associated with an increase in depression, which requires a period of adaptation or adjustment (Livneh & Antonak, 2005; Rovner, Zisselman, & Shmuely-Dulitzki, 1996). Assuming that adjustment does eventually take place following the onset of DSL, we would expect to see a decrease in depression over time, after the initial increase. Whether the level of depression will return to pre-DSL levels is unknown. If it does return to its initial level, we do not know the average length of time taken for this process to occur.
This study proposes to fill these gaps in the existing research by investigating the trajectory of depression for older adults who develop a DSL. Two primary hypotheses were investigated:
Growth patterns in depressive symptoms will be associated with the development of DSL.
Vision loss and hearing loss status prior to the development of DSL will be associated with growth patterns in depressive symptoms.
Methods
Data
Data were obtained from the Health and Retirement Study (HRS) and the Aging and Health Dynamics study (AHEAD). These nationally representative panel studies were initially conducted separately, but data collection was combined in 1998 and since referred to as HRS. HRS is an ongoing study conducted by the Institute for Social Research at the University of Michigan. Its focus is economic resources and retirement, but data collection covers a wide range of topics, including physical and functional health, disability, employment, cognitive status, and housing. Data collection has occurred every two years since 1992 for HRS. Data collection for AHEAD began in 1993, was repeated in 1995, then was combined with HRS in 1998. HRS includes 22,000 participants who were born between the years of 1931-1941 or before 1923 and their spouses. Data from the years 1993, 1994, 1995, 1996, 1998, 2000, 2002, 2004, and 2006 were used for this research.
Sample
The population of primary interest is older persons who experienced a dual sensory loss (DSL) during the course of the study. A small number of participants reported a DSL at the first data collection time. As the purpose of this study is assessing the effects of developing a DSL on depression, these people were excluded from the analyses. The sample consists of two groups: (a) persons who developed DSL during the study and did not at a later time report improved hearing or vision (the DSL group) and (b) an equal number of persons who did not report sensory loss during the study, matched to the DSL group based on age and gender (the comparison group). The purpose of the comparison group was to illustrate differences in the experience of depressive symptoms over time between persons who develop DSL and those who do not experience sensory loss. Stratified random sampling (with gender and age at the first observation point for the DSL group being the strata) was used to select the comparison group. Age was used for sample stratification because those who experience DSL are on average much older than the rest of the HRS sample and gender was used because it is known to be related to depression and the experience of sensory loss. A total of 1380 people who developed persistent DSL during the course of the study and who had depression data available were identified for the DSL group. Because sensory loss is common in old age, there were not enough people without sensory loss to match to the DSL group in the oldest age groups. All available sample members without sensory loss over the age of 75 were included in the comparison sample; this resulted in 1309 people for the comparison group. Rather than adding additional younger people to the comparison group, unequal group sizes were used. Approximately 12% of persons eligible for the DSL sample and 15% of persons eligible for the comparison sample had to be excluded due to missing depression data.
The total sample size was 2689, and a total of 13,460 observations were used in the analyses. Number of observations per person ranged from 2 to 7. A variety of intermittent missing data patterns were present in the data for a small percentage of participants; however, the most commonly occurring missing data pattern was dropout from the study. Because the HRS data includes information on why people are not present in the data by waves, it was possible to obtain reasons for missing data in the majority of cases. The most common reason for missing data for both groups was death, followed by use of a proxy to complete the interview for persons in the DSL group. (In cases of a proxy interview, CES-D items are not asked, but other information is obtained. Therefore, persons who developed DSL were more likely to have missing depression data after the DSL was reported, due to proxy interviews.) Inspection of the patterns of missingness based on depression score were conducted and no differences were found. The data are assumed to be missing at random, based on these analyses and on the knowledge that depression scores are highly correlated over time.
Variables and Measures
Time
Time was measured in terms of months since baseline. Months were then converted to years for these analyses. The time variable associated with the first available data for each person (regardless of which wave it is from) was assigned a value of zero. The next time point was assigned an exact value based on the number of years and months since the previous data was collected. The second time point was approximately “2” for most participants; the third was approximately “4”, etc., as the waves are spaced approximately two years apart. One caveat to this rule was for participants who were originally in the AHEAD data sample, which had a three-year difference in one wave administration.
A second time-related variable associated with the development of DSL was included in the models. This time-varying predictor, labeled “Time-Post DSL,” documents the passage of time after the development of DSL. For all time points prior to DSL and the first time reporting DSL, its value is zero. After the development of DSL, each individual's values on Time and Time-Post DSL increase at the same rate (Singer & Willet, 2003). This variable provides the difference in slopes of depression after a person experiences DSL.
Sensory Impairments
All sensory impairment measures were self-report. The questions related to eyesight and hearing remained the same throughout HRS data collection. The question used to measure vision loss was: “(With your glasses), Is your eyesight excellent, very good, good, fair, or poor?” Legally blind was a sixth category available for this question, if the person volunteered that information. Vision loss was identified by a report of fair eyesight, poor eyesight, or legal blindness. The question used to measure hearing loss was: “(With your hearing aid) Is your hearing excellent, very good, good, fair, or poor?” A report of fair or poor hearing was identified as a hearing loss. Vision loss and hearing loss were modeled as time-invariant variables, measured at the time point prior to the report of DSL. Almost two-thirds of the DSL sample experienced one sensory loss prior to the DSL: 32% had a vision loss and 33% experienced a hearing loss. The remaining 35% reported both sensory losses at the same time point.
Persons were identified with DSL when they reported both vision loss and hearing loss at the same time point. This dichotomous variable had a value of “0” prior to the person reporting both hearing and vision loss and a value of “1” at and after this initial report. It provides the magnitude of the shift in elevation in depression when a person experiences DSL.
Depression
Depression was measured with the shortened Center for Epidemiologic Studies Depression scale (CES-D). Only respondents who answered items for themselves (rather than by proxy) were asked these questions. The original CES-D, one of the most widely-used measures of depression, contains 20 items that are rated on a four-level frequency scale (Radloff, 1977). The shortened version of the instrument used in HRS consists of 8 of these 20 items, rated with a yes-no response. Rather than asking how often the person experiences the feelings (i.e., symptoms of depression), the respondent is asked whether the statements are true for him or her much of the time during the past week. Responses to these eight items were summed, with responses indicative of depression given a score of 1. Therefore, scores ranged from 0 to 8 with higher scores associated with greater depression. The HRS Health Working Group (Steffick, 2000) evaluated the psychometric properties of this abbreviated CES-D scale. They determined that the scale shows good internal consistency, with Cronbach's alphas ranging from .77 to .83. Using principal components analysis, two factors were identified: depressed mood and somatic complaints. Analyses conducted by the group documented that non-response was not a large problem with the CES-D items. However, to maximize the sample size, responses for respondents who missed only one item (n = 91) were imputed with individual mean score substitution for the missing item.
Covariates
Several person-level variables that are not of focal interest to this study are known to be related to depression in older adults. Generally the use of a longitudinal design precludes the need to include variables in the analyses that remain relatively stable over time (Fitzmaurice, Laird, & Ware, 2004). However, three time-invariant variables were included as covariates in the models: minority status, gender, and age. The prevalence of visual impairments and specific conditions that cause visual impairment are known to differ based on race/ethnicity and gender (Massof, 2002), and it is relevant to determine if differences exist in the experience of depressive symptoms for these populations. Minority status was a dichotomous variable, with White persons coded as “0” and persons of any other race or Hispanic origin coded as “1.” Gender was included to determine whether the experience of DSL on depression is the same in males and females, and to control for it if an effect was found. Age at first time point in the study was included as there is a wide age range in the sample and this may influence the experience of depression.
Analytic techniques
Data Analysis Method
The statistical technique used to analyze the data and test the hypotheses was multilevel modeling. Two primary advantages to this method are that it allows for an estimation of individual change trajectories as a function of person-specific parameters and random error, and it allows for the number and timing of observations to vary randomly across participants (Raudenbush & Bryk, 2002). In other words, with multilevel modeling the researcher can determine the average rate of change and individual variability in change over time, and can utilize all observations in the estimation of parameters, if they include at least one time point.
The statistical models have two levels: (a) the level-1 model, referred to as the individual growth model, which represents the change in the outcome measure experienced by each respondent over time and (b) the level-2 model which represents differences in changes in the outcome measure across respondents. SAS version 9.2 (SAS Institute, Inc., Cary, NC), and specifically the PROC MIXED procedure with full maximum likelihood estimation, was used for the analyses. Prior to initiating hypothesis testing, the HRS dataset was converted from its current person-level format to a person-period format. Exploratory analyses of individual respondent's change over time on the outcome variable were conducted to guide specification of the type of level-1 growth trajectory appropriate for the models.
Hypothesized Change Trajectories
This research was focused on an event occurring during the study (i.e., development of DSL), which was expected to cause an increase in the outcome variable. It was therefore hypothesized that individual change in depressive symptoms would be discontinuous at this event. In other words, the models proposed here do not predict smooth individual change trajectories for persons who develop DSL, but rather individual change trajectories that are discontinuous at the development of DSL. These are also referred to as piecewise linear growth models (Fitzmaurice, Laird, & Ware, 2004; Raudenbush & Bryk, 2002). Because participants in the study developed DSL at different times, the model involved person-specific discontinuity. Development of DSL is hypothesized to cause an increase in both elevation and slope of the trajectories. The inclusion of the dichotomous variable “DSL” allowed for the assessment of an increase in elevation, while the time-related variable “Time-Post DSL” allowed for determination of whether the rate of change in depressive symptoms increases after DSL (Singer & Willett, 2003).
Results
Descriptive statistics for the two groups that comprise the sample are provided in Table 1. The groups have a similar gender and age make-up as they were matched on these variables. Because of the necessity to add additional comparison group members in the oldest age groups, regardless of gender, they do not match exactly on the two variables. Differences were noted between the groups in terms of race and education (more minority group members and lower levels of education in the DSL group).
Table 1. Descriptive Statistics of DSL and Comparison Group Samples.
| Variable | DSL Group | Comparison Group |
|---|---|---|
| Gender – Male | 45.9% | 46.6% |
| Age range | 38 – 95 | 40 – 93 |
| Average age | 69.15 (10.91) | 67.86 (10.14) |
| Race/ethnicity | ||
| White, Non-Hispanic | 74.8% | 84.1% |
| Black/African American | 13.6% | 10.5% |
| Hispanic | 10.3% | 3.4% |
| Other | 1.3% | 2.0% |
| Education level | ||
| Less than high school | 40.1% | 20.6% |
| High school/GED | 46.8% | 53.3% |
| College degree (2 or 4 year) | 9.3% | 16.2% |
| Master's or Professional degree | 3.8% | 9.9% |
The model-fitting method recommended by Singer and Willett (2003) was followed to investigate the hypotheses. Two simple models, the unconditional means model and the unconditional growth model, were examined first (results for some of the models are provided in Table 2, referenced by number). The unconditional means model (Model 1), which does not include any predictors, partitions the total variance in depression. The unconditional growth model (Model 2), which includes time and its quadratic as its only predictors, determines whether depression significantly changes over time and whether between-person differences in change are due to individual differences in initial status or rate of change. Both models serve as baselines for comparison to later models. Exploratory analyses of the data suggested that the change trajectory for depression might be curvilinear; therefore the necessity of a quadratic term in the model was evaluated. Group membership was entered into the model first, followed by DSL and Time-Post DSL. The covariates were entered into the model as the last step in model building to test Hypothesis 1. To test the second hypothesis, the best fitting model from Hypothesis 1 was used as the base model, and prior hearing loss and vision loss were added to it.
Table 2. Results of Model Building: Estimates of Fixed and Random Effects.
| Parameter estimates (SE) for Depression | |||
|---|---|---|---|
| Model 2 | Model 3 | Model 5 | |
| Fixed effects | |||
| Intercept | 1.31** (0.03) |
0.79** (0.04) |
0.47** (0.05) |
| Time | 0.085** (0.01) |
0.075** (0.01) |
0.083** (0.01) |
| Time2 | -0.003* (0.001) |
-0.004** (0.001) |
-0.004** (0.001) |
| Group | 1.02** (0.05) |
0.86** (0.06) |
|
| DSL | 0.37** (0.06) |
0.39** (0.06) |
|
| Time-post DSL | 0.19** (0.04) |
0.19** (0.04) |
|
| Time-post DSL2 | -0.02* (0.006) |
-0.02* (0.006) |
|
| Gender | 0.46** (0.05) |
||
| Minority | 0.48** (0.07) |
||
| Minority* time | -0.04** (0.01) |
||
| Vision Loss | 0.34** (0.08) |
||
| Variance components | |||
| Within- Person | 1.62** (0.03) |
1.61** (0.03) |
1.61** (0.03) |
| Intercept | 1.86** (0.09) |
1.60** (0.07) |
1.50** (0.07) |
| Time | 0.06** (0.01) |
0.009** (0.001) |
0.008** (0.001) |
| Time2 | 0.0004** (<0.001) |
--a | --a |
| DSL | 1.36** (0.18) |
1.37** (0.18) |
|
| Time-post DSL | 0.062* (0.02) |
0.062* (0.02) |
|
| Goodness of fit | |||
| -2LL | 51151.7 | 50532.4 | 50390.4 |
| AIC | 51171.7 | 50568.4 | 50434.4 |
p < .01,
p < .001,
Models could not be estimated with Time2 included.
Results from the unconditional means model indicate that approximately half of the total variation in depression is attributable to differences among people. Inclusion of a quadratic term for time was necessary to improve model fit of the unconditional growth model. All fixed and random effects were statistically significant. Results from fitting this model indicate that the average person began the study with a depression score of 1.32 (on a scale of 0 to 8). This depression score increased slightly over time, but this increase slowed as time went on, resulting in a slight curvilinear, rather than linear, pattern to the growth in depression. The small value of the parameter associated with Time2 indicated that the curve is very slight, and mathematical calculations were used to determine that the quadratic trajectory peaks at approximately 14 years after the first observation. The significant random effects indicated that variation exists in both initial depression scores and in growth rates of depression scores across people.
Hypothesis 1: The effect of DSL on growth patterns in depression
To determine the effects of experiencing a DSL on depression over time, three additional predictors were added to the model: DSL, time since experiencing the DSL (Time-post DSL), and the quadratic term associated with time since experiencing DSL (Time-post DSL2). In addition, a variable identifying the group the person belonged to (would experience DSL – the DSL group – or would not experience sensory loss – the comparison group) was included in the model to determine whether differences in levels of depression existed between those groups of people prior to the development of DSL. Models were tested with and without variance components associated with DSL and time after experiencing DSL. Time-post DSL2 was also tested as a random effect but the model could not be estimated with the data. Including these variance components (random effects) for DSL and Time-post DSL provided a much better fit of the model (difference in deviance statistics of 45.1, which far exceeds the .001 critical value of the chi-square distribution with 7 degrees of freedom). This model is presented in Table 2 as Model 3. All fixed and random effects retained in Model 3 were significant. Covariates used for control were added to the model next (Model 4). Interaction terms between DSL and gender, age, and minority status were also included in the models to determine whether the experience of DSL differed based on these factors. None of these interaction terms were significant and were therefore not retained. Women and minorities were found to have a higher initial score on depression, and minorities were also found to have a smaller increase in depression over time compared to Whites.
Results indicated that experiencing DSL causes a vertical displacement of the depression trajectory, as well as an increase in the slope of the trajectory. This vertical displacement was a significant increase of 0.39 in depression (at the first time point reporting it), and depression increased at a rate much faster after developing DSL than it did for those who did not experience DSL. This rate of increase was large initially, but gradually decreased over time, as illustrated by the significant quadratic parameter. Because of the curvilinear nature of the growth curve, after a long period of time, persons with DSL actually had a slower rate of increase in depression than those without DSL. The values of both parameters associated with the time since experiencing DSL and its quadratic were substantially larger than the values associated with time. This indicates that the increase in depression after experiencing a DSL was greater initially following the DSL, but that the size of this effect decreased more quickly over time. This increase peaked at approximately 5 years and 3 months after the first report of DSL for White subjects. The increase peaked sooner for minority subjects, at approximately 4 years and 7 months. Of interest also was the large value of the parameter associated with Group. Persons who would develop DSL at a later time point started out with a depression score that was on average .86 points higher than those who did not develop sensory loss during the study. The significant variance components associated with DSL and Time-post DSL indicated that the effects of experiencing DSL vary randomly across people, including both the initial effects and the effects over time.
Hypothesis 2: The effect of hearing loss and vision loss prior to DSL
To determine whether having one sensory loss prior to developing DSL (as opposed to developing both sensory losses during the same time period) had an effect on growth patterns in depression, two time-invariant variables representing vision loss and hearing loss were incorporated into the level-2 models of the best fitting model from Hypothesis 1 (i.e., Model 4, without age, which was nonsignificant). These variables were measured at the time point immediately preceding a person's report of DSL and were incorporated into each level-2 model. Only one parameter was significant: vision loss was significantly associated with initial status on depression. Persons who experienced a vision loss prior to developing a DSL had an initial depression score that was an average of 0.34 points higher. They did not experience any difference in change over time in depression compared to others with DSL. Experiencing hearing loss prior to a DSL was not associated with initial status or change over time in depression. Neither variable was related to the effect of DSL on depression. Model 5 represents the final model developed from testing the hypotheses, with only significant variables retained. The fit of this model, which includes vision loss but not age, was a significant improvement over the fit of Model 4.
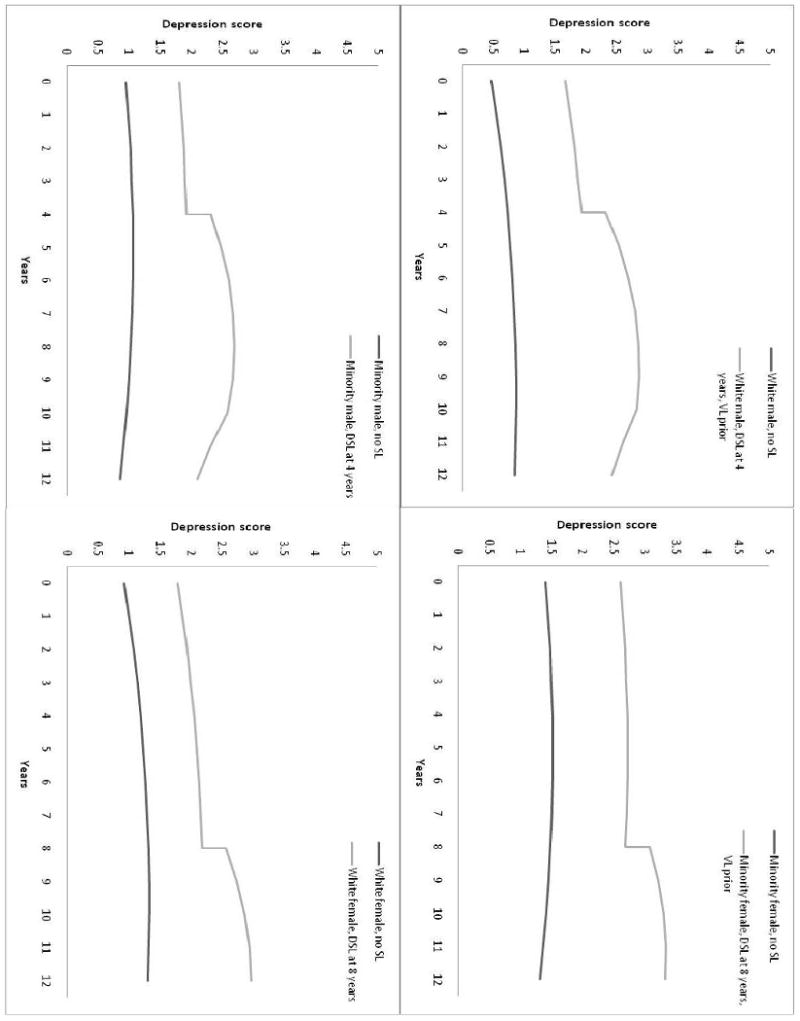
The figure illustrates the results of fitting Model 5 to data. Results are presented by race and gender as these groups exhibited different levels or trajectories of depression, although the impact of DSL was the same for each subgroup. In panel 1, growth trajectories of depression for white males with and without DSL are displayed. The men in this example developed DSL at 4 years after the initial time point in the study, after first experiencing vision loss prior to the DSL. (The average length of time for DSL to develop in white males in this study was approximately 7 years, but for illustrative purposes an earlier point of development was selected.) As illustrated on the graph, there is an increase in depression at the point of first report of DSL, and a substantially faster increase in depression from that point forward until approximately 5 years later, compared to males without sensory loss. After 5 to 6 years, the growth rate of depression for men with DSL slows, and in fact becomes slower than men without DSL at approximately 12 years (8 years post-DSL). In panel 2, growth trajectories of depression for minority females with and without DSL are displayed. In this example, the females who developed DSL had a vision loss prior to the DSL, and their DSL was first reported at 8 years post study origin (which is the average length of time until report of DSL for females in the study). The increase in depression at the point of first reporting DSL is seen again (as this effect was the same for males and females), and growth in depression proceeds in the same way, although at a slightly slower rate of increase. The primary differences are the higher levels of depression for them compared to the males, as minorities and females both had higher initial depression values, and the flatter slope for minorities. In panels 3 and 4 results for minority males and white females are provided.
Figure 1. Effects of DSL and Pre-existing VL on Depression, by Race and Gender.
Discussion
The primary purpose of this study was to determine the growth trajectory of depression for older adults who develop a DSL in later life. This growth trajectory was compared to the trajectory of depression for a matched sample of older adults who did not experience sensory loss. Results indicate that people experience a significant increase in depression at the first time period reporting DSL. This effect remained even if they experienced a vision loss prior to the DSL, although a pre-existing vision loss did increase initial depression scores. Having a hearing loss that pre-existed the DSL did not have any effect on initial depression scores, and neither variable had an influence on change in depression over time. Having a single-sensory loss prior to experiencing DSL also did not change the impact the DSL had on depression. In addition to the increase in depression at the first report of DSL, these persons also experienced a larger increase in depression over time, compared to their increase prior to DSL and to persons who did not develop sensory loss. The trajectory of this growth was curvilinear, peaking approximately 5 years after the initial report of DSL.
Significant variability in the effect of acquiring a DSL and its effect over time also existed, indicating that the effect of DSL on depression varies randomly across people. The fixed effect values represent the average initial effect of the DSL and its effect over time, but these effects exhibited marked variability across individuals. Some people likely will experience little or no effect on depression from developing DSL, while others will experience much larger effects than the average. The presence of this variability indicates that additional explanatory variables would be useful in evaluating the characteristics of persons likely to experience a negative effect from developing a DSL. Results from this study indicate that the effect of DSL did not differ based on gender, race, or age, but other explanatory variables should be investigated.
One variable that may explain some of this variability is receipt of rehabilitation services. Prior research has indicated that training in blindness skills and the use of aids (such as hearing aids and low vision optical devices) are associated with a reduction in depression and improvement in other areas of psychological functioning for consumers with a single sensory loss (e.g., Horowitz, Reinhardt, & Boerner, 2005; Mulrow, Tuley, & Aguilar, 1992). Research involving persons with DSL should be conducted to determine if receiving rehabilitation services impacts their trajectory of depression also.
Although experiencing a DSL did cause an increase in depression initially and over time as expected, a large difference in depression scores between those who develop DSL and those without sensory loss occurred prior to the development of the DSL. This difference existed between the groups at the outset of the study, many years prior to the time the majority of participants developed DSL. This suggests that there are significant pre-existing differences between the groups, in other areas in addition to depression. An insignificant decrease in this group effect occurred when the covariates were added to the model, and a larger, but still relatively small, decrease occurred when vision loss was added to the model. Previous studies, with the exception of one (Sloan, Ostermann, Brown, & Lee, 2005), that investigated the relationship between visual impairment or DSL and depression included people who began the study with the impairments. These studies have documented the strong association between these sensory impairments and depression, but this is the first study to show that those with DSL have higher levels of depression years prior to developing the impairments.
Why these differences in depression exist, even prior to experiencing a DSL, is a pertinent question. As noted previously, persons in this sample who developed DSL were less educated and more likely to be members of a minority group than persons who did not develop sensory loss. Previous research has documented that persons with DSL or visual impairments are more disadvantaged than those without sensory loss (Capella-McDonnall, 2005; Horowitz, Brennan, & Reinhardt, 2005; Klein, Klein, & Jensen, 1994; Tielsch, Sommer, Katz, Quigley, & Ezrine, 1991; Horowitz, Reinhardt, & Kennedy, 2005). Persons with visual impairments or combined visual and hearing impairments are more likely to have lower income and education levels, poorer health, and be members of racial/ethnic minority groups. They are also more likely to have additional chronic conditions, diseases, or disabilities, including major depression. In addition to these differences, research has documented that persons with visual impairment and DSL have lower levels of social support available to them (Capella-McDonnall, 2005; Horowitz, Brennan, & Reinhardt, 2005). Relationships between all of these variables and depression are well-established (Lynch, Kaplan, & Shema, 1997; Kennedy, Kelman, & Thomas, 1989; Taylor & Lynch, 2004; Jang, Haley, & Small, 2002). It is possible that the effect of group membership on depression seen in this study can, at least in part, be explained by differences in these areas. Previous research has documented that persons with single sensory loss and DSL are more likely than persons without sensory loss to experience symptoms of depression, even with many of these socioeconomic and social factors controlled for (Capella-McDonnall, 2005), which may represent the effect of experiencing the DSL illustrated in this study, or may indicate that other differences between the groups exist.
Implications
The curvilinear growth pattern exhibited in depression following DSL is indicative of an adjustment process taking place. Experiencing the DSL causes an initial spike in depression, as expected with the onset of a disability, which is often a traumatic experience that causes increased stress for the individual (Livneh & Antonak, 2005). Often people with sensory losses try to continue to function as they did prior to the loss, even when the methods they used previously are no longer effective. With mild sensory deficits, traditional modes of functioning will frequently work, even if they are not the most effective way of doing things. As deficits increase, which they often do as the person ages, these methods usually become ineffective. Over time, depression increases, which may be associated with further deterioration of hearing and vision and with the increased inability to function as one did prior to the sensory losses. However, as the person lives with DSL over the course of many years, adjustment will usually take place, in terms of a cognitive acceptance of the losses and their permanency and an affective acceptance of oneself as a person with sensory losses (Livneh & Antonak, 2005). As adjustment occurs, depression decreases.
How this adjustment occurs and methods that could be used to expedite the process are of interest. Adjustment may possibly be hastened by the use of two coping modes: assimilative and accommodative (Brandtstadter & Rothermund, 2002). Learning about and using alternative techniques to accomplish activities (an example of the assimilative coping mode), may help individuals adjust to sensory losses. Alternatively, accepting that one can no longer perform certain activities, such as driving (the accommodative coping mode), may also help in the adjustment process. Research with persons with visual impairments has provided support for the importance of these modes of coping to positive mental health outcomes, including lower levels of depression (Boerner, 2004).
Future Research
As previously mentioned, it is important to investigate the reason for the pre-existing differences in depression between persons who eventually develop DSL and those who do not. In addition, further investigation of what factors contribute to the increase in depression caused by developing a DSL is warranted. Developing a DSL was shown in this study to cause an increase in depression, both initially and over time. However, these effects were also shown to vary significantly across people. It is important to identify variables that influence this effect. Many variables could potentially moderate the relationship between DSL and depression, and some of the variables that have a positive impact are likely associated with personal characteristics of individuals, such as optimism or marital status. The positive effects of other factors, especially factors that can be changed or influenced by the individual, should also be considered. Research has not yet been conducted on the ability of adaptable factors to moderate the relationship between DSL and depression. The focus on adaptable variables is important because of their potential to be included in interventions and their potential ability to offer individuals some control in reducing their experience of depressive symptoms. Variables such as receipt of rehabilitation services, physical status (exercise and physical condition), and productive activities (employment, volunteer work and informal helping) should be evaluated as to their ability to mitigate the negative impact of DSL on depression. Future research will focus on the impact of these variables on depression for this population. Finally, research that focuses on the adjustment process associated with adapting to DSL is warranted, to increase our understanding of this process and identify coping methods and strategies than can expedite this process.
Limitations
A limitation of the study is the use of self-report sensory data rather than clinically measured data. It is recognized that there will be some differences in the DSL population identified by self-report as opposed to measured acuities. Based on previous research findings, one can assume that persons identified with DSL through self-report will include a number of individuals who experience a functional vision loss, rather than an actual (i.e., uncorrectable) visual impairment (Massof, 2002; Klein, Klein, Linton, & De Mets, 1991), and a number of people misclassified as to hearing loss, with a greater likelihood of excluding those with a measured hearing impairment than including persons with normal hearing (Nondahl, Cruickshanks, & Wiley, 1998; Valete-Rosalino & Rozenfeld, 2005). In addition to the differences suspected based on research, other unknown differences could exist between the two populations. Self-report sensory data is considered valuable because it represents the functional sensory experience of the individual. A response of “fair” or “poor” in either sensory modality is indicative of the individual's perception of some amount of loss. The use of self-report sensory data in this study creates an evaluation of the relationship between self-perceived functional dual sensory loss and depression rather than clinically measured dual sensory loss and depression, and this distinction must be considered in the interpretation of results. The fact that both depression and sensory loss data are self-report also allows for the possibility that the sensory loss data could be impacted by the presence of depression, rather than the reverse which is assumed in this study.
Another limitation of the study is the fact that a larger percentage of persons with DSL do not have depression data available, due to proxy interviews. As the HRS data is collected by face-to-face or phone interviews, proxy interviews were likely conducted because of the hearing loss of the participants with DSL. (Note that prior to developing the DSL, percentages with missing depression data were similar for the two groups.) If this assumption is correct, those persons with the most significant hearing losses did not provide depression data. Therefore, this study should be considered an evaluation of the effects of developing DSL that is mild enough to allow conversation. Effects for persons who have a DSL that is more severe may be different.
Acknowledgments
This research was supported by National Institute on Aging grant number R03-AG029355.
References
- Blazer DG. Depression in late life: Review and commentary. Journal of Gerontology: Medical Sciences. 2003;58A(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Boerner K. Adaptation to disability among middle-aged and older adults: The role of assimilative and accommodative coping. Journals of Gerontology B: Psychological Sciences. 2004;59B:P35–P42. doi: 10.1093/geronb/59.1.p35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtstadter J, Rothermund K. The life-course dynamics of goal pursuit and goal adjustment: A two-process framework. Developmental Review. 2002;22:117–150. [Google Scholar]
- Brennan M, Horowitz A, Su Y. Dual sensory loss and its impact on everyday competence. The Gerontologist. 2005;45(3):337–346. doi: 10.1093/geront/45.3.337. [DOI] [PubMed] [Google Scholar]
- Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, Dolnak D, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900. doi: 10.1016/s0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- Caban AJ, Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Prevalence of concurrent hearing and visual impairment in US adults: The National Health Interview Survey, 1997-2002. American Journal of Public Health. 2005;95:1940–1942. doi: 10.2105/AJPH.2004.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults – United States, 1993-1997. Morbidity & Mortality Weekly Report. 1999;48(SS8):131–156. [PubMed] [Google Scholar]
- Capella-McDonnall ME. The effects of single and dual sensory loss on symptoms of depression in the elderly. International Journal of Geriatric Psychiatry. 2005;20:855–861. doi: 10.1002/gps.1368. [DOI] [PubMed] [Google Scholar]
- Casten RJ, Rovner BW, Edmonds SE. The impact of depression in older adults with age-related macular degeneration. Journal of Visual Impairment and Blindness. 2002;96(6):399–406. [Google Scholar]
- Chia E, Mitchell P, Rochtchina E, Foran S, Golding M, Wang JJ. Association between vision and hearing impairments and their combined effects on quality of life. Archives of Ophthalmology. 2006;124:1465–1470. doi: 10.1001/archopht.124.10.1465. [DOI] [PubMed] [Google Scholar]
- Chou K. Combined effects of vision and hearing impairment on depression in older adults: Evidence from the English Longitudinal Study of Ageing. Journal of Affect Disorder. 2008;106:191–196. doi: 10.1016/j.jad.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Chou K, Chi I. Combined effects of vision and hearing impairment on depression in elderly Chinese. International Journal of Geriatric Psychiatry. 2004;19:825–832. doi: 10.1002/gps.1174. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, NJ: Lawrence Erlbaum Assoc; 2003. [Google Scholar]
- Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. American Journal of Public Health. 2004;94(5):823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Horowitz A, Brennan M, Reinhardt JP. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Research on Aging. 2005;27:307–325. [Google Scholar]
- Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging and Mental Health. 2005;9(6):563–570. doi: 10.1080/13607860500193500. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Reinhardt JP, Kennedy GJ. Major and subthreshold depression among older adults seeking vision rehabilitation services. American Journal of Geriatric Psychiatry. 2005;13(3):180–187. doi: 10.1176/appi.ajgp.13.3.180. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Reinhardt JP, McInerney R, Balistreri E. Age-related vision loss: Factors associated with adaptation to chronic impairment over time (Final Report) New York: The Lighthouse Research Institute; 1994. [Google Scholar]
- Jang Y, Haley WE, Small BJ. The role of mastery and social resources in the association between disability and depression in later life. Gerontologist. 2002;42:807–813. doi: 10.1093/geront/42.6.807. [DOI] [PubMed] [Google Scholar]
- Kennedy GJ, Kelman HR, Thomas C. Hierarchy of characteristics associated with depressive symptoms in an urban elderly sample. American Journal of Psychiatry. 1989;146:220–225. doi: 10.1176/ajp.146.2.220. [DOI] [PubMed] [Google Scholar]
- Kramer SE, Kapteyn TS, Kuik DJ, Deeg DJH. The association of hearing impairment and chronic diseases with psychosocial health status in older age. Journal of Aging and Health. 2002;14(1):122–137. doi: 10.1177/089826430201400107. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Jensen SC. The relation of socioeconomic factors to eye-related cataract, maculopathy, and impaired vision. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1969–1979. doi: 10.1016/s0161-6420(13)31077-x. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: Visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- LaForge RG, Spector WD, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. Journal of Aging and Health. 1992;4(1):126–148. [Google Scholar]
- Lee P, Smith JP, Kington R. The relationship of self-rated vision and hearing to functional status and well-being among seniors 70 years and older. American Journal of Ophthalmology. 1999;127(4):447–452. doi: 10.1016/s0002-9394(98)00418-8. [DOI] [PubMed] [Google Scholar]
- Livneh H. A unified approach to existing models of adaptation to disability: A model of adaptation. In: Marinelli RP, Dell Orto AE, editors. The psychological and social impact of disability. 3rd. New York: Springer; 1991. pp. 111–138. [Google Scholar]
- Livneh H, Antonak RF. Psychosocial adaptation to chronic illness and disability: A primer for counselors. Journal of Counseling & Development. 2005;83:12–20. [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. New England Journal of Medicine. 1997;337:1889–1895. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- Massof RW. A model of the prevalence and incidence of low vision and blindness among adults in the U.S. Optometry and Vision Science. 2002;79(1):31–38. doi: 10.1097/00006324-200201000-00010. [DOI] [PubMed] [Google Scholar]
- McDonnall MC. Risk factors for depression among older adults with dual sensory loss. Aging and Mental Health. 2009;3(4):569–576. doi: 10.1080/13607860902774410. [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Tuley MR, Aguilar C. Sustained benefits of hearing aids. Journal of Speech and Hearing Research. 1992;35:1402–1405. doi: 10.1044/jshr.3506.1402. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein R, Klein BE. Accuracy of self-reported hearing loss. Audiology. 1998;37(5):295–301. doi: 10.3109/00206099809072983. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Reuben DB, Mui S, Damesyn M, Moore AA, Greendale GA. The prognostic value of sensory impairment in older persons. Journal of the American Geriatrics Society. 1999;47:930–935. doi: 10.1111/j.1532-5415.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ, Tasman WS. Effect of depression on vision function in age-related macular degeneration. Archives of Ophthalmology. 2002;120(8):1041–1044. doi: 10.1001/archopht.120.8.1041. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Zisselman PM, Shmuely-Dulitzki Y. Depression and disability in older people with impaired vision: A follow-up study. Journal of the American Geriatrics Society. 1996;44(2):181–184. doi: 10.1111/j.1532-5415.1996.tb02436.x. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Sloan FA, Ostermann J, Brown DS, Lee PP. Effects of changes in self-reported vision on cognitive, affective, and functional status and living arrangements among the elderly. American Journal of Ophthalmology. 2005;140(4):618–627. doi: 10.1016/j.ajo.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study (HRS/AHEAD Documentation Report) Ann Arbor, MI: Survey Research Center, University of Michigan; 2000. [Google Scholar]
- Taylor MG, Lynch SM. Trajectories of impairment, social support, and depressive symptoms in later life. Journals of Gerontology B: Social Sciences. 2004;59(B):S238–S246. doi: 10.1093/geronb/59.4.s238. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Sommer A, Katz J, Quigley H, Ezrine S. Socioeconomic status and visual impairment among urban Americans. Baltimore Eye Survey Research Group. Archives of Ophthalmology. 1991;109:637–641. doi: 10.1001/archopht.1991.01080050051027. [DOI] [PubMed] [Google Scholar]
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Chu RM, Brown SI. The psychosocial impact of macular degeneration. Archives of Ophthalmology. 1998;116:514–520. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- Valete-Rosalino CM, Rozenfeld S. Auditory screening in the elderly: Comparison between self-report and audiometry. Revista Brasileira de Otorrinolaringologia. (English) 2005;71(2):193–200. doi: 10.1016/S1808-8694(15)31310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


