Abstract
Although personality disorders are best understood in the context of lifetime development, there is a paucity of work examining their longitudinal trajectory. An understanding of the expected course and the genetic and environmental contributions to these disorders is necessary for a detailed understanding of risk processes that lead to their manifestation. The current study examined the longitudinal course and heritability of borderline personality disorder (BPD) over a period of 10 years starting in adolescence (age 14) and ending in adulthood (age 24). In doing so, we built on existing research by using a large community sample of adolescent female twins, a sensitive dimensional measure of BPD traits, an extended follow-up period, and a longitudinal twin design that allowed us to investigate the heritability of BPD traits at four discrete ages spanning mid-adolescence to early adulthood. Results indicated that mean-level BPD traits significantly decline from adolescence to adulthood but rank order stability remained high. BPD traits were moderately heritable at all ages with a slight trend for increased heritability from age 14 to age 24. A genetically-informed latent growth curve model indicated that both the stability and change of BPD traits are highly influenced by genetic factors and modestly by non-shared environmental factors. Our results indicate that as is the case for other personality dimensions, trait BPD declines as individuals mature from adolescence to adulthood and that this process is influenced in part by the same genetic factors that influence BPD trait stability.
Personality disorders are hypothesized to be genetically influenced forms of psychopathology that have their onset in adolescence or early adulthood and show a pattern of dysfunction throughout the lifespan (APA, 1994, 2000). Personality disorders are thus developmental constructs that are best understood within a lifespan perspective. However, a number of theorists have raised concerns about the potential deleterious effects of premature labeling that could arise as a consequence of diagnosing children and adolescents with personality disorders. Indeed, earlier versions of the current diagnostic system (the Diagnostic and Statistical Manual-III-R, APA, 1987) required that an individual reach adulthood before a diagnosis of a personality disorder could be made. Because of such concerns and restrictions, much of the extant personality disorder research focuses on adults. The focus on adult samples, in turn, limits our understanding of the developmental origins of personality disorders as well as their normative trajectories across the life course. Current theoretical frameworks such as the developmental psychopathology perspective (e.g., Cicchetti & Rogosch, 2002) argue that in order to understand psychopathology at a given endpoint (i.e., in adulthood), it is necessary to examine the course and variability of dysfunction beginning at a much earlier time point, such as adolescence or childhood. Such work would enable researchers to understand the factors underlying the continuity or discontinuity of psychopathology trajectories through the lifespan (e.g., Stattin & Magnusson, 1996).
Current conceptual and empirical work holds that the understanding of normative personality development can assist in understanding the development of personality pathology (Cicchetti & Rogosch, 2002). Specifically, multiple studies provide data for the notion that personality disorders are extreme variants on normal personality dimensions rather than distinct, independent categories (Edens, Marcus, & Ruiz, 2008; Rothschild, Cleland, Haslam, & Zimmerman, 2003; Trull et al. 2003; Wilberg et al. 1999; O’Connor & Dyce, 2001). Moreover, recent work indicates etiologic (genetic and environmental) overlap (Jang & Livesley, 1999; Markon, Krueger, Bouchard, & Gottesman, 2002) and a common structural model (Markon, Krueger, & Watson, 2005; O’Connor, 2002) between normal and abnormal personality. Given this overlap between normal personality dimensions and personality disorders, it is beneficial to draw upon research documenting the course and heritability of normal personality in order to understand maladaptive developmental processes (Cicchetti, 1984, 1990; Sroufe, 1990). Current research documenting the course of normal personality dimensions shows that these dimensions show significant mean-level change but high stability (Roberts & DelVecchio, 2000) over the lifespan. In particular, traits such as negative affectivity and behavioral disinhibition show a pattern of mean-level decline over the lifespan, with the largest decline evidenced in the period between adolescence and adulthood (Blonigen, Carlson, Hicks, Krueger, & Iacono 2008; Roberts, Caspi, & Moffitt, 2001; McGue, Bacon, & Lykken, 1993), reflecting an increased maturity and flexibility (Roberts et al, 2001).Finally, processes contributing to both the stability and change in personality traits are heritable (Blonigen et al, 2008). Specifically, Blonigen et al (2008) found that the factors that influence personality traits at both age 17 and 24 (i.e., contributions to stability) have an average heritability of about .30, whereas the factors that influence age 24 personality only (contributing to change since age 17) have a heritability of about .20.
Beyond mean-level changes and stability indices of individual differences, work on how heritability changes throughout adolescence and early adulthood can also inform researchers about risk processes and person-environment transactions. Again, it is useful to draw on work with normal personality dimensions in order to inform hypotheses about character pathology. In particular, personality traits such as aggression, fearfulness, approach, and religiosity (Koenig, McGue, Krueger, & Bouchard, 2005; Matheny, 1989; Miles & Carey, 1997) as well as some forms of personality pathology (i.e., antisocial personality disorder, Jacobson et al, 2002) show an age-related linear increase in heritability and a decrease in environmental influences. Similarly, Bergen et al (2007) reported evidence for cross-time heritability increases for externalizing/antisocial behavior, mood and anxiety disorder symptoms, and substance use. In part, these changes in heritability may be accounted for by gene expression changes, as genes are switched on or off in response to environmental context (Whitelaw & Whitelaw, 2006). As an additional reason, behavior during childhood and pre-adolescence is more strongly influenced by social and/or familial environment (e.g., parental guidance) than in late adolescence and adulthood. With the transition into adolescence, however, individuals have an increased opportunity to actively select their own environments, experiences and behavior, in turn leading to increased expression of the latent genotypes. Together, this analysis indicates that examining the change in heritability rather than simply relying on cross-sectional snapshots is likely to better inform our understanding of how changes in gene-environment interplay contribute to the development of personality.
This large research literature on the normative development and genetic and environmental influences on normal-range personality constructs can inform and guide research on the development of personality disorders. One disorder in which this type of extrapolation might be especially useful is borderline personality disorder (BPD). BPD is characterized by persistent problems with emotional (e.g., emotional lability), behavioral (e.g., deliberate self-harm and suicidal behavior), cognitive (e.g., dissociation), and interpersonal (e.g., chaotic relationships) functioning (APA, 1994). Individuals with BPD exhibit heightened levels of numerous health-compromising behaviors, including deliberate self-harm and suicidal behaviors, drug and alcohol abuse, unsafe sexual behavior, and disrupted eating behaviors (APA, 1994; Frankenburg & Zanarini, 2004; Links et al., 1995; Skodol et al., 2002, 2005). Moreover, BPD frequently co-occurs with several Axis I disorders including mood, anxiety, eating, and substance use disorders (Zanarini et al., 1998; Wonderlich, Swift, Slomik, & Goodman, 1990; Trull, Sher, Minks-Brown, Durbin, & Burr, 2000). Because of the public health costs and distress to individuals and families of those with BPD, there is an especially strong need for an understanding of the etiological factors involved in its development and persistence.
As is the case for most personality disorders, BPD is considered to be a genetically influenced disorder that has its etiologic roots in childhood and its onset in adolescence. Because of this developmental pattern, it is logical to begin investigating the stability and genetic and environmental influences at this window of time. However, a number of commentaries have questioned whether adolescent BPD is meaningful, in the light of findings indicating the diagnosis may not be temporally stabile. Specifically, studies of both community and hospitalized adolescents have reported that BPD in adolescence has low diagnostic stability over a period of 2–3 years (Meijer et al, 1998; Mattanah, Becker, Levy, Edell, & McGlashan, 1995). For instance, Bernstein et al (1993) followed a large sample of community adolescents over a period of two years and found that less than a third of those originally diagnosed with BPD met criteria for the disorder at the follow-up assessment. A similar pattern of findings was reported for longitudinal studies of adult psychiatric inpatients, such that across studies only about a third of patients originally diagnosed with BPD met the criteria for the disorder at 1–3 year follow-up assessments (Zanarini, Frankenburg, Hennen, and Silk, 2003; Paris, Brown, and Nowlis, 1987; Shea et al., 2002). Because clinical lore suggests that once a personality disorder has been diagnosed, an individual is likely to meet the diagnosis indefinitely (Clark, 2009), these disappointing findings have lead researchers to conclude that adolescent BPD is not a valid construct.
However, the low temporal stability in these studies is not surprising given that in every study a dichotomous diagnosis of BPD (presence or absence of a disorder) was used. As pointed out in a recent review (Clark, 2009), a dichotomous diagnosis artificially widens the gap between individuals who are just above threshold and those that present with a subclinical level of symptoms (e.g., 4 out of 5 BPD symptoms). In turn, this categorization scheme renders it easy to switch from a clinical to a non-clinical group, leading to low diagnostic stability over time. A method that will yield more sensitive and precise measures of longitudinal stability is a dimensional assessment of BPD traits. The use of dimensional BPD scales allows the detection of slight variations in the level of symptom or trait expression. Moreover, scores on a dimensional scale can be compared to an individual’s peer group, taking into account the normative adolescent behavior and emotional functioning at a given age. As an example of this methodology, Chanen et al (2004) examined the temporal stability of BPD traits and other psychiatric disorders over a period of two years, using both a categorical and dimensional assessment of BPD. The results of this study indicated that the stability of the categorical BPD diagnosis was rather low, but the stability of BPD measured dimensionally was considerably higher (see also Lenzenweger, 1999 and Crick, Murray–Close, & Woods, 2005 for similar results). Taken together, these results suggest the utility of dimensional assessments in longitudinal BPD research.
In terms of etiological influences relatively few investigations have examined the genetic and environmental contributions to BPD characteristics. Studies testing the heritability of the temperamental vulnerability to BPD, such as the traits of affective dysregulation and behavioral undercontrol, however, have reported moderate heritability estimates of .40 to .60 (Livesley et al, 1993, 1998; Jang et al, 1996). To date, only four cross-sectional twin studies have examined the heritability of BPD traits per se and reported inconsistent results. A study focusing on a small sample of pre-adolescent twins (Coolidge et al, 2001) reported a BPD heritability estimate of 76%. However, recent studies focused on adult samples report heritability estimates ranging from zero to 70% (Torgersen 1984, 2000; Distel et al, 2008; Torgersen et al 2008; Kendler et al, 2008). The most recent studies provide a heritability estimates around 40%. For instance, in a large-scale study (2748 adult twin pairs), Distel et al (2008) investigated the heritability of BPD using a well-validated self-report measure, the Personality Assessment Inventory-Borderline Scale (Morey et al, 1991). The authors reported that BPD features are influenced by a combination of genetic and non-shared environmental factors, with a heritability estimate of 42%. Finally, in a recent large-scale study (2794 adult twin pairs) Torgersen et al (2008) reported that the heritability of BPD as measured by a diagnostic interview was 35% (also see Kendler et al 2008 for similar results).
These cross-sectional studies represent excellent steps toward determining the genetic and environmental influences on BPD. But because these studies focused almost exclusively on adults, it is difficult to make inferences about the changing nature of risk processes throughout development. Indeed, as noted above, for most behavioral phenotypes, heritability increases in the transition from adolescence to adulthood. The examination of this change in heritability affords excellent opportunities to make inferences about the transactions between personality and environment. Specific to BPD, adolescence is a period of time in which the prodromal condition or the actual disorder first manifests. As such, there is a clear need for the use of longitudinal designs that examine the genetics and the unfolding of BPD characteristics in the period between adolescence and early adulthood (cf. Lenzenweger & Cicchetti, 2005).
Current Study
The current study examined the longitudinal course and heritability of BPD traits over a period of 10 years starting in adolescence (age 14) and ending in adulthood (age 24). In doing so, we extended prior work which mostly utilized small adult and/or clinical samples, categorical measures of BPD, and relatively short (2–3 year) follow-up intervals. We took advantage of a large community sample of adolescent twins; a new, well-validated dimensional measure of BPD traits; and an extended follow-up period to investigate the heritability of BPD traits over the critical age span during which they typically first become manifest.
In examining the longitudinal course of BPD characteristics, we examined two indices of stability and change. The first was mean-level stability, an analysis examining how the level of expressed BPD varies with age. As a second index of stability and change, we explored the differential or rank-order stability of BPD traits. This index refers to the retention of an individual’s relative placement in the group over a period of time. We also examined the genetic and environmental influences on trait BPD at each of four assessment ages (14, 17, 20, & 24). Finally, we used a genetically-informed latent growth curve model to examine the genetic and environmental influences on the change and stability of BPD traits over time. Based on the studies examining the trajectories of normal personality, we expected that the mean-level BPD scores would diminish from adolescence to adulthood, whereas rank-order stability would remain moderate to high. Consistent with previous data on externalizing, internalizing, and substance use disorders (Bergen et al, 2007), we expected an increase in heritability from adolescence to adulthood. Finally, we expected a moderate to strong genetic contribution to the stability and change of BPD traits over time.
Method
Sample
Participants were adolescent female twins taking part in the Minnesota Twin Family Study (MTFS), an ongoing population-based, longitudinal study of twins and their families (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). Birth records and public databases were used to locate more than 90% of families that included a twin birth in the state of Minnesota from 1975 to 1984 and 1988 to 1994. Eligible twins and their families were a) living within a day’s drive of Minneapolis with at least one biological parent, and b) had no mental or physical handicap precluding participation. All protocols were approved by the institutional review board. Parents and children gave informed consent or assent as appropriate.
The MTFS intake sample includes an 11-year-old and a 17-year-old cohort consisting of male and female twins. However, the current study focused on the female twins, as the male twins only had BPD data at two assessment time points. Intake and follow-up assessments are scheduled to coincide with major transitions in the lives of adolescents and young adults. The younger cohort (age 11 at intake) was recruited using two methods. Approximately sixty percent of this sample was drawn from the general community with no exclusions other than the ones described above. The remaining 40% of families (enrichment sample, ES) were recruited using a procedure designed to enrich the participation of high-risk adolescents, i.e., adolescents who are at risk for the development of a childhood disruptive disorder by age 14. Specifically, half of the families were screened via an interview with the mother and retained if at least one twin exhibited elevated symptoms of ADHD or CD. These screener variables were chosen based on data from the larger MTFS study, as they serve as predictors for the onset of a childhood disruptive disorders by age 14. Previous investigations have successfully used a similar sampling method (Kim-Cohen, Moffitt, Taylor, Pawlby, & Caspi, 2005). The other half of this subset was recruited using the same methods as the larger study. As this sampling procedure was successful in elevating the prevalence of psychopathology in this cohort, a weighting procedure was used to adjust for the non-random recruitment for the portion of the sample that was screened prior to inclusion. To adjust for the unequal selection probabilities of families in this sample, we weighted each pair by the inverse of its probability of inclusion in the sample. Because selection occurred at the level of the twin pair, weights are applied at this level as well. These weights were normalized to sum to the number of families in the sample. The original MTFS sample was an equal probability sample; all twin pairs are therefore equally weighted.
BPD traits were first assessed at age 14 for the younger cohort, and at age 17 for the older cohort. Follow-up assessments of BPD traits were conducted at age 17 and 24 in the younger cohort and at ages 20 and 24 in the older cohort. Attrition rates ranged from 5–10% for any given assessment; however, if an individual dids not participant in a given follow up assessment attempts were made to recruit that individual to participate in later follow up assessments. Table 1 provides a schematic representation of the available data. Specifically, at age 14, BPD data were available for the younger cohort and the 11-year old ES twins. At age 17, data were combined for the older and younger cohorts as well as for the portion of the ES twin sample that had completed their second follow up assessment (with assessments ongoing for the majority of ES twins). At age 20, data were available for the older cohort twins, and a small number of younger cohort twins for whom personality was not assessed at the age 17 follow-up. At age 24, BPD data were available for the older and younger cohorts. In the current study, the cohorts were combined and matched by age of assessment (see Table 1 for breakdown of Ns across cohorts). It should be noted that despite the fact that subjects did not all have data available at each time point, the analytic procedures used in the current study (see below for description) provide an optimal representation of the data, as long as the data were missing at random (Little & Rubin, 1987; see Johnson et al, 2006 and Carlson & Iacono, 2006 for similar methods and analyses).
Table 1.
Schedule of Assessments across Cohorts
| Cohort | Assessment Time Point | |||
|---|---|---|---|---|
| Age 14 | Age 17 | Age 20 | Age 24 | |
| 11-year old | X | X | X * | |
| 11-year old Enrichment | X | X * | ||
| 17-year old | X | X | X | |
| Total |
N = 1118 (339 MZ pairs, 218 DZ pairs) |
N = 1492 (471 MZ pairs, 280 DZ pairs) |
N = 617 (204 MZ pairs, 107 DZ pairs) |
N = 1014 (331 MZ pairs, 176 DZ pairs) |
indicates that data are still being collected for this assessment point;
Zygosity was determined by agreement among 3 estimates: MTFS staff evaluations of the twins’ physical similarity; parents’ completion of a standard zygosity questionnaire; and twin similarity on an algorithm of ponderal and cephalic indices and fingerprint ridge count. A serological analysis was performed if the 3 estimates did not agree.
Consistent with the demographics of Minnesota for the birth years sampled, over 95% of the twins were Caucasian. Although the assessments were scheduled at specific ages, there was still slight variability in age at each assessment. Because preliminary analyses of the current data indicated that the scores on the MPQ-BPD are negatively associated with age, we used a centering procedure to regress out the age effects on BPD traits within a particular time frame. For instance, scores at the age 14 assessment actually ranged from 13.5 to 17. In order to center the BPD scores around age, we regressed these variations out of the age 14 personality assessment.
Measures
Multidimensional Personality Questionnaire- -Borderline Personality Disorder Scale (MPQ-BPD, Bornovalova et al, 2009)
The MPQ (Tellegen et al, 1982, 1988; Tellegen & Waller, 2008) is a self-report inventory that was developed through factor analysis to assess a variety of personality traits and temperament constructs frequently identified in the personality literature. The MPQ includes 11 primary trait scales which load onto three higher-order factors. The traits of Well-Being, Achievement, Social Closeness, and Social Potency load onto the higher-order factor of Positive Emotionality (predisposition to experience positive affect); the traits of Stress Reactivity, Alienation, and Aggression make up the higher-order factor of Negative Emotionality (the predisposition to experience negative affect); the traits of Control, Harm Avoidance, and Traditionalism load on the higher-order factor of Constraint (predisposition to behavioral self-control, the converse of disinhibition); and the trait of Absorption (the tendency to experience vivid and compelling images and become easily engrossed in sensory stimuli) does not load preferentially on any of the higher-order factors. Scores from the traits scales of the MPQ have demonstrated high internal consistency (Cronbach’s alphas range from .74 to .84; Tellegen, 1982; Tellegen & Waller, 2008). The 198-item version of the MPQ was administered at ages 17 and 24. A shorter version (133 items), administered at ages 14 and 20, included 6 of the 11 scales: Well-Being, Stress Reaction, Alienation, Aggression, Control, and Harm Avoidance (see Johnson et al, 2007 for further description of this measure). Each MPQ item was answered “definitely true,” “probably true,” “probably false,” or “definitely false” and assigned a score from 1–4.
The MPQ-BPD is a 19-item scale developed through item and content analysis of the 198-item version of the MPQ, and was designed to be similar to the Personality Assessment Inventory-Borderline Scale (PAI-BOR, Morey et al, 1991), the BPD measures used in the Distel et al (2008) heritability study. Items on the MPQ-BPD were rated on the 4-point scale and keyed such that higher scores indicated a higher level of trait BPD. Hence, a total BPD trait score was calculated by adding the ratings on the 19 items, with possible scores ranging from 19–76.
The MPQ-BPD was developed and validated in 5 samples. Final scale items were identified after conducting various psychometric analyses in a sample of undergraduate students (n=274) and cross-validation analyses in a large community sample of twins in late adolescence (n=1132). The resulting item set was drawn from the stress reaction, alienation, control, aggression, well-being, and absorption scales of the MPQ, and scores on this scale were strongly related to the PAI-BOR in the undergraduate sample (r = .80).
A valid measure of the BPD construct should show a theoretically expected pattern of associations with childhood trauma, post-traumatic stress symptoms, substance use disorders, internalizing distress, and externalizing behaviors, in line with well-established findings (Zanarini et al, 1997; Zanarini et al, 1998; Skodol et al., 2002; Siever, Torgersen, Gunderson, Livesley, & Kendler, 2002). Consistent with expectation, the MPQ-BPD was correlated with indices of depression, anxiety, substance use disorders, and antisocial behavior in the adolescent twin sample.
To further explore the construct validity of the MPQ-BPD, we examined the relationship of the MPQ-BPD to a number of external/clinical correlates in three clinical samples that are known to have elevated rates of BPD: female prisoners, male prisoners, and urban substance users. The MPQ-BPD showed an expected pattern of associations and was significantly related to the external variables that should be associated with the latent construct of BPD: a history of traumatic exposure (r = .27); symptoms of post-traumatic stress disorder (r = .56); indices of behavioral disinhibition including a diagnosis of conduct disorder and adult antisocial behavior, criminal charges before age 17, a violent behavior composite, and measures of trait-impulsivity (rs = .19–.42); indices of internalizing distress including symptoms of depression and anxiety as well as history of past suicide attempts (rs = .31–.48); and drug/alcohol use severity including alcohol abuse and dependence scales, substance use frequency, and a number of substance dependence diagnoses (rs = .25–.42). Additionally, the MPQ-BPD was significantly related to scores on normal-range personality scales tapping negative affect (including the dimensions of distress, fearfulness, anger, neuroticism, and negative affectivity rs = .47–.64); positive affect (r = −.39) and disinhibition (including the dimensions of [lack of] socialization; sensation-seeking and impulsivity, rs = .26–.32).
As a further index of construct validity, the MPQ-BPD scores were strongly related to another continuous measure of BPD (the IIP-BPD, Lejuez et al, 2003, r = .62) as well as the DSM-based diagnosis of BPD (point-biserial correlation = .60). Beyond this general correlation, we examined the incremental validity of our new measure by testing if it was predictive of clinical/external variables even after controlling for the diagnosis of BPD. Our findings revealed that the MPQ-BPD was predictive of childhood trauma (R2Δ = .058, p < .001), depression symptoms (R2Δ = .126, p < .001), impulsivity (R2Δ = .254, p < .001), drug use frequency (R2Δ = .028, p < .01), and the self-report measure of BPD (IIP-BPD; R2Δ = .275, p < .001). This pattern of findings held even when a BPD symptom count rather than a diagnostic variable was used as a covariate. Similarly, we examined whether the MPQ-BPD was adding predictive utility beyond simply negative affectivity. The MPQ-BPD was predictive of depression symptoms (R2Δ = .076, p < .001), impulsivity (R2Δ = .179, p < .001), number of substance dependence diagnoses (R2Δ = .030, p < .05), and the IIP-BPD (R2Δ = .051, p < .001). These results suggest that the MPQ-BPD is capturing something over and above negative emotionality.
Internal consistency was high across the community and clinical samples (Cronbach’s alphas ranged from .81–.83). Thus, the MPQ-BPD demonstrates substantial construct validity across a number of criteria and shows excellent promise for use in studies of development and etiology (Bornovalova et al, 2009).
As noted above, at age 14 and 20, participants received a 133-item shortened version of the MPQ. Because this version lacked 2 items on the MPQ-BPD scale (these items are part of the original Absorption scale), scores were prorated for the age 14 and 20 assessments (mean of scores on 17 items multiplied by 19).
Statistical Analyses
Mean-level Change and Rank-Order Stability of BPD traits
We conducted two sets of analyses to assess developmental change in BPD traits: mean-level change, and rank-order stability. Mean-level change refers to the magnitude of change in the average scale scores over time for a given population. Mean-level effects were evaluated by calculating effect sizes for the change in mean score relative to the age 14 mean (see above discussion about centering age at 14). Our second index of developmental change was rank-order stability. This index refers to the consistency of the relative ordering of individuals over time and provides an indicator of the extent to which participants maintain their relative position in a group over time. In the current study, rank-order stability was assessed via the test–retest Pearson correlation coefficients for the MPQ-BPD over all follow-up points. Significance levels were adjusted with linear mixed models in SPSS to account for the nonindependence of the twin observations.
Biometric Analyses
Standard biometric models were used to estimate the influence of additive genetic, shared environmental, and non-shared environmental factors on MPQ-BPD scores at each time point. In all biometric models, the additive genetic component (A) refers to the additive effect of individual genes summed over loci on trait variance. Genetic influences are inferred if the MZ correlation is greater than the DZ correlation for a given trait. Shared environmental (C) effects refer to environmental influences that increase similarity between members of a twin pair. Shared environmental effects are inferred if the DZ correlation is more than ½ the MZ correlation. Non-shared environmental (E) effects refer to environmental factors that contribute to differences between members of a twin pair. Measurement error is also included in the estimate of e.
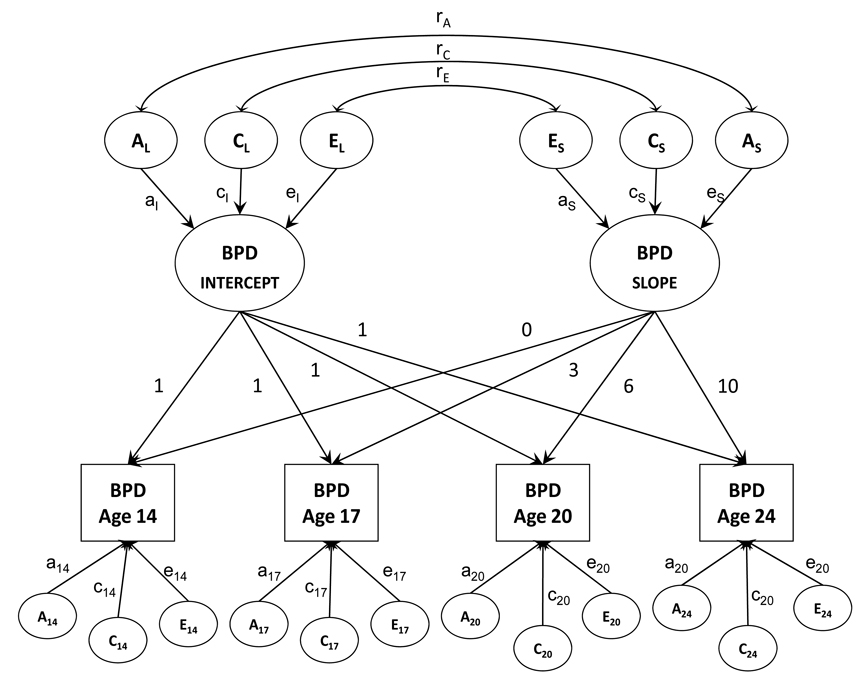
Finally, a biometric growth model was fit to the multiple waves of BPD trait data to examine the genetic and environmental effects on change and stability in BPD traits over time. All models were fit in the computer program Mx (Neale, Boker, Xie, & Maes, 1999) using raw data maximum likelihood estimation. The biometric growth model extends the latent growth model to twin data (Neale & McArdle, 2000). Figure 1 provides a graphical depiction of the model. In the growth model, BPD traits at each age are a function of an intercept (initial status) or overall level effect, and a slope or rate of change effect. The variance of the latent intercept and slope variables can be further decomposed into additive genetic, shared environment, and nonshared environmental effects. The intercept and slope variables are also allowed to correlate, and the source of their covariance can also be decomposed into genetic and environmental effects (ra, rc, re). In addition to the general intercept and slope effects, BPD traits at each age are also influenced by occasion-specific residual effects, which can also be decomposed into genetic and environmental effects. Latent growth models can easily accommodate missing data as long as the data is missing at random, a reasonable assumption for the current study. Previous work indicates that maximum likelihood estimation that uses all available data results in less bias in parameter estimates and fewer convergence failures in structural equation models (SEM) than common alternative methods such as listwise deletion, pairwise deletion, regression based imputation, or mean imputation (Enders & Bandalos, 2001) under the assumption of data as missing at random. Moreover, simulation studies (Newman, 2003) support the idea that using maximum likelihood estimation with all available data under the assumption that data are missing at random results in solutions with less bias, smaller standard errors, more stable estimates of population parameters over successive repetitions, and fewer inadmissible solutions than these other methods. This is true even when 50% of the data are missing at random. Even if the data are not missing at random, a maximum likelihood approach using all available data is no worse than and often better than other approaches when as much as 50% of data are missing.
Figure 1.
Note: Initial biometric growth model (all paths included) for initial BPD status and BPD change over time. BPD refers to borderline personality disorder traits. The model allows for BPD scores to be observed at four assessment points (age 14–17). Each assessment point is assumed to reflect an effect of initial status (intercept), change (slope), and assessment-specific additive genetic (A), shared environmental (C), and nonshared envoronmental effects (E). The intercept and slope effects are also decomposed into correlated additive genetic, shared environmental, and nonshared environmental effects. Subscripted numbers refer to age of measurement; numbers above the paths from initial level and slope to occasion-specific scores refer to years from initial assessment.
Model fit was evaluated using the likelihood ratio test. That is, the −2 x loglikelihood (−2lnL) of the full growth model was compared to the −2lnL of nested model that removed non-significant parameters from the model. Differences between models in the likelihood are distributed as a χ2 with degrees of freedom equal to the difference in the number of model parameters. In addition to the χ2 statistics, we also used several information theoretic fit indices that balance overall fit with model parsimony such that lower values are indicative of better fit. These fit indices included Akaike’s Information Criterion (AIC), the Bayesian Information Criterion, the sample-size adjusted BIC, and the Deviance Information Criterion (DIC). The AIC, BIC and DIC are not interpreted in isolation; rather they are interpreted collectively to compare alternative models such that lower values are indicative of better fit.
Results
Mean-Level Change and Rank-Order Stability Over Four Time Points
Table 2 provides the means, standard deviations, and effect sizes of change for the MPQ-BPD scores for four time points. In order to estimate these means and effect sizes in spite of the missing data patterns, we utilized an EM (expectation-maximization) algorithm in SPSS to impute missing values. In order to obtain effect size indices, the age 17, 20, and 24 scores were compared to age 14 scores. The overall pattern indicates steady declines in MPQ-BPD scores from age 14 to age 24. Specifically, there was no meaningful change from age 14 to age 17, moderate change from age 14 to 20, and a large change from age 14 to 24.
Table 2.
Means and Longitudinal Change of BPD Traits as Measured by the MPQ-BPD across Five Time Points
| Mean | SD | ES (d) of difference from age 14 |
|
|---|---|---|---|
| BPD Age 14 | 41.26 | 8.12 | N/A |
| BPD Age 17 | 40.86 | 8.03 | −0.05 |
| BPD Age 20 | 37.21 | 6.58 | −0.55 |
| BPD Age 24 | 35.19 | 6.41 | −0.83 |
Table 3 provides the correlations among the MPQ-BPD scores across the five time points. The MPQ-BPD scores evidenced high rank-order stability, as indexed by the high correlations between all time points (rs = .53 to .73, all ps < .001). .
Table 3.
Rank Order Stability of BPD symptoms from Age 14 to Age 24.
| BPD Age 17 | BPD Age 20 | BPD Age 24 | |
|---|---|---|---|
| BPD Age 14 | .60** | .73** | .53** |
| BPD Age 17 | --- | .63** | .57** |
| BPD Age 20 | --- | .68** |
Univarite Biometric Analyses
Table 4 presents the genetic and environmental contributions to the MPQ-BPD scores across the four time points. The univariate biometric models revealed that, at age 14, there was a modest effect of additive genetic and shared environmental factors and a large effect of non-shared environmental factors. In contrast, at ages 17, 20, and 24, the influence of additive genetic factors tended to increase, whereas the effects of shared environmental factors gradually fell to zero. The effects of non-shared environmental factors became slightly stronger from age 17 to 24.
Table 4.
Variance Component Estimates for Each Assessment Time Point
| Variable | MZ | DZ | a2 | c2 | e2 |
|---|---|---|---|---|---|
| BPD Age 14 | .48 (.39,.56) | .38 (.26,.49) | .31 (.06,.56) | .20 (0,.41) | .50 (.42,.58) |
| BPD Age 17 | .50 (.43,.57) | .30 (.19,.40) | .38 (.15,.55) | .12 (0,.32) | .51 (.45,.58) |
| BPD Age 20 | .43 (.30,.53) | .25 (.06,.43) | .35 (.00,.53) | .08 (0,.41) | .57 (.47,.70) |
| BPD Age 24 | .48 (.38..56) | .22 (.07,.36) | .46 (.14,.54) | .00 (0,.28) | .54 (.46,.62) |
Biometic Growth Curve Modeling
We began by fitting a full model that included all possible parameters, including general genetic and environmental effects on both intercept and slope; occasion (assessment time point)-specific genetic and environmental effects; and genetic and environmental correlations between the general genetic and environmental influences on intercept and slope. This model was used as a “standard” of model fit. Next, consistent with previous investigations using these modeling techniques (e.g., McGue & Christensen, 2003; Carlson & Iacono, 2006), we began removing non-significant parameters from the model. All subsequent models were compared to the full model to see if they fit the observed data as well or better than the full model. These reduced-growth models allowed us to test for necessity of genetic and environmental factors to the intercept, slope, and occasion-specific effects.
The fit statistics for these reduced models are listed in Table 5. Several reduced models yielded improved fit relative to the full growth model. Model fit improved (decreases in fit indices) when shared environmental effects to the latent intercept and slope variables were removed (model 4), when occasion-specific additive genetic and shared environment effects were removed (models 7 and 8, see also model 9), and when covariance between the intercept and slope variables was restricted to genetic factors only (i.e., nonshared environmental effects are set equal to zero, model 10). Removal of any other parameters decreased model fit (models 2, 3, 5 and 6). Because taking out the specific genetic and shared environmental factors simultaneously resulted in a slightly worse model fit, we examined the residuals as well as the univariate models. Together, these suggested that adding occasion-specific shared environmental effects at age 14 and 17 might improve model fit. Putting these parameters back into the model significantly improved model fit, with all three fit indices favoring model 11 which yielded the best fitting model in terms of balancing overall fit with model parsimony.
Table 5.
Model Fit Statistics for Biometric Growth Model.
| Model | −2LL | df | AIC | BIC adj | DIC | BIC | Δχ2 | df | p | AIC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Full Growth | 29,246.48 | 4265 | 20,716.48 | 6799.33 | 3945.95 | 26.67 | ||||
| 2. | No intercept-slope correlation | 29,335.55 | 4268 | 20,799.55 | 6838.37 | 3982.97 | 60.94 | 89.07 | 3 | .000 | 83.07 |
| 3. | No general A | 29,264.00 | 4268 | 20,728.00 | 6802.59 | 3947.19 | 25.16 | 17.52 | 3 | .001 | 11.52 |
| 4. | No general C | 29,248.45 | 4268 | 20,712.45 | 6794.82 | 3939.42 | 17.39 | 1.97 | 3 | .579 | −4.03 |
| 5. | No general A or C | 29,458.90 | 4271 | 20,916.90 | 6894.54 | 4037.13 | 112.35 | 212.42 | 6 | .000 | 200.42 |
| 6. | No general E | 29,438.23 | 4268 | 20,902.23 | 6889.71 | 4034.31 | 112.28 | 191.75 | 3 | .000 | 185.75 |
| 7. | No specific A | 29,246.49 | 4269 | 20,708.49 | 6792.00 | 3934.94 | 12.99 | .01 | 4 | 1.000 | −7.99 |
| 8. | No specific C | 29,248.49 | 4269 | 20,710.49 | 6793.00 | 3936.94 | 13.99 | 2.01 | 4 | .734 | −5.99 |
| 9. | No specific A or C | 29,264.70 | 4273 | 20,718.70 | 6793.77 | 3935.03 | 8.40 | 18.22 | 8 | .020 | 2.22 |
| 10. | No general C | 29,268.78 | 4277 | 20,714.78 | 6788.47 | 3927.05 | −3.25 | 22.30 | 12 | .034 | −1.70 |
| No E to correlation | |||||||||||
| No specific A or C | |||||||||||
| 11. | No general C | 29,250.48 | 4275 | 20,700.48 | 6782.99 | 3922.91 | −5.56 | 4.00 | 10 | .947 | −16.00 |
| No specific A | |||||||||||
| Specific C at age 14 and 17 | |||||||||||
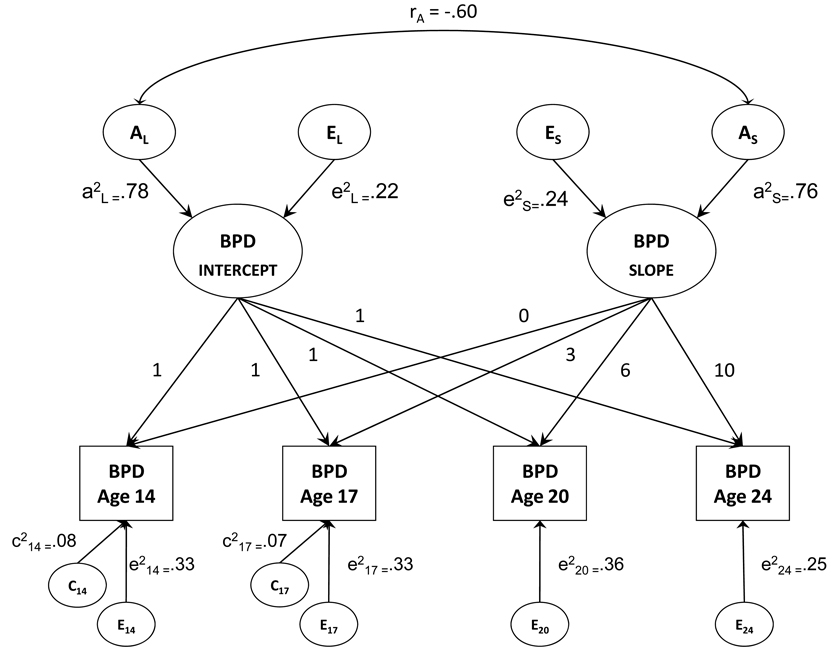
Figure 2 provides a graphical depiction of the best fitting model that included additive genetic and nonshared environmental effects on the latent intercept and slope variables, the correlation between the intercept and slope due to genetic factors, occasion-specific shared environmental effects at ages 14 and 17, and occasion-specific non-shared environmental effects present at each time point. In terms of how well the model approximated the observed data, the model estimated mean intercept corresponded to a BPD trait score of 42.1, and the mean slope indicated that the trait score declined by −.69 units on the MPQ-BPD scale per year. These model estimates are similar to the observed mean MPQ-BPD score at age 14 (41.3) followed by a linear decline in scores over the succeeding 10 years [42.1 – 10(−.69) = 35.2, similar to the observed mean MPQ-BPD score of 35.19 at age 24]. The correlation between the intercept and slope was −.44, indicating a tendency for female twins with higher initial scores to exhibit greater declines in their MPQ-BPD scores over time.
Figure 2.
Note: Final biometric growth model after removal of non-significant paths for initial BPD status (level) and BPD change over time (slope). BPD refers to borderline personality disorder traits; A refers to genetic influence; C refers to shared environmental influence; E refers to nonshared environmental influence; subscripted numbers refer to age of measurement. Coefficients on the diagram are standardized variance component estimates (i.e., paths squared).
The biometric results showed that both the intercept (a2 = .78, 95% CI: .53, 1.00) and slope (a2 = .76, 95% CI: .69, .82) were highly heritable with modest nonshared environmental effects. This indicates that both a person’s stable or average-level of BPD traits at different time points and their rate of change across time was highly heritable. Additionally, there was a high genetic correlation between initial status and slope (r = .60), indicating that the same genetic factors that influence BPD trait stability also influence change. Notably, the heritability of the latent intercept and slope variables was much higher than the heritability estimate for any given specific time point (see Table 4). In terms of occasion-specific effects, there were modest shared environmental effects at age 14 and 17, but these dropped to zero at the older assessments in young adulthood. Occasion-specific nonshared environmental effects were present at each time point, indicating both time-specific environmental influences and measurement error.
Discussion
Although personality disorders are considered to be problems that are best understood in the context of lifetime development, there is a paucity of work examining their stability and change during the key period from adolescence to young adulthood, and little regarding how genetic and environmental influences contribute to their development. In order to understand the unfolding of the underlying risk processes contributing to the development of personality disorders, it is necessary to examine the onset, course, duration and stability of PDs using repeated assessment of youths as they mature to adulthood. This is especially true in the case of BPD. Indeed, although BPD is considered to onset in adolescence or young adulthood, there is a limited literature examining its developmental course. The studies that do focus on adolescents or longitudinal course are mostly limited by the predominant use of clinical samples, small sample sizes, and relatively short follow-up times – as well as the use of dichotomous/diagnostic measures of BPD that limit the ability to examine subtle changes over time. Finally, no studies have yet examined the gene-environment interplay of BPD traits in the window between adolescence and adulthood – a necessary step in understanding (and in turn, influencing) the pathways of risk and resilience (Lenzenweger & Cicchetti, 2005; Cicchetti & Curtis, 2007).
In the current study, we aimed to fill in these conceptual and methodological gaps in the literature. In particular, we aimed to provide more definitive answers about the longitudinal course and stability of BPD characteristics over the period of adolescence to adulthood – that is, the period when BPD traits become manifest (Bernstein et al, 1993). In doing so, we utilized a overlapping sequential cohort longitudinal design that improves considerably on previous studies. Specifically, we utilized a large representative community sample of adolescent twins, a sensitive dimensional measure of BPD traits, and repeated assessments over an extended follow-up period (spanning 10 years after the initial assessment). In addition, we examined how genetic and environmental influences contributing the trait BPD vary over this developmental period, providing novel findings that set the stage for future work that examines the exact nature of gene-environment interplay in BPD over the course of development.
In exploring the course of BPD traits, we examined the degree to which the mean level of trait BPD traits as well as its rank-ordering over the span of 10 years. The results indicated that mean-level MPQ-BPD declined from adolescence to adulthood. Although there was little change from age 14 to 17, thereafter, the mean-level BPD traits declined significantly at each assessment point. This leveling off of trait BPD from mid to late adolescence is not necessarily an intuitive finding. Indeed, given the overlap between normal personality and personality pathology, it would instead be reasonable to predict that BPD traits would first increase from age 14 to 17, and only then decrease at follow-up assessments – a pattern found with the normal personality dimension of negative affect (DiRaggio, 2009; Johnson et al, 2007). On the other hand, two studies on personality pathology proper report that BPD traits as well as the absolute prevalence of the disorder are highest around puberty (ages 12–14) and decline thereafter (Johnson et al, 2000; Bernstein et al, 1993). The fact that our results are consistent with the latter two studies suggests that we are capturing the “true state of affairs.”
Additionally, the MPQ-BPD showed moderate rank-order stability from age 14 to 24 – a pattern of results similar to those of Chanen et al (2004) who found a BPD stability index of .54 (albeit over two rather than ten years). The current pattern of stability and change are in line with previous longitudinal studies of normal personality dimensions. Indeed, a large number of empirical studies and meta-analyses report that the degree of distress, dysfunction, and behavioral undercontrol as measured by traits such as negative affect and disinhibition decline from adolescence to adulthood (Roberts, Walton, & Viechtbauer, 2006; Blonigen, Carlson, Hicks, Krueger, & Iacono 2008; Roberts, Caspi, & Moffitt, 2001), reflecting a trend toward an increased level of maturity and ability to adjust to progressively more challenging environmental demands. These and other studies also provide evidence for an increase in the stability of personality from childhood to adulthood (Caspi et al, 2005; Roberts & DelVecchio, 2000). Thus, the similarity of the trajectory of BPD traits with the longitudinal pattern evidenced by normal personality dimensions provides some evidence for the previously-suggested notion that BPD is an extreme version on a continuum of normal personality functioning (e.g., Trull et al, 2003).
As another approach to the study of risk trajectories, we also examined the genetic and environmental influences on BPD traits at the four time points. This type of longitudinal examination of heritability affords the opportunity to make inferences about the transactions between personality and environment during the window between adolescence and adulthood. First, we found evidence for the average heritability of approximately .3–.5, consistent with the results of Trull et al (2008). Yet unlike the results of Distel et al and of others (Coolidge, 2001; Torgersen, 2000), we found some limited evidence for a shared environmental influence, although this effect failed to reach significance. Additionally, the strength of shared environment declined with increasing age. The disparity between the studies is not surprising. Indeed, previous work indicates that the influence of shared environment on many behavioral phenotypes declines over time (Bergen et al, 2007), and the Distel et al (2008) and Torgersen (1984, 2000) studies mainly utilized samples that were generally late into adulthood. Finally, we found evidence for consistently strong effects of non-shared environment. The exact origin of this variance remains to be investigated; however, it most likely stems from factors such as exposure to childhood abuse and other traumatic life events, differential parental treatment (or perceptions of such), and nonsystematic events (e.g., accidents).
Our results also indicated a trend for increasing heritability of trait BPD over the course of 10 years. This finding is similar to that observed in studies concerning other forms of psychopathology (i.e., externalizing behavior, anxiety symptoms, depressive symptoms, substance use). This trend might be due to the declining influence of shared environmental factors, gene expression changes, or a transaction between environment and personality (i.e., gene-environment correlations), such that over time, individuals have more opportunities to choose environments in which their genetic risk is more likely to become expressed (Bergen et al, 2007; Fruzzetti, Shenk, & Hoffman, 2005). These competing hypotheses remain to be disentangled in follow-up studies.
Finally, we fit a series of biometric latent growth models in order to examine the genetic and environmental influences on the stability and change in BPD traits over time. The final reduced model revealed that there were strong additive genetic effects and modest non-shared environmental effects on both stability and change of BPD traits. Thus, although BPD scores at any given time point were only moderately heritable, an individual’s overall, stable level of trait BPD as well as the degree to which one changes in her level of BPD are strongly heritable. The most likely reason for the difference between the moderate univariate heritability and the large latent factor heritability is reduced measurement error in trait BPD when all four assessment time points are accounted for in the biometric model. Additionally, the high negative correlation between the genetic effects on the intercept and slope suggests that the genes influencing the latent BPD trait stability overlap highly with the genes influencing change in BPD trait levels. Finally, examination of the residual or occasion-specific effects revealed an absence of specific genetic influences for any given time point. Instead, the best-fitting model revealed a limited presence of occasion-specific shared environmental factors that disappear by age 20 and a stable presence of non-shared environment or idiosyncratic factors plus error.
The sum of the current findings has a number of clinical implications that should be noted. First, the current findings on the stability and change of BPD traits belie the clinical myth of “once a personality disorder, always a personality disorder”. Instead, our results indicate that personality disorders in general and BPD in particular are developmental processes that have normative increases and decreases throughout development (Clark, 2009). In other words, an adolescent who scores in the top ranges on BPD scales at age 14 will not necessarily have the same level of pathology and dysfunction at age 24. Instead, this same adolescent will most likely show the steepest decrease in symptoms/traits at a later age. Second, the current findings establish that the critical risk period (the window or point in time where symptoms are at their peak) for BPD characteristics is around ages 14–17. In the tradition of successful interventions during periods of highest risk (e.g., prevention of alcohol use disorders and antisocial behavior), it may be most useful to intervene at this critical time in order to influence the trajectory of BPD in a positive direction.
Finally, the increasing influences of genetic factors over time and the strong influence of such factors on both stability and change do not mean that environmental influences (i.e., family) “don’t matter” at later ages. As noted in the introduction, environment is likely to influence gene expression (Bergen et al, 2007). In turn, this implies that an intervention at the level of, for instance, family might ensure an environment that serves as a protective factor against the expression of pathological traits.
Despite these interesting and informative results and implications, a number of methodological constraints should be acknowledged. First, the current study focused on a sample of female twins. As such, we were not able to examine gender differences in the course and heritability of the MPQ-BPD. As a second limitation, the current study relied on a novel self-report of BPD levels. Although the construct validity of this measure is supported by a host of findings derived from studies of five different community and clinical samples, future studies would benefit from the use of a multi-assessment, multi-informer design, as previous work suggests that different assessment methods and informants eachprovide unique and valid information (Oltmanns & Turkheimer, 2009). Finally, the current study followed the participants only up to age 24. It will be informative to continue following the current participants through middle adulthood and even further in order to continue examining the course and heritability over the entire lifespan.
In summary, the current study was successful in indexing the longitudinal course and heritability of BPD levels in the time period between adolescence and adulthood. This work provides a basis for a number of follow-up studies. For instance, future work might aim to address the question of “what makes the developmental challenges of mid and late adolescence (e.g., identity development, orientation to romantic relationships) so challenging?” (Lenzenweger & Cicchetti, 2005). As an example of this line of research, it would be interesting to attempt to account for the variance in the initial status and change in BPD traits using several predictors such as environmental stress (e.g., childhood trauma) or the presence of co-occurring distress and dysfunction (e.g., substance use, depression). Such variables might also be used to predict the initial status and slope of BPD trait levels in structural models such as one used in the current study (e.g., Lenzenweger & Castro, 2005). Likewise, future work might examine whether protective factors including environmental, neurobiological, and molecular genetic variables (Curtis & Cicchetti, 2007; Cicchetti & Gunnar, 2008; Cicchetti, Rogosch & Sturge-Apple, 2007; Charney, 2004) can be used to predict the rate of change in BPD traits over time – a research avenue that is in line with the need to understand competent adaptation despite adversity at multiple levels of analysis (Cicchetti & Garmezy, 1993 Cicchetti & Curtis, 2007). Moreover, the current study sets the basis for testing sophisticated models that include not only genetic and environmental influences on BPD traits, but gene-environment correlations as well (Fruzzetti, et al, 2005). In other words, future work might examine how, across different ages, at-risk individuals choose their environments. Work of this kind is likely to contribute substantially to knowledge of the etiology of BPD, and in turn to methods for preventing and treating this disorder.
Acknowledgments
Data for this project were collected at the University of Minnesota. This work was supported by National Institute of Drug Abuse Grants DA05147 and DA 13240, National Institute on Alcohol Abuse and Alcoholism grant AA09367, and National Institute on Mental Health Grant MH017069. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
No conflict of interest exists for any of the authors.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition. Washington, DC: Vol. American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington D. C.: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fourth edition. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Cohen P, Velez CN, Schwabstone M, Siever LJ, Shinsato L. Prevalence and stability of the dsm-iii-r personality-disorders in a community-based survey of adolescents. American Journal of Psychiatry. 1993;150(8):1237–1243. doi: 10.1176/ajp.150.8.1237. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Carlson MD, Hicks BM, Krueger RF, Iacono WG. Stability and change in personality traits from late adolescence to early adulthood: A longitudinal twin study. Journal of Personality. 2008;76(2):229–266. doi: 10.1111/j.1467-6494.2007.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant H, Greenfield B, Tse SM. Construct validity of the adolescent borderline personality disorder: a review. Can Child Adolesc Psychiatr Rev. 2004;13(3):53–57. [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Patrick C, Iacono WG, McGue M. Development and Validation of the Multidimensional Personality Questionnaire Borderline Personality Disorder Scale (MPQ-BPD) Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Rosenthal MZ, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clinical Psychology Review. 2005;25:790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bunce SC, Coccaro E. Factors differentiating personality-disordered individuals with and without a history of unipolar mood disorder. Depression and Anxiety. 1999;10(4):147–157. [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43(5):470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: Stability and Change. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Chanen AM, Jackson HJ, McGorry PD, Allot KA, Clarkson V, Yuen HP. Two-year stability of personality disorder in older adolescent outpatients. Journal of Personality Disorders. 2004;18(6):526–541. doi: 10.1521/pedi.18.6.526.54798. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological and vulnerability : Implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. The emergence of developmental psychopathology. Child Development. 1984;55:1–7. [PubMed] [Google Scholar]
- Cicchetti D. An historical perspective on the discipline of developmental psychopathology. In: Rolf J, Masten A, Cicchetti D, Nuechterlein K, Weintraub S, editors. Risk and protective factors in the development of psychopathology. New York: Cambridge University Press; 1990. pp. 2–28. [Google Scholar]
- Cicchetti D, Curtis WJ. Multilevel perspectives on pathways to resilient functioning. Development and Psychopathology. 2007;19(3):627–629. doi: 10.1017/s0954579407000314. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR. Integrating biological measures into the design and evaluation of preventive interventions. Development and Psychopathology. 2008;20(3):737–743. doi: 10.1017/S0954579408000357. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: Depressive symptornatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Clark LA. Stability and Change in Personality Disorder. Current Directions in Psychological Science. 2009;18(1):27–31. [Google Scholar]
- Coolidge FL, Thede LL, Jang KL. Heritability of personality disorders in childhood: A preliminary investigation. Journal of Personality Disorders. 2001;15(1):33–40. doi: 10.1521/pedi.15.1.33.18645. [DOI] [PubMed] [Google Scholar]
- Crick NR, Murray-Close D, Woods K. Borderline personality features in childhood: A short-term longitudinal study. Development and Psychopathology. 2005;17(4):1051–1070. [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Development and Psychopathology. 2007;19(3):811–840. doi: 10.1017/S0954579407000405. [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, et al. Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 2008;38(9):1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Huckabee HCG, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Research. 1999;85(3):315–326. doi: 10.1016/s0165-1781(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Ruiz MA. Taxometric analyses of borderline personality features in a large-scale male and female offender sample. Journal of Abnormal Psychology. 2008;117(3):705–711. doi: 10.1037/0021-843X.117.3.705. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. Journal of Clinical Psychiatry. 2004;65:1660–1665. doi: 10.4088/jcp.v65n1211. [DOI] [PubMed] [Google Scholar]
- Fruzzetti AE, Shenk C, Hoffman PD. Family interaction and the development of borderline personality disorder: A transactional model. Development and Psychopathology. 2005;17(4):1007–1030. doi: 10.1017/s0954579405050479. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-case disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Development and Psychopathology. 2002;14(2):395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ. Why do measures of normal and disordered personality correlate? A study of genetic comorbidity. Journal of Personality Disorders. 1999;13:10–17. doi: 10.1521/pedi.1999.13.1.10. [DOI] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA, Jackson DN. Heritability of personality disorder traits: A twin study. Acta Psychiatrica Scandinavica. 1996;94(6):438–444. doi: 10.1111/j.1600-0447.1996.tb09887.x. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Skodol AE, Hamagami F, Brook JS. Age-related change in personality disorder trait levels between early adolescence and adulthood: a community-based longitudinal investigation. Acta Psychiatrica Scandinavica. 2000;102:265–275. doi: 10.1034/j.1600-0447.2000.102004265.x. [DOI] [PubMed] [Google Scholar]
- Johnson W, Hicks BM, McGue M, Iacono WG. Most of the girls are alright but some aren't: Personality trajectory groups from ages 14 to 24 and some associations with outcomes. Journal of Personality and Social Psychology: Personality Processes and Individual Differences. 2007;93:266–284. doi: 10.1037/0022-3514.93.2.266. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Developmental Psychology. 2006;42:514–532. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, et al. The Structure of Genetic and Environmental Risk Factors for DSM-IV Personality Disorders A Multivariate Twin Study. Archives of General Psychiatry. 2008;65(12):1438–1446. doi: 10.1001/archpsyc.65.12.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children's antisocial behavior - Nature and nurture effects. Archives of General Psychiatry. 2005;62(2):173–181. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- Koenig LB, McGue M, Krueger RF, Bouchard TJ. Genetic and environmental influences on religiousness: Findings for retrospective and current religiousness ratings. Journal of Personality. 2005;73(2):471–488. doi: 10.1111/j.1467-6494.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Stability and change in personality disorder features - The longitudinal study of personality disorders. Archives of General Psychiatry. 1999;56(11):1009–1015. doi: 10.1001/archpsyc.56.11.1009. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Castro DD. Predicting change in borderline personality: Using neurobehavioral systems indicators within an individual growth curve framework. Development and Psychopathology. 2005;17(4):1207–1237. doi: 10.1017/s0954579405050571. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Cicchetti D. Toward a developmental psychopathology approach to borderline personality disorder. Development and Psychopathology. 2005;17(4):893–898. doi: 10.1017/s095457940505042x. [DOI] [PubMed] [Google Scholar]
- Levy KN, Becker DF, Grilo CM, Mattanah JJF, Garnet KE, Quinlan DM, et al. Concurrent and predictive validity of the personality disorder diagnosis in adolescent inpatients. American Journal of Psychiatry. 1999;156(10):1522–1528. doi: 10.1176/ajp.156.10.1522. [DOI] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- Links PS, Heslegrave RJ, Mitton JE, van Reekum R, Patrick J. Borderline personality disorder and substance abuse: Consequences of comorbidity. Canadian Journal of Psychiatry. 1995;40:9–14. doi: 10.1177/070674379504000105. [DOI] [PubMed] [Google Scholar]
- Little RTA, Rubin DB. New York: Wiley; 1987. Statistical analysis with missing data. [Google Scholar]
- Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. Archives of General Psychiatry. 1998;55(10):941–948. doi: 10.1001/archpsyc.55.10.941. [DOI] [PubMed] [Google Scholar]
- Livesley WJ, Jang KL, Jackson DN, Vernon PA. Genetic and environmental contributions to dimensions of personality-disorder. American Journal of Psychiatry. 1993;150(12):1826–1831. doi: 10.1176/ajp.150.12.1826. [DOI] [PubMed] [Google Scholar]
- Ludolph PS, Westen D, Misle B, Jackson A, Wixom J, Wiss FC. The borderline diagnosis in adolescents: symptoms and developmental history. American Journal of Psychiatry. 1990;147(4):470–476. doi: 10.1176/ajp.147.4.470. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: An integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88(1):139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Bouchard TJ, Gottesman II. Normal and abnormal personality traits: Evidence for genetic and environmental relationships in the Minnesota Study of Twins Reared Apart. Journal of Personality. 2002;70:661–693. doi: 10.1111/1467-6494.05020. [DOI] [PubMed] [Google Scholar]
- Matheny AP. Childrens behavioral inhibition over age and across situations - genetic similarity for a trait during change. Journal of Personality. 1989;57(2):215–235. doi: 10.1111/j.1467-6494.1989.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Mattanah JJF, Becker DF, Levy KN, Edell WS, McGlashan TH. Diagnostic stability in adolescents followed up 2 years after hospitalization. American Journal of Psychiatry. 1995;152(6):889–894. doi: 10.1176/ajp.152.6.889. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Grilo CM, Skodol AE, Gunderson JG, Shea MT, Morey LC, et al. The Collaborative Longitudinal Personality Disorders Study: baseline axis I/II and II/II diagnostic co-occurrence. Acta Psychiatrica Scandinavica. 2000;102(4):256–264. doi: 10.1034/j.1600-0447.2000.102004256.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of depression symptoms in elderly Danish twins: Occasion-specific versus general effects. Behavior Genetics. 2003;33(2):83–93. doi: 10.1023/a:1022545600034. [DOI] [PubMed] [Google Scholar]
- McGue M, Bacon S, Lykken DT. Personality stability and change in early adulthood: A behavioral genetic analysis. Developmental Psychology. 1993;29:96–109. [Google Scholar]
- Meijer M, Goedhart AW, Treffers PDA. The persistence of borderline personality disorder in adolescence. Journal of Personality Disorders. 1998;12(1):13–22. doi: 10.1521/pedi.1998.12.1.13. [DOI] [PubMed] [Google Scholar]
- Miles DR, Carey G. Genetic and environmental architecture of human aggression. Journal of Personality and Social Psychology. 1997;72(1):207–217. doi: 10.1037//0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- Miller AL, Muehlenkamp JJ, Jacobson CM. Fact or fiction: Diagnosing borderline personality disorder in adolescents. Clinical Psychology Review. 2008;28(6):969–981. doi: 10.1016/j.cpr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Morey LC. Personality Assessment Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Neale MC, McArdle JJ. Structured latent growth curves for twin data. Twin Res. 2000;3(3):165–177. doi: 10.1375/136905200320565454. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 5th ed. Available Department of Psychiatry, Medical College of South Carolina; 1999. [Google Scholar]
- Newman DA. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organizational Research Methods. 2003;6:328–362. [Google Scholar]
- Nurnberg G, Raskin M, Pollack S, Levine S. Borderline Personality Disorder as a Negative Prognostic Factor in Anxiety Disorder. Journal of Personality Disorders. 1989;3:205–216. [Google Scholar]
- O'Connor BP. The search for dimensional structure differences between normality and abnormality: A statistical review of published data on personality and psychopathology. Journal of Personality and Social Psychology. 2002;83:962–982. [PubMed] [Google Scholar]
- O'Connor BP, Dyce JA. Rigid and externe: A geometric representation of personality disorders in five-factor model space. Journal of Personality and Social Psychology. 2001;81:1119–1130. doi: 10.1037//0022-3514.81.6.1119. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Turkheimer E. Person Perception and Personality Pathology. Current Directions in Psychological Science. 2009;18(1):32–36. doi: 10.1111/j.1467-8721.2009.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J, Brown R, Nowlis D. Long-term follow-up of borderline patients in a general hospital. Comprehensive Psychiatry. 1987;28(6):530–535. doi: 10.1016/0010-440x(87)90019-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychological Assessment. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pinto A, Grapentine WL, Francis G, Picariello CM. Borderline personality disorder in adolescents: Affective and cognitive features. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(10):1338–1343. doi: 10.1097/00004583-199610000-00021. [DOI] [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Caspi A, Moffitt TE. The kids are alright: Growth and stability in personality development from adolescence to adulthood. Journal of Personality and Social Psychology. 2001;81(4):670–683. [PubMed] [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132(1):1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Rothschild L, Cleland C, Haslam N, Zimmerman M. A taxometric study of borderline personality disorder. Journal of Abnormal Psychology. 2003;112(4):657–666. doi: 10.1037/0021-843X.112.4.657. [DOI] [PubMed] [Google Scholar]
- Rothschild L, Cleland C, Haslam N, Zimmerman M. A taxometric study of borderline personality disorder. Journal of Abnormal Psychology. 2003;112(4):657–666. doi: 10.1037/0021-843X.112.4.657. [DOI] [PubMed] [Google Scholar]
- Shea MT, Stout R, Gunderson J, Morey LC, Grilo CM, McGlashan T, et al. Short-term diagnostic stability of schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders. American Journal of Psychiatry. 2002;159(12):2036–2041. doi: 10.1176/appi.ajp.159.12.2036. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. American Journal of Psychiatry. 1991;148(12):1647–1658. doi: 10.1176/ajp.148.12.1647. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Torgersen S, Gunderson JG, Livesley WJ, Kendler KS. The borderline diagnosis III: Identifying endophenotypes for genetic studies. Biological Psychiatry. 2002;51:964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: Psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: Psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: Psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Oldham JM, Bender DS, Dyck IR, Stout RL, Morey LC, et al. Dimensional Representations of DSM-IV Personality Disorders: Relationships to Functional Impairment. American Journal of Psychiatry. 2005;162:1919–1925. doi: 10.1176/appi.ajp.162.10.1919. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Oldham JM, Hyler SE, Stein DJ, Hollander E, Gallaher PE, et al. Patterns of anxiety and personality disorder comorbidity. Journal of Psychiatric Research. 1995;29(5):361–374. doi: 10.1016/0022-3956(95)00015-w. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Considering normal and abnormal together: The essence of developmental psychopathology. Development and Psychopathology. 1990;2:335–347. [Google Scholar]
- Stattin H, Magnusson D. Antisocial development: A holistic approach. Development and Psychopathology. 1996;8:617–645. [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the multidimensional personality questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. Handbook of personality theory and testing. Personality measurement and assessment. Vol. II. London: Sage; 2008. pp. 261–292. [Google Scholar]
- Tellegen A. Brief manual for the Multidimensional Personality Questionnaire. Minneapolis: University of Minnesota; 1982. Unpubhshed manuscript. [Google Scholar]
- Tellegen A, Lykken DT, Bouchard TJ, Jr, Wilcox K, Segal N, Rich S. Personality similarity in twins reared apart and together. Journal of Personality and Social Psychology. 1988;54:1031–1039. doi: 10.1037//0022-3514.54.6.1031. [DOI] [PubMed] [Google Scholar]
- Torgersen S. Genetic and nosological aspects of schizotypal and borderline personality-disorders: a twin study. Archives of General Psychiatry. 1984;41(6):546–554. doi: 10.1001/archpsyc.1984.01790170020003. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Roysamb E, Neale MC, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychological Medicine. 2008;38(11):1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Lygren S, Oien PA, Skre I, Onstad S, Edvardsen J, et al. A twin study of personality disorders. Comprehensive Psychiatry. 2000;41(6):416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clinical Psychology Review. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: a review and integration. Clinical Psychology Review. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Widiger TA, Lynam DR, Costa PT. Borderline personality disorder from the perspective of general personality functioning. Journal of Abnormal Psychology. 2003;112(2):193–202. doi: 10.1037/0021-843x.112.2.193. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. How lifetimes shape epigenotype within and across generations. Human Molecular Genetics. 2006;1:R131–R137. doi: 10.1093/hmg/ddl200. (Spec. No. 2) [DOI] [PubMed] [Google Scholar]
- Wilberg T, Urnes O, Friis S, Pedersen G, Karterud S. Borderline and avoidant personality disorders and the five-factor model of personality: A comparison between DSM-IV diagnoses and NEO-PI-R. Journal of Personality Disorders. 1999;13(3):226–240. doi: 10.1521/pedi.1999.13.3.226. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Swift WJ, Slotnick HB, Goodman S. DSM-III-R personality disorders in eating-disorder subtypes. International Journal of Eating Disorders. 1990;9(6):607–616. [Google Scholar]
- Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, et al. Axis II comorbidity of borderline personality disorder. Comprehensive Psychiatry. 1998;39:296–302. doi: 10.1016/s0010-440x(98)90038-4. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, et al. Axis I comorbidity of borderline personality disorder. American Journal of Psychiatry. 1998;155:1733–1739. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Silk KR. The longitudinal course of borderline psychopathology: 6-year prospective follow-up of the phenomenology of borderline personality disorder. American Journal of Psychiatry. 2003;160(2):274–283. doi: 10.1176/appi.ajp.160.2.274. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, et al. Reported pathological childhood experiences associated with the development of borderline personality disorder. American Journal of Psychiatry. 1997;154:1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]