Abstract
Abnormalities of kidney and urinary tract development are the most common cause of end-stage kidney failure (ESKD) in childhood in the U.S. (NAPRTCS 2006). Over the past 20 years, the advent of mutant and transgenic mice and manipulation of gene expression in other animal models has resulted in major advances in identification of the cellular and molecular mechanisms that direct kidney morphogenesis, providing insights into the pathophysiology of renal and urologic anomalies. This review focuses on the molecular mechanisms that define kidney progenitor cell populations, induce nephron formation within the metanephric mesenchyme, initiate and organize ureteric bud branching, and participate in terminal differentiation of the nephron. Highlighted are common signaling pathways that function at multiple stages during kidney development, including signaling via Wnts, bone morphogenic proteins (BMPs), fibroblast growth factor (FGF), sonic hedgehog (shh), GDNF/Ret, and notch pathways. Also emphasized are the roles of transcription factors Odd1, Eya1, Pax2, Lim1 and WT-1 in directing renal development. Areas requiring future investigation include the factors which modulate signaling pathways to provide temporal and site specific effects. The evolution of our understanding of the cellular and molecular mechanisms of kidney development may provide methods for improved diagnosis of renal anomalies and, hopefully, targets for intervention for this common cause of childhood ESKD.
Keywords: genes, kidney development, metanephric mesenchyme, ureteric bud, progenitor, nephron, differentiation
Abnormalities of kidney and urinary tract development, including aplasia/dysplasia, vesicoureteral reflux (VUR) and obstructive uropathies are the most common cause of renal failure in childhood in the U.S., comprising 31% of children with end-stage kidney disease (NAPRTCS 2006). Over the past 20 years, the advent of manipulation of gene expression in mice and other animal models has resulted in major advances in identification of the cellular and molecular mechanisms that direct kidney morphogenesis, providing insight into the pathogenesis of these anomalies. Yet many discoveries remain to be made, and questions are still unanswered.
The original studies of kidney development involved descriptive studies of the morphologic changes, including seminal work by Edith Potter on human fetal kidneys.1,2 There are two embryonic kidney precursors, the pronephros and mesonephros. Remarkably, these precursors take form and then involute and yet are necessary for development of the definitive kidney, as interruption of their development results in renal agenesis. The pronephros, composed of simple tubules that empty into a pronephric duct, originates from nephrogenic cords of intermediate mesoderm. Subsequently, as the pronephros begins to regress, the mesonephros arises at its caudal end and matures into well developed nephrons with vascular glomeruli connected to proximal and distal tubules that drain into the mesonephric duct (also known as the Wolffian duct). The mesonephros will ultimately fuse with the cloaca, and contributes to formation of the urinary bladder. The last embryonic kidney, the metanephros, is formed as the ureteric bud branches out of the caudal end of the Wollfian duct (Figure 1). Reciprocal interactions between the ureteric bud and the metanephric mesenchyme result in nephron induction, and a subset of cells within the mesenchyme coalesce (forming “condensates”) and develop an epithelial phenotype (known as a mesenchymal-epithelial transition). The ureteric bud branches in a highly reproducible manner, and nephrons are induced at each ureteric bud tip. These branches will form the collecting system, including collecting ducts, renal pelvis, ureter, and bladder trigone. At the same time, the epithelial cells undergo a stereotyped sequence of morphologic changes, starting as a sphere of cells (the vesicle), becoming a comma, and then an S-shape body. Three segments of the S-shape body emerge, oriented with the distal segment adjacent to the ureteric bud tips: the proximal segment differentiates into glomerular epithelial cell (podocytes), the mid-section forms the proximal tubule and loop of Henle, and the distal segment becomes the distal tubule and joins with the ureteric bud branches.
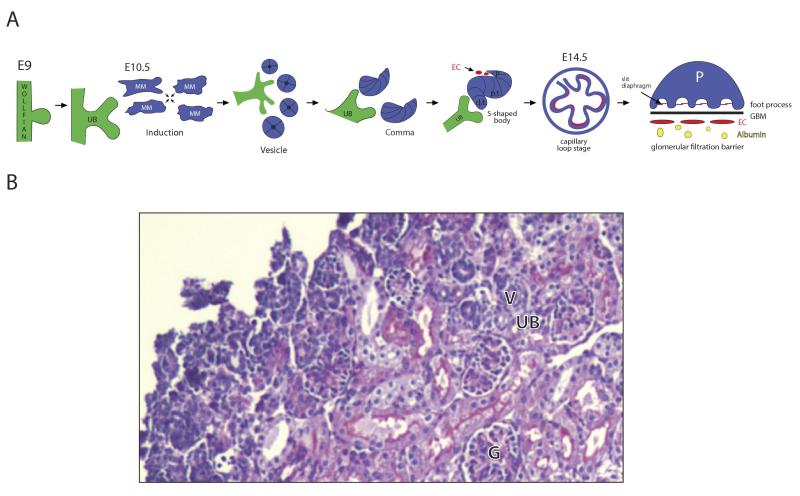
Figure 1.
(A) schema of metanephric kidney development: The ureteric bud (UB) arises from the Wolfian duct (part of the mesonephros) at embryonic day 9 (E9) in mice. Nephrons are induced at tips of the ureteric bud branches; the serial branching of the ureteric bud establishes the radial structure of the kidney architecture. Wnt signals provide the major signal for induction, stimulating aggregation of metanephric mesenchymal (MM) cells and transformation to an epithelial cell phenotype (MET). The epithelial cells form a vesicle, then comma and S-shaped body. Notch2 defines cell fate of the proximal segments of the S-shaped body, the future podocytes and proximal tubule, while the distal segment form the distal tubule. By E14.5, the first glomeruli are formed, with podocytes with foot processes, slit diaphragms, glomerular basement membrane (GBM) and a fenestrated endothelium (EC). (B) PAS of newborn mouse kidney exhibiting developing nephrons (ureteric bud (UB) and renal vesicles (V)) in the outer nephrogenic cortex, and a mature glomeruli (G) in the deep cortex.
Vascular development in the kidney occurs concurrent with glomerular development. There is evidence that the vasculature may arise from both progenitor cells within the metanephric mesenchyme (angiogenesis) as well as penetration of developing mesenchyme by existing vessels (via vasculogenesis)3,4. Endothelial cells migrate into the vascular cleft of the S-shape body and differentiate to form the fenestrated glomerular endothelium.4 While neurogenic factors are expressed in the developing kidney and have been shown to play a role in ureteric bud branching,5 little is known about the factors which induce kidney innervation, although a recent description of renal nerves may provide a platform to investigate which genes are involved6.
Human kidney development begins as early as the third week of embryonic development, with formation of the pronephros, followed by the mesonephros at 4 weeks and the metanephros at 5 weeks gestation. The first glomeruli appear at nine weeks in humans, and nephrogenesis is complete by 36 weeks gestation. In mice, in whom gestation lasts approximately 21 days, the ureteric bud forms from the Wollfian duct at embryonic day 9 (E9), nephron induction begins at E10.5, the first glomeruli appear at E14.5, and nephrogenesis continues after birth for two weeks.
The central question of renal development is how does one “make” a kidney. To answer this, investigators have used genetic manipulation in a variety of animal models to dissect out the cellular and molecular signals which define the morphologic transitions. This review focuses on major signaling pathways and transcription factors that have been identified which coordinate cell fate determination, migration, proliferation and differentiation required for kidney development. It is notable that the same signaling pathways and transcription factors may play distinct roles depending on the spatial and temporal context; for example, Wnt/β-catenin signaling plays different roles in the metanephric mesenchyme and ureteric bud, while Pax2 signaling functions early as a determinant of nephron progenitors and late in terminal differentiation of specific nephron segments.7-11 Thus, relatively few molecular mechanisms are capable of directing a diverse sequence of events during kidney organogenesis; the current challenge is to identify the molecular modifiers that provide the temporal and cell specific effects.
What genes define kidney progenitor cells?
One of the earliest genetic markers of kidney progenitor cells in both chickens and mice is the transcription factor Odd skipped related1 (Odd1 or Osr1).12,13 Chickens, like mammals, form 3 embryonic kidneys; their rapid development and accessibility of developing embryos have made them a useful model for studying gene expression profiles. Osr1 expression in developing chick kidneys localizes to intermediate mesoderm and then to the mesenchyme surrounding the mesonephros13. Recently, genetic fate mapping has clarified further the gene expression patterns of Osr1; inducible genetic labeling of Osr-1 expressing cells demonstrated that early in development (before E9.5) Osr-1 cells are multipotent, and contribute to nephron and collecting duct epithelia and the cortical interstitium; however, later in development Osr-1 transcription becomes gradually restricted to the developing cap mesenchyme (the mesenchyme at the tip of the ureteric bud which undergoes active nephrogenesis).14 Gene deletion of Odd1(Osr1) in mice results in failure to form a metanephric mesenchyme12. The function of Odd1(Osr1) was further examined in zebrafish. Zebrafish are a useful model for studying the genetic determinants of intermediate mesoderm cell fate, for while they develop solely a pronephric stucture, their development occurs rapidly (within 2 days), visibly (embryos are translucent), and genes can be manipulated with morpholinos in a temporal and spatial specific manner.15 In the zebrafish model, Odd1(Osr1) acted as a transcriptional repressor and directed cell fate towards kidney rather than vascular phenotype; loss of Odd1(Osr1) function resulted in either complete absence of a pronephric structure, or increased vascular cells at the expense of renal progenitor cells.16,17 In mice, Odd1 expression was required for expression of other transcription factors, including Eya1, Pax2, Six2, Sall1, and GDNF, indicating that Odd1 acts upstream of these pathways12.
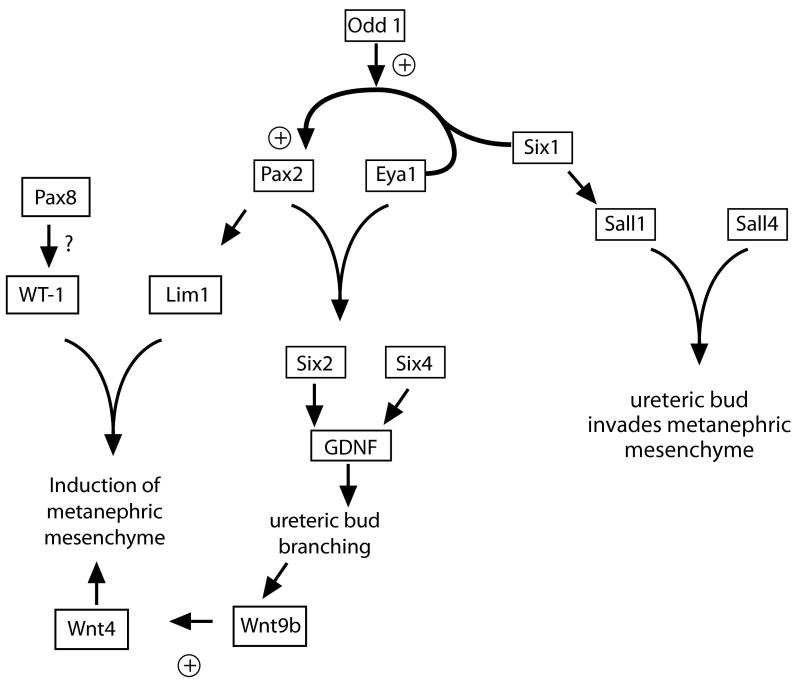
It was shown by tissue recombination experiments that a subpopulation of cells within the mesonephros and metanephric mesenchyme formed renal tubules in the presence of inductive signals, suggesting that this subset of cells may express specific genes that define them as nephron progenitors18. Several transcription factors are expressed in these nephron progenitors, including LIM-class homeodomain transcription factor Lim1, Pax2, Eya1, homeobox family members Six 1, 2, and 4, Sall1 and WT-1. These factors are required for nephron development, and loss of their function in the developing metanephric mesenchyme results in either renal agenesis or hypoplasia. There is an apparent hierarchy amongst the transcription factors, and several physically interact, resulting in complex regulation of DNA binding and transcriptional activation versus repression (figure 2). For example, Eya1 is capable of binding both Pax2 and Six1: the Eya1-Pax2 complex upregulates Six2 and GDNF, while the binding of Eya1 to Six1 turns Six1 from a repressor to an activator and upregulates Pax2, resulting in a positive feedback loop.19,20 Together, Six1-Eya1-Pax2 act synergistically and upregulate GDNF.21,22 Both Six1 and Six4 are required for GDNF expression, and Six1 induces transcription of Sall1.20,23,24 Sall1 deletion in the metanephric mesenchyme results in renal agenesis, not because of a mesenchymal cell autonomous role for Sall1 (Sall1−/− mesenchyme can be induced in vitro), but rather because Sall1 is required to allow invasion of the ureteric bud into the metanephric mesenchyme that provides the signals for ongoing nephrogenesis25. Interestingly, Six2 upregulates GDNF and appears to maintain the metanephric mesenchyme in a dedifferentiated state; this is important because differentiation of the metanephric mesenchyme results in the cessation of GDNF stimulated ureteric bud branching (see below)26. Thus, Six2 maintains the population of renal progenitor cells required for nephron formation27. The homeobox transcription factor Lim1 is a downstream target of Pax2; gene deletion of Lim1 in the metanephric mesenchyme halts development at the renal vesicle stage and its own targets include Notch patterning genes.10,28,29 The gene mutated in Wilms tumor, WT-1, is a transcription factor with functions both early and late in nephron development; it is required in metanephric mesenchyme but also plays later roles in podocyte differentiation.30 In the metanephric mesenchyme, Pax-8 may activate WT-1 expression31. WT-1 is required to for metanephric mesenchymal survival, but the mechanism is unclear: it has been proposed that WT-1 contributes to maintaining Pax-2 expression via stimulation of VEGF-A, as Flk1 signaling upregulates Pax2 in metanephric mesenchyme, yet the metanephric mesenchyme of WT-1−/− mutant embryos express Pax2.32,33
Figure 2.
Hierarchy of transcription factors in nephron progenitor cells
Remarkably, gene mutations in several of these transcription factors have been identified in syndromes characterized by renal hypodysplasia, including Sall1 (Townes-Brocks syndrome), Eya1-Six1 (branchial-oto-renal syndrome), and Pax2 (renal-coloboma syndrome).34 A mutation was also identified in a related transcription factor, Sall4, in Okihiro syndrome; Sall1 was found to be required for proper localization of Sall4, indicating that the Sall1 phenotype (Townes-Brocks) may be mediated in part by loss of Sall4 function.35
Stromal and vascular progenitors in the metanephric mesenchyme
The cells of the metanephric mesenschyme cells which do not coalesece to form the developing nephron are generally held to form the stroma and the renal capsule. FoxD1 was recently identified as marking the progenitors of the renal capsule. Remarkably, loss of FoxD1 function resulted in fused pelvic kidneys that lost their radial organization, suggesting that FoxD1 signaling contributes to patterning by defining the boundaries that limit nephron induction to appropriate zones within the metanephric mesenchyme36.
Vascular progenitors within the metanephric mesenchyme or angioblasts, have been identified by their expression of the VEGF-A receptor, VEGFR2 (Flk1); VEGF-A acts as a chemo-attractant to these vascular progenitors, directing cell migration towards the developing nephron.37,38 Recently, a subpopulation of stromal cells that are FoxD1 negative have been shown to express C-kit, a receptor for stem cell factor (SCF); SCF secreted from the ureteric bud may expand this cell population39. These C-kit positive cells appear to be a source of vascular progenitors, as a subset also express Flk1.
Notably, while multiple progenitors cell populations have been identified within metanephic mesenchyme, there is a relative paucity of data regarding genes that define ureteric bud progenitors within the Wollfian duct, as the focus has been on molecular signals which organize ureteric budding and branching; further investigation will be required to identify the genes which define ureteric bud progenitors.
Molecular patterning in the metanephric mesenchyme and ureteric bud
There are conserved pathways for spatial organization of morphogenesis that contribute to kidney patterning. The homeobox (Hox) genes (1-13) are a family of transcription factors involved in body segmentation. Hox11 is expressed in the intermediate mesoderm and loss of function results in kidney agenesis as a result of failure to form a ureteric bud21. Studies of 4 Hox11 paralogues (Hoxa11-Hoxd11) demonstrated functional redundancy between Hoxa11 and Hoxad11, with dual loss of Hoxa11 and Hoxad11 recapitulating the phenotype of complete loss of Hox11. Hoxa11 and Hoxd11 are expressed in a restricted region within intermediate mesoderm, thereby controlling development of the dorso-ventral renal axis.40,41 Hox11 paralogues are required for Six2 and GDNF expression in the metanephric mesenchyme, indicating a mechanism by which they may affect the position of ureteric bud outgrowth (see below).40 Of note, the restricted expression of another Hox gene, HoxB7, to the branching ureteric bud has proved useful for visualization of the branching both in vivo and in vitro with the HoxB7-GFP mouse and for generating ureteric bud lineage specific transgenic mice.42,43
What are the molecular signals that induce the ureteric bud to arise from the Wollfian duct?
The ureteric bud emerges as a single outgrowth from a stereotyped site of the Wollfian duct caudal to the hindlimb. GDNF signaling via its receptor Ret is the major trophic factor for ureteric budding: GDNF−/− and Ret−/− mutants fail to form a ureteric bud and die perinatally with agenesis of both kidneys and ureters.5,44-49 As indicated above, GDNF expression in the metanephric mesenchyme is upregulated by multiple transcription factors (Eya1, Pax2, and Six 1,2 and 4). GDNF is a secreted growth factor, and its receptor, Ret is a protooncogene and tyrosine kinase receptor expressed in the Wollfian duct, with the highest expression level at the site of ureteric bud outgrowth and later at the tips of the ureteric bud.49 Vitamin A (or retinoids) are required for Ret expression.50 Signaling via Ret stimulates cell proliferation (via PI-3K/AKT and ERK pathways) and migration, resulting in invasion of the ureteric bud into the metanephric mesenchyme51-53. Mutations in Ret are associated with renal tumors, but also have been recently identified in fetuses with renal agenesis.54,55
Several other genes contribute to specifying the unique origin of the ureteric bud. Sprouty, a tyrosine kinase inhibitor, acts to modulate sensitivity of Ret cells to GDNF signals, and is required for a unique origin of the ureteric bud56. Gene deletion of FoxC1 resulted in multiple ureteric buds, leading to duplicated ureters with abnormal bladder insertion and hydronephrosis57. FoxC1 was determined to restrict GDNF expression within the intermediate mesoderm. A chemo-repellent ligand-receptor pair involved in neural guidance, Slit2-Robo2, also functions to limit the expression of GDNF, and gene deletion of either Slit or Robo resulted in multiple, rather than a single, ureteric bud.58 The relevance of these genes to human kidney disease was confirmed by finding of mutations in FOXC1 and ROBO2 associated with congenital anomalies of the kidney and urologic tract (CAKUT) and vesicoureteral reflux (VUR), respectively (VUR is held to result from abnormal insertion of the ureter into the bladder).59,60
What are the molecular signals that contribute to ureteric bud branching and patterning?
The ureteric bud outgrowth undergoes serial branching, defining the kidney architecture. Nephron are induced at the tips of the branching bud, so that ureteric bud branching also determines nephron number. GDNF is an important stimulant of ureteric bud branching; remarkably, localization of its receptor Ret, initially expressed throughout the ureteric bud, becomes restricted to a tip region of the ureteric bud.61,62 This appears to regulate sites of cell proliferation and branching. Overexpression of Ret in ureteric bud resulted in small, cystic kidneys and VUR63. While GDNF is a major trophic factor for bud branching, its effects are modulated by several other growth factors as well as inhibitors of bud branching. Bud branching is stimulated by Angiotensin-II, VEGF, Protein kinase X and inhibited by transforming growth factor β (TGF-β) and Semaphorin3a.64 Angiotensin II activates both angiotensin receptors type 1 and 2 on the ureteric bud to stimulate branching and is also required for elongation of the collecting duct. Renin-angiotensin system (RAS) blockade results in renal agenesis, and mutations in the RAS have also been identified in renal tubular dysgenesis and congenital obstructive uropathy.65,66 Interactions between cells and the extracellular matrix contribute to both ureteric bud branching and nephron induction (see below). Gene deletion of Glypican3, a cell surface heparin sulfate, stimulates early excessive ureteric bud branching and later in development induces apoptosis and loss of medullary collecting ducts.67,68 Other pathways (Wnts, sonic hedgehog (shh), bone morphogenic proteins (BMPs) and fibroblast growth factors (FGFs)) which control ureteric bud branching are discussed separately below, emphasizing the central role of these signaling mechanisms in renal development.
What are the molecular signals that result in nephron induction?
Reciprocal interactions between the ureteric bud and the metanephric mesenchyme are required for nephron induction with transformation of mesenchyme to an epithelial cell phenotype. Signaling via Wnts provide major molecular signals of this transition. Early studies identified that the metanephric mesenchyme could be induced to form nephron epithelia by multiple tissues in vitro, including spinal cord; spinal cords secrete several Wnts. In vivo, the tips of the ureteric bud are the source of signals for induction of nephrons: Wnt9b is produced by ureteric tip cells and stimulates Wnt-4 expression in the metanephric mesenchyme. Wnt4 is require by the metanephric mesenchyme for differentiation into nephron epithelia in vivo. 69 Other Wnts, including Wnt 1,3,7 and 11 can replicate Wnt4 induction of metanephric mesenchyme in vitro.70 Wnt-4 expression is stimulated by the transcription factor Pax-2.71 As indicated above, the extracellular matrix may contribute to Wnt4 induction, as inhibition of glycosaminoglycans prohibits epithelial transformation in vitro.72 Furthermore, loss of heparan sulfate 2-sulfotransferase (HS2ST) expression in vivo inhibits aggregation of the metanephric mesenchyme and results in renal agenesis.73
Growth factors also are capable of inducing nephrons, and may augment epithelial induction, including fibroblast growth factors, FGF2 and FGF7, leukemia inhibitory factor (LIF) and TGFβ2. The contribution of TGFβ signaling contribution is highlighted by the fact that deletion of its intracellular effector Smad4 from mesenchyme impaired nephron induction. The transcription factor Lim1 is also required for nephron induction, and may act to upregulate other genes involved in nephron segmentation and differentiation such at the Notch pathways.28
A few molecular signaling pathways coordinate multiple aspects of renal development
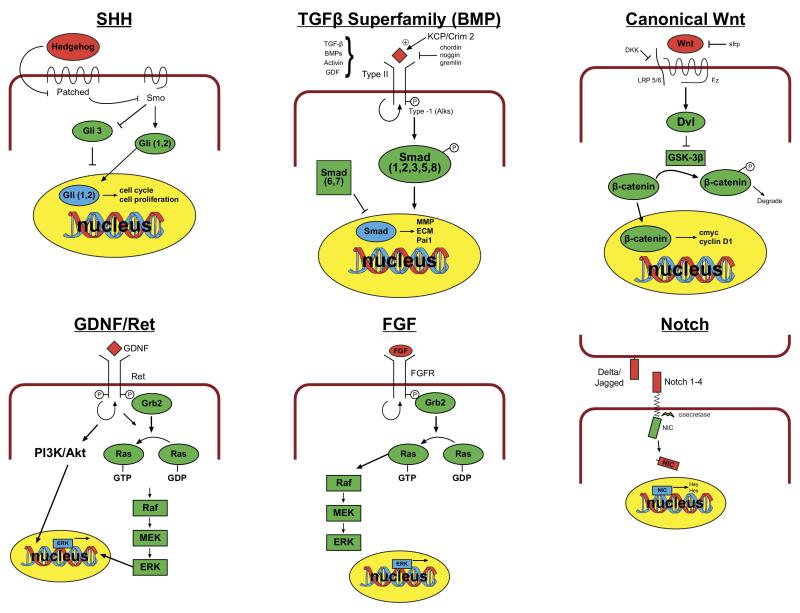
Common molecular signaling pathways with conserved roles in cell fate determination, proliferation, migration and differentiation during tissue morphognesis regulate kidney organogenesis. These pathways have distinct site and time specific effects. Thus, signaling by canonical Wnt/β-catenin and sonic hedgehog (shh) pathways, as well as by bone morphogenic proteins (BMP) and fibroblast growth factors (both members of the TGF-β signaling superfamily) contribute both to nephrogenesis within the metanephric mesenchyme and to ureteric bud branching (figure 3).
Figure 3.
Schema of major signaling pathways involved in renal development (for space and clarity, not all factors in each pathway shown)
Canonical Wnt/β-catenin signaling
As discussed above, Wnt signals play key roles in induction of the mesenchymal-epithelial transformation during nephrogenesis. Wnts are ligands for transmembrane frizzled receptors74. Binding of Wnts to Frizzleds and co-receptors LRP 5/6 recruits Disheveled to the cell membrane. The activated Disheveled inhibits glycogen synthase kinase (GSK-3β) a serine threonine kinase that phosphorylates β-catenin and marks it for degradation. Thus, canonical signaling by Wnts results in stabilization of β-catenin, which translocates to the nucleus to initiate transcription of downstream mediators, including cell proliferation factors CyclinD1 and C-myc. Canonical Wnt signaling plays a role in induction of the metanephric mesenchyme and in ureteric bud branching.7,8,69 The tissue specific roles of β-catenin were elegantly demonstrated by gene deletion in the metanephric mesenchyme and ureteric bud, respectively, by breeding mice with Cre-recombinase under control of tissue specific promoters with mice with β-catenin flanked by lox-P sites, allowing for recombination in the presence of the Cre-recombinase. Gene deletion in the ureteric bud lineage (HoxB7-Cre) resulted in renal agenesis and hypoplasia secondary to impaired ureteric bud branching; this appeared to be a result of expression of premature differentiation with failure to maintain the cap mesenchyme in the precursor necessary state.7,8,75 Inhibition of canonical Wnt signaling by Dickkopf1 (an inhibitor of LRP 5/6) in vitro also impaired ureteric bud branching.11 Gene deletion of β-catenin in the nephron progenitors (Six2-Cre) prevented formation of the renal vesicle and tubulogenesis, resulting in hypoplastic kidneys.8 Congruent with this finding, competitive inhibition of Wnt signaling by secreted Frizzled Related Proteins (sFRP) impairs nephron differentiation in vitro (sFRP can compete with membrane bound Frizzled receptors for their shared ligand, Wnt).76
Sonic hedgehog (Shh) signaling
Sonic hedgehog is an inhibitory ligand for Patched receptor, which constituitively inhibits Smoothened.77 On binding of Hedgehog, Smoothened becomes activated, inhibits the processing of full length Gli3 to a shorter protein that represses gene transcription, and stimulates translocation of Gli 1 and 2 to the nucleus where they activate transcription of multiple downstream effectors, including patterning genes such as Pax2 and Sall1 and cell cycle regulators, cyclin D1 and n-myc. In the absence of hedgehog binding, the repressor Gli-3 is instead dominant. Truncating mutations in Gli-3 that activate its repressor functions are associated with Pallister-Hall Syndrome and renal hypo/dysplasia.78,79 Mutations in Shh are associated with a VACTERYL-like syndrome in mice, with midline defects and hypoplastic kidneys.80 Deletion of Shh from the ureteric bud lineage using the HoxB7-Cre resulted in hypoplastic kidneys with hydronephrosis and hydroureter, associated with decreased proliferation of ureteral mesenchyme and impaired the ureteral smooth muscle differention.
The TGFβ Superfamily: Bone Morphogenic Proteins (BMPs)
BMPs, activin and growth/differentiation factor (GDF) are all members of the TGFβ superfamily, which act as ligands for transmembrane serine-threonine kinase receptors, the activin-like receptor kinases (Alks).81 Binding of BMP can be facilitated by extracellular activators (Krim/KCP) and inhibited by extracellular inhibitors such as Gremlin, Chordin and Noggin. On binding of their ligands, Alks dimerize and phosphorylate their mediators, the Smad proteins, which are also involved in TGFβ signaling. BMPs signal via activating Smads 1,5 and 8 while TGFβ signals vial Smads 2 and 3. Activating Smads translocate to the nucleus and stimulate transcription of metallomatrix proteases (MMPs), extracellular matrix proteins (ECM), Pai1, and other modulators of cell adhesion and differentiation. BMPs 4 and 7 are highly expressed in both the ureteric bud and cap mesenephric mesenchyme. Tight regulation of these proteins appears to be critical, as demonstrated by the finding that both complete loss of BMP7 expression and loss of inhibition of BMP by gremlin results in renal agenesis.82 This phenotype may in part result from effects on ureteric bud branching. In vitro, low levels of BMPs 4 and 7 may stimulate bud branching, while at high levels they inhibit bud branching, in part by activation of inhibitory Smad1.83 In vivo, gene deletion of the BMP receptor Alk3 from ureteric bud cell causes early increased and abnormal ureteric bud branching, resulting in fewer branches at later stages, indicating that dysregulation of bud branching can impair renal development.84 Another BMP expressed in the kidney is BMP2. BMP2 gene deletion is embryonic lethal; it inhibits ureteric bud branching in vitro and in vivo.83,85
There appears to be some functional redundancy between BMP4 and 7, as expression of BMP4 under the control of the BMP7 promotor rescues the null mutant phenotype.86 However, recent studies have identified podocyte specific roles for BMP4 and 7 during development. Podocyte BMP4 is required for glomerular capillary formation, while podocyte BMP7 is required for proximal tubular cell proliferation and growth during nephrogenesis; gene deletion of either BMP from developing podocytes resulted in a hypodysplastic renal phenotype.87,88
Several other ligands in the TGFβ superfamily may contribute to specification of the site of ureteric bud outgrowth. GDF11, BMP4 and Activin A are endogenous inhibitors that restrict the site of outgrowth of the ureteric bud to one location.89,90,91
Fibroblast Growth Factors (FGFs)
The family of FGFs bind the receptor tyrosine kinases (FGFR1 and 2) ;FGF binding stimulates homodimerization and phosphorylation of the receptor, leading to recruitment of the Grb2 adaptor and activation of Ras GTP proteins and ERK activation and cell proliferation. Binding by FGF to FGFR is facilitated by heparin sulfate proteoglycans.92 FGFs are critical mitogens in development of multiple organs, and deletion of FGF ligands and receptors are embryonic lethal. In vitro, FGF2 can promote condensation, induce WT-1 expression and inhibit apoptosis in metanephric mesenchyme.93,94 Studies of FGF in zebrafish demonstrated an early requirement of FGF in the intermediate mesoderm, with a late requirement for FGF8 in condensation of the metanephric mesenchyme.62 FGFs also function in the ureteric bud, and deletion of FGF7 and 10, and their receptor isoform FGFR2-IIIb results in decreased ureteric bud branching and smaller kidneys with decreased nephron number.95
Conditional deletion mutants have further defined lineage specific roles for FGFs. 95 In the metanephric mesenchyme, loss of FGF8 interrupts nephron development, with failure to express important mediators of nephron epithelial development, such as Wnt4 and Lim1.96 Deletion of the receptors FGFR1 and 2 in metanephric mesenchyme resulted in renal aplasia; remarkably this was also associated with decreased ureteric bud branching, providing further evidence that FGFs function in reciprocal signaling between metanephric mesenchyme and ureteric bud.97 The FGF receptors site-specific roles were further dissected in vivo: gene deletion of FGFR2 in mesenchymal stromal cells (by Pax-3-cre) resulted in formation of more than one ureteric bud, leading to duplicated collecting systems with ectopic ureteral insertion and hydroureter. Meanwhile, loss of FGFR1 expression in these mesenchymal cells had no phenotypic consequences. This indicates that signaling via mesenchymal FGFR2 defines the site of ureteric bud outgrowth, either secondary to effects of FGFR2 on the metanephric mesenchyme or possibly as a direct effect of stromal cells on the ureteric bud.98 In comparison, gene deletion of FGFR2 from ureteric bud by conditional targeting with Hox-B7-cre resulted in thin UB stalks, decreased branching, decreased nephrons and increased stroma. Remarkably, these FGFR effects were independent of changes in GDNF, Ret, Sprouty, Slit/Robo, and BMP-4 expression level.98
What are the factors that contribute to overall renal patterning and nephron number?
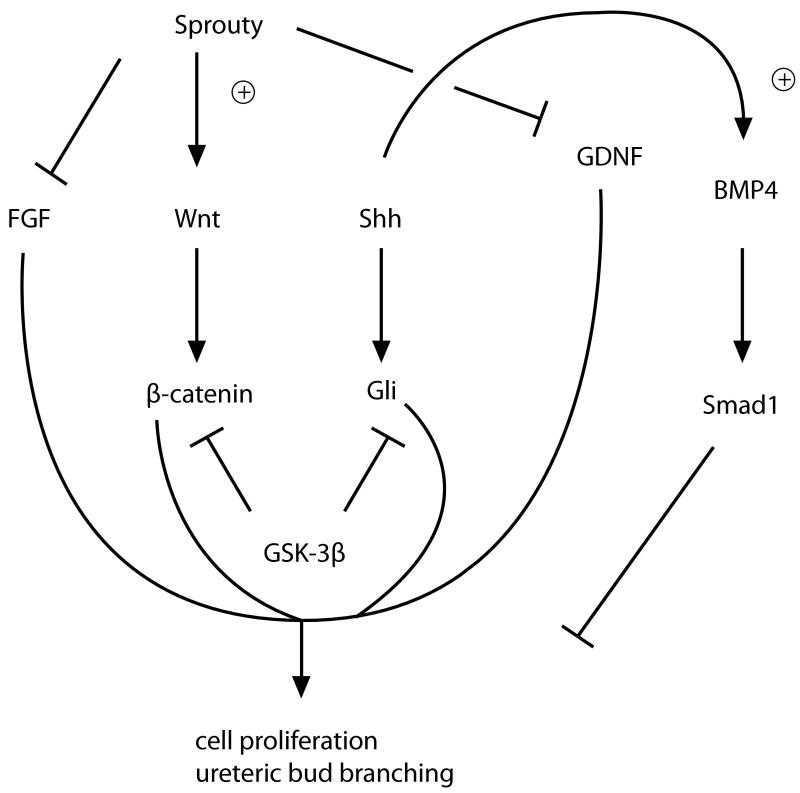
Serial branching of the ureteric tree with nephron induction at the ureteric tips generates the pattern of adult renal architecture and determines nephron number. It has been demonstrated that the above signaling pathways converge and modulate each other, provide complex regulation of overall renal patterning (figure 4). Shh, Wnt and BMP pathways can interact, with GSK-3β stimulating degradation of Gli effectors and Shh up-regulating BMP4 in the ureteric bud, providing evidence for complex modulation of signaling pathways.77 Shh signaling may also inhibit FGF signaling. Sprouty has also been shown to coordinate Wnt11, FGF7 and GDNF expression and sprouty can inhibit the intracellular signaling of both FGF and GDNF pathways.99
Figure 4.
Crosstalk between sonic hedgehog (Shh), bone morphogenic protein (BMP), fibroblast growth factor (FGF) and GDNF/ret signaling pathways modulates ureteric bud branching
The signals which terminate bud branching and regulate cell proliferation and apoptosis during kidney development are not fully understood. Tight control of apoptotic rate is critical during renal development, as evidence by renal dyplasia in salt stressed kallikrein/bradykinin B2 receptor deficient mice.100 Increased epithelial cell apoptosis and decreased ureteric bud branching was induced via a p53 dependent pathway resulting in renal dysplasia.100 Inappropriate cell proliferation may also contribute to the pathogenesis of renal tumors and cysts.101 The tumor suppressor protein von Hippel-Lindau gene (pVHL) downregulates the hypoxia induced factor (HIF 2α), which is upregulated in cysts and renal carcimomas from patients with VHL. In support of this pathophysiologic mechanism of cyst formation, specific gene deletion of pVHL from the proximal tubule resulted in cyst formation, composed of dedifferentiated cells with an increased rate of cell proliferation. Bcl-2 and cut related homeobox (cux-1) also contribute to regulation of apoptosis during development.102 Aberrant tubular flow as a result of impaired ciliary function is also thought to contribute to cell proliferation and cyst formation. Mutations in ciliary genes have been associated with cystic kidney diseases including polycystin 1 and 2 (PKD1 and 2, mutated in autosomal dominant polycystic kidney disease), polyductin (Pkhd1), nephronopthisis (NPHS 1-6), and the oral facial digital syndrome gene (ODS1). Signaling via growth factors may also contribute to cyst formation. Kidney specific deletion of hepatocyte nuclear factor-1β led to renal failure and cyst formation, associated with decreased Pkhd1 expression103. EGF and VEGF may also play a role; blockade of VEGFR2 also led to cysts.104
What are the molecular mechanisms that control terminal differentiation the nephron segments?
Notch genes encode single transmembrane proteins that mediate short-range signaling between cells by their ligands delta and jagged. Binding of their ligands stimulates Notch receptor cleavage by a proteolyte enzyme, γ-secretase, releasing the cleaved intracellular notch portion. The cleaved intracellular notch translocates to the nucleus and forms a complex with Cbf1/Rbp-J DNA binding proteins, whereupon it activates transcription of downstream targets. Notch signaling defines the podocyte and proximal tubular cell fates during S-shape segmentation.105 By using cultured kidney explants removed at various stages of nephron development, time sensitivity to Notch signaling could be established. Early explants (E12.5) exposed to Notch inhibitor failed to form proximal tubules and podocytes; whereas podocyte development in late explants (E14.5) was preserved. In vivo studies demonstrated Notch2, but not Notch1, was required for segmentation and differentiation of the proximal tubular segments and podocytes.
The signaling mechanisms by which podocytes differentiate are incompletely understood and are an area of active investigation; they will not be reviewed in depth here. In brief, Pax-2 is required for aggregation of the metanephic mesenchyme. Subsequent differentiation of epithelial cells into podocytes requires WT-1 induced downregulation of Pax-2.106 It is also known that WT-1 induces transcription characteristic of differentiated podocytes, ie. the slit diaphragm protein, nephrin.107 Gene deletion studies have demonstrated that several proteins are involved in podocyte differentiation and form foot processes, including Lmx1b, podocalyxin, pod1, kreisler and GLEPP1. The proteins that contribute to forming the slit diaphragms that link adjacent foot processes, including podocin, nephrin, CD2AP are also critical for normal differentiated podocyte structure. The GBM is secreted by podocytes and endothelial cell and is composed in part by collagen and laminin chains which undergo a switch during development. The GBM provides a framework for podocyte development, and can provide intracellular signals via binding of laminin components to integrins. It is notable that glomerular development is disturbed in integrin deficient mice.108 Signaling via growth factors contribute to migration of mesangial and endothelial cells during glomerular development, with PDGFR-β and VEGF required for mesangial and endothelial cell migration, respectively. The stimulus of low oxygen tension of the developing kidney may induce expression of Hypoxic inducible factor (HIF) and lead to expression of VEGF; in addition, podocyte WT1 also upregulates VEGF expression. Endothelial cell migration is further modulated by angiopoeitin-Tie2 and angiotensin II signaling pathways.
What genes control maturation of the ureteric bud into the ureter and bladder?
Even less is known about the factors involved in terminal differentiation of the ureteric bud. Notably, Tbx18 is required for development of ureteral mesenchyme, and loss of Tbx18 function results in phenotype similar to the clinical syndrome of megaureter, with abnormal peristalsis of the ureteric musculature. Shh and another protein, teashirt, which is upregulated by BMP4, have both been recently shown to play a role in ureteral smooth muscle differentiation.109
In summary, complex interactions between a relative few signaling pathways regulate the multiple steps of renal development. The ongoing challenge is to identify the modulating factors which provide time and site specific specificity to the actions of these signaling pathways. The evolution of our understanding of the cellular and molecular mechanisms of kidney development may provide methods for improved diagnosis of renal anomalies and, hopefully, targets for intervention for this common cause of childhood ESKD.
Table 1.
Genes expressed by progenitor cell populations
| Renal Progenitor Cell Populations | |
|---|---|
| Pronephros | |
| Iroquois110 | Required for pronephros |
| Kohtalo/trap230111 | Required for pronephros |
| XTRAP-gamma112 | Required for pronephros |
| Odd1 | Pronephros vs. vascular cell fate |
| Metanephric Mesenchyme Progenitors | |
| Eya1 | Branchio-oto-renal syndrome |
| Six 1,2,4 | Renal hypodysplasia113 |
| Pax2 | Renal-coloboma syndrome |
| Sall1 | Townes-B rocke/ Okihiro syndrome |
| Lim1 | Stimulate ureteric bud cell differentiation? |
| Emx2 | |
| WT-1 | |
| Stroma | |
| foxD1 (Bf2) | |
| Pax3 | Pax3 expression is suppressed in nephrogenic precursors by W T-1 and remains persistently expressed in stroma. |
| Vascular Progenitors | |
| VEGFR2 (Flk1) | |
| ckit | |
Table 2.
Factors involved in ureteric bud outgrowth and branching
| Ureteric Bud (UB): Origin and Branching | |
|---|---|
| FoxC1 | Single origin of UB; mutated in CAKUT |
| Slit2/Robo2 | Single origin of UB; mutated in VUR |
| GDNF/c-Ret | UB formation/ branching |
| Retinoic acid receptors (RARαβγ) | Maintains c-ret expression in UB tips |
| FGFR2 | Single origin of UB(in mesenchyme);branching |
| BMP2 & glypican3, BMP 4/7, Alk3, Gremlin, smad1 | Patterning of ureteric bud branching |
| Sonic Hedgehog (Shh) & Gli effectors | |
| Wnt 11/ β-catenin | |
| Activin | |
| GDF11 | |
| Sprouty | |
| Angiotensin II/ angiotensin receptors 1 & 2 | Stimulate ureteric bud branching |
| VEGF | |
| Protein kinase X | |
| Semaphorin 3a | Inhibit ureteric bud branching |
| TGFβ | |
Table 3.
Factors involved in induction and differentiation of metanephric mesenchyme
| Metanephric Mesenchyme: Induction / Mesenchymal-Epithelial Transition (MET) | |
|---|---|
| Wnt 4 (MM)/ Wnt 9b (UB) [Wnts 1,3,7,11] β-catenin |
Induction |
| BMP4/7 | Induction |
| FGF2 | Aggregation |
| Leukemia inhibitory factor (LIF) | Induction |
| TGFβ2/ Smad4 | |
| FGF 7,8/ FGFR1/2 | |
| Pax 2/8 | Epithelial differentiation |
| WT-1/Lim1/Pax 2/8 | Epithelial differentiation |
| Lim1 | Epithelial differentiation |
Table 4.
Factors involved in differentiation of nephron segments and glomerular differentiation
| Nephron Differentiation and Glomerular Development | |
|---|---|
| Segmentation | |
| Notch2 | Proximal tubular and podocyte cell fate |
| Podocyte BMP7 | Proximal tubular cell proliferation |
| Podocyte differentiation | |
| WT-1 | |
| Pax-2 | |
| Lmx1b | |
| Pod-1(Tcf21) | |
| GLEPP1 | |
| lim1 | |
| Pod1 | |
| Kreisler (Mafb) | |
| Podocalyxin | |
| FAT1 | |
| Slit diaphragm proteins | |
| Nephrin | |
| Neph 1,2,3 | |
| Podocin | |
| CD2AP | |
| GBM | |
| s-laminin/ laminin beta2 | |
| Alpha 3 integrin | |
| Alpha 8 integrin | |
| dystroglycan | |
| laminin | |
| entactin | |
| fibronectin | |
| Type IV collagen | |
| heparan sulfate proteoglycan | |
| vitronectin | |
| Glomerular capillaries | |
| VEGF/ VEGFR2 (Flk) | |
| Podocyte BMP4/ Id1 | |
| Eph family tyrosine kinases ELK/LERK | |
| Angiopoietin II/ Tie2 | |
| Mesangial cells | |
| Pdgfβ/ Pdgfβ receptor | |
| Stromal cells | |
| foxC1/C2 | |
References
- 1.Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1(3):385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 2.Holliday MA, Barratt TM, Avner ED. Pediatric nephrology. 3rd ed i. Williams & Wilkins; Baltimore: 1994. pp. 3–24. [Google Scholar]
- 3.Sariola H. Interspecies chimeras: an experimental approach for studies on embryonic angiogenesis. Med Biol. 1985;63(2):43–65. [PubMed] [Google Scholar]
- 4.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF--a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106(2):32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 5.Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 6.Fazan VP, Ma X, Chapleau MW, Barreira AA. Qualitative and quantitative morphology of renal nerves in C57BL/6J mice. Anat Rec. 2002;268(4):399–404. doi: 10.1002/ar.10174. [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134(13):2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 8.Bridgewater D, Cox B, Cain J, et al. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317(1):83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119(3):711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 10.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18(4):1121–1129. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias DM, Hueber PA, Chu L, et al. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007;293(2):F494–500. doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- 12.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133(15):2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 13.Stricker S, Brieske N, Haupt J, Mundlos S. Comparative expression pattern of Odd-skipped related genes Osr1 and Osr2 in chick embryonic development. Gene Expr Patterns. 2006;6(8):826–834. doi: 10.1016/j.modgep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond IA. The zebrafish pronephros: a genetic system for studies of kidney development. Pediatr Nephrol. 2000;14(5):428–435. doi: 10.1007/s004670050788. [DOI] [PubMed] [Google Scholar]
- 16.Tena JJ, Neto A, de la Calle-Mustienes E, et al. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301(2):518–531. doi: 10.1016/j.ydbio.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 17.Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135(20):3355–3367. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vize PD, Seufert DW, Carroll TJ, Wallingford JB. Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev Biol. 1997;188(2):189–204. doi: 10.1006/dbio.1997.8629. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Oghi KA, Zhang J, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 20.Xu PX, Zheng W, Huang L, et al. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130(14):3085–3094. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27(21):7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruf RG, Xu PX, Silvius D, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101(21):8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007;124(4):290–303. doi: 10.1016/j.mod.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Chai L, Yang J, Di C, et al. Transcriptional activation of the SALL1 by the human SIX1 homeodomain during kidney development. J Biol Chem. 2006;281(28):18918–18926. doi: 10.1074/jbc.M600180200. [DOI] [PubMed] [Google Scholar]
- 25.Nishinakamura R, Matsumoto Y, Nakao K, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128(16):3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- 26.Self M, Lagutin OV, Bowling B, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25(21):5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi A, Valerius MT, Mugford JW, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi A, Kwan KM, Carroll TJ, et al. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132(12):2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 29.Potter SS, Hartman HA, Kwan KM, Behringer RR, Patterson LT. Laser capture-microarray analysis of Lim1 mutant kidney development. Genesis. 2007;45(7):432–439. doi: 10.1002/dvg.20309. [DOI] [PubMed] [Google Scholar]
- 30.Kreidberg JA, Sariola H, Loring JM, et al. WT-1 is required for early kidney development. Cell. 1993;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 31.Dehbi M, Pelletier J. PAX8-mediated activation of the wt1 tumor suppressor gene. EMBO J. 1996;15(16):4297–4306. [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Chen X, Taglienti M, et al. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132(24):5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 33.Donovan MJ, Natoli TA, Sainio K, et al. Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev Genet. 1999;24(34):252–262. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<252::AID-DVG8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Weber S, Moriniere V, Knuppel T, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17(10):2864–2870. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 35.Sakaki-Yumoto M, Kobayashi C, Sato A, et al. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development. 2006;133(15):3005–3013. doi: 10.1242/dev.02457. [DOI] [PubMed] [Google Scholar]
- 36.Levinson RS, Batourina E, Choi C, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132(3):529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 37.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10(10):2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 38.Tufro A. VEGF spatially directs angiogenesis during metanephric development in vitro. Dev Biol. 2000;227(2):558–566. doi: 10.1006/dbio.2000.9845. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt-Ott KM, Chen X, Paragas N, et al. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299(1):238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16(11):1423–1432. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugford JW, Sipila P, Kobayashi A, Behringer RR, McMahon AP. Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol. 2008;319(2):396–405. doi: 10.1016/j.ydbio.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271(1):98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Bridgewater D, Cox B, Cain J, et al. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317(1):83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Moore MW, Klein RD, Farinas I, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 45.Pichel JG, Shen L, Sheng HZ, et al. GDNF is required for kidney development and enteric innervation. Cold Spring Harb Symp Quant Biol. 1996;61:445–457. [PubMed] [Google Scholar]
- 46.Sanchez MP, Silos-Santiago I, Frisen J, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 47.Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci U S A. 1996;93(20):10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durbec P, Marcos-Gutierrez CV, Kilkenny C, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 49.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 50.Moreau E, Vilar J, Lelievre-Pegorier M, Merlet-Benichou C, Gilbert T. Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am J Physiol. 1998;275(6 Pt 2):F938–945. doi: 10.1152/ajprenal.1998.275.6.F938. [DOI] [PubMed] [Google Scholar]
- 51.Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243(1):128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- 52.Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol. 2007;307(2):290–299. doi: 10.1016/j.ydbio.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang MJ, Worley D, Sanicola M, Dressler GR. The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol. 1998;142(5):1337–1345. doi: 10.1083/jcb.142.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet. 2008;82(2):344–351. doi: 10.1016/j.ajhg.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DC, Chan KW, Chan SY. RET receptor tyrosine kinase isoforms in kidney function and disease. Oncogene. 2002;21(36):5582–5592. doi: 10.1038/sj.onc.1205741. [DOI] [PubMed] [Google Scholar]
- 56.Basson MA, Akbulut S, Watson-Johnson J, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8(2):229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127(7):1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- 58.Grieshammer U, Le M, Plump AS, et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6(5):709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 59.Nakano T, Niimura F, Hohenfellner K, Miyakita E, Ichikawa I. Screening for mutations in BMP4 and FOXC1 genes in congenital anomalies of the kidney and urinary tract in humans. Tokai J Exp Clin Med. 2003;28(3):121–126. [PubMed] [Google Scholar]
- 60.Bertoli-Avella AM, Conte ML, Punzo F, et al. ROBO2 gene variants are associated with familial vesicoureteral reflux. J Am Soc Nephrol. 2008;19(4):825–831. doi: 10.1681/ASN.2007060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28(2):117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 62.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8(1):65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Yu OH, Murawski IJ, Myburgh DB, Gupta IR. Overexpression of RET leads to vesicoureteric reflux in mice. Am J Physiol Renal Physiol. 2004;287(6):F1123–1130. doi: 10.1152/ajprenal.00444.2003. [DOI] [PubMed] [Google Scholar]
- 64.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125(56):558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gribouval O, Gonzales M, Neuhaus T, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37(9):964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 66.Niimura F, Kon V, Ichikawa I. The renin-angiotensin system in the development of the congenital anomalies of the kidney and urinary tract. Curr Opin Pediatr. 2006;18(2):161–166. doi: 10.1097/01.mop.0000193288.56528.40. [DOI] [PubMed] [Google Scholar]
- 67.Cano-Gauci DF, Song HH, Yang H, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146(1):255–264. doi: 10.1083/jcb.146.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol. 2001;231(1):31–46. doi: 10.1006/dbio.2000.0127. [DOI] [PubMed] [Google Scholar]
- 69.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372(6507):679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 70.Herzlinger D, Qiao J, Cohen D, Ramakrishna N, Brown AM. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev Biol. 1994;166(2):815–818. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- 71.Torban E, Dziarmaga A, Iglesias D, et al. PAX2 activates WNT4 expression during mammalian kidney development. J Biol Chem. 2006;281(18):12705–12712. doi: 10.1074/jbc.M513181200. [DOI] [PubMed] [Google Scholar]
- 72.Davies JA. Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat (Basel) 1996;156(3):187–201. doi: 10.1159/000147846. [DOI] [PubMed] [Google Scholar]
- 73.Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12(12):1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merkel C, Karner C, Carroll T. Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatr Nephrol. 2007;22(11):1825–1838. doi: 10.1007/s00467-007-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marose T, Merkel C, McMahon A, Carroll T. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314(1):112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshino K, Rubin JS, Higinbotham KG, et al. Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev. 2001;102(12):45–55. doi: 10.1016/s0925-4773(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 77.Gill P, Rosenblum N. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle. 2006;5(13):1426–1430. doi: 10.4161/cc.5.13.2928. [DOI] [PubMed] [Google Scholar]
- 78.Hu MC, Mo R, Bhella S, et al. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133(3):569–578. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- 79.Bose J, Grotewold L, Ruther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet. 2002;11(9):1129–1135. doi: 10.1093/hmg/11.9.1129. [DOI] [PubMed] [Google Scholar]
- 80.Kim PC, Mo R, Hui Cc C. Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36(2):381–384. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- 81.Oxburgh L, Chu GC, Michael SK, Robertson EJ. TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131(18):4593–4605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- 82.Michos O, Panman L, Vintersten K, et al. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 83.Piscione TD, Phan T, Rosenblum ND. BMP7 controls collecting tubule cell proliferation and apoptosis via Smad1-dependent and -independent pathways. Am J Physiol Renal Physiol. 2001;280(1):F19–33. doi: 10.1152/ajprenal.2001.280.1.F19. [DOI] [PubMed] [Google Scholar]
- 84.Hartwig S, Bridgewater D, Di Giovanni V, et al. BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol. 2008;19(1):117–124. doi: 10.1681/ASN.2007010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartwig S, Hu MC, Cella C, et al. Glypican-3 modulates inhibitory Bmp2-Smad signaling to control renal development in vivo. Mech Dev. 2005;122(78):928–938. doi: 10.1016/j.mod.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Oxburgh L, Dudley AT, Godin RE, et al. BMP4 substitutes for loss of BMP7 during kidney development. Dev Biol. 2005;286(2):637–646. doi: 10.1016/j.ydbio.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 87.Kazama I, Mahoney Z, Miner JH, et al. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19(11):2181–2191. doi: 10.1681/ASN.2007111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ueda H, Miyazaki Y, Matsusaka T, et al. Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol. 2008;19(4):685–694. doi: 10.1681/ASN.2006090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esquela AF, Lee SJ. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol. 2003;257(2):356–370. doi: 10.1016/s0012-1606(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 90.Maeshima A, Vaughn DA, Choi Y, Nigam SK. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol. 2006;295(2):473–485. doi: 10.1016/j.ydbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 91.Michos O, Goncalves A, Lopez-Rios J, et al. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134(13):2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- 92.Slack JM, Darlington BG, Gillespie LL, et al. Mesoderm induction by fibroblast growth factor in early Xenopus development. Philos Trans R Soc Lond B Biol Sci. 1990;327(1239):75–84. doi: 10.1098/rstb.1990.0044. [DOI] [PubMed] [Google Scholar]
- 93.Urban AE, Zhou X, Ungos JM, et al. FGF is essential for both condensation and mesenchymal-epithelial transition stages of pronephric kidney tubule development. Dev Biol. 2006;297(1):103–117. doi: 10.1016/j.ydbio.2006.04.469. [DOI] [PubMed] [Google Scholar]
- 94.Plisov SY, Yoshino K, Dove LF, et al. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development. 2001;128(7):1045–1057. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- 95.Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol. 2007;22(3):343–349. doi: 10.1007/s00467-006-0239-7. [DOI] [PubMed] [Google Scholar]
- 96.Grieshammer U, Cebrian C, Ilagan R, et al. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005;132(17):3847–3857. doi: 10.1242/dev.01944. [DOI] [PubMed] [Google Scholar]
- 97.Poladia DP, Kish K, Kutay B, et al. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291(2):325–339. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 98.Hains D, Sims-Lucas S, Kish K, et al. Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e318187cc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chi L, Zhang S, Lin Y, et al. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131(14):3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- 100.El-Dahr SS, Aboudehen K, Dipp S. Bradykinin B2 receptor null mice harboring a Ser23-to-Ala substitution in the p53 gene are protected from renal dysgenesis. Am J Physiol Renal Physiol. 2008 doi: 10.1152/ajprenal.90378.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong AC, Wheatley DN. Polycystic kidney disease--the ciliary connection. Lancet. 2003;361(9359):774–776. doi: 10.1016/S0140-6736(03)12662-1. [DOI] [PubMed] [Google Scholar]
- 102.Heuvel GB Vanden, Bodmer R, McConnell KR, Nagami GT, Igarashi P. Expression of a cut-related homeobox gene in developing and polycystic mouse kidney. Kidney Int. 1996;50(2):453–461. doi: 10.1038/ki.1996.336. [DOI] [PubMed] [Google Scholar]
- 103.Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68(5):1944–1947. doi: 10.1111/j.1523-1755.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 104.McGrath-Morrow S, Cho C, Molls R, et al. VEGF receptor 2 blockade leads to renal cyst formation in mice. Kidney Int. 2006;69(10):1741–1748. doi: 10.1038/sj.ki.5000314. [DOI] [PubMed] [Google Scholar]
- 105.Cheng HT, Kim M, Valerius MT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134(4):801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan G, Steele-Perkins V, Morris JF, Rauscher FJ, 3rd, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995;121(3):867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- 107.Wagner N, Wagner KD, Xing Y, Scholz H, Schedl A. The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol. 2004;15(12):3044–3051. doi: 10.1097/01.ASN.0000146687.99058.25. [DOI] [PubMed] [Google Scholar]
- 108.Kanasaki K, Kanda Y, Palmsten K, et al. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313(2):584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caubit X, Lye CM, Martin E, et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135(19):3301–3310. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- 110.Alarcon P, Rodriguez-Seguel E, Fernandez-Gonzalez A, Rubio R, Gomez-Skarmeta JL. A dual requirement for Iroquois genes during Xenopus kidney development. Development. 2008;135(19):3197–3207. doi: 10.1242/dev.023697. [DOI] [PubMed] [Google Scholar]
- 111.Hong SK, Haldin CE, Lawson ND, et al. The zebrafish kohtalo/trap230 gene is required for the development of the brain, neural crest, and pronephric kidney. Proc Natl Acad Sci U S A. 2005;102(51):18473–18478. doi: 10.1073/pnas.0509457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li DH, Chan T, Satow R, et al. The role of XTRAP-gamma in Xenopus pronephros development. Int J Dev Biol. 2005;49(4):401–408. doi: 10.1387/ijdb.052005dl. [DOI] [PubMed] [Google Scholar]
- 113.Weber S, Taylor JC, Winyard P, et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol. 2008;19(5):891–903. doi: 10.1681/ASN.2006111282. [DOI] [PMC free article] [PubMed] [Google Scholar]