Abstract
Over the past decade, advances in the ventilatory management of acute lung injury (ALI) and ARDS have improved outcomes; however, until recently the search for other therapies has been less fruitful. Recently, the Acute Respiratory Distress Syndrome Network Fluid and Catheter Treatment Trial reported that a conservative fluid management strategy, compared with a fluid liberal strategy, increased the mean (± SE) number of ventilator-free days in patients with ALI (14.6 ± 0.5 vs 12.1 ± 0.5 days, respectively; p < 0.001). In addition to this beneficial effect on outcomes, the study found that the conservative fluid strategy did not increase the incidence of renal failure or the development of shock. Other studies have demonstrated that albumin and furosemide therapy may be beneficial in hypoproteinemic patients with lung injury, though data on outcomes is still lacking. Although several pharmacologic therapies, such as corticosteroids, surfactant, and nitric oxide, have been demonstrated to be ineffective in improving outcomes, several promising new treatments are being investigated in ongoing or upcoming clinical trials. This article reviews these developments and other recent research on the optimal nonventilatory management of patients with ALI.
Keywords: acute lung injury, fluid therapy, management, pulmonary edema
Advances in the ventilatory management of acute lung injury (ALI) and ARDS over the past decade have been dramatic. In particular, the use of a low-tidal volume (6 mL/kg predicted body weight), plateau pressure-limited strategy has been demonstrated to reduce mortality from 40 to 31%.1 Further, a large, multicenter, randomized, controlled trial2 demonstrated the equivalence of higher and lower levels of positive end-expiratory pressure. Over this time period, a number of nonventilatory therapies for ALI/ARDS have been investigated, many of which have not proven to be effective, while others appear more promising.
This article will review the most recent and relevant evidence regarding nonventilatory treatments for ALI/ARDS, including advances in fluid management and pharmacotherapy. In addition, we will briefly survey promising new and investigational therapies for ALI/ARDS that are the focus of current and upcoming clinical trials.
FLUID MANAGEMENT
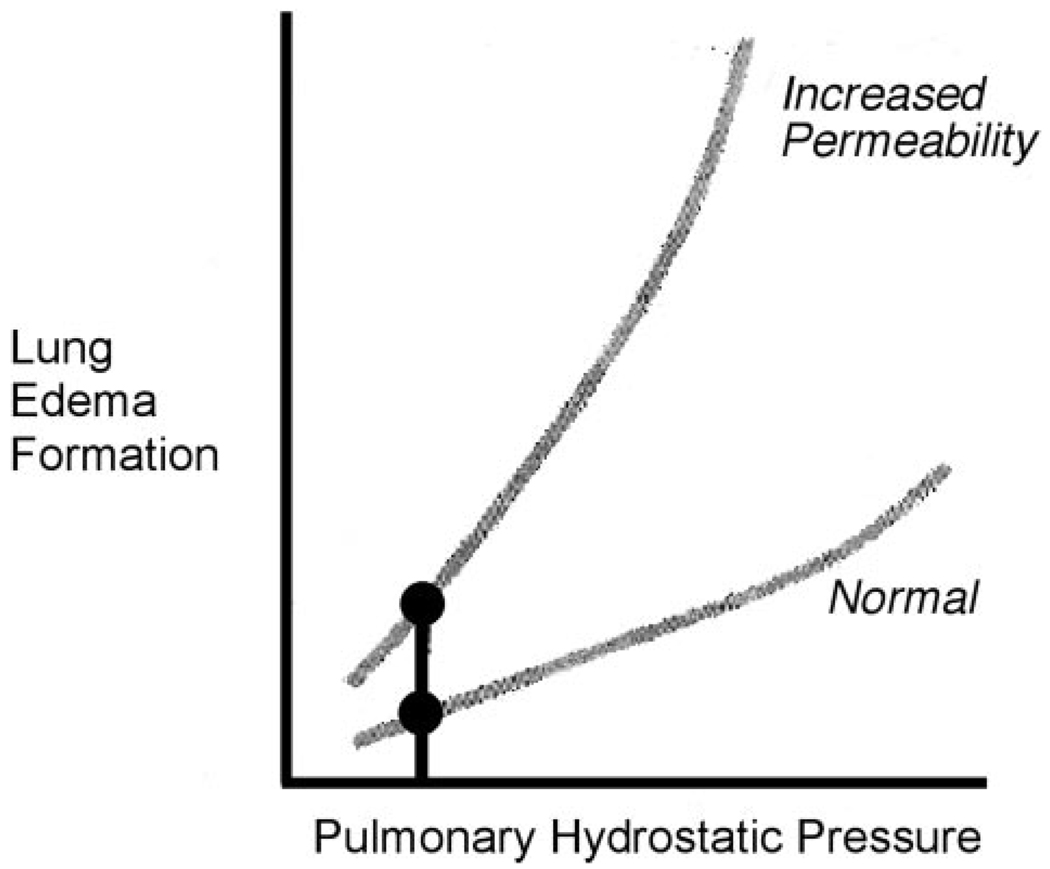
Until recently, the optimal strategy for fluid management in patients with ALI/ARDS was unclear. Pulmonary edema, even when noncardiogenic in origin, increases with a rise in hydrostatic pressures. Experimental studies demonstrated that a modest decrease in pulmonary vascular pressure could reduce the quantity of pulmonary edema in oleic acid-induced permeability pulmonary edema in dogs.3 Norman Staub recommended in this journal in 1978 a clinical strategy of lowering microvascular pressures in the setting of increased permeability pulmonary edema (Fig 1).4 Increased extravascular lung water has been associated with poor outcome in ARDS patients,5 and likewise, a reduction in pulmonary capillary wedge pressure has been associated with increased survival in ARDS patients.6 However, balancing the risks of increased edema vs those of decreased vital organ perfusion with a lower intravascular pressure has remained difficult.
FIGURE 1.
Relationship between pulmonary hydrostatic pressure and lung edema formation under normal conditions and increased permeability. Even under normal conditions, an increase in pulmonary hydrostatic pressure results in increased lung edema formation. This relationship, however, is dramatically accentuated under conditions of increased lung permeability. Adapted with permission from Staub.4
Recently, the National Heart, Lung and Blood Institute (NHLBI) Acute Respiratory Distress Syndrome Network published the results of the Fluid And Catheter Treatment Trial (FACTT),7 a large randomized trial comparing a liberal fluid management strategy to a conservative fluid management strategy in patients with ALI. Fluid and diuretic management were dictated by a highly protocolized regimen, which is described in detail in the original publication, and all patients were managed with a low-tidal volume, plateau pressure-limited ventilation strategy.1 The fluid management strategies were tested in a factorial design that also evaluated the utility of catheterization with a central venous catheter (CVC) vs a pulmonary artery catheter (PAC)8; the two types of catheters were equivalent in terms of clinical outcomes (mortality rate: PAC group, 27.4%; CVC group, 26.3%; p = 0.69; 95% confidence interval for difference, –4.4 to 6.6%). In contrast, there was a clear difference in outcome between the liberal fluid management and conservative fluid management arms of the study. Patients in the conservative fluid management arm had significantly more ventilator-free days than those in the liberal fluid management arm (mean [± SE], 14.6 ± 0.5 vs 12.1 ± 0.5 days, respectively; p < 0.001) and concordant improvements in pulmonary physiology (Table 1).69 Likewise, patients in the conservative fluid management arm had more ICU-free days than those in the liberal fluid management arm (13.4 ± 0.4 vs 11.2 ± 0.4, respectively; p < 0.001). There was also a 2.9% reduction in the 60-day mortality rate in the conservative fluid management arm compared with the liberal fluid management arm, though the comparison was not statistically significant (25.5% vs 28.4%, respectively; p = 0.30; 95% confidence interval for the difference, −2.6 to 8.4%).
Table 1.
Pulmonary Outcomes and Physiologic Variables in the FACTT*
| Fluid Management |
|||
|---|---|---|---|
| Variables | Conservative Group | Liberal Group | p Value† |
| Ventilator-free days, No. | 14.6 ± 0.5 | 12.1 ± 0.5 | < 0.001 |
| PEEP, cm H2O | 7.5 ± 0.3 | 8.2 ± 0.2 | 0.008 |
| Plateau pressure, cm H2O | 24.2 ± 0.6 | 25.7 ± 0.5 | 0.002 |
| Pao2/Fio2 | 198 ± 8 | 183 ± 6 | 0.07 |
| Oxygenation index‡ | 10.1 ± 0.8 | 11.8 ± 0.7 | 0.003 |
| Lung injury score§ | 2.03 ± 0.07 | 2.27 ± 0.06 | < 0.001 |
Values are given as the mean ± SE, unless otherwise indicated. Fio2 = fraction of inspired oxygen; PEEP = positive end-expiratory pressure.
p Values for the physiologic variables are for comparison of trends over time using repeated-measures analysis of variance, though only day 7 values are shown for simplicity.
Oxygenation index was calculated as (mean airway pressure × Fio2/Pao2) × 100, with a lower number indicating better gas exchange.
Lung injury score was calculated as previously described by Murray et al.69
The study found no differences between the two fluid strategies in the incidence or prevalence of shock or in the need for renal replacement therapy, although there was a strong trend in the latter toward a benefit in the conservative fluid management arm (10% required dialysis; liberal fluid management arm, 14%; p = 0.06). Similarly, the mean number of days of renal support required did not differ between the two groups (conservative fluid management group 11.0 ± 1.7 days; liberal fluid management group, 10.9 ± 1.4 days; p = 0.96). These data provide reassurance that the conservative fluid management strategy did not negatively impact renal function.
Translation to Clinical Practice
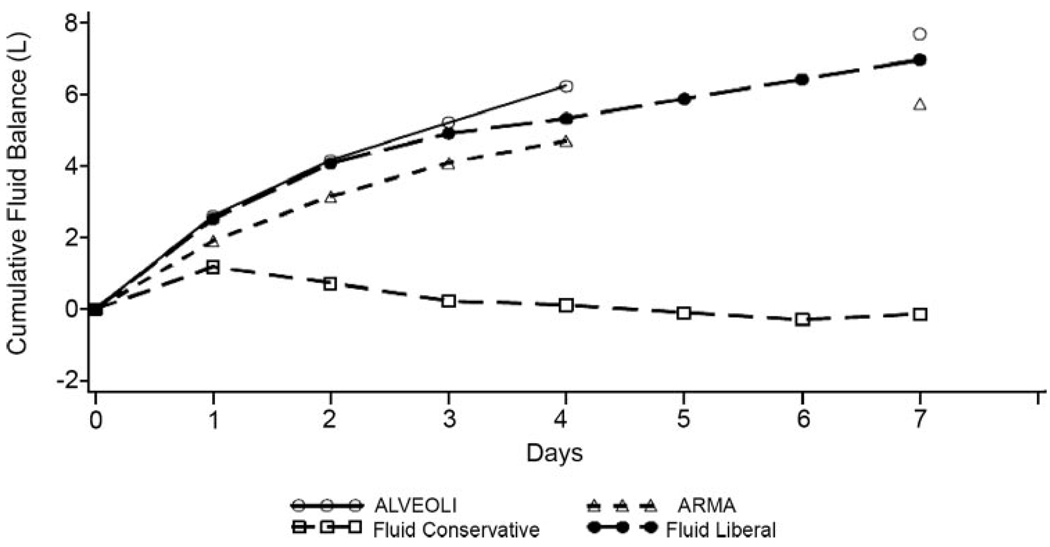
When considering how to translate the results of the FACTT into clinical practice, several factors should be considered. First, only patients who were not experiencing cardiopulmonary shock were managed by the protocol. If the mean arterial pressure was < 60 mm Hg or the patient required therapy with vasopressors (other than dopamine at a dose of < 5 µg/kg/min), fluid management was left to the judgment of the managing physician. Thus, the finding that a conservative fluid management strategy was associated with better outcomes in this study does not imply that patients who are in shock ought to have fluids restricted. Second, the mean time from ICU admission to protocol implementation was approximately 43 h. Therefore, as pointed out by Rivers in the editorial accompanying the trial,9 the results of the FACTT do not conflict with the finding that early goal-directed therapy in patients with sepsis (with its concomitant aggressive resuscitation) improves outcomes.10 In the trial that first established the benefits of early goal-directed therapy,10 patients with early severe sepsis or septic shock were enrolled on average 1.5 h after arrival in the emergency department and were treated for at least 6 h with aggressive crystalloid resuscitation, using central venous pressure monitoring, and optimization of oxygen delivery using vasopressors, RBC transfusions, and inotropes as necessary. This strategy decreased the in-hospital mortality rate in that single-center study from 46.5 to 30.5% (p = 0.009) and is now widely accepted as appropriate initial management of early severe sepsis. Third, the liberal fluid management strategy would be more accurately called a usual fluid management strategy, since the average daily fluid gain of approximately 1 L in this group is quite similar to that observed in prior studies1,2 from the Acute Respiratory Distress Syndrome Network in which fluid management was not protocolized (Fig 2). In contrast, the conservative fluid management group had a net fluid balance of approximately zero over the first 7 days of the protocol. These data may provide useful benchmarks to guide the fluid management of critically ill patients in the real world, with the important caveats that electrolytes must be closely monitored and the many safety features of the FACTT (like holding back therapy with diuretics until patients had been out of shock for at least 12 h) must be noted. Finally, patients with an established need for dialysis were excluded from the trial, and it remains unclear what volume management strategy should be followed in this population.
FIGURE 2.
Cumulative fluid balance in patients enrolled in the FACTT compared to patients in prior Acute Respiratory Distress Syndrome Network studies. Fluid administration in the liberal fluid management arm of the FACTT mimicked that in two prior Acute Respiratory Distress Syndrome Network studies of ventilator management strategy and resulted in a gain of approximately 1 L/d. In contrast, fluid administration in the conservative fluid management arm resulted in a net even fluid balance. ARMA = Acute Respiratory Distress Syndrome Network trial of 6 vs 12 mL/kg tidal volume ventilation1; ALVEOLI = Acute Respiratory Distress Syndrome Network trial of low vs high positive end-expiratory pressure.2 Adapted with permission from the Acute Respiratory Distress Syndrome Network.7 Copyright 2006 Massachusetts Medical Society. All rights reserved.
The Acute Respiratory Distress Syndrome Network has generated a simplified version of the conservative fluid management protocol that will be used for future trials (Table 2). This more practical version of the protocol is greatly simplified from the one used in the FACTT and published with the original study; however, it provides useful targets for the monitoring of intravascular pressure and urine output, and suggests therapies to help reach those goals once the patient has been out of shock for at least 12 h. Although the FACTT demonstrated the equivalence of CVCs and PACs, the protocol includes guidelines for use with a PAC for those physicians who remain more comfortable with this type of monitoring or for situations in which a PAC is needed for another purpose. In addition, suggested guidelines for continuous furosemide infusion are provided, as some authorities have suggested that this method of administration provides equivalent or superior diuresis with lower doses of medication,11 though this approach was not specifically evaluated in the Acute Respiratory Distress Syndrome Network trial.
Table 2.
Simplified Algorithm for Conservative Management of Fluids in Patients With ALI, Based on Protocol Used in the FACTT*
| CVP, mm Hg (Recommended) |
PAOP, mm Hg (Optional) |
MAP ≥ 60 mm Hg and Not Receiving Vasopressors for ≥ 12 h |
|
|---|---|---|---|
| Average Urine Output < 0.5 mL/kg/h | Average Urine Output ≥ 0.5 mL/kg/h | ||
| > 8 | > 12 | Furosemide†; reassess in 1 h | Furosemide; reassess in 4 h |
| 4–8 | 8–12 | Fluid bolus as fast as possible‡; reassess in 1 h | Furosemide; reassess in 4 h |
| < 4 | < 8 | Fluid bolus as fast as possible‡; reassess in 1 h | No intervention; reassess in 4 h |
CVP = central venous pressure; PAOP = pulmonary artery occlusion pressure; MAP = mean arterial pressure. Reprinted with the courtesy of the NHLBI Acute Respiratory Distress Syndrome Network. Patients must have had a MAP of > 60 mm Hg without requiring vasopressors for at least 12 h before this protocol is initiated.
Furosemide dosing: begin with a 20-mg bolus, 3 mg/h infusion, or last known effective dose. Double each subsequent dose until the goal is achieved (oliguria reversal or intravascular pressure target), with a maximal dose of 160-mg bolus or 24 mg/h. Do not exceed 620 mg/d. If the patient has heart failure, treatment with dobutamine may be considered. Diuretic therapy should be withheld for patients with renal failure, which is defined as dialysis dependence, oliguria with a serum creatinine level of > 2 mg/dL, or oliguria with a serum creatinine level of < 2 mg/dL but with urinary indices indicative of acute renal failure.
Fluid bolus: 15 mL/kg crystalloid (round to nearest 250 mL) or 1 unit of packed RBCs or 25 g of albumin.
Other Fluid Management Strategies
The debate over whether to resuscitate critically ill patients with crystalloid or colloid solutions has been ongoing in the critical care literature for years. Early metaanalyses12 suggested that albumin resuscitation was associated with increased mortality in critically ill patients, although these results were called into question by later metaanalyses.13 More recently, a large randomized controlled trial14 in a mixed population of 7,000 critically ill patients (the Saline versus Albumin Fluid Evaluation trial) reported that albumin resuscitation was equivalent to saline solution resuscitation, and a similar trial15 of crystalloid vs other colloids is ongoing. Addressing this controversy, a 2004 consensus panel convened by the American Thoracic Society.16 concluded that the use of colloids should generally be reserved for specific conditions in which there was clear evidence of benefit. The subset of lung injury patients with hypoproteinemia was included in this consensus statement as one in which the judicious use of albumin (with furosemide) may be beneficial. Hypoproteinemia is a documented risk factor for the development of ALI and for poor outcome in critical illness in general.17,18 Martin and colleagues19 randomized 37 atients with ALI and a serum protein concentration of < 5.0 mg/dL to receive either furosemide and albumin every 8 h for 5 days or double placebo. The intervention group had improved oxygenation, fluid balance, and hemodynamics; no differences in mortality were seen, although the study was not powered to assess this outcome. In a follow-up study20 comparing the administration of furosemide with albumin to that of furosemide without albumin, the combination of the two agents again proved to be superior, using similar end points. Thus, in the particular setting of hypoproteinemia and ALI, the combination of furosemide and albumin may be useful in improving pulmonary physiology; whether outcomes will be improved by this therapy remains to be seen.
Continuous hemofiltration with a zero fluid balance has been proposed as a potential therapy for ALI patients due to the theoretical benefits of removing humoral mediators of lung injury from the circulation and reducing lung vascular pressures. In an animal model21 of oleic acid-induced lung injury, continuous hemofiltration reduced pulmonary edema by reducing both lung vascular pressures and epithelial permeability to protein, with the latter apparently achieved in part by decreasing plasma levels of the inflammatory cytokines interleukin-6 and interleukin-8. Similar results have been reported in other animal models22–24; however, studies in humans have reported conflicting results. One single-center trial25 administered continuous venovenous hemodiafiltration to 10 pediatric oncology patients with ARDS and found that 9 of the children were successfully extubated. In contrast, another observational trial26 of 37 adult patients with acute renal failure and ALI found that the therapy had little beneficial impact on pulmonary physiology end points. Further clinical trials on this subject are needed.
PHARMACOTHERAPY
The search for an effective pharmacologic therapy for ALI/ARDS has continued over the past decade without major success; to date, no pharmacologic agent has been demonstrated to reduce mortality among patients with this condition.27 Several treatments, however, merit brief discussion due to historical interest as a therapy for ALI, conclusive evidence of lack of benefit, or potentially intriguing analyses of subgroups or secondary outcomes. In addition, we will cover several promising new therapies that are the focus of ongoing or upcoming clinical trials.
Pharmacologic therapies recently investigated27 as possible treatments for ALI include surfactant, inhaled nitric oxide (NO), corticosteroids, antifungal drugs, and phosphodiesterase inhibitors (Table 3).28–33,35–40,42–48,51,70–73 Exogenous surfactant administration was first administered as a therapy for lung injury in the late 1980s.28 Several phase I and II trials in humans showed promising trends in outcomes,29,30 but a larger randomized controlled trial31 of aerosolized synthetic surfactant in patients with sepsis-related lung injury did not demonstrate a benefit. Since then, further trials32,33 of endotracheal delivery of natural or recombinant surfactants have also found no benefit in adult populations. One randomized controlled trial34 in a pediatric population, however, found that exogenous surfactant improved both oxygenation and mortality in children with ALI.
Table 3.
Pharmacotherapies Investigated as Possible Treatment for ALI/ARDS
| Therapy | Outcomes | References |
|---|---|---|
| Surfactant | No significant mortality benefit (adult populations) | 28–33 |
| NO | Improves oxygenation but no mortality benefit | 35–40 |
| Corticosteroids (preventative) | Not effective in preventing ALI/ARDS | 42–45 |
| Corticosteroids (therapeutic) | No mortality benefit; may increase risk in patients with ARDS of ≥ 14 d duration |
46–48,51 |
| Antifungal agents (-azoles) | No mortality benefit in treating established ARDS; may help prevent development of ARDS |
70–72 |
| Phosphodiesterase inhibitors (eg, lisofylline and pentoxifylline) |
No mortality benefit in ALI/ARDS | 73 |
Inhaled NO has been considered a promising therapy for lung injury due to its ability to provide selective pulmonary vasodilatation and improve ventilation-perfusion mismatch. Unfortunately, although several trials35–40 have now demonstrated some improvements in oxygenation and pulmonary hemodynamics with the use of NO, the lack of mortality benefit has been just as consistent. Further, while nearly 60% of patients who receive inhaled NO will respond clinically with improved oxygenation, these benefits are typically short-lived, fading after the first 1 to 2 days of administration.41 Thus, although the use of NO may be considered as a rescue therapy in patients who are exceptionally difficult to oxygenate, it has no role in standard therapy for ALI.
Similarly, corticosteroids seemed to be an ideal therapy for lung injury, given their potent antiinflammatory and antifibrotic properties. Clinical trials have evaluated the utility of corticosteroids in preventing ALI/ARDS42–45 and in treating either early-stage (inflammatory) or late-stage (fibrotic) ALI/ARDS46–48; none have demonstrated a mortality benefit. In addition, the use of corticosteroids has been limited by concerns over their contributions to neuromuscular disorders associated with critical illness, particularly when combined with neuromuscular blocking agents.49,50
Most recently, the NHLBI Acute Respiratory Distress Syndrome Network published the results of a randomized controlled trial51 of methylprednisolone in ARDS patients of at least 7 days duration. Although therapy with methylprednisolone increased the number of ventilator-free days, shock-free days, and ICU-free days during the first month, it was associated with a significant increase in 60-day and 180-day mortality rates among patients enrolled > 13 days after the onset of ARDS. This discrepancy may be related to the finding that patients who were treated with methylprednisolone were more likely to return to assisted ventilation after extubation than those treated with placebo (28% vs 9%, respectively; p = 0.006). Methylprednisolone did, however, reduce the 60-day mortality rate in patients with elevated BAL fluid levels of procollagen peptide III, which is a biological marker of collagen synthesis in the lung previously demonstrated to have prognostic value in ARDS.52 Although the overall rate of neuromyopathy at 180 days did not differ between the corticosteroid and placebo groups, all nine reports of serious adverse events associated with neuromyopathy were in the methylprednisolone group (p = 0.001). Of note, the subgroup interaction analyses of outcomes up to day 60 were defined a priori per the study investigators, while those analyses focusing on 180-day outcomes were post hoc. Thus, although the results of this large, randomized, controlled trial are complex, on balance the evidence still argues against treating ALI/ARDS with corticosteroids, particularly in patients with ALI/ARDS of > 13 days duration, given the lack of beneficial effect on long-term outcomes and concerns about neuromuscular side effects.
Novel Potential Therapies for ALI
Though none of the aforementioned agents have proved to be effective in improving ALI-related mortality, several promising new therapies are being evaluated in ongoing or upcoming clinical trials. Activated protein C, available commercially as drotrecogin alfa, has been demonstrated to significantly reduce mortality in patients with severe sepsis.53 Recognition of the potential role of disordered coagulation and fibrinolysis in the pathogenesis of lung injury is growing.54,55 In patients with lung injury, lower levels of endogenous protein C are associated with poor clinical outcomes.56 Furthermore, studies57,58 in healthy human subjects have demonstrated that the administration of activated protein C decreases lung inflammation and inhibits coagulation after endotoxin exposure. These discoveries prompted the design of a multicenter phase II trial of activated protein C in patients with ALI, which is expected to finish enrollment in 2008.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is another novel therapy currently being evaluated as a possible therapy for lung injury. GM-CSF plays an important role in the development and homeostasis of alveolar macrophages as well as in the prevention of apoptosis in the alveolar epithelium.59 Animal models60 first suggested a benefit from GM-CSF therapy in experimentally induced lung injury, and a randomized controlled phase II trial61 of GM-CSF in 10 human patients with lung injury found an improvement in oxygenation over a 5-day period. A randomized controlled trial is ongoing now at the University of Michigan to determine whether a 14-day course of GM-CSF improves clinical outcomes, including ventilator-free days and mortality, in patients with ALI/ARDS.
Alveolar fluid clearance is a critical component of the resolution of lung injury.62 β-agonists accelerate alveolar fluid clearance in isolated human lung models63 and in rat models of lung injury,64 and salmeterol has been demonstrated to decrease the incidence of high-altitude pulmonary edema in an at-risk population.65 β-agonists may also decrease lung inflammation, as demonstrated by both in vivo experiments66 and in vitro experiments.67 A small, single-center, randomized, controlled trial68 recently demonstrated that therapy with IV salbutamol (albuterol) reduced the amount of extravascular lung water, though there was a trend toward a higher incidence of arrhythmias in the treatment group. As a result of these findings, the NHLBI Acute Respiratory Distress Syndrome Network is initiating a large, multicenter, randomized, controlled trial of the efficacy and safety of aerosolized β-agonist therapy in ALI/ARDS patients.
CONCLUSIONS
New evidence strongly suggests that we should follow a conservative fluid management strategy for most patients with established ALI/ARDS who are not in shock, with the goal of keeping the patient’s fluid balance net even. Managing fluids with this approach increases the number of ventilator-free days in patients with ALI with no increase in the rates of shock or renal failure. Electrolyte values must be closely monitored when following this strategy, and patients in shock should still receive aggressive volume resuscitation. Therapy with albumin and furosemide may be beneficial in selected patients with hypoproteinemia and ALI, although conclusive data are still lacking on patient outcomes.
Although there have been no dramatic advances in the pharmacologic treatment of ALI/ARDS over the past decade, several new and promising treatments, including activated protein C, GM-CSF, and inhaled β-agonists, are currently being evaluated in clinical trials. Notably, the latest advances in the treatment of ALI, such as ventilation with lower tidal volumes and conservative management of fluids, constitute major improvements in supportive care. Assiduous attention to other elements of the supportive care of critically ill patients such as the prevention of ventilator-associated pneumonia and adequate nutritional support should also be provided to ALI patients in hopes of further improving outcomes.
ACKNOWLEDGMENT
This research was supported by National Heart, Lung, and Blood Institute grants HL74005, HL58156, and HL51854.
The authors thank Xiaohui Fang, MD, for assistance with preparation of the figures.
Abbreviations
- ALI
acute lung injury
- CVC
central venous catheter
- FACTT
Fluid and Catheter Treatment Trial
- GM-CSF
granulocyte macrophage colony-stimulating factor
- NHLBI
National Heart, Lung, and Blood Institute
- NO
nitric oxide
- PAC
pulmonary artery catheter
Footnotes
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
Reprints Information about ordering reprints can be found online: http://www.chestjournal.org/site/misc/reprints.xhtml
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.The Acute Respiratory Distress Syndrome Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 3.Prewitt RM, McCarthy J, Wood LD. Treatment of acute low pressure pulmonary edema in dogs: relative effects of hydrostatic and oncotic pressure, nitroprusside, and positive end-expiratory pressure. J Clin Invest. 1981;67:409–418. doi: 10.1172/JCI110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staub NC. Pulmonary edema: physiologic approaches to management. Chest. 1978;74:559–564. doi: 10.1378/chest.74.5.559. [DOI] [PubMed] [Google Scholar]
- 5.Sakka SG, Klein M, Reinhart K, et al. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;122:2080–2086. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey H, Hall J, Sznajder I, et al. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97:1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 7.Acute Respiratory Distress Syndrome Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 9.Rivers EP. Fluid-management strategies in acute lung injury: liberal, conservative, or both? N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMe068105. [DOI] [PubMed] [Google Scholar]
- 10.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 11.Martin SJ, Danziger LH. Continuous infusion of loop diuretics in the critically ill: a review of the literature. Crit Care Med. 1994;22:1323–1329. doi: 10.1097/00003246-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkes MM, Navickis RJ. Patient survival after human albumin administration: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:149–164. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- 14.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 15.Cook D. Is albumin safe? N Engl J Med. 2004;350:2294–2296. doi: 10.1056/NEJMe048095. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Evidence-based colloid use in the critically ill: American Thoracic Society consensus statement. Am J Respir Crit Care Med. 2004;170:1247–1259. doi: 10.1164/rccm.200208-909ST. [DOI] [PubMed] [Google Scholar]
- 17.Mangialardi RJ, Martin GS, Bernard GR, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis: Ibuprofen in Sepsis Study Group. Crit Care Med. 2000;28:3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Freire AX, Bridges L, Umpierrez GE, et al. Admission hyperglycemia and other risk factors as predictors of hospital mortality in a medical ICU population. Chest. 2005;128:3109–3116. doi: 10.1378/chest.128.5.3109. [DOI] [PubMed] [Google Scholar]
- 19.Martin GS, Mangialardi RJ, Wheeler AP, et al. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med. 2002;30:2175–2182. doi: 10.1097/00003246-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Martin GS, Moss M, Wheeler AP, et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–1687. doi: 10.1097/01.ccm.0000171539.47006.02. [DOI] [PubMed] [Google Scholar]
- 21.Su X, Bai C, Hong Q, et al. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med. 2003;29:2034–2042. doi: 10.1007/s00134-003-2017-3. [DOI] [PubMed] [Google Scholar]
- 22.Yan XW, Li WQ, Wang H, et al. Effects of high-volume continuous hemofiltration on experimental pancreatitis associated lung injury in pigs. Int J Artif Organs. 2006;29:293–302. doi: 10.1177/039139880602900307. [DOI] [PubMed] [Google Scholar]
- 23.Ullrich R, Roeder G, Lorber C, et al. Continuous venovenous hemofiltration improves arterial oxygenation in endotoxin-induced lung injury in pigs. Anesthesiology. 2001;95:428–436. doi: 10.1097/00000542-200108000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Stein B, Pfenninger E, Grunert A, et al. The consequences of continuous haemofiltration on lung mechanics and extravascular lung water in a porcine endotoxic shock model. Intensive Care Med. 1991;17:293–298. doi: 10.1007/BF01713941. [DOI] [PubMed] [Google Scholar]
- 25.DiCarlo JV, Alexander SR, Agarwal R, et al. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol. 2003;25:801–805. doi: 10.1097/00043426-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Hoste EA, Vanholder RC, Lameire NH, et al. No early respiratory benefit with CVVHDF in patients with acute renal failure and acute lung injury. Nephrol Dial Transplant. 2002;17:2153–2158. doi: 10.1093/ndt/17.12.2153. [DOI] [PubMed] [Google Scholar]
- 27.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richman PS, Spragg RG, Robertson B, et al. The adult respiratory distress syndrome: first trials with surfactant replacement. Eur Respir J Suppl. 1989;3:109s–111s. [PubMed] [Google Scholar]
- 29.Reines HD, Silverman H, Hurst J. Effects of two concentrations of nebulized surfactant (Exosurf) in sepsis-induced adult respiratory distress syndrome (ARDS) [abstract] Crit Care Med. 1992;20:S61. [Google Scholar]
- 30.Weg JG, Balk RA, Tharratt RS, et al. Safety and potential efficacy of an aerosolized surfactant in human sepsis-induced adult respiratory distress syndrome. JAMA. 1994;272:1433–1438. [PubMed] [Google Scholar]
- 31.Anzueto A, Baughman RP, Guntupalli KK, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome: Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 32.Spragg RG, Lewis JF, Wurst W, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 33.Spragg RG, Lewis JF, Walmrath HD, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 34.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 35.Rossaint R, Falke KJ, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 36.Lundin S, Mang H, Smithies M, et al. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study: the European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911–919. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 37.Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial: Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 39.Michael JR, Barton RG, Saffle JR, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. 1998;157:1372–1380. doi: 10.1164/ajrccm.157.5.96-10089. [DOI] [PubMed] [Google Scholar]
- 40.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 42.Weigelt JA, Norcross JF, Borman KR, et al. Early steroid therapy for respiratory failure. Arch Surg. 1985;120:536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 43.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med. 1984;311:1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 44.Bone RC, Fisher CJ, Jr, Clemmer TP, et al. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92:1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 45.Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 46.Ashbaugh DG, Maier RV. Idiopathic pulmonary fibrosis in adult respiratory distress syndrome: diagnosis and treatment. Arch Surg. 1985;120:530–535. doi: 10.1001/archsurg.1985.01390290012002. [DOI] [PubMed] [Google Scholar]
- 47.Bernard GR, Luce JM, Sprung CL, et al. High-dose cortico-steroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 48.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 49.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 50.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 51.The Acute Respiratory Distress Syndrome Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 52.Chesnutt AN, Matthay MA, Tibayan FA, et al. Early detection of type III procollagen peptide in acute lung injury: pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 53.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 54.Ware LB, Camerer E, Welty-Wolf KE, et al. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L307–L311. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 55.Sapru A, Wiemels JL, Witte JS, et al. Acute lung injury and the coagulation pathway: potential role of gene polymorphisms in the protein C and fibrinolytic pathways. Intensive Care Med. 2006;32:1293–1303. doi: 10.1007/s00134-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 56.Ware LB, Fang X, Matthay MA. Protein C and thrombo-modulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L514–L521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 57.Nick JA, Coldren CD, Geraci MW, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 58.van der Poll T, Levi M, Nick JA, et al. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med. 2005;171:1125–1128. doi: 10.1164/rccm.200411-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paine R, III, Wilcoxen SE, Morris SB, et al. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol. 2003;163:2397–2406. doi: 10.1016/S0002-9440(10)63594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lechner AJ, Lamprech KE, Potthoff LH, et al. Recombinant GM-CSF reduces lung injury and mortality during neutropenic Candida sepsis. Am J Physiol. 1994;266:L561–L568. doi: 10.1152/ajplung.1994.266.5.L561. [DOI] [PubMed] [Google Scholar]
- 61.Presneill JJ, Harris T, Stewart AG, et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 62.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 63.Sakuma T, Okaniwa G, Nakada T, et al. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 64.McAuley DF, Frank JA, Fang X, et al. Clinically relevant concentrations of β2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 65.Sartori C, Allemann Y, Duplain H, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;346:1631–1636. doi: 10.1056/NEJMoa013183. [DOI] [PubMed] [Google Scholar]
- 66.Maris NA, de Vos AF, Dessing MC, et al. Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med. 2005;172:878–884. doi: 10.1164/rccm.200503-451OC. [DOI] [PubMed] [Google Scholar]
- 67.Matthay MA, Abraham E. β-adrenergic agonist therapy as a potential treatment for acute lung injury. Am J Respir Crit Care Med. 2006;173:254–255. doi: 10.1164/rccm.rccm2511003. [DOI] [PubMed] [Google Scholar]
- 68.Perkins GD, McAuley DF, Thickett DR, et al. The β-Agonist Lung Injury Trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 69.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 70.The Acute Respiratory Distress Syndrome Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 71.Slotman GJ, Burchard KW, D’Arezzo A, et al. Ketoconazole prevents acute respiratory failure in critically ill surgical patients. J Trauma. 1988;28:648–654. doi: 10.1097/00005373-198805000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Yu M, Tomasa G. A double-blind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med. 1993;21:1635–1642. doi: 10.1097/00003246-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 73.The Acute Respiratory Distress Syndrome Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]