Abstract
The fibroproliferative response to acute lung injury (ALI) results in severe, persistent respiratory dysfunction. We have reported that IL-1β is elevated in pulmonary edema fluid in those with ALI and mediates an autocrine-acting, fibroblast mitogenic pathway. In this study, we examine the role of IL-1β-mediated induction of cyclooxygenase-2 and PGE2, and evaluate the significance of individual E prostanoid (EP) receptors in mediating the fibroproliferative effects of IL-1β in ALI. Blocking studies on human lung fibroblasts indicate that IL-1β is the major cyclooxygenase-2 mRNA and PGE2-inducing factor in pulmonary edema fluid and accounts for the differential PGE2 induction noted in samples from ALI patients. Surprisingly, we found that PGE2 produced by IL-1β-stimulated fibroblasts enhances fibroblast proliferation. Further studies revealed that the effect of fibroblast proliferation is biphasic, with the promitogenic effect of PGE2 noted at concentrations close to that detected in pulmonary edema fluid from ALI patients. The suppressive effects of PGE2 were mimicked by the EP2-selective receptor agonist, butaprost, by cAMP activation, and were lost in murine lung fibroblasts that lack EP2. Conversely, the promitogenic effects of mid-range concentrations of PGE2 were mimicked by the EP3-selective agent, sulprostone, by cAMP reduction, and lost upon inhibition of Gi-mediated signaling with pertussis toxin. Taken together, these data demonstrate that PGE2 can stimulate or inhibit fibroblast proliferation at clinically relevant concentrations, via preferential signaling through EP3 or EP2 receptors, respectively. Such mechanisms may drive the fibroproliferative response to ALI.
Acute lung injury is a heterogeneous syndrome of unclear etiology with an annual incidence of 20–85 per 100,000 and an overall mortality of 30–50% (1–3). A significant subpopulation of patients with acute inflammatory lung injury develop a severe fibroproliferative response that is characterized by the formation of alveolar granulation tissue composed of mesen-chymal cellular proliferation and a provisional matrix composed of fibrin, fibronectin, vitronectin, and collagen (4–8). It is thought that the fibroproliferative response is initiated by activation of lung fibroblasts within hours of the acute lung injury (ALI).3 For example, a finding of elevated levels of type III procollagen peptide in alveolar edema fluid within hours of endotracheal intubation is associated with the development of a fibroproliferative response, and a poor outcome (9–13). Similarly, the presence of soluble indicators of proliferative fibroblast activity and a proliferative cell phenotype in the alveolus in ALI are associated with the development of a fibroproliferative response, and a poor outcome (14–16).
Several cytokines and chemokines that may modulate fibroblast proliferation have been identified in pulmonary edema fluid (or bronchoalveolar lavage) from patients at risk for, or with established, ALI, including TNF-α, ILs 1 and 8 (IL-1, IL-8), growth-related oncogenes (gro-β, MIP-2-α), epithelial neutrophil-activating protein 78, and platelet-derived growth factor (1, 17–20). The finding that transient overexpression of IL-1β using an adenoviral vector induces pulmonary fibrosis in rat lungs indicates that IL-1β acts as a potent profibrotic cytokine in vivo (21); however, its mechanism of action has not been elucidated.
We found recently that pulmonary edema fluid from patients with early ALI induces a greater mitogenic effect than pulmonary edema fluid from patients with hydrostatic pulmonary edema and that this effect was mediated largely by IL-1β (22). IL-1β was found to induce IL-6, which acted in an autocrine manner in concert with IL-1β, to stimulate fibroblast proliferation (22). The observed IL-1β/IL-6 responses did not, however, account for all of the proliferative bioactivity associated with the ALI edema fluid (22). Rather, these responses accounted for ~40% of this activity, suggesting the existence of other IL-1β-initiated mitogenic pathways.
In the course of investigation of other IL-1β-initiated mitogenic pathways, we found evidence presented here that IL-1β-induction of PGE2 and/or the response of lung fibroblasts to PGE2 may play a role in the induction of a fibroproliferative response after ALI.
It is well-established that PGE2 is an important downstream effector of IL-1β; however, it is found to mediate suppression of cell proliferation in many systems (23, 24). PGE2 is produced through enzymatic catalysis of membrane-derived arachidonic acid by cyclooxygenases (COX), of which there are two well-characterized isoforms, COX-1 and COX-2 (25). PGE2 is the major prostanoid product in lung tissue and lung fibroblasts (24, 26, 27). Although fibroblasts generally express COX-1 constitutively, COX-2 expression is regulated (24, 26). It has been shown that fibroblasts derived from patients with idiopathic pulmonary fibrosis exhibit an enhanced proliferative capacity, together with down-regulated expression of COX-2, reduced PGE2 production, and an insensitivity to the COX-2-stimulating properties of IL-1β (28–31).
PGE2 can initiate intracellular signals through binding to several G protein-coupled, prostanoid receptors, E prostanoid (EP)1, EP2, EP3, and EP4, that mediate a variety of physiologic responses (24, 25, 32–34). Gs-coupled EP2 and EP4 initiate intracellular signals through increases in intracellular cAMP, whereas Gi-coupled EP3 decreases cAMP (25, 33). Gq-coupled EP1 signals via increases in intracellular calcium (25, 33, 35). Recent studies suggest that, in experimental pulmonary fibrosis, changes in the relative amounts of EP2 and EP3 can sway the balance between PGE2-induced suppression or stimulation of fibroblast proliferation (36). Our current findings indicate that the high levels of IL-1β seen in ALI up-regulate PGE2 to an extent that stimulates the fibroproliferative response. Furthermore, that PGE2 can act to stimulate or suppress adult human lung fibroblast proliferation with the outcome being dependent on the concentration of PGE2 and the relative signaling through EP2 and EP3 receptors.
Materials and Methods
Reagents
Selective EP receptor agonists sulprostone (EP1/EP3 – PGE2 binding inhibition Ki (dissociation constant) = 0.35 nM; IC50 = 10 nM), butaprost (EP2 – PGE2 binding inhibition Ki = 4.5 µM), SC-19220 (EP-1-selective antagonist), and rabbit polyclonal Abs for EP1, EP2, EP3, and EP4 were purchased from Cayman Chemical (32). IL-1β-neutralizing Ab was obtained from R&D Systems. PGE2-neutralizing Ab was provided by Pfizer (37).
Patient selection and collection of pulmonary edema fluid
All patient protocols were approved by the Committee for Human Research of the University of California at San Francisco and the Institutional Review Board at the University of Alabama. Patients were eligible for inclusion in this study if they had acute pulmonary edema from either ALI or hydrostatic causes and required endotracheal intubation for positive pressure ventilation. Pulmonary edema fluid was obtained from patients within 4 h of intubation through gentle luminal suction applied to a 14 French catheter passed into the distal airways, as described previously (22, 38). Samples were centrifuged (3000 × g, 10 min, 4°C) and the supernatants were stored at −80°C until analysis. The patients were identified as having pulmonary edema secondary to ALI or hydrostatic causes based on the edema fluid/plasma total protein ratio, as determined by the Biuret method, by clinical criteria (22, 38, 39), and by the simplified acute physiology score (SAPS II), which was calculated as described (40). Patients were identified as having ALI if the edema fluid/plasma total protein ratio was >0.65 and the patients had clinical criteria of bilateral infiltrates on a chest radiograph, PaO2/FIO2 of <300 and a pulmonary capillary wedge pressure of <18 mm Hg, if measured, with clinical risk factor(s) for the development of clinical ALI (22, 41). Patients were identified as having hydrostatic edema (HYDRO) if the edema fluid/plasma total protein ratio was <0.65 and the patients had a wedge pressure of >18 mm Hg or a two-dimensional echocardiogram demonstrating a reduced left ventricular ejection fraction and a clinical history consistent with cardiac dysfunction (22, 41). As we have done previously, equal volumes of each patient’s sample within the same group were pooled for the gene expression studies, due to limited amounts of patient-derived material (22).
Cell proliferation assays
Normal diploid adult human lung fibroblasts (CCL-210) were obtained from American Type Culture Collection, and primary isolates of wild-type and EP2 knockout murine lung fibroblasts were generated as described (36). The fibroblasts were propagated in Eagle’s MEM with l-glutamine and penicillin/streptomycin (Mediatech/Cellgro; Shared Media Facility) with 10% FBS (Sigma-Aldrich). For proliferation measurements (including the effect of PGE2), lung fibroblasts plated at a low density (18,000 cells/cm2 growth area) were rendered quiescent by incubation in 0.2% serum-containing medium (SCM) for 48 h, before addition of either pooled pulmonary edema fluid diluted in serum-free medium (SFM), or the indicated reagents diluted in SFM/1% BSA or 2% SCM for the indicated times. Where indicated, DNA synthetic rates were measured after exposure to agonists/antagonists for 18 h as BrdU incorporation by ELISA, according to the manufacturer’s protocol (Roche Applied Science). Cell number was determined by cell counting in a hemocytometer. For growth inhibition assays, lung fibroblast cell monolayers or test solutions were preincubated with the indicated reagents (20 min, 37°C). For study of the effects of pertussis toxin-sensitive G proteins, fibroblasts were preincubated with pertussis toxin for 4 h before stimulation with 2% SCM ± PGE2 The ND50 of the IL-1 receptor antagonist (IL-1ra) is 1 ng/ml to neutralize the bioactivity of 50 pg/ml IL-1β (R&D Systems). The ND50 for the PGE2 Abs was found to be equimolar with PGE2 in terms of abrogation of PGE2-mediated suppression of T cell proliferation (37). Preliminary experiments revealed that a 4-fold molar excess of neutralizing PGE2 Abs abrogated the anti-proliferative effect of PGE2.
Quantitative RT-PCR
To quantify COX-1, COX-2, and GAPDH mRNA levels, quantitative RT-PCR was performed using a dsDNA-specific fluorochrome (SYBR Green-I) and the LightCycler Instrument (Roche Molecular Biochemicals) as described (22, 42). Briefly, the reverse transcriptase reaction was performed as described previously (22). The gene-specific PCR primer sets were obtained commercially (BD Clontech). Reaction conditions were optimized for each primer set and each reaction was validated individually for generation of a specific, single product (melting temperature/ size homogeneity) using a melt curve and gel electrophoresis. To determine relative RNA levels, the second derivative maximum of the fluorescence/cycle number plot was calculated. The linear relationship (r2 > 0.99) between the starting DNA concentration and the log of the concentration over a large concentration range (6–8 logs in duplicate) was verified for each primer set, and similar efficiencies of PCR among the fibroblast RNA samples and the individual cDNA-containing plasmids were verified. Recovery of spiked cDNA into the fibroblast RNA samples was routinely >95% with an intersample coefficient of variation of <5%. The difference in individual starting mRNA amounts in each sample was calculated by comparison with a known amount of cDNA standards, and to serially diluted unknown samples run in parallel. As we have shown, these validation steps support robust and accurate quantification of RNA levels among samples (22).
PGE2 assay
Lung fibroblasts were seeded at subconfluent density (1 × 105 cells/2 cm2 area), starved of sera for 48 h, then exposed to edema fluid (1/20 diluted in SFM) ± specific neutralizing Abs or control IgG (15 µg/ml) for 24 h. To assess IL-1β induction of PGE2, lung fibroblasts were serum starved for 48 h, and exposed to the indicated concentrations of IL-1β in SFM or SCM for 24 h. Conditioned medium was assayed for PGE2 using a competitive immunoassay according to the manufacturer’s instructions (R&D Systems). No interfering substances were detected and addition of a known amount of exogenous PGE2 to edema fluid obtained from patients with either ALI or edema due to hydrostatic causes or to cell culture medium resulted in appropriate (within 15%) PGE2 detection rates.
cAMP assay
Subconfluent lung fibroblasts were serum starved, and incubated with the indicated concentrations of PGE2, butaprost, or sulprostone for 10 min. Cells were lysed in 0.1 M hydrochloric acid, and cAMP levels were measured using an enzyme immunoassay kit according to the manufacturer’s instructions (Cayman Chemical).
EP receptor Western blotting
Subconfluent human lung fibroblasts were lysed (1% Nonidet P-40/TBS/ (pH 8.0) with protease inhibitors), centrifuged, and assayed for total protein using the BCA kit according to the manufacturer’s instructions (Pierce Biotechnology). Either 5 or 50 µg of protein was loaded per lane on SDS-PAGE (10%) gels, electrophoresed under reducing conditions, transferred to polyvinylidene difluoride membranes (Immobilon; Millipore) and immu-noblotted with ECL detection (Amersham/GE Healthcare), using the indicated primary EP receptor or β-actin loading control Abs, as published (8, 43).
Statistical analysis
The continuous variables were compared using an unpaired Student t test or a Mann-Whitney U test if the variables were not normally distributed. The effects of ALI and HYDRO fluids, and the effects of inhibitory molecules on COX-2 and PGE2 production were compared by ANOVA, followed by the Student-Newman-Keuls or the Dunnett’s multiple comparison procedures. All proportional values were compared by a Fisher’s exact test (44). Statistical significance was accepted at the p ≤ 0.05 level.
Results
Patient characteristics at time of pulmonary edema fluid collection
The edema fluid/plasma total protein ratio was 1.0 ± 0.2 for the patients with ALI and 0.45 ± 0.1 for the patients with HYDRO (p < 0.001) (see Table I). Underlying infection was the most common cause of ALI and was the primary cause in 70% of the patients. The mean ages, gender distributions, and SAPS (SAPS II; ALI, 60 ± 21; HYDRO, 52 ± 16) of the patients with ALI and with HYDRO were similar (see Table I).
Table I.
Patient demographics a
| ALI | HYDRO | p Value | |
|---|---|---|---|
| Number of patients | 15 | 15 | |
| Initial edema fluid to plasma protein ratio |
1.0 ± 0.2 | 0.45 ± 0.1 | <0.001 |
| Male | 61% | 65% | 0.83 |
| Age | 49 ± 17 | 54 ± 21 | 0.18 |
| Current smoker | 20% | 44% | 0.25 |
| SAPS II score | 60 ± 21 | 52 ± 16 | 0.10 |
| Infectious etiology for lung injury | 70% | 0% | <0.001 |
Data as mean ± SD or percent of patients.
PGE2 levels are higher in the edema fluid of patients with ALI than patients with HYDRO
We have found that the pulmonary edema fluid of patients with ALI contains higher levels of IL-1β and has greater IL-1β-dependent mitogenic activity than the pulmonary edema fluid of patients with HYDRO (22). Prior reports demonstrate that IL-1β can modulate proliferation through its effects on PGE2 production. Measurement of the levels of PGE2 in the edema fluids by competitive enzyme immunoassay (EIA) indicated that the PGE2 levels were 4-fold higher in the edema fluid samples from patients with ALI (1.11 ± 0.27 ng/ml) compared with that from patients with HYDRO (0.27 ± 0.071 ng/ml;p = 0.005, n = 15/group). The level of PGE2 in the edema fluid also correlated positively with that of IL-1β in the ALI patients (r = 0.78, p = 0.05; data not shown).
IL-1β up-regulates COX-2 mRNA and induces PGE2 production by adult human lung fibroblasts
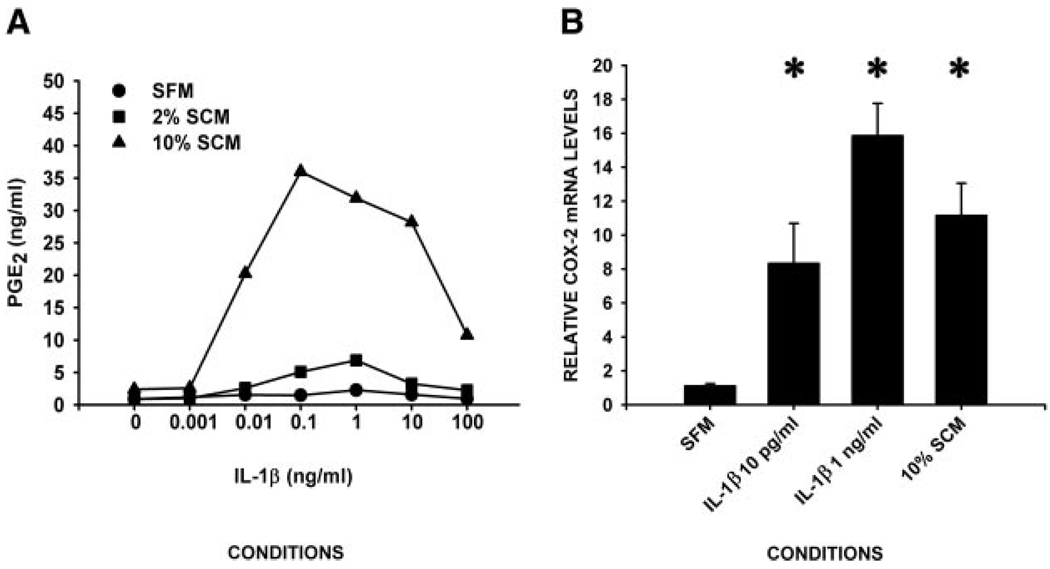
To determine whether fibroblasts have the capacity to contribute to the elevated PGE2 in the edema fluid in response to IL-1β, we first measured the effect of exogenous IL-1β on the production of PGE2 by adult lung fibroblasts using a competitive EIA. IL-1β enhanced production of PGE2 by adult human fibroblasts in a dose-dependent manner. The peak response of 35 ng/ml PGE2 was observed upon addition of 0.1 ng/ml IL-1β. The effect was also serum dependent, as indicated by an EC50 of 0.01 ng/ml IL-1β in 10% SCM and an EC50 of 0.1 ng/ml IL-1β in 2% SCM (Fig. 1A). Importantly, the concentration range of IL-1β for the EC50 and peak PGE2 response overlapped with the measured IL-1β levels in pulmonary edema fluids obtained from patients with hydrostatic pulmonary edema or ALI (22).
FIGURE 1.
IL-1β induces COX-2 mRNA and PGE2 in human lung fibroblasts. Subconfluent human lung fibroblasts were incubated in the indicated medium ± the indicated concentrations of IL-1β for 24 h (for PGE2 measurements) or 8 h (for RNA measurements) (n ≥ 3 in triplicate/condition). A, IL-1β induces PGE2 in a concentration-dependent manner. Conditioned medium (1.2 × 104 cells in 100 µl of medium for 24 h) was assayed for PGE2 by competitive EIA. Data are plotted as mean of nanograms of PGE2 per milliliter of conditioned medium. B, IL-1β induces COX-2 mRNA in a concentration-dependent manner. Total RNA was isolated and subjected to real-time quantitative RT-PCR for COX-2. Data are plotted as the mean ± SD COX-2 mRNA in relative to that seen in the absence of IL-1β. *, p < 0.01 by ANOVA/p < 0.05 Dunnett’s test vs SFM values.
The effect of clinically relevant concentrations (22) of exogenous IL-1β on expression of COX-1 and COX-2 mRNA, relative to GAPDH, was determined by real-time, quantitative RT-PCR. Addition of 0.01 or 1 ng/ml IL-1β had no effect on the levels of COX-1 mRNA (data not shown). In contrast, the levels of COX-2 mRNA were enhanced 8- to 16-fold, in a dose-dependent manner (Fig. 1B).
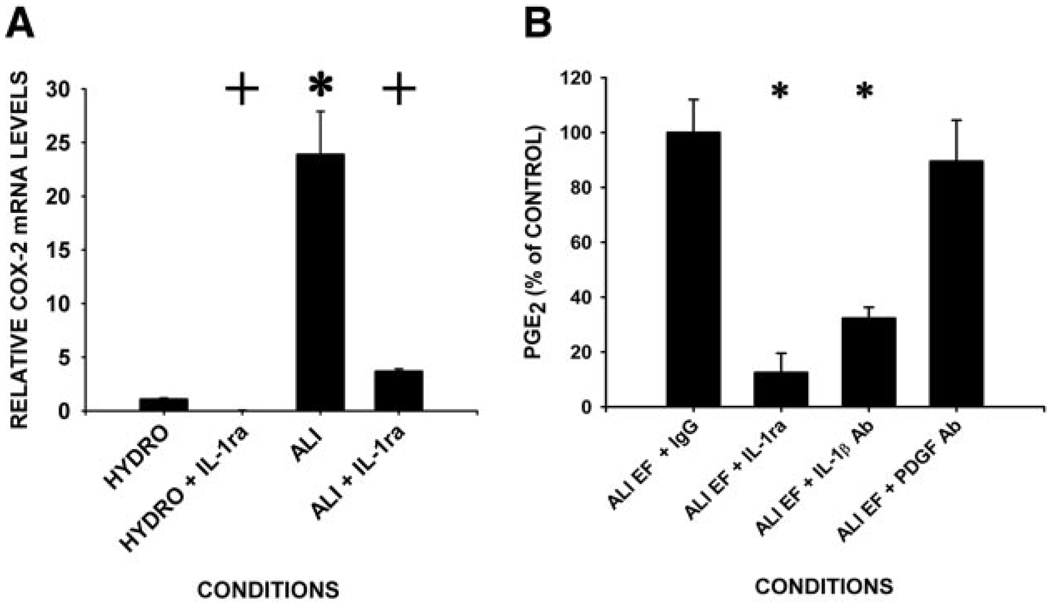
Incubation of adult lung fibroblasts of pulmonary edema fluids (1/20 dilution in SFM) enhanced both COX-2 mRNA production and secretion of PGE2 (Fig. 2). However, the levels of induction of COX-2 mRNA in fibroblasts incubated with edema fluids from patients with ALI were 20-fold greater than that seen in fibroblasts incubated with HYDRO fluids (Fig. 2A). As other substances in edema fluid (e.g., TNF-α, IL-6) may modify the net cellular response to IL-1β, we examined the effect of pulmonary edema fluid on adult lung fibroblasts in the presence or absence of the IL-1-specific signaling antagonist, IL-1ra. Preincubation of the cells with IL-1ra (500-fold molar excess over IL-1β, 30 min) blocked 90% of the COX-2 mRNA induction in edema fluid-exposed cells (Fig. 2A). Concordant with these observations, IL-1ra blocked 90% of the PGE2 (of 3.4 ± 0.6 ng/ml) secreted into the medium upon incubation with ALI edema fluid (Fig. 2B). As both IL-1α and IL-1β can bind the same receptors with overlapping cellular effects, we verified that IL-1β was the predominant PGE2-inducing bioactive species in ALI edema fluid by addition of an IL-1β-specific neutralizing Ab. This Ab inhibited PGE2 production in edema fluid from patients with ALI by 70% (Fig. 2B).
FIGURE 2.
Induction of COX-2 mRNA and PGE2 in response to ALI edema fluid is greater than that of HYDRO fluid and is dependent on IL-1β. Subconfluent serum starved human lung fibroblasts were incubated with pulmonary edema fluid (diluted 1/20 in SFM) from patients with either ALI or HYDRO ± pretreatment with IL-1ra (200 ng/ml) or IL-1β-neutralizing Ab. A, COX-2 mRNA induction is greater in response to ALI than HYDRO, and is dependent on IL-1β. RNA (after 8 h) was quantified by real-time, quantitative RT-PCR for COX-2. Data are plotted as mean ± SD COX-2 mRNA level relative to the value observed in the presence of HYDRO fluid. *, p < 0.01 compared with HYDRO values, while + denotes p < 0.05 compared with values obtained ± IL-1ra by ANOVA. B, PGE2 secretion is greater in response to ALI than HYDRO and is dependent on IL-1β. Conditioned medium (24 h) was assayed for PGE2 by competitive EIA. Data are plotted as mean ± SD PGE2 relative to ALI + IgG control levels (3.4 ± 0.6 ng/ml). *, p < 0.001 ANOVA/p < 0.05 Dunnett’s test post hoc.
PGE2 is a downstream effector of the IL-1β-mediated proliferative effects of pulmonary edema fluid
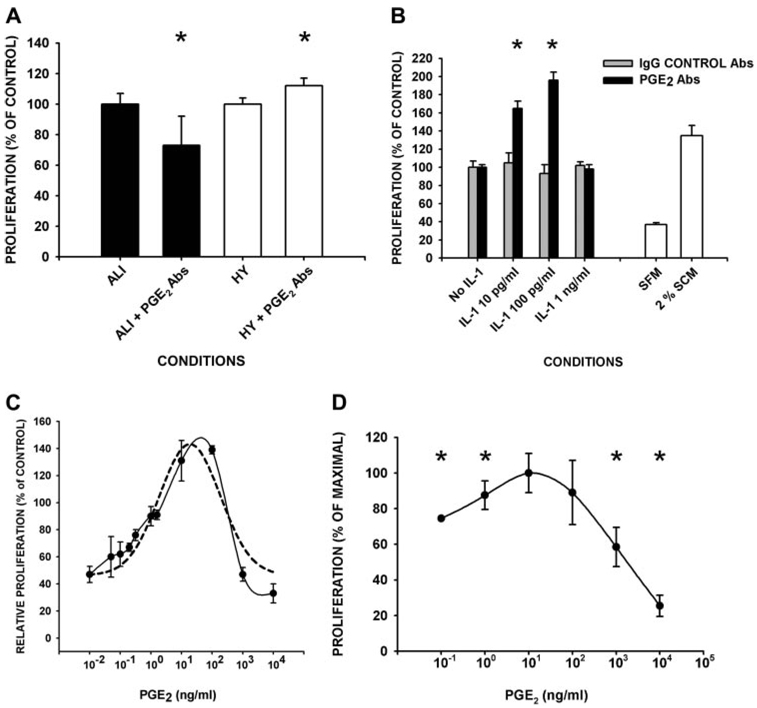
To investigate whether PGE2 in edema fluids possesses proliferative activity, we compared the proliferative response of human lung fibroblasts to edema fluid samples ± excess PGE2 neutralizing Abs. Neutralization of PGE2 blunted the proliferative response to ALI edema fluid by 27 ± 19% (p = 0.03 by t test), and minimally enhanced (by 8 ± 4%, p= 0.05 by t test) the proliferative response to hydrostatic pulmonary edema in comparison with IgG controls (Fig. 3A). For these experiments, the patient edema fluids were diluted 1/4 in SFM resulting in a starting range of 0.07–0.400 ng/ml (10−10 to 10−9 M) of PGE2. These data indicate that the PGE2 in ALI edema fluid enhances fibroblast proliferation, while the PGE2 in HYDRO has a small, opposing, suppressive effect.
FIGURE 3.
PGE2 mediates proliferation of fibroblasts in pulmonary edema fluid samples, is the downstream effector of low concentrations of IL-1β-induced proliferative suppression, and induces a biphasic proliferative response. A, Serum-starved, human lung fibroblasts (105 cells) were incubated with the indicated edema fluid samples (1/4 diluted in SFM) ± neutralizing PGE2 Ab (10 µg/ml; ALI + PGE2 Abs or HY + PGE2 Abs) or control IgG (10 µg/ml; ALI or HY; n ≥ 3 in quadruplicate/condition). B, Serum-starved, human lung fibroblasts (105 cells) were incubated with the indicated edema fluid samples (1/4 diluted in SFM, A) or the indicated concentrations of IL-1β in 2% SCM for 24 h. The 24-h conditioned medium was harvested and transferred to fresh fibroblast monolayers (1/3 diluted with SFM) ± preincubation with either PGE2 neutralizing Ab (▪) or IgG controls Ab ( ) (at 10 µg/ml; n ≥ 3 in triplicate/condition). C, Biphasic effect of PGE2 on cell proliferation. Fibroblasts were incubated with the indicated concentrations of PGE2 in 2% SCM for 24 h (n ≥ 3 in quadruplicate/condition). Dashed line, mathematical regression; solid line, raw data. D, As in C with a second, independent primary lung cell strain from normal lungs. In A–D, cell proliferation was measured as DNA synthesis by BrdU incorporation. Data are plotted as proliferation relative to the values obtained upon preincubation of the same edema fluid (A) or conditioned medium (B) with control IgG, or with 2% SCM in the absence of PGE2 (B–D). *, Difference between PGE2 Ab and IgG control Ab, or ± added PGE2 at p < 0.05 by t test (A), or ANOVA and post-hoc Student-Newman-Keuls test or Dunnett’s test (B and D).
) (at 10 µg/ml; n ≥ 3 in triplicate/condition). C, Biphasic effect of PGE2 on cell proliferation. Fibroblasts were incubated with the indicated concentrations of PGE2 in 2% SCM for 24 h (n ≥ 3 in quadruplicate/condition). Dashed line, mathematical regression; solid line, raw data. D, As in C with a second, independent primary lung cell strain from normal lungs. In A–D, cell proliferation was measured as DNA synthesis by BrdU incorporation. Data are plotted as proliferation relative to the values obtained upon preincubation of the same edema fluid (A) or conditioned medium (B) with control IgG, or with 2% SCM in the absence of PGE2 (B–D). *, Difference between PGE2 Ab and IgG control Ab, or ± added PGE2 at p < 0.05 by t test (A), or ANOVA and post-hoc Student-Newman-Keuls test or Dunnett’s test (B and D).
Prior reports indicate that PGE2 can function as a downstream mediator of IL-1β-induced effects on lung fibroblasts. This possibility was investigated by measuring the effect of neutralizing PGE2 on the proliferative activity of IL-β-stimulated, fibroblast-conditioned medium. The addition of low concentrations of IL-1β (0.10 or 0.100 ng/ml; as seen in HYDRO edema fluid) resulted in final conditioned medium levels of PGE2 of 0.1 ng/ml (10−10 M) and 0.4 ng/ml (10−9 M), respectively. Neutralization of PGE2 in the conditioned medium from fibroblasts that had been exposed to low concentration IL-1β enhanced the proliferation of a second well of fibroblasts by 50–100%. This observation demonstrates that PGE2 can act in an autocrine/paracrine manner to suppress proliferation of adult lung fibroblasts (Fig. 3B; p < 0.05 by ANOVA). In contrast, neutralization of PGE2 in conditioned medium from cells that were exposed to a higher concentration of IL-1β (1 ng/ml; as seen in ALI edema fluid; final conditioned medium PGE2 > 1 ng/ml) or medium alone controls, did not affect the proliferative response of fibroblasts. These data indicate that PGE2 can act as a downstream, autocrine/paracrine mediator of the suppressive effect of IL-1β on proliferation, but selectively, upon stimulation by low (≤100 pg/ml) concentrations of IL-1β. These suppressive IL-1β concentrations were in the range of HYDRO patient samples, but were 10-fold below that seen in ALI patient edema samples.
The effects of PGE2 on proliferation of adult human lung fibroblasts are dose dependent and biphasic
To resolve the observed disparate effects of PGE2 on fibroblast proliferation, we examined the direct proliferative activity of exogenous PGE2 on serum-induced fibroblast proliferation over a broad concentration range. Serum was added to the medium at 2% as this was the EC50 for serum-induced proliferation for our cells (data not shown). We found that the activity of PGE2 on proliferation was biphasic with maximal suppressive activity at concentrations ≤0.1 ng/ml (≤0.3 nM) and ≥1000 ng/ml (≥3 µM). In contrast, PGE2 concentrations between ~1 and 100 ng/ml (30–300 nM) exhibited a proliferation-stimulating effect with a maximal effect at 10 ng/ml (Fig. 3C). Upon detailed examination in a narrow concentration range of PGE2, 1 ng/ml (3 nM) PGE2 was found to be the threshold below which PGE2 mediates a suppressive effect on fibroblast proliferation (Fig. 3C). This biphasic proliferative response to varying the concentration of PGE2 was confirmed using a second independently derived, primary fibroblast lung cell strain from normal lungs (at P3–P5; Fig. 3D).
To evaluate the contextual dependence of PGE2-mediated proliferation, fibroblast responses to patient-derived edema fluid samples (1/10 diluted in SFM) ± PGE2 were measured. PGE2 suppressed proliferation (43%) at high (1000 ng/ml) concentrations, and induced proliferation (37%) at mid-range (10 ng/ml) concentrations, as compared with unspiked edema fluid samples (data not shown). Thus, the ability of PGE2 to modulate proliferation is biphasic in the context of both patient-derived pulmonary edema fluids, and in serum. Prior work suggests that PGE2 may induce an S-phase arrest in some cells (45). Thus, the DNA synthesis-based proliferation assay results were repeated (PGE2 at 0.1–1000 ng/ml for 72 h) and confirmed using actual cell counts, as a measure of proliferation (data not shown).
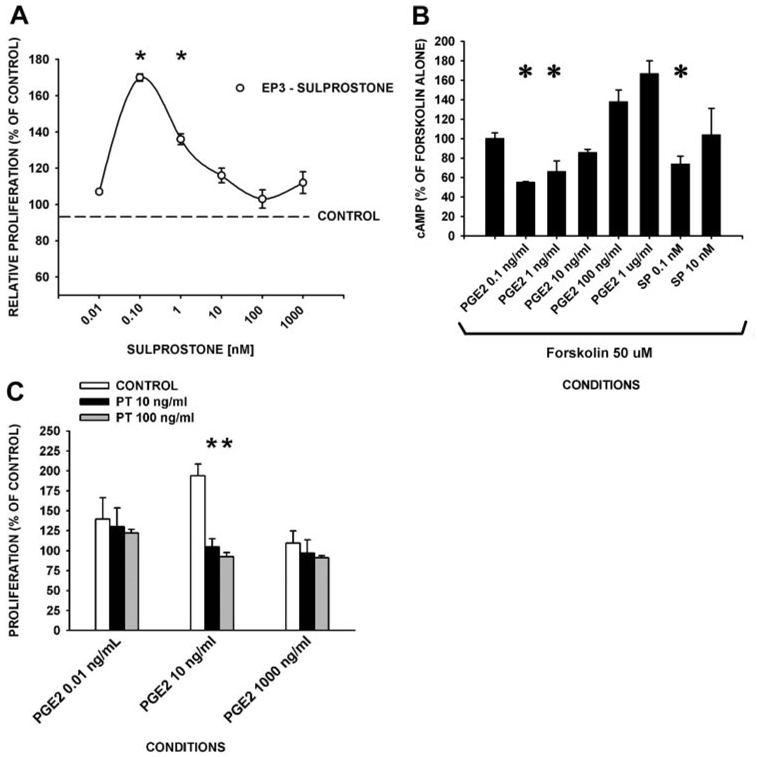
PGE2 mediates suppression of proliferation of adult human lung fibroblasts through EP2 receptors and stimulation of proliferation through EP3 receptors
To define the pathways involved in the biphasic proliferative response to PGE2 in human lung fibroblasts, we evaluated the expression and intracellular signaling actions of the PGE2 (EP) receptors. Western blot analysis indicated that human lung fibroblasts express all four receptors (EP1, EP2, EP3, and EP4) (Fig. 4).
FIGURE 4.
Human lung fibroblasts express all four EP receptors. Subcon-fluent monolayers of lung fibroblasts were lysed (1% Nonidet P-40/protease inhibitors), assayed for total protein, and either 5 or 50 µg protein/lane, as indicated, were subjected to SDS-PAGE under reducing conditions followed by immunoblotting with Abs directed against the indicated EP receptors.
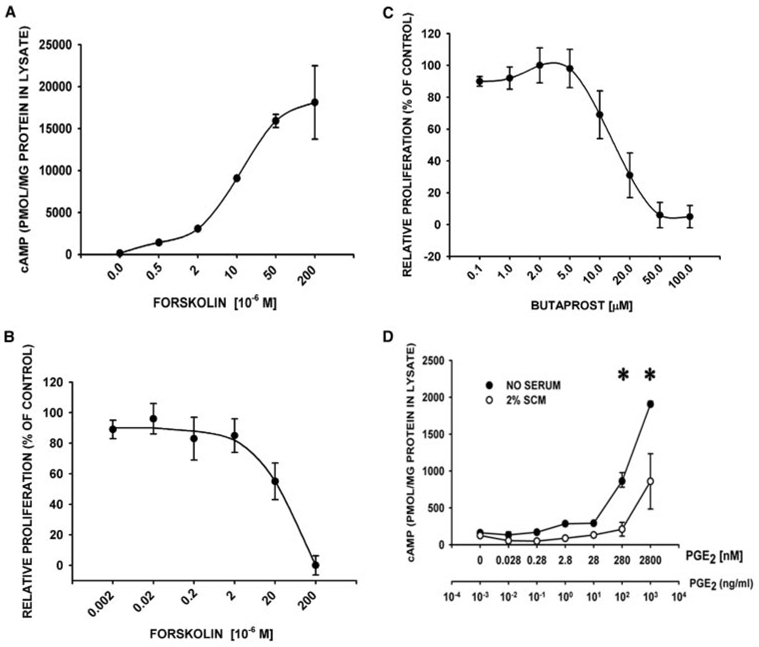
EP2 receptors act via activation of adenyl cyclase through Gs. We found that forskolin, an inducer of adenylyl cyclase activity, blunted serum-induced proliferation of the adult human lung fibroblasts in a dose-dependent manner, with an EC50 that approximated its measured EC50 for cAMP induction in these cells (10–20 µM) (Fig. 5, A and B). Similarly, the EP2-selective agonist, butaprost, suppressed proliferation in a dose-dependent manner with an EC50 at concentrations near its Ki (5 µM) for inhibition of PGE2 binding to EP2 (Fig. 5C). Butaprost increased the cAMP levels 4-fold (835 ± 11 pM/mg protein at 50 µM butaprost vs 236 ± 13 pM/mg protein at 2 µM butaprost, p < 0.001) but only when added at butaprost’s proliferative-suppressive concentrations (>5 µM; data not shown). PGE2 itself induced cAMP (maximum 12.1 ± 0.2-fold; p < 0.001 by ANOVA) in adult human lung fibroblasts, but only at concentrations at which the physiologic effect of PGE2 is to suppress proliferation (≥ 100 ng/ml; 280 nM) (Fig. 5D). Furthermore, the proliferative suppressing effects of high (≥100 ng/ml; 280 nM) PGE2 concentrations were selectively lost in primary isolates of lung fibroblasts derived from mice lacking EP2 receptors, compared with those derived from wild-type mice (EP2 knockout fibroblasts: 48 ± 12% vs wt controls: 105 ± 9%, p < 0.001). In contrast, the proliferative responses of EP2 knockout lung fibroblasts were indistinguishable from that seen in wild-type cells using a broad range of lower concentrations of PGE2 (0.001–10 ng/ml; 0.003–28 nM; data not shown). Together, these data verify the robust nature of the EP2 receptor-cAMP induction-proliferative suppressive pathway in human and murine lung fibroblasts, and indicate that high PGE2 concentrations (>100 ng/ml; 280 nM) uniquely initiate this signaling cascade.
FIGURE 5.
The EP2 receptor signaling pathway is intact and mediates the proliferative-suppressive actions of high concentration (≥200 ng/ml, ≥10−6 M) PGE2. A, Forskolin induces cAMP in a concentration-dependent manner. Serum-starved, subconfluent human lung fibroblasts were incubated with the indicated concentrations of forskolin (adenylyl cyclase activator) and the phosphodiesterase inhibitor IBMX (100 µM) for 10 min (n ≥ 3 in duplicate/concentration). cAMP levels in cell lysates were measured by competitive EIA. B, Induction of cAMP by forskolin abrogates fibroblast proliferation. Serum-starved, subconfluent human lung fibroblasts were incubated with the indicated concentrations of forskolin in 2% SCM for 24 h (n ≥ 3 in duplicate/concentration). Cell proliferation was measured by BrdU ELISA. Data are plotted relative to values obtained with an inactive forskolin analog. C, The EP2 selective agonist, butaprost, abrogates proliferation at its Ki (5 µM). Serum-starved, subconfluent human lung fibroblasts were incubated with the indicated concentrations of butaprost (EP2-selective agonist) in 2% SCM for 24 h (n ≥ 3 in triplicate/condition). Cell proliferation values were measured as above, and data are plotted as the mean ± SD, relative to 2% SCM alone. D, PGE2 induces cAMP at proliferative-suppressive concentrations (≥100 ng/ml; ≥280 nM). Serum-starved, subconfluent human lung fibroblasts were incubated with the indicated concentrations of PGE2 in SFM or 2% SCM for 10 min (n ≥ 3 in duplicate/condition). cAMP levels were measured in acid cell lysates by commercially available competitive EIA, and plotted as picomoles of cAMP per milligram of lysate total protein. ●, SFM; ○, 2% SCM. *, p < 0.05 ANOVA/Dunnett’s test vs cAMP in the absence of PGE2.
In contrast, the selective EP1/EP3 agonist, sulprostone, exhibited a stimulating effect on proliferation of adult human lung fibroblasts at concentrations (0.1 nM) near its Ki (0.1–0.35 nM for EP3) (Fig. 6A). Concordantly, cAMP levels were reduced (p = 0.003), when forskolin-stimulated cells were incubated with midrange concentrations of PGE2 (1–10 ng/ml), or with sulprostone at concentrations that stimulate proliferation (0.1 nM) (Fig. 6B). The effects of pertussis toxin on PGE2-dependent fibroblast proliferation were measured as a probe for EP3-dependent, Gαi protein-coupled signaling. Pertussis toxin was found to completely abrogate (>95%) the fibroblast proliferative stimulatory effects of 10 ng/ml PGE2, but had no effect on the fibroblast suppressive response to either 0.01 or 100 ng/ml PGE2, or of serum (Fig. 6C; serum (2%)-alone proliferation was 160 ± 8%). Furthermore, the EP1 receptor antagonist (SC-19220) had no discernable effect on proliferation at up to 10 times its Kd for EP1 (4.5 µM) (data not shown). Taken together, these data suggest that EP3 receptors are primarily responsible for the ability of PGE2 to stimulate proliferation at PGE2 concentrations (≥1–10 ng/ml; 2.8–28 nM), whereas EP2 receptors mediate the suppression of proliferation at high concentrations of PGE2 (≥100 ng/ml; 2800 nM) in adult human lung fibroblasts.
FIGURE 6.
The EP3 receptor signaling pathway mediates the proliferative-stimulating actions of mid-range concentration (1–100 ng/ml; 2.8–280 nM) PGE2. A, The EP3-selective agonist, sulprostone, induces fibroblast proliferation (Ki = 0.1 nM). Serum-starved, subconfluent human lung fibroblasts were incubated with the indicated concentrations of sulprostone (EP3-selective agonist) in 2% SCM for 24 h (n ≥ 3 in triplicate/condition). Cell proliferation values were measured as DNA synthesis by BrdU ELISA. B, cAMP levels are reduced by mid-range PGE2 concentrations and proliferative-suppressive concentrations of sulprostone. Fibroblasts were incubated with forskolin (50 µM, 5 min) followed by the indicated concentrations of sulprostone (SP; EP3 selective agonist) or PGE2 for 10 min (n ≥ 3 in triplicate/condition). Cell lysates were assayed for cAMP and data are reported as the percent of cAMP values from forskolin-alone controls. C, Mid-range (10 ng/ml) PGE2-related proliferation is sensitive to pertussis toxin. Fibroblasts were preincubated with pertussis toxin (indicated concentrations) and stimulated with serum ± the indicated PGE2 concentrations for 24 h followed by a BrdU proliferation assay. A–C, Data are plotted as proliferation (mean ± SD) relative to controls. SCM (2%) alone resulted in a proliferation of 160 ± 8% of that of SFM. *, p < 0.05 ± sulprostone (A), ± PGE2 or sulprostone (B), ± pertussis toxin (C) compared with controls.
Discussion
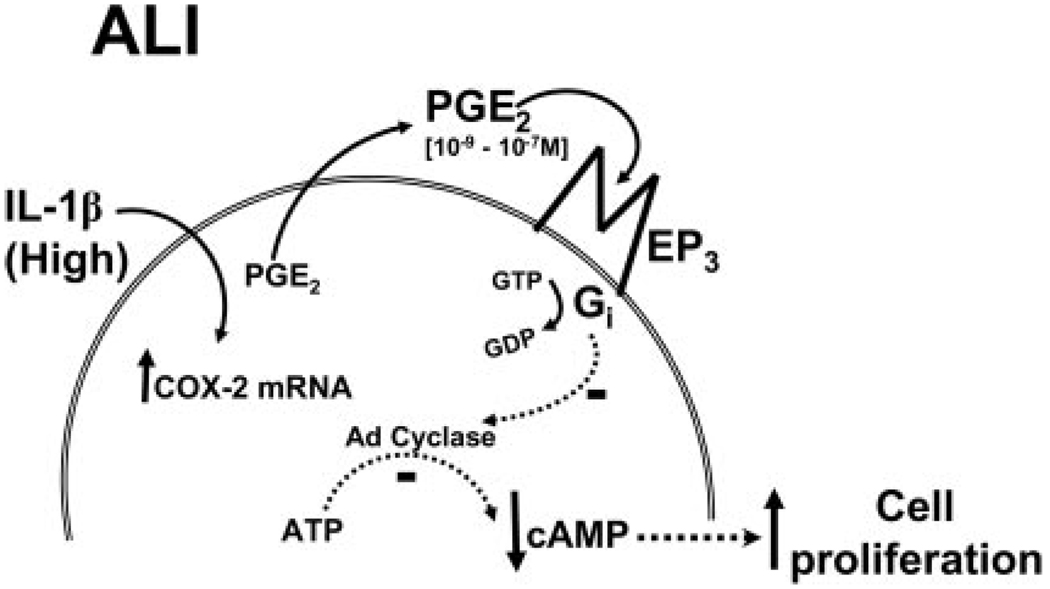
Our studies suggest a model of fibroproliferative disease following ALI in which the proliferative responses of the fibroblasts are initiated by a dramatic increase in IL-1β in the pulmonary edema fluid within hours of ALI. The high level of IL-1β acts to induce IL-6 and PGE2, both of which can contribute to the fibroproliferative response. The ability of IL-1β to up-regulate PGE2-mediated enhancement of the fibroproliferative response appears to be regulated at several levels. First, IL-1β can act to enhance the level of PGE2 through up-regulation of COX-2 in the adult human lung fibroblasts. Second, these IL-1β-dependent up-regulations are concentration dependent, with an EC50 (0.01–1 ng/ml) spanning the observed range of IL-1β measured in patient-derived edema fluids (22). Third, the levels of production of PGE2 are critical, as PGE2 can either stimulate or suppress fibroblast proliferation, depending on its concentration. PGE2 at concentrations of 10−9 to 10−7 M enhance proliferation of adult human lung fibroblasts, whereas PGE2 at concentrations of ≤10−10 M or ≥10−6 M suppresses proliferation of adult human lung fibroblasts. Furthermore, the switch point threshold for proliferative induction vs suppression of ~1 ng/ml PGE2 is within the range of clinical samples derived from ALI patients. The mechanism underlying the differential response of the lung fibroblasts to the PGE2 involves differential use of the PGE2 receptors, EP2 and EP3. Taken together, these data indicate that fibroblast proliferation can be modulated by IL-1β-induced, physiological range PGE2 concentrations in an autocrine manner in ALI (see Fig. 7). Differences in IL-1β-induced fibroblast PGE2 production may underlie the fibroproliferative response to ALI.
FIGURE 7.
Model of IL-1β-induced, PGE2-dependent effects on proliferation in ALI. In ALI, high concentrations of IL-1β highly induce COX-2, thereby up-regulating PGE2 production. The PGE2 (1–10 ng/ml range, 10−9 to 10−7 M) thereby produced selectively activates EP3 receptors, leading to the activation of a pertussis toxin-sensitive Gi protein, which inhibits adenyl cyclase, resulting in a reduction in cAMP and an increase in fibroblast proliferation.
This study is the first to show that PGE2 mediates both proliferative induction and suppression in the same cells in a concentration-dependent manner, and further, to identify the individual EP receptors involved in its inductive and suppressive effects. Although we and others have demonstrated increases in IL-1β in the alveolar compartment in ALI (22, 46), this study is the first to demonstrate that PGE2 can mechanistically link proliferative induction and suppression as a function of IL-1β levels in patient samples.
It is well-established that PGE2 concentration is greater in lung than in plasma, and that PGE2 is the major eicosanoid product of fibroblasts (27, 47). Lung fibroblast PGE2 production has been reported previously to be up-regulated at the level of COX-2 in response to mediators of direct relevance to lung injury including LPS, TGF-β, and IL-1β (29, 48). However, our data using purified IL-1β, and inhibitors of IL-1β signaling (i.e., IL-1ra and IL-β-neutralizing Abs) clearly demonstrate that IL-1β is the predominant mediator of COX-2 induction and resultant PGE2 production in adult human lung fibroblasts, in the context of ALI. The observations that PGE2 levels were significantly higher (4-fold) in pulmonary edema fluid from patients with early ALI compared with HYDRO, and the positive correlation of edema fluid IL-1β Ag levels with that of PGE2 Ag, further supports the in vivo relevance of our findings, and extends the increases in PGE2 previously noted in a smaller group of patients (49).
Although tissue/disease-specific differences in the fibroblast responses to IL-1β have been reported, the preponderance of prior evidence using either embryonic or adult human lung fibroblasts indicates that PGE2 is the primary downstream effector of IL-1β-mediated suppression of proliferation (26, 47, 50–53). In this study, we show that at extreme high and low concentrations (≥200 and ≤1 ng/ml; ≥10−6 and ≤10−10 M), PGE2 can act as an autocrine inhibitor of proliferation while at mid-range concentrations (≥ 1–200 ng/ml; 10−9 to 10−7 M), PGE2 acts to enhance proliferation of nontransformed human lung fibroblasts. The biphasic PGE2 proliferative response is unlikely to be a methodologic artifact as it was observed using several different lots of serum, and when using either BrdU incorporation (DNA synthesis) or increment in cell number as the measure of proliferation.
The pleiotropic physiologic responses ascribed to PGE2 are increasingly recognized to depend largely on the signals initiated by actions of the four known G protein-coupled prostanoid receptors (EP1–4) (25, 51, 52, 54–58). These EP receptors vary in their affinities for PGE2, their intracellular signaling pathways, and their induced physiologic responses (25, 33, 34, 59, 60). We found that adult lung fibroblasts express all four of these receptors. However, EP2 selectively initiates a proliferative suppressive signal at high concentrations (≥200 ng/ml; ≥10−6 M) of PGE2, while EP3 selectively signals proliferative stimulation at mid-range concentrations (≥ 1–200 ng/ml; 10−9 to 10−7 M) of PGE2.
To our knowledge, a concentration-dependent, opposing action of PGE2 in the same cells has been reported only once, for PGE2-induced macrophage phagocytosis, and furthermore, the mechanisms/receptors used for these effects were not determined (61, 62). Several lines of evidence in our study point to a PGE2 concentration-dependent, differential use of EP2 and EP3 leading to opposite effects on proliferation. First, both the proliferative stimulatory effect, and cAMP-reducing effects, of mid-range PGE2 concentrations (10−9 to 10−7 M) are mimicked by the EP3 selective agonist, sulprostone. Second, the selective inhibition of 10−8 M (1–10 ng/ml) PGE2-induced proliferation by pertussin toxin, known to block Gαi protein-coupled EP3, and lack of effect of the EP1 antagonist (SC-19220), identify EP3 as the key proliferative stimulatory receptor. In contrast, PGE2 at high concentrations (≥10−6 M) inhibits proliferation through EP2 receptor-mediated increases in cAMP. EP2 signaling was demonstrable by the findings that PGE2 induced cAMP only at high (≥10−6 M) concentrations, the EP2 selective agonist, butaprost, similarly induced cAMP, and that butaprost- or forskolin-mediated increases in cAMP inhibit proliferation. Concordantly, lung fibroblasts derived from EP2-deficient mice, selectively lost the PGE2-mediated suppression seen at high concentrations (≥10−6 M) PGE2 in fibroblasts derived from wild-type mice.
Several plausible molecular events may explain our observed differential use of EP2 and EP3. Most simply, the reported binding affinity of PGE2 for EP3 (Ki ≅ 100 pM) of 30-fold lower than that of EP2 (3 nM), supports a differential PGE2 concentration-dependent ligation of EP2 and EP3 (25, 59). Alternatively, relative over-expression of EP3 over EP2 may favor an EP3-type response to lower concentrations of PGE2. Changes in EP receptor expression profiles can be mediated by several factors known to be increased in ALI including LPS, serum, stress, and IFN-γ, and PGE2 itself (33, 63, 64). In fact, fibroblasts isolated from wild-type mice after experimentally induced lung fibrosis exhibit a relative increase in the proportion of EP3 over EP2 receptors, and a switch from a proliferative-suppressive to a proliferative-stimulating response to PGE2 (36). These observations suggest that baseline variability or agonist-induced changes in fibroblast EP receptor profiles might support a profibrotic (high EP3-expressing) clonal selection of fibroblasts. Furthermore, mice deficient in EP2 receptors exhibit a greater profibrotic response to bleomycin than wild-type mice, suggesting that changes in EP receptor profile can control in vivo fibrotic responses (36). Although we have clearly shown that EP2 and EP3 receptors are coupled to increases and decreases in cAMP, respectively, it remains possible that cross-talk among other downstream signaling intermediates, and/or transactivation of other receptors, may condition the response to PGE2 and/or changes in cAMP (65–68). Future studies will be directed at defining the precise explanation for our novel observations.
This work identifies a new molecular mechanism whereby IL-1β in the alveolar compartment in ALI can initiate/maintain the fibroproliferative response. The observation that transient overexpression of IL-1β in murine lungs induces a fibrotic response is further proof of concept (21). Although we are limited in individual patient data correlating IL-1β levels to in vivo fibrosis, IL-1β did positively correlate with PGE2 levels in edema fluid. Prior work demonstrates that IL-1β positively tracks with biomarkers of increased collagen synthesis (procollagen peptides), and procollagen peptide increases track with fibroblast mitogenic bioactivity of alveolar fluid and with overall outcome, in individual patients with ALI (13, 14).
PGE2 has been shown to inhibit fibroblast migration, collagen synthesis, transdifferentiation of fibroblasts into a myofibroblast phenotype, including gel contraction, as well as fibroblast proliferation (51, 52, 54–58, 69–71) so the precise mechanism of its effect(s) on in vivo fibrosis remain to be elucidated. Nonetheless, available evidence in more chronic fibroproliferative disorders of the lung underscores a key pathogenic role for PGE2. For example, fibroblasts derived from patients with idiopathic pulmonary fibrosis exhibit down-regulated COX-2 levels and PGE2 synthetic capacity, and bronchoalveolar lavage PGE levels are lower than in normals (28–30). Furthermore, reductions in PGE2, through deletion of one COX-2 allele or administration of indomethacin, worsens pulmonary fibrosis in experimentally induced pulmonary fibrosis in mice (6, 72–74). Interestingly, mice are not protected from experimental pulmonary fibrosis under conditions of increased PGE2, consistent with the possibility of the existence of a biphasic PGE2-fibrosis response in this model (72).
We have confirmed the biphasic, proliferative response to PGE2 in a second primary lung cell line. The use of a single-cell strain for most of the studies surmounts the issue of phenotypic heterogeneity noted in lung fibroblasts, thus supporting the detection of more subtle cell responses. In contrast, it is possible that fibroblasts may modulate their PGE2 response to IL-1β, or their EP receptor expression profile, due to longer term stimulation, as has been noted in fibroblast from pulmonary fibrosis patients.
In summary, we show that IL-1β induces PGE2 production in human lung fibroblasts through COX-2 induction and, furthermore, that IL-1β is the key mediator of COX-2 induction and PGE2 production in fibroblasts exposed to ALI pulmonary edema fluid. Surprisingly, the fibroblast proliferation inhibitory activity of PGE2 is biphasic within the range of the ALI clinical samples. EP2 receptor signaling of increased cAMP mediates the proliferative suppressive effects of high concentrations of PGE2, while EP3 receptor signaling of cAMP reduction mediates the proliferative stimulatory effects of mid-range concentrations of PGE2. Taken together, these data indicate that fibroblast proliferation can be modulated by IL-1β-induced, physiological range PGE2 concentrations in an autocrine manner in ALI. Differences in IL-1β-in-duced fibroblast PGE2 production may underlie the fibroproliferative response to ALI.
Acknowledgments
We thank Dr. Jerome Pugin for providing TNF-α-binding protein. We also thank Janice Buffett for her secretarial assistance.
Footnotes
This work was supported by grants from the Veterans Administration MERIT Review and National Institutes of Health Grants HL-58655 (to M.A.O.), HL-51856 and HL-51854 (to M.A.M.), HL-70521 (to L.B.W.), HL-56402 and HL-071586 (to B.B.M.), and HL-56402 (to M.P.-G.), and the American Heart Association (to Q.D.).
Abbreviations used in this paper: ALI, acute lung injury; HYDRO, hydrostatic edema; COX, cyclooxygenase; IL-1ra, IL-1 receptor antagonist; SCM, serum-conditioned medium; SFM, serum-free medium; SAPS, simplified acute physiology score; EP, E prostanoid; EIA, enzyme immunoassay.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Ware LB, Matthay MA. Medical progress: the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit. Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabory E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am. J. Med. 1992;92 doi: 10.1016/0002-9343(92)90606-c. 39S-43S. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Striker LJ, Hudson LD, Striker GE. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Resp. Dis. 1985;131:281–289. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 6.Olman MA. The fibroproliferative phase of acute lung injury. In: Matthay M, Lenfant C, editors. Acute Respiratory Distress Syndrome. Washington: Marcel Dekker, Seattle; 2003. pp. 313–354. [Google Scholar]
- 7.Olman M, Mackman N, Gladson C, Moser K, Loskutoff D. Changes in procoagulant and fibrinolytic gene expression during bleomycin induced lung injury in the mouse. J. Clin. Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olman MA, Simmons WL, Pollman DJ, Loftis AY, Bini A, Miller EJ, Fuller GM, Rivera K. Polymerization of fibrinogen in murine bleomycin-induced lung injury. Am. J. Physiol. 1996;271:L519–L526. doi: 10.1152/ajplung.1996.271.4.L519. [DOI] [PubMed] [Google Scholar]
- 9.Bachofen A, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am. Rev. Resp. Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am. J. Pathol. 1987;126:171–182. [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Ann. Intern. Med. 1995;122:17–23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Am. J. Respir. Crit. Care Med. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 13.Pugin J, Verghese GM, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit. Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 14.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am. J. Respir. Crit. Care Med. 2000;162:1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 15.Synder LS, Hertz MI, Peterson MS, Harmon KR, Marinelli WA, Henke CA, Greenheck JR, Chen B, Bitterman PB. Acute lung injury: pathogenesis of intraalveolar fibrosis. J. Clin. Invest. 1991;88:663–673. doi: 10.1172/JCI115351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, Polunovsky V, White J, Blazar B, Nakhleh R, Jessurun J, Peterson M, Bitterman P. Mesenchymal cells isolated after acute lung injury manifest an enhanced proliferative phenotype. J. Clin. Invest. 1992;90:1778–1785. doi: 10.1172/JCI116052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am. J. Respir. Crit. Care Med. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 18.Suter PM, Suter SM, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Resp. Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 19.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 20.Gaggar A, Olman MA. Biologic markers of mortality in acute lung injury. Clin. Chimica Acta. 2006;372:24–32. doi: 10.1016/j.cca.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olman MA, White KE, Ware L, Simmons WL, Pugin J, Benveniste EN, Matthay MA, Zhu S. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1β-induced IL-6 expression. J. Immunol. 2004;172:2668–2677. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- 23.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am. J. Physiol. 2003;284:L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez T, Moreno JJ. Role of EP1 and EP4 PGE2 subtype receptors in serum-induced 3T6 fibroblast cycle progression and proliferation. Am. J. Physiol. 2002;282:C280–C288. doi: 10.1152/ajpcell.00128.2001. [DOI] [PubMed] [Google Scholar]
- 25.Narumiya S, Sugimoto Y, Ushkubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 26.Endo T, Ogushi F, Sone S, Ogura T, Taketani Y, Hayashi Y, Uedo N, Yamamoto S. Induction of cyclooxygenase-2 is responsible for interleukin-1β-dependent prostaglandin E2 synthesis by human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1995;12:358–365. doi: 10.1165/ajrcmb.12.3.7873203. [DOI] [PubMed] [Google Scholar]
- 27.Ozaki T, Rennard SI, Crystal RG. Cyclooxygenase metabolites are compartmentalized in the human lower respiratory tract. J. Appl. Physiol. 1987;62:219–222. doi: 10.1152/jappl.1987.62.1.219. [DOI] [PubMed] [Google Scholar]
- 28.Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, Rennard SI, Crystal RG. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am. Rev. Respir. Dis. 1991;144:1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 29.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J. Clin. Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vancheri C, Sortino MA, Tomaselli V, Mastruzzo C, Condorelli F, Bellistri G, Pistorio MP, Canonico PL, Crimi N. Different expression of TNF-α receptors and prostaglandin E2 production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am. J. Respir. Cell Mol. Biol. 2000;22:628–634. doi: 10.1165/ajrcmb.22.5.3948. [DOI] [PubMed] [Google Scholar]
- 31.Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am. Rev. Resp. Dis. 1988;137:579–584. doi: 10.1164/ajrccm/137.3.579. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RJ, Rhodes SA, Wood RL, Shield VJ, Noel LS, Gray DW, Giles H. Functional pharmacology of human prostanoid EP2 and EP4 receptors. Eur. J. Pharmacol. 2004;501:49–58. doi: 10.1016/j.ejphar.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Breyer RM, Bagdassarian C, Myers S, Breyer M. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 35.Funk CD, Furci L, Fitzgerald GA, Grygorczy R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- 36.Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J. Immunol. 2005;174:5466–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 37.Mnich SJ, Veenhuizen AW, Monahan JB, Sheehan KCF, Lynch KR, Isakson PC, Portanova JP. Characterization of a monoclonal antibody that neutralizes the activity of prostaglandin E2. J. Immunol. 1995;155:4437–4444. [PubMed] [Google Scholar]
- 38.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am. Rev. Resp. Dis. 1999;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 39.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky M, Spracc R, Suter PM. The American-European consensus conference on ARDS, part 2. Am. J. Respir. Crit. Care Med. 1998;157:1332–1347. doi: 10.1164/ajrccm.157.4.ats2-98. [DOI] [PubMed] [Google Scholar]
- 40.Le Gall JR, Lemenshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. J. Am. Med. Assoc. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 41.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J. Appl. Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 42.Petering H, Kohl J, Weyergraf A, Dulkys Y, Kimmig D, Smolarski R, Kapp A, Elsner J. Characterization of synthetic C3a analog peptides on human eosinophils in comparison to the native complement component C3a. J. Immunol. 2000;164:3783–3789. doi: 10.4049/jimmunol.164.7.3783. [DOI] [PubMed] [Google Scholar]
- 43.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am. J. Physiol. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 44.Zar JH. Biostatistical Analysis. Englewood Cliffs: Prentice-Hall; 1984. [Google Scholar]
- 45.Sanchez T, Moreno JJ. GR 63799X, an EP3 receptor agonist, induced S phase arrest and 3T6 fibroblast growth inhibition. Eur. J. Pharmacol. 2006;529:16–23. doi: 10.1016/j.ejphar.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 47.Elias JA. Tumor necrosis factor interacts with interleukin-1 and interferons to inhibit fibroblast proliferation via fibroblast prostaglandin-dependent and -independent mechanisms. Am. Rev. Respir. Dis. 1988;138:652–658. doi: 10.1164/ajrccm/138.3.652. [DOI] [PubMed] [Google Scholar]
- 48.Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-β in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthay MA, Eschenbacher WL, Goetzl EJ. Elevated concentrations of leukotriene D4 in pulmonary edema fluid of patients with adult respiratory distress syndrome. J. Clin. Immunol. 1984;4:479–483. doi: 10.1007/BF00916578. [DOI] [PubMed] [Google Scholar]
- 50.Lonnemann G, Shapiro L, Engler-Blum G, Muller GA, Koch KM, Dinarello CA. Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis-derived kidney fibroblasts. Kidney Int. 1995;47:837–844. doi: 10.1038/ki.1995.126. [DOI] [PubMed] [Google Scholar]
- 51.Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J. Clin. Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elias JA, Rossman MD, Zurier RB, Daniele RP. Human alveolar macrophage inhibition of lung fibroblast growth: a prostaglandin-dependent process. Am. Rev. Respir. Dis. 1985;131:94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Ostrom RS, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am. J. Physiol. 2004;286:C1089–C1099. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- 54.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am. J. Respir. Cell Mol. Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 55.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am. J. Respir. Cell Mol. Biol. 2002;27:752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 56.White ES, Atrasz RG, Dickie EG, Arnonff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin E2 inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am. J. Respir. Cell Mol. Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E2 inhibits fibroblast chemotaxis. Am. J. Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- 58.Zhu YK, Liu XD, Skold MC, Umino T, Wang H, Romberger DJ, Spurzem JR, Kohyama T, Wen F-Q, Rennard SI. Cytokine inhibition of fibroblast-induced gel contraction is mediated by PGE2, and NO acting through separate parallel pathways. Am. J. Respir. Cell Mol. Biol. 2001;25:245–253. doi: 10.1165/ajrcmb.25.2.4383. [DOI] [PubMed] [Google Scholar]
- 59.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugimoto Y, Narumiya S, Ichikawa A. Distribution and function of prostanoid receptors: studies from knockout mice. Prog. Lipid Res. 2000;39:289–314. doi: 10.1016/s0163-7827(00)00008-4. [DOI] [PubMed] [Google Scholar]
- 61.Coquette A, Boeynaems JM, Vray B. Eicosanoids modulate CR1-and Fc-dependent bacterial phagocytosis. Eur. J. Pharmacol. 1992;226:1–4. doi: 10.1016/0922-4106(92)90075-7. [DOI] [PubMed] [Google Scholar]
- 62.Razin E, Bauminger S, Globerson A. Effect of prostaglandins on phagocytosis of sheep erythrocytes by mouse peritoneal macrophages. J. Recticul. Soc. 1978;23:237–242. [PubMed] [Google Scholar]
- 63.Nasrallah R, Laneuville O, Ferguson S, Hebert RL. Effect of COX-2 inhibitor NS-398 on expression of PGE2 receptor subtypes in M-1 mouse CCD cells. Am. J. Physiol. 2001;281:F123–F132. doi: 10.1152/ajprenal.2001.281.1.F123. [DOI] [PubMed] [Google Scholar]
- 64.Ikegami R, Sugimoto Y, Segi E, Katsuyama M, Karahashi H, Amano R, Maruyama T, Yamane H, Tsuchiya S, Ichikawa A. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipo-polysaccharide in C3H/HeN peritoneal macrophages. J. Immunol. 2001;166:4689–4696. doi: 10.4049/jimmunol.166.7.4689. [DOI] [PubMed] [Google Scholar]
- 65.Handler JA, Danilowicz RM, Eling TE. Mitogenic signaling by epidermal growth factor (EGF), but not platelet-derived growth factor, requires arachidonic acid metabolism in BALB/c 3T3 cells. J. Biol. Chem. 1990;265:3669–3673. [PubMed] [Google Scholar]
- 66.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 67.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J. Biol. Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 68.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 69.Clark JG, Kostal KM, Marino BA. Modulation of collagen production following bleomycin-induced pulmonary fibrosis in hamsters: presence of a factor in lung that increases fibroblast prostaglandin E2 and cAMP and suppresses fibroblast proliferation and collagen production. J. Biol. Chem. 1982;257:8098–8105. [PubMed] [Google Scholar]
- 70.Fine A, Poliks CF, Donahur LP, Smith BD, Golstein RH. The differential effect of prostaglandin E2 on transforming growth factor-β and insulin-induced collagen formation in lung fibroblasts. J. Biol. Chem. 1989;264:16988–16991. [PubMed] [Google Scholar]
- 71.Green JA, Stockton RA, Johnson C, Jacobson BS. 5-Lipoxy-genase and cyclooxygenase regulate wound closure in NIH/3T3 fibroblast monolayers. Am. J. Physiol. 2004;287:C373–C383. doi: 10.1152/ajpcell.00509.2003. [DOI] [PubMed] [Google Scholar]
- 72.Hodges RJ, Jenkins RG, Wheeler-Jones CPD, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E2 production. Am. J. Pathol. 2004;165:1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, Toews GB. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J. Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 74.Charbeneau RY, Christensen PJ, Chrisman CJ, Paine R, Toews GB, Peters-Golden M, Moore BB. Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/− mice: implications for fibroproliferation. Am. J. Physiol. 2003;284:L1103–L1111. doi: 10.1152/ajplung.00350.2002. [DOI] [PubMed] [Google Scholar]