Abstract
A phospholipase C/ sphingomyelinase from Pseudomonas aeruginosa has been assayed on vesicles containing phosphatidylcholine, sphingomyelin, phosphatidylethanolamine and cholesterol, at equimolar ratios. The enzyme activity modifies the bilayer chemical composition giving rise to diacylglycerol (DAG) and ceramide (Cer). Assays of enzyme activity, enzyme-induced aggregation and fusion have been performed. Ultrastructural evidence of vesicle fusion at various stages of the process is presented, based on cryo-EM observations. The two enzyme lipidic end-products, DAG and Cer, have opposite effects on the bilayer physical properties, the former abolishes lateral phase separation, while the latter generates a new gel phase [Sot et al., FEBS Lett. 582, 3230–3236 (2008)]. Addition of either DAG, or Cer, or both to the liposome mixture causes an increase in enzyme binding to the bilayers and a decrease in lag time of hydrolysis. These two lipids also have different effects on the enzyme activity, DAG enhancing enzyme-induced vesicle aggregation and fusion, Cer inhibiting the hydrolytic activity. These effects are explained in terms of the different physical properties of the two lipids. DAG increases bilayers fluidity and decreases lateral separation of lipids, thus increasing enzyme activity and substrate accessibility to the enzyme. Cer has the opposite effect mainly because of its tendency to sequester sphingomyelin, an enzyme substrate, into rigid domains, presumably less accessible to the enzyme.
Introduction
Membrane fusion is an essential event in a variety of cellular processes, e.g. neurotransmitter release, certain viral infections (influenza, HIV) or membrane biogenesis. The mechanism by which the two involved bilayers transiently break down their structures, to become a single continuous bilayer is not fully understood yet. The most widely accepted model of membrane fusion involves the formation of a highly curved semitoroidal intermediate, “the stalk”, between the two membranes [1]. It is known that the presence of diacylglycerol in the membrane greatly facilitates membrane fusion [2–4].
Phospholipases are essential enzymes in maintaining membrane homeostasis and in the generation of metabolic signals. They are also powerful tools for bacteria to infect and eventually destroy eukaryotic cells, helping in the degradation of the target cell membranes. Phospholipases C (PLC) cleave the phosphodiester bond between the glycerophospholipid DAG moiety and the phosphoryl groups. Most sphingomyelinases hydrolyze the equivalent bond between ceramide and phosphorylcholine in sphingomyelin (SM). From the point of view of their enzyme activity, phospholipases, and lipases in general, are rather unique among enzymes in that their substrates and most end-products do not occur free in solution, but are found instead making part of cell membranes [5–8].
PlcHR2 is a novel phsopholipase C/sphingomyelinase from Pseudomonas aeruginosa [9] equally active on PC and on SM when these lipids are in the monomeric form, or in mixed micelles with detergents. In a previous paper [10] we found that, when acting on vesicles, the enzyme produces both DAG and Cer, that are in turn responsible for vesicle aggregation, fusion. Aggregation occurs when DAG and/or Cer are formed in the membrane above a certain level. Aggregation is necessary but not sufficient for fusion to occur. DAG, an end-product of the enzyme, induces membrane fusion [2, 11–13]. In the present work we have applied our previous knowledge to study the effects of the end-products DAG and Cer on PlcHR2-induced membrane fusion assayed in LUV in suspension.
Materials and methods
Materials
PlcHR2 was purified as previously described [9]. Egg phosphatidylcholine (PC), egg phosphatidylethanolamine (PE) and egg diacylglycerol (DAG) were purchased from Lipid Products (South Nutfield, UK). Egg SM, egg Cer and cholesterol were from Avanti Polar Lipids (Alabaster, AL). NBD-PE and Rho-PE were supplied by Molecular Probes, Inc. (Eugene, OR).
LUV preparation
LUV of diameters 100–150 nm were prepared by the extrusion method [14] using Nuclepore filters 0.1 µm or 0.2 µm pore diameter at room temperature, in 25 mM HEPES, 100 mM NaCl, pH 7.2. Quantitative analysis of the lipid composition of our LUV preparations, as described by Ruiz-Argüello et al. [12], showed that it did not differ significantly from the initial lipid mixture. All experiments were performed at 37° C. Lipid concentration was 0.3 mM, and PlcHR2 was used at 0.5 µg/ml.
Cryo-electron microscopy
Samples were prepared under controlled conditons (37°C, 99% humidity) in a Vitrobot (Eindhoven, Nederland). 3 µl of a suspension containing 10 mg phospholipid/ml were applied on a grid. After careful spreading of the drop, excess liquid was immediately blotted with filter paper. A 3 µl droplet creates a cylinder of about 0.5mm thick on a grid and by blotting away excess liquid this cylinder is reduced to a layer of about 100nm. After blotting, the sample was immediately plunged into liquid ethane held at its freezing point [15]. The specimen was transferred to a cryoholder of a microscope. After a thermal equilibration period (20 min), images are taken at 100kV and low-dose conditions in a Philips CM 12 microscope (Philips, Eindhoven, the Netherlands).
PlcHR2 binding to vesicles
The intrinsic fluorescence spectra of PlcHR2 either alone or in the presence of vesicles were recorded in an Aminco Bowman Series 2 luminescence spectrometer equipped with a thermostated cell holder and a magnetic stirrer. Small aliquots of a concentrated LUV suspension were added to a fixed concentration of enzyme solution (3.5 µg/ ml) in a cuvette with continuous stirring, exciting the samples at 295 nm and collecting emission between 300 and 420 nm. The slit widths were 5 nm for both excitation and emission. The intrinsic fluorescence of PlcHR2 was titrated with increasing amounts of lipid, in the 0–60µM concentration range, added at 1.5 min intervals.
Spectra were corrected for the light scattered by the LUVs of identical composition and concentration, but in the absence of protein. The signal was also corrected using the soluble Trp analogue, NATA, which does not partition into membranes. The apparent mole fraction coefficients, Kx(app), were determined by fitting the experimental values to the hyperbolic function:
where [L] is the lipid concentration and K is the lipid concentration at which the bound enzyme fraction is 0.5. Therefore, Kx(app) = [W]/K, where [W] is the molar concentration of water.
The fraction of PlcHR2 bound to the membranes was estimated according to the equation
Enzyme activity
PlcHR2 activity was assayed by determining phosphorous contents [16]. The liposome concentration was 0.3 mM in all experiments. For optimal catalytic activity, the enzyme was assayed at 37° C, in 25mM HEPES, 100 mM NaCl, pH 7.2. Enzyme concentration was 0.5µg/ml. Enzyme activity was assayed by determining water-soluble phosphorous. Aliquots (50 µl) were removed from the reaction mixture at regular intervals and extracted with 250 µl of a chloroform/methanol/hydrochloric acid mixture (66/33/1, v/v/v) and the aqueous phase was assayed for phosphorous content.
Aggregation and fluorescence measurements
Liposome aggregation was estimated as an increase in absorbance at 500 nm, measured in a Uvikon 922 (Kontron instruments, Groβ-Zimmern, Germany.). Vesicle-vesicle fusion was assayed as mixing of inner monolayer lipids by the resonance energy transfer method [17] modified by Montes et al. [10], using NBD-PE and Rho-PE. Vesicles containing 2 % NBD-PE and 2 % Rho-PE are briefly treated with sodium dithionite, a reagent that does not penetrate the membrane and that bleaches the fluorescence of the probes located in the outer monolayers. The resulting vesicles, containing fluorescent probes only in the inner monolayer composition were mixed with probe-free liposomes at 1:4 ratio. When vesicles fuse the inner monolayers of the fusing vesicles become into contact, lipid mixing ensues, and the subsequent probe dilution leads to decreased resonance energy tranfers from NBD to rhodamine, and increased NBD emission. NBD emission was followed at 530 nm (excitation wavelength at 465 nm) with a cut off filter at 515 nm. Zero percent mixing was established as the equilibrium fluorescence emission in the absence of enzyme. 100% percent mixing was set after addition of 1 mM Triton X-100. These experiments were performed in an Aminco Bowman Series 2 luminescence spectrometer.
Statistics
Unless otherwise indicated, data are average values of three independent measurements ± one standard deviation. Student’s t-test was used in order to assess the significance of observed differences.
Results and Discussion
Cryo-electron microscopy. Membrane fusion
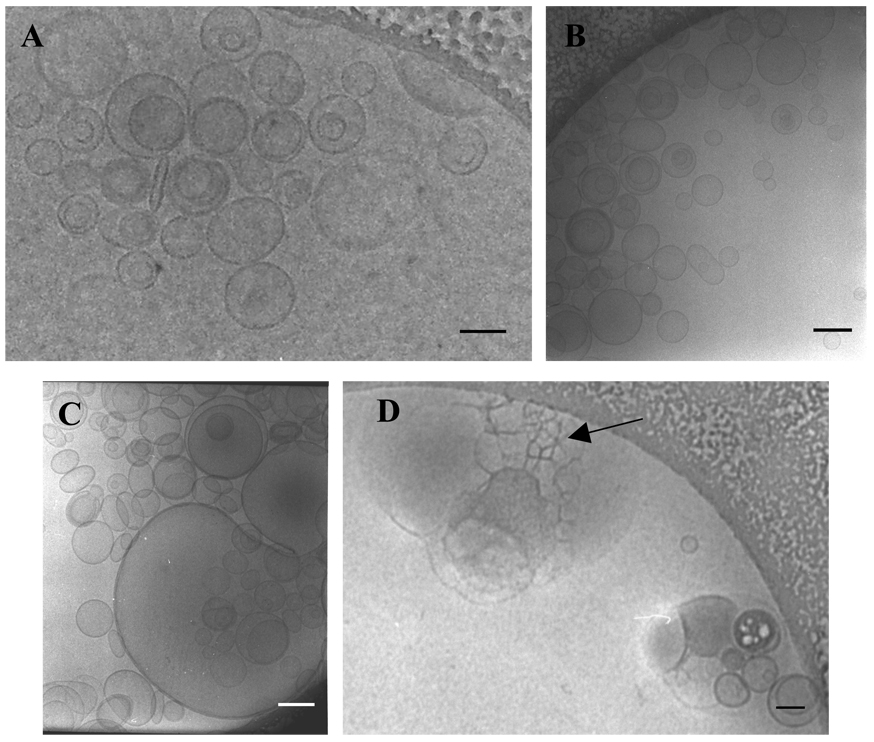
As shown by Montes et al. on the basis spectroscopic technique [18], the addition of PlcHR2 to LUVs composed by PC:PE:SM:Ch (1:1:1:1) leads to extensive membrane fusion. Ultrastructural evidence for this phenomenon is presented here using, cryo-electron micrographs taken various times after enzyme addition (Fig.1). At 0 min the size of the vesicles is 162±27nm. After 2 min the vesicle size does not change, probably because of the lag time of the enzyme activity [7]. At longer times (5 min) some very large vesicles are seen, together with others whose size has not changed. Finally, after 10 min huge vesicles and vesicles aggregates (1156±285nm) can be observed. Inside these aggregates some of the honeycomb structures, marked by arrows, arising from vesicles undergoing fusion events, are found. These honeycomb patterns had been observed in the fusion of LUV induced by pshopholipase C from B. cereus [4, 19] using freeze-fracture and cryo-transmission electron microscopies.
Fig. 1.
Cryo-TEM micrographs of LUV of PC:PE:SM:Ch (1:1:1:1) incubated with PlcHR2 at 37°C for (A) 0 min, (B) 2 min, (C) 5 min and (D)10 min. Arrow in (D) indicates the “honeycomb structures”.
Enzyme binding to vesicles
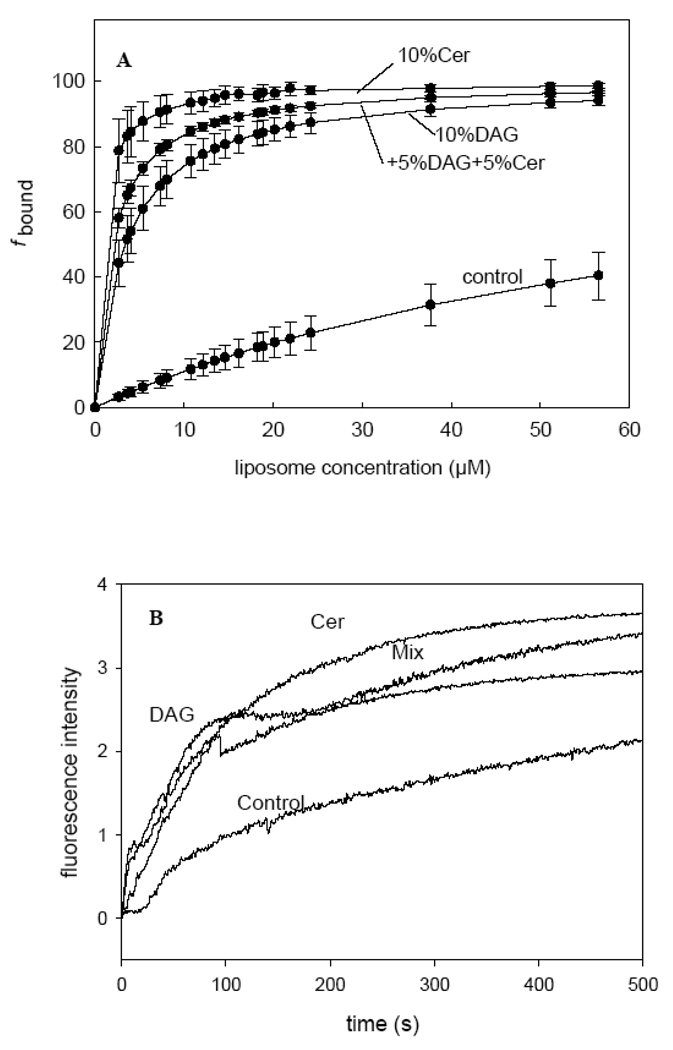
In order to estimate the enzyme affinity for the various bilayer compositions a fixed concentration of enzyme, PlcHR2, (3.5 µg/ ml) was titrated with increasing concentrations of lipid in the form of LUVs up to 60µM. The increase in fluorescence was measured 1 min after enzyme addition, at 37° C. Plots of fluorescence intensity vs. lipid concentration are shown in Fig. 2A. The enzyme binds the lipid with much higher affinity when DAG and/or Cer are present in the bilayer (Table 1). The enzyme being catalycally active under these conditions, it was possible that the data in Fig. 2A were influenced by the presence of increasing amounts of DAG+Cer in the bilayer. In order to overcome this difficulty, the initial rates of binding (or maximum rates, when there was a lag time) were computed from time-resolved studies of the increase in enzyme intrinsic fluorescence secondary to membrane binding. The results (Fig. 2B and Table 1) confirm that both DAG and Cer, either alone or in combination, facilitate PlcHR2 binding to the lipid bilayers.
Fig. 2.
PlcHR2 binding to LUV of different lipid composition. (A) The enzyme has been titrated with increasing concentrations of lipids. See main text for experimental details. Control: basic lipid composition, consisting of an equimolar mixture of PC:PE:SM:Ch. Average values ±S.D. (n=3).(B)The enzyme binding depending of the time.
Table 1.
Effects of ceramide and/or diacylglycerol in bilayers originally composed of PC: SM: PE: Ch (1: 1: 1: 1, mol ratio). Data are average ±S. D.
In a previous work where the lag time of the PC-PLC from B. cereus [20] was studied, it was suggested that the enzyme worked at very low rates during the initial period. Then, after a given concentration of DAG had been reached in the bilayers, a kinetic transition occurred and maximum rates of hydrolysis were observed. The same paper described experiments showing that lag times became almost zero when DAG was present in the membrane before enzyme addition. The data in Fig. 2 indicate that DAG or Cer increase dramatically PlcHR2 binding. With yet another enzyme, PI-PLC from Bacillus cereus, Ahyayauch et al. [21] observed that including DAG in bilayers increased enzyme binding. Thus, it may be a general phenomenon that adding the lipidic end-products of a lipase to a bilayer facilitates enzyme binding and decreases the lag times of enzyme activity. The mechanism by which addition of Cer or DAG enhances PlcHR2 binding cannot be ascertained at present. In fact, the possibility of various coexisting mechanisms cannot be ruled out. The data by Ahyayauch et al. [21] suggest that, in general, increasing membrane fluidity, e. g. by adding DAG [22], facilitates enzyme binding; however, Cer gives rise to less fluid domains [22], yet it also enhances binding (Fig. 2). In the absence of direct evidence, the hypothesis can be proposed that the enzyme has an affinity for the interfaces between the Cer-rich and – poor domains. There are several examples of proteins that became prefentially inserted at rigid-fluid interfaces [20, 23]
Enzyme activity. Vesicle aggregation and fusion
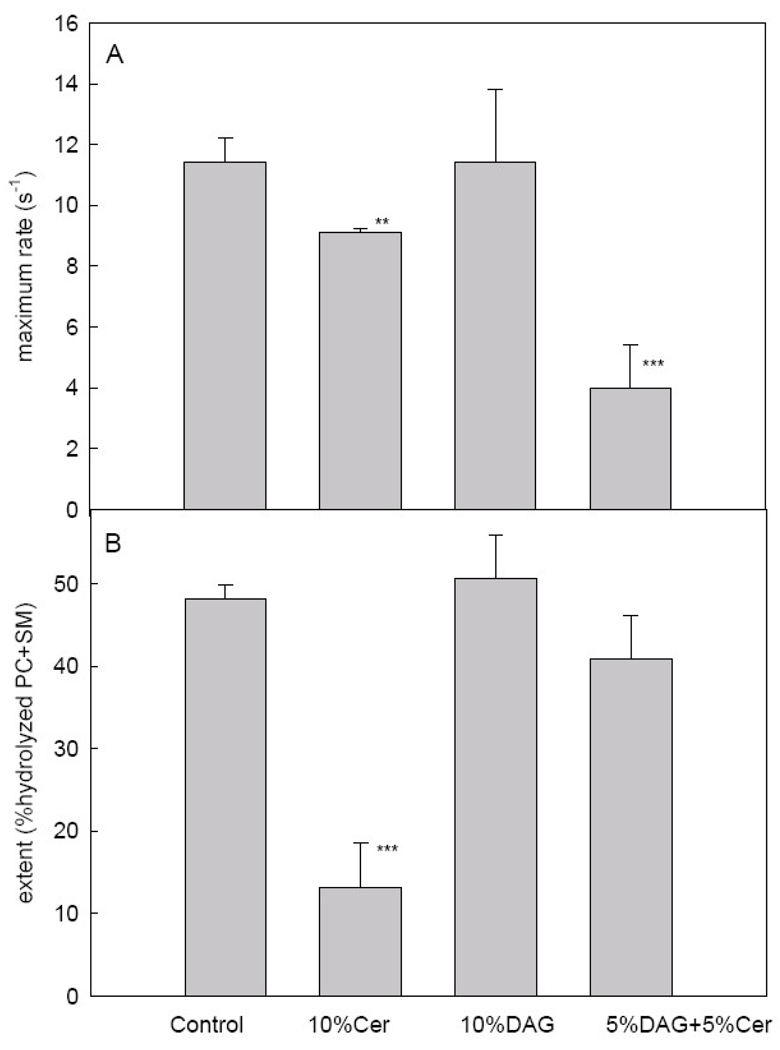
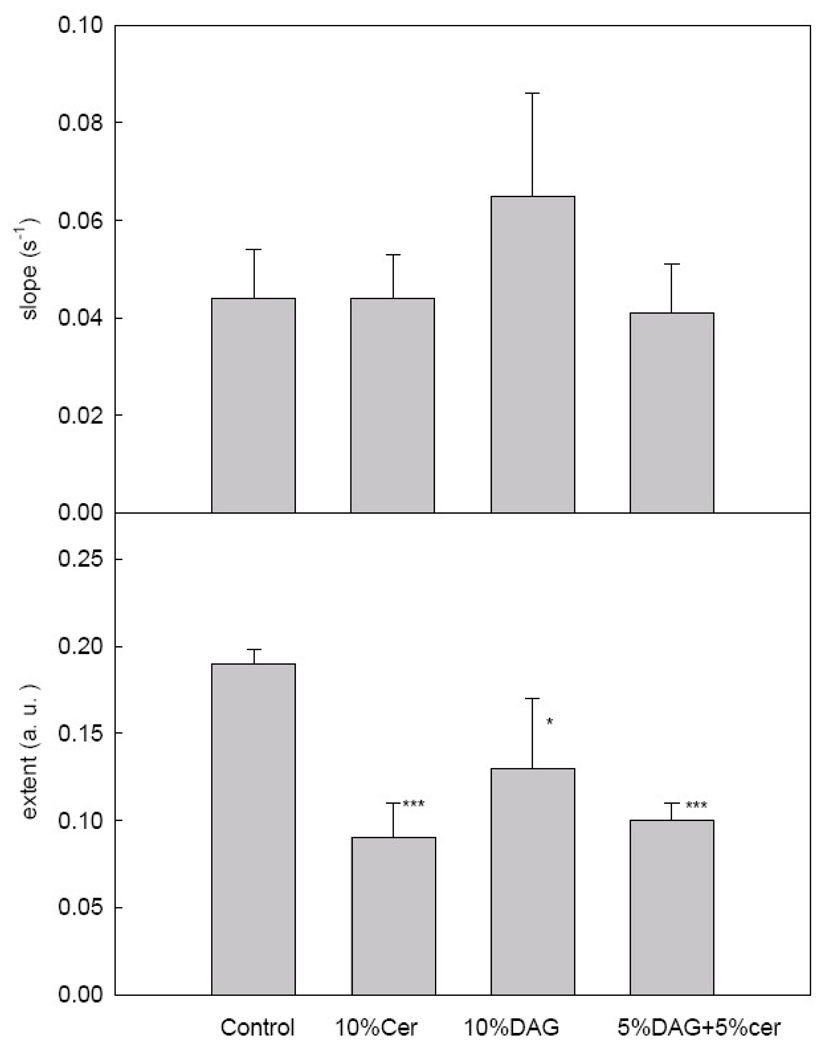
Montes et al. [18] have shown that PlcHR2 activity induces aggregation and fusion of phospholipid vesicles (LUV) composed of PC: SM: PE: Ch (1: 1: 1: 1, mol ratio). In the present paper, we describe the effects of including DAG and/or Cer in the initial composition. As shown in Fig. 2A, the maximum rates of hydrolysis decrease when Cer is present in the vesicle composition. Maximum rates rather than initial rates are given because, as discussed by Montes et al. [18], a latency period, or lag time, is observed with this enzyme, as with many other lipases. When the extent of hydrolysis after an intermediate stage in the fusion process (Fig. 1C) 5 min is measured (Fig. 3B) Cer, but not DAG, is clearly seen to act as an enzyme inhibitor. Our interpretation for the data in Fig. 3 is that, beyond their role as end-product inhibitors, Cer and DAG have opposite physical properties that influence enzyme activity. Cer separates into rigid SM-Cer domains [22, 24], thus presumably a large fraction of the substrate SM is not available to the enzyme. Recent evidence [25, 26] with B. cereus sphingomyelinase has shown that the enzyme acts in the fluid phase by producing over-saturation of ceramide which causes end-product inhibition of the enzyme activity and eventually segregation of ceramide-enriched domains.
Fig. 3.
Activity of PlcHR2 for the four different lipid mixtures. (A) Maximum slopes. (B) Extent of the activity. Average values ±S.D. (n=3). The asterisk denotes the results of the Student´s t-test. (*)0.01<p<0.05; (**) 0.001<p<0.01; (***) P<0.001.
On the contrary, DAG increases membrane fluidity, and this facilitates enzyme activity and compensates end-product inhibition. Ahyayauch et al. [21] have shown for another PLC (PI-PLC from Bacillus cereus) that the enzyme is activated by an increase in membrane fluidity. The results with vesicles containing initially 5 % Cer + 5 % DAG can be interpreted in the same way. DAG homogenizes or solubilizes Cer-enriched domains, thus the observed decrease in enzyme rate (Fig. 3A) is probably due to end-product inhibition. However, most of the lipid is in the fluid state, thus accessible to the enzyme, and this explains the small difference in the amount of lipid cleaved after 5 min between the “control” and the “5 % DAG + 5 % Cer” samples. Moreover, it should be noted, from the comparison of Fig. 2 and Fig. 3, that there is no correlation between enzyme binding and rates of hydrolytic activity.
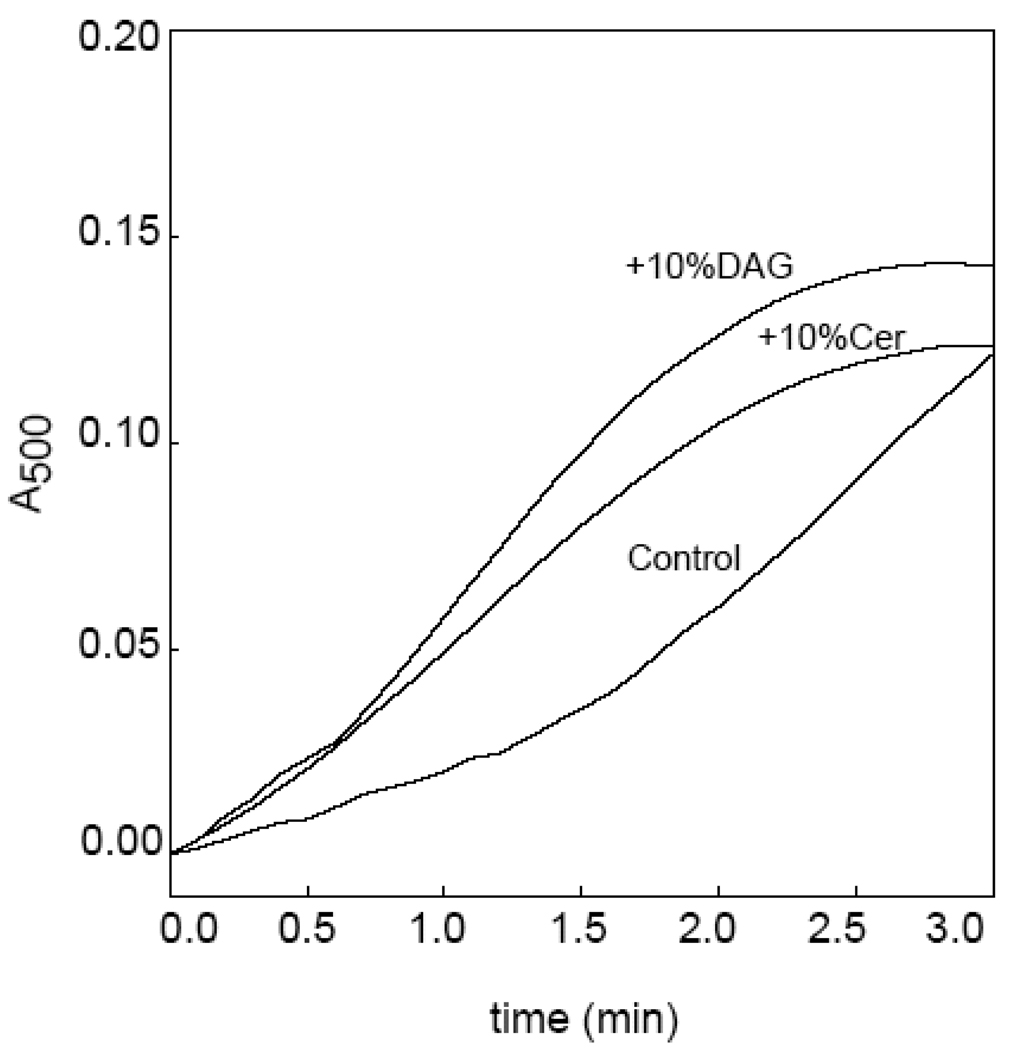
PC and SM hydrolysis by PlcHR2 leads to vesicle aggregation. Lipid hydrolysis and vesicle aggregation progress in parallel with this [10] and other lipases [27, 28]. Aggregation is assayed as an increase in light scattering by the vesicle suspension [18], thus light scattering measurements provide a convenient method to measure lag times. Time-courses of enzyme-induced LUV aggregation are shown in Fig. 4. It is clear that both Cer and DAG reduce greatly the latency period after which maximum aggregation rates are observed. Aggregation lag times for the four populations of LUV under study are given in Table 1. There is a very good parallelism between enzyme affinity for the vesicles (data from experiments in Fig. 2) and lag times (data from experiments in Fig. 3). We conclude that PlcHR2 behaves as shown previously for Bacillus PC-PLC [27]: the latency period is decreased when a small proportion of the end-product is present from the beginning of the enzyme assay. Moreover, the combined observations of Fig. 2 and Fig. 4 indicate that the transition from the slow to the fast regime of enzyme activity is accompanied (caused) by the increased binding of enzyme. Note also the lack of correlation between lag times (Table 1) and maximum enzyme activity (Fig. 3). A ceramide-increased binding of B. cereus sphingomyelinase and subsequent enhancement of the pre-catalytic steps (lag time shortening) was thoroughly described and kinetically analysed by Fanani et al. [29] working on lipid monolayers at the air-water interface.
Fig. 4.
Time-course PlcHR2-induced LUV aggregation, for the three different lipid compositions. Aggregation is assayed as an increase in suspension turbidity (A500). A latency period is clearly observed for the control vesicles.
The rates and extents of LUV aggregation induced by PlcHR2 are shown in Fig. 5. The maximum rates (slopes) appear to be independent of lipid composition, the increase observed for the mixture containing DAG being statistically non-significant. The extents of aggregation after 5 min, when an apparent equilibrium has been reached, follow a somewhat similar pattern as the corresponding data for lipid hydrolysis (Fig. 3B), ceramide inhibition being the main result. A ceramide-dependent decrease in enzyme activity was also found by Fanani et al. [29] for B. cereus sphingomyelinase. The data in Fig. 5 confirm a trend that has been observed previously for other PLC´s, namely that aggregation appears to be a direct effect of lipid cleavage.
Fig.5.
PlcHR2-induced LUV aggregation. (A) Maximum slopes. (B) Extent of aggregation (A500) after 5 min, under apparent steady-state conditions. Average values ± S.D. (n=3–4). The asterisk denotes the results of the Student´s t-test. (*)0.01<p<0.05; (**) 0.001<p<0.01; (***) P<0.001.
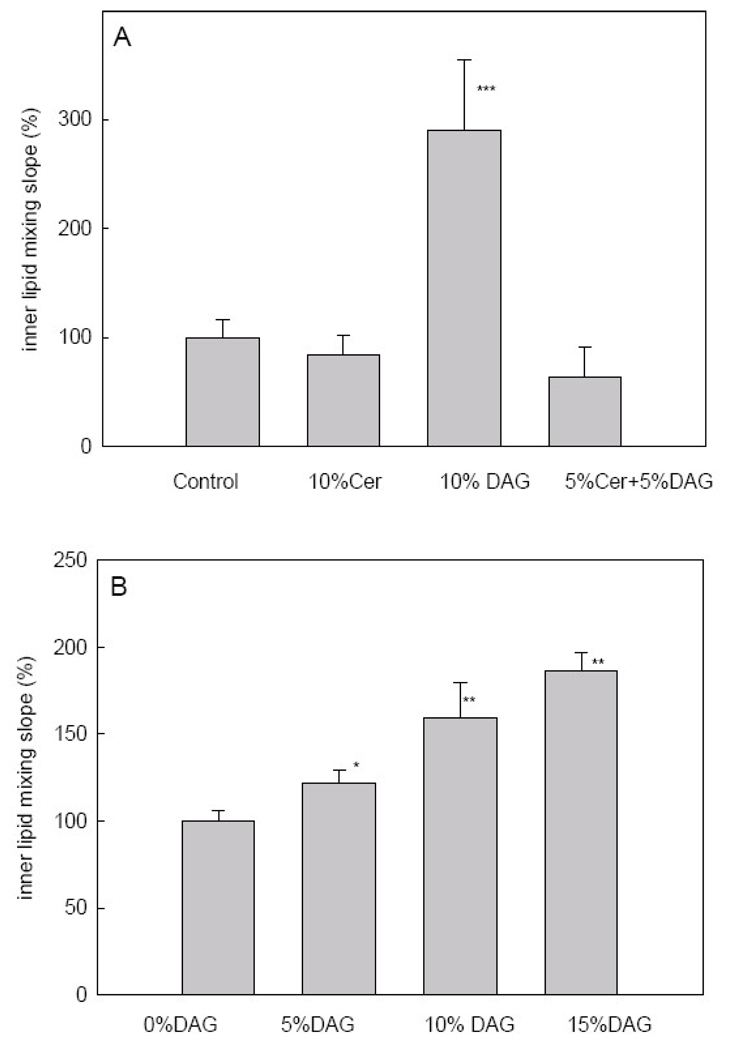
As shown for other PLC´s [12, 28, 30–32] enzyme-induced LUV aggregation may lead, under certain circumstances, to vesicle fusion. This is also the case for PlcHR2, as shown by Montes et al. [18] and in Fig. 1. Among the various available fusion assays, the inner monolayer lipid mixing assay is particularly specific for intervesicular fusion, as distinct from hemifusion [18, 28]. Results for vesicles containing DAG and/or Cer are summarized in Fig. 6A. Only maximum slopes are given, because in this kind of assay the total extent of fusion cannot be reliably ascertained. As shown in Fig. 6A, 10%DAG increases significantly the fusion rate, while Cer, or DAG + Cer mixtures are not effective. The data in Fig. 6B show that the enhancing effect of DAG is dose – dependent. This confirms our previous observations [2, 11, 12, 32–35] that DAG, but not Cer, is a potent fusogen.
Fig. 6.
PlcHR2-induced intervesicular mixing of inner monolayer lipids. (A) Maximum slopes for the various lipid compositions. (B) Dose-response effect of DAG. Average values ± S.D. (n=3). The asterisk denotes the results of the Student´s t-test. (*)0.01<p<0.05; (**) 0.001<p<0.01; (***) P<0.001.
Previous studies have pointed out the mutual modulation of phospholipase C and sphingomyelinases when these enzymes are added separately to a lipid bilayer [32, 36]. In our case, we have both activity associated to a single protein, but the interplay of bilayer physical properties and enzyme activities is probably very similar. In our former study with PlcHR2 [18] we described that the catalytic activity of the enzyme, that cleaves PC and SM at about the same rates, induced vesicle fusion in the absence of vesicular leakage. In the present contribution we have explored in further detail the effects of the enzyme end-products DAG and Cer. From the various assays of enzyme activity and enzyme-induced vesicle aggregation and fusion we conclude that both DAG and Cer decrease the lag times of lipid hydrolysis, that Cer inhibits the PlcHR2 hydrolytic activity, and that DAG increases fusion rates. The observed decrease in lag times confirms our previous observation that, in PLC´s, the latency period is used to accumulate a certain amount of lipid end-product, and that the lag time can be substantially decreased if the corresponding end-product is already present in the bilayer at the time of enzyme addition [27]. Cer inhibition of the enzyme hydrolytic activity appears to be due mainly to lateral segregation of rigid SM-Cer domains [22] thus to a decrease in accessible substrate. Moreover, activation of fusion by DAG is probably related to the ability of DAG to give rise to the inverted non-lamellar phases whose structure is reproduced in the fusion intermediate “stalk” [1, 11, 13].
Whether the mechanisms demonstrated in our model system would also be applicable to cell membranes remains an open question. The observed effects, including gel-phase separations, that require a relatively high concentration of enzyme end-products could indeed exist, although certainly limited in space and time to a particular region (nanodomain?) of the membrane, and for a short time. However, the current technologies are still unable to detect these small, transient domains in an unambiguous way.
Finally, the observations reported herein that ceramide formation inhibits PlcHR2 hydrolitic activity, seems to be counter intuitive in the context of the recently reported biological activities [37] associated with this protein. That is, the cytotoxicity of PlcHR2 appears to be mediated primarily by its sphingomyelinase activity, rather than its PC-PLC activity. This is the consequence of the fact that PlcHR2 induces a calcium signalling-mediated apoptosis in the endothelial cells, which strongly suggest that its cytotoxicity is mediated by the generation of ceramide, rather through the generation of DAG. On the other hand, this interpretation may be overly simplistic since PlcHR2, besides being PC-PLC and a SMase, also has sphingomyelin synthase activity [38]. That is it can remove the phosphorylcholine head group of PC, then transfer it to ceramide, resulting in a net synthesis of sphingomyelin. Accordingly, further studies of the biochemistry and biophysics as described this report may help in dissecting the mechanisms by which very low concentrations of PlcHR2 (i. e. picomolar) are selectively cytotoxic to endothelial cell and the mechanisms by which this protein causes the hemolysis of sheep erythrocytes, which have virtually no PC in their outer leaflet of their membranes, which in terms of their lipids are essentially composed only of shimgomyelin [39].
Acknowledgements
This work was supported in part by grants from Spanish Ministerio de Educación y Ciencia (BFU 2008-01637/BMC) (A. A.), (BFU 2007-62062) (F. M. G.), the Basque Government (IT461-07) (F. M. G.), ETORKET (07/26) (A. A.) and NIH (HL062608) (M. L. V.). M. I. was a graduate student supported by the Basque government.
Abbreviations
- Cer
egg ceramide
- DAG
diacylglycerol
- LUV
large unilamellar vesicles
- NBD-PE
N-(7-nitrobenzen-2-oxa-1,3-diazol-4-ol)-phsophatidylethanolamine
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PLC
phospholipase C
- PlcHR2
phospholipase C/sphingomyelinase from Pseudomonas aeruginosa
- Rho-PE
rhodamine phosphatidylethanolamine
- SM
sphingomyelin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chernomordik L, Chanturiya A, Green J, Zimmerberg J. The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys J. 1995;69:922–929. doi: 10.1016/S0006-3495(95)79966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goñi FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res. 1999;38:1–48. doi: 10.1016/s0163-7827(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 3.Nieva JL, Alonso A, Basanez G, Goñi FM, Gulik A, Vargas R, Luzzati V. Topological properties of two cubic phases of a phospholipid:cholesterol:diacylglycerol aqueous system and their possible implications in the phospholipase C-induced liposome fusion. FEBS Lett. 1995;368:143–147. doi: 10.1016/0014-5793(95)00631-i. [DOI] [PubMed] [Google Scholar]
- 4.Basáñez G, Nieva JL, Rivas E, Alonso A, Goñi FM. Diacylglycerol and the promotion of lamellar-hexagonal and lamellar-isotropic phase transitions in lipids: implications for membrane fusion. Biophys J. 1996;70:2299–2306. doi: 10.1016/S0006-3495(96)79795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goñi FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46. doi: 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- 6.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50 Suppl:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahyayauch H, Villar AV, Alonso A, Goni FM. Modulation of PI-specific phospholipase C by membrane curvature and molecular order. Biochemistry. 2005;44:11592–11600. doi: 10.1021/bi050715k. [DOI] [PubMed] [Google Scholar]
- 8.Bailey RW, Olson ED, Vu MP, Brueseke TJ, Robertson L, Christensen RE, Parker KH, Judd AM, Bell JD. Relationship between membrane physical properties and secretory phospholipase A2 hydrolysis kinetics in S49 cells during ionophore-induced apoptosis. Biophys J. 2007;93:2350–2362. doi: 10.1529/biophysj.107.104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stonehouse MJ, Cota-Gomez A, Parker SK, Martin WE, Hankin JA, Murphy RC, Chen W, Lim KB, Hackett M, Vasil AI, Vasil ML. A novel class of microbial phosphocholine-specific phospholipases C. Mol Microbiol. 2002;46:661–676. doi: 10.1046/j.1365-2958.2002.03194.x. [DOI] [PubMed] [Google Scholar]
- 10.Montes LR, Goñi FM, Johnston NC, Goldfine H, Alonso A. Membrane fusion induced by the catalytic activity of a phospholipase C/sphingomyelinase from Listeria monocytogenes. Biochemistry. 2004;43:3688–3695. doi: 10.1021/bi0352522. [DOI] [PubMed] [Google Scholar]
- 11.Nieva JL, Alonso A, Basáñez G, Goñi FM, Gulik A, Vargas R, Luzzati V. Topological properties of two cubic phases of a phospholipid:cholesterol:diacylglycerol aqueous system and their possible implications in the phospholipase C-induced liposome fusion. FEBS Lett. 1995;368:143–147. doi: 10.1016/0014-5793(95)00631-i. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Argüello MB, Basáñez G, Goñi FM, Alonso A. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem. 1996;271:26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- 13.Basáñez G, Ruiz-Arguello MB, Alonso A, Goñi FM, Karlsson G, Edwards K. Morphological changes induced by phospholipase C and by sphingomyelinase on large unilamellar vesicles: a cryo-transmission electron microscopy study of liposome fusion. Biophys J. 1997;72:2630–2637. doi: 10.1016/S0006-3495(97)78906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 15.Frederik PM, Hubert DH. Cryoelectron microscopy of liposomes. Methods Enzymol. 2005;391:431–448. doi: 10.1016/S0076-6879(05)91024-0. [DOI] [PubMed] [Google Scholar]
- 16.Bottcher CSF, Van Gent CM, Pries C. A rapid sensitive submicro phosphorous determination. Anal. Chim. Acta. 1961;24:203–204. [Google Scholar]
- 17.Struck DK, Hoekstra D, Pagano RE. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- 18.Montes LR, Ibarguren M, Goñi FM, Stonehouse M, Vasil ML, Alonso A. Leakage-free membrane fusion induced by the hydrolytic activity of PlcHR(2), a novel phospholipase C/sphingomyelinase from Pseudomonas aeruginosa. Biochim Biophys Acta. 2007;1768:2365–2372. doi: 10.1016/j.bbamem.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Burger KN, Nieva JL, Alonso A, Verkleij AJ. Phospholipase C activity-induced fusion of pure lipid model membranes. A freeze fracture study. Biochim Biophys Acta. 1991;1068:249–253. doi: 10.1016/0005-2736(91)90216-u. [DOI] [PubMed] [Google Scholar]
- 20.Basáñez G, Nieva JL, Goni FM, Alonso A. Origin of the lag period in the phospholipase C cleavage of phospholipids in membranes. Concomitant vesicle aggregation and enzyme activation. Biochemistry. 1996;35:15183–15187. doi: 10.1021/bi9616561. [DOI] [PubMed] [Google Scholar]
- 21.Ahyayauch H, Villar AV, Alonso A, Goñi FM. Modulation of PI-specific phospholipase C by membrane curvature and molecular order. Biochemistry. 2005;44:11592–11600. doi: 10.1021/bi050715k. [DOI] [PubMed] [Google Scholar]
- 22.Sot J, Ibarguren M, Busto JV, Montes LR, Goñi FM, Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Barlic A, Gutierrez-Aguirre I, Caaveiro JM, Cruz A, Ruiz-Arguello MB, Perez-Gil J, Gonzalez-Manas JM. Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J Biol Chem. 2004;279:34209–34216. doi: 10.1074/jbc.M313817200. [DOI] [PubMed] [Google Scholar]
- 24.Sot J, Bagatolli LA, Goñi FM, Alonso A. Detergent-resistant, ceramide-enriched domains in sphingomyelin/ceramide bilayers. Biophys J. 2006;90:903–914. doi: 10.1529/biophysj.105.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Tullio L, Maggio B, Fanani ML. Sphingomyelinase acts by an area-activated mechanism on the liquid-expanded phase of sphingomyelin monolayers. J Lipid Res. 49;2008:2347–2355. doi: 10.1194/jlr.M800127-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Fanani ML, De Tullio L, Hartel S, Jara J, Maggio B. Sphingomyelinase-induced domain shape relaxation driven by out-of-equilibrium changes of composition. Biophys J. 2008 doi: 10.1529/biophysj.108.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basánez G, Nieva JL, Goñi FM, Alonso A. Origin of the lag period in the phospholipase C cleavage of phospholipids in membranes. Concomitant vesicle aggregation and enzyme activation. Biochemistry. 1996;35:15183–15187. doi: 10.1021/bi9616561. [DOI] [PubMed] [Google Scholar]
- 28.Villar AV, Alonso A, Goñi FM. Leaky vesicle fusion induced by phosphatidylinositol-specific phospholipase C: observation of mixing of vesicular inner monolayers. Biochemistry. 2000;39:14012–14018. doi: 10.1021/bi992515c. [DOI] [PubMed] [Google Scholar]
- 29.Fanani ML, Maggio B. Kinetic steps for the hydrolysis of sphingomyelin by Bacillus cereus sphingomyelinase in lipid monolayers. J Lipid Res. 2000;41:1832–1840. [PubMed] [Google Scholar]
- 30.Nieva JL, Goñi FM, Alonso A. Liposome fusion catalytically induced by phospholipase C. Biochemistry. 1989;28:7364–7367. doi: 10.1021/bi00444a032. [DOI] [PubMed] [Google Scholar]
- 31.Villar AV, Goñi FM, Alonso A. Diacylglycerol effects on phosphatidylinositol-specific phospholipase C activity and vesicle fusion. FEBS Lett. 2001;494:117–120. doi: 10.1016/s0014-5793(01)02333-x. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Argüello MB, Goñi FM, Alonso A. Vesicle membrane fusion induced by the concerted activities of sphingomyelinase and phospholipase C. J Biol Chem. 1998;273:22977–22982. doi: 10.1074/jbc.273.36.22977. [DOI] [PubMed] [Google Scholar]
- 33.Nieva JL, Goñi FM, Alonso A. Phospholipase C-promoted membrane fusion. Retroinhibition by the end-product diacylglycerol. Biochemistry. 1993;32:1054–1058. doi: 10.1021/bi00055a009. [DOI] [PubMed] [Google Scholar]
- 34.Walter A, Yeagle PL, Siegel DP. Diacylglycerol and hexadecane increase divalent cation-induced lipid mixing rates between phosphatidylserine large unilamellar vesicles. Biophys J. 1994;66:366–376. doi: 10.1016/s0006-3495(94)80786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel DP. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984;45:399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidsen J, Jorgensen K, Andresen TL, Mouritsen OG. Secreted phospholipase A(2) as a new enzymatic trigger mechanism for localised liposomal drug release and absorption in diseased tissue. Biochim Biophys Acta. 2003;1609:95–101. doi: 10.1016/s0005-2736(02)00659-4. [DOI] [PubMed] [Google Scholar]
- 37.Vasil ML, Stonehouse MJ, Vasil AI, Wadsworth SJ, Goldfine H, Bolcome RE, 3rd, Chan J. A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells. PLoS Pathog. 2009;5:e1000420. doi: 10.1371/journal.ppat.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luberto C, Stonehouse MJ, Collins EA, Marchesini N, El-Bawab S, Vasil AI, Vasil ML, Hannun YA. Purification, characterization, and identification of a sphingomyelin synthase from Pseudomonas aeruginosa. PlcH is a multifunctional enzyme. J Biol Chem. 2003;278:32733–32743. doi: 10.1074/jbc.M300932200. [DOI] [PubMed] [Google Scholar]
- 39.Montes LR, Lopez DJ, Sot J, Bagatolli LA, Stonehouse MJ, Vasil ML, Wu BX, Hannun YA, Goni FM, Alonso A. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry. 2008;47:11222–11230. doi: 10.1021/bi801139z. [DOI] [PMC free article] [PubMed] [Google Scholar]