ABSTRACT
Rectal cancer staging provides critical information concerning the extent of the disease. The information gained from staging is used to determine prognosis, to guide management, and to assess response to therapy. Accurate staging is essential for directing the multidisciplinary approach to therapy. This article focuses on the evolution of staging systems, the rational for staging, and current methods used to stage rectal cancer.
Keywords: Rectal cancer, staging, endorectal ultrasound, magnetic resonance imaging, positron emission tomography, computed tomography
In the United States, colorectal cancer is the third most common malignancy with ~41,420 new cases estimated for 2007.1 Rectal cancer staging provides information about the extent of disease. The information gained from staging is used to determine prognosis, guide management, and assess response to therapy. This article focuses on the evolution of staging systems, the rationale for staging, and current methods used to stage rectal cancer.
The following classification of cancer of the rectum is of interest in relation to the general pathology of malignant disease and has proved of value in the prognosis of rectal cancer.2
Cuthbert E. Dukes
Pathologist to St. Mark's Hospital
1932
EVOLUTION OF RECTAL CANCER STAGING
Rectal cancer staging has had a long evolution. In 1926, Lockhart-Mummery3,4 proposed a staging system for rectal cancer. In this system, the depth of invasion and lymph node positivity detected in specimens removed at surgery were identified as important prognostic factors. In 1932, Dukes2 summarized current opinion, stating that in its earliest stages, rectal cancer begins as an epithelial proliferation rising from the surface and that carcinoma develops from a preexisting adenoma. The cancer metastasizes through the bowel wall to the lymphatics. Cases in which the carcinoma is limited to the wall of the rectum were designated A. Those in which the cancer has spread by direct continuity to the extrarectal tissue were designated B. Cases in which metastases are present in the regional lymph nodes were called C (Fig. 1). A more advanced pathologic stage was associated with a worse prognosis.
Figure 1.
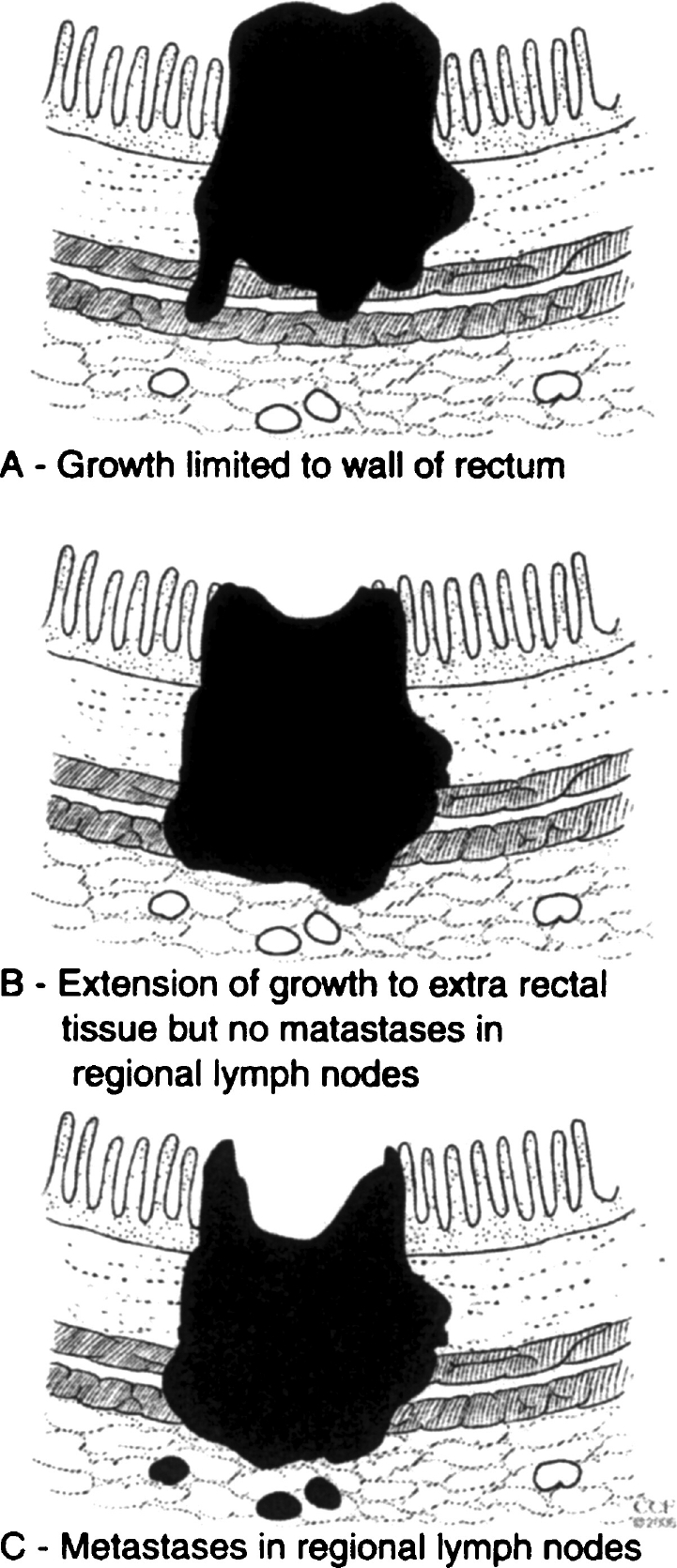
Rectal cancer staging system proposed by Dukes in 1932. (From the Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.)
In 1949, Kirklin, Dockerty, and Waugh5 proposed a modification of Dukes' classification. The authors preserved the A, B, C framework and added, for B lesions, the subscript designation “1” for lesions that have extended into, but not through the muscularis propria and “2” for tumors that have penetrated the muscularis propria. In 1954, Astler and Coller6 reported on specimens of the rectum and colon removed at surgery and classified them using Dukes' classification as modified by Kirklin et al.5 The modified Astler–Coller (MAC) classification is listed below:
Type A—Lesions limited to the mucosa
Type B1—Lesions extending into the muscularis propria, but not penetrating it, with negative nodes
Type B2—Lesions penetrating the muscularis propria, with negative nodes
Type C1—Lesions extending into the muscularis propria, but not penetrating it, with positive nodes
Type C2—Lesions penetrating the muscularis propria with positive nodes
The yearly survival of patients demonstrated a progressive decline proportional to the depth of penetration of the colonic wall. Lymph node involvement decreased the 5-year survival rate even more drastically. In 1963, Turnbull et al7 designated stage D to identify tumors that have metastasized to the liver, lung, bone, parietes, and adjacent organs. In addition, the histologic grade of each tumor was recorded even though it was not included in pathologic staging.
In 1987, the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (IUCC) introduced a cancer staging system based on local tumor depth of invasion (T), the presence and number of nodal metastases (N), and the presence of distant metastases (M).8 The 2002-updated TNM criteria for colorectal cancer shown in Tables 1A,B are used most commonly by clinicians today.9,10 The influence of earlier staging classifications on the TNM staging systems is unmistakable.
Table 1A.
Tumor, Node, Metastases (TNM) Classification of Colorectal Cancer
| T-Primary tumor |
| Tx Primary tumor cannot be assessed |
| T0 No evidence of primary tumor |
| Tis Carcinoma in situ: intraepithelial or invasion of lamina propria |
| T1 Tumor invades submucosa |
| T2 Tumor invades muscularis propria |
| T3 Tumor invades through muscularis propria into subserosa or into non-peritonealized pericolic or perirectal tissues |
| T4 Tumor directly invades other organs or structures and/or perforates visceral peritoneum |
| N-Regional lymph nodes |
| NX Regional lymph nodes cannot be assessed |
| N0 No regional lymph node metastasis |
| N1 Metastasis in one to three regional lymph nodes |
| N2 Metastasis in four or more regional lymph nodes |
| M-Distant metastasis |
| MX Distant metastasis cannot be assessed |
| M0 No distant metastasis |
| M1 Distant metastasis |
Table 1B.
Stage Grouping
| Stage | T | N | M | Dukes | MAC |
|---|---|---|---|---|---|
| 0 | Tis | N0 | M0 | — | — |
| I | T1 | N0 | M0 | A | A |
| T2 | N0 | M0 | A | B1 | |
| IIA | T3 | N0 | M0 | B | B2 |
| IIB | T4 | N0 | M0 | B | B3 |
| IIIA | T1, T2 | N1 | M0 | C | C1 |
| IIIB | T3, T4 | N1 | M0 | C | C2/C3 |
| IIIC | Any T | N2 | M0 | C | C1/C2/C3 |
| IV | Any T | Any N | M1 | — | D |
Unlike the 1932 Duke's staging system, which is based on histopathology alone, the TNM staging system permits assessment of T, N, and M categories by other clinical criteria including physical examination, imaging, endoscopy, and/or surgical exploration. Previously, information gained from pathologic staging was used primarily to determine prognosis. Current rectal cancer staging has assumed additional roles, namely, the determination of optimal therapy and assessment of response to therapy.
RATIONALES FOR RECTAL CANCER STAGING
The modern treatment of rectal cancer is multidisciplinary and involves a cooperative effort between medical oncologists, radiation therapists, endoscopists, radiologists, and surgeons. Information concerning the extent of disease guides therapy. Decisions made based on staging information include curative versus palliative operation, radical versus local excision, preoperative chemoradiation therapy, and postsurgical adjuvant therapy.11,12,13,14
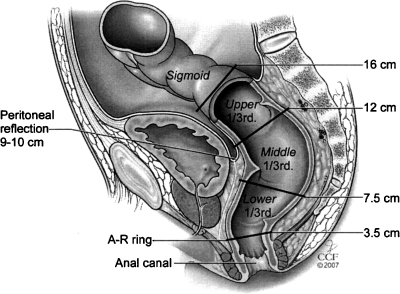
Accurate information about tumor anatomy is particularly important. The rectum extends proximally from the rectosigmoid junction where the taenia coli coalesce distally to the anorectal ring.15 Goligher et al16 divided the rectum into thirds beginning at the anorectal ring at 3.5 cm: lower rectum 3.5 cm to 7.5 cm; midrectum 7.5 cm to 12 cm; upper rectum 12 cm to 16 cm (Fig. 2). The most proximal rectum is intraperitoneal. The middle and lower rectum is partially or wholly extraperitoneal. Cancers of the upper rectum (above 12 cm) behave like colon cancers with regard to recurrence.17 For upper third lesions, resection with partial mesorectal excision generally is accepted surgical therapy.18,19 Treatment for middle and lower third rectal cancers in the absence of distant metastases varies. Proctosigmoidectomy with total mesorectal excision (TME) is recommended in most cases.20 However, local excision may be appropriate for selected early distal rectal cancers.21,22 Chemoradiation may be given preoperatively or postoperatively.
Figure 2.
Diagrammatic representation of rectal thirds as defined by sigmoidoscopic distance from the anal canal (after Goligher et al16). (From the Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.)
In 1990, the National Institutes of Health Consensus Conference recommended the use of combined postoperative chemotherapy and radiation therapy to improve local control and survival in stage II and III patients.23 Patients with cancers of the middle and lower rectum and preoperative evidence of locally advanced disease (T4, T3, N) are considered candidates for preoperative neoadjuvant therapy.24,25 Because the pathologic stage is not known with certainty in this situation, overtreatment is possible. It is desirable, therefore, to choose tests that can estimate the stage as accurately as possible. Patients found to have distant metastatic disease on initial staging receive individualized care programs that may or may not involve surgery.
STAGING INVESTIGATIONS
Physical examination, endoscopy, and imaging are used to define local tumor characteristics and to identify distant disease. Locally, information is sought concerning tumor location, depth of invasion, lymph node positivity, proximity to neighboring structures, integrity of the mesorectal envelope,26 lateral margin,27 local peritoneal involvement by tumor,28 and venous invasion.29 These features have prognostic significance and can influence management decisions. Investigations intended to detect distant disease are directed routinely to common metastatic sites such as the liver and selectively to additional sites based on symptoms and physical findings.
With digital rectal exam (DRE), the size, location, and degree of fixation of most low and some middle third rectal tumors can be detected and assessed. Assessment of the extent of local disease by DRE is imprecise, however, and provides only a rough estimate of local rectal cancer stage.30,31 Endoscopy is done not only to detect the tumor and obtain tissue for histologic diagnosis, but also to assess tumor dimensions, distance from the anal sphincter, and relation to landmarks such as the prostate or vagina with a view toward future surgery. The entire large intestine is examined by colonoscopy, proctosigmoidoscopy combined with contrast enema, or computed tomography (CT) colonography to exclude synchronous lesions.32
Carcinoembryonic antigen (CEA), if elevated, can be followed after treatment to detect recurrent disease.33,34 Chest films are done to screen for pulmonary metastatic disease. Computed tomography (CT) of the abdomen and pelvis provides information concerning the primary tumor, its relation to neighboring pelvic structures and intraabdominal metastases, especially involving the liver. Although conventional CT is accurate in identifying invasion of neighboring structures, it is not able to demonstrate the layers of the rectal wall accurately. This drawback limits the role of conventional CT in the local determination of rectal cancer stage.35 Investigations currently best suited for the determination of T classification and N status are endorectal ultrasound (EUS) and magnetic resonance imaging (MRI).
Endorectal Ultrasound
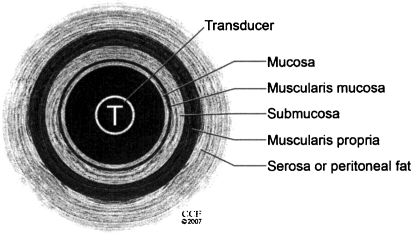
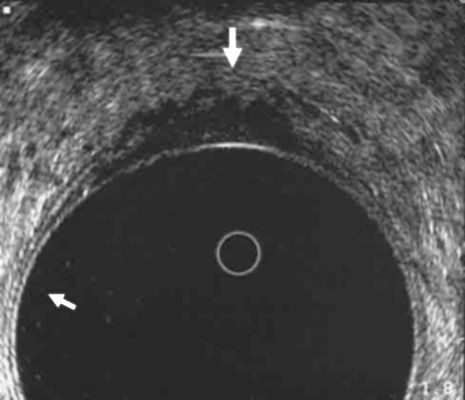
EUS is commonly used to determine the extent of local wall penetration for middle- and lower-third rectal cancers. Examination is performed using a rotating endosonic transducer, which is placed directly over the tumor with an intervening water acoustic interface. Orrom and colleagues36 described a five-layer model of the rectal wall as seen by ultrasound with three echogenic (white lines) and two echo-poor (dark lines) as shown in Fig. 3. The ultrasonic staging system is based on depth of invasion and lymph node positivity. An example of a uT3 lesion is shown in Fig. 4.
Figure 3.
Five-layer model for the interpretation of endorectal ultrasound. Three echogenic (white lines) and two echo-poor (dark lines) are seen (after Orrom et al36). (From the Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.)
Figure 4.
Endorectal ultrasound of a uT3 rectal cancer. The outer black line is breached. The scalloped pattern of the outer tumor edge is consistent with penetration of the muscularis mucosa (large arrow). Note the five layers of the normal rectal wall (small arrow). (From the Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.)
EUS is advantageous in that the exam can be done as an office procedure with minimal preparation. However, there are several disadvantages: During the exam, the transducer must be held at a right angle to the tumor, which may not be possible to achieve, especially for upper rectal or stenotic tumors37; EUS does not completely assess the mesorectum or the mesorectal envelope; the ability of EUS to detect lymph nodes is only fair; and interpretation of EUS images depends on the experience of the operator.
The accuracy of EUS in predicting pathologic stage is defined best by large studies. In 2004, Kauer38 described 458 patients with rectal cancer in whom the EUS stage was compared with the pathologic stage. The overall rate for correctly classified patients was 69% with respect to the T category and 68% with respect to the N category. The T3 category tumors were the most accurately identified (86%), and the T4 tumors were the least accurately identified (36%). Tumor overstaging (19%) was more frequent that understaging (12%). A high interobserver variability was noted. For pathologic stage T1 tumors, the 10-MHz scan was almost 2 times more accurate that the 7.5-MHz scan (71% versus 36%). The authors concluded that the accuracy of EUS staging of rectal carcinoma depends on the T-category. They also found that a high-resolution scanner and an experienced examiner give the best results, especially for early carcinoma.
In 2005, Knaebel et al39 described 424 patients undergoing EUS for rectal cancer who underwent surgery. They compared the preoperative EUS findings with postoperative pathology and found a T category accuracy of 81% and an N category accuracy of 76%. In a second series of an additional 332 patients with rectal tumors (including adenomas), the authors found that endosonography was most accurate when done by experienced individuals and that EUS is inaccurate when used for staging in patients who have undergone chemoradiation.
In 2005, Harewood40 performed a MEDLINE search for all published estimates of EUS accuracy in staging rectal cancer between 1985 and 2003. Two-hundred two abstracts were reviewed. The EUS findings of 4118 subjects were reported with an overall mean T-staging accuracy of 85% and N-staging accuracy of 75%. There was a paucity of smaller studies expressing low EUS accuracy rates. The accuracy rates both of T-staging and N-staging declined over time with the lowest rates reported in more recent literature. The author concluded that the performance of EUS in staging rectal cancer may be overestimated in the literature due to publication bias and that this inflated estimate of the capability of EUS may lead to unrealistic expectations for this technology.
In 2006, Ptok et al41 studied the feasibility and accuracy of EUS in the pretreatment staging of rectal cancer. Overall, 13,610 patients with rectal cancer were enrolled in the study. Five thousand fifty-six subjects (37%) underwent EUS. In 3501 patients, EUS findings (uT-stage) would be compared with the definitive histologic investigation (pT-stage). EUS accuracy in all T categories was 66%. The highest sensitivity was achieved in the T3 category (75%); for T2, T1, and T4, it was 59%, 59%, and 31%, respectively. In this large study, successful completion of EUS and diagnostic accuracy were not as good as that reported in the earlier studies.
In 2004, Shami et al42 described the use of EUS-guided fine-needle aspiration (FNA) biopsy in patients with rectal cancer and its effect on rectal cancer staging. A total of 21 EUS-guided FNA biopsies were done. Of 16 patents with non-juxtatumoral lymph nodes detected by EUS who underwent EUS-guided FNA, cytology was negative in 4 patients. Based on these negative FNA findings, 3 patients went directly to surgery instead of to neoadjuvant chemotherapy and were found to be negative for lymph node metastases. Thus, EUS-guided FNA changed management in 3 of 16 (19%) of patients deemed node-positive by EUS alone. All five cases of recurrent carcinoma were correctly diagnosed by fine-needle aspiration.
In 2002 Valentini et al43 used EUS to help determine whether downstaging after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer predicts improved outcome. One hundred sixty-five patients with locally advanced extraperitoneal cancer of the rectum received preoperative chemoradiation. Four to 5 weeks after chemoradiation, patients were restaged by EUS. The authors found that the clinical response and tumor/nodal pathologic downstaging showed a close correlation with improved outcomes. The 5-year survival and local control was better in the patients with T0 to T2 tumors. The cancer responded in a manner that was similar to rectal cancer diagnosed at clinical stage T1 to T2.
MAGNETIC RESONANCE IMAGING
Early MRI studies in the 1980s that attempted local rectal cancer staging were unable to define tumor T-stage or N-stage with high accuracy.44,45 The introduction of an endorectal coil positioned directly over the lesion improved the accuracy of rectal wall MRI and in some studies, the T category accuracy was comparable to that of EUS.
In 1994, Schnall et al46 correlated rectal cancers staged by endorectal coil MRI and pathologic findings in 36 patients. Endorectal coil MRI visualized all layers of the rectal wall in all patients. The MRI tumor stage agreed with pathologic findings in 29 of 36 (81%) of cases. Although MRI was able to detect lymph nodes as small as 2 mm, the specificity for N1 disease was only 72%. In 2002, Torricelli et al47 described 43 patients with rectal cancer evaluated by the endorectal coil MRI technique. In 14 of 43 patients, neoadjuvant therapy was administered. The tumor was detected by endorectal coil MRI in 38 of 43 patients. Tumor nondetection was associated with inability to position the endorectal probe over the tumor, motion artifacts, and patient intolerance of the examination. Overall, endorectal coil MRI findings agreed with the pathological results in 34 of 38 of patients (89%). MRI evaluation of perirectal lymph node metastases revealed N0 (17 cases), N1 (14 cases), and N2 (7 cases). The comparison of MRI nodal findings with pathology showed a 63% accuracy, 82% sensitivity, and 55% specificity.
In 2004, Akin et al48 prospectively assessed the accuracy of endorectal MRI imaging in the preoperative local staging of rectal cancer in 20 patients. MRI was able to determine accurately the T stage in 17 of these patients (85%). The authors noted no significant morphological or signal characteristics on endorectal MRI imaging that differentiated metastatic lymph nodes from normal or inflamed ones. When all lymph nodes that measured greater than 0.5 cm were considered metastatic, the sensitivity and specificity of MR imaging were 91% and 56%, respectively. When 1.0 cm was accepted as the upper limit for non-metastatic lymph nodes, the sensitivity decreased to 80%, but the specificity increased to 70%.
In 2006, Tatli et al49 described 51 patients with rectal cancer who underwent combined preoperative endorectal and pelvic phased-array coil MRI and surgical resection. Overall, MRI-based staging was identical to pathology-based T staging in 45 of 51 patients (88%). MRI correctly identified 14 of 15 (93%) T3 tumors with a sensitivity of 93% and a specificity of 86%. MRI correctly predicted lymph node metastases in 11 of 13 patients with a sensitivity of 85% and a specificity of 69%. The authors concluded that combined endorectal and pelvic phased-array coil MRI was highly predictive in terms of excluding T3 tumors, but still had limitations in predicting lymph node metastases.
A disadvantage of the endorectal coil technique is the need for coil placement adjacent to the tumor, which may not be possible with stenotic tumors or tumors of the upper rectum. Improvements in MRI technology including phase arrayed multielement surface coils and advances in magnetic field gradients have enabled high-spatial-resolution, high-contrast-resolution scanning, which provides information similar to that obtained with an endorectal coil.50
In 2000, Kim et al51 described 217 patients with histologically proven rectal cancer staged preoperatively by MRI, which accurately determined the depth of invasion in 176 of 217 patients (81%) and regional lymph node invasion in 110 of 217 patients (63%). MRI also predicted levator ani muscle tumor involvement in 8 of 11 patients (72%) and metastatic lateral pelvic lymph nodes in 4 of 14 patients (29%), who had undergone lateral pelvic lymph node dissection. The authors of this study cited the advantages of MRI, including the fact that its accuracy for determining the depth of tumor invasion is comparable with that of EUS. They also noted that it provides clear imaging of the cancer and adjacent structures in multiple planes and can demonstrate lateral pelvic lymph node and levator ani invasion.
In 2003, Brown et al52 reported on 98 patients undergoing TME for biopsy-proven rectal cancer. These patients were assessed preoperatively using high-resolution MRI for T and N, depth of extramural tumor spread, the presence or absence of extramural venous invasion, a threatened circumferential resection margin, and serosal involvement at or above the peritoneal reflection. MRI assessment of these prognostic factors was compared with the histopathologic findings in matched whole-mount sections of the resection specimen. Overall, there was a 94% weighted agreement (weighted κ = 0.67) between MRI and pathology assessment of T stage. Ten patients were understaged for the T component of the TMN classification and 13 patients were overstaged. Nearly all disagreements in staging were between T1 and T2 tumors and between T2 and T3 tumors. Forty of 98 patients had positive lymph nodes on histological examination and 33 of these were correctly identified by MRI. Conversely 50 of 58 node-negative specimens were correctly identified by MRI. Although tumor involvement of small veins was not discernable using MRI, large extramural venous invasion was identified correctly in 15 of 18 patients. Seven of 9 patients with peritoneal perforation by tumor (T4) were identified correctly using MRI.
Martling et al53 used MRI to assess the prognostic impact of an involved circumferential resection margin (CRM) in patients with rectal cancer. Preoperative MRI was performed on 115 patients with rectal cancer. The shortest distance from the tumor to the CRM was measured, compared with histopathologic findings, and correlated with patient outcome. The risk of any recurrence in patients with or without a tumor-involved margin on MRI was 9 of 29 and 9 of 57 patients, respectively (p = 0.036). Overall survival at 5 years was 43% and 77%, respectively (p = 0.012). Twenty-four of 30 patients who had an involved CRM on histopathology were correctly identified by MRI.
It is important to note that the endorsement of MRI for rectal cancer T- and N-staging is not entirely positive. In 2004, Branagan et al54 described 40 patients with rectal cancer who were staged preoperatively using MRI. These authors found that preoperative MRI scans provided poor predictive data as to subsequent pathologic tumor and node stage. However, MRI did correctly report the status of the CRM margin in 39 patients; one was understaged. MRI findings of upper rectal cancers also can influence management.
In 2006, Burton et al55 assessed 75 patients with distal sigmoid, rectosigmoid, and upper rectal tumors preoperatively by MRI. If the tumor extended beyond the planned surgical resection plane, then chemoradiation was offered. Fifty-seven patients underwent primary surgery. In this group, the T-stage agreement was 63%. Nodal staging by MRI criteria was accurate in 65% of cases (37 of 57). The other 18 patients underwent neoadjuvant therapy for poor prognostic features with predicted surgical resection margin involvement (T4 invading adjacent organs and/or potentially CRM positive).
Following chemoradiation therapy the accuracy of MRI may decrease. In a 2005, Kuo et al56 reported on 36 patients with locally advanced rectal cancer who underwent neoadjuvant therapy followed 6 to 8 weeks later by radical surgery. Preoperative MRI findings were compared with the histopathologic staging. T-level downstaging occurred in 10 of 36 patients (28%), and N-level downstaging occurred in 29 of 36 patients (80%) after completion of chemoradiation therapy.
In 2005, Chan et al57 evaluated the prognostic value of the posttreatment TNM stage as a predictor of outcome in locally advanced rectal cancer treated with preoperative chemoradiotherapy (CRT). One hundred twenty-eight patients with tethered (103) or fixed (25) rectal cancers were treated with combined preoperative CRT therapy. Postoperatively, the patients underwent post-CRT staging. The pretreatment tumor status (fixed versus tethered), the post-CRT TNM stage, T-category downstaging, pathologic T4 tumors, node-positive disease after CRT, and lymphovascular or perineural invasion all were found to be statistically significant prognostic indicators of disease-specific survival and relapse-free survival. In multivariate analysis, the post-CRT TNM stage was the most statistically significant independent predictor of survival and relapse-free survival.
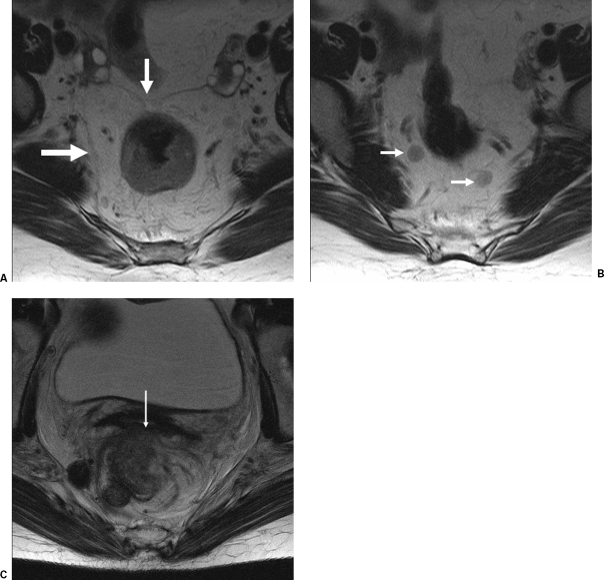
MRI scans demonstrating the circumferential mesorectal envelope, lymph nodes, and locally advanced disease are shown in Figs. 5A–C.
Figure 5.
(A) Magnetic resonance imaging (MRI) scan of a rectal cancer showing the circumferential mesorectal envelope. (B) MRI scan of the rectal cancer patient depicted in (A) showing lymph nodes. (C) MRI scan showing locally advanced rectal cancer that has invaded the posterior wall of the vagina (arrow). (From the Cleveland Clinic Foundation, Cleveland, Ohio. Reprinted with permission.)
Comparative Studies
In 2004, Brown et al58 compared the accuracy of DRE, EUS, and high-resolution MRI in staging 98 patients with rectal cancer who underwent resection. Agreement between the preoperative stage and pathology was 94% for MRI, 69% for EUS, and 65% for DRE. In addition, MRI gave better results than both DRE and EUS with regard to cost and clinical effectiveness. Kwok et al59 reviewed 83 studies from 78 papers that included 4,897 patients and compared methods used for preoperative staging of rectal cancer. They found that in determining the wall penetration of the tumor, the sensitivity for CT, EUS, MRI, and EMRI with endorectal coil were 78%, 93%, 86% and 89%, respectively. The specificity was 63%, 78%, 77% and 79%, respectively, and the accuracy was 73%, 87%, 82% and 84%, respectively. In determining nodal involvement by tumor, the sensitivity values for CT, EUS, MRI and MRI with endorectal coil were 52%, 71%, 65% and 82%, respectively. The specificity was 78%, 76%, 80% and 83%, respectively, and the accuracy was 66%, 74%, 74% and 82%, respectively. The authors concluded that MRI with an endorectal coil was the single investigation that most accurately predicted pathological stage in rectal cancer.
NEW TECHNIQUES
Positron Emission Tomography
In 2004, Heriot et al60 studied 46 patients with advanced primary rectal cancer referred for consideration of adjuvant preoperative therapy who underwent a positron emission tomography (PET) scan. The PET findings then were compared with subsequent staging and actual management implemented to determine the appropriateness of the PET-induced changes noted on subsequent follow-up. The overall tumor stage was changed in 18 of 46 patients (39%). In 8 of 46 cases (17%), management was altered because of the PET study. The authors concluded that PET scanning appeared to accurately change the stage or appropriately alter the therapy in almost a third of patients with advanced primary rectal cancer.
In 2006, Gearhart et al61 examined the influence of 18F-fluorodeoxyglucose-(FDG-) PET/CT on the initial evaluation of primary rectal cancer staging. Thirty-seven patients with a previously untreated rectal cancer underwent EUS or MRI, spiral CT, and FDG-PET/CT. Discordant findings between spiral CT and FDG-PET/CT were confirmed by histological analysis or follow-up imaging. FDG-PET/CT identified discordant findings in 14 patients (38%) and resulted in upstaging in 7 patients (50%) and downstaging of 3 patients (21%). Discordant PET/CT findings were significantly more common in the patients with a low rectal cancer than in those with middle or high rectal cancer. The most common discordant finding was lymph node metastasis. Histologic confirmation of discordant FDG-PET/CT findings was performed in 7 patients, and in no case did FDG-PET/CT prove to be inaccurate. Discordant PET/CT findings resulted in a deviation in the proposed treatment plan in 27% of patients (n = 10). The authors concluded that FDG-PET/CT frequently yields additional staging information in patients with low rectal cancer, and the improved accuracy of pretreatment imaging with FDG-PET/CT will allow for more appropriate stage-specific therapy.
Metabolic Classification
In 2003, Walenta et al62 devised a metastasis and survival-related metabolic classification in human rectal carcinomas. Tissue concentrations of ATP, glucose, and lactate in viable tumor regions were measured at a microscopic level using imaging bioluminescence. In metastatic carcinomas, lactate levels were significantly higher and glucose levels significantly lower than those in nonmetastatic carcinomas. No patient had distant metastasis with tumor lactate levels below the population median of 8.0 μmol/g or glucose levels above 0.9μmol/g. The authors concluded that these results supported the hypothesis that lactate and glucose levels measured in human primary carcinomas may serve as early prognostic tools with regard to formation of metastasis and patient survival.
SUMMARY
Rectal cancer staging defines local and distant extent of disease. Postsurgical pathologic staging provides information on prognosis and may indicate the need for additional therapy. Pretreatment staging determines management. Accurate assessment of local T, N, and M categories is crucially important for determining the best stage-specific treatment. Current assessment techniques include physical examination, plain radiographs, CT, EUS, MRI, and MRI with endorectal coil. Digital rectal exam gives information concerning tumor location and fixation, but is not accurate for staging. Conventional CT detects local invasion of neighboring structures and distant metastases. High-resolution MRI images obtained in multiple planes with high soft tissue contrast resolution provides detailed information concerning the relation of the tumor to the circumferential resection margin and adjacent structures, extramural venous invasion, lateral pelvic lymphadenopathy, and peritoneal surface involvement by tumor. The two best tests to determine local tumor T-stage are EUS and MRI with endorectal coil. EUS has the advantages of a relatively low expense and convenience and remains a common first choice for local T staging of low and middle third rectal cancers. Disadvantages of EUS include limited sensitivity for detecting metastatic regional lymph nodes, inability to assess stenotic lesions or lesions of the upper rectum, results that are operator-dependent, and low accuracy for tumors that have received radiotherapy. Endorectal coil MRI can provide T-stage information that is comparable to EUS. However, its high cost compared with EUS may limit its routine use. Neither EUS nor MRI can predict nodal stage with great accuracy. High-resolution MRI with external phased array coils has increased the accuracy of tumor staging information.
Methods used in rectal cancer staging are continually improving. Early reports of additional techniques such as PET, FDG-PET/CT, EUS with lymph nodes biopsy, and innovative approaches to staging such as metabolic analysis appear to provide additional information that can influence management. Optimal rectal cancer staging continues to evolve. Further improvements of existing technologies and the discovery of new techniques that will better define the extent of disease and best treatment for rectal cancer will most certainly occur.
ACKNOWLEDGMENTS
Grateful acknowledgment is given to Charles M. O'Malley, M.D., and Joseph C. Veniero, M.D., Ph.D., of the Department of Radiology, Cleveland Clinic Foundation, for the selection and interpretation of MRI images. The author wishes to acknowledge Amy Moore, Cleveland Clinic Foundation, Department of Editorial Services, for her meticulous review of this manuscript; Joe Pangrace, Cleveland Clinic Foundation, Department of Medical Illustration, for his excellent drawings; and Joyce Balliet for her overall support of this project.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun M J. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Dukes C E. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35:323–332. [Google Scholar]
- 3.Zinkin L D. A critical review of the classifications and staging of colorectal cancer. Dis Colon Rectum. 1983;26:37–43. doi: 10.1007/BF02554677. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart-Mummery J P. Two hundred cases of cancer of the rectum treated by perineal excision. Br J Surg. 1926–1927;14:110–124. [Google Scholar]
- 5.Kirklin J W, Dockerty M B, Waugh J M. The role of the peritoneal reflection in the prognosis of carcinoma of the rectum and sigmoid colon. Surg Gynecol Obstet. 1949;88:326–331. [PubMed] [Google Scholar]
- 6.Astler V B, Coller F A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139(6):846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbull R B, Jr, Kyle K, Watson F R, Spratt J. Cancer of the colon: The influence of the no-touch isolation technique on survival rates. Ann Surg. 1967;166:420–427. doi: 10.1097/00000658-196709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutter R VP. At last-worldwide agreement on the staging of cancer. Arch Surg. 1987;122:1235–1239. doi: 10.1001/archsurg.1987.01400230021002. [DOI] [PubMed] [Google Scholar]
- 9.Greene F L, Page D L, Fleming I D, et al. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag; 2002. pp. 113–123.
- 10.UICC International Union Against Cancer In: Sobin LH, Wittekind Ch, editor. TNM Classification of Malignant Tumours. 6 ed. New York, NY: Wiley-Liss, New York; 2002. pp. 72–76.
- 11.Påhlman L. Optimal staging and treatment of localized rectal cancer. Ann Oncol. 2002;13(suppl 4):251–255. doi: 10.1093/annonc/mdf667. [DOI] [PubMed] [Google Scholar]
- 12.Wiggers T. Staging of rectal cancer. Br J Surg. 2003;90:895–896. doi: 10.1002/bjs.4279. [DOI] [PubMed] [Google Scholar]
- 13.Enker W E. The elusive goal of preoperative staging in rectal cancer. Ann Surg Oncol. 2004;11:245–246. doi: 10.1245/aso.2004.12.941. [DOI] [PubMed] [Google Scholar]
- 14.Balch G C, De Meo A, Guillem J G. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12:3186–3195. doi: 10.3748/wjg.v12.i20.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry A C, Simmang C L, Boulos P, et al. Consensus statement of definitions for anorectal physiology and rectal cancer. Dis Colon Rectum. 2001;44:915–919. doi: 10.1007/BF02235475. [DOI] [PubMed] [Google Scholar]
- 16.Goligher J C, Duthie H L, Dedombal F T, Watts J McK. Abomino-anal pull-through excision for tumours of the mid-third of the rectum. Br J Surg. 1965;52:323–335. doi: 10.1002/bjs.1800520504. [DOI] [PubMed] [Google Scholar]
- 17.Pilipshen S J, Heilweil M, Quan S H, Sternberg S S, Enker W E. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984;53:1354–1362. doi: 10.1002/1097-0142(19840315)53:6<1354::aid-cncr2820530623>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Kostner F, Lavery I C, Hool G R, Rybicki L A, Fazio V W. Total mesorectal excision is not necessary for cancers of the upper rectum. Surgery. 1998;124:612–617. doi: 10.1067/msy.1998.91361. [DOI] [PubMed] [Google Scholar]
- 19.Hermanek P, Hohenberger W, Klimpfinger M, Köckerling F, Papadopoulos T. The pathological assessment of mesorectal excision: implications for further treatment and quality management. Int J Colorectal Dis. 2003;18:335–341. doi: 10.1007/s00384-002-0468-6. [DOI] [PubMed] [Google Scholar]
- 20.Heald R J, Husband E M, Ryall R DH. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 21.Madbouly K M, Remzi F H, Erkek B A, et al. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum. 2005;48:711–721. doi: 10.1007/s10350-004-0666-0. [DOI] [PubMed] [Google Scholar]
- 22.Idrees K, Paty P B. Early rectal cancer: transanal excision or radical surgery? Adv Surg. 2006;40:239–248. doi: 10.1016/j.yasu.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 24.Minsky B D. Role of adjuvant therapy in adenocarcinoma of the rectum. Semin Surg Oncol. 1999;17:189–198. doi: 10.1002/(sici)1098-2388(199910/11)17:3<189::aid-ssu8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Valentini V, Glimelius B, Minsky B D, et al. The multidisciplinary rectal cancer treatment: main convergences, controversial aspects and investigational areas which support the need for an European consensus. Radiother Oncol. 2005;76:241–250. doi: 10.1016/j.radonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 26.McAnena O J, Heald R J, Lockhart-Mummery H E. Operative and functional results of total mesorectal excision with ultralow anterior resection in the management of carcinoma of the lower one-third of the rectum. Surg Gynecol Obstet. 1990;170:517–521. [PubMed] [Google Scholar]
- 27.Quirke P, Durdey P, Dixon M F, Williams N S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd N A, Baxter K J, Love S B. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995;48:849–855. doi: 10.1136/jcp.48.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot I C, Ritchie S, Leighton M H, Hughes A O, Bussey H JR, Morson B C. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- 30.Nicholls R J, Mason A Y, Morson B C, Dixon A K, Fry I K. The clinical staging of rectal cancer. Br J Surg. 1982;69:404–409. doi: 10.1002/bjs.1800690716. [DOI] [PubMed] [Google Scholar]
- 31.Rafaelsen S R, Kronborg O, Fenger C. Digital rectal examination and transrectal ultrasonograghy in staging of rectal cancer. A prospective, blind study. Acta Radiol. 1994;35:300–304. [PubMed] [Google Scholar]
- 32.Halligan S, Altman D G, Taylor S A, et al. CT colonography in the detection of colorectal polyps and cancer: systemic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893–904. doi: 10.1148/radiol.2373050176. [DOI] [PubMed] [Google Scholar]
- 33.Ohlsson B, Palsson B. Follow-up after colorectal cancer surgery. Acta Oncol. 2003;42:816–826. doi: 10.1080/02841860310019016. [DOI] [PubMed] [Google Scholar]
- 34.Li Destri G, Di Cataldo A, Puleo S. Colorectal cancer follow-up: useful or useless. Surg Oncol. 2006;15:1–12. doi: 10.1016/j.suronc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Heriot A G, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. doi: 10.1046/j.1365-2168.1999.00996.x. [DOI] [PubMed] [Google Scholar]
- 36.Orrom W J, Wong W D, Rothenberger D A, Jensen L L, Goldberg S M. Endorectal ultrasound in the preoperative staging of rectal tumors. Dis Colon Rectum. 1990;33:654–659. doi: 10.1007/BF02150740. [DOI] [PubMed] [Google Scholar]
- 37.Nesbakken A, Løvig T, Lunde O C, Nygaard K. Staging of rectal carcinoma with transrectal ultrasonography. Scand J Surg. 2003;92:125–129. doi: 10.1177/145749690309200203. [DOI] [PubMed] [Google Scholar]
- 38.Kauer W KH, Prantl L, Dittler H J, Siewart J R. The value of endosonographic rectal carcinoma staging in routine diagnostics. Surg Endosc. 2004;18:1075–1078. doi: 10.1007/s00464-003-9088-7. [DOI] [PubMed] [Google Scholar]
- 39.Knaebel H-P, Koch M, Feise T, Benner A, Kienle P. Diagnostics of rectal cancer: endorectal ultrasound. Recent Results Cancer Res. 2005;165:46–57. doi: 10.1007/3-540-27449-9_7. [DOI] [PubMed] [Google Scholar]
- 40.Harewood G C. Assessment of publication bias in the reporting EUS performance in staging rectal cancer. Am J Gastroenterol. 2005;100:808–816. doi: 10.1111/j.1572-0241.2005.41035.x. [DOI] [PubMed] [Google Scholar]
- 41.Ptok H, Marusch F, Meyer F, et al. Feasibility and accuracy of TRUS in the pre-treatment staging for rectal carcinoma in general practice. Eur J Surg Oncol. 2006;32:420–425. doi: 10.1016/j.ejso.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Shami V M, Parmar K S, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59–65. doi: 10.1007/s10350-003-0001-1. [DOI] [PubMed] [Google Scholar]
- 43.Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 44.Butch R J, Stark D D, Wittenberg J, et al. Staging rectal cancer by MR and CT. AJR. Am J Roentgenol. 1986;146:1155–1160. doi: 10.2214/ajr.146.6.1155. [DOI] [PubMed] [Google Scholar]
- 45.Hodgman C G, MacCarty R L, Wolff B G, et al. Preoperative staging of rectal carcinoma by computed tomography and 0.15T magnetic resonance imaging. Dis Colon Rectum. 1986;29:446–450. doi: 10.1007/BF02561581. [DOI] [PubMed] [Google Scholar]
- 46.Schnall M D, Furth E E, Rosato E F, Kressell H Y. Rectal tumor stage: correlation of endorectal MR imaging and pathologic findings. Radiology. 1994;190:709–714. doi: 10.1148/radiology.190.3.8115616. [DOI] [PubMed] [Google Scholar]
- 47.Torricelli P, Lo Russo S, Pecchi A, Luppi G, Cesinaro A M, Romagnoli R. Endorectal coil MRI in local staging of rectal cancer. Radiol Med (Torino) 2002;103:74–83. [PubMed] [Google Scholar]
- 48.Akin O, Nessar G, Agildere A M, Aydog G. Preoperative local staging of rectal cancer with endorectal MR imaging. Comparison with histopathologic findings. Clin Imag. 2004;28:432–438. doi: 10.1016/S0899-7071(03)00314-0. [DOI] [PubMed] [Google Scholar]
- 49.Tatli S, Mortele K J, Breen E L, Bleday R, Silverman S G. Local staging of rectal cancer using combined pelvic phased-array and endorectal coil MRI. Magn Reson Imag. 2006;23:534–540. doi: 10.1002/jmri.20533. [DOI] [PubMed] [Google Scholar]
- 50.Brown G, Daniels L R. Preoperative staging of rectal cancer. The MERCURY research project. Recent Results Cancer Res. 2005;165:58–74. doi: 10.1007/3-540-27449-9_8. [DOI] [PubMed] [Google Scholar]
- 51.Kim N K, Kim M J, Park J K, Park S I, Min J S. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000;7:732–737. doi: 10.1007/s10434-000-0732-3. [DOI] [PubMed] [Google Scholar]
- 52.Brown G, Radcliffe A G, Newcombe R G, Dallimore N S, Bourne M W, Williams G T. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 53.Martling A, Holm T, Bremmer S, Lindholm J, Cedermark B, Blomqvist L. Prognostic value of preoperative magnetic resonance imaging of the pelvis in rectal cancer. Br J Surg. 2003;90:1422–1428. doi: 10.1002/bjs.4276. [DOI] [PubMed] [Google Scholar]
- 54.Branagan G, Chave H, Fuller C, McGee S, Finnis D. Can magnetic resonance imaging predict circumferential margins and TNM stage in rectal cancer? Dis Colon Rectum. 2004;47:1317–1322. doi: 10.1007/s10350-004-0594-z. [DOI] [PubMed] [Google Scholar]
- 55.Burton S, Brown G, Daniels I, et al. MRI identified prognostic features of tumors in distal sigmoid, rectosigmoid and upper rectum: treatment with radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:445–451. doi: 10.1016/j.ijrobp.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 56.Kuo L-J, Chern M-C, Tsou M-H, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23–28. doi: 10.1007/s10350-004-0787-5. [DOI] [PubMed] [Google Scholar]
- 57.Chan A KP, Wong A, Jenken D, Heine J, Buie D, Johnson D. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:665–677. doi: 10.1016/j.ijrobp.2004.06.206. [DOI] [PubMed] [Google Scholar]
- 58.Brown G, Davies S, Williams G T, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging. Br J Cancer. 2004;91(1):23–29. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwok H, Bissett I P, Hill G L. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 60.Heriot A G, Hicks R J, Drummond E GP, et al. Does positron emission tomography change management in primary rectal cancer? A prospective study. Dis Colon Rectum. 2004;47:451–458. doi: 10.1007/s10350-003-0089-3. [DOI] [PubMed] [Google Scholar]
- 61.Gearhart S L, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 62.Walenta S, Chau T-V, Schroeder T, et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol. 2003;129:321–326. doi: 10.1007/s00432-003-0450-x. [DOI] [PubMed] [Google Scholar]