Abstract
Soybean transformation by ovary-drip was improved by optimizing the length of the transformation pathway by cutting the styles. These modifications facilitated soybean transformation manipulation and improved transformation reproducibility and efficiency. Using a linear minimal gus gene cassette as the foreign DNA, a maximum transformation frequency of 11% was obtained in flowers of the soybean cultivar ‘Liaodou 14’ with their styles mostly removed, whereas removal of only the stigma, partial style cutting and partial ovary cutting gave transformation frequencies of 0%, 1%, and 2%, respectively. An average transformation frequency of 8.2% was obtained when 619 flowers from three soybean cultivars (‘Liaodou 14’, ‘Liaodou 13’, and ‘Tiefeng 29’) were transformed by this optimized method. Southern blotting analysis showed that the gus reporter gene (encoding β-glucuronidase) was stably inherited with a simple pattern. Reverse transcription-polymerase chain reaction (RT-PCR) and GUS staining confirmed the expression of the gus gene in transgenic plants.
Keywords: Soybean, Glycine max (L.) Merrill, In planta ovary-drip transformation, Optimization, Style cutting, Minimal gus reporter gene cassette
INTRODUCTION
Agrobacterium-mediated transformation of cotyledonary nodes and gene gun particle bombardment transformation of embryogenic cultures are currently the methods most commonly used for soybean transformation (Paz et al., 2004; 2006; Xue et al., 2006; Sato et al., 1993; Droste et al., 2002); however, both require sophisticated regeneration methods. Great challenges remain in soybean transformation because of difficulties in tissue culture and plant regeneration, and low transformation efficiency (Bent, 2000; Mello-Farias and Chaves, 2008). The development of an in planta soybean transformation method with high transformation efficiency that avoids tissue culture and regeneration, would be of great interest. It may also avoid the constraints of genotype specificity, tissue culture-induced genetic variations, and reductions in fertility (Hu and Wang, 1999).
Agrobacterium-mediated in planta floral-dip transformation has been developed and successfully applied in the model plant Arabidopsis thaliana and also attempted in the legume model plant Medicago truncatula (Clough and Bent, 1998; Bent, 2006; Zhang et al., 2006; Trieu et al., 2000). This Agrobacterium-mediated inflorescence transformation method involves the simple dipping of developing floral tissues into a transformation solution containing plasmid DNA. The primary target of floral transformation has been demonstrated to be female reproductive tissues, including the ovary (Ye et al., 1999; Bechtold et al., 2000; Bent, 2000; Desfeux et al., 2000). A transformation frequency of at least 1% was obtained for Arabidopsis thaliana. In the case of Medicago truncatula, a transformation frequency of from 4.7% to 76% was reported (Trieu et al., 2000). However, the transformation of M. truncatula using this technique has not been reproduced elsewhere or used again in the same lab (Chabaud et al., 2007). Agrobacterium-mediated floral transformation frequently resulted in the occurrence of undesirable sibling transformants and unhealthy plants (Trieu et al., 2000). Thus a method for plant transformation that could dispense with the use of Agrobacterium as well as vector with potential integration of backbone and undesirable DNA sequences would be ideal.
Recently, direct transformation of a minimal linear gene cassette by stigma or ovary-drip without using bacteria has been reported in corn and soybean (Gao et al., 2007; Wu et al., 2008; Yang et al., 2009a; 2009b; Liu et al., 2009). This simple direct transformation method does not require the Agrobacterium Ti plasmid or vector, and does not result in any unnecessary backbone or selective marker in the transgenic products. Fluorescein isothiocyanate (FITC)-labelled DNA tracing studies have shown that exogenous DNA could enter the soybean embryo sac more readily via a shortened pathway created by ovary-cutting (Liu et al., 2009). Direct transformation of a linear gene cassette in onion lower epidermal cells revealed that, although lacking the mediation of the Agrobacterium plasmid, the exogenous gene could enter the host cell nucleus and be successfully expressed (Cheng et al., 2009). The frequency of successful soybean ovary-drip transformation is about 3% (Liu et al., 2009). Current ovary-drip protocols are limited to only a few soybean cultivars. Therefore, more detailed analysis of the transformation pathway and application of this technique to more cultivars are required to confirm its transformation efficiency and reproducibility.
In this study, we further investigated the soybean ovary transformation method using a minimal linear gus gene cassette, by optimizing the length of the transformation pathway through varying the length of the style by cutting. Molecular and biochemical analyses of the transformants and their progeny plants were carried out. These modifications facilitated transformation manipulation and improved transformation reproducibility and efficiency.
MATERIALS AND METHODS
Soybean cultivars
Three elite soybean cultivars from Northeastern China were used in this study: ‘Liaodou 14’, ‘Liaodou 13’, and ‘Tiefeng 29’, provided by the Liaoning Academy of Agricultural Sciences, China.
Preparation of DNA cassettes for ovary transformation
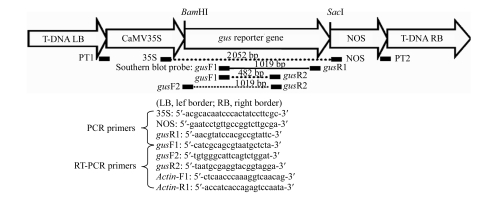
The linear minimal gus reporter gene cassette was generated by polymerase chain reaction (PCR) using pBI121 (Clontech, USA) as a template and the primers PT1 (5′-tggcaggatatattgtggtgtaaactgcctgcaggtccccagattagcctt-3′) and PT2 (5′-gtttacccgccaatatatcctgtcaccgatctagtaacatatgagacaccgc-3′), according to Gao et al.(2007). The relative positions of all primers on the minimal gus reporter gene cassette are shown in Fig.1.
Fig.1.
Schematic diagram of minimal gus reporter gene cassette
The minimal gus reporter gene cassette contains four basic elements: a 35S promoter, the gus reporter gene, a NOS terminator, and T-DNA border repeats (LB: left border; RB: right border). The binding sites of the various primers and the unique sites, BamHI and SacI, within the minimal gus gene cassette, are shown. Primer pairs, 35S/NOS and gusF1/gusR1, were used for PCR screening; gusF1/gusR2 and gusF2/gusR2 were used for RT-PCR analysis. The 1019 bp PCR fragment amplified with gusF1 and gusR1 was used as a probe for Southern blotting analysis. The Actin gene was used as an internal reference
Soybean ovary transformation
The soybean transformation procedure was modified from that of Liu et al.(2009). ‘Liaodou 14’ was used to investigate the effects of style cutting on transformation. After removing the two wing petals and one keel petal, the stigmas and styles were exposed. The styles were then cut off at various lengths using dissecting scissors (Fig.2).
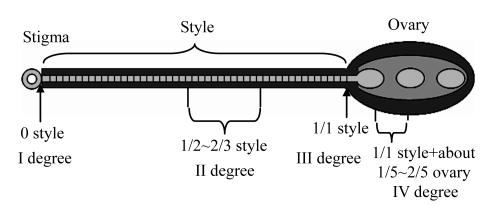
Fig.2.
Schematic representation of a soybean pistil showing the four style cutting sites
First degree cutting corresponds to the stigma removal only; second degree corresponds to the cutting of half of the style exposed above the hidden ovary; third degree style cutting is complete removal of the whole style, but no ovary wounding; fourth degree cutting wounds a part of the ovary
For ease of reference, the extent of cutting was designated as first, second, third, or fourth degree cutting: first degree cutting removed only the stigma, the style remaining intact; second degree cutting removed about 1/2~2/3 of the style; third degree cutting completely removed the style, without wounding the ovary; fourth degree cutting completely removed the style and also removed about 1/5~2/5 from the top of the ovary. Seven microliters of a DNA solution containing 200 μg/ml of the gene cassette in 0.1× SSC (1.5 mmol/L Na3C6H5O7, 15 mmol/L NaCl) was immediately applied to the cut site. The transformed flowers were tagged, whereas untreated flowers, or buds at the same node, were gently removed. The seeds from transformed flowers were harvested and planted for subsequent analysis. Seeds harvested from untreated flowers at different nodes were used as controls in further molecular and biochemical analyses.
The cutting length that gave the maximum transformation frequency was chosen for large-scale transformation of three Chinese soybean cultivars, ‘Liaodou 14’, ‘Liaodou 13’ and ‘Tiefeng 29’. The seeds from transformed flowers with linear gene cassette were harvested and planted for subsequent analysis. The seeds from flowers treated with only 0.1× SSC solution were harvested as the controls for each cultivar in further molecular and biochemical analyses.
Selecting soybean transformants by PCR
Genomic DNA was extracted from fresh leaves of T0 or T1 plants using the cetyltrimethylammonium bromide (CTAB) method (Murray and Thompson, 1980). Initial PCR screening was performed with genomic DNA using the primers gusF1 (5′-catcgcagcgtaatgctcta-3′) and gusR1 (5′-aacgtatccacgccgtattc-3′), which were derived from an internal region of the gus gene. The PCR conditions were: 98 °C/2 min; 30 cycles of 98 °C/30 s, 55 °C/1 min, and 72 °C/45 s, followed by holding at 72 °C for 10 min. For a positive PCR control, pBI121 was used as a template instead of genomic DNA. The expected PCR product size was 1019 bp. The positive transformants were then screened by a second PCR with the primer pair 35S/NOS (35S: 5′-acgcacaatcccactatccttcgc-3′; NOS: 5′-gaatcctgttgccggtcttgcga-3′) under the same conditions but with the extension time changed to 2 min during the 30-cycle step. The expected product size was 2052 bp.
Southern blotting analysis
Genomic DNA was isolated from young leaves of PCR positive T0 or T1 plants using the plant DNA extraction kit (Roche, USA). To investigate the transgene integration site, the genomic DNA was digested with the single cut enzyme BamHI, SacI, or BamHI/SacI, and separated on a 0.8% (w/v) agarose gel, transferred to Hybond N+ membranes (Amersham Biosciences, USA) and subjected to Southern blotting analysis performed according to standard protocol at 50 °C (Sambrook et al., 1989). A PCR fragment of the gus gene was generated as a probe, using pBI121 as a template and the primers gusF1/gusR1, and then labelled with 32P using a random primer DNA labelling kit (TaKaRa, Japan). Autoradiography was performed with Kodak X-Omat AK films according to standard procedures.
Reverse transcription-polymerase chain reaction
The expression of the gus reporter gene was determined by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was isolated from fresh leaves of T1 transgenic plants with Trizol-reagent (GibcoBRL, USA) according to the manufacturer’s protocol. The isolated RNA was pretreated with RNase-free DNAse I (TaKaRa, Dalian, China) and then precipitated by ethanol. cDNA was synthesized from 1 μg of RNA with AMV reverse transcriptase and random primers (RNA PCR kit ver. 2.1, TaKaRa, Dalian, China). The cDNA was used for further PCR with primers gusF2 (5′-tgtgggcattcagtctggat-3′) and gusR2 (5′-taatgcgaggtacggtagga-3′) or gusF1/gusR2 to obtain a 1019 bp or 482 bp fragment of the gus reporter gene. The actin gene was used as an internal reference, using the primer pair Actin-R1 (5′-ctcaacccaaaggtcaacag-3′) and Actin-F1 (5′-accatcaccagagtccaata-3′).
GUS staining
RT-PCR positive transgenic plants were also analyzed by GUS staining, according to the procedure described by Jefferson (1987) with some modifications. Leaves or roots from two-week-old plants were pretreated with 0.5 g/L cefotaxime sodium for 3 min followed by 1 min of washing with sterile water, and were then stained with 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) solution (0.5 mg/ml X-Gluc dissolved in 50 mmol/L NaPO4, pH 7.0, containing 0.1 mol/L ferricyanide, 0.1 mol/L ferrocyanide, 10 mmol/L Na2EDTA, 0.1 % (v/v) Triton X-100, and 20% (v/v) methanol) at 37 °C overnight. The stained leaves were bleached in 95% (v/v) ethanol for 4 h, followed by 75% (v/v) ethanol overnight.
Statistical analysis
All statistical analyses were performed using SPSS program version 11.0 (SPSS, Chicago, IL, USA). Data were first analyzed by one-way analysis of variance (ANOVA). When differences were found, Duncan’s multiple range tests were used to compare the means of transformation frequencies.
RESULTS
Effects of different style cutting treatments on soybean ovary transformation
The length of the pathway by which foreign DNA enters the embryo sac is regarded as one of the key factors affecting transformation efficiency (Liu et al., 2009). A shortened pathway, by partial ovary cutting, facilitated the entry of DNA molecules into the embryo sac (Liu et al., 2009), but also resulted in reduced pod-bearing rates (Gao et al., 2007). In this study, we compared the effects of four types of style cutting on transformation efficiency in detail. First degree cutting that removed only the stigma yielded few transformants; second degree cutting that removed 1/2~2/3 of the whole style yielded a transformation frequency of 1.0%; while, fourth degree cutting, with partial ovary removal and the shortest passageway, yielded a frequency of 2.0% (Table 1). The complete removal of the style without wounding the ovary (third degree cutting) yielded a maximum transformation frequency of 11.0% (Table 1). We assume that third degree cutting created a shorter pathway but a higher pod-bearing rate compared with fourth degree cutting in which ovary wounding might increase the risk of bacterial infection and pod abortion. Thus third degree cutting, which had the optimum transformation frequency, was selected for subsequent transformations.
Table 1.
Effects of different style cuttings on transformation frequencies and pod bearing rates in Chinese soybean cultivar genotype Liaodou 14 transformed with minimal gus reporter gene cassette
| Style cutting | Flowers | Pods | PR (%) | T0 plants | PPP | TF (%) * |
| Uncut | 200 | 110 | 55.0 | 310 | 0 | 0 |
| First | 213 | 97 | 45.5 | 179 | 0 | 0 a |
| Second | 206 | 90 | 43.7 | 175 | 2 | 1.0 b |
| Third | 209 | 81 | 38.8 | 135 | 23 | 11.0 d |
| Fourth | 197 | 50 | 25.3 | 20 | 4 | 2.0 c |
PR: pod-bearing rate; PPP: PCR positive plants; TF: transformation frequency
Transformants were screened by PCR using gus specific primers gusF1 and gusR1
indicates significance difference at the 0.05 probability level
indicates significance difference at the 0.05 probability level
indicates significance difference at the 0.05 probability level
indicates significance difference at the 0.05 probability level
Analysis of genotype effect on transformation efficiency
Following the first successful transformation experiment (Table 1), in which the treatment with the highest transformation efficiency was determined, one additional experiment was undertaken to detect the effect of genotype on the transformation efficiency. A total of 619 flowers from three soybean cultivars were transformed with the linear DNA cassette using the optimized ovary-drip method, yielding from 7.1% to 11.3% transformed plants among the T0 seedlings. The transformation efficiencies among the different cultivars showed no significant differences, with an average transformation frequency of 8.2% (Table 2). The PCR results from representative positive plants are shown in Fig.3.
Table 2.
Large-scale transformation using third degree style cutting in three Chinese soybean cultivar genotypes
| Recipient cultivars | Treated flowers | T0 plants | PCR positive plants | Transformation frequency (%) |
| Tiefeng 29 | 295 | 125 | 21 | 7.1 a |
| Liaodou 13 | 174 | 84 | 13 | 7.5 a |
| Liaodou 14 | 150 | 93 | 17 | 11.3 a |
| Total | 619 | 302 | 51 | 8.2 |
indicates that the frequencies are not significantly different among the three soybean cultivars at the 0.05 probability level
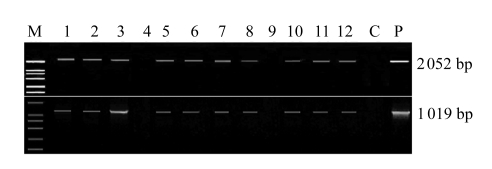
Fig.3.
PCR analysis of T0 plants
Genomic DNA, isolated from the leaves, was screened for the integrity of the minimal gus reporter gene cassette by PCR using two primer pairs: 35S/NOS (2052 bp) and gusF1/gusR1 (1019 bp). M: DNA marker DL2000 (TaKaRa); Samples 1~12: transformed soybean plants (positive results are shown except in Sample 4 and Sample 9); C: the plants from the untreated flowers; P: pBI121. A similar PCR analysis was carried out in large-scale transformation with soybean plants from the flowers transformed with 0.1× SSC as the control
Transgenic integration and inheritance
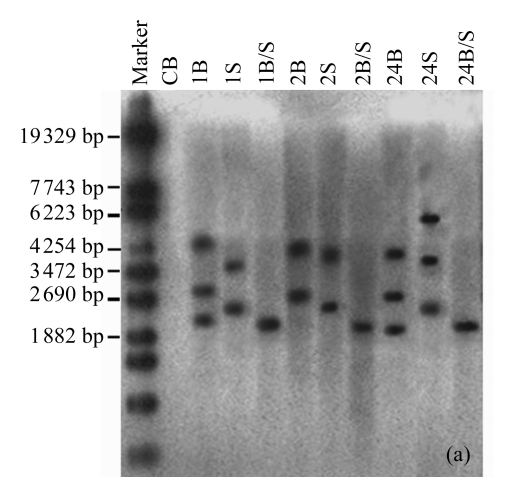
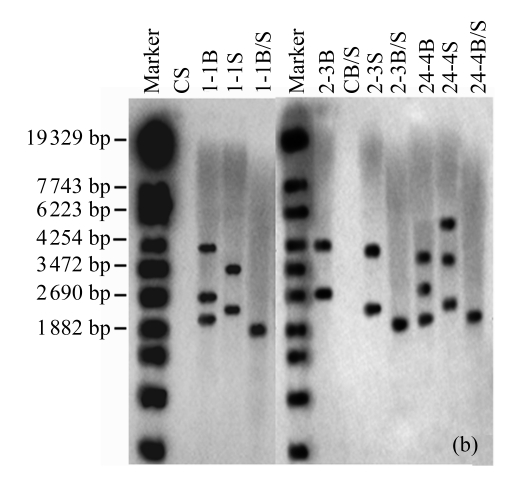
The existence of the gus reporter gene in the host soybean genome was confirmed by Southern blotting analysis. Total genomic DNA of T0 plants from three independent transformants was digested with BamHI, SacI, or BamHI/SacI and probed with a 1019 bp gus gene fragment. A low number of bands were observed, indicating a low number of insertion sites (Fig.4a). For sample 1, three bands were obtained with BamHI and two bands with SacI, indicating one insertion site with two copies in a tail-tail orientation, and another insertion site with one independent copy. Sample 2 showed two bands with BamHI or SacI, indicating two insertion sites, each with one copy. Sample 24 showed three copies inserted at independent sites. Similar results were observed for their progenies (Fig.4b). The results showed that at least one copy of an intact gus reporter gene had been inserted into the host genome and was stably inherited by the progeny plants.
Fig.4.
Southern blotting analysis in T0 and T1 plants
The blots show the results of (a) three T0 plants (samples 1, 2, 24) of Liaodou 14 and (b) their progeny T1 plants (samples 1-1, 2-3, 24-4) with BamHI (B) and SacI (S), or BamHI/SacI (B/S). C: soybean plants from the flowers transformed with 0.1× SSC
Transgene expression
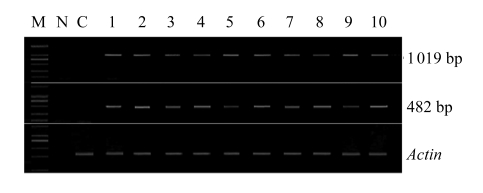
The expression of the gus gene in T1 plants from different independent lines was confirmed by RT-PCR with two pairs of gus specific primers, gusF1/gusR2 and gusF2/gusR2. All positive plants showed the expected bands of 482 bp (for gusF1/gusR2) and 1019 bp (for gusF2/gusR2) (Fig.5).
Fig.5.
RT-PCR analysis of gus expression in T1 plants
Total RNA of young leaves was extracted from ten independent transgenic plants. Reversed transcription (RT) was carried out with random primers according to the manufacturer’s protocol using RT-PCR kit (TaKaRa, Dalian, China). The primers gusF2/gusR2 and gusF1/gusR2 were used to generate the PCR fragments of 1019 bp and 482 bp. M: DNA marker DL2000 (TaKaRa); Samples 1~10: T1 plants; C: soybean plants from the flowers treated with 0.1× SSC; N: negative control using RT solution without total RNA as the template. An Actin gene was used as a reference molecule with the primer pair Actin-R1 and Actin-F1
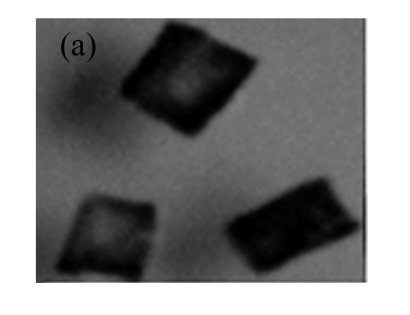
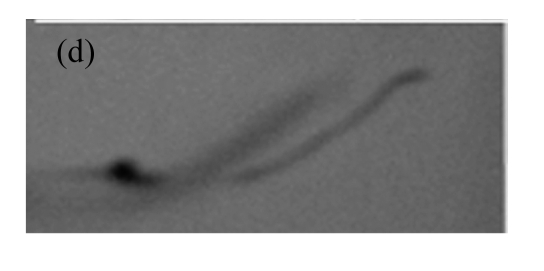
The expression of gus was also confirmed by GUS staining. Fig.6 shows the GUS staining of different tissues of the transgenic plants from one representative line. These results show that the transgene was stably inherited and effectively expressed in T1 plants.
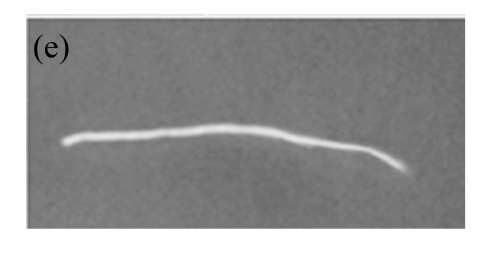
Fig.6.
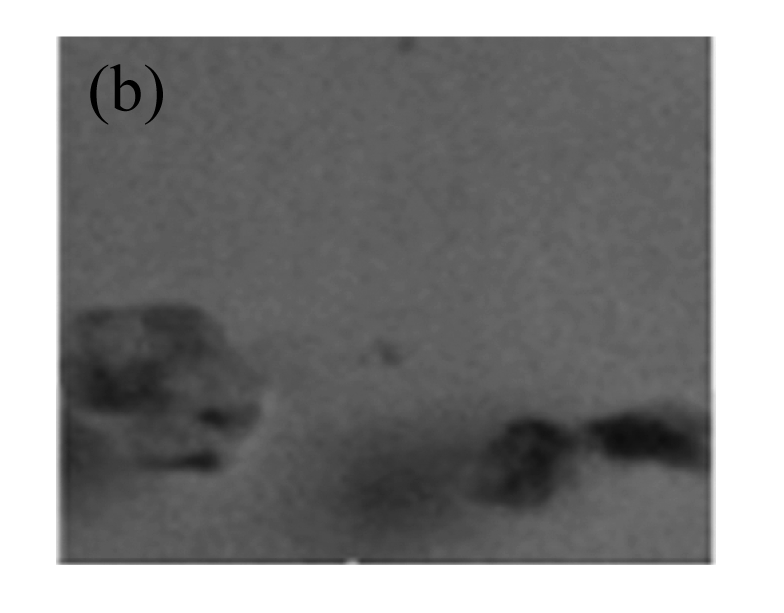
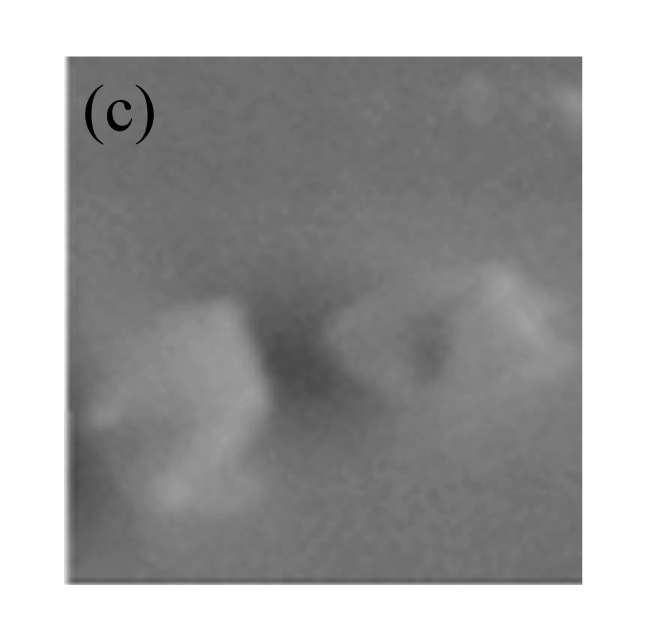
GUS expression in T1 plants
(a) & (b) Leaves of T1 plants derived from RT-PCR positive plants; (c) Leaves of control soybean plant; (d) A root from a T1 plant; (e) A root of control soybean plant
DISCUSSION
A variety of in planta transformation procedures have been developed for model plants, including Agrobacterium infiltration into seeds, shoots, and flowers (Clough and Bent, 1998; Zhang et al., 2006). However, few in planta approaches have been developed for other plant species, especially legume crops. The ovary transformation method reported here has been successfully used for soybean transformation, and might also be suitable for other plants. Compared with the earlier tissue culture-based transformation methods for soybean (McCabe et al., 1988; Santarém and Finer, 1999), this transformation procedure is tissue culture-independent and easier to perform. It also differs from the floral dip method used in the transformation of Arabidopsis (Clough and Bent, 1998; Zhang et al., 2006) as it does not use Agrobacterium and the Vir machinery to transfer and integrate T-DNA in the plant genome. It makes use of the shortened pathway that the foreign DNA needs to pass along to reach the ovary. The optimization was a compromise between the length of this pathway and the yield of seeds, resulting in a higher transformation frequency.
The passage of exogenous DNA through the stigma-style and its entry into the host reproductive ovary cells is the key to successful transformation by floral infiltration (Bechtold et al., 2000; Bent, 2000). A shortened pathway facilitates entry of the DNA molecules into the embryo sac (Liu et al., 2009). The ovary transformation pathway is a kind of in situ reproductive transformation system; hence the physiological traits of the host soybean plants, such as the pod-bearing rate, determine the final transformation yield. Excessive wounding of the flowers causes its abortion, thereby reducing pod-bearing rates but insufficient penetration does not sufficiently expose target tissues for DNA delivery; therefore there must be a transformation pathway length which optimizes the transformation frequency and pod-bearing rates, thereby maximizing the recovery of transformed plants. In our study, the complete removal of the style without ovary wounding resulted in a maximum transformation frequency (Table 1). An average transformation frequency of 8.2% was obtained from the transformation of three soybean cultivars (Table 2). The optimization of the ovary-drip transformation method described here is therefore genotype-independent, and should work for most soybean cultivars.
In conclusion, by optimizing the length of the cut style, in planta transformation of soybean by the ovary transformation method can improve the efficiency and reproducibility of transformation. The transgene can be maintained and expressed in transgenic soybean plants and their progeny.
Acknowledgments
We thank Alan K CHANG of Dalian University of Technology, China for his help with the revision of the manuscript.
Footnotes
Project (No. JY03-B-18-02) supported by the National R & D Project of Transgenic Crops of Ministry of Science and Technology of China
References
- 1.Bechtold N, Jaudeau B, Jolivet S, Maba B, Vezon D, Voisin R, Pelletier G. The maternal chromosome set is the target of the T-DNA in the in planta transformation of Arabidopsis thaliana . Genetics. 2000;155(4):1875–1887. doi: 10.1093/genetics/155.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bent AF. Arabidopsis in planta transformation. Uses, mechanisms, and prospects for transformation of other species. Plant Physiol. 2000;124(4):1540–1547. doi: 10.1104/pp.124.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bent AF. Arabidopsis thaliana floral dip transformation method. Methods Mol Biol. 2006;343:87–103. doi: 10.1385/1-59745-130-4:87. [DOI] [PubMed] [Google Scholar]
- 4.Chabaud M, Ratet P, de Sousa Araújo S, et al. Agrobacterium tumefaciens-Mediated Transformation and in vitro Plant Regeneration of M. truncatula . 2007. [Oct. 8, 2009]. Available from http://www.noble.org/MedicagoHandbook/
- 5.Cheng YQ, Yang J, Xu FP, An LJ, Liu JF, Chen ZW. Transient expression of minimum linear gene cassettes in onion epidermal cells via direct transformation. Appl Biochem Biotechnol. 2009;159(3):739–749. doi: 10.1007/s12010-009-8554-7. [DOI] [PubMed] [Google Scholar]
- 6.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 7.Desfeux C, Clough SJ, Bent AF. Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol. 2000;123(3):895–904. doi: 10.1104/pp.123.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droste A, Pasquali G, Bodanese-Zanettini HM. Transgenic fertile plants of soybean [Glycine max (L.) Merrill] obtained from bombarded embryogenic tissue. Euphytica. 2002;127(3):367–376. doi: 10.1023/A:1020370913140. [DOI] [Google Scholar]
- 9.Gao XR, Wang GK, SU Q, Wang Y, AN LJ. Phytase expression in transgenic soybeans: stable transformation with a vector-less construct. Biotechnol Lett. 2007;29(11):1781–1787. doi: 10.1007/s10529-007-9439-x. [DOI] [PubMed] [Google Scholar]
- 10.Hu CY, Wang LZ. In planta soybean transformation technologies developed in China: procedure, confirmation and field performance. In Vitro Cell Dev Biol Plant. 1999;35(5):417–420. doi: 10.1007/s11627-999-0058-1. [DOI] [Google Scholar]
- 11.Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5(4):387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- 12.Liu JF, Su Q, An LJ, Yang AF. Transfer of a minimal linear marker-free and vector-free smGFP cassette into soybean via ovary-drip transformation. Biotechnol Lett. 2009;31(2):295–303. doi: 10.1007/s10529-008-9851-x. [DOI] [PubMed] [Google Scholar]
- 13.McCabe DE, Swain WF, Martinell BJ, Christou P. Stable transformation of soybean (Glycine max) by particle acceleration. Bio/Technology. 1988;6(8):923–926. doi: 10.1038/nbt0888-923. [DOI] [Google Scholar]
- 14.Mello-Farias P, Chaves A. Advances in Agrobacterium-mediated plant transformation with emphasis on soybean. Sci Agric (Piracicaba, Braz) 2008;65(1):95–106. doi: 10.1590/S0103-90162008000100014. [DOI] [Google Scholar]
- 15.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paz MM, Shou H, Guo Z, Zhang Z, Banerjee AK, Wang K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica. 2004;136(2):167–179. doi: 10.1023/B:EUPH.0000030669.75809.dc. [DOI] [Google Scholar]
- 17.Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006;25(3):206–213. doi: 10.1007/s00299-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Tritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Santarém ER, Finer JJ. Transformation of soybean (Glycine max (L.) Merrill) using proliferative embryogenic tissue maintained on semi-solid medium. In Vitro Cell Dev Biol Plant. 1999;35(6):451–455. doi: 10.1007/s11627-999-0067-0. [DOI] [Google Scholar]
- 20.Sato S, Newell C, Kolacz K, Tredo L, Finer JJ, Hinchee M. Stable transformation via particle bombardment in two different soybean regeneration systems. Plant Cell Rep. 1993;12(7-8):408–413. doi: 10.1007/BF00234702. [DOI] [PubMed] [Google Scholar]
- 21.Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, et al. Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium . Plant J. 2000;22(6):531–541. doi: 10.1046/j.1365-313x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Su Q, Xia XY, Wang Y, Luan YS, An LJ. The Suaeda liaotungensis Kitag betaine aldehyde dehydrogenase gene improves salt tolerance of transgenic maize mediated with minimum linear length of DNA fragment. Euphytica. 2008;159(1-2):17–25. doi: 10.1007/s10681-007-9451-1. [DOI] [Google Scholar]
- 23.Xue RG, Xie HF, Zhang B. A multi-needle-assisted transformation of soybean cotyledonary node cells. Biotechnol Lett. 2006;28(19):1551–1557. doi: 10.1007/s10529-006-9123-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang AF, Su Q, An LJ, Liu JF, Wu W, Qiu Z. Detection of vector- and selectable marker-free transgenic maize with a linear GFP cassette transformation via the pollen-tube pathway. J Biotechnol. 2009;139(1):1–5. doi: 10.1016/j.jbiotec.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Yang AF, Su Q, An L. Ovary-drip transformation: a simple method for directly generating vector- and marker-free transgenic maize (Zea mays L.) with a linear GFP cassette transformation. Planta. 2009;229(4):793–801. doi: 10.1007/s00425-008-0871-5. [DOI] [PubMed] [Google Scholar]
- 26.Ye GN, Stone D, Pang SZ, Creely W, Gonzalez K, Hinchee M. Arabidopsis ovule is the target for Agrobacterium in planta vacuum infiltration transformation. Plant J. 1999;19(3):249–257. doi: 10.1046/j.1365-313X.1999.00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]