Abstract
The effects of process variables such as enzyme types, enzyme ratio, reaction temperature, pH, time, and ethanol concentration on the extraction of unripe apple polyphenol were investigated. The results indicated that Viscozyme L had the strongest effect on polyphenols extraction and was selected to study the polyphenol composition. The ratio of enzyme (Viscozyme L) to substrate (2 fungal beta-glucanase units (FBG)) at 0.02, reaction at pH 3.7, 50 °C for 12 h, and ethanol concentration of 70% were chosen as the most favorable extraction condition. Total phenolic content (TPC), reducing sugar content (RSC), and extraction yield increased by about 3, 1.5, and 2 times, respectively, compared with control. The contents of p-coumaric acid, ferulic acid, and caffeic acid increased to 8, 4, and 32 times, respectively. The enzyme-aided polyphenol extraction process from unripe apples might be applied to food industry for enhancing bioactive compound production.
Keywords: Carbohydrate-hydrolyzing enzymes, Unripe apples, Polyphenol extraction, Caffeic acid
INTRODUCTION
Apples and apple products are widely considered as important parts of the human diet, because they contain several nutritional and health-benefit constituents including organic acid, dietary fiber, vitamins, and polyphenol (Schieber et al., 2001). The beneficial effects of these constituents have been attributed to polyphenol and its antioxidant capacities. The main classes of polyphenol in apples are flavan-3-ols (catechin, epicatechin, and proanthocyanidin), hydroxycinnamic acids (chlorogenic acid and p-coumaroylquinic acid), dihydrochalcones (phloridzin and phloretin glucoside), and flavonols (quercetin and rutin) (Schieber et al., 2001; Alonso-Salces et al., 2005). Interestingly, the contents of these polyphenols are strongly dependent on their varieties and maturity (Akiyama et al., 2005; Wu et al., 2007). Especially, total phenolic content (TPC) (Park et al., 2004; Renard et al., 2007), proanthocyanidin (Akiyama et al., 2005), and flavonoid (Awad et al., 2001) are contained in unripe apples 10 times higher than in the ripe.
Unripe apples, which are discarded in the orchards (Park et al., 2004) by thinning out or falling, have accounted for 20%~30% of total apple production in Korea (479 000 Mt, Korea National Statistical Office). A small part of them are utilized as ingredients for animal feed and fertilizer, although this practice poses some environmental and ecological problems (Sudha et al., 2007). Unripe apples contain fairly high levels of polyphenol (Adil et al., 2007; Sudha et al., 2007), which has been reported to have various physiological functions including anti-allergic activity (Kojima et al., 2000; Akiyama et al., 2005), anti-cancer activity (Yanagida et al., 2000), and anti-arteriosclerosis activity (D′Angelo et al., 2007). Some of the agricultural solid by-products like fruit pomace and peel had also been studied for their potential exploitation because of the high contents of polyphenol (Makris et al., 2007). In order to improve plant polyphenol extraction efficiency, the use of carbohydrate-hydrolyzing enzymes, such as pectinase, cellulase, hemicellulase, and glucanase has been recently introduced to release cell wall complex polyphenol (Meyer et al., 1998; Landbo and Meyer, 2001; Sørensen et al., 2005), as well as the extraction technology such as ultrasound-assisted extraction (Liyana-Pathirana and Shahidi, 2005; Wang et al., 2008). These enzymes might play a role in disintegrating the plant cell wall matrix and facilitating the polyphenol extraction (Renard et al., 2001; Pinelo et al., 2006; Stalikas, 2007; Le Bourvellec et al., 2009). Dozens of processing variables, such as enzymes, substrates, solvents, temperature, etc. may affect the polyphenol extraction efficiency in plant (Meyer et al., 1998). We had also tried to utilize pectinase for enhancing the extraction efficiency of apple pomace polyphenol (Zheng et al., 2008). However, there have been few relative reports on the utilization of carbohydrate-hydrolyzing enzymes in the polyphenol extraction of unripe apples, although it is a rich source of plant polyphenol.
In this study, in order to achieve more efficient conditions for carbohydrate-hydrolyzing enzymes, introduction to the polyphenol extraction of the unripe apples, the TPC, and extraction yield were examined for reaction and extraction variables, such as the ratio of enzymes to substrates, reaction pH, temperature, and time. In addition, the polyphenol composition of unripe apples was also studied.
MATERIALS AND METHODS
Materials and chemicals
Unripe apples (Malus pumila cv. Fuji) were collected from the orchard of Kyungpook National University, Korea, in July, 2007, and stored in a freezer at −70 °C until the experiment. Viscozyme L (from Aspergillus aculeatus, 100 fungal β-glucanase units (FBG)/ml, Novozymes, Bagsvaerd, Denmark), Celluclast 1.5L (from Trichoderma reesei, 700 endoglucanase units (EGU)/ml, Novozymes, Bagsvaerd, Denmark), and Pectinex 5XL (from Aspergillus niger, 5 000 ferment depectinization units (FDU)/ml, Novozymes, Bagsvaerd, Denmark) were used in this study. Folin-Ciocalteu phenol reagent, (±)-catechin, (−)-epicatechin, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, quercetin, phloretin, and phloridzin were obtained from Sigma Co. (St. Louis, MO, USA), 5-hydroxymethylfurfural was purchased from Fluka Co. (Buchs, Switzerland), and procyanidin B1 was from Extrasynthese Co. (Lyon, France). Quercetin-3-galactoside, quercetin 3-glucoside, and quercetin 3-rhamnoside were obtained from Plantech Co. (Reading, UK). All organic solvents were at the analytical grade from Duksan Co. (Seoul, Korea), except for high performance liquid chromatography (HPLC; J. T. Baker, Phillpsburg, NJ).
Instruments
Ultraviolet-visible (UV-vis) spectrophotometer (UV 1601 PC, Shimadzu, Co., Kyoto, Japan) and HPLC (LC-10A, Shimadzu, Co., Kyoto, Japan) were used for the determination of TPC, reducing sugar content (RSC), and the polyphenol composition of unripe apples.
Treatment of enzyme-aided extraction
Five hundred grams of unripe apples were cut into cylindrical shapes without peeling, added with an equivalent volume of solution, and then homogenized. For enzyme-aided extraction, 8 g of apple homogenate was put into a 30 ml vial, added with a certain enzyme solution, and then incubated after nitrogen flushing. The polyphenols of the hydrolyzed samples were extracted with aqueous ethanol solutions. Briefly, reaction variables of enzyme to substrate ratio from 0 to 0.1 (from 0 to 10 FBG for Viscozyme L, from 0 to 70 EGU for Celluclast 1.5L, and from 0 to 500 FDU for Pectinex 5XL, respectively), reaction solution pH from 3.0 to 6.0, reaction temperature from 40 to 55 °C, and reaction time from 6 to 36 h were selected at a certain level with regard to the enzyme supplier’s manual. In solvent extraction, the extraction solvent concentrations from 60% to 80% were also studied.
Determination of total phenolic content
TPC was examined using Folin-Ciocalteu reagent with some modifications (Singleton et al., 1999), and is expressed as gallic acid equivalents (GAE) in milligrams per 100 g fresh weight (mg GAE/100 g).
Determination of reducing sugar content
The RSC of test solution was determined by the dinitrosalicylic acid (DNS) method (Miller, 1959) with some modifications and is expressed as glucose equivalents in gram per 100 g fresh weight. Briefly, 500 μl of DNS assay reagent and 150 μl of sample were added to the test solution, boiled at 98 °C for 5 min, and measured for absorbance at 550 nm.
Determination of unripe apple polyphenol extraction yield
After being extracted with aqueous ethanol solutions, the sample solutions were evaporated, freeze-dried, and weighed (unripe apple polyphenol). The polyphenol extraction yield was calculated as the percent ratio of the weight of unripe apple polyphenol to that of the unripe apple sample.
HPLC analysis of the polyphenol composition of unripe apples
Twelve microliters of unripe apple polyphenol solution was injected onto HPLC equipped with an ODS-HG-5 (Develosil, 150×4.6 mm i.d.) column, in a mobile phase of 2% (v/v) acetic acid in water (solvent A) and 0.5% (v/v) acetic acid and 49.5% (v/v) acetonitrile in water (solvent B) at a flow rate of 1.0 ml/min, and monitored at 290 nm. In addition, in order to validate the enzymatic hydrolysis of chlorogenic acid to caffeic acid by Viscozyme L, the following experiment was executed. Briefly, 1 ml of 3 mmol/L chlorogenic acid solution was prepared in acetate buffer (pH 3.7) and added with 20 μl (2 FBG) Viscozyme L, and after nitrogen flushing, the reaction solution was incubated at the same reaction condition with the unripe apples for 0.5 h to 12 h. After filtrated (0.45 μm), the reaction solution was diluted to 10 times, and 20 μl was injected onto HPLC.
RESULTS AND DISCUSSION
Effects of variables on polyphenol extraction
In general, the efficiency of the enzyme-aided extraction for polyphenol is influenced by several variables, such as enzymes, reaction temperature, time, and pH (Landbo and Meyer, 2001; Sørensen et al., 2005; Pinelo et al., 2006). Therefore, the effects of some important variables on TPC were investigated for determining the best condition for the polyphenol extraction from unripe apples. TPC assay, as a routine assay, is a very convenient, simple, and reproducible analytical method to measure the total content of polyphenol in fruit and vegetables (Huang et al., 2005). The TPC profile is well-correlated with antioxidant capacities in the plant.
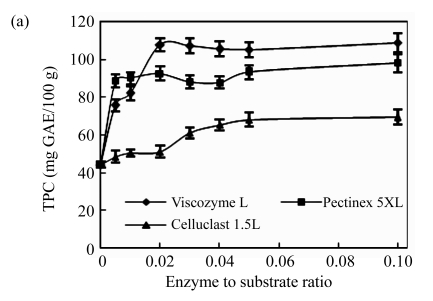
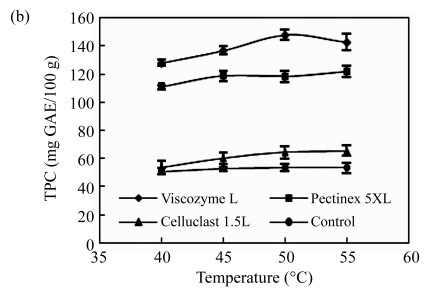
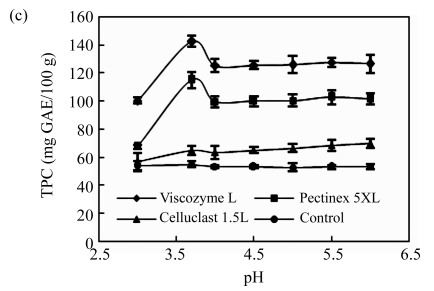
First, the effects of the ratio of enzyme to substrate on the polyphenol extraction from unripe apples were investigated and are shown in Fig.1a. Three kinds of commercial carbohydrate-hydrolyzing enzymes, Viscozyme L, Celluclast 1.5L, and Pectinex 5XL, were used, and the TPC was measured as the total contents of unripe apple polyphenol at all of the treatments. All TPC values of enzyme treatments sharply increased, but maintained invariant at the ratio of 0.02 (Viscozyme L to substrate, 2 FBG), 0.01 (Pectinex 5XL to substrate, 50 FDU), and 0.05 (Celluclast 1.5L to substrate, 35 EGU), respectively. Especially, the Viscozyme L treatment exhibited higher TPC values at higher overall ratios than any other enzyme treatments. The effects of the reaction temperature on the polyphenol extraction from unripe apples were investigated (Fig.1b). All TPC values of enzyme treatments increased smoothly, but maintained invariant or gradually decreased over temperature around 50 °C. In addition, the Viscozyme L treatment exhibited the highest TPC values. These results suggest that an increase of reaction temperature may favor enzyme activities and enhance the decomposition activity during the enzyme reaction. In addition, according to our previous study, an excess decrease or increase of reaction temperature partly inhibited enzyme activity, thereby decreasing the TPC values. The similar results were reported by Landbo and Meyer (2001) and Pinelo et al.(2006), who found that a proper given temperature may mobilize certain antioxidants and promote possible concurrent decomposition of antioxidants, while too high or low temperature had a significantly negative effect on the polyphenol extraction. The pH of the reaction solution also plays an important role in cell wall hydrolysis and polyphenol extraction in plant (Weinberg et al., 1999; Ahn et al., 2005; Zheng et al., 2008). The effects of reaction solution pH on the polyphenol extraction from unripe apples are shown in Fig.1c. The reaction solution pH was controlled with acetate and/or NaOH solution. All the TPC values sharply increased and reached a peak at the reaction solution pH of 3.7, and then sharply decreased to the pH of 4.0, and maintained invariant at all of the treatments.
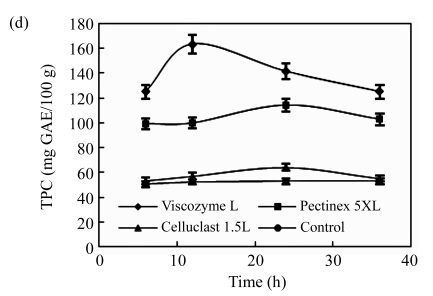
Fig.1.
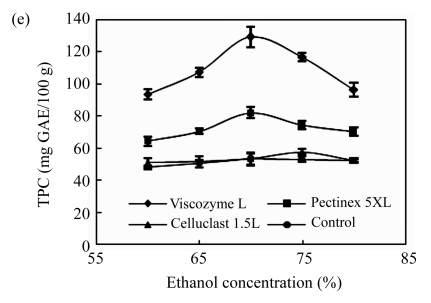
Effects of (a) the ratio of enzyme to substrate, (b) reaction temperature, (c) reaction solvent pH, (d) reaction time, and (e) ethanol concentration on the polyphenol extraction from unripe apples
Reaction: (a) pH 4.5, 40 °C, 12 h; (b) the ratio of enzyme to substrate 0.02, pH 4.5, 12 h; (c) the ratio of enzyme to substrate 0.02, 40 °C, 12 h; (d) the ratio of enzyme to substrate 0.02, pH 3.7, 50 °C; (e) the ratio of enzyme to substrate 0.02, pH 4.5, 40 °C, 12 h. Extraction: (a)~(d) 80% ethanol, 85 °C, 12 h; (e) 85 °C, 12 h. Values are the means of triplicates. TPC: total phenolic content
The effects of the reaction time on the polyphenol extraction from unripe apples are shown in Fig.1d. The TPC value increased sharply along with the reaction time, and reached a peak at the reaction time of 12 h, and smoothly decreased in Viscozyme L treatment. However, in Celluclast 1.5L and Pectinex 5XL treatments, the TPC values smoothly increased, and reached a peak at the reaction time of 24 h. These results, possibly due to the decomposition of active compounds during the excessive heat treatment, support the conclusion that long enzyme reaction time has a significantly negative effect on the polyphenol extraction (Pinelo et al., 2008; Zheng et al., 2008).
The effects of the enzyme type on the polyphenol extraction from unripe apples were also examined together with other variables and are shown in Fig.1. Viscozyme L showed a higher set of TPC values than other enzyme treatments at all stages of process variables. Ahn et al.(2005) also reported that Viscozyme L has an excellent effect on flavonoid extraction from Korean citrus peel. Celluclast 1.5L has low activity, which is consistent with the findings of Meyer et al.(1998), who explained that plant cell wall material retarded by nonproductive adsorption of Celluclast 1.5L to lignin or polysaccharides in the wall matrix. Although the yield of TPC varied in response to the process variables, the efficient reaction conditions have been determined to be a ratio of 0.02 of Viscozyme L to substrate (2 FBG), 50 °C for the reaction temperature, 3.7 for the reaction pH, and 12 h for reaction time. Furthermore, several reports suggested that extraction solvent concentration was the other important variable contributing to the extraction of bioactive components, such as wheat (Liyana-Pathirana and Shahidi, 2005), grape pomace (Pinelo et al., 2005), and peach pomace (Adil et al., 2007). Although methanol is the best solvent for polyphenol extraction (Adil et al., 2007), ethanol was selected as the solvent for extraction due to its low toxicity. The effects of the ethanol concentration on the polyphenol extraction from unripe apples were investigated and are shown in Fig.1e. As the ethanol concentration goes up, TPC values of at all enzyme treatment increased, and reached the highest level at 70% of ethanol concentration, but decreased slightly above 70%. In aqueous alcoholic solution, solvent concentrations may affect the solubility of the phenolic compounds, especially in case of the glycosidic compounds (Silva et al., 2007).
Extraction efficiency of unripe apple polyphenol with the enzyme treatment
We determined that the most favorable conditions of polyphenol extraction from unripe apples were a ratio of 0.02 for enzyme (Viscozyme L) to substrate (2 FBG), 12 h hydrolysis reaction at 50 °C, pH 3.7, and 70% ethanol extraction. Our results are in accordance with Zheng et al.(2008) and Park and Kim (2009), who reported that cellulase and pectinase significantly enhanced the total phenolic content extraction from the apple pomace and peel at the dosage of 2%~10%. The TPC, RSC, and polyphenol extraction yield comparisons between the most favorable extraction condition and control treatments are shown in Table 1. The results indicate that TPC, RSC, and extraction yield increased by about 3, 1.5, and 2 times, respectively, compared with the control treatment, indicating the occurrence of more active hydrolysis reaction at the most favorable condition. Briefly, the apple cell wall was decomposed by Viscozyme L and released polyphenol and related sugars, therefore improving the TPC, RSC, and the polyphenol extraction yield (Table 1). Furthermore, our results are consistent with the reports of Khanizadeh et al.(2008) and Silvina and Balz (2004), who obtained similar TPC from apple fruits. The polyphenolic compounds and glucose also increased in the fruits and lentils treated by carbohydrate-hydrolyzing enzymes (Dueñas et al., 2007). In addition, Benoit et al.(2006) speculated about a positive correlation between glucose and total phenolic compound in agro-industrial by-products treated by feruloyl esterases.
Table 1.
TPC, RSC and yield of unripe apple polyphenol extracted with Viscozyme L-aided extraction condition a
| Treatment | TPC b (mg GAE/100 g) | RSC c (g/100 g) | Extraction yield (%) |
| Viscozyme L | 163.43±1.55 d | 11.19±0.05 | 7.08±0.30 |
| Control e | 45.56±0.86 | 7.43±0.04 | 3.20±0.17 |
Reaction: the ratio of Viscozyme L to substrate 0.02 (2 FBG), pH 3.7, 50 °C, 12 h; Extraction: 70% ethanol, 85 °C, 12 h
TPC: total phenolic content
RSC: reducing sugar content
Values are the mean±SD of triplicates
Without enzyme treatment
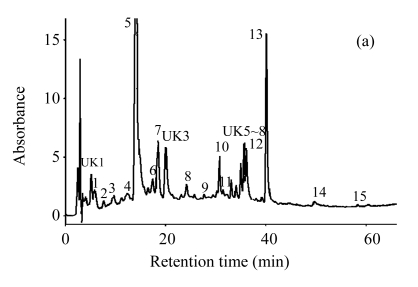
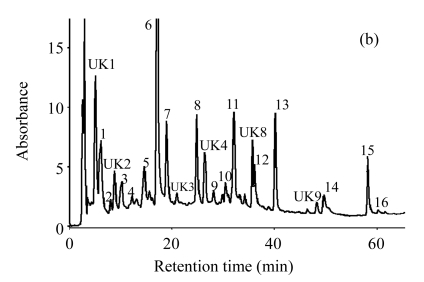
The composition of unripe apple polyphenol extracted with Viscozyme L at the most favorable condition was analyzed by HPLC, and compared with that of the control treatment. The 15 polyphenols, including 3 flavan-3-ols, 4 hydroxycinnamic acids, 4 flavonols, 2 dihydrochalcones, protocatechuic acid, and kaempferol were separated and their contents were compared between the Viscozyme L treatment and the control treatment. Procyanidin B1, (−)-epicatechin, and chlorogenic acid were the predominant polyphenols in unripe apples, and their contents reached about 70% of the total polyphenol contents. However, the polyphenol composition of the unripe apples with enzyme treatment was slightly different, which reflects the decrease of chlorogenic acid content associated with the increase of caffeic acid content. The caffeic acid content increased 32 times, whereas the chlorogenic acid content decreased to 1/7 (Table 2 and Fig.2). Compared with the control treatment, the contents of p-coumaric acid and ferulic acid increased about 8 and 4 times, respectively. Furthermore, the contents of phloretin, quercetin-3-glucoside, and quercetin also increased about 25, 45, and 5 times, respectively. Other compounds showed no significant difference of the contents between the enzyme treatment and the control.
Table 2.
Composition of unripe apple polyphenol extracted with and without Viscozyme L-aided extraction condition a (mg/kg)
| Class | Compound | Viscozyme L | Control b |
| Flavan-3-ols | Procyanidin B1 | 328.84 | 118.44 |
| (±)-catechin | 76.10 | 78.11 | |
| (−)-epicatechin | 272.29 | 235.93 | |
| Hydroxycin-namic acids | Chlorogenic acid | 18.70 | 129.07 |
| Caffeic acid | 55.66 | 1.76 | |
| p-coumaric acid | 14.70 | 1.83 | |
| Ferulic acid | 2.40 | 0.48 | |
| Flavonols | Quercetin-3-D-galactoside | 14.99 | 34.54 |
| Quercetin-3-glucoside | 66.66 | 1.49 | |
| Quercetin-3-rhamnoside | 27.67 | 30.41 | |
| Quercetin | 21.08 | 4.17 | |
| Dihydro-chalcones | Phloridzin | 27.41 | 54.89 |
| Phloretin | 25.28 | Trace | |
Reaction: the ratio of Viscozyme L to substrate 0.02 (2 FBG), pH 3.7, 50 °C, 12 h; Extraction: 70% ethanol, 85 °C, 12 h
Without enzyme treatment
Fig.2.
HPLC chromatograms of unripe apple polyphenol extracted (a) without and (b) with Viscozyme L-aided condition
1: 5-hydroxymethyl furfural; 2: protocatechuic acid; 3: procyanidin B1; 4: (±)-catechin; 5: chlorogenic acid; 6: caffeic acid; 7: (−)-epicatechin; 8: p-coumaric acid; 9: ferulic acid; 10: quercetin-3-D-galactoside; 11: quercetin-3-glucoside; 12: quercetin-3-rhamnoside; 13: phloridzin; 14: quercetin; 15: phloretin; 16: kaempferol; UK1~9: unknown. Reaction: the ratio of Viscozyme L to substrate 0.02 (2 FBG), pH 3.7, 50 °C, 12 h; Extraction: 70% ethanol, 85 °C, 12 h
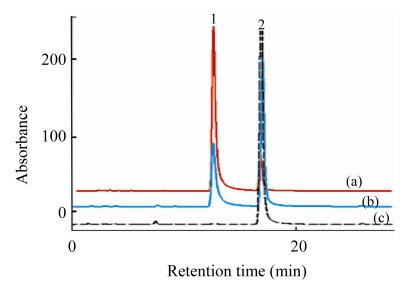
In order to validate the above hydrolysis process, the hydrolysis experiment of chlorogenic acid to caffeic acid by Viscozyme L was performed under the same condition as the unripe apples (Table 3 and Fig.3). The chlorogenic acid contents decreased linearly depending on the reaction time, and 98% of chlorogenic acid was hydrolyzed after 12 h, showing the similar hydrolysis pattern to that of unripe apples.
Table 3.
Hydrolysis ratio of chlorogenic acid to caffeic acid by Viscozyme L depending on the reaction time a
| Reaction time (h) | Chlorogenic acid (mmol/L) | Caffeic acid (mmol/L) | Hydrolysis Ratio (%) |
| 0 | 3.00 | 0 | 0 |
| 0.5 | 2.63 | 0.45 | 15.00 |
| 3.0 | 1.12 | 1.80 | 60.00 |
| 6.0 | 0.19 | 2.72 | 90.67 |
| 12.0 | 0.02 | 2.94 | 98.00 |
Reaction: the ratio of Viscozyme L to substrate 0.02 (2 FBG), pH 3.7, 50 °C
Fig.3.
HPLC chromatograms of hydrolysis of chlorogenic acid to caffeic acid by Viscozyme L
1: chlorogenic acid; 2: caffeic acid. Reaction: the ratio of Viscozyme L to substrate 0.02 (2 FBG), pH 3.7, 50 °C, with the reaction time of 0.5 h (a), 3.0 h (b) and 12.0 h (c)
The apple cell wall is a complex network that is composed of polysaccharides, such as cellulose, hemicelluloses, pectin, and lignin (Sun-Waterhouse et al., 2008; Le Bourvellec et al., 2009). Especially, lignin is the major phenolic constituent, deposited together with hydroxycinnamic acids (p-coumaric and ferulic acid), simple flavonoids, and procyanidin B2 (Landbo and Meyer, 2001; Pinelo et al., 2008). Hence, p-coumaric acid and ferulic acid have been recognized as indicators of decomposition of lignin during the enzyme hydrolysis process (Meyer et al., 1998). Furthermore, chlorogenic acid (3- or 5-caffeoylquinic acid) is the ester compound of caffeic acid and quinic acid, and the increase of caffeic acid could be observed by the carbohydrate-hydrolyzing enzyme treatment (Renard et al., 2001; Benoit et al., 2006; Zheng et al., 2008).
CONCLUSION
The most favorable condition of polyphenol extraction from unripe apples with enzyme treatment was determined to be the ratio of 0.02 for enzyme (Viscozyme L) to substrate (2 FBG), 50 °C, pH 3.7, 12 h hydrolysis reaction and extraction with 70% ethanol in the present study. Compared with the control treatment, TPC, RSC, and extraction yield of unripe apples increased by about 3, 1.5, and 2 times, respectively, and caffeic acid content increased by 32 times, associated with the decrease of chlorogenic acid. In order to obtain the optimum condition of carbohydrate-hydrolyzing enzyme treatment of the polyphenol extraction from unripe apples, further research using response surface methodology (RSM) is needed.
Footnotes
Project (No. GBTA2009-04) supported by Technology Development Program for Agriculture and Fishery, Gyeongsangbuk-Do, Korea
References
- 1.Adil İH, Çetin Hİ, Yener ME, Bayındırlı A. Subcritical (carbon dioxide+ethanol) extraction of polyphenol from apple and peach pomaces, and determination of the antioxidant activities of the extracts. J Supercrit Fluids. 2007;43(1):55–63. doi: 10.1016/j.supflu.2007.04.012. [DOI] [Google Scholar]
- 2.Ahn SC, Kim MS, Lee SY, Kang JH, Kim BH, Oh WK, Kim BY, Ahn JS. Increase of bioactive flavonoid aglycone extractable from Korean citrus peel by carbohydrate-hydrolyzing enzymes. Kor J Microbiol Biotechnol. 2005;33(4):288–294. (in Korea) [Google Scholar]
- 3.Akiyama H, Yuji S, Takahiro W, Megumi HN, Yasuo Y, Toshihiko S, Tomomasa K, Kiyoshi Y, Mamoru T, Reiko T, et al. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005;579(20):4485–4491. doi: 10.1016/j.febslet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Alonso-Salces RM, Herrero C, Barranco A, Berrueta LA, Gallo B, Vicente F. Classification of apple fruits according to their maturity state by the pattern recognition analysis of their polyphenolic compositions. Food Chem. 2005;93(1):113–123. doi: 10.1016/j.foodchem.2004.10.013. [DOI] [Google Scholar]
- 5.Awad MA, de Jager A, van der Plas LHW, van der Krol AR. Flavonoid and chlorogenic acid changes in skin of ‘Elstar’ and ‘Jonagold’ apples during development and ripening. Sci Hortic. 2001;90(1):69–83. doi: 10.1016/S0304-4238(00)00255-7. [DOI] [Google Scholar]
- 6.Benoit I, Navarro D, Marnet N, Rakotomanomana N, Meessen LL, Sigoillot JC, Asther M. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohyd Res. 2006;341(11):1820–1827. doi: 10.1016/j.carres.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Dueñas M, Hernández T, Estrella I. Changes in the content of bioactive polyphenolic compounds of lentils by the action of exogenous enzymes. effect on their antioxidant activity. Food Chem. 2007;101(1):90–97. doi: 10.1016/j.foodchem.2005.11.053. [DOI] [Google Scholar]
- 8.D′Angelo S, Cimmino A, Raimo M, Salvatore A, Zappla V, Galletti P. Effect of reddening-ripening on the antioxidant activity of polyphenol extracts from cv. ‘Annurca’ apple fruits. J Agric Food Chem. 2007;55(24):9977–9985. doi: 10.1021/jf071773a. [DOI] [PubMed] [Google Scholar]
- 9.Huang DJ, Ou BX, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 10.Khanizadeh S, Tsao R, Rekika D, Yang R, Charles MT, Rupasinghe HPV. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J Food Compos Anal. 2008;21(5):396–401. doi: 10.1016/j.jfca.2008.03.004. [DOI] [Google Scholar]
- 11.Kojima T, Akiyama S, Taniuchi S, Goda Y, Toyoda M, Kobayashi Y. Anti-allergic effect of apple polyphenol on patients with atopic dermatitis: a pilot study. Allergol Int. 2000;49(1):69–73. doi: 10.1046/j.1440-1592.2000.00161.x. [DOI] [Google Scholar]
- 12.Landbo AK, Meyer AS. Enzyme-assisted extraction of antioxidative phenols from black currant juice press residues (Ribes nigrum) J Agric Food Chem. 2001;49(7):3169–3177. doi: 10.1021/jf001443p. [DOI] [PubMed] [Google Scholar]
- 13.Le Bourvellec C, Guyot S, Renard CMGC. Interactions between apple (Malus x domestica Borkh.) polyphenol and cell walls modulate the extractability of polysaccharides. Carbohyd Polym. 2009;75(2):251–261. doi: 10.1016/j.carbpol.2008.07.010. [DOI] [Google Scholar]
- 14.Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93(1):47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- 15.Makris DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Compost Anal. 2007;20(2):125–132. doi: 10.1016/j.jfca.2006.04.010. [DOI] [Google Scholar]
- 16.Meyer AS, Jepsen SM, Sørensen NS. Enzymatic release of antioxidants for human low-density lipoprotein from grape pomace. J Agric Food Chem. 1998;46(7):2439–2446. doi: 10.1021/jf971012f. [DOI] [Google Scholar]
- 17.Miller GL. Modified DNS method for reducing sugars. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 18.Park MK, Kim CH. Extraction of polyphenols from apple peel using cellulase and pectinase and estimation of antioxidant activity. J Korean Soc Food Sci Nutr. 2009;38(5):535–540. doi: 10.3746/jkfn.2009.38.5.535. (in Korea) [DOI] [Google Scholar]
- 19.Park MW, Park YK, Kim ES. Properties of phenolic compounds in unripe apples. J East Asian Soc Dietary Life. 2004;14(4):343–347. (in Korea) [Google Scholar]
- 20.Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- 21.Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Tech. 2006;17(11):579–590. doi: 10.1016/j.tifs.2006.05.003. [DOI] [Google Scholar]
- 22.Pinelo M, Zornoza B, Meyer AS. Selective release of phenols from apple skin: mass transfer kinetics during solvent and enzyme-assisted extraction. Sep Purif Technol. 2008;63(3):620–627. doi: 10.1016/j.seppur.2008.07.007. [DOI] [Google Scholar]
- 23.Renard CMGC, Baron A, Guyot S, Drilleau JF. Interactions between apple cell walls and native apple polyphenol: quantification and some consequences. Int J Biol Macromol. 2001;29(2):115–125. doi: 10.1016/S0141-8130(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 24.Renard CMGC, Dupont N, Guillermin P. Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry. 2007;68(8):1128–1138. doi: 10.1016/j.phytochem.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910(2):265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 26.Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55(3):381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- 27.Silvina BL, Balz F. Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Radical Bio Med. 2004;36(2):201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenolic and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 29.Sørensen HR, Pedersen S, Anders VN, Meyer AS. Efficiencies of designed enzyme combinations in releasing arabinose and xylose from wheat arabinoxylan in an industrial ethanol fermentation residue. Enzyme Microb Tech. 2005;36(5-6):773–784. doi: 10.1016/j.enzmictec.2005.01.007. [DOI] [Google Scholar]
- 30.Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30(18):3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 31.Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenol and its effect on the rheological characteristics and cake making. Food Chem. 2007;104(2):686–692. doi: 10.1016/j.foodchem.2006.12.016. [DOI] [Google Scholar]
- 32.Sun-Waterhouse D, Melton LD, O′Connor CJ, Kilmartin PA, Smith BG. Effect of apple cell walls and their extracts on the activity of dietary antioxidants. J Agric Food Chem. 2008;56(1):289–295. doi: 10.1021/jf072670v. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Sun BG, Cao YP, Tian Y, Li XH. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106(2):804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 34.Weinberg ZG, Akiri B, Potoyevski E, Kanner J. Enhancement of polyphenol recovery from rosemary (Rosmarinus officinalis) and sage (Salvia officinalis) by enzyme-assisted ensiling (ENLAC) J Agric Food Chem. 1999;47(7):2959–2962. doi: 10.1021/jf981317+. [DOI] [PubMed] [Google Scholar]
- 35.Wu JH, Gao HY, Zhao L, Liao XJ, Chen F, Wang ZF, Hu XS. Chemical compositional characterization of some apple cultivars. Food Chem. 2007;103(1):88–93. doi: 10.1016/j.foodchem.2006.07.030. [DOI] [Google Scholar]
- 36.Yanagida A, Kanda T, Tanabe M, Matsudaira F, Oliveira CJG. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J Agric Food Chem. 2000;48(11):5666–5671. doi: 10.1021/jf000363i. [DOI] [PubMed] [Google Scholar]
- 37.Zheng HZ, Lee HR, Lee SH, Kim CS, Chung SK. Pectinase assisted extraction of polyphenol from apple pomace. Chin J Anal Chem. 2008;36(3):306–310. (in Chinese) [Google Scholar]