Abstract
Objective: To clarify the association of IL-6 polymorphisms and periodontitis, a meta-analysis of case-control studies and a systemic review were conducted. Material and methods: We performed a literature search using PubMed and Medline database to May 2009, with no restrictions. We also reviewed references from all retrieved articles. Six case-control studies involving 1 093 periodontitis cases and 574 controls were selected for meta-analysis to assess the purported associations between IL-6 polymorphisms and the risk of periodontitis. IL-6 −174 G/C and −572 C/G polymorphisms were included in the present meta-analysis, and the association between IL-6 −6331 T/C polymorphism and the risk of periodontitis was adequately reviewed as well. Results and conclusion: The present meta-analysis indicates that the IL-6 −174 G allele could not modify the risk of chronic periodontitis, but increased the risk of aggressive periodontitis. And −572 C/G polymorphism is associated with the pathogenesis of periodontitis, including chronic periodontitis or aggressive periodontitis.
Keywords: Interleukin-6, Polymorphism, Periodontitis, Risk, Meta-analysis
INTRODUCTION
Periodontitis is an opportunistic inflammatory disease of the periodontium. It is widely regarded as one of the most common diseases worldwide, with a prevalence of 10%~15% (Albandar and Rams, 2002). The pathogenesis of periodontitis involves more than virulent microorganisms (D′Aiuto et al., 2005; Kornman, 2008; Salvi et al., 2008). The systemic immune response, genetic factors, and environmental factors also affect the risk of developing periodontitis (Agrawal et al., 2006; Corey et al., 1993; van Dyke and Sheilesh, 2005). In recent years, studies have demonstrated that periodontitis is associated with elevated levels of a variety of inflammatory biomarkers (Loos, 2005; Loos et al., 2000; Shi et al., 2008; Slade et al., 2003). Furthermore, genetic variants of some cytokines confer susceptibility to periodontitis (Holla et al., 2008; Nibali et al., 2008a; Trevilatto et al., 2003).
Interleukin-6 (IL-6) is produced by many types of cells and is one of the most important mediators of the inflammatory response, in which it may play proinflammatory or anti-inflammatory role (Seruga et al., 2008; Woods et al., 2000). Several inflammatory diseases, including periodontitis, have been associated with elevated levels of IL-6 (Kishimoto, 2006; Loos et al., 2000). As the −174 G/C and −572 C/G polymorphisms of the IL-6 gene increase IL-6 expression (Fishman et al., 1998; Gu et al., 2008), they may be associated with susceptibility to periodontitis.
Trevilatto et al.(2003) first investigated the association between the IL-6 −174 G/C polymorphism and chronic periodontitis, and observed differences between controls and periodontitis patients in genotype and allele frequencies. Subsequently, many studies were published on whether the IL-6 −174 G/C polymorphism predisposes to periodontitis, but the results are contradictory (Babel et al., 2006; Moreira et al., 2007; Nibali et al., 2009). A similar issue exists concerning the relationship between the −572 C/G polymorphism and periodontitis (Holla et al., 2004; Komatsu et al., 2005; Nibali et al., 2009). As statistical power is limited by small sample sizes in case-control studies, we conducted a meta-analysis using pooled published data to examine the association between IL-6 polymorphisms and the risk of periodontitis.
MATERIALS AND METHODS
Search strategy
To identify all studies on the association between IL-6 polymorphisms and periodontitis, we conducted a systematic search of literature published before May 2009 using the Medline database (US National Library of Medicine, Bethesda, Maryland) and PubMed (National Center for Biotechnology, National Library of Medicine) and the key words, interleukin-6, IL-6, polymorphism, polymorphisms, and periodontitis. The full texts of the candidate articles were examined to determine whether they contained sufficient information on the association of the IL-6 −174 G/C and −572 C/G polymorphisms and the risk of periodontitis. Furthermore, references cited in the retrieved articles were screened to trace additional relevant studies.
Inclusion criteria
Articles were included in the meta-analysis if they met all the following criteria: (1) unrelated case-control experimental design, (2) genotype frequency documentation, and (3) frequency distributions for genotypes that do not deviate from Hardy-Weinberg equilibrium (HWE).
Data extraction
Two investigators independently reviewed all studies and extracted the data using a standardized form. Data were collected on the authors, year of publication, country of origin, study design (hospital based, population based, or nested), numbers of cases and controls with GG, GC, or CC genotypes, and type of periodontitis (Table 1).
Table 1.
Characteristics of studies focusing on IL-6 polymorphisms and periodontitis
| Study | Country | Study design | Case (GG/GC/CC) | Control (GG/GC/CC) | Form of disease |
| −174 polymorphism | |||||
| Trevilatto et al.(2003) | Brazil | Hospital c/c | 29/15/4 | 12/21/3 | Chronic |
| Holla et al.(2004) | Czech | Population c/c | 43/71/34 | 37/53/17 | Chronic |
| Brett et al.(2005) | UK | Population c/c | 52/37/17 | 55/19/25 | Aggressive, chronic |
| Wohlfahrt et al.(2006) | US | Hospital c/c | 48/63/26 | 32/36/14 | Chronic |
| Babel et al.(2006) | Germany | Population c/c | 72*/52 | 84*/32 | Chronic |
| Moreira et al.(2007) | Brazil | Hospital c/c | 98/48/9 | 30/18/6 | Aggressive, chronic |
| Tervonen et al.(2007) | Finland | Population c/c | 11/40† | 37/141† | Chronic |
| Nibali et al.(2008b) | UK | Hospital c/c | 139/66/17 | 110/91/30 | Aggressive |
| Nibali et al.(2009) | UK | Hospital c/c | 273/166/54 | 102/87/29 | Aggressive, chronic |
| −572 polymorphism | |||||
| Holla et al.(2004) | Czech | Population c/c | 139/9/0 | 86/21/0 | Chronic |
| Komatsu et al.(2005) | Japan | Hospital c/c | 5/36/71 | 4/32/41 | Chronic |
| Nibali et al.(2009) | UK | Population c/c | 11/36/220 | 3/30/185 | Chronic |
c/c: case/control
Total number of individuals carrying GG or GC genotype
Total number of individuals carrying GC or CC genotype
Statistical analysis
HWE analysis for genotype distribution among cases and controls was carried out using Pearson’s goodness-of-fit chi-squared test. The chi-square-based Q statistic test (Lau et al., 1997), which measures homogeneity between studies, was used to assess the presence of heterogeneity. A value of 0% for I 2 indicates the absence of heterogeneity and increasing percentages indicate increasing heterogeneity (Higgins et al., 2003). When heterogeneity was not significant, the results were pooled using a fixed effect model and the Mantel-Haenszel method, or a random effect model and the DerSimonian and Laird method (Petitti, 1994). Funnel plots were used to examine the publication bias of reported associations. The strength of associations between IL-6 polymorphisms and periodontitis was assessed according to the odds ratio (OR), which was calculated using the method of Woolf (1955). The significance of the pooled OR was determined using the Z-test. Analyses were performed using Review Manage version 5.0 software (RevMan, Oxford, England). A P value of <0.05 was considered statistically significant.
RESULTS
Eleven studies focusing on the topic of the association of IL-6 polymorphisms with priodontitis were detected (Babel et al., 2006; Brett et al., 2005; Holla et al., 2004; Jansson et al., 2006; Komatsu et al., 2005; Moreira et al., 2007; Nibali et al., 2008b; 2009; Tervonen et al., 2007; Trevilatto et al., 2003; Wohlfahrt et al., 2006). Of the 11 studies, 10 containing genotyping data were listed in Table 1. However, three studies were excluded from the present analysis because the number of individuals who carried a GG or CC genotype was not supplied (Babel et al., 2006; Jansson et al., 2006; Tervonen et al., 2007). Two studies (Brett et al., 2005; Nibali et al., 2008b) were also excluded, as they have overlapping patients with the study performed by Nibali et al.(2009), as clearly described in the paper. Finally, six studies composing of 1 093 periodontitis patients and 574 controls met the inclusion criteria. None of the frequency distributions for the genotypes studied deviated from the HWE of the controls (data not shown).
IL-6 −174 G/C polymorphism and periodontitis
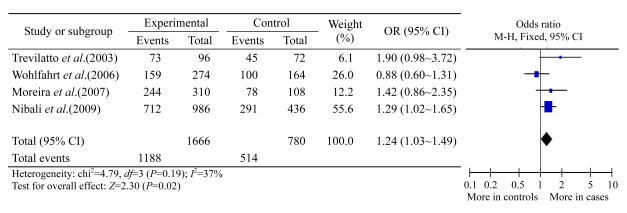
For IL-6 −174 G/C polymorphism, there are overlapping patients among three studies (Brett et al., 2005; Nibali et al., 2008b; 2009). Therefore, only the last study (Nibali et al., 2009) was included in the overall analysis, as it contains information on all of the above patients. Finally, five studies (Holla et al., 2004; Moreira et al., 2007; Nibali et al., 2009; Trevilatto et al., 2003; Wohlfahrt et al., 2006) composing of 981 periodontitis patients and 497 controls were involved in the following meta-analysis. Overall, no significant difference in allele frequency was found between the periodontitis cases and the healthy controls (OR: 1.13, 95% confidence interval (CI): 0.85~1.51). However, the pooled analysis showed strong heterogeneity (P=0.04, I 2=61%). This was caused by the study of Holla et al.(2004), which is an outlier in the funnel plots. After excluding this study, the heterogeneity of the meta-analysis decreased to be unremarkable (P=0.19, I 2=37%). As shown in Fig.1, the −174 G allele frequency was significantly higher for the pooled cases than for the corresponding controls (Z=2.30, P=0.02). The pooled OR was 1.24 (95% CI: 1.03~1.49), suggesting that carriers of the −174 G allele have a significantly higher risk of being predisposed to periodontitis. This result was confirmed by pooled analyses of genotypic frequency. Individuals carrying the GG genotype have a remarkably increased risk of periodontitis compared with individuals with the GC or CC genotype (OR: 1.35, 95% CI: 1.06~1.73, P=0.02), and there was no significant difference between study heterogeneities (P=0.12, I 2=49%). Of the 981 periodontitis patients and 497 controls mentioned above, 806 patients and 423 controls were Caucasians. The meta-analysis data were similar to the overall result: the −174 G allele frequency was significantly higher for the pooled cases than for the controls (P=0.04, OR: 1.24, 95% CI: 1.01~1.51). Furthermore, a significantly increased risk was associated with the GG genotype compared with the GC or CC genotype (OR: 1.39, 95% CI: 1.05~1.85, P=0.10 for heterogeneity). Overall, the IL-6 −174 G allele increased the risk of periodontitis.
Fig.1.
Meta-analysis of the association between the IL-6 −174 G allele and periodontitis
The numbers depicted under “events” and “total” represent the number of −174 G alleles out of all allele
Chronic periodontitis (CP) and aggressive periodontitis (AP) have distinct pathophysiological differences and may have different genetic predispositions; therefore, we performed separate analyses for the two forms of periodontitis. The data of Moreira et al.(2007) were excluded in the following meta-analysis, as they did not report the genotype data of CP subgroup or AP subgroup. Therefore, four studies (including 604 patients and 443 controls) were involved in the analysis of the association of IL-6 −174 G/C polymorphism with risk of CP. The pooled OR of the G allele versus the C allele was 0.98 (95% CI: 0.82~1.17, P=0.82) and there was no significant difference between study heterogeneities (P=0.11, I 2=50%) in the CP subgroup. In Caucasian population, the pooled analyses of allele frequency between CP cases and controls are similar (OR: 1.02, 95% CI: 0.84~1.23, P=0.07 for heterogeneity). Furthermore, there was no significant difference in genotypic frequency between the healthy controls and the CP cases (data not shown), suggesting that the −174 G allele and the −174 G/C polymorphism do not confer a risk of CP. Up to date, only one case-control study on 224 AP patients and 231 healthy controls was performed in order to detect the association of IL-6 −174 G/C polymorphism with risk of AP (Nibali et al., 2008b). The genotyping result indicated that IL-6 −174 G allele significantly increased the risk of AP in comparison with −174 C allele in subjects of all ethnicities (OR: 1.69, 95% CI: 1.26~2.27, P=0.0005). Compared with GC or CC genotype, the genotype GG was associated with a diagnosis of AP (OR: 1.86, 95% CI: 1.28~2.70, P=0.001). Similar results were obtained in Caucasian patients and controls (data not shown). In brief, the IL-6 −174 G allele could not modify the risk of CP, but increased the risk of AP. The association of IL-6 −174 G/C polymorphism and periodontitis risk is summarized in Table 2.
Table 2.
Association of IL-6 −174 G/C polymorphism and state of periodontitis*
| State of periodontitis | G vs C |
GG vs GC/CC |
||||||
| OR (95% CI) | P | I2 (%) | P† | OR (95% CI) | P | I2 (%) | P† | |
| Chronic periodontitis | 0.98 (0.82~1.17) | 0.82 | 50 | 0.11 | 0.84 (0.59~1.18) | 0.32 | 0 | 0.78 |
| Aggressive periodontitis | 1.69 (1.26~2.27) | 0.0005 | – | – | 1.86 (1.28~2.70) | 0.001 | – | – |
| Overall | 1.24 (1.03~1.49) | 0.02 | 37 | 0.19 | 1.35 (1.06~1.73) | 0.02 | 49 | 0.12 |
Most subjects involved in meta-analysis were Caucasians
P value for heterogeneity
IL-6 −572 C/G polymorphism and periodontitis
Three original investigations, including 527 patients and 402 controls, were involved in studies on the IL-6 −572 C/G polymorphism among CP cases (Holla et al., 2004; Komatsu et al., 2005; Nibali et al., 2009). The meta-analysis showed that individuals carrying the GG genotype have a remarkably increased risk of developing CP compared with individuals with the GC or CC genotype (OR: 2.65, P=0.002; 95% CI: 1.44~4.87, P=0.18 for heterogeneity). No significant difference was detected in allelic frequency distribution between CP cases and controls. However, the pooled analysis was strongly heterogeneous (P=0.004). This was caused by the study of Holla et al.(2004), which is an outlier in the funnel plots with a high OR. When this study was excluded, the heterogeneity of the meta-analysis decreased (P=0.07). The second meta-analysis also showed that there was no significant difference in allelic frequency distribution and genotypic frequency distribution (under the dominant or co-dominant genetic model) between CP patients and controls (data not shown). We did not conduct a meta-analysis of the association between the −572 C/G polymorphism and AP because only one relevant study was detected (Nibali et al., 2008b).
DISCUSSION
It is widely accepted that cytokines and oral pathogens play central roles in the inflammatory process associated with the etiology of periodontitis (Taylor et al., 2004; Wu et al., 2007; He and Shi, 2009). As a pleiotropic cytokine, IL-6 is induced in response to several inflammatory stimuli (Terry et al., 2000). IL-6 also regulates the expression of cytokines such as IL-1, IL-10, and tumor necrosis factor-α (Opal and DePalo, 2000; Woods et al., 2000). IL-6 is present in endothelial cells, fibroblasts, and macrophages in periodontitis patients, but is absent from these cells in healthy individuals (Takahashi et al., 1994). Clinical studies indicate that IL-6 plays a crucial role in the inflammatory response to Gram-negative bacteria (Dalrymple et al., 1996) by affecting the composition of the subgingival microbiota and increasing the susceptibility to colonization with periodontopathogenic bacteria (Cooke and Hill, 2001; Ishihara et al., 1997; Coats et al., 2009). IL-6 is also a potent stimulator of osteoclast differentiation and bone resorption (Gemmell et al., 1997; Hughes et al., 2006), which are typical of periodontitis. The intensity of IL-6 expression is positively correlated with attachment loss (Moreira et al., 2007) and IL-6 is associated with continuous tissue destruction in periodontitis (McCauley and Nohutcu, 2002). Furthermore, levels of IL-6 are elevated in periodontitis patients (Loos, 2005; Loos et al., 2000) and decreased after successful periodontal therapy (D′Aiuto et al., 2004a; 2004b). Thus, the available evidence indicates that IL-6 is involved in the pathogenesis of periodontitis.
Because of the notion that genetic factors may predispose to periodontitis, several studies had been conducted to determine whether IL-6 gene polymorphisms predispose to periodontitis. However, the results of studies on the associations between these polymorphisms and clinical forms of periodontitis are contradictory. By and large, our meta-analysis showed that carriers of the −174 G allele had an increased risk of developing periodontitis. And specifically, the IL-6 −174 G allele could not modify the risk of CP, but increased the risk of AP. Furthermore, compared with −572 C allele carriers (genotypes GC and CC), individuals carrying the GG genotype had a significantly higher risk of CP. These findings suggest that common polymorphisms in the IL-6 promoter region modify the risk of periodontitis.
A recent study also reviewed the association between the IL-6 −174 G/C polymorphism and periodontitis (Nikolopoulos et al., 2008). It is difficult to compare their results directly with ours for three reasons. First, the previous review focused on the pathogenesis of CP, whereas we paid more attention to whether common IL-6 polymorphisms increase the risk of aggressive or chronic types of periodontitis. Second, our review is the more comprehensive one of the two, as we identified 6 studies on the association between IL-6 polymorphisms and periodontitis compared with four studies in the other report. Finally, our study is the first to summarize the association between the IL-6 −572 C/G polymorphism and periodontitis.
After the initial meta-analysis of the −174 G/C polymorphism and CP, a case-control study (Nibali et al., 2009) involving 271 CP patients and 144 controls was published and was included in a subsequent analysis. There was no significant association between this polymorphism and CP, which is in accordance with the results of the previous analysis (Nikolopoulos et al., 2008). Notably, in the study Caucasian Brazilians genotype GG was statistically associated with susceptibility to severe form of CP (Trevilatto et al., 2003). The result reminds us that the association of IL-6 −174 G/C polymorphism with severe form of CP should be paid more attention to in subsequent investigations. Moreover and interestingly, one association study performed by Nibali et al.(2008b), which involved 224 AP patients and 231 controls, revealed that the −174 G allele is statistically significantly associated with an increased risk of AP. Although the sample size was not large enough, the results nevertheless indicated that the IL-6 −174 G allele is associated with susceptibility to AP. Compared with the −174 C allele, the −174 G allele significantly increased the expression of IL-6 (Fishman et al., 1998), which explains the positive relationship between the −174 G/C polymorphism and AP. Furthermore, Nibali et al.(2008c) revealed that the IL-6 −174 GG genotype is associated with an increased OR of concomitant detection of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. These results imply that the IL-6 −174 G/C polymorphism cause periodontitis by altering immune competence.
The IL-6 −572 C/G polymorphism has also been studied in the context of susceptibility to periodontitis (Holla et al., 2004; Komatsu et al., 2005; Nibali et al., 2009). When we pooled data from previous studies, there was no remarkable difference in allelic frequency distribution between CP cases and controls. However, compared with C allele carriers (genotypes GC and CC), the GG genotype significantly increased the risk of CP, suggesting that the −572 G allele is associated with the pathogenesis of CP under the recessive genetic model. A meta-analysis of the association between the −572 C/G polymorphism and AP was not conducted because only one relevant study was detected (Nibali et al., 2008b). According to genotyping data (Nibali et al., 2008b), the −572 G allele significantly increased the risk of developing AP compared with the −572 C allele (OR: 1.79, 95% CI: 1.18~2.72, P=0.006). Therefore, on the basis of the aforementioned evidence, we concluded that the −572 C/G polymorphism is associated with the pathogenesis of periodontitis, as it predisposes to either CP or AP. However, as one study has shown that the −572 G polymorphism reduces the transcriptional activity of the IL-6 promoter (Gu et al., 2008), the mechanism of this polymorphism predisposed to periodontitis seems too complicated. Herein, functional investigations on the association of IL-6 −572 C/G polymorphism with periodontitis are warranted. Besides the well-discussed IL-6 −174 G/C and −572 C/G polymorphisms, the −6331 T/C polymorphism in the promoter region of the gene could also alter the transcriptional activity of IL-6 (Smith et al., 2008). Actually, the IL-6 −6331 T/C polymorphism is in complete linkage disequilibrium with the −6106 A/T polymorphism. Nibali et al.(2009; 2008b) recently reported that −6106 TT increased the risk of localized AP in Caucasian population. Therefore, we could conclude that IL-6 −6331 T/C polymorphism is involved in the pathogenesis of periodontitis among Caucasians. However, the association of IL-6 −6331 T/C polymorphism with risk of periodontitis requires to be confirmed in other ethnics.
The power of mapping and characterization analyses of disease-causing genes is significantly increased when association studies are based on haplotypes instead of genotypes (Akey et al., 2001). Holla et al.(2004) and Nibali et al.(2009) hypothesized that IL-6 haplotypes confer susceptibility to periodontitis. As a result, some haplotypes of IL-6 gene were discovered to be associated with the risk of developing periodontitis. However, it is difficult to determine the role of a particular haplotype in disease susceptibility using meta-analysis.
In conclusion, our meta-analysis indicates that the IL-6 −174 G allele could not modify the risk of CP, but increased the risk of AP. And −572 C/G polymorphism is associated with the pathogenesis of periodontitis, as it predisposes to either CP or AP. The association of IL-6 −174 G/C polymorphism with severe form of CP should be paid more attention to in future investigations.
Acknowledgments
We thank the authors of the studies used for this meta-analysis for providing evidence of the association between IL-6 polymorphisms and periodontitis. None of the authors have any conflict of interest in relation to this study.
References
- 1.Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J Periodontol. 2006;77(9):1515–1521. doi: 10.1902/jop.2006.050427. [DOI] [PubMed] [Google Scholar]
- 2.Akey J, Jin L, Xiong M. Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet. 2001;9(4):291–300. doi: 10.1038/sj.ejhg.5200619. [DOI] [PubMed] [Google Scholar]
- 3.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontology 2000. 2002;29(1):7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 4.Babel N, Cherepnev G, Babel D, Tropmann A, Hammer M, Volk HD, Reinke P. Analysis of tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-10, IL-6, and interferon-gamma gene polymorphisms in patients with chronic periodontitis. J Periodontol. 2006;77(12):1978–1983. doi: 10.1902/jop.2006.050315. [DOI] [PubMed] [Google Scholar]
- 5.Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D′Aiuto F, Tonetti M. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005;84(12):1149–1153. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- 6.Coats SR, To TT, Jain S, Braham PH, Darveau RP. Porphyromonas gingivalis resistance to polymyxin B is determined by the lipid A 4′-phosphatase, PGN_0524. Int J Oral Sci. 2009;1(3):126–135. doi: 10.4248/IJOS.09062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2(12):967–977. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 8.Corey LA, Nance WE, Hofstede P, Schenkein HA. Self-reported periodontal disease in a Virginia twin population. J Periodontol. 1993;64(12):1205–1208. doi: 10.1902/jop.1993.64.12.1205. [DOI] [PubMed] [Google Scholar]
- 9.D′Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 10.D′Aiuto F, Parkar M, Brett PM, Ready D, Tonetti MS. Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine. 2004;28(1):29–34. doi: 10.1016/j.cyto.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.D′Aiuto F, Graziani F, Tete S, Gabriele M, Tonetti MS. Periodontitis: from local infection to systemic diseases. Int J Immunopathol Pharmacol. 2005;18(3 Suppl.):1–11. [PubMed] [Google Scholar]
- 12.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64(8):3231–3235. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontology 2000. 1997;14(1):112–143. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 15.Gu W, Du DY, Huang J, Zhang LY, Liu Q, Zhu PF, Wang ZG, Jiang JX. Identification of interleukin-6 promoter polymorphisms in the Chinese Han population and their functional significance. Crit Care Med. 2008;36(5):1437–1443. doi: 10.1097/CCM.0b013e31816a0adb. [DOI] [PubMed] [Google Scholar]
- 16.He XS, Shi WY. Oral microbiology: past, present and future. Int J Oral Sci. 2009;1(2):47–58. doi: 10.4248/IJOS.09029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holla LI, Fassmann A, Stejskalova A, Znojil V, Vanek J, Vacha J. Analysis of the interleukin-6 gene promoter polymorphisms in Czech patients with chronic periodontitis. J Periodontol. 2004;75(1):30–36. doi: 10.1902/jop.2004.75.1.30. [DOI] [PubMed] [Google Scholar]
- 19.Holla LI, Fassmann A, Augustin P, Halabala T, Znojil V, Vanek J. The association of interleukin-4 haplotypes with chronic periodontitis in a Czech population. J Periodontol. 2008;79(10):1927–1933. doi: 10.1902/jop.2008.080035. [DOI] [PubMed] [Google Scholar]
- 20.Hughes FJ, Turner W, Belibasakis G, Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontology 2000. 2006;41(1):48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara Y, Nishihara T, Kuroyanagi T, Shirozu N, Yamagishi E, Ohguchi M, Koide M, Ueda N, Amano K, Noguchi T. Gingival crevicular interleukin-1 and interleukin-1 receptor antagonist levels in periodontally healthy and diseased sites. J Periodontal Res. 1997;32(6):524–529. doi: 10.1111/j.1600-0765.1997.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 22.Jansson H, Lyssenko V, Gustavsson A, Hamberg K, Soderfeldt B, Groop L, Bratthall G. Analysis of the interleukin-1 and interleukin-6 polymorphisms in patients with chronic periodontitis. A pilot study. Swed Dent J. 2006;30(1):17–23. [PubMed] [Google Scholar]
- 23.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl. 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu Y, Tai H, Galicia JC, Shimada Y, Endo M, Akazawa K, Yamazaki K, Yoshie H. Interleukin-6 (IL-6) −373 A9T11 allele is associated with reduced susceptibility to chronic periodontitis in Japanese subjects and decreased serum IL-6 level. Tissue Antigens. 2005;65(1):110–114. doi: 10.1111/j.1399-0039.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 25.Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79(8 Suppl.):1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 26.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl.):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 28.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71(10):1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 29.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J Periodontol. 2002;73(11):1377–1391. doi: 10.1902/jop.2002.73.11.1377. [DOI] [PubMed] [Google Scholar]
- 30.Moreira PR, Lima PM, Sathler KO, Imanishi SA, Costa JE, Gomes RS, Gollob KJ, Dutra WO. Interleukin-6 expression and gene polymorphism are associated with severity of periodontal disease in a sample of Brazilian individuals. Clin Exp Immunol. 2007;148(1):119–126. doi: 10.1111/j.1365-2249.2007.03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibali L, Donos N, Brett PM, Parkar M, Ellinas T, Llorente M, Griffiths GS. A familial analysis of aggressive periodontitis—clinical and genetic findings. J Periodontal Res. 2008;43(6):627–634. doi: 10.1111/j.1600-0765.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 32.Nibali L, Griffiths GS, Donos N, Parkar M, D′Aiuto F, Tonetti MS, Brett PM. Association between interleukin-6 promoter haplotypes and aggressive periodontitis. J Clin Periodontol. 2008;35(3):193–198. doi: 10.1111/j.1600-051X.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 33.Nibali L, Tonetti MS, Ready D, Parkar M, Brett PM, Donos N, D′Aiuto F. Interleukin-6 polymorphisms are associated with pathogenic bacteria in subjects with periodontitis. J Periodontol. 2008;79(4):677–683. doi: 10.1902/jop.2008.070453. [DOI] [PubMed] [Google Scholar]
- 34.Nibali L, D′Aiuto F, Donos N, Griffiths GS, Parkar M, Tonetti MS, Humphries SE, Brett PM. Association between periodontitis and common variants in the promoter of the interleukin-6 gene. Cytokine. 2009;45(1):50–54. doi: 10.1016/j.cyto.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Nikolopoulos GK, Dimou NL, Hamodrakas SJ, Bagos PG. Cytokine gene polymorphisms in periodontal disease: a meta-analysis of 53 studies including 4178 cases and 4590 controls. J Clin Periodontol. 2008;35(9):754–767. doi: 10.1111/j.1600-051X.2008.01298.x. [DOI] [PubMed] [Google Scholar]
- 36.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 37.Petitti DB. Statistical Methods in Meta-Analysis. In: Petitti DB, editor. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis. New York: Oxford University Press; 1994. pp. 90–114. [Google Scholar]
- 38.Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl.):398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 39.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 40.Shi D, Meng H, Xu L, Zhang L, Chen Z, Feng X, Lu R, Sun X, Ren X. Systemic inflammation markers in patients with aggressive periodontitis: a pilot study. J Periodontol. 2008;79(12):2340–2346. doi: 10.1902/jop.2008.080192. [DOI] [PubMed] [Google Scholar]
- 41.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163(10):1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 42.Smith AJ, D′Aiuto F, Palmen J, Cooper JA, Samuel J, Thompson S, Sanders J, Donos N, Nibali L, Brull D, et al. Association of serum interleukin-6 concentration with a functional IL6 −6331T>C polymorphism. Clin Chem. 2008;54(5):841–850. doi: 10.1373/clinchem.2007.098608. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Takashiba S, Nagai A, Takigawa M, Myoukai F, Kurihara H, Murayama Y. Assessment of interleukin-6 in the pathogenesis of periodontal disease. J Periodontol. 1994;65(2):147–153. doi: 10.1902/jop.1994.65.2.147. [DOI] [PubMed] [Google Scholar]
- 44.Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontology 2000. 2004;35(1):158–182. doi: 10.1111/j.0906-6713.2004.003561.x. [DOI] [PubMed] [Google Scholar]
- 45.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275(24):18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 46.Tervonen T, Raunio T, Knuuttila M, Karttunen R. Polymorphisms in the CD14 and IL-6 genes associated with periodontal disease. J Clin Periodontol. 2007;34(5):377–383. doi: 10.1111/j.1600-051X.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 47.Trevilatto PC, Scarel-Caminaga RM, de Brito RBJr, de Souza AP, Line SR. Polymorphism at position −174 of IL-6 gene is associated with susceptibility to chronic periodontitis in a Caucasian Brazilian population. J Clin Periodontol. 2003;30(5):438–442. doi: 10.1034/j.1600-051X.2003.20016.x. [DOI] [PubMed] [Google Scholar]
- 48.van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7(1):3–7. [PMC free article] [PubMed] [Google Scholar]
- 49.Wohlfahrt JC, Wu T, Hodges JS, Hinrichs JE, Michalowicz BS. No association between selected candidate gene polymorphisms and severe chronic periodontitis. J Periodontol. 2006;77(3):426–436. doi: 10.1902/jop.2006.050058. [DOI] [PubMed] [Google Scholar]
- 50.Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J. 2000;21(19):1574–1583. doi: 10.1053/euhj.1999.2207. [DOI] [PubMed] [Google Scholar]
- 51.Woolf B. On estimating the relationship between blood group and disease. Ann Human Genet. 1955;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu YM, Yan J, Chen LL, Gu ZY. Association between infection of different strains of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival plaque and clinical parameters in chronic periodontitis. J Zhejiang Univ Sci B. 2007;8(2):121–131. doi: 10.1631/jzus.2007.B0121. [DOI] [PMC free article] [PubMed] [Google Scholar]