Abstract
The ascomycete Cymadothea trifolii, a member of the Dothideomycetes, is unique among obligate biotrophic fungi in its capability to only partially degrade the host cell wall and in forming an astonishingly intricate interaction apparatus (IA) in its own hyphae, while the attacked host plant cell is triggered to produce a membranous bubble opposite the IA. However, no sequence data are currently available for this species. Based on molecular phylogenetic results obtained from complete SSU and partial LSU data, we show that the genus Cymadothea belongs to the Mycosphaerellaceae (Capnodiales, Dothideomycetes). This is the first report of sequences obtained for an obligate biotrophic member of Mycosphaerellaceae.
Keywords: biotrophy, Capnodiales, Cymadothea trifolii, Dothideomycetes, GenomiPhi, LSU, Mycosphaerella kilianii, Mycosphaerellaceae, sooty/black blotch of clover, SSU
INTRODUCTION
The obligate biotrophic ascomycete Cymadothea trifolii (Dothideomycetes, Ascomycota) is the causal agent of sooty/black blotch of clover. Although the fungus is not regarded as a serious agricultural pathogen, it has a significant impact on clover plantations used for animal nutrition, and is often found at natural locations. In one study it was observed that sooty blotch was “the most frequently recorded disease” at sampling sites in England and Wales, with the number of leaves damaged ranging from 4–21 % (Lewis & Thomas 1991). Cymadothea trifolii is widespread on Trifolium species (Fabaceae), but has also been reported on alfalfa (Medicago sativa) (Puschner 2005). The fungus is considered a likely cause of edema, erythema, vesiculation and necrosis of the light pigment areas of horses grazing infected clover (Puschner 2005). It is characterised by small black pustules, the stromata, on the lower side of clover leaflets. The asexual state (Polythrincium trifolii) can easily be identified by thick-walled, melanised, sympodially growing conidiophores with a spiral appearance. In its sexual state it produces pseudothecial ascomata and spermatogonia. Infection of the host occurs via stomata (Roderick 1993, Simon et al. 2005b). Inside the leaf the fungus proliferates intercellularly, but forms an intricate interaction apparatus (IA) to obtain nutrients from its host (Simon et al. 2004). During the interaction with the attacked host cell the wall of the latter is partially degraded. Pectins are dissolved while cellulose and xyloglucans remain intact (Simon et al. 2005a). This structure is thus far unique among ascomycetes.
Although the morphology of C. trifolii has been accurately documented (Wolf 1935), no molecular evidence is currently available to clarify its taxonomy. Due to the unique interaction this obligate pathogen has with its host, the aim of the present study was to obtain DNA sequence data to resolve its phylogenetic position.
MATERIALS AND METHODS
Sampling
Infected leaves of Trifolium repens were collected at the edge of an alfalfa (Medicago sativa) field near Hohenentringen (Tübingen, Baden-Württemberg, Germany) on 31 July 2007 (Herbarium CBS H-20110). Furthermore, 53 species from the CBS culture collection were included to supplement sequences obtained from GenBank due to the paucity of complete small subunit (SSU) data of related fungal nuclear ribosomal DNA in GenBank (Table 1).
Table 1.
Species names, culture collection and GenBank accession numbers of fungal strains used in this study. The GenBank accession number is for the concatenated 18S rDNA, ITS1, 5.8S rDNA, ITS2 and partial 28S rDNA sequence, unless otherwise indicated.
| Species | Strain no.1 | GenBank no. |
|---|---|---|
| Ascochyta fabae | CBS 114.36 | EU167566 |
| Ascochyta pisi var. pisi | CBS 108.26 | EU167557 |
| Ascochyta vicia-pannonicae | CBS 254.92 | EU167559 |
| Ascochyta viciae-villosae | CBS 255.92 | EU167560 |
| Asteroma alneum | CBS 109840 | EU167609 |
| Bagnisiella examinans | CBS 551.66 | EU167562 |
| Cercospora beticola | CBS 116456 | AY840527 |
| Cladosporium sp. 1 | CBS 280.49 | EU167574 |
| Cladosporium sp. 2 | CBS 282.49 | EU167586 |
| Cladosporium sp. 3 | CBS 266.53 | EU167592 |
| Cymadothea trifolii | Herbarium CBS H-20110 | EU167612 (SSU) |
| Herbarium CBS H-20110 | EU167613 (SSU) | |
| Herbarium CBS H-20110 | EU167610 (LSU) | |
| Herbarium CBS H-20110 | EU167611 (LSU) | |
| Davidiella macrospora | CBS 138.40 | EU167591 |
| Davidiella tassiana | CBS 723.79 | EU167558 |
| Didymella bryoniae | CBS 233.52 | EU167573 |
| Didymella exitialis | CBS 446.82 | EU167564 |
| Didymella phacae | CBS 184.55 | EU167570 |
| Didymella rabiei | CBS 237.37 | EU167600 |
| Dothidea berberidis | CBS 186.58 | EU167601 |
| Dothidea muelleri | CBS 191.58 | EU167593 |
| Guignardia vaccinii | CBS 114751 | EU167584 |
| Kabatiella caulivora | CBS 242.64 | EU167576 |
| Kabatiella microsticta | CBS 342.66 | EU167608 |
| Mycosphaerella aleuritidis | CBS 282.62 | EU167594 |
| Mycosphaerella arbuticola | CBS 355.86 | EU167571 |
| Mycosphaerella berberidis | CBS 324.52 | EU167603 |
| Mycosphaerella brassicicola | CBS 174.88 | EU167607 |
| Mycosphaerella coacervata | CBS 113391 | EU167596 |
| Mycosphaerella crystallina | CBS 681.95 | EU167579 |
| Mycosphaerella flageoletiana | CBS 114302 | EU167597 |
| Mycosphaerella fragariae | CBS 719.84 | EU167605 |
| Mycosphaerella gregaria | CBS 110501 | EU167580 |
| Mycosphaerella handelii | CBS 113302 | EU167581 |
| Mycosphaerella harthensis | CBS 325.52 | EU167602 |
| Mycosphaerella laricina | CBS 326.52 | EU167595 |
| Mycosphaerella linorum | CBS 261.39 | EU167590 |
| Mycosphaerella microsora | CBS 100352 | EU167599 |
| Mycosphaerella milleri | CBS 541.63 | EU167577 |
| Mycosphaerella punctata | CBS 113315 | EU167582 |
| Mycosphaerella populicola | CBS 100042 | EU167578 |
| Mycosphaerella pseudoellipsoidea | CBS 114709 | EU167585 |
| Mycosphaerella punctiformis | CBS 113265 | EU167569 |
| Mycosphaerella pyri | CBS 100.86 | EU167606 |
| Mycosphaerella grossulariae | CBS 235.37 | EU167588 |
| Mycosphaerella rosigena | CBS 330.51 | EU167587 |
| Mycosphaerella rubi | CBS 238.37 | EU167589 |
| Mycosphaerella stromatosa | CBS 101953 | EU167598 |
| Phaeosphaeria rousseliana | CBS 580.86 | EU167604 |
| Phoma exigua var. exigua | CBS 118.94 | EU167567 |
| Phoma medicaginis var. medicaginis | CBS 533.66 | EU167575 |
| Phoma pinodella | CBS 110.32 | EU167565 |
| Phoma sojicola | CBS 567.97 | EU167568 |
| Pleiochaeta ghindensis | CBS 552.92 | EU167561 |
| Pleiochaeta setosa | CBS 496.63 | EU167563 |
| Pseudocercospora vitis | CPC 11595 | DQ073923 |
| Ramichloridium cerophilum | CBS 103.59 | EU041798 |
| Schizothyrium pomi | CBS 486.50 | EF134948 |
| CBS 406.61 | EF134949 | |
| Teratosphaeria fibrillosa | CPC 1876 | EU019282 |
| Teratosphaeria microspora | CBS 101951 | EU167572 |
| Teratosphaeria molleriana | CBS 118359 | EU167583 |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
DNA extraction and amplification
Approximately 12 conidial stromata of C. trifolii were dissected from one spot of an infected leaf with a sterilised razor blade, and washed in 20 μL of AE-buffer (Qiagen, Hilden, Germany). Each stroma was examined with a light microscope to check for possible contaminations with other fungi. Apparently uncontaminated stromata were collected in a fresh drop of AE-buffer, gently washed, and re-examined before placing them onto another 20 μL drop of AE-buffer in an Eppendorf tube (1.5 mL). The procedure was repeated from another spot on the same leaf. This method was chosen because earlier attempts to isolate DNA of this fungus had always resulted in contaminations with other species of fungi (not shown). Secondly, it allowed us to exclude plant material.
To break up the thick melanised cell walls of conidiophores and conidia, the cups containing fungal material were placed in liquid nitrogen for 5 min and heated immediately afterwards for 5 min at 96 °C in a heating block (Dri-block DB-2A, Techne, Cambridge, UK). This step was repeated twice. Because little DNA was present in the samples, the whole genome was amplified using the GenomiPhi kit (GE Healthcare, Munich, Germany) according to the manufacturer’s protocol: 1 μL of each sample was placed into 9 μL of sample buffer and placed in a heating block (Techne) at 96 °C for 3 min, cooled down on ice and mixed with 10 μL of a prepared solution consisting of 9 μL reaction buffer and 1 μL enzyme mix. The resulting 20 μL solution was incubated for 24 h at 30.5 °C in a thermal cycler (model 2720, Applied Biosystems, Foster City, CA, USA) equipped with a heated lid. Afterwards, the samples were heated up to 96 °C for 10 min in a heating block (Techne) and subsequently cooled on ice to stop polymerase activity of the kit. DNA was extracted with the DNAeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions.
Additionally, DNA was isolated from 52 fungi of the class Dothideomycetes and one fungus of the class Sordariomycetes from the CBS culture collection (Table 1) using a CTAB-based method modified from Möller et al. (1992) as described in Gams et al. (2007).
Polymerase chain reactions (PCRs) were performed in a total volume of 50 μL containing 5 μL 10 × PCR-buffer (Life Technologies, Eggenstein, Germany), 34.1 μL H2O, 2 μL MgCl2 (50mM, Life Technologies), 2 μL dNTPs (5 mM, Life Technologies), 1 μL forward and 1 μL reverse primers (25 pmol/μL each), 0.2 μL Bovine Serum Albumin (1 %, BSA, Sigma-Aldrich, Munich, Germany), 0.2 μL Taq polymerase (Life Technologies) and 5 μL DNA extract diluted 1 : 10. The following primers were used for amplification: SSU: a) forward: NS17, NS19, NS21, NS23, b) reverse: NS18, NS20, NS22, NS24 (Gargas & Taylor 1992); LSU: a) forward: LR0R (Rehner & Samuels 1994), b) reverse: LR5 (Vilgalys & Hester 1990). PCR was carried out on a 2720 Thermal Cycler (Applied Biosystems) equipped with a heated lid. Initial denaturation and enzyme activation took place at 94 °C for 5 min and was followed by amplification for 35 cycles. The parameters were as follows: 30 s at 94 °C, 90 s at either 50, 55, 60 or 65 °C (depending on primers), 4 min at 72 °C, plus a final 7 min extension at 72 °C with subsequent cooling down to 4 °C.
Sequencing
For cycle sequencing the same primers were applied as for PCR using the ABI PRISM BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer’s protocol, but with a reaction volume of 10 μL, and the enzyme diluted 1 : 6 with the supplied dilution buffer. Electrophoresis and data sampling were performed on an ABI 3100 Genetic Analyser (Applied Biosystems). Sequences were manually edited with SEQUENCHERTM v. 4.1.2 (Gene Codes Corporation, Ann Arbor, Mi, USA).
Phylogenetic analyses
DNA sequences were assembled, added to the outgroup and complemented with further GenBank sequences using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002). Manual adjustments for improvement were made by eye where necessary. Any large insertions were excluded. Phylogenetic analyses of sequence data were done with PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003) and consisted of neighbour-joining analysis with the uncorrected (“p”), the Kimura 2-parameter and the HKY85 substitution model. Alignment gaps were treated as missing data and all characters were unordered and of equal weight. Any ties were broken randomly when encountered. For parsimony analysis, alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1 000 bootstrap replications (Hillis & Bull 1993). Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC) were calculated and the resulting trees were printed with TreeView v. 1.6.6 (Page 1996).
Bayesian analysis was conducted on the same aligned dataset after MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model for each data partition (18S rDNA and 28S rDNA). Phylogenetic analyses were performed with MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003) applying a general time-reversible (GTR) substitution model with gamma (G) and proportion of invariable site (I) parameters to accommodate variable rates across sites and with inverse gamma rates and dirichlet base frequencies. The Markov Chain Monte Carlo (MCMC) analysis of four chains started with a heating parameter of 0.1 from a random tree topology and lasted 1 031 000 generations. Trees were saved each 100 gene- rations, resulting in 10 311 saved trees. Burn-in was set at 2 500 generations after which the likelihood values were stationary, leaving 7 811 trees from which the 50 % majority rule consensus trees and posterior probabilities were calculated. All trees were rooted with Asteroma alneum (Sordariomycetes) as outgroup taxon.
RESULTS
Complete SSU and partial LSU sequences were obtained for Cymadothea trifolii using the GenomiPhi Kit (GE Healthcares). Despite repeated attempts and specific primer design we were unable to obtain ITS sequences for this species. Sequence comparisons of the two isolations showed that both isolates were 100 % identical. Additionally, we were able to generate sequences from another 19 species thus far absent from GenBank (checked both by CoreNucleotide search of names and by using BLAST searches against the NCBI database, (http://www.ncbi.nlm.nih.gov/)). GenBank accession numbers are provided in Table 1.
The manually adjusted alignment contains 60 taxa plus the outgroup sequence. 2 419 characters including alignment gaps (available in TreeBASE) were used for phylogenetic analyses. Of these, 371 were parsimony-informative, 154 were variable and parsimony-uninformative, and 1 894 were constant. Neighbour-joining analyses using the three substitution models on the sequence data yielded trees with similar overall topology and bootstrap support values. The parsimony analysis yielded 141 equally most parsimonious trees (TL = 1 438 steps; CI = 0.524; RI = 0.855; RC = 0.448), the first of which is shown in Fig. 1. These trees differed mainly with regard to the order of taxa in the Pleosporales and in the Mycosphaerellaceae (see thickened consensus lines in Fig. 1). Also, the Schizothyriaceae was placed within the Mycosphaerellaceae in some of these trees. MrModeltest identified the same substitution parameters for both data partitions. The Bayesian analysis resulted in a consensus tree (Fig. 2) with the same topology and clades as that obtained from the parsimony analysis.
Fig. 1.
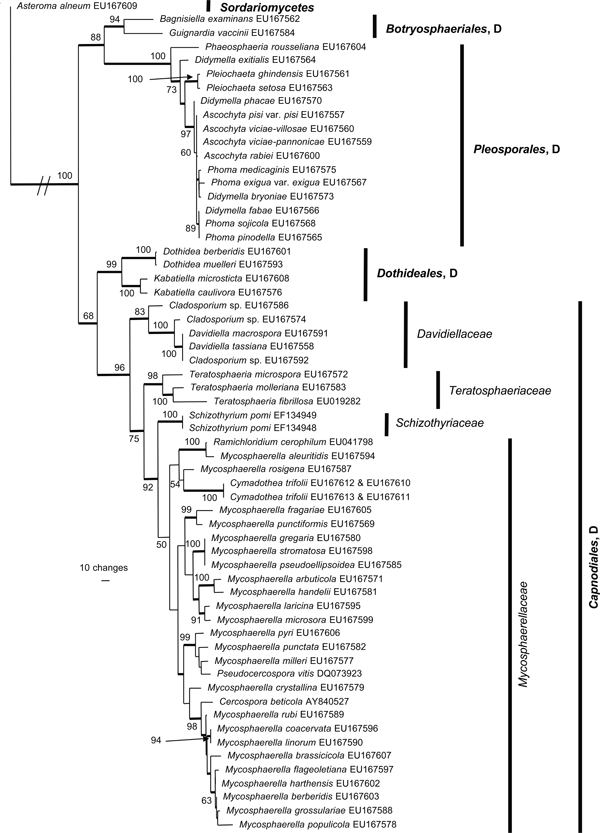
One of 141 equally most parsimonious trees obtained from the combined SSU and LSU sequence alignment. The scale bar shows 10 changes and bootstrap support values from 1 000 replicates are shown at the nodes. The tree was rooted to Asteroma alneum. The relevant order and class names are given on the right with family designations included for Capnodiales. D = Dothideomycetes.
Fig. 2.
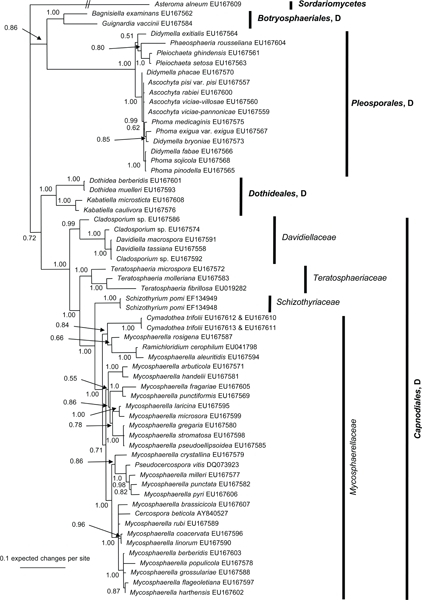
The 50 % majority rule tree of 7 811 trees obtained from a Bayesian analysis of the combined SSU and LSU sequence alignment. Bayesian posterior probabilities are given at the nodes and the scale bar shows 0.1 expected changes per site. The tree was rooted to Asteroma alneum. D = Dothideomycetes.
Both analyses show that C. trifolii unequivocally belongs to Mycosphaerellaceae s.str. (Dothideomycetes, Capnodiales) with M. aleuritidis, M. rosigena and Ramichloridium cerophilum as nearest relatives. However, the Bayesian analyses supported this cluster with a posterior probability value of 0.84 (Fig. 2), whereas the parsimony analysis failed to provide bootstrap or consensus support for the association (Fig. 1). The Mycosphaerellaceae as family was highly supported only in the Bayesian analysis while many subgroupings received only little support or appeared paraphyletic in both analyses. In the parsimony analysis, the clustering of Mycosphaerellaceae and Schizothyriaceae were well supported but not the Mycosphaerellaceae in itself.
DISCUSSION
Our analyses based upon whole nuclear ribosomal SSU and partial LSU (D1-D3) sequence data show that Cymadothea trifolii belongs to the Mycosphaerellaceae s.str. However, the position of this clade within the Mycosphaerellaceae remains uncertain due to the lack of high MP bootstrap support values or Bayesian posterior probabilities. Furthermore, recent studies have led to the conclusion that the genus Mycosphaerella is polyphyletic (Crous et al. 2007a), and that this morphology type occurs in several families within the Capnodiales, including Mycosphaerellaceae, Schizothyriaceae (Batzer et al. 2008) and Davidiellaceae (Crous et al. 2007b, Schubert et al. 2007). Within the Mycosphaerellaceae, however, several genera other than Mycosphaerella can be distinguished. Although these are chiefly recognised based on their anamorphs, the fact that these anamorph genera are also paraphyletic within the order is cause for more confusion (Arzanlou et al. 2007, 2008, Cheewangkoon et al. 2008, Crous et al. 2007a, 2008a, Crous et al. b).
On a general scale, the phylogenetic placement of the included orders is congruent with the multi-gene phylogeny for Dothideomycetes published recently (Schoch et al. 2006). The relatively comprehensive representation of Pleosporales is due to the fact that some of our preliminary analyses had pointed to the genus Didymella as the group to which C. trifolii might belong. Later we discovered that these earlier findings were due to contaminations (see below). We were also able to contribute sequences for 19 species hitherto unrepresented in GenBank, including M. aleuritidis and M. rosigena, which have turned out to be most closely related to C. trifolii. Morphologically, C. trifolii is a typical member of the Mycosphaerellaceae (Fig. 3), having spermatogonia, and hyaline, 1-septate ascospores in 8-spored, bitunicate asci, formed in fascicles in pseudothecial ascomata. Its anamorph, which is placed in the monotypic genus Polythrincium, is passalora-like (Crous & Braun 2003), but should be retained as separate due to the unique morphology of its conidiophores and arrangement of its conidial scars. Subsequently, based on its phylogeny and unique anamorph, the genus Cymadothea should be regarded as a distinct genus within the Mycosphaerellaceae. Apparently, the closest relatives of Cymadothea have yet to be found. Since this species is an obligate biotroph, other members of its group may turn out to be well-known biotrophic Mycosphaerellaceae that could thus far not be grown on agar media. In our analyses, only the Bayesian analysis provided strong support for the Mycosphaerellaceae with little resolution within the family. Using only LSU data, Crous et al. (2007a) obtained bootstrap support of 76 % and a posterior probability value of 0.83 for parsimony and Bayesian analyses respectively for the Mycosphaerellaceae. The lack of support for this family obtained during this study could be due to the high homoplasy because of the taxon sampling and/or selected gene regions adversely influencing the phylogenetic signal. Using only LSU and representatives of the Mycosphaerellaceae and Teratosphaeriaceae, Cheewangkoon et al. (2008) obtained a bootstrap support value of 88 % for the Mycosphaerellaceae.
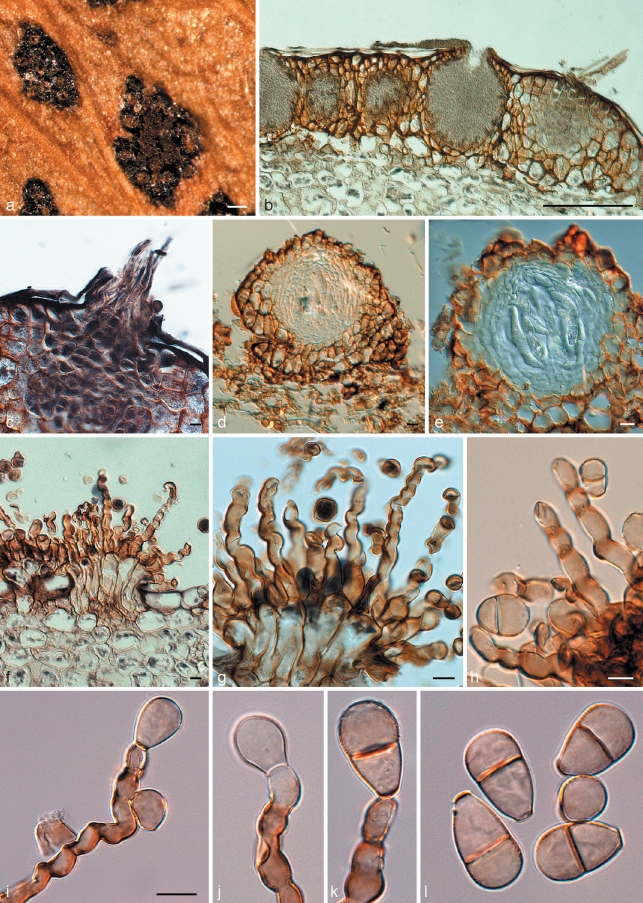
Fig. 3.
Cymadothea trifolii and its anamorph Polythrincium trifolii. a. Ascomata and spermatogonia on the leaf surface; b. vertical section through spermatogonia; c. trichogynes arising from developing ascoma; d, e. vertical section through ascomata; f–h. fasciculate conidiophores arising from leaf tissue. Basal part consisting of tightly aggregated subcylindrical cells that give rise to one or more curved conidiogenous cells with flattened, darkened scars along the length of one side of each conidiogenous cell; i–k. conidiogenous cells with developing conidia; l. mature (0–)1-septate conidia. — Scale bars = 10 μm, except a = 150 μm, b = 120 μm.
To our knowledge, this is the first report of sequence data for a truly obligate biotrophic member of this economically important family, which contains thousands of serious plant pathogens, including some of which the genomes are, or soon will be, available, such as M. graminicola (septoria leaf and glume blotch of wheat) and M. fijiensis (black leaf streak of banana). During this study it was extremely difficult to obtain uncontaminated DNA of C. trifolii – a problem well known to all researchers working with obligate biotrophs. While the SSU sequences were relatively easy to obtain, more than 50 previous attempts to generate clear LSU sequences with a variety of primer combinations tested were unsuccessful. Even cloning produced no obvious results because contaminations were so abundant. Only after extremely careful removal, washing and light microscopic examination of conidiostromata and then applying the GenomiPhi kit (GE Healthcares), which allows amplification of DNA from very small samples (see Tan & Murray 2006), we succeeded in obtaining clear sequences. Thus, we strongly recommend using this kit in combination with the cleaning procedure described in this manuscript when there is little DNA present, or when there is a high risk of contamination.
Cymadothea trifolii has a hitherto unique mode of nutrient acquisition via an extremely complex IA as documented in previous work (Simon et al. 2004, 2005b). Applying immunocytochemical methods it was found that C. trifolii differentially dissolves the host cell wall at the contact area: skeletal elements (cellulose and xyloglucans) are left intact, while the pectin matrix gets degraded (Simon et al. 2005b). Thereby the pathogen presumably increases the host cell wall pore size without totally disrupting the integrity of the attacked cell. Both the cellular interaction and the highly localised differential host wall degradation make C. trifolii at present unique among fungi.
Such an intricate cellular interaction is unlikely to have evolved without intermediate forms. Accordingly, there should be fungi producing structures resembling those of C. trifolii as shown by Bauer et al. (1997) for a somewhat similar kind of interaction in the Exobasidiales (Basidiomycota). However, these species have yet to be discovered. Only by widening the sampling, especially of biotrophic species, will we be able to tell whether or not this mode of interaction is mirrored in phylogenetic relationships and evolutionary trends.
Acknowledgments
We want to express our gratitude to Sigisfredo Garnica and Michael Weiß for extremely valuable advice with phylogenetic analyses and for critically reading earlier drafts of the manuscript. U.K. Simon is grateful to the German Research Foundation (DFG) for financial support. Morphological photos were taken from permanent slides prepared from the type specimen by Wolf (1935), kindly made available by the Farlow Herbarium (FH).
This work is dedicated to Dr Robert Bauer for 25 years of outstanding electron microscopic documentation of peculiar microfungi, and Prof. Dr Franz Oberwinkler for 50 years of restlessly promoting fungi as being central organisms in earth’s ecosystems.
REFERENCES
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater M-F, Buddenhagen IW, Viljoen A, Crous PW. 2008. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19 – 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. 2008. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 232 – 244 [DOI] [PubMed] [Google Scholar]
- Bauer R, Oberwinkler F, Vànky K. 1997. Ultrastructural markers and systematics in smut fungi and allied taxa. Canadian Journal of Botany 75: 1273 – 1314 [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-anan C. 2008. Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77 – 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U. 2003. Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1 – 571 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1 – 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33 – 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ. 2008a. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59 – 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ. 2008b. Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135 – 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams W, Verkleij GJM, Crous PW. 2007. CBS Course of Mycology, 5th ed.Centraalbureau voor Schimmelcultures, Utrecht, Netherlands: [Google Scholar]
- Gargas A, Taylor JW. 1992. Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84: 589 – 592 [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 [Google Scholar]
- Lewis GC, Thomas BJ. 1991. Incidence and severity of pest and disease damage to white clover foliage at 16 sites in England and Wales. Annals of Applied Biology 118: 1 – 8 [Google Scholar]
- Möller EM, Bahnweg G, Sandermann H, Geiger HH. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Research 20: 6115 – 6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA. 2004. MrModeltest v 2.2. Program distributed by the author Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden: [Google Scholar]
- Page RDM. 1996. TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357 – 358 [DOI] [PubMed] [Google Scholar]
- Puschner B. 2005. Problem weeds in hay and forages for lifestock. 35th California Alfalfa & Forage Symposium, Visalia, California: 12–14 Department of Agronomy and Range Science Extension, University of California, Davis, USA: [Google Scholar]
- Rambaut A. 2002. Sequence Alignment Editor. Version 2.0a11 Department of Zoology, University of Oxford, Oxford, United Kingdom: [Google Scholar]
- Rehner SA, Samuels GJ. 1994. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625 – 634 [Google Scholar]
- Roderick HW. 1993. The infection of white clover (Trifolium repens) by conidia of Cymadothea trifolii. Mycological Research 97: 227 – 232 [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041 – 1052 [DOI] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, Hoog GS de, Crous PW. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105 – 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon UK, Bauer R, Oberwinkler F. 2004. The unique cellular interaction between the leaf pathogen Cymadothea trifolii and Trifolium repens. Mycologia 96: 1210 – 1218 [PubMed] [Google Scholar]
- Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F. 2005a. The intercellular biotrophic leaf pathogen Cymadothea trifolii locally degrade pectins, but not cellulose or xyloglucan in the cell walls of Trifolium repens. New Phytologist 165: 243 – 260 [DOI] [PubMed] [Google Scholar]
- Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F. 2005b. The vegetative life cycle of the clover pathogen Cymadothea trifolii as seen in the electron microscope. Mycological Research 109: 764 – 778 [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP* Phylogenetic analysis using parsimony (* and other methods), version 4.0b10 Sinauer Associates, Sunderland, MA, USA: [Google Scholar]
- Tan M-K, Murray GM. 2006. A molecular protocol using quenched FRET probes for the quarantine surveillance of Tilletia indica, the causal agent of Karnal bunt of wheat. Mycological Research 110: 203 – 210 [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238 – 4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FA. 1935. Morphology of Polythrincium, causing sooty blotch of clover. Mycologia 27: 58 – 73 [Google Scholar]