Abstract
A new Phytophthora species, isolated from rhizosphere soil of declining or dead trees of Eucalyptus gomphocephala, E. marginata, Agonis flexuosa, and another 13 plant species, and from fine roots of E. marginata and collar lesions of Banksia attenuata in Western Australia, is described as Phytophthora multivora sp. nov. It is homothallic and produces semipapillate sporangia, smooth-walled oogonia containing thick-walled oospores, and paragynous antheridia. Although morphologically similar to P. citricola, phylogenetic analyses of the ITS and cox1 gene regions demonstrate that P. multivora is unique. Phytophthora multivora is pathogenic to bark and cambium of E. gomphocephala and E. marginata and is believed to be involved in the decline syndrome of both eucalypt species within the tuart woodland in south-west Western Australia.
Keywords: decline, dieback, forest, jarrah, phylogeny, Phytophthora citricola, tuart
INTRODUCTION
The oomycete genus Phytophthora includes many well-known species that contribute to and often drive tree declines worldwide. Knowledge about the diversity and significance of Phytophthora species in forest ecosystems has significantly increased in recent years as research has focussed on new and devastating tree declines in natural ecosystems in Europe and the Americas (Jung et al. 1999, 2000, 2002, Vettraino et al. 2001, 2002, Rizzo et al. 2002, Jung & Blaschke 2004, Brasier et al. 2005, Balci et al. 2007, Greslebin et al. 2007, Jung 2008) and advances in molecular techniques have improved our phylogenetic understanding of the genus (Cooke et al. 2000, Kroon et al. 2004). Since the discovery of P. cinnamomi in the south-west of Western Australia (WA) (Podger et al. 1965), this introduced pathogen has become renowned for its unparalleled impact on flora biodiversity with 40 % of the 5 710 species in the south-west Botanical Province found to be susceptible and 14 % highly susceptible (Shearer et al. 2004).
As a result of the wide scale forest quarantine and management of P. cinnamomi in WA, extensive and regular testing of soil and plant tissue samples for P. cinnamomi at the Vegetation Health Service (VHS) laboratory of the Department of Environment and Conservation has led to the isolation of a large range of Phytophthora spp. and undescribed Phytophthora taxa (Stukely et al. 1997, 2007a, Stukely et al. b, Burgess et al. 2009). The recovery of Phytophthora taxa other than P. cinnamomi from some sites with declining vegetation in WA has recently focussed attention onto their role in the decline of these woodland and forest ecosystems (Shearer & Smith 2000).
Across the south-west of WA there are a number of recently observed and significant forest declines occurring. In particular, the declines of Corymbia calophylla (Paap et al. 2008), Eucalyptus wandoo (Hooper & Sivasithamparam 2005), E. gomphocephala (tuart, Fig. 1a, b), E. marginata (jarrah, Fig. 1c, d), E. rudis, Agonis flexuosa and Banksia spp. (Fig. 1e, f) are causing concern to land managers and the general community. Within the tuart woodland of Yalgorup National Park on the Swan Coastal Plain south of Perth, a significant decline and substantial numbers of deaths of E. gomphocephala have been observed together with localised declines and mortality of E. marginata since the 1990s and A. flexuosa since 2006. A range of biotic and abiotic factors has been shown to contribute to tuart decline (Edwards 2004, Archibald 2006), although as yet, no satisfactory aetiology has been established. The progressive canopy thinning and dieback, and the heterogeneous distribution of the decline, are similar to Jarrah dieback (Shearer & Tippett 1989) and suggest the potential involvement of a Phytophthora species.
Fig. 1.

a. Severe dieback and mortality of a forest stand of Eucalyptus gomphocephala; b. crown symptoms of a declining E. gomphocephala including thinning, clustering of leaves, and dieback of branches and parts of the crown; c. dieback and mortality of a forest stand of E. marginata; d. crown symptoms of a declining E. marginata including thinning, clustering of leaves and dieback of branches and parts of the crown; e, f. collar rot of Banksia attenuata caused by Phytophthora multivora; e. sudden wilting and death due to the girdling of the collar; f. tongue-shaped, orange-brown necrosis of the inner bark.
In May and June 2007 Phytophthora isolates were recovered from the rhizosphere of declining E. gomphocephala, E. marginata and A. flexuosa in Yalgorup National Park. These isolates morphologically resembled P. citricola, which has been recovered over the past three decades throughout the south-west of WA by the VHS (Stukely et al. 1997). However, recent re-evaluation of the VHS culture collection using molecular techniques has identified most of these isolates as a new taxon (Phytophthora sp. 4) in the P. citricola complex (Burgess et al. 2009). DNA sequence data from the internal transcribed spacer regions (ITS1 and ITS2) and 5.8S gene of the rRNA operon and the mitochondrial cox1 gene were used in combination with morphological and physiological characteristics to characterise these isolates and compare them to the ex-type isolate of P. citricola as described by Sawada (1927). Due to their unique combination of morphological and physiological characters, and ITS and cox1 sequences, these semipapillate homothallic isolates from the south-west of WA are described here as a new species, P. multivora sp. nov.
MATERIAL AND METHODS
Sampling and Phytophthora isolation
Phytophthora isolates sampled from the tuart forest were obtained using soil sampling, baiting and isolation techniques modified from Jung et al. (1996, 2000). Soils were sampled beneath trees of E. gomphocephala, E. marginata or A. flexuosa from 32 sites (four trees per site). Sites sampled included 24 sites with all stages of crown dieback, and 8 sites without visible signs of canopy decline. From each tree a total of 4 L of soil was collected from 4 points, at a distance of 50–150 cm from the stem base. Soils were sampled below the upper 5 cm organic layer to a depth of 30 cm, with attention paid to sampling along main lateral roots. The four subsamples from each tree were bulked and baited in 35 × 35 cm plastic trays. Samples were pre-moistened for 12 h before flooding with distilled water to 3–4 cm in depth above the soil line. Floating organic material was moved to the side of the baiting tray with flyscreen meshing and any remaining organic material floating on the surface of the baiting water was removed with paper towelling. Juvenile leaves of Quercus ilex, Q. suber and Pittosporum undulatum were floated on the water as baits. Leaves with brownish lesions appearing after 48–96 h were examined for the presence of Phytophthora sporangia using a light microscope. Leaflets with sporangia were blotted dry, and the lesions cut into 1–2 mm 2 sections and plated onto Phytophthora selective PARPNH medium (Jung et al. 2000). Colonies growing from the plated lesion sections were transferred to V8 agar for confirmation as Phytophthora isolates.
Phytophthora isolates
In addition to the five semi-papillate Phytophthora isolates (WAC13200–WAC13204) collected in the present study, another two isolates were used for morphological and physiological comparisons including a semi-papillate isolate from the VHS collection (DCE 236, WAC13205) previously isolated from fine roots of a recently dead E. marginata in the jarrah forest near Jarrahdale in 1981, and the ex-type isolate of P. citricola (IMI 021173) recovered from Citrus sinensis fruits in Taiwan (Sawada 1927) (Table 1).
Table 1.
Isolates of Phytophthora multivora, P. citricola and P. ‘inflata’ considered in the morphological, physiological and phylogenetic studies.
| Culture no.1 | Identification | Host | Location | Reference | GenBank Accession No. |
|
|---|---|---|---|---|---|---|
| ITS | cox1 | |||||
| WAC132012 | P. multivora (ex-type) | Eucalyptus marginata | Yalgorup, Western Australia (WA) | This study | FJ237521 | FJ237508 |
| WAC132002 | E. gomphocephala | Yalgorup, WA | This study | FJ237522 | FJ237509 | |
| WAC132022 | E. gomphocephala | Yalgorup, WA | This study | FJ237520 | ||
| WAC132032 | Agonis flexuosa | Yalgorup, WA | This study | FJ237519 | ||
| WAC132042 | E. gomphocephala | Yalgorup, WA | This study | FJ237518 | FJ237507 | |
| WAC13205, DCE2362 | E. marginata | Jarrahdale, WA | This study | FJ237517 | FJ237506 | |
| VHS16158 | Banksia menziesii | Wanneroo, WA | This study | FJ237514 | FJ237503 | |
| VHS16168 | B. grandis | Pemberton, WA | This study | FJ237513 | FJ237502 | |
| DDS1450, IMI 329674 | Soil | Walpole, WA | This study | FJ237515 | FJ237504 | |
| VHS16439 | B. littoralis | Mandarah, WA | This study | FJ237516 | FJ237505 | |
| P777 | E. marginata | Western Australia | This study | FJ237525 | ||
| P15946 | P. citricola | Cistus canariensis | Mallorca, Spain | Moralejo et al. (2008) | EU244846 | |
| 77a | Quercus sp. | Hungary | Lakatos & Szabo (unpubl.) | EU594606 | ||
| Citri-P1817 | Japan | Uddin et al. (unpubl.) | AB367494 | |||
| P1817 | Medicago sativa | South Africa | Kroon et al. (2004) | AY564170 | ||
| Ps-5 | Rhododendron sp. | Asturias, Spain | Moralejo et al. (2008) | EU194425 | ||
| 83–185 | Antirrhinum majus | Switzerland | Lefort et al. (unpubl.) | EU000083 | ||
| KACC40184 | Zizyphus jujuba | Korea | Hong et al. (unpubl.) | AF228080 | ||
| CH455 | Mangifera indica | Spain | Zea-Bonilla et al. (2007) | AM235209 | ||
| P16246 | – | Balearic Islands, Spain | Belbahri et al. (unpubl.) | EF153674 | ||
| P142 | Rhododendron | Switzerland | Belbahri et al. (unpubl.) | EF193230 | ||
| IF031016 | P. ‘sojae’ | – | Japan | Villa et al. (2006) | AB217685 | |
| BR514 | Phytophthora sp. | – | Canada | Rose et al. (unpubl.) | DQ821185 | |
| IMI 021173, CBS 221.882 | P. citricola (ex-type) | Citrus sinensis fruit | Taiwan, 1927 | This study | FJ237526 | FJ237512 |
| CBS 295.29 | P. citricola (authentic) | Citrus leaf | Japan, 1929 | This study | ||
| CIT9 | Quercus robur | Pulling, Germany | This study | FJ237523 | FJ237510 | |
| CIT35 | Q. petraea | Tivoli, Slovenia | This study | FJ237524 | FJ237511 | |
| CIT7 | Q. robur | Pulling, Germany | Schubert et al. (1999) | AJ007370 | ||
| MN21HH | Rhododendron | USA | Schwingle et al. (2007) | DQ486661 | ||
| UASWS0208 | Soil from declining alder stand | Poland | Calmin et al. (unpubl.) | DQ396420 | ||
| 92–198 | Taxus sp. | Geneva, Switzerland | Belbahri et al. (unpubl.) | EF418946 | ||
| P131 | Rhododendron | Switzerland | Belbahri et al. (unpubl.) | EF193216 | ||
| 112 | – | Switzerland | Bragante et al. (unpubl.) | EU263906 | ||
| Citri-P0713 | – | Japan | Uddin et al. (unpubl.) | AB367492 | ||
| BR518 | – | Canada? | Rose et al. (unpubl.) | DQ821180 | ||
| IMI 031372 | Rubus idaeus | Ireland | Cooke et al. (2000) | AF266788 | ||
| 6f | P. ‘inflata’ | – | Poland | Cordier et al. (unpubl.) | EU240195 | |
| P44 | – | Slovenia | Munda et al. (unpubl.) | EF423556 | ||
| InfGaul | Gaultheria shalon | Scotland | Schlenzig (2005) | AY879291 | AY894685 | |
| InfRhod2 | Rhododendron sp. | Scotland | Schlenzig (2005) | AY879293 | ||
| InfVacc | Vaccinium vitis-idaea | Scotland | Schlenzig (2005) | AY879292 | AY894684 | |
| 804 | Soil from declining alder stand | Poland | Cordier et al. (unpubl.) | EU240058 | ||
| IMI 342898 | Syringa vulgaris | UK | Cooke et al. (2000) – ITS | |||
| Kroon et al. (2004) – cox1 | AF266789 | AY564187 | ||||
1Abbreviations of isolates and culture collections: CBS = Centraalbureau voor Schimmelcultures Utrecht, Netherlands; IMI = CABI Bioscience (Imperial Mycological Institute), UK; WAC = Department of Agriculture and Food Western Australia Plant Pathogen Collection, Perth, Australia; VHS = Vegetation Health Service of the Department of Environment and Conservation, Perth, Australia; DDS, DCE = earlier prefixes of VHS collection. Other isolate names and numbers are as given on GenBank.
2Isolates used in the morphological and growth-temperature studies.
Immediately prior to the present study, all isolates maintained in 90 mm Petri dishes on V8A media and as 9 mm V8A discs stored in 20 mL sterile water in McCartney bottles, were passaged through juvenile leaves of Q. suber used as baits on colonised agar discs flooded with sterile deionised water, and re-isolated using PARPNH selective medium.
Colony morphology, growth rates and cardinal temperatures
Hyphal morphology and colony growth patterns were described from 7 d old cultures grown at 20 °C in the dark on V8A, malt extract agar (MEA), corn-meal agar (CMA) and potato-dextrose agar (PDA) (all from BBL, Becton, Dickinson & Co, Sparks MD 21152 USA). Colony morphologies were described according to Brasier & Griffin (1979), Erwin & Ribeiro (1996) and Jung et al. (2003). Radial growth rate was recorded 5–7 d after the onset of linear growth along two lines intersecting the centre of the inoculum at right angles (Jung et al. 1999). The growth test was repeated once. For temperature growth studies, all isolates were subcultured onto V8A plates and incubated for 24 h at 20 °C to initiate growth. Three replicate plates for each isolate and temperature were then transferred to incubators set at 10, 15, 17.5, 20, 22.5, 25, 30 and 32.5 °C, and radial colony growth was measured as above after 5–7 d.
Morphology of sporangia and gametangia
Sporangia and gametangia were produced on V8A and measurements were made as described by Jung et al. (1999). Sporangia were obtained by flooding 5 × 5 mm square agar discs taken from growing margins of 3–5 d old colonies with non-sterile soil extract in 90 mm Petri dishes and incubating them in the dark at 18–22 °C for 12–16 h. The non-sterile soil extract was obtained by flooding 100 mL of commercial composted potting mix (Richgro, Jandakot, WA) with 1 L of deionised water. After 24 h at 10–25 °C, the soil extract was removed from the water surface with a pipette and diluted to 10 % with deionised water. Dimensions and characteristic features of 50 mature sporangia chosen at random were determined at ×400 magnification (BH-Olympus) for each isolate. For each isolate dimensions and characteristic features of 50 mature oogonia, oospores and antheridia, and diameters of 25 primary hyphae chosen at random were measured at ×400 magnification at the surface of 15 mm discs cut from the centre of 14–22 d old V8A cultures grown in the dark at 20 °C. For each isolate the oospore wall index was calculated as the ratio between the volume of the oospore wall and the volume of the entire oospore (Dick 1990).
DNA isolation, amplification and sequencing
The Phytophthora isolates were grown on half strength PDA (Becton, Dickinson and Company, Sparks, USA, 19.5g PDA, 7.5g of agar and 1L of distilled water) at 20 °C for 2 wk and the mycelium was harvested by scraping from the agar surface with a sterile blade and placed in a 1.5 mL sterile Eppendorf® tube. Harvested mycelium was frozen in liquid nitrogen, ground to a fine powder and genomic DNA was extracted according to Andjic et al. (2007). The region spanning the internal transcribed spacer (ITS)1-5.8S-ITS2 region of the ribosomal DNA was amplified using the primers ITS-6 (5’ GAA GGT GAA GTC GTA ACA AGG 3’) (Cooke et al. 2000) and ITS-4 (5’TCC TCC GCT TAT TGA TAT GC 3’) (White et al. 1990). The PCR reaction mixture, PCR conditions, the clean-up of products and sequencing were as described by Andjic et al. (2007).
The mitochondrial gene cox1 was amplified with primers Fm84 (5’TTT AAT TTT TAG TGC TTT TGC) and Fm83 (5’CTC CAA TAA AAA ATA ACC AAA AAT G) (Martin & Tooley 2003). The PCR reaction mixture was the same as for the ITS region, but the PCR conditions were as described previously (Martin & Tooley 2003). Templates were sequenced in both directions with primers used in amplification, as well as primers FM 85 (5’AAC TTG ACT AAT AAT ACC AAA) and FM 50 (5’GTT TAC TGT TGG TTT AGA TG) (Martin & Tooley 2003). The clean-up of products and sequencing were the same as for the ITS region.
Phylogenetic analysis
In order to compare Phytophthora isolates used in this study with other closely related species (ITS clade 2, Cooke et al. 2000), additional sequences were obtained from GenBank (Table 1). Sequences were also obtained for species representing other ITS clades (Cooke et al. 2000). Sequence data for the ITS region were initially assembled using Sequence Navigator v. 1.01 (Perkin Elmer) and aligned in Clustal X (Thompson et al. 1997). Manual adjustments were made visually by inserting gaps where necessary in BioEdit v. 5.0.6 (Hall 2001). There were no gaps in the cox1 alignment. All sequences derived in this study were deposited in GenBank and accession numbers are shown in Table 1.
Parsimony analysis was performed in PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003). The most parsimonious trees were obtained using heuristic searches with random stepwise addition in 100 replicates, with the tree bisection-reconnection branch-swapping option on and the steepest-descent option off. Maxtrees were unlimited, branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. Estimated levels of homoplasy and phylogenetic signal (retention and consistency indices) were determined (Hillis 1992). Branch and branch node support was determined using 1 000 bootstrap replicates (Felsenstein 1985).
Bayesian analysis was conducted on the same individual dataset as that used in the parsimony analysis. First, MrModeltest v. 2.5 (Nylander J.A.A. 2004. Program distributed by the author. Evolutionary Biology Centre, Uppsala University) was used to determine the best nucleotide substitution model. Phylogenetic analyses were performed with MrBayes v. 3.1 (Ronquist & Huelsenbeck 2003) applying a general time reversible (GTR) substitution model with gamma (G) and proportion of invariable site (I) parameters to accommodate variable rates across sites. Two independent runs of Markov Chain Monte Carlo (MCMC) using 4 chains were run over 1 000 000 generations. Trees were saved each 1 000 generations, resulting in 10 001 trees. Burn-in was set at 101 000 generations (i.e. 101 trees), well after the likelihood values converged to stationary, leaving 9 900 trees from which the consensus trees and posterior probabilities were calculated.
Statistical analysis
Analyses of Variances were carried out using Statistica v. 5.1 (Statsoft Inc., Tulsa, Oklahoma) to determine whether physiological and morphological measurements were different between isolates.
RESULTS
Phylogenetic analysis
The ITS dataset consisted of 894 characters of which 420 were parsimony informative. The dataset contained significant phylogenetic signal compared to 1 000 random trees (p < 0.01, g1 = −1.38). Heuristic searches resulted in 10 most parsimonious trees of 848 steps (CI = 0.72, RI = 0.89). The topology of the Bayesian tree was very similar (TreeBASE SN4153) (Fig. 2). Several ITS sequences from GenBank are identical to P. multivora (AB217685, AB367494, AF228080, AM235209, DQ821185, EF153674, EF193230, EU000083, EU194425, EU244846, EU594606). These sequences were not all included in the phylogenetic analysis, but information on origin and studies they have derived from is given in Table 1 and the position of polymorphic nucleotides indicating their similarity to P. multivora in Table 2. In addition, on GenBank there are several isolates of P. multivora, originally designated as P. sp. 4 by Burgess et al. (2009) (EU301126–32 and EU0869194–99).
Fig. 2.
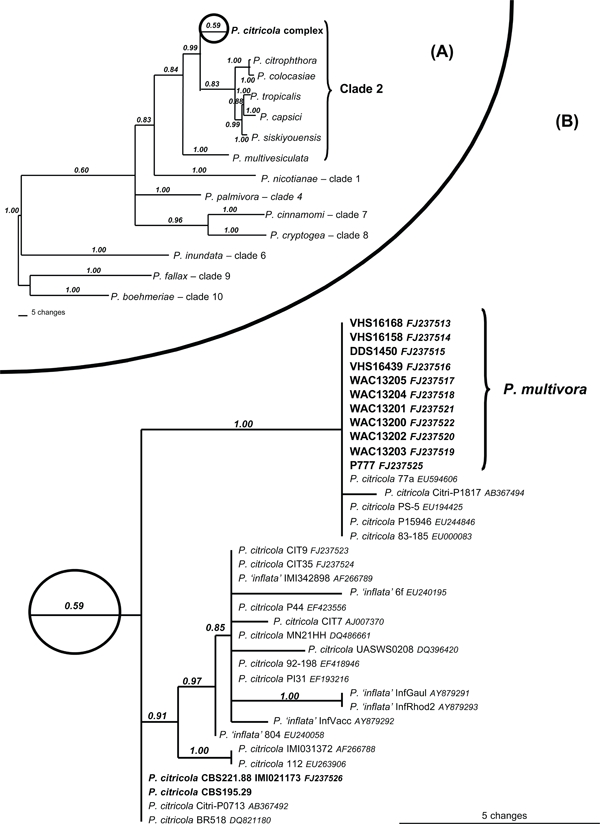
Bayesian inference tree using rDNA ITS sequences showing phylogenetic relationships between (A) clade 2 species and representative species from other clades and (B) isolates from the P. citricola complex. Numbers above branches represent posterior probability based on Bayesian analysis of the dataset. Both trees result from a single analysis as given in TreeBASE (SN4153). For tree A, clades were collapsed to show the relationship between isolates from P. citricola complex and other species in clade 2. Tree B shows the finer details within the P. citricola complex (node enclosed in circle on tree A) and the relationship between P. multivora and other P. citricola and P. ‘inflata’ isolates including the ex-type of P. citricola (IMI 021173).
Table 2.
Positions of polymorphic nucleotides (bp) from aligned sequence data of the ITS gene region showing the variation between Phytophthora multivora, P. citricola and P. ‘inflata’ isolates. Polymorphisms that differ from the type of P. multivora (WAC13201) are in blue.
| Isolate no. | 4 | 15 | 20 | 43 | 54 | 67 | 154 | 397 | 412 | 485 | 543 | 633 | 650 | 704 | 736 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. multivora | |||||||||||||||
| WAC13201 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| VHS16439 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| WAC13205 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| WAC13204 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| WAC13203 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| WAC13202 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| WAC13200 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| P777 | A | A | C | C | C | – | T | T | A | C | G | A | G | C | T |
| VHS16168 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| VHS16158 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| DDS1450 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| P15946 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| PS-5 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| 77a | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| Citri-P1817 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| 83–185 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| KACC40184 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| IF031016 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| BR514 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| CH455 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| P16246 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| P142 | A | – | C | C | C | – | T | T | A | C | G | A | G | C | T |
| P. citricola | |||||||||||||||
| IMI 021773* | A | – | C | T | T | T | T | T | G | T | G | G | G | T | T |
| CBS 295.29 | A | – | C | T | T | T | T | T | G | T | G | G | G | T | T |
| Citri-P0713 | A | – | C | T | T | T | T | T | G | T | G | G | G | T | T |
| BR518 | A | – | C | T | T | T | T | T | G | T | G | G | G | T | T |
| CIT7 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| CIT9 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| CIT35 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| P44 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| MN21HH | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| UASWS0208 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| 92–198 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| P131 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| IMI 031372 | A | – | T | T | T | – | T | T | G | T | A | G | A | T | T |
| 112 | A | – | T | T | T | – | T | T | G | T | A | G | A | T | T |
| P. ‘inflata’ | |||||||||||||||
| IMI 342898 | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| 804 | A | – | T | T | T | T | C | T | G | T | G | G | G | T | T |
| 6f | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
| Infgaul | C | – | T | T | T | T | C | C | G | T | G | G | G | T | A |
| InfRhod2 | C | – | T | T | T | T | C | C | G | T | G | G | G | T | A |
| InfVacc | A | – | T | T | T | T | C | C | G | T | G | G | G | T | T |
All isolates of P. multivora reside in a strongly supported terminal clade clearly distinct from the ex-type and authentic type of P. citricola (IMI 021173 and CBS 295.29) within ITS clade 2 (Cooke et al. 2000). Two additional isolates listed on GenBank (Citri-P0713 and BR518) have identical sequence to the ex-type of P. citricola (IMI 021173) (Fig. 2). There are seven fixed polymorphisms that are different between P. multivora and IMI 021173 (Table 2). Isolates listed on GenBank as P. citricola from the Northern Hemisphere (CIT7, CIT9, CIT35, P44, MN21HH, UASWS0208, 92–198, P131, IMI031372, 112) differ by at least 10 bp from P. multivora. (Fig. 2, Table 2). Isolates listed on GenBank as P. ‘inflata’ (6f, P44, InfGaul, InfRhod2, InfVacc, 804, IMI 342898) are dispersed among the northern hemisphere isolates of P. citricola (Fig. 2, Table 2) and it is unclear whether any of these isolates represent the original P. inflata.
The cox1 dataset consisted of 742 characters of which 107 were parsimony informative. The dataset contained significant phylogenetic signal compared to 1 000 random trees (p < 0.01, g1 = −0.66). Heuristic searches resulted in 12 most parsimonious trees of 301 steps (CI = 0.50, RI = 0.67). The topology of the Bayesian tree was very similar (TreeBASE SN4153) (Fig. 3). Species from ITS clade 2 group together with strong support. Isolates of P. multivora were in a separate clade from the ex-type isolate of P. citricola (IMI 021173) and two European isolates (CIT9 and CIT35) also sequenced in this study, and the isolates listed on GenBank as P. inflata. There was another single sequence of P. citricola, P1817, available from the study of Kroon et al. (2004). This sequence was distinct from our sequences for P. citricola and P. multivora, all GenBank sequences for P. inflata and other ITS clade 2 species, and, based on the findings of this study, must be either an incorrectly identified isolate or an incorrect sequence.
Fig. 3.
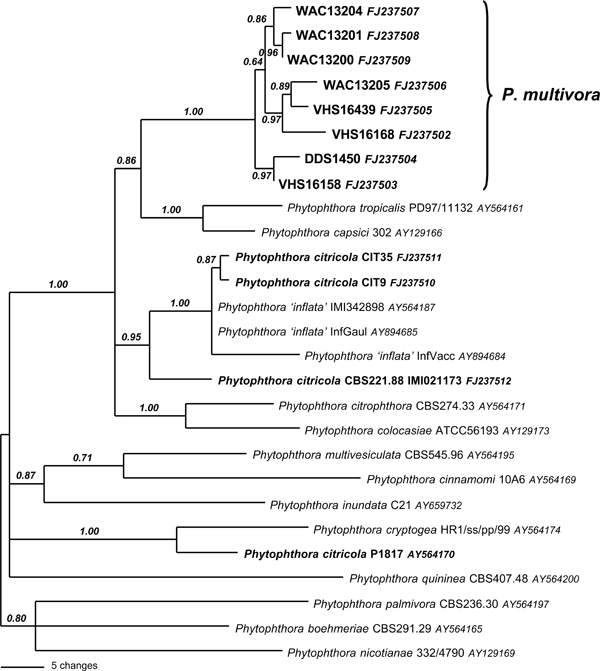
Bayesian inference tree using sequences of mitochondrial gene cox1 showing phylogenetic relationships between P. multivora and P. citricola, including the ex-type of P. citricola (IMI 021173).
Taxonomy
Phytophthora multivora P.M. Scott & T. Jung, sp. nov. — MycoBank MB512497; Fig. 4, 5
Fig. 4.
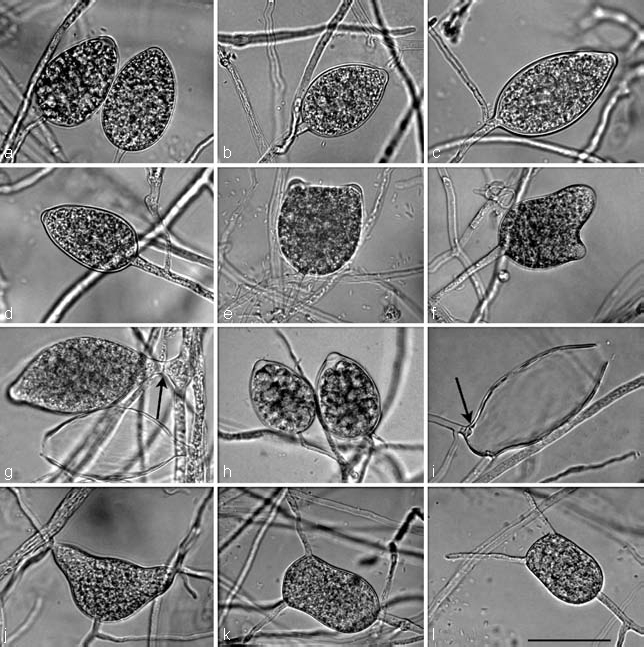
Semipapillate sporangia of Phytophthora multivora on V8 agar. — a–i after 12–24 h flooding with soil extract. a. Ovoid, the left sporangium with swollen papilla shortly before release of the already differentiated zoospores; b. ovoid; c. limoniform; d. obpyriform; e. bipapillate; f. bipapillate, bilobed; g. limoniform, laterally inserted to the sporangiophore (arrow); h. ovoid, shortly before release of zoospores; i. limoniform, intercalary inserted, with conspicuous basal plug (arrow) protruding into empty sporangium after release of zoospores. — j–l direct germination after 48 h flooding. j. Bipapillate, bilobed with several germ tubes growing from each papilla; k, l. bipapillate, bell-shaped with one germ tube growing from each papilla. — Scale bar = 50 μm, applies to a–l.
Fig. 5.
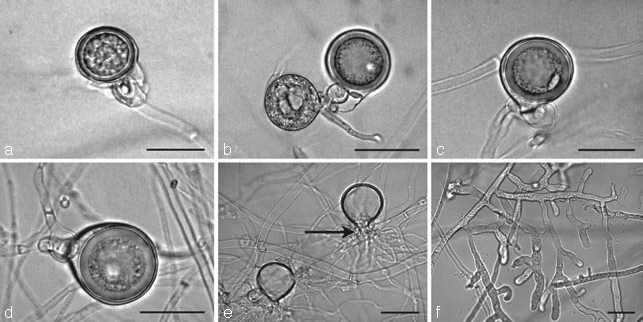
a–d. Oogonia of Phytophthora multivora with paragynous antheridia and plerotic oospores on V8 agar. a. Juvenile oogonium with thin-walled oospore and undifferentiated cytoplasm; b–d. mature oogonia with thick-walled oospores and ooplast; b. oogonium on the left side is aborted; e. direct germination of oospores with several germtubes through the oogonial bases (arrow) after 5 wk incubation at 20 °C; f. tubular, irregular lateral hyphae. — Scale bars = 25 μm.
Sporangia abundantia in cultura liquida, persistentia, terminalia, semi-papillata, ovoidea aut limoniformia, rare distorta vel bipapillata, 53 ± 10.1 × 31.8 ± 6.2 μm, ratio longitudo ad altitudinem 1.7 ± 0.2 μm. Systema sexus homothallica; oogonia globosa vel rare subglobosa, 27.1 ± 2.1 μm. Oosporae fere pleroticae, 23.9 ± 2 μm, paries 2.6 ± 0.5 μm. Antheridia paragynosa, 13 ± 2.2 × 8.8 ± 1.1 μm. Chlamydosporae et inflationes hypharum non observatae. Temperaturae crescentiae in agaro ‘V8A’, optima c. 25 °C et maxima 30–32.5 °C. Coloniae in agaro ‘V8A’ stellatae cum mycelio aerio restricto et margine submersa. Regiones ‘rDNA ITS’ et ‘cox1’ cum unica sequentia (GenBank FJ237508, FJ237521).
Etymology. Name refers to the wide host range (multi L. = many, -vora L. = feeding).
Sporangia (Fig. 4) — Sporangia were rarely observed on solid agar but were produced abundantly in non-sterile soil extract. The majority of sporangia for all P. multivora isolates and the ex-type of P. citricola (IMI 021173) were formed between 7–12 h after flooding with soil extract. Little variation in sporangial shapes was observed between the P. multivora isolates. The majority of sporangia were semipapillate and either ovoid, limoniform, ellipsoid or obpyriform (Fig. 4a–d, g–i), sometimes with just a very shallow apical thickening (Fig. 4f), non-caducous, occasionally forming a conspicuous basal plug (Fig. 4i) that protruded into the empty sporangium. Sporangia with two or three papillae or distorted shapes were occasionally formed by all isolates (Fig. 4e, f, j–l). Sporangia were typically borne terminally (Fig. 4a–f, j–l) but some were laterally attached (Fig. 4g) or intercalary (Fig. 4i). External proliferation was regularly observed (Fig. 4a–d, j, l), either irregular or in lax or dense sympodia. The majority of sporangia of each isolate had released zoospores between 15–20 h after flooding. Compared to P. citricola, sporangia of P. multivora showed a higher proportion of abortion or direct germination (Fig. 4j–l) after 24–48 h within the same soil extract. After 24–48 h, bell-shaped sporangia were formed by all six isolates of P. multivora which germinated directly from two points without prior formation of papillae (Fig. 4k, l). The mean sporangial dimensions of the six P. multivora isolates were 51.0 ± 10.4 × 30.0 ± 5.1 μm (overall range of 25–97 × 13–63 μm) with a length/breadth ratio of 1.7 ± 0.22 (overall range 1.3–3.3). The mean sporangial dimensions of the ex-type of P. citricola (IMI 021173), at 50.9 ± 6.9 × 29.9 ± 5.1 μm (range 39–70 × 22–40 μm) and a length/breadth ratio of 1.7 ± 0.3 (overall range 1.3–2.6), were within the range of the P. multivora isolates (Table 3). In contrast to P. multivora, sporangia of P. citricola were generally more variable and often showed distorted shapes including: multiple papillae, curved apices and hyphal beaks. Twelve percent of sporangia of P. citricola were distorted compared to 5 % in P. multivora. With 9 % and 10 %, respectively, P. multivora and P. citricola had a similar proportion of sporangia with lateral attachment to the sporangiophore. Isolate WAC13204 was different from all other P. multivora isolates by forming significantly (p < 0.05) larger sporangia with a mean size of 62.3 ± 10.8 × 34.0 ± 4.9 μm.
Table 3.
Morphological dimensions (μm) and temperature-growth relations of Phytophthora multivora and P. citricola.
|
P. multivora |
P. citricola |
||||||
|---|---|---|---|---|---|---|---|
| Isolate no.1 | WAC13200 | WAC132012 | WAC13202 | WAC13203 | WAC13204 | WAC13205 | IMI 0211732 |
| Sporangia | |||||||
| L×b mean | 56.5 ± 7 × 31.8 ± 4 | 53 ± 10.1 × 31.8 ± 6.2 | 44.2 ± 4.4 × 26.2 ± 3.1 | 44.5 ± 7.8 × 28.9 ± 4.2 | 62.3 ± 10.8 × 34.0 ± 4.9 | 45.7 ± 5.2 × 27.9 ± 3.6 | 50.9 ± 6.9 × 29.9 ± 5.1 |
| Range | 38−72 × 25−41 | 38−97 × 24−63 | 36−58 × 13−33 | 33−65 × 24−45 | 25−86 × 18−44 | 37−58 × 20−34 | 39−70 × 22−40 |
| l/b ratio | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.1 | 1.8 ± 0.3 | 1.6 ± 0.1 | 1.7 ± 0.3 |
| Oogonia | |||||||
| Mean diam | 25.6 ± 1.3 | 27.1 ± 2.1 | 25.5 ± 1.4 | 26.2 ± 1.5 | 26.8 ± 1.5 | 27.8 ± 2.5 | 30.3 ± 2.7 |
| diam range | 23−28 | 23−31 | 21−28 | 21−29 | 23−30 | 19−37 | 22−34 |
| Oospore | |||||||
| mean diam | 22.7 ± 1.3 | 23.9 ± 2 | 22.8 ± 1.4 | 23.3 ± 1.5 | 23.8 ± 1.7 | 24.8 ± 2.2 | 27.3 ± 2.6 |
| diam range | 20−26 | 19−28 | 18−26 | 19−26 | 21−28 | 17−31 | 20−30 |
| wall diam | 2.6 ± 0.4 | 2.6 ± 0.5 | 2.7 ± 0.4 | 2.7 ± 0.3 | 2.3 ± 0.4 | 2.5 ± 0.5 | 1.9 ± 0.3 |
| Antheridia | |||||||
| L×b mean | 13.1 ± 1.8 × 8.8 ± 1.3 | 13 ± 2.2 × 8.8 ± 1.1 | 11.7 ± 1.9 × 7.9 ± 1 | 13.5 ± 1.6 × 9 ± 1.4 | 12.4 ± 1.6 × 8.7 ± 1.4 | 13.8 ± 1.8 × 8.5 ± 1.2 | 13.2 ± 2.5 × 8.1 ± 1.8 |
| L×b range | 9−17 × 6−13 | 8−18 × 7−12 | 8−16 × 5−10 | 10−16 × 6−14 | 9−17 × 5−12 | 10−20 × 6−11 | 7−19 × 5−14 |
| Maximum temperature (°C) | 30−32.5 | 30−32.5 | 30−32.5 | 30−32.5 | > 32.5 | 30−32.5 | 30−32.5 |
| Optimum temperature (°C) | 25 | 25 | 25 | 25 | 25 | 25 | 22.5 |
| Growth rate on V8A at optimum (mm/d) | 4.7 | 5.7 | 5.7 | 5.7 | 6.1 | 5.4 | 5.7 |
| Growth rate at 20 °C (mm/d) | |||||||
| V8A | 4.0 | 4.0 | 4.4 | 4.5 | 4.7 | 3.8 | 5.2 |
| MEA | 4.1 | 3.8 | 3.4 | 3.6 | 4.7 | 3.4 | 4.0 |
| CMA | 4.4 | 4.5 | 3.3 | 3.3 | 4.5 | 3.5 | 4.8 |
| PDA | 3.1 | 2.9 | 2.6 | 2.5 | 3.7 | 2.9 | 2.3 |
1For isolate details see Table 1.
2Ex-type isolate.
Oogonia, oospores and antheridia (Fig. 5a–e) — Gametangia were readily produced in single culture by all P. multivora isolates and the ex-type of P. citricola (IMI 021173) on V8A within 4 d. Oogonia of both P. multivora and P. citricola were borne terminally, had smooth walls and were globose to slightly subglobose (Fig. 5a–d). With a mean diam of 26.5 ± 1.9 μm (overall range 19–37 μm and range of isolate means 25.5–27.8 μm) the oogonia of the six P. multivora isolates were on average smaller than those of P. citricola (30.3 ± 2.7 μm, range 22–34 μm), although the ranges were broadly overlapping (Table 3). Oospores of both P. multivora (Fig. 5b–d) and P. citricola were globose and nearly plerotic. The P. multivora isolates produced significantly (p < 0.05) thicker oospore walls (2.6 ± 0.5 μm, overall range 1.4–4.6 μm) than P. citricola (1.9 ± 0.3 μm, overall range 1.2–2.6). Due to the smaller oospore size of P. multivora the oospore wall index was significantly higher (p < 0.0001) in P. multivora (0.52 ± 0.07) than in P. citricola (0.36 ± 0.05). Antheridia of both species were obovoid, club-shaped or irregular, almost exclusively paragynous, diclinous and typically attached close to the oogonial stalk. Intercalary and amphigynous antheridia were only rarely observed. After 4 wk in V8A at 20 °C, more than 90 % of all P. multivora oospores had germinated directly. Since the thick inner oospore wall of Phytophthora species erodes during the germination process due to enzymatic digestion of its major components, the glucans, (Erwin & Ribeiro 1996) only the thin outer oospore wall surrounded by the thin oogonial wall was left (Fig. 5e). No direct germination was observed in cultures of the ex-type of P. citricola (IMI 021173) growing under the same conditions.
Colony morphology, growth rates and cardinal temperatures — Colony growth patterns of two isolates of P. multivora (WAC13201 and WAC13205) and the ex-type isolate of P. citricola (IMI 021173) are shown in Fig. 6. All P. multivora isolates except isolate WAC13204 produced similar colony growth patterns on the four different types of media. On V8A, CMA and MEA P. multivora isolates produced limited aerial mycelium and distinct growth patterns, while isolate WAC13204 formed fluffy to felty, uniform colonies without distinct growth pattern. The colony morphology on V8A and MEA of all P. multivora isolates clearly differed from the colony morphology of the ex-type isolate of P. citricola (IMI 021173). Phytophthora multivora isolates produced stellate growth patterns with a clearly delimited, submerged margin on V8A and faintly stellate to dendroid patterns on MEA while P. citricola formed a typical chrysanthemum pattern on both media. On CMA, P. multivora isolates formed appressed to submerged colonies with a faintly stellate to petaloid pattern while P. citricola produced even sparser submerged colonies with a faintly stellate pattern. On PDA, the P. multivora isolates and P. citricola produced petaloid felty to fluffy colonies. Diameters of primary hyphae varied from 3.8–4.6 μm. Lateral hyphae of P. multivora were often tubular and slightly inflated (Fig. 5f). No substantial differences were observed between hyphae of P. multivora and P. citricola.
Fig. 6.
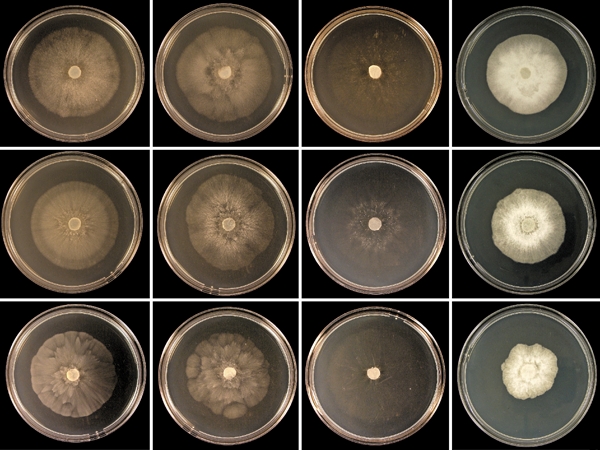
Colony morphology of isolates WAC13201 (ex-type) and WAC13205 of Phytophthora multivora, and the ex-type isolate of P. citricola (from top to bottom) after 6 d growth at 20 °C on V8 agar, malt extract agar, cornmeal agar and potato-dextrose agar (from left to right).
Temperature growth relations of P. multivora and the ex-type isolate of P. citricola are shown in Fig. 7. The maximum growth temperature for isolates of both P. multivora and the ex-type of P. citricola (IMI 021173) on V8A was between 30–32.5 °C. All isolates of P. multivora except isolate WAC13204 were unable to grow at 32.5 °C, but started re-growth within 12 h when plates that were incubated for 7 d at 32.5 °C were transferred to 25 °C. All six P. multivora isolates had a growth optimum at 25 °C with growth rates ranging from 4.7–6.1 mm/d while P. citricola showed a broad growth optimum between 22.5 °C (5.7 mm/d) and 30 °C (5.5 mm/d). Compared to all P. multivora isolates the growth rate of P. citricola at 20 °C was higher on V8A and CMA and lower on PDA (Table 2). On V8A, over the whole temperature range except at 25 °C, all P. multivora isolates were markedly slower growing than P. citricola (Fig. 7).
Fig. 7.
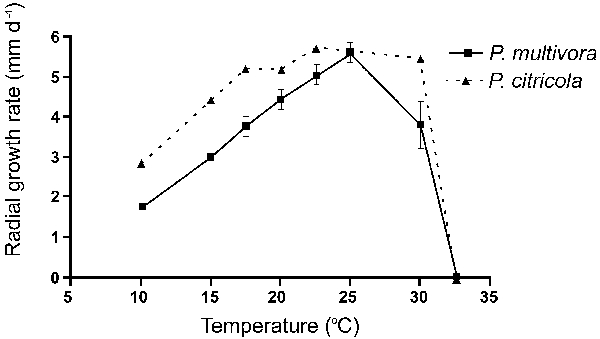
Radial growth rates of Phytophthora multivora (means and standard errors calculated from six isolates), solid line, and the ex-type isolate of P. citricola, dashed line, on V8 agar at different temperatures.
Specimens examined. Western Australia, Yalgorup, from rhizosphere soil of declining Eucalyptus marginata, May 2007, P. Scott & T. Jung, holotype MURU 434 (dried culture on V8A, Herbarium of Murdoch University, Western Australia), culture ex-type WAC13201; from rhizosphere soil of declining Eucalyptus gomphocephala, May 2007, T. Jung & P. Scott, WAC13200; from rhizosphere soil of declining Eucalyptus gomphocephala, June 2007, P. Scott, WAC13202; from rhizosphere soil of declining Agonis flexuosa, June 2007, P. Scott, WAC13203; from rhizosphere soil of declining Eucalyptus gomphocephala, June 2007, P. Scott, WAC13204; North Jarrahdale, from rhizosphere soil of declining Eucalyptus marginata, 1980, unknown, WAC13205.
Notes — In previous studies P. multivora is referred to as P. citricola (Shearer et al. 1987, 1988, Shearer & Tippett 1989, Bunny 1995, Stukely et al. 1997), and more recently as P. sp. 4 (Burgess et al. 2009). Many isolates from a wide range of host species in WA that had been identified as P. citricola in the past must be reassigned to P. multivora. As indicated above, P. multivora has been isolated from the south-west of WA from rhizosphere soil of E. gomphocephala, E. marginata and A. flexuosa in Yalgorup National Park. It has also been recovered by the VHS from soil and root samples collected beneath dying, Phytophthora-sensitive ‘indicator species’ in native ecosystems in the south-west of WA by the VHS over the last 30 yr, which extends the host list to include Banksia attenuata, B. grandis, B. littoralis, B. menziesii, B. prionotes, Conospermum sp., Leucopogon verticillatus, Xanthorrhoea gracilis, Podocarpus drouyniana, Patersonia sp., Bossiaea sp., Gastrolobium spinosum and Pinus radiata (plantation) (Burgess et al. 2009). Phytophthora multivora has also recently been isolated from large girdling stem lesions of B. attenuata in Injidup, WA (G. Hardy unpubl. data, Fig. 1e, f), and from fine roots of declining E. marginata in the Jarrah forest near Jarrahdale in 1981 (M. Stukely unpubl. data) and near Dwellingup in 2008 (T. Jung unpubl. data).
DISCUSSION
Phytophthora multivora was previously identified as P. citricola in WA based solely on morphological characters including homothallic breeding behaviour, production of paragynous antheridia, semipapillate persistent sporangia and oogonia with dimensions in the correct range, absence of catenulate hyphal swellings in liquid culture, and similar growth rates at 25 °C. Phylogenetic analyses of the ITS and cox1 gene regions show that P. multivora is unique and comprises a discrete cluster within the major ITS clade 2 of Cooke et al. (2000) with its present closest relative being P. citricola.
Morphological and molecular studies using a broad range of P. citricola isolates have demonstrated that P. citricola is very diverse (Oudemans et al. 1994, Bhat & Browne 2007, Moralejo et al. 2008), and that many of the differences are associated with host and geography (Oudemans et al. 1994, Bhat & Browne 2007). In the isozyme study of Oudemans et al. (1994) a global collection of 125 isolates of P. citricola clustered into five distinct subgroups suggesting P. citricola is a species complex instead of a single species which is to be expected considering the broad geographic and host range of P. citricola isolates (Fontaneto et al. 2008).
Even though multiple P. citricola sequences have been submitted to GenBank, sequence data for the ex-type of P. citricola (IMI 021173) from Citrus sinensis fruits in Taiwan (Sawada 1927) has not previously been available, and this has led to confusion in the phylogeny. Besides the ex-type culture, an authentic type of P. citricola (CBS 295.29) isolated from Citrus leaves in Japan was submitted to CBS in 1929 by Sawada. The present study is the first to provide sequence data of these isolates, and our results clearly demonstrate that the isolates from WA constitute a new species, P. multivora. Isolates designated as P. ‘inflata’ were distributed through the P. citricola complex demonstrating the difficulty in distinguishing between P. inflata and P. citricola. The original P. inflata ex-type from elm trees in the United States (Caroselli & Tucker 1949) has been lost and it has been suggested that designated isolates of P. inflata from other hosts (Hall et al. 1992) are conspecific with P. citricola (Cooke et al. 2000). Among isolates from the P. citricola complex, isolates now described as P. multivora are the most distant to the ex-type of P. citricola, differing in the ITS region by 7 bp. However, there appear to be many subclades within the P. citricola complex which may correspond to additional new taxa. Further study of this important species complex is required to elucidate the host and geographic range and phylogeny of isolates within the complex and to determine if they constitute new species.
In GenBank, 11 ITS sequences, designated as P. citricola, are identical to P. multivora. Seven are from unpublished studies in Hungary, Canada, Switzerland, Korea and Japan, and two sequences are from isolates of Moralejo et al. (2008) from ornamental nurseries in Spain, an isolate from Mangifera indica in Spain (Zea-Bonilla et al. 2007). In addition, an isolate designated as P. sojae in a study from Japan also has identical sequence (Villa et al. 2006). This low number of very recent submissions of P. multivora sequences as compared to the high number of other sequences from the P. citricola complex indicates that P. multivora may have been introduced to these countries. Due to the widespread distribution of P. multivora across natural ecosystems in WA, it is likely that WA may be a source of dispersal, possibly via the nursery trade (Brasier 2008).
In the cox1 analysis P. multivora and the ex-type isolate of P. citricola grouped together with other ITS clade 2 species, although the distance between P. multivora and P. citricola was greater in the cox1 analysis than in the ITS analysis. In the ITS sequence there was only 1bp difference between all isolates of P. multivora. In the cox1 analysis there were more differences resulting in the formation of subclades. The greater phylogenetic variation and presence of subclades in the cox1 analysis reflects the expected faster rate of mitochondrial than genomic DNA evolution (Kroon et al. 2004). The observed variability, however, strongly supports the hypothesis that P. multivora in WA is not a recent clonal introduction, but rather was introduced long ago or is endemic. This is also reflected by the phenotypic variability observed among isolates of P. multivora. There was generally some variation in the colony growth patterns and growth rates, and in the dimensions of morphological structures of the different P. multivora isolates. However, isolate WAC13204 was particularly different from the other five isolates of P. multivora, having significantly larger sporangia, a higher maximum growth temperature and faster growth rates.
A cox1 sequence for P. citricola was available on GenBank from the study of Kroon et al. (2004). In their study, this putative P. citricola was closest to P. cryptogea and they discussed this incongruency, as it was one of the few species that did not fall into the same clades in both the mitochondrial and nuclear gene analysis. With our new sequences for P. citricola, including the ex-type isolate, it is clear that the sequence used for P. citricola by Kroon et al. (2004) was incorrect.
Morphological similarities between taxa, as observed between P. multivora and P. citricola, are increasingly found in the unravelling of different species complexes within the genus Phytophthora using molecular methods (Brasier et al. 2003, 2004, Jung et al. 2003). This study therefore highlights the importance of using ex-type cultures where available and the value of using molecular tools to unravel the ambiguity of species previously identified solely on morphological characteristics. Over the last 30 yr, in the absence of sufficient molecular techniques, P. multivora has been routinely identified in the south-west of WA as P. citricola using morphological characteristics. Similar misidentification of Phytophthora species has occurred with the identification of P. pseudosyringae isolates as P. syringae (Jung et al. 2003).
Despite the similarities, there are clear morphological and physiological differences between P. multivora and the ex-type isolate of P. citricola. If more isolates of P. citricola were to be examined the morphological differences between the two species may be less resolved. Phytophthora multivora and P. citricola produce different colony growth patterns on V8A, MEA and CMA with the most distinct variation observed on V8A. Phytophthora multivora has a clear optimum growth temperature of 25 °C while the optimum growth rate of P. citricola is at 22.5 °C and decreases by only 0.2 mm/d between 22.5–30 °C. Over the whole temperature range, except of the optimum temperature of 25 °C, P. multivora isolates are slower growing than P. citricola. Sporangial shapes of P. multivora are generally more uniform while in P. citricola sporangia are more variable and the frequency of distorted shapes is significantly higher. A high variability of sporangial shapes was also found by Zentmyer et al. (1974) studying P. citricola isolates from Persea americana in California. Although most morphological measurements of the ex-type isolate of P. citricola fell within the range of P. multivora isolates there were clear differences between both species. All six P. multivora isolates produced on average significantly smaller oogonia and oospores, and significantly thicker oospore walls than P. citricola. This is reflected by the oospore wall index, which is the ratio between the volume of the oospore wall and the volume of the entire oospore (Dick 1990). The oospore wall index of P. multivora (0.52 ± 0.07) was almost 50 % higher than that of the ex-type isolate of P. citricola (0.36 ± 0.05). A calculation of the oospore wall index using the original datasets of Jung et al. (1999, 2002, 2003) for P. europaea (0.37 ± 0.07), P. ilicis (0.41 ± 0.11), P. pseudosyringae (0.27 ± 0.09), P. psychrophila (0.42 ± 0.06), P. quercina (0.45 ± 0.08), P. syringae (0.24 ± 0.07) and P. uliginosa (0.46 ± 0.09) demonstrated that P. multivora had the highest oospore wall index of all nine species examined. The thick oospore wall of P. multivora is most likely an adaptation to the seasonally extremely dry soil conditions in WA. This survival mechanism was also suggested for P. quercina in European oak forests (Jung et al. 1999, 2000). After 4 wk in V8A at 20 °C, in all six P. multivora isolates, more than 90 % of the oospores had germinated directly. This lack of dormancy had previously been observed for oospores of P. medicaginis (Erwin & Ribeiro 1996), however, this does not preclude dormancy occurring under different conditions. No direct germination was observed in cultures of the ex-type isolate of P. citricola growing under the same conditions. This result corresponds to the low oospore germination rates observed in European isolates of the P. citricola complex (Delcan & Brasier 2001). Whether these differences in germination rates reflect different survival mechanisms of the two species requires further investigation.
Phytophthora multivora can easily be distinguished from other homothallic Phytophthora species with paragynous antheridia and semipapillate sporangia by its unique combination of morphological and physiological characters, and DNA sequences. Phytophthora multivora is separated from P. syringae by the absence of hyphal swellings, the occurrence of distorted and bipapillate sporangia, thicker oospore walls, different colony growth patterns on V8A, MEA and CMA, higher optimum and maximum temperatures for growth, and different ITS and cox1 sequences (Waterhouse & Waterston 1964, Erwin & Ribeiro 1996, Jung et al. 2003). Phytophthora multivora can be distinguished from P. pseudosyringae by the absence of hyphal swellings and caducity of sporangia, the occurrence of distorted bipapillate sporangia, thicker oospore walls, different colony growth patterns on V8A, MEA and CMA, higher optimum and maximum temperatures for growth, and different ITS and cox1 sequences (Jung et al. 2003). Phytophthora multivora is discriminated from the original P. inflata of Caroselli & Tucker (1949) by having larger sporangia, markedly smaller oogonia and thinner oospore walls, and by the absence of inflated irregular antheridia which are often twining or twisted around the oogonial stalk in P. inflata.
Under the original morphological identification as P. citricola, an isolate of P. multivora was used in an underbark inoculation test, and caused significantly longer lesions on stems of E. marginata and C. calophylla than P. cinnamomi (Shearer et al. 1988). Experiments are currently underway to determine pathogenicity of P. multivora towards E. gomphocephala and E. marginata.
Phytophthora multivora has been isolated in WA from natural forest and heath-land stands for the last 30 yr from beneath dead and dying plants of 16 species from seven families. Phytophthora multivora is very widespread in south-west WA with a distribution similar to that known for P. cinnamomi. The VHS uses detection methods developed specifically for P. cinnamomi and even under these conditions, P. multivora is the next most commonly isolated taxon after P. cinnamomi. There is now evidence that in some sites it may be P. multivora and not P. cinnamomi that is responsible for tree mortality, while the latter is driving the collapse of whole ecosystems known as Phytophthora dieback. These findings may have direct implications for forest management and biosecurity, and our study highlights the potential importance of new and yet undescribed Phytophthora taxa in natural ecosystems in the south-west of WA (Burgess et al. 2009), and the need for continued research.
Acknowledgments
The authors are grateful to Murdoch University for a Murdoch University Research Scholarship for the first author, the Australian Research Council and industry partners for generous financial support (LP0346931 and LP0668195), Janet Webster and Juanita Ciampini (VHS) for providing P. multivora isolates for DNA sequencing, Diane White (CPSM) for the sequencing of isolates, Amanda Hewson (CPSM) for assistance in field work, Dang Tan Dang (CPSM) for assistance in morphological and physiological measurements, Alex Rea (CPSM) for providing the cox1 sequence of the VHS isolates, and Bernard Dell, William Dunstan, Daniel Hüberli (CPSM), and Chris Dunne and Colin Crane (Deptartment of Environment and Conservation, WA) for constructive discussions.
REFERENCES
- Andjic V, Barber PA, Carnegie AJ, Hardy GEStJ, Wingfield MJ, Burgess TI. 2007. Phylogenetic reassessment supports accommodation of Phaeophleospora and Colletogloeopsis from eucalypts in Kirramyces. Mycological Research 111: 1184 – 1198 [DOI] [PubMed] [Google Scholar]
- Archibald R. 2006. Fire and the persistence of tuart woodlands. PhD thesis, Murdoch University, Perth, Australia
- Balci Y, Balci S, Eggers J, MacDonald WL, Juzwik J, Long RP, Gottschalk KW. 2007. Phytophthora spp. associated with forest soils in eastern and north-central U.S. oak ecosystems. Plant Disease 91: 705 – 710 [DOI] [PubMed] [Google Scholar]
- Bhat RG, Browne GT. 2007. Genetic diversity in populations of Phytophthora citricola associated with horticultural crops in California. Plant Disease 91: 1556 – 1563 [DOI] [PubMed] [Google Scholar]
- Brasier CM. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57: 792 – 808 [Google Scholar]
- Brasier CM, Beales PA, Kirk SA, Denman S, Rose J. 2005. Phytophthora kernoviae sp. nov., an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in the UK. Mycological Research 109: 835 – 859 [DOI] [PubMed] [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM, Hansen EM. 2003. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides–P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycological Research 107: 277 – 290 [DOI] [PubMed] [Google Scholar]
- Brasier CM, Griffin MJ. 1979. Taxonomy of ‘Phytophthora palmivora’ on cocoa. Transactions of the British Mycological Society 72: 111 – 143 [Google Scholar]
- Brasier CM, Kirk SA, Delcan J, Cooke DEL, Jung T, Man in ’t Veld WA. 2004. Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycological Research 108: 1172 – 1184 [DOI] [PubMed] [Google Scholar]
- Bunny FJ. 1995. The biology, ecology and taxonomy of Phytophthora citricola in native plant communities in Western Australia. PhD thesis, Murdoch University, Perth, Australia
- Burgess TI, Webster JL, Ciampini JA, White D, Hardy GEStJ, Stukely MJC. 2009. Re-evaluation of Phytophthora species isolated during 30 years of vegetation health surveys in Western Australia using molecular techniques. Plant Disease 93: 215 – 223 [DOI] [PubMed] [Google Scholar]
- Caroselli NE, Tucker CM. 1949. Pit canker of elm. Phytopathology 39: 481 – 488 [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology 30: 17 – 32 [DOI] [PubMed] [Google Scholar]
- Delcan J, Brasier CM. 2001. Oospore viability and variation in zoospore and hyphal tip derivatives of the hybrid alder Phytophthoras. Forest Pathology 31: 65 – 83 [Google Scholar]
- Dick MW. 1990. Keys to Pythium. University of Reading Press, Reading, United Kingdom
- Edwards T. 2004. Environmental correlates and associations of tuart (Eucalyptus gomphocephala DC.) decline. Masters thesis, Edith Cowen University, Perth, Australia
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide APS Press, American Phytopathological Society, St. Paul, Minnesota: [Google Scholar]
- Felsenstein J. 1985. Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39: 783 – 791 [DOI] [PubMed] [Google Scholar]
- Fontaneto D, Barraclough TG, Chen K, Ricci C, Herniou EA. 2008. Molecular evidence for broad-scale distributions in bdelloid rotifers: everything is not everywhere but most things are very widespread. Molecular Ecology 17: 3136 – 3146 [DOI] [PubMed] [Google Scholar]
- Greslebin AG, Hansen EM, Sutton W. 2007. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentinia). Mycological Research 111: 308 – 316 [DOI] [PubMed] [Google Scholar]
- Hall GS, Dobson S, Nicholls C. 1992. First record of Phytophthora inflata in the United Kingdom. Plant Pathology 41: 95 – 97 [Google Scholar]
- Hall T. 2001. BioEdit version 5.0.6. Department of Microbiology, North Carolina State University. http://www.mbio.ncsu.edu/BioEdit/bioedit.html.
- Hillis DM. 1992. Signal, noise and reliability in molecular phylogenetic analysis. Journal of Heredity 83: 189 – 195 [DOI] [PubMed] [Google Scholar]
- Hooper RJ, Sivasithamparam K. 2005. Characterization of damage and biotic factors associated with the decline of Eucalyptus wandoo in southwest Western Australia. Canadian Journal of Forest Research 35: 2589 – 2602 [Google Scholar]
- Jung T. 2008. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. Forest Pathology 38: doi: 10.1111/j.1439–0329.2008.00566.x [Google Scholar]
- Jung T, Blaschke H, Neumann P. 1996. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. European Journal of Forest Pathology 26: 253 – 272 [Google Scholar]
- Jung T, Blaschke H, Oßwald W. 2000. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathology 49: 706 – 718 [Google Scholar]
- Jung T, Blaschke M. 2004. Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies. Plant Pathology 53: 197 – 208 [Google Scholar]
- Jung T, Cooke DEL, Blaschke H, Duncan JM, Oßwald W. 1999. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycological Research 103: 785 – 798 [Google Scholar]
- Jung T, Hansen EM, Winton L, Oßwald W, Delatour C. 2002. Three new species of Phytophthora from European oak forests. Mycological Research 106: 397 – 411 [Google Scholar]
- Jung T, Nechwatal J, Cooke DEL, Hartmann G, Blaschke M, Oßwald WF, Duncan JM, Delatour C. 2003. Phytophthora pseudosyringae sp. nov., a new species causing root and collar rot of deciduous tree species in Europe. Mycological Research 107: 772 – 789 [DOI] [PubMed] [Google Scholar]
- Kroon LPNM, Bakker FT, Bosch GBM van den, Bonants PJM, Flier WG. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology 41: 766 – 782 [DOI] [PubMed] [Google Scholar]
- Martin FN, Tooley PW. 2003. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 95: 269 – 284 [PubMed] [Google Scholar]
- Moralejo E, Pérez-Sierra AM, Álavez LA, Belbahri L, Lefort F, Descals E. 2008. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathology 57: 1 – 11 [Google Scholar]
- Oudemans P, Förster H, Coffey MD. 1994. Evidence for distinct isozyme subgroups within Phytophthora citricola and close relationships with P. capsici and P. citrophthora. Mycological Research 98: 189 – 199 [Google Scholar]
- Paap T, Burgess TI, McComb JA, Shearer BL, Hardy GEStJ. 2008. Quambalaria species, including Q. coyrecup sp. nov., implicated in canker and shoot blight diseases causing decline of Corymbia species in the southwest of Western Australia. Mycological Research 112: 57 – 69 [DOI] [PubMed] [Google Scholar]
- Podger FD, Doepel RF, Zentmyer GA. 1965. Association of Phytophthora cinnamomi with a disease of Eucalyptus marginata forest in Western Australia. Plant Disease Reporter 49: 943 – 947 [Google Scholar]
- Rizzo DM, Garbelotto M, Davidson JM, Slaughter GW, Koike T. 2002. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Disease 86: 205 – 214 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Sawada K. 1927. Descriptive catalogue of the Formosan fungi. Report of the Government Research Institute Department of Agriculture Formosa 27: 21 – 24 [Google Scholar]
- Schlenzig A. 2005. First report of Phytophthora inflata on nursery plants of Rhododendron spp., Gaultheria shalon and Vaccinium vitis-idaea in Scotland. Plant Pathology 54: 582 [Google Scholar]
- Schubert R, Bahnweg G, Nechwatal J, Jung T, Cooke DEL, Duncan JM, Müller-Starck G, Langebartels C, Sandermann H, Jr, Oßwald W. 1999. Detection and quantification of Phytophthora species which are associated with root-rot diseases in European deciduous forests by species-specific polymerase chain reaction. European Journal of Forest Pathology 29: 169 – 188 [Google Scholar]
- Schwingle BW, Smith JA, Blanchette RA. 2007. Phytophthora species associated with diseased woody ornamentals in Minnesota nurseries. Plant Disease 91: 97 – 102 [DOI] [PubMed] [Google Scholar]
- Shearer BL, Crane CE, Cochrane A. 2004. Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52: 435 – 443 [Google Scholar]
- Shearer BL, Michaelsen BJ, Somerford PJ. 1988. Effects of isolate and time of inoculation on invasion of secondary phloem of Eucalyptus spp. and Banksia grandis by Phytophthora spp. Plant Disease 72: 121 – 126 [Google Scholar]
- Shearer BL, Michaelsen BJ, Warren HJ. 1987. Comparative behaviour of Phytophthora species in the secondary phloem of stems and excised roots of Banksia grandis and Eucalyptus marginata. Australian Journal of Botany 35: 103 – 110 [Google Scholar]
- Shearer BL, Smith IW. 2000. Diseases of Eucalyptus caused by soilborne species of Phytophthora and Pythium. In: Keane PJ, Kile GA, Podger FD, Brown BN. (eds), Diseases and pathogens of Eucalyptus: 259 – 291 CSIRO Publishing, Melbourne, Australia: [Google Scholar]
- Shearer BL, Tippett JT. 1989. Jarrah dieback: the dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forest of south-western Australia. Research Bulletin No. 3. Department of Conservation and Land Management, Como, Western Australia.
- Stukely MJC, Shearer BL, Tay FCS, Hart RM, Hart RP. 1997 Phytophthora species in natural vegetation in Western Australia. In: ‘Programme and Summaries’, 11th Biennial Conference of the Australasian Plant Pathology Society: 199 Perth, Western Australia [Google Scholar]
- Stukely MJC, Webster JL, Ciampini JA, Brown E, Dunstan WA, Hardy GEStJ, Woodman J, Davison EM, Tay FCS. 2007a. Phytophthora inundata isolated from native vegetation in Western Australia. Australasian Plant Pathology 36: 606 – 608 [Google Scholar]
- Stukely MJC, Webster JL, Ciampini JA, Kerp NL, Colquhoun IJ, Dunstan WA, Hardy GEStJ. 2007b. A new homothallic Phytophthora from the jarrah forest in Western Australia. Australian Plant Disease Notes 2: 49 – 51 [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (* and other methods), version 4.0b 10. Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876 – 4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettraino AM, Barzanti GP, Bianco MC, Ragazzi A, Capretti P, Paoletti E, Luisi N, Anselmi N, Vannini A. 2002. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. Forest Pathology 32: 19 – 28 [Google Scholar]
- Vettraino AM, Natili G, Anselmi N, Vannini A. 2001. Recovery and pathogenicity of Phytophthora species associated with a resurgence of ink disease in Castanea sativa in Italy. Plant Pathology 50: 90 – 96 [Google Scholar]
- Villa NO, Kageyama K, Asano T, Suga H. 2006. Phylogenetic relationships of Pythium and Phytophthora species based on ITS rDNA, cytochrome oxidase II and beta-tubulin gene sequences. Mycologia 98: 410 – 422 [DOI] [PubMed] [Google Scholar]
- Waterhouse GM, Waterston JM. 1964. Phytophthora syringae. CMI Descriptions of Pathogenic Fungi and Bacteria 32: 1 – 2 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315 – 321 Academic Press, San Diego, California: [Google Scholar]
- Zea-Bonilla T, Martin-Sánchez PM, Hermoso JM, Carmona MP, Segundo E, Pérez-Jimenéz RM. 2007. First report of Phytophthora citricola on Mangifera indica in Spain. Plant Pathology 56: 356 [Google Scholar]
- Zentmyer GA, Jefferson L, Hickman CJ, Chang-Ho Y. 1974. Studies of Phytophthora citricola, isolated from Persea americana. Mycologia 66: 830 – 845 [PubMed] [Google Scholar]


