Abstract
Receptor-mediated endocytosis is a pivotal function of renal proximal tubule epithelial cells (PTECs) to reabsorb and metabolize substantial amounts of proteins and other substances in glomerular filtrates. The function accounts for the conservation of nutrients, including carrier-bound vitamins and trace elements, filtered by glomeruli. Impairment of the process results in a loss of such substances and development of proteinuria, an important clinical sign of kidney disease and a risk marker for cardiovascular disease. Megalin is a multiligand endocytic receptor expressed at clathrin-coated pits of PTEC, playing a central role in the process. Megalin cooperates with various membrane molecules and interacts with many intracellular adaptor proteins for endocytic trafficking. Megalin is also involved in signaling pathways in the cells. Megalin-mediated endocytic overload leads to damage of PTEC. Further studies are needed to elucidate the mechanism of megalin-mediated endocytosis and develop strategies for preventing the damage of PTEC.
1. Introduction
Renal proximal tubular epithelial cells (PTECs) are involved in a variety of vital functions. Of these, receptor-mediated endocytosis is a pivotal function of the cells to reabsorb and metabolize proteins and other substances in glomerular filtrates. Megalin is a membrane receptor that plays a central role in the endocytic functions of PTEC. Megalin cooperates with various molecules in the cells, taking up ligands into the endocytic pathway to lysosomes, as well as mediating signal transduction. In this review, we focus on recent progress in the research on megalin and its associated molecules. We also discuss how impaired or overloaded endocytosis induces PTEC damage which is tightly associated with the onset of proteinuria and the development of chronic kidney disease (CKD).
2. Megalin: A Major Endocytic Receptor in PTEC
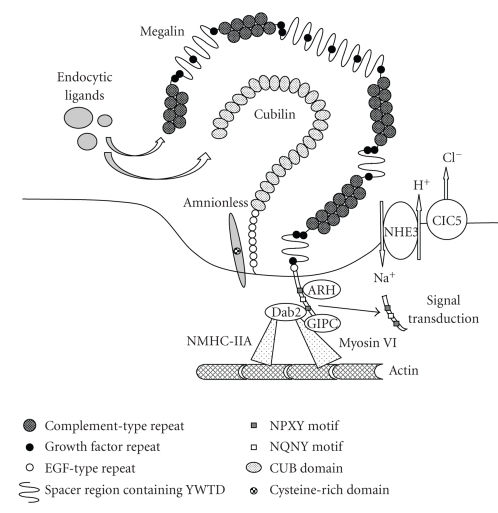
Megalin is a large (~600 kDa) glycoprotein member of the low-density lipoprotein (LDL) receptor family [1, 2] that is primarily expressed at clathrin-coated pits and partly at microvilli of PTEC (Figure 1) [3, 4]. Megalin contains a huge extracellular domain responsible for its multispecific properties. The domain consists of 4398 amino acids (in humans) and is made by three types of repeats which are characteristic of the LDL receptor family: (1) 36 cysteine-rich complement-type repeats organized in four clusters, (2) 16 growth factor repeats separated by 8 YWTD containing spacer regions involved in pH dependent release of ligands in endosomal compartments [5], and (3) a single epidermal growth factor-like repeat. The extracellular domain is followed by a single transmembrane segment and a cytoplasmic domain of 209 amino acids. The cytoplasmic tail contains two endocytic motifs (NPXY) mediating clustering into clathrin coated pits and an NPXY-like motif (NQNY) involved in apical sorting of the receptor [6] as well as other protein interaction motifs (SH3 and PDZ domains) and phosphorylation sites [1, 2]. The physiological potential of these regulatory motifs has not yet been fully understood.
Figure 1.
Megalin and its associated molecules involved in receptor-mediated endocytosis in PTEC. On the apical membrane of PTEC, various molecules are involved in the process of receptor-mediated endocytosis. Megalin, playing a central role in the process, cooperates with other membrane proteins such as the cubilin-amnionless complex (CUBAM), NHE3, and ClC5. Megalin and CUBAM directly bind a variety of ligands, whereas NHE3 and ClC5 are involved in endosomal acidification, which is important for further processing of endocytosed proteins. Megalin also interacts with intracellular adaptor proteins such as ARH, Dab2, and GIPC. Dab2 binds to motor proteins, myosin VI, and NMHC IIA, which may mediate endocytic trafficking of the molecular complexes through actin filaments. The cytoplasmic tail of megalin is released from the membrane by γ-secretase and is involved in intracellular signal transduction.
Megalin plays a critical role in the reabsorption of glomerular-filtered substances including albumin and low-molecular-weight proteins. Also, megalin may take up proteins that are released by PTEC to the apical tubular space. Megalin knockout mice display low-molecular-weight proteinuria and albuminuria [7]. Furthermore, patients with Donnai-Barrow and facio-oculo-acoustico-renal syndromes, caused by mutations in the megalin gene, show increased urinary excretion of albumin and low-molecular-weight proteins [8]. In this process, meglin mediates the conservation of carrier bound vitamins and trace elements filtered by glomeruli, including vitamin D [9], vitamin A [10], vitamin B12 [11], and iron [12]. Megalin cooperates with a variety of molecules at the apical membranes and also in the cytoplasm of PTEC (Figure 1) as described in the next section.
3. Molecules Associated with Megalin's Functions in PTEC
3.1. Cubilin-Amnionless Complex (CUBAM)
Cubilin is a 460-kDa peripheral glycoprotein, thus lacking transmembrane and intracellular segments, but anchored to the apical membranes in PTEC. It was originally identified as the receptor for intrinsic factor-vitamin B12 [13, 14], and its gene defects are the causes of hereditary megaloblastic anaemia 1 or Imerslund-Gräsbeck syndrome (selective vitamin B12 malabsorption with proteinuria) [15]. Cubilin is also involved in the absorption of various protein ligands present in glomerular filtrates, including albumin, transferrin, and vitamin D-binding protein [4]. Cubilin is known to interact with megalin for its endocytic functions [12, 16]; however, it is bound more firmly by a protein called amnionless, forming a complex named CUBAM, to be translocated to the plasma membrane [17, 18]. Amnionless, a 38–50 kDa membrane protein with a single transmembrane domain, was initially identified as a component for the normal development of the trunk mesoderm derived from the middle streak [19]. Its gene defects also cause hereditary megaloblastic anaemia [20]. However, the role of amnionless in PTEC is not fully identified.
3.2. Na+/H+ Exchanger Isoform 3 (NHE3)
NHE3, the main NHE isoform in PTEC, mediates isotonic reabsorption of approximately two thirds of the filtered NaCl and water, the reabsorption of bicarbonate, and the secretion of ammonium [21]. It also contributes to the reabsorption of filtered citrate, amino acids, and oligopeptides by providing H+ used for the H+-coupled cotransporters. Enhanced NHE3 activity is assumed to be a factor for increased Na+ reabsorption and the development of hypertension in diabetes. NHE3 was reported to interact with megalin in intermicrovillar clefts of PTEC [22, 23]. After endocytosis with megalin, NHE3 is postulated to utilize the outward transvesicular Na+ gradient of endocytic vesicles and early endosomes to drive inward movement of H+ and endosomal acidification, which is important for dissociating reabsorbed ligand proteins from megalin for further processing.
3.3. ClC-5
ClC-5 is a 746-amino acid protein originally assumed to belong to the voltage-gated chloride channel family [24], but more recent evidence suggests that it may function as an H+/Cl− exchanger [25]. In kidney, ClC-5 is highly expressed in PTEC and α and β intercalated cells of collecting ducts [26]. In PTEC, ClC-5 is located at the apical endosomes together with electrogenic V-type H+-ATPases [26], where it has a complementary function in endosomal acidification [27]. The physiological relevance of ClC-5 in renal functions came into view when mutations in the CLCN5 gene were identified in patients with Dent's disease, an X-linked renal tubular disorder [26]. This disorder is characterized by low molecular weight proteinuria, hypercalciuria, nephrocalcinosis, nephrolithiasis, aminoaciduria, phosphaturia, glycosuria, and renal failure [28]. The precise mechanism of this abnormality is not entirely clear but possibly results from defective acidification and/or reduced expression of megalin and cubilin in PTEC [29, 30].
3.4. Intracellular Adaptor Proteins
Various sorting and signaling proteins bind to megalin's cytoplasmic tail such as JIP1 and JIP2, SEMCAP-1 (GIPC), ANKRA, Dab2, PDS-95, MegBP, and ARH [31–37]. ARH and Dab2 are components of the clathrin coat, and they bind to the first and third NPXY motif of megalin, respectively, through their PTB domains [33, 37]. ARH and Dab2 are known to interact with motor proteins as described below. Dab2 is also known to mediate signal transduction [38, 39].
4. Regulation of Megalin Expression
Cellular expression of megalin was found to be downregulated by the action of TGFß [40]. We also found that megalin expression is upregulated in cultured PTEC by treatment with insulin or high-concentration glucose (17.5 mM), whereas it is downregulated by angiotnsin II [41]. Furthermore, we demonstrated that there is competitive cross talk between anigotensin II type 1 receptor- and insulin-mediated signaling pathways in the regulation of megalin expression in the cells, suggesting a counter-balanced mechanism that regulates megalin expression and functions in PTEC [41].
Decreased megalin expression in PTEC has been found in the early diabetic stages in experimental animals [40, 42]. It is also suggested that the functions of megalin are impaired in patients in the early stages of diabetic nephropathy, since low-molecular-weight proteinuria are frequently observed in patients at these stages [43, 44]. Thus, the altered regulation of megalin expression and functions must be significantly responsible for the early development of proteinuria/albuminuria in diabetic patients. The mechanisms of the regulation remain to be further investigated.
5. Regulation of Megalin Transport by Motor Proteins
The mechanisms of intracellular transport of megalin are largely unknown. Reverse-direction molecular motor myosin IV was found to be linked to Dab2 and GIPC, which binds to the cytoplasmic tail of megalin, and is assumed to be involved in the endocytosis in PTEC [45]. However, myosin VI knockout mice, used as an animal model for deafness, showed no apparent renal manifestation presenting proteinuria [46].
We recently identified that another motor protein, nonmuscle myosin heavy chain IIA (NMHC IIA), binds to Dab2 and is involved in megalin-mediated endocytosis [47]. Genetic alterations of NMHC-IIA are known to cause inherited human diseases, known as MYH9 disorders, which are characterized by giant platelets, thrombocytopenia, and granulocyte inclusions [48, 49]. The spectrum of diseases due to mutations in the gene includes May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome [48–51]. It has been also reported that all of these disorders are related to development of kidney disease [50, 52]. The manifestation of kidney disease in MYH9 disorders indicates the importance of NMHC-IIA in maintaining normal kidney functions, which has been also verified by two recent genomewide scan analyses [53, 54].
Another megalin-binding adaptor protein ARH also associates with motor and centrosomal proteins and is involved in centrosome assembly and cytokinesis [55]. The relevance of the adaptor protein's association with such molecules in the regulation of megalin transport remains undetermined.
6. Overloaded Endocytosis-Induced PTEC Injury in CKD
Overloaded endocytosis in PTEC due to increased glomerular protein filtration has been postulated to be a cause of tubulointerstitial injury. Megalin is identified as the key molecule to initiate the pathogenic process [56]. In diabetes, advanced glycation endproducts (AGEs) are generated in the circulation and involved in a variety of cellular damage [57]. Megalin also mediates the endocyosis of glomerular-filtered AGE in PTEC [58, 59], which causes toxicity in the cells [60, 61]. In metabolic syndrome or dyslipidemia, free fatty acids are delivered to PTEC with the carrier proteins such as albumin or liver-type fatty acid binding protein [62]. Metabolically overloaded PTECs are activated to express proinflammatory cytokines, such as MCP1 and TNFα, and lead to apoptosis [56] or epithelial-mesenchymal transition [63, 64].
7. Handling of Albumin in PTEC, Related to the Mechanism of Albuminuria
Albumin (~69 kDa) is the most abundant circulating protein, carrying a variety of substances in plasma. Glomerular albumin filtration is assumed to be 3–6 g/d in humans [65]. Only negligible amounts of albumin are detected in urine, and the substantial remaining of glomerular-filtered albumin is reabsorbed in PTEC via endocytosis, mediated by megalin and CUBAM. Albuminuria is an important clinical sign of kidney disease such as diabetic nephropathy [66, 67] as well as a risk marker of cardiovascular disease (CVD) [68, 69]. Impaired endocytic functions of PTEC for albumin are relevant to the mechanisms of albuminuria.
After endocytosis, albumin is considered to be transferred to lysosomes for degradation to amino acids [70]. On the contrary, the presence of a retrieval or transcytic pathway of albumin in PTEC is suggested [71]. A recent analysis using neonatal Fc receptor knockout mice supports the retrieval pathway in PTEC where the receptor appears to play a critical role to reclaim albumin from the glomerular filtrates [72].
The association of albuminuria with the development of CVD may be related to the impairment of metabolic or synthetic functions of PTEC that may contribute to systemic vascular damage. For instance, vitamin D deficiency, which is caused by megalin dysfunction, is independently associated with increased cardiovascular mortality [73, 74]. Selenoprotein P, a major carrier of selenium, is taken up by megalin [75] and provides selenium for synthesizing glutathione peroxidase 3 (GPx3) in PTEC [76, 77]. GPx3 is secreted into the extracellular space from where it enters the blood and acts as antioxidant [78]. Therefore, reduced uptake of selenoprotein P in PTEC due to impaired megalin function may result in decreased GPx3 synthesis by the cells and may be associated with the development of vascular diseases.
8. Megalin-Mediated Signaling
Biemesderfer and his colleagues identified that megalin undergoes regulated intramembrane proteolysis as some other membrane proteins such as those belonging to the Notch and amyloidal precursor protein families [79, 80]. They showed (1) that high levels of γ-secretase are expressed in the brush border and endocytic pathway of PTEC where it colocalizes with megalin, (2) that megalin is subjected to PKC-regulated, metalloprotease-mediated ectodomain shedding that produces a 35 to 40 kDa megalin COOH-terminal fragment (MCTF), and (3) that the MCTF is membrane bound and is constitutively processed by γ-secretase activity [81]. They also found evidence suggesting that the COOH-terminal domain of megalin regulates megalin and NHE3 gene expression [82]. These findings strongly indicate that megalin is not only involved in scavenging functions in PTEC but also participate in the signal transduction in the cells.
9. Conclusions
Megalin, an endocytic receptor, mediates the conservation of nutrients and carrier bound vitamins and trace elements in glomerular filtrates via interaction with various molecules in PTEC. Megalin also plays a critical role in the uptake of pathological substances or overloaded endocytosis that may lead to the cellular damage. Megalin-mediated signaling transduction may be also involved in the process. Further studies are needed to elucidate the molecular mechanism fully and develop strategies for preventing PTEC damage.
Acknowledgments
The authors thank Taeko Soma and Ryoko Niizuma for their help with preparing the manuscript.
References
- 1.Saito A, Pietromonaco S, Loo AK-C, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hjälm G, Murray E, Crumley G, et al. Cloning and sequencing of human gp330, a Ca2+-binding receptor with potential intracellular signaling properties. European Journal of Biochemistry. 1996;239(1):132–137. doi: 10.1111/j.1432-1033.1996.0132u.x. [DOI] [PubMed] [Google Scholar]
- 3.Verroust PJ, Kozyraki R, Hammond TG, Moestrup SK, Christensen EI. Physiopathologic role of cubilin and megalin. Advances in Nephrology from the Necker Hospital. 2000;30:127–145. [PubMed] [Google Scholar]
- 4.Christensen EI, Verroust PJ, Nielsen R. Receptor-mediated endocytosis in renal proximal tubule. Pflügers Archiv European Journal of Physiology. 2009;458(6):1039–1048. doi: 10.1007/s00424-009-0685-8. [DOI] [PubMed] [Google Scholar]
- 5.Davis CG, Goldstein JL, Sudhof TC, Anderson RG, Russell DW, Brown MS. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987;326(6115):760–765. doi: 10.1038/326760a0. [DOI] [PubMed] [Google Scholar]
- 6.Takeda T, Yamazaki H, Farquhar MG. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. American Journal of Physiology. 2003;284(5):C1105–C1113. doi: 10.1152/ajpcell.00514.2002. [DOI] [PubMed] [Google Scholar]
- 7.Leheste J-R, Rolinski B, Vorum H, et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. American Journal of Pathology. 1999;155(4):1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarci S, Al-Gazali L, Hill RS, et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nature Genetics. 2007;39(8):957–959. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nykjaer A, Fyfe JC, Kozyraki R, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen EI, Moskaug JO, Vorum H, et al. Evidence for an essential role of megalin in transepithelial transport of retinol. Journal of the American Society of Nephrology. 1999;10(4):685–695. doi: 10.1681/ASN.V104685. [DOI] [PubMed] [Google Scholar]
- 11.Moestrup SK, Birn H, Fischer PB, et al. Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8612–8617. doi: 10.1073/pnas.93.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozyraki R, Fyfe J, Verroust PJ, et al. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seetharam B, Levine JS, Ramasamy M, Alpers DH. Purification, properties, and immunochemical localization of a receptor for intrinsic factor-cobalamin complex in the rat kidney. Journal of Biological Chemistry. 1988;263(9):4443–4449. [PubMed] [Google Scholar]
- 14.Seetharam B, Christensen EI, Moestrup SK, Hammond TG, Verroust PJ. Identification of rat yolk sac target protein of teratogenic antibodies, gp280, as intrinsic factor-cobalamin receptor. Journal of Clinical Investigation. 1997;99(10):2317–2322. doi: 10.1172/JCI119411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aminoff M, Carter JE, Chadwick RB, et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nature Genetics. 1999;21(3):309–313. doi: 10.1038/6831. [DOI] [PubMed] [Google Scholar]
- 16.Yammani RR, Seetharam S, Seetharam B. Identification and characterization of two distinct ligand binding regions of cubilin. Journal of Biological Chemistry. 2001;276(48):44777–44784. doi: 10.1074/jbc.M106419200. [DOI] [PubMed] [Google Scholar]
- 17.Fyfe JC, Madsen M, Højrup P, et al. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103(5):1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- 18.Coudroy G, Gburek J, Kozyraki R, et al. Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. Journal of the American Society of Nephrology. 2005;16(8):2330–2337. doi: 10.1681/ASN.2004110925. [DOI] [PubMed] [Google Scholar]
- 19.Kalantry S, Manning S, Haub O, et al. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nature Genetics. 2001;27(4):412–416. doi: 10.1038/86912. [DOI] [PubMed] [Google Scholar]
- 20.Tanner SM, Aminoff M, Wright FA, et al. Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nature Genetics. 2003;33(3):426–429. doi: 10.1038/ng1098. [DOI] [PubMed] [Google Scholar]
- 21.Bobulescu IA, Moe OW. Luminal Na+/H+ exchange in the proximal tubule. Pflügers Archiv European Journal of Physiology. 2009;458(1):5–21. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. Journal of Biological Chemistry. 1999;274(25):17518–17524. doi: 10.1074/jbc.274.25.17518. [DOI] [PubMed] [Google Scholar]
- 23.Biemesderfer D, DeGray B, Aronson PS. Active (9.6 s) and inactive (21 s) oligomers of NHE3 in microdomains of the renal brush border. Journal of Biological Chemistry. 2001;276(13):10161–10167. doi: 10.1074/jbc.M008098200. [DOI] [PubMed] [Google Scholar]
- 24.Uchida S. In vivo role of CLC chloride channels in the kidney. American Journal of Physiology. 2000;279(5):F802–F808. doi: 10.1152/ajprenal.2000.279.5.F802. [DOI] [PubMed] [Google Scholar]
- 25.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436(7049):424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 26.Jentsch TJ. Chloride transport in the kidney: lessons from human disease and knockout mice. Journal of the American Society of Nephrology. 2005;16(6):1549–1561. doi: 10.1681/ASN.2005020207. [DOI] [PubMed] [Google Scholar]
- 27.Günther W, Lüchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. CIC-5, the chloride channel mutated in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrong OM, Norden AGW, Feest TG. Dent's disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Quarterly Journal of Medicine. 1994;87(8):473–493. [PubMed] [Google Scholar]
- 29.Christensen EI, Devuyst O, Dom G, et al. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanuma A, Sato H, Takeda T, et al. Functional characterization of a novel missense CLCN5 mutation causing alterations in proximal tubular endocytic machinery in Dent's disease. Nephron Physiology. 2007;107(4):p87–p97. doi: 10.1159/000111253. [DOI] [PubMed] [Google Scholar]
- 31.Gotthardt M, Trommsdorff M, Nevitt MF, et al. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. Journal of Biological Chemistry. 2000;275(33):25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 32.Rader K, Orlando RA, Lou X, Farquhar MG. Characterization of ANKRA, a novel ankyrin repeat protein that interacts with the cytoplasmic domain of megalin. Journal of the American Society of Nephrology. 2000;11(12):2167–2178. doi: 10.1681/ASN.V11122167. [DOI] [PubMed] [Google Scholar]
- 33.Oleinikov AV, Zhao J, Makker SP. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochemical Journal. 2000;347, part 3:613–621. [PMC free article] [PubMed] [Google Scholar]
- 34.Lou X, Mcquistan T, Orlando RA, Farquhar MG. GAIP, GIPC and Gαi3 are concentrated in endocytic compartments of proximal tubule cells: putative role in regulating megalin's function. Journal of the American Society of Nephrology. 2002;13(4):918–927. doi: 10.1681/ASN.V134918. [DOI] [PubMed] [Google Scholar]
- 35.Larsson M, Hjälm G, Sakwe AM, et al. Selective interaction of megalin with postsynaptic density-95 (PSD-95)-like membrane-associated guanylate kinase (MAGUK) proteins. Biochemical Journal. 2003;373(2):381–391. doi: 10.1042/BJ20021958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen HH, Hilpert J, Militz D, et al. Functional interaction of megalin with the megalin-binding protein (MegBP), a novel tetratrico peptide repeat-containing adaptor molecule. Journal of Cell Science. 2003;116(3):453–461. doi: 10.1242/jcs.00243. [DOI] [PubMed] [Google Scholar]
- 37.Nagai M, Meerloo T, Takeda T, Farquhar MG. The adaptor protein ARH escorts megalin to and through endosomes. Molecular Biology of the Cell. 2003;14(12):4984–4996. doi: 10.1091/mbc.E03-06-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hocevar BA, Smine A, Xu X-X, Howe PH. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO Journal. 2001;20(11):2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prunier C, Hocevar BA, Howe PH. Wnt signaling: physiology and pathology. Growth Factors. 2004;22(3):141–150. doi: 10.1080/08977190410001720860. [DOI] [PubMed] [Google Scholar]
- 40.Russo LM, del Re E, Brown D, Lin HY. Evidence for a role of transforming growth factor (TGF)-β1 in the induction of postglomerular albuminuria in diabetic nephropathy: amelioration by soluble TGF-β type II receptor. Diabetes. 2007;56(2):380–388. doi: 10.2337/db06-1018. [DOI] [PubMed] [Google Scholar]
- 41.Hosojima M, Sato H, Yamamoto K, et al. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor—and insulin-mediated signaling cross talk. Endocrinology. 2009;150(2):871–878. doi: 10.1210/en.2008-0886. [DOI] [PubMed] [Google Scholar]
- 42.Tojo A, Onozato M, Ha H, et al. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochemistry and Cell Biology. 2001;116(3):269–276. doi: 10.1007/s004180100317. [DOI] [PubMed] [Google Scholar]
- 43.Pontuch P, Jensen T, Deckert T, Ondrejka P, Mikulecky M. Urinary excretion of retinol-binding protein in type 1 (insulin-dependent) diabetic patients with microalbuminuria and clinical diabetic nephropathy. Acta Diabetologica. 1992;28(3-4):206–210. doi: 10.1007/BF00779000. [DOI] [PubMed] [Google Scholar]
- 44.Hong C-Y, Hughes K, Chia K-S, Ng V, Ling S-L. Urinary α1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26(2):338–342. doi: 10.2337/diacare.26.2.338. [DOI] [PubMed] [Google Scholar]
- 45.Hasson T. Myosin VI: two distinct roles in endocytosis. Journal of Cell Science. 2003;116(17):3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- 46.Avraham KB, Hasson T, Steel KP, et al. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nature Genetics. 1995;11(4):369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- 47.Hosaka K, Takeda T, Iino N, et al. Megalin and nonmuscle myosin heavy chain IIA interact with the adaptor protein Disabled-2 in proximal tubule cells. Kidney International. 2009;75(12):1308–1315. doi: 10.1038/ki.2009.85. [DOI] [PubMed] [Google Scholar]
- 48.Kelley MJ, Jawien W, Ortel TL, Korczak JF. Mutation of MYH9, encoding non-muscle myosin heavy chain A, in May-Hegglin anomaly. Nature Genetics. 2000;26(1):106–108. doi: 10.1038/79069. [DOI] [PubMed] [Google Scholar]
- 49.Seri M, Cusano R, Gangarossa S, et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. Nature Genetics. 2000;26(1):103–105. doi: 10.1038/79063. [DOI] [PubMed] [Google Scholar]
- 50.Heath KE, Campos-Barros A, Toren A, et al. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and alport-like syndromes. American Journal of Human Genetics. 2001;69(5):1033–1045. doi: 10.1086/324267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. Journal of the American Society of Nephrology. 2002;13(1):65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 52.Seri M, Savino M, Bordo D, et al. Epstein syndrome: another renal disorder with mutations in the nonmuscle myosin heavy chain 9 gene. Human Genetics. 2002;110(2):182–186. doi: 10.1007/s00439-001-0659-1. [DOI] [PubMed] [Google Scholar]
- 53.Kao WHL, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nature Genetics. 2008;40(10):1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nature Genetics. 2008;40(10):1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehtonen S, Shah M, Nielsen R, et al. The endocytic adaptor protein ARH associates with motor and centrosomal proteins and is involved in centrosome assembly and cytokinesis. Molecular Biology of the Cell. 2008;19(7):2949–2961. doi: 10.1091/mbc.E07-05-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motoyoshi Y, Matsusaka T, Saito A, et al. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney International. 2008;74(10):1262–1269. doi: 10.1038/ki.2008.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nature Clinical Practice Endocrinology and Metabolism. 2008;4(8):444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 58.Saito A, Nagai R, Tanuma A, et al. Role of megalin in endocytosis of advanced glycation end products: implications for a novel protein binding to both megalin and advanced glycation end products. Journal of the American Society of Nephrology. 2003;14(5):1123–1131. doi: 10.1097/01.asn.0000062962.51879.f8. [DOI] [PubMed] [Google Scholar]
- 59.Saito A, Takeda T, Sato K, et al. Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Annals of the New York Academy of Sciences. 2005;1043:637–643. doi: 10.1196/annals.1333.072. [DOI] [PubMed] [Google Scholar]
- 60.Sebeková K, Schinzel R, Ling H, et al. Advanced glycated albumin impairs protein degradation in the kidney proximal tubules cell line LLC-PK1. Cellular and Molecular Biology. 1998;44(7):1051–1060. [PubMed] [Google Scholar]
- 61.Verbeke P, Perichon M, Friguet B, Bakala H. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochimica et Biophysica Acta. 2000;1502(3):481–494. doi: 10.1016/s0925-4439(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 62.Oyama Y, Takeda T, Hama H, et al. Evidence for megalin-mediated proximal tubular uptake of L-FABP, a carrier of potentially nephrotoxic molecules. Laboratory Investigation. 2005;85(4):522–531. doi: 10.1038/labinvest.3700240. [DOI] [PubMed] [Google Scholar]
- 63.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs. 2007;185(1–3):222–231. doi: 10.1159/000101323. [DOI] [PubMed] [Google Scholar]
- 64.Strutz FM. EMT and proteinuria as progression factors. Kidney International. 2009;75(5):475–481. doi: 10.1038/ki.2008.425. [DOI] [PubMed] [Google Scholar]
- 65.Gekle M. Renal tubule albumin transport. Annual Review of Physiology. 2005;67:573–594. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 66.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1(8287):1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 67.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. New England Journal of Medicine. 1984;310(6):356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 68.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Journal of the American Medical Association. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 69.Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Annals of Internal Medicine. 2003;139(11):901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 70.Maunsbach AB. Absorption of I125-labeled homologous albumin by rat kidney proximal tubule cells. A study of microperfused single proximal tubules by electron microscopic autoradiography and histochemistry. Journal of Ultrasructure Research. 1966;15(3-4):197–241. doi: 10.1016/s0022-5320(66)80108-9. [DOI] [PubMed] [Google Scholar]
- 71.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. American Journal of Physiology. 2008;295(6):F1589–F1600. doi: 10.1152/ajprenal.00142.2008. [DOI] [PubMed] [Google Scholar]
- 72.Sarav M, Wang Y, Hack BK, et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. Journal of the American Society of Nephrology. 2009;20(9):1941–1952. doi: 10.1681/ASN.2008090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. Journal of Clinical Endocrinology and Metabolism. 2008;93(10):3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 74.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Archives of Internal Medicine. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 75.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. Journal of Biological Chemistry. 2008;283(11):6854–6860. doi: 10.1074/jbc.M709945200. [DOI] [PubMed] [Google Scholar]
- 76.Avissar N, Ornt DB, Yagil Y, et al. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. American Journal of Physiology. 1994;266(2):C367–C375. doi: 10.1152/ajpcell.1994.266.2.C367. [DOI] [PubMed] [Google Scholar]
- 77.Maser RL, Magenheimer BS, Calvet JP. Mouse plasma glutathione peroxidase. cDNA sequence analysis and renal proximal tubular expression and secretion. Journal of Biological Chemistry. 1994;269(43):27066–27073. [PubMed] [Google Scholar]
- 78.Whitin JC, Bhamre S, Tham DM, Cohen HJ. Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. American Journal of Physiology. 2002;283(1):F20–F28. doi: 10.1152/ajprenal.00014.2001. [DOI] [PubMed] [Google Scholar]
- 79.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100(4):391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 80.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature Reviews Neuroscience. 2006;7(2):93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 81.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. Journal of Biological Chemistry. 2004;279(33):34302–34310. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Cong R, Biemesderfer D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. American Journal of Physiology. 2008;295(2):C529–C537. doi: 10.1152/ajpcell.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]