Abstract
Objective
By magnetic resonance imaging (MRI), subchondral bone attrition (SBA) can be seen in early osteoarthritis (OA), but the significance of this is unknown. We therefore evaluated whether SBA was associated with cartilage loss within the same subregion of the knee.
Methods
The Multicenter Osteoarthritis Study is a cohort of individuals who have or are at high risk for knee OA. At baseline and 30 months, participants’ knee MRIs were graded using the Whole-Organ Magnetic Resonance Imaging Score in the 10 subregions of the tibiofemoral joint for cartilage morphology and SBA. We conducted analyses within a knee to eliminate between-person confounding, using an M:N (cases:controls) matched case–control approach with the 10 subregions of a person’s knee forming a matched set. Cases within a knee were defined as subregions with cartilage loss, while controls were subregions in that same knee without cartilage loss. We evaluated the association of cartilage loss over 30 months with the presence of baseline SBA in the same subregion within that knee using conditional logistic regression.
Results
SBA was associated with an odds ratio of 7.5 (95% confidence interval 5.6 –9.9, P < 0.0001) for cartilage loss in the same subregion compared with subregions without any baseline SBA in our sample of 459 knees from participants, 64% of whom were women, with a mean age of 63 years and a mean body mass index of 30.5 kg/m2.
Conclusion
SBA is strongly associated with cartilage loss within the same subregion of a knee. SBA may directly influence overlying cartilage loss or serve as a marker of an area undergoing great compressive stress and in which cartilage loss is inevitable.
INTRODUCTION
The exact role of bone in the pathogenesis of osteoarthritis (OA) is not well understood (1), with much focus to date placed on the articular cartilage. Because bone may act as the major shock absorber in the joint by attenuating forces through the joint, it may thereby protect cartilage from damage by excessive loads, although this contention is controversial. Nonetheless, if there are alterations in the subchondral bone, it may be less able to absorb and dissipate energy, thereby increasing forces transmitted through the joint. Indeed, subchondral bone changes have been demonstrated to be common in surgical specimens of OA (2). One such subchondral bone abnormality commonly noted in persons with advanced OA is subchondral bone attrition (SBA), which is a loss of subchondral bone resulting in a change of the articular bone shape such as flattening or depression of the bony surface, unrelated to gross fracture.
Although traditionally SBA was thought to be a late finding in OA, magnetic resonance imaging (MRI) studies have revealed that SBA is also present in knees with early OA and even preradiographic OA (3). Animal models have demonstrated that subchondral bone changes can occur early in the course of OA, and that loading-related damage to subchondral bone can lead to cartilage abnormalities (4–10). Therefore, alterations in subchondral bone could perhaps even precede cartilage abnormalities in OA. Whether SBA is associated with overlying cartilage loss in human knee OA is not known. If such an association was demonstrated, it would provide evidence to support the importance of subchondral bone in OA progression. It may be that cartilage and bone subjacent to that cartilage are changing together either from load transmitted onto both tissues, or there may be effects of bone on cartilage. Identifying colocalized abnormalities would suggest an extremely focal process, one perhaps not even spread across a compartment.
Our current methods of evaluating the relationship of bone change and cartilage change, examining whether they occur in the same knee or even the same compartment, are not sufficient for studying the geographic matching of interest. One would ideally study the local effects of SBA on cartilage loss within the same region of the knee rather than studying whether SBA is associated with cartilage loss across individuals. Assessment of the association of SBA and cartilage loss within the same subregions of individual knees using a matched design would allow one to determine the relative frequency with which cartilage loss occurs in regions with SBA versus those without SBA within the same knee.
We propose using an M:N matched case–control approach in which matched sets consist of a varying number of cases (M) and controls (N) (11). Specifically, in this analysis, the subregions in a knee will comprise a matched set, in which “M” number of subregions are cases (i.e., with cartilage loss) and “N” (10 eligible subregions within a knee – M) is the number of control subregions. This method eliminates between-person and between-knee confounding, an important threat to validity, particularly in observational studies. It does so because the comparisons are within the subregions of each knee, which experience the same knee-level factors such as genetics, sex, malalignment, physical activity, etc. Furthermore, this method allows one to examine the specific geographic colocalization of pathologic features. Using such an approach, we examined the association between the baseline presence of SBA with cartilage loss over time in a large cohort of individuals with or at high risk for knee OA.
SUBJECTS AND METHODS
Study sample
The sample for this study included eligible participants drawn from the Multicenter Osteoarthritis (MOST) Study, a prospective cohort study of 3,026 individuals ages 50–79 years whose goal is to identify risk factors for incident symptomatic knee OA and progressive OA in a sample either with or at high risk of OA. For this sample, eligible participants had to have had knee MRIs at baseline and followup, with no missing data for both SBA and cartilage MRI readings, and fulfill the requirements for the M:N matched case–control analysis (see below).
All of the MOST subjects were recruited from 2 communities in the US: Birmingham, Alabama, and Iowa City, Iowa. Details of the study population have been published elsewhere (12). Briefly, those considered at high risk of developing knee OA included persons who were overweight or obese, those with knee pain, aching, or stiffness on most days of the last 30 days, a history of knee injury that made it difficult to walk for at least one week, or previous knee surgery. Persons were excluded if they 1) had bilateral total knee replacement, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis, or a history of cancer (except for nonmelanoma skin cancer); 2) required dialysis; 3) were unable to walk without the help of another person or walker; or 4) planned to move out of the area in the subsequent 3 years. The study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco, and Boston University Medical Center.
MRI assessments
At baseline and 30 months, knee MRIs were performed using a 1.0T OrthOne extremity scanner (ONI Medical Systems, Wilmington, MA) with axial and sagittal proton-density fat-suppressed and coronal STIR sequences. MRIs were scored for SBA on a 0–3 scale and for cartilage morphology on a 0–6 scale using the Whole-Organ Magnetic Resonance Imaging Score (WORMS) (13) by 2 musculoskeletal radiologists (AG, FR) in 5 subregions within each of the medial and lateral tibiofemoral joints: the anterior, central, and posterior tibia, and the central and posterior femur (i.e., a total of 10 subregions within the tibiofemoral joint). The presence of SBA within a subregion was defined as an SBA score >0 at baseline. Cartilage loss was defined as any worsening of the WORMS score between baseline and 30 months by ≥1 grade, except that a baseline score of 0 had to increase by ≥2 grades.
Other covariates
Age and sex were ascertained at the baseline visit. Weight was measured at baseline in light-weight clothing without shoes or heavy jewelry using a standard balance beam scale. Height was measured at baseline without shoes at the peak of inhalation using a Harpenden stadiometer (Holtain, Pembrokeshire, UK). Body mass index (BMI) was calculated as weight in kg divided by height in m2.
Statistical analyses
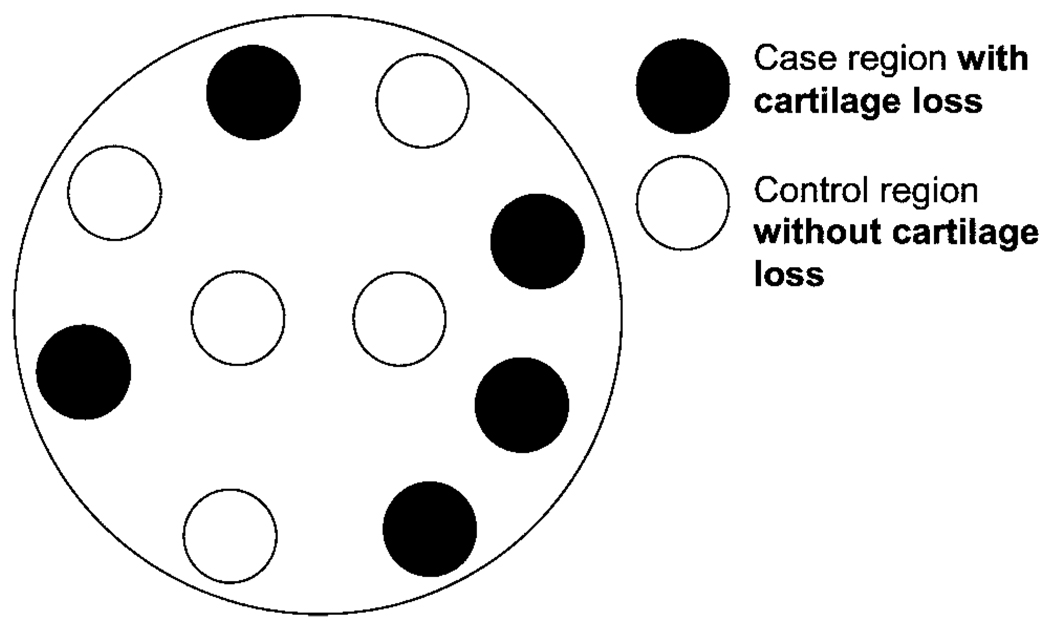
For the M:N matched case–control analysis in which 10 subregions within the tibiofemoral joint formed a matched set, we matched subregions with cartilage loss (cases; M) to sites without cartilage loss (controls; N) over 30 months within each knee (Figure 1). The risk factor, SBA, was defined as present (exposed) or absent (unexposed) in each of those 10 subregions at baseline. We examined the relationship of SBA to cartilage loss using conditional logistic regression (11). We also evaluated these associations using a higher threshold for defining the presence of SBA in a subregion (score ≥2 versus score 0 or 1), as well as categorized as absent (score 0), score 1, and score ≥2. We repeated the analyses in a compartment-specific manner (i.e., the medial and lateral compartments), and then limited analyses to subregions that would be considered to undergo greater loading and to those that would be considered to undergo less loading to determine if the association between SBA and cartilage loss was modified. Finally, for comparison, we also repeated analyses using 2 more commonly used traditional approaches of evaluating whether the presence of SBA is associated with cartilage loss: 1) the association was evaluated within subregions across all of the studied knees using generalized estimating equations (GEEs) to account for correlations between subregions within a knee (subregion-based analysis), and 2) the association was evaluated as the presence of SBA and cartilage loss occurring anywhere in the knee or compartment (knee- or compartment-based analysis). Both such traditional approaches require the adjustment for potential between-person confounders due to the analysis being conducted across individuals’ knees.
Figure 1.
Example of case and control regions within a knee (each of the 10 circles represents each of the 10 subregions within a knee). The exposure status (i.e., the presence or absence of subchondral bone attrition) within each of these subregions is determined for each case and control subregion.
RESULTS
Of the 1,229 knees that had both baseline and followup knee MRIs with no missing MRI readings for SBA and cartilage morphology, 459 knees (1 knee per person) met the eligibility criteria for the M:N matched case–control analysis. The excluded knees were not eligible either because none of the subregions were eligible for any further cartilage loss, all 10 subregions within the knee had baseline SBA (i.e., only exposed subregions present), all 10 subregions had no baseline SBA (i.e., only unexposed subregions present), all 10 subregions had cartilage loss (i.e., only case subregions present), or all 10 subregions had no cartilage loss (i.e., only control subregions present). The mean ± SD age of these individuals was 63.0 ± 7.8, with a mean ± SD BMI of 30.5 ± 5.1 kg/m2. Sixty-four percent were women. SBA was present in at least one subregion in 45% of knees, and 59% of knees had radiographic OA at baseline, as defined by a Kellgren/Lawrence scale grade of >2.
The crude prevalences of baseline SBA and of cartilage loss over 30 months by subregion are shown in Table 1. Both features occurred in all subregions of the knee, but some subregions had a higher prevalence of these features. For example, both SBA and cartilage loss occurred most frequently in the femoral medial central and tibial medial central subregions (Table 1).
Table 1.
Crude prevalences of SBA and cartilage loss by subregion (n = 4,446 subregions in 459 knees)*
| Subregion location | Prevalence of SBA, n/N (%) |
Prevalence of cartilage loss, n/N (%) |
|---|---|---|
| Femoral lateral central | 33/446 (7.4) | 104/446 (23.3) |
| Femoral lateral posterior | 22/454 (4.9) | 75/454 (16.5) |
| Femoral medial central | 87/410 (21.2) | 227/410 (55.4) |
| Femoral medial posterior | 40/459 (8.7) | 138/459 (30.1) |
| Tibial lateral anterior | 10/456 (2.2) | 14/456 (3.1) |
| Tibial lateral central | 38/433 (8.8) | 88/433 (20.3) |
| Tibial lateral posterior | 32/445 (7.2) | 53/445 (11.9) |
| Tibial medial anterior | 3/456 (0.7) | 44/456 (9.7) |
| Tibial medial central | 78/429 (18.2) | 173/429 (40.3) |
| Tibial medial posterior | 11/458 (2.4) | 57/458 (12.5) |
SBA = subchondral bone attrition; n = number of subregions with the feature; N = number of knees that had information available for that subregion.
In the M:N matched case–control analysis, the baseline presence of SBA (grade >0) within a subregion was associated with a 7.5 times higher risk (95% confidence interval [95% CI] 5.6 –9.9, P < 0.0001) of having cartilage loss over 30 months than a subregion without any SBA at baseline. When using a stricter definition for the presence of definite SBA (grade ≥2) within a subregion, the corresponding odds ratio (OR) was 5.5 (95% CI 3.0 –10.0, P < 0.0001) in comparison with subregions with SBA grade 0 (Table 2).
Table 2.
Relationship of increasing severity of SBA to cartilage loss (n = 459 knees, 4,446 subregions)*
| Cartilage loss subregions, N |
Non-loss subregions, N |
OR (95% CI)† | |
|---|---|---|---|
| Baseline SBA grade | |||
| SBA grade 0 (none) | 781 | 3,311 | 1.0 (reference group) |
| SBA grade 1 | 165 | 131 | 7.9 (5.8–10.6) |
| SBA grade ≥2 | 27 | 31 | 5.5 (3.0–10.0) |
SBA = subchondral bone attrition; N = number of subregions with (case) cartilage loss and without (control) cartilage loss; OR = odds ratio; 95% CI = 95% confidence interval.
OR for cartilage loss in the same subregion as SBA compared with subregions without SBA.
When evaluating these associations in a compartment-specific manner, similar magnitudes of effect for the relationship of SBA to cartilage loss were seen in both the medial and lateral tibiofemoral compartments (Table 3). However, when analyses were limited to certain subregions, some differences were noted. For example, when analyses were limited to the central subregions of the medial tibiofemoral joint, an OR of 8.0 (95% CI 1.8 –34.8) was noted. On the other hand, when analyses were limited to the posterior subregions of the medial tibiofemoral joint, the magnitude of effect was lower, with an OR of 3.3 (95% CI 1.3–8.3). The presence of these features was also lower in these subregions (Table 1). Therefore, it appears that the strength of the association may vary depending on the specific subregion studied. Analyses stratified by the presence of radiographic OA demonstrated no effect measure modification and similar effect estimates as those for the main analyses, with an OR of 7.5 (95% CI 5.5–10.2) for knees with OA and an OR of 7.1 (95% CI 3.4 –14.6) for knees without OA.
Table 3.
Compartment-specific relationship of SBA to cartilage loss*
| Cartilage loss subregions, N |
Non-loss subregions, N |
OR (95% CI)† | P | |
|---|---|---|---|---|
| Medial compartment | 5.4 (3.6–8.1) | < 0.0001 | ||
| (n = 330 knees, 1,589 subregions) | ||||
| No SBA | 477 | 925 | ||
| SBA | 126 | 61 | ||
| Lateral compartment | 5.5 (2.4–12.7) | < 0.0001 | ||
| (n = 196 knees, 950 subregions) | ||||
| No SBA | 101 | 779 | ||
| SBA | 33 | 37 |
SBA = subchondral bone attrition; N = number of subregions with (case) cartilage loss and without (control) cartilage loss; OR = odds ratio; 95% CI = 95% confidence interval.
OR for cartilage loss in the same subregion as SBA compared with subregions without SBA.
When analyses were repeated using the more commonly used traditional approaches, 968 knees were eligible for evaluation given the less stringent eligibility criteria (Table 4). Using a subregion-based approach, the presence of SBA within a subregion was associated with a 5.4 times higher risk of cartilage loss over 30 months compared with a subregion without SBA (95% CI 4.1–7.0) across all of the knees. Using the knee-based approach, the presence of SBA was associated with a 3.0 times higher risk of cartilage loss in a knee compared with knees without SBA (95% CI 2.2–4.2).
Table 4.
Effect estimates for the association of SBA and cartilage loss using 3 different methodologic approaches*
| Methodologic approach | OR (95% CI)† |
|---|---|
| M:N matched case–control approach (n = 459 knees, 4,446 subregions) | 7.5 (5.6–9.9) |
| Subregion-based approach (n = 968 knees, 9,680 subregions) | 5.4 (4.1–7.0)‡ |
| Knee-based approach (n = 968 knees) | 3.0 (2.2–4.2)‡ |
SBA = subchondral bone attrition; OR = odds ratio; 95% CI = 95% confidence interval; M:N = cases:controls.
OR for the association of SBA with cartilage loss.
Adjusted for potential confounders (age, sex, body mass index) because these analyses are across individuals. The M:N matched case–control approach does not require such adjustments since the analyses are within knee.
DISCUSSION
Using a method that allows for evaluation of the association between SBA and directly overlying cartilage loss within a subregion of the knee, thereby eliminating between-person confounding, we were able to demonstrate that the baseline presence of SBA was strongly associated with cartilage loss over time occurring within the same subregion of a knee. These results were consistent in both compartments of the knee, although there was a suggestion that the effects were of a greater magnitude in specific subregions that experience greater loading.
The current method has advantages over the more standard methods of analyses in its ability to eliminate between-person and between-knee confounding. As illustrated, if one were to conduct these analyses by simply evaluating the association of any baseline SBA with any cartilage loss over time in knees across all of the individuals using generalized GEEs, adjusting for age, sex, and BMI, the OR would be 3.0, in contrast to our finding of 7.5, which is an effect estimate that is biased toward the null by more than 2-fold. Even if one were to assess the relationship between baseline SBA in a given subregion with cartilage loss within the same subregion across all of the knees using GEEs, adjusting for age, sex, and BMI, the OR would be 5.4 (95% CI 4.1–7.0). In this latter GEE approach, although the sample size is increased 10-fold compared with the former GEE approach (since the analysis is subregion based rather than knee based), the upper bound of the 95% CI excludes the effect estimate obtained in the M:N matched case–control approach. In general, one is interested in causal relationships between a risk factor and disease. As such, conditional logistic regression and random-effects models both allow the assessment of subject-specific effects while eliminating between-person confounding. Therefore, such methods generate less biased results compared with approaches using GEEs, since the latter provides a population average across the study population and remains prone to between-person bias.
These results may suggest that SBA influences overlying cartilage loss due to alterations in bone surface shape that may make cartilage more vulnerable to altered load. SBA may therefore serve as a marker of an area undergoing compressive stress in which cartilage loss is inevitable. However, a limitation to this study is that causal relationships are difficult to discern. It is possible that cartilage loss and SBA may both be features of severe disease, and that a more proximal insult has resulted in both the presence of SBA and cartilage loss. For example, bone marrow lesions have also been noted to be associated with both cartilage loss and SBA (14–16). When we attempted to identify knees without any cartilage abnormalities at baseline, very few had SBA present, indicating that both seem to occur concurrently.
When we limited our analyses to different subregions, we noted that some subregions had higher associations between SBA and cartilage loss than others. This may reflect the fact that the curvature of the femur differs for the different subregions. For example, SBA may be more difficult to discern in the posterior femur. On the other hand, this may point to a stronger association occurring in specific subregions that experience greater load.
One proximal factor that may influence both SBA and cartilage loss is malalignment. Neutral limb alignment distributes load widely across an articular surface. Medial tibiofemoral knee OA is more common than lateral knee OA, likely in part because it is subject to more load than the lateral compartment. When a knee has varus malalignment, more stress is transmitted across the medial compartment. Malalignment is associated with an increased risk of structural radiographic progression in knees with existing OA and with cartilage loss in the compartment loaded by the malalignment (17). Malalignment is also associated with ipsilateral bone marrow lesions, which are probably lesions of bone trauma deep to the subchondral plate (18,19). Finally, malalignment has been associated with subchondral bone attrition on MRI cross-sectionally in a small sample of knees with existing OA (18), as well as in the MOST cohort, in which malalignment was also associated with incident SBA in the more loaded compartment (20). Because malalignment leads to altered stress in a joint, subchondral bone remodeling with resultant attrition can occur at those points of increased stress in response to those loads and in response to local micro-fractures as a result of those loads (21). Therefore, the co-occurrence of SBA and cartilage loss within the same subregions of a knee, particularly for subregions thought to experience greater loading, may point to abnormal mechanical loading as a common cause of these pathologies. On the other hand, alterations in the bone surface shape resulting from SBA as well as cartilage loss changing the articular surface can in and of themselves also lead to malalignment and altered mechanical load through those regions. These intricate and complicated temporal relationships are difficult to disentangle.
In summary, the use of advanced methodology that has broad applicability to other studies of relationships between local pathologies within the knee has demonstrated that SBA is strongly associated with cartilage loss over time within the same subregion of a knee. Further studies into the temporal relationship of these findings along with the potential role of malalignment are warranted to provide additional insight into the relationship of bone pathology and cartilage loss in OA.
Acknowledgments
Supported by the NIH (grant AR47785). Dr. Neogi’s work was supported by an American College of Rheumatology Research and Education Foundation/Association of Specialty Professors Junior Career Development Award in Geriatrics (T. Franklin Williams Scholar Award), an Arthritis Foundation Arthritis Investigator Award, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K23-AR055127). Dr. Felson’s work was supported by an NIH grant from the National Institute on Aging (U01-AG18820). Dr. Nevitt’s work was supported by an NIH grant from the National Institute on Aging (U01-AG19069). Dr. Lewis’s work was supported by an NIH grant from the National Institute on Aging (U01-AG18947).
Footnotes
Dr. Guermazi has received consultant fees, speaking fees, and/or honoraria (less than $10,000) from Facet Solutions and (more than $10,000) from Merck Sorono, owns stock and/or holds stock options in Synarc, Inc., and is the President of Boston Imaging Core Lab, LLC. Dr. Roemer is a shareholder in Boston Imaging Core Lab, LLC. Dr. Wallace has received honoraria (less than $10,000 each) from Merck and Novartis.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Neogi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Neogi, Felson, Niu, Nevitt, Zhang.
Acquisition of data. Felson, Lynch, Nevitt, Guermazi, Roemer, Lewis, Wallace.
Analysis and interpretation of data. Neogi, Felson, Niu, Zhang.
REFERENCES
- 1.Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? [editorial] Arthritis Rheum. 2004;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 2.Bullough PG. Osteoarthritis and related disorders: pathology. In: Klippel JH, Dieppe P, editors. Rheumatology. 2nd ed. London: Mosby; 1998. pp. 1–8. [Google Scholar]
- 3.Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, et al. Prevalence of bone attrition on knee radio-graphs and MRI in a community-based cohort. Osteoarthritis Cartilage. 2008;16:1005–1010. doi: 10.1016/j.joca.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331–339. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 5.Anderson-MacKenzie JM, Quasnichka HL, Starr RL, Lewis EJ, Billingham ME, Bailey AJ. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int J Biochem Cell Biol. 2005;37:224–236. doi: 10.1016/j.biocel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972;1:519–522. doi: 10.1016/s0140-6736(72)90179-1. [DOI] [PubMed] [Google Scholar]
- 8.Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res. 1984;2:221–234. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- 9.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;213:34–40. [PubMed] [Google Scholar]
- 10.Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8:572–585. doi: 10.1002/jor.1100080414. [DOI] [PubMed] [Google Scholar]
- 11.Stokes ME, Davis CS, Koch GG. Conditional logistic regression. In: Stokes ME, Davis CS, Koch GG, editors. Categorical data analysis using the SAS System. 2nd ed. Cary: SAS Institute; 2000. pp. 271–322. [Google Scholar]
- 12.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 13.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 15.Roemer FW, Guermazi A, Neogi T, Zhu Y, Zhang Y, Javaid MK, et al. The association of MRI-detected tibiofemoral subchondral bone marrow lesions with prevalent and incident subchondral bone attrition: the MOST Study [abstract] Arthritis Rheum. 2009;58 Suppl:S697. [Google Scholar]
- 16.Wluka AE, Hanna F, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68:850–855. doi: 10.1136/ard.2008.092221. [DOI] [PubMed] [Google Scholar]
- 17.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol. 2005;32:2192–2199. [PubMed] [Google Scholar]
- 19.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 20.Neogi T, Nevitt M, Niu J, Sharma L, Lewis CE, Torner J, et al. Subchondral bone attrition is a reflection of compartment-specific mechanical load: the MOST Study [abstract] Arthritis Rheum. 2008;58 Suppl:S425. doi: 10.1136/ard.2009.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004;70:77–80. [PubMed] [Google Scholar]