Abstract
Nicotine causes dose-dependent alterations in accuracy on the differential-reinforcement of low-rate responding (DRL) 29.5-s schedule in rats. The current investigation evaluated whether nicotine-associated contextual cues can produce nicotine-like perturbations in DRL-schedule performance in the absence of nicotine. Nicotine and saline administrations occurred just prior to DRL 29.5-s schedule responding for sucrose solution, and two different experimental contexts (differentiated by visual, olfactory, and tactile cues) were utilized. All subjects (N = 16) experienced two consecutive daily sessions of DRL-schedule responding per day. The experimental group (n = 8) was exposed to saline immediately prior to the first session and 0.3mg/kg nicotine before the second session, and the context was changed between sessions. This sequence of saline and then nicotine administration, paired with two reliable contexts, persisted for 12 consecutive days and successive nicotine administrations corresponded with increasingly poorer performance on the DRL 29.5-s schedule. No nicotine was administered for days 13–20 during context testing, and the nicotine-associated context produced response disinhibition on the DRL schedule. Two control groups were included in the design; subjects in one control group (n = 4) received saline in each context to verify that the contexts themselves were not exerting control over operant responding. To assess how explicit and non-explicit pairings of nicotine and contextual cues influenced DRL behavior, subjects in a second control group (n = 4) were given nicotine prior to the second session, but the contexts were not altered between sessions. The results from this experiment suggest that environmental stimuli associated with nicotine exposure can come to elicit nicotine-induced performance decrements on a DRL 29.5-s schedule.
Keywords: Impulsivity, Response disinhibition, DRL schedule, Nicotine, Context, Sensitization
1. Introduction
Cue-induced reinstatement of psychomotor stimulant self-administration is a model that has generated a tremendous amount of data regarding the neurobiology of learning and substance dependence (Shaham et al. 2003 for a review), but the clinical relevance of this model of relapse has been debated (e.g. Katz and Higgins, 2003). With regard to cue-induced drug craving, although functional imaging (Brody et al. 2007; Due et al. 2002; Franklin et al. 2007; Kilts et al. 2001; McBride et al. 2006; Smolka et al. 2006) and genotyping (Hutchison et al. 2002; McClernon et al. 2007; 2008) methods have yielded strong evidence of neurobiological correlates and predictors, neither cue reactivity nor a patient's ability to attenuate cue reactivity have been demonstrated to be good predictors of abstinence (Sterling et al. 2004). Although the surmounting neurobiological evidence is quite provocative, without a strong connection between cue-evoked craving and relapse, further investigation on this matter may be of limited utility to clinical treatment.
Evidence exists to suggest that impulsivity is an important variable governing treatment outcomes for nicotine-dependent individuals (Dallery and Raiff, 2007; Krishnan-Sarin et al., 2007; Yoon et al. 2007). Doran, Spring, and McChargue (2007) utilized a delay-discounting paradigm and discovered that higher discounting (greater impulsivity) was found to be a significant predictor of greater cue-induced craving by cigarette-related stimuli. Plausible is the hypothesis that environmental cues associated with cigarette consumption trigger both craving and impulsive-behavior tendencies and thereby increase the likelihood of a relapse.
Drug-associated contextual cues have the capacity to reinstate both place preference and drug self-administration post extinction (e.g. Di Pietro, Black, and Kantak, 2006; Wing and Shoaib, 2008). In the same vein, contextual cues associated with psychomotor-stimulant administration may be able to produce impulsive behavior in the absence of the direct effects of the drug. A review of the literature fails to yield evidence of context-dependent impulsivity despite strong evidence that contextual cues can renew appetitive and avoidance responses (e.g. Bouton 2002; 2004), and facilitate both drug sensitization (Badiani, Anagnostaras, and Robinson, 1995; Badiani, Browman, and Robinson, 1995; Crombag, Badiani, Maren, and Robinson, 2000; Damianopoulos and Carey, 1992; Stewart and Vezina, 1988) and tolerance (Baker and Tiffany, 1985; Carter and Tiffany, 1996; Capeda-Benito, Davis, Reynoso, and Harriad, 2005; Siegel, Hinson, and Krank, 1978).
The investigators of the present study sought to develop an animal model of context-induced impulsivity, and this objective necessitated a review of the paradigms used in the laboratory to evaluate impulsive behavior. To be used in the present study, the paradigm needed to demonstrate consistent and predictable effects of psychomotor stimulant administration; therefore, delay discounting would not have been an appropriate measure of impulsivity because the paradigm has not demonstrated consistent effects. Some researchers (Anderson and Woolverton, 2003; Dallery and Locey, 2005; Helms, Reeves, and Mitchell, 2006; Simmons, Mendez, and Setlow, 2007; Woolverton, Myerson, and Green, 2007) report that stimulant exposure promotes a greater degree of delay discounting, and others report that stimulant administration leads to less impulsive responding (de Wit, Enggasser, and Richards, 2002; Diller et al. 2008; Pitts and Febbo, 2004; Richards, Sabol, and de Wit; Wade, de Wit, and Richards, 2000). Furthermore, Field et al. (2007) found no evidence of cue-elicited impulsivity on a delay-discounting task.
Another category of impulsivity, separate but perhaps related to delay discounting, is response disinhibition (Evenden, 1999; Perry and Carroll, 2008; Sonuga-Barke, 2002; Solanto et al. 2001; Swann et al. 2002; Winstanley, Eagle, and Robbins, 2006). Sufficient evidence exists to suggest that response disinhibition is among the constellation of behaviors that are altered as a consequence of psychomotor-stimulant administration; however, the relationship between response disinhibition and psychomotor stimulant administration is multifaceted, and dependent on the specific task utilized.
Pre-session, acute psychomotor-stimulant treatment consistently fosters response disinhibition on differential-reinforcement of low-rate responding (DRL) schedules (Bacells-Olivero, Richards, and Seiden, 1999; Kirshenbaum, Brown, Hughes and Doughty, 2008; McClure and McMillan, 1997; Morrison, 1968; Popke et al., 2000; Pradhan and Dutta, 1970; Sanger, 1978; Saulsgiver, McClure, and Wynne, 2007; Schuster and Zimmerman, 1961; Segal 1962; Wiley, Compton and Golden, 2000; Zimmerman and Schuster, 1962). Furthermore, the degree to which psychomotor stimulants produce poorer performance accuracy is dependent upon the length of the time interval used to reinforce operant responding and the drug dose; longer schedules and higher doses typically produce poorer performance and there are interactive effects of these two variables (Kirshenbaum et al, 2008; McClure and McMillan, 1997). In contrast to other behavioral models of response disinhibition (the five-choice serial-reaction-time task: Bizarro et al. 2004; Navarra et al. 2008; Semenova, Stolerman, and Markou, 2007; van Gaalen et al. 2006; the go/no-go task: de Wit, Enggasser and Richards, 2002; Paine and Olmstead, 2004; Fillmore, Rush and Marczinski, 2003; and the stop-signal task: Eagle and Robbins, 2003; Feola, de Wit, and Richards, 2000; Tannock, Schachar, and Logan, 1995) the findings from investigations utilizing DRL schedules indicate unanimously that acute psychomotor-stimulant exposure leads to performance decrements.
In order to experimentally demonstrate context-dependent response disinhibition, the following experiment involves performance on the DRL schedule. Subjects operating on a DRL schedule are typically required to withhold a response for a predetermined time interval (X); only after X amount of time has elapsed will the completion of a response result in a reinforcing event. Thus, DRL schedules require the inhibition of a response and maintain low rates of responding, and are sometimes referred to as an inter-response time (IRT) greater than X schedule. The density of reinforcement over the course of an experimental session can be used as an index of performance accuracy and the DRL schedule has been proposed as a behavioral assay of impulsivity (Evenden, 1999; Pothuizen et al. 2005; Monterosso and Anslie, 1999; Sanabria and Killeen, 2008; Stoffle and Cunningham, 2008; Van den Broek, Bradshaw, and Szabody, 1987; Ven den Bergh et al. 2005).
In the present investigation, context-elicited response disinhibition was evaluated by performance on a DRL 29.5-s schedule. During the context-training sessions, rats were administered nicotine and the effects of the drug were directly paired with the presentation of specific contextual cues. During testing in the absence of nicotine administration, performance on the DRL schedule was assessed in response to the nicotine-associated context. Although conditioned suppression of DRL-schedule behavior has been demonstrated previously (Randich, Jacobs, LoLordo, and Sutterer, 1978), and Balcells-Olivero et al. (1999) did not find evidence that cues associated with amphetamine exposure facilitated a sensitized response to amphetamine on the DRL schedule, the present experiment is the first to rigorously test the phenomenon of context-dependent response disinhibition on DRL-schedule performance.
2. Methods
2.1. Subjects
The subjects were 16 male Sprague-Dawley rats approximately 90 days old at the beginning of the experiment (Rattus Norvegicus, obtained from Charles River Labs, Inc., Montreal, Quebec). They were housed in groups of four in a 20 inch × 30 inch plastic home cage, experiencing a 12-hr light/dark schedule. Experimentation occurred during the light portion of the cycle. The rats had continuous access to water 21 hours daily in their home cages; water was removed 1 hour prior to testing, and experimentation lasted for 1–2 hrs daily. Food was restricted to 15 grams of rat chow per rat per day, available in their home cages at least 1 hr after daily experimental testing terminated. A 6-in X 12-in PVC tube and a Nylabone® chew also were placed in their home cages. All care and experimental procedures were approved by the Saint Michael's College IACUC prior to the initiation of the study.
2.2. Apparatus
During the initial shaping period (operant training), behavior was assessed using four identical operant test chambers (MED Associates, Inc., Saint Albans, Vermont, model number ENV-007). Each operant chamber was housed in a PVC sound-attenuated cubicle with ventilating fan, a houselight located at the top-center of the back wall of the chamber, and two tri-color nosepoke response devices on either side of the reinforcer-delivery well. To earn a mixture of sucrose and water (30 ml granulated sugar per 240 ml water), subjects were required to operate the active (right side) tri-color cue-light nosepoke with infrared photobeam interrupts (green, red, and yellow LEDs situated in an equilateral-triangle pattern within the nose poke, MED Associates, model number ENV-114M). Left nosepoke responses were recorded but did not result in reinforcer delivery, and these responses served as a measure of adjunctive responding during the testing sessions. A liquid-reinforcement dipper (model number ENV-202M) was located equidistant from the two nose-poke operanda in the center of the front wall. The liquid dipper delivered 0.04ml of sucrose solution per reinforcing event. Head-entry responses into the food-well receptacle were monitored using an infrared photobeam interrupts. All operant conditioning hardware were interfaced with a PC computer with MED-PC IV software (MED Associates, model SOF-735), and all four chambers were operated simultaneously during the experimental sessions.
Following operant training (during context training), the experimental chambers were modified to provide distinct contextual cues differentiated by visual, tactile, and olfactory stimuli. Chambers 1 and 2 (referred to as `CONTEXT A') were surrounded by newspaper on the outside of the plexiglass chamber walls. The floors were the standard rat grid floor (MED Associates, model ENV-005), and the removable stainless steel trays under the grid floors were scented with Vicks Vapo Rub (roughly 0.6 ml) prior to each day's experimental sessions. Operant chambers 3 and 4 (referred to as `CONTEXT B') also contained three specific contextual cues to differentiate it from CONTEXT A; 0.3 ml of vanilla-soap scent was placed on the tray beneath an opaque white plexi-glass floor, and a 7 watt light-bulb candlestick was located outside of the operant chamber but inside the sound-attenuating cubicle to provide ambient light.
2.3. Procedure
2.3.1. Operant Training
All training sessions lasted 50 min and each session occurred once daily. All subjects were shaped to respond on the right nosepoke using a progression of reinforcement schedules. Subjects first experienced two sessions of a variable-time 15-s schedule followed by 2 shaping sessions of a fixed-ratio 1 schedule. During the FR1 sessions, the green LED was illuminated in the right nosepoke. If a response in the right nosepoke was detected, the green LED terminated, the red LED was simultaneously illuminated and the dipper lifted to provide reinforcement. If a consumption response was detected in the dipper well, the subjects were given 3 s to consume the reinforcer before the dipper became inaccessible. The red LED terminated to signal that the reinforcer was no longer available. A new trial began with the illumination of the green LED, and a 5-s inter-trial interval was used throughout experimentation during which only the houselight was illuminated. The FR1 was increased to an FR2, and subjects experienced two more operant-training sessions.
The DRL-shaping program/schedule was introduced in the seventh session, and the program began each session at DRL schedule of 0.5-s. The DRL protocol used was a response-initiated, discrete-trial DRL schedule, or essentially an FR2 with an imposed delay, and to reiterate, the imposed delay at the beginning of the shaping program was 0.5 s. A trial began with the illumination of the green LED in the right nosepoke, and when a response was made, the interval timer began to elapse. If the subject waited for 0.5 s before responding again, reinforcement was delivered in a manner identical to that of FR shaping. If the subjects failed to wait 0.5-s before responding, a 5-s inter-trial blackout period initiated during which both the green LED and the houselight were extinguished. Responses during the inter-trial blackout period were recorded (as a frequency-of-response measure) but were not factored into DRL-schedule responding and did not result in the delivery of reinforcement. Trials were defined as time period from the initiation of the DRL schedule to either the delivery of reinforcement or the initiation of the inter-trail blackout. The 5-s ITI was used for every trial, regardless of whether or not reinforcement was delivered. If the subject was able to earn 5 reinforcers, the delay interval between responses was increased by 0.5 s. Each successive daily session was catered to each subject so that it began with the final interval achieved by each subject on the previous day. Following the completion of DRL-shaping, subjects were maintained on a DRL 29.5-s schedule of reinforcement. The DRL 29.5-s shaping process occurred over 14 sessions and an additional 10 sessions of DRL 29.5-s schedule responding were required to establish a consistent baseline before progressing to the context-training sessions.
2.3.2. Context Training
For the remainder of experimentation, all subjects experienced two consecutive 50-minute DRL 29.5-s sessions daily. All sessions occurred between 8am and 6pm seven days a week and context-training occurred for 12 consecutive days. A 10-min pre-session blackout period, during which responses were recorded but did not result in the delivery of reinforcement, preceded each 50-min DRL session. Subjects were randomly assigned to one of three groups (experimental, control-group α, and control-group β). All groups were given 1 ml/kg of 0.9% saline solution injected subcutaneously prior to the first of two sessions. After the saline was administered, all subjects were placed in the operant chambers such that half of the subjects were placed into CONTEXT A and the other half, CONTEXT B.
The experimental group (n=8) received 0.3mg/kg nicotine prior to the second session. The nicotine ditartrate dihydrate (Sigma Chemical Co., St. Louise, Missouri nicotine) was delivered subcutaneously as a base and the concentrations varied across subjects to keep a standardized volume of solution at 1 ml/kg. After the delivery of nicotine, the operant-chamber contexts were altered such that if the subjects were exposed to CONTEXT A for the first session, they were exposed to CONTEXT B after receiving the nicotine injection. Alternatively, those subjects that experienced CONTEXT B during the saline session were placed into CONTEXT A for the nicotine session. Therefore, each subject paired the effects of nicotine with one reliable context and the effects of saline with a different context. The contexts were counterbalanced to remove the possibility of interactive effects involving the administration of nicotine and the particular stimuli used in either context.
Control-group α (n = 4) served as a secondary control for the unanticipated effects of a particular context on DRL performance. Subjects in control-group α received only saline injections prior to all operant responding, and the contexts were switched between sessions in a manner similar to that of the experimental group. Control group β (n = 4) served to determine if the sequence and timing of saline and nicotine injections influence DRL schedule performance. Subjects in control group β received saline prior to the first session and nicotine prior to the second session, but the contexts were not altered between sessions. The contextual cues temporally surrounding all injections were tightly controlled such that the same researcher technician delivered all injections and the sessions began at the same time of day (within 10 min) each day.
2.3.3. Context Testing
During the testing phase, 0.9% saline (1 ml/kg) was administered prior to all 50-min sessions for every subject in every group (N = 16) and no nicotine was administered. A 10-min pre-session blackout period preceded all sessions, and responses were recorded but did not result in the delivery of reinforcement. For the experimental group (n = 8), the order of context presentation was counterbalanced during testing such that, for 50% of the subjects (n=4), the sequence of context presentations was reversed, see table 1. The order of context presentation was counterbalanced during testing sessions, and context testing lasted for eight consecutive days.
Table 1.
Experimental design for the experimental group (n = 8). During context training and testing, each subject experienced two operant sessions daily. Nicotine (0.3mg/kg) was delivered to subjects during the second session of context training; no nicotine was administered during testing sessions. The numbers indicate the operant-chamber I.D. (1–4) and the letter indicates context (A or B). Context training lasted for 12 consecutive days, and context testing for 8 consecutive days. Testing is listed in “odd” and “even” days in the table because the sequence of context presentation during the context testing phase alternated daily. Data are presented in 2-day averages, or “blocks,” in Figures 2 and 3 to represent the means obtained from the alternating sequences on odd and even-testing days.
| Context Training | Context Testing, Odd days | Context Testing, Even days | ||||||
|---|---|---|---|---|---|---|---|---|
| Session1 | Session2 | Session1 | Session2 | Session1 | Session2 | |||
| Ss | SALINE | NICOTINE | SALINE | SALINE | Sequence of presentation | SALINE | SALINE | Sequence of presentation |
| 09 | 1A | 3B | 1A | 3B | Same | 3B | 1A | Reverse |
| 10 | 2A | 4B | 4B | 2A | Reverse | 2A | 4B | Same |
| 11 | 3B | 1A | 3B | 1A | Same | 1A | 3B | Reverse |
| 12 | 4B | 2A | 2A | 4B | Reverse | 4B | 2A | Same |
| 13 | 1A | 3B | 1A | 3B | Same | 3B | 1A | Reverse |
| 14 | 2A | 4B | 4B | 2A | Reverse | 2A | 4B | Same |
| 15 | 3B | 1A | 3B | 1A | Same | 1A | 3B | Reverse |
| 16 | 4B | 2A | 2A | 4B | Reverse | 4B | 2A | Same |
Subjects in control-group α were treated in the same manner during context testing as they were during the context-training sessions. Subjects in control-group β had received nicotine during the second daily session of context training, but the contexts were not altered during training. Therefore, nicotine was not exclusively paired with contextual cues and, theoretically, the contextual cues for group β were not reliable predictors of the presence of nicotine. During testing, control β received only saline prior to each session and no nicotine was administered and these subjects experienced the same context as they had during training.
2.4. Data analysis
2.4.1. Cumulative IRT Frequency Distribution Analysis
The degree to which nicotine, and the nicotine-associated context, altered the distribution of inter-response times (IRT's) was among the most important primary dependent measures. The cumulative IRT frequency distribution (see Fig. 1 and McClure and McMillan, 1997) is often used as an indicator of the pattern of responding on the DRL schedule. In previous experimentation, nicotine administration (0.3mg/kg) has led to a shortening of IRT's on the DRL 29.5-s schedule compared to saline conditions (Kirshenbaum et al., 2008). If in the present experiment, the nicotine-associated context assumed the properties of nicotine, then the distribution of IRT's between nicotine and saline-associated contexts should differ during context testing. More specifically, the cumulative IRT distribution for the nicotine-associated context should include a greater frequency of short IRT's than the saline context.
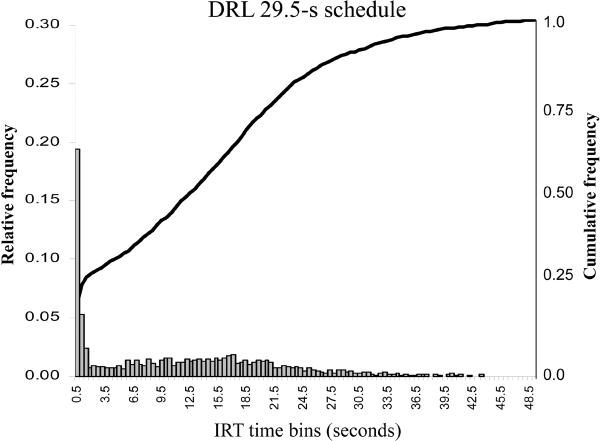
Figure 1.
The relative and cumulative inter-response time (IRT) distributions for the DRL 29.5-s schedule. The relative IRT distribution is a histogram of the average number of inter-responses times per 0.5-s time bin. The cumulative distribution (see also McClure and McMillan, 1997) shows that the majority of inter-response times are emitted prior to the 29.5-s schedule requirement. The data depicted in the figure represent the mean IRT distributions for all subjects (N =16) prior to the context-training sessions.
Cumulative IRT frequency distributions were constructed for each subject using the first two-day means (Block 7) of context testing. The first step in constructing the cumulative IRT frequency distribution was to obtain relative-frequency histograms per session (Doughty and Richards, 2002; Richards et al., 1993; Schuster and Zimmerman, 1961) for each subject; relative-frequency histograms included the proportion of total IRT's per time bin (see Richards et al. 1993).
The second step involved the transformation of the relative-frequency histograms into curvilinear, cumulative IRT frequency distributions (Kirshenbaum et al., 2008; McClure and McMillan, 1997; Sanabria and Killeen, 2008). To analyze differences between the saline and nicotine-associated contexts, a non-linear regression analysis was performed on each subject's mean (across two consecutive sessions, “Block 7”) cumulative IRT frequency distribution for each context (e.g. saline vs. nicotine) during the first two days of context testing. The non-linear regression was performed using a cumulative Gaussian distribution-for-proportions as the model (Kirshenbaum et al. 2008), illustrated by:
μ and σ (the mean and standard deviation) are for the cumulative distribution function Φ(x) of the Gaussian distribution. The raw cumulative IRT frequency distributions followed the sigmoidal shape of a cumulative Gaussian curve and the distribution included an asymptote at 1.0. The median IRT time bin between 0.0-s and the asymptote is represented by μ in the equation (0.5 on the y-axis). Large values for μ indicated better DRL performance. The slope of the cumulative IRT frequency distributions was indicated by the standard deviation (σ) of the equation and smaller numbers yielded steeper slopes. Steeper slopes necessarily mean tighter distributions. Smaller μ and σ parameters values compared to saline conditions are a consequence of nicotine administration (Kirshenbaum et al. 2008).
The nonlinear regression analysis was performed using the “cumulative Gaussian function-for-fractions” analysis in Prism 5 (Graphpad Software, San Diego, California, 2007). The non-linear regression yielded values of both free parameters ( μ and σ ) and a goodness-of-fit value (R2). Since the values of μ andσ did not violate the homogeneity-of-variance assumption of parametric statistics (based on Mauchly's test of sphericity), an ANOVA was performed to determine whether the μ, σ, and R2 values differed between the saline and nicotine-associated contexts. A 3 (group) × 2 (session) mixed-design ANOVA was performed to evaluate changes in the cumulative IRT frequency distribution across experimental conditions during the first two days of context testing.
2.4.2. General performance measures
Mixed-design ANOVAs were also used to evaluate the effects of experimental context and drug administration on general DRL-schedule performance measures, these included: proportion-correct trials (number of reinforcing events divided by the number of total trials), frequency of head entries into the dipper well, and the absolute number of responses per session on both the active nose-hole-poke and the inactive (adjunctive) nose-hole-poke operants. Proportion-correct trials have been used as a method of assessing DRL-schedule performance accuracy (McClure and McMillan, 1997; Kirshenbaum et al., 2008). The data used for the ANOVA were obtained from two-day means, or “blocks.” Given proportion-correct trials were based on a total number of trials that were free to vary from session-to-session, these data were arcsine-transformed to improve homogeneity of variance for the parametric analyses; the raw data are depicted in Figure 2 and Table 2.
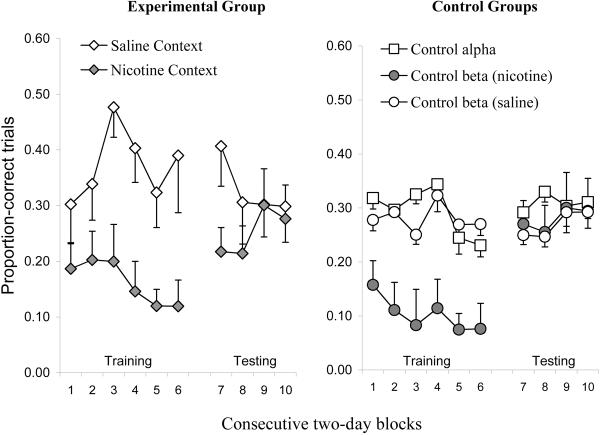
Figure 2.
DRL-schedule performance accuracy, as measured by proportion-correct trials per two-day block means, is illustrated in the graph. Standard-error bars are included. The figure on the left illustrates performance accuracy for the experimental group across context-training (blocks 1–6) and testing (blocks 7–10) phases. The figure on the right illustrates the performance for both control groups (α and β) across the same time period as the experimental group. Control group alpha's data has been collapsed because exposure to contexts A and B resulted in no significant differences in any phase of experimentation.
Table 2.
General performance variables averaged across the first two days, “Block 7,” of context testing; means and standard errors are reported. Operant (procurement) and head-entry (consumption) responses are represented as absolute frequencies emitted during each 50-min operant session. Proportion-correct trials (accuracy) were obtained by dividing the number of reinforcing events per session by the total number of trials.
| Group | Session or Context | Proportion correct trails | Operant responses | Head-entry responses |
|---|---|---|---|---|
| Experimental n = 8 | Nicotine | 0.19 ± 0.03 | 138.37 ± 15.70 | 187.75 ± 20.52 |
| Saline | 0.35 ± 0.06 | 113.25 ± 18.51 | 166.00 ± 23.26 | |
| Alpha control n = 4 | Session 1 | 0.29 ± 0.08 | 131.52 ± 22.14 | 174.75 ± 21.21 |
| Session 2 | 0.23 ± 0.06 | 141.75 ± 26.17 | 194.50 ± 32.02 | |
| Beta control n = 4 | Session 1 | 0.25 ± 0.08 | 139.00 ± 25.11 | 181.66 ± 29.93 |
| Session 2 | 0.24 ± 0.04 | 163.01 ± 42.34 | 168.21 ± 23.45 | |
In the experimental group, to evaluate sensitization to nicotine and contextual control over non-schedule controlled responding, activity during the 10-min pre-session blackout period was recorded each session. Head-entry responses and operant responses were compared across saline and nicotine-associated contexts using a repeated measures ANOVA.
To determine whether stability in DRL-schedule responding was achieved during context training (and therefore when to initiate context testing), a repeated-measures MANOVA was performed on proportion-correct trials, head entries in the dipper well, operant and adjunctive responses across the last four days (Blocks 5 and 6) of context training.
3. Results
3.1. Context-Training Stability
Proportion-correct trials, frequency of operant and adjunctive responses, and frequency of head entries were assessed throughout the duration of the experiment. A repeated-measures MANOVA was performed using the data from the experimental group across the final four consecutive days (Blocks 5 & 6) of context training. Data from the saline and nicotine sessions were analyzed independently and no monotonic increases or decreases were apparent across all general performance measures; F's (6, 54) ~ 1.0, p's > 0.1.
3.2. Final Four Days of Context Training, General Performance Measures
The final four-day means (context-training days 9–12, Blocks 5 & 6), per subject and per session (first/saline vs. second/nicotine session), of the general-performance data were analyzed to determine whether: (1) the delivery of nicotine in the second session occasioned DRL-schedule performance decrements compared to saline for the experimental and control-group β, (2) the experimental and control-group β differed in regard to their response to nicotine, and (3) the performance of control-group α differed between contexts.
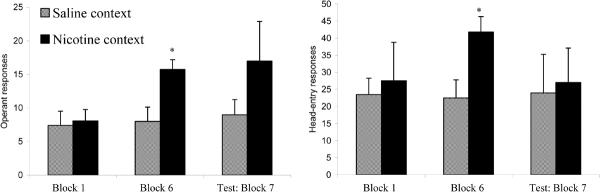
Figure 2 illustrates that the proportion of correct trials per session per group during context training were greatly influenced by the delivery of nicotine, and that proportion-correct trials diminished across training days. The diminishing proportion correct, or the DRL schedule accuracy measure, demonstrates sensitization of response disinhibition due to repeated nicotine administration. A 2 (group: experimental vs. control β) × 2 (session: saline vs. nicotine administration) mixed-design ANOVA was performed using the four aforementioned general-performance variables, and this analysis revealed a main effect of session for proportion-correct trials (sal > nic: F(1,10) = 32.41, p < 0.01, partial-η2 = 0.76), operant responses on the active nose-hole poke (nic > sal: F(1,10) = 77.84, p < 0.01, partial-η2 = 0.87), and head entries into the dipper well (nic > sal: F(1,10) = 7.01, p < 0.05, partial-η2 = 0.41), but not for adjunctive responses in the inactive nose-hole poke (F(1,10) = 2.73, p > 0.1). Main effects of group (experimental vs. β control) were not discovered for any general performance measure (F's(1,10) < 2.94, p's > 0.10) nor were there significant session × group interactions (F's(1,10) < 1.21, p's > 0.10). These results suggest that 0.3mg/kg nicotine administration promoted poorer accuracy as measured in proportion-correct trials and higher rates of procurement and consumption responses. Furthermore, these measures did not differ between the experimental group and β control (see Fig 2). Therefore, for the experimental group, switching the contexts between sessions resulted in no discernable differences in DRL-schedule performance compared to when the contexts were not altered between sessions (β-control group). There were few apparent differences in the rate of operant responding between the experimental and β-control groups, see Fig. 4.
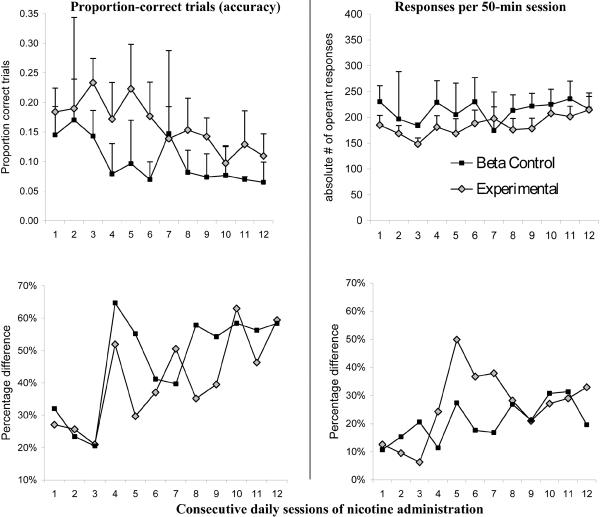
Figure 4.
The top two graphs show that the experimental group and control-group β failed to differ with regards to the speed at which sensitization developed. Nicotine delivery was always followed by a different and reliable context compared to the administration of saline for the experimental group. For the control group, saline and nicotine injections were always followed by exposure to the same context, so the effects of nicotine were not exclusively paired with a particular context. The figure on the top-left depicts mean performance accuracy (and S.E.M.) following nicotine administration across the 12 consecutive context-training days. The figure on the top-right show the mean-absolute number of responses emitted during a session, and an escalation in mean response frequency was not apparent. The bottom two graphs show the percentage difference between nicotine and saline sessions across context-training days for each group (saline sessions not represented in graph). The bottom two graphs demonstrate that the degree and rate of induction of sensitization is not relative to saline performance.
For the α-control group, planned comparison paired-samples t-tests were used to determine whether the four general performance variables differed between CONTEXTS A and B. To reiterate, control-group α received only saline prior to each session but the contexts were altered between sessions to determine whether the particular contexts would produce differential response patterns. Each general performance variable was averaged across the final four consecutive days of context training. All paired t-tests, performed on each general performance measure, failed to reveal significant differences between CONTEXTS A and B; t's (3) < 2.61, all p's > 0.05. Thus, the contextual cues themselves did not alter the general performance measures under saline conditions. Control-group α's performance across both contexts was collapsed in Fig. 2.
3.3. Context Testing, General Performance Measures
For the experimental group, in order to determine whether the nicotine-associated context exerted control over DRL-schedule performance in the absence of nicotine, planned comparison paired t-tests were performed on two-day averages obtained from the first two days of context testing (Block 7). Proportion-correct responses differed significantly across the two contexts (sal > nic: t (7) = 3.05, p < 0.05). The other general performance variables (head-entry, operant and adjunctive responses) did not significantly differ; p's > 0.15. One might expect operant-response rate and performance accuracy on the DRL to be interchangeable, or at least correlated, variables. Worth noting is that operant responses emitted during the inter-trial intervals and inter-trial blackouts were recorded by the computer and were therefore factored into the operant-response measures; therefore, operant responses -- as a dependent variable -- are not representative of schedule-controlled behavior. The results of the analysis indicate that extra-schedule responses may be obscuring context-dependent effects on schedule-controlled response rate. Head-entry responses, or procurement responses into the dipper well, were also tabulated during the inter-trial periods, yet these responses were not influenced by the nicotine-associated context over the first two days of testing. Therefore, the only variable that was altered significantly by the nicotine-associated context was performance accuracy, or proportion-correct trials (see Fig. 2).
The mean proportion-correct-trials measure for the experimental group in the saline context during testing was higher than for the performance for the control groups, see Table 2. Furthermore, there were many inter-response times (IRTs) that extended beyond the 60-s time bin for the experimental group in the saline context; in fact, during context testing, Fig 3 illustrates that all IRTs had not occurred by the 80-s time bin. Therefore, the proportion-correct measure for the experimental group may be inflated due to extraordinary long IRTs that occurred well beyond the schedule requirement. These long IRTs are still reinforced and they may represent something other than schedule-controlled response inhibition. Therefore, a secondary analysis was conducted in which all IRTs beyond the 60-s time bin were removed from the proportion-correct variable. A planned-comparison, paired-samples t-test was performed comparing the experimental group's two-day averages (Block 7) across the saline and nicotine contexts; sal > nic, t(7) = 2.29, p = 0.056. The elimination of the long IRTs brought down the means for both contexts (M's = 0.28 & 0.18, for the saline and nicotine contexts, respectively) because there was a proportion of > 60-s IRTs in both contexts, but a significant difference between the two contexts during testing was still evident.
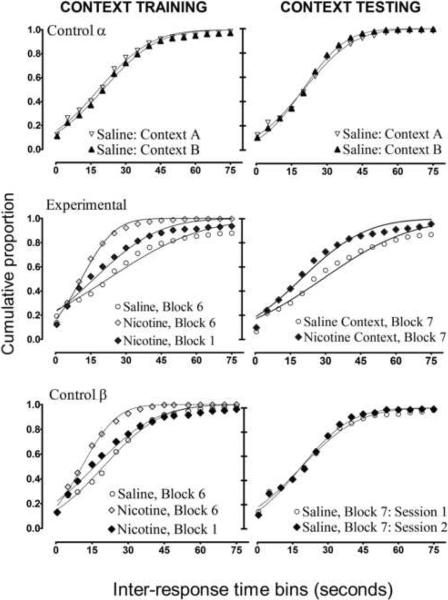
Figure 3.
Cumulative inter-response time (IRT) frequency distributions for all groups per experimental condition. Each curve represents the data for all members of each group per experimental condition. The lines fitted to each curve on each figure are the results of the nonlinear regression analysis. The top two figures show the IRT distributions for control-group α(n = 4), and this figure shows that the particular experimental contexts failed to produce differential DRL-schedule performance in any phase of experimentation. The middle two figures represent the data for the experimental group (n=8). The results for context training demonstrated an effect of sensitization; the IRT distribution became more abrupt after 12 consecutive-daily dosing of 0.3mg/kg nicotine (compare Block 1 vs. Block 6). During context testing (Block 7) in the absence of nicotine, the nicotine-associated context (i.e. “nicotine context') elicited a higher proportion of short IRT's. The bottom two figures depict the results from the control group that received nicotine during the 12 days of context training, but nicotine administration was not exclusively paired with a reliable context. The results for this group during testing show the absence of a lingering effect of nicotine once nicotine administration was terminated.
During context testing, the sequence of nicotine-context presentation was altered (with respect to that of training) for 50% of subjects in the experimental group per testing day, see table 1. The purpose for this change in sequence was to avoid a possible temporal confound since the context switch between sessions was tied inextricably to the order of context presentation during training. In hindsight, the change in sequence during the test may have not been necessary because of the presence of control-group β. The possibility exists that altering the sequence of the nicotine-context presentation during the test was responsible for the decrements in DRL-schedule performance accuracy occasioned by the nicotine-associated context. In other words, presenting the nicotine-associated context first (rather than second) in the sequence of testing sessions may have evoked poor performance-accuracy, and therefore, represents a confound in interpretation. To test this possibility, the experimental-group's data from the nicotine-associated context during the first testing day were analyzed; the accuracy of those subjects that received the nicotine context in the usual training order (second session, n = 4) was contrasted against those subjects that received the nicotine context in the reverse order (first session, n = 4). An independent t-test revealed no significant differences between these two groups (t(6) = 1.06, p > 0.05) although the mean-accuracy for those subjects that received the nicotine context in the training sequence was lower than that of those subjects that received the presentation in reverse to the training sequence (M = 0.189 and 0.233, respectively). Therefore, the change in the sequence of context presentation during the test did not significantly alter testing performance.
To identify whether significant differences existed amongst the α and β control groups during testing, the collapsed data obtained from the first two-days (Block 7) of context testing was used in a 2(control groups) × 2 (session) ANOVA performed on general performance measures. No main effects of session were discovered for any measure (F's(1,6) < 1.26, p's > 0.05) nor were there main effects of group (F's(1,6) < 1.67, p's > 0.05). Furthermore, no group × session interactions were discovered (F's(1,6) < 4.03, all p's > 0.05). Thus, the control groups failed to differ with respect to one another during testing. Furthermore, control-group β's performance did not demonstrate residual effects of nicotine delivered during the previous context-training phase.
DRL-schedule performance accuracy was compared across all three groups using the proportion-correct trials from the first two days of testing (Block 7). A one-way ANOVA was conducted to compare the experimental group (saline context) to first-session data from control groups α and β. No main effect of group was discovered; F (2,15) = 0.35, p > 0.05. Thus, the saline-context performance of the experimental group was not significantly different to that of the control groups' during the test. Therefore, the differences in the experimental group's behavior between the saline and nicotine-associated contexts during the test cannot be attributable to unusually better saline-performance accuracy. A second one-way ANOVA was performed to compare the experimental group (nicotine context) to the second-session performance of control groups α and β, and a significant difference was discovered; F (2,15) = 4.21, p < 0.05. Tukey's post-hoc multiple comparison revealed that the experimental group (nicotine context) differed significantly from the α control (p < 0.05) and the β control (p = 0.08). The α and β-control groups did not differ from one another (p = 0.39).
Figure 2 shows that performance accuracy for the experimental group was poorer for the nicotine-associated context than the saline-associated context during the test, and this performance decrement persisted for at least two consecutive days. However, the nicotine-associated context failed to exert differential control over DRL-schedule performance after five days of testing. Also, the performance decrement occasioned by the nicotine-associated context on days one and two of testing was less pronounced than the performance decrement produced by the direct effects of nicotine during contextual training. Figure 3 (middle row) illustrates that the performance perturbations occasioned by the nicotine context during the test were more similar to the acute effects of the first dose of nicotine during training than the sensitized effects of nicotine after 12 consecutive days of training.
3.4. Cumulative IRT Frequency Distribution Analysis: Experimental Group
For the experimental group, the mean IRT distributions from the last two days of context training (Block 6) and the first two days of context testing (Block 7) were used in the non-linear regression analysis. More specifically, each subject's cumulative IRT frequency-distribution data for each session (saline or nicotine) and each condition (training or testing) were included in the analysis to obtain the parameter values μ and σ as well as the goodness-of-fit value (R2). Figure 3 includes the mean raw cumulative IRT frequency distributions for the experimental and control groups per session and condition, as well as the regression curve fit to each data set.
Mean parameter (μ and σ) and goodness-of-fit (R2) values per session and condition are listed in table 3 for the experimental group. The parameter and goodness-of-fit values for each subject were loaded into a 2 (context: nicotine vs. saline) × 2 (condition: training block 6 vs. testing block 7) repeated- measures ANOVA. A main effect of context was discovered for both μ and σ values (sal > nic: F's (1, 7) > 14.89, p's < 0.01, partial-η2 > 0.68), but not R2 values (F (1, 7) = 3.54, p > 0.05). A main effect of condition was not discovered for both free parameters μ and σ (F's (1, 7) = 1.48 and 0.001, p's > 0.05, for μ and σ, respectively), nor was there a main effect for R2 values (F (1, 7) = 1.29, p > 0.05). A condition × context interaction was also revealed for σ (sal > nic: F (1, 7) = 10.40, p < 0.05, partial-η2 > 0.59), but not μ nor R2 values (F's (1, 7) < 4.12, p's > 0.05). Thus, the σ parameter changed significantly across contexts and conditions, μ value differed across contexts, but the fit (R2) of the Gaussian equation was not significantly altered across contexts or conditions.
Table 3.
Mean parameter values (and standard errors), per session and condition, for the experimental group (n = 8) are listed in the table
| Parameter Values | Condition | Training: Block 1 | Training: Block 6 | Testing: Block 7 |
|---|---|---|---|---|
| μ | Saline | 26.32 ± 7.12 | 28.11 ± 3.81 | 29.43 ± 4.12 |
| Nicotine | *13.10± 3.86 | *10.72 ± 2.58 | *19.76± 2.67 | |
| σ | Saline | 18.84 ± 3.83 | 32.45 ± 6.45 | 29.48 ± 4.03 |
| Nicotine | 17.96± 3.19 | *13.52 ± 1.27 | *21.66± 2.38 | |
| R2 | Saline | 0.98 ± 0.03 | 0.97 ± 0.00 | 0.96 ± 0.01 |
| Nicotine | 0.99± 0.03 | 0.98 ± 0.01 | 0.97± 0.03 | |
indicates a significant difference (p < 0.05) based upon paired t-tests comparing nicotine to saline conditions. The parameter μ represents the median IRT bin, from 0.0–0.05 s to the asymptote of the cumulative IRT frequency distribution curve; σ represents the standard deviation of the cumulative function (the slope of the curve). The values below were obtained from applying the Gaussian model to the IRT distributions for all subjects in the experimental group.
The fact that the free-parameter values (μ and σ) were significantly different across contexts in the experimental group is not very surprising because nicotine was delivered during the training phase, so the main effect reported above could be due to the direct effects of nicotine rather than that of context. Therefore, a planned comparison paired-samples t-test was performed on the parameter values associated with each context during the first day of testing. The context alone (in the absence of nicotine during the testing phase) produced significant differences for the μ and σ parameters (t(7) = 4.80 and 3.05, respectively, p's < 0.05). Thus, the steepness of the curve (σ) and the median IRT value (μ) differed between nicotine and saline-associated contexts, suggesting that a higher proportion of short IRT's were emitted in the nicotine-associated context and all IRT's were emitted by the 60-s time bin. Again, no differences were discovered for the goodness-of-fit parameter; t(7) = 1.44, p > 0.05. Taken together, these results suggest that the pattern of IRT distributions created by each context was significantly different, and the integrity of the Gaussian model to represent the IRT distributions was not compromised across contexts.
3.5. Pre-session Blackout Responding
To assess nicotine sensitization and contextual control over non-schedule controlled behavior, head-entry (consumption) responses into the dipper well and operant (procurement) responses were recorded during the 10-min pre-session blackout period. Responses during the pre-session blackout were not followed by any consequences. Only the data from the experimental group were analyzed, and Fig. 5 depicts the means for the saline and nicotine contexts (n = 8) for the first two days of context training (Block 1), the last two days of context training (Block 6), and the first two days of context testing (Block 7). A 2 (context) × 3 (condition: Blocks 1 vs. 6 vs. 7) ANOVA was performed, finding no significant differences for head-entry responses, but a main effect of condition for operant responses (F(2, 14) = 6.79, p < 0.01, partial-η2 = 0.49), and a significant condition × context interaction for operant responses (F(2, 14) = 5.68, p < 0.05, partial-η2 = 0.45). Operant responses and head-entry responses were loaded into six separate, planned comparison, paired-samples t-tests to identify differences between the saline and nicotine context for each of the three conditions depicted in Figure 5. Significant differences were discovered for both behavioral measures on the last two days of context training (Block 6; t's (7) = 2.37 and 3.11, for head-entry and operant responses, respectively, p's < 0.05). This result is not surprising given that the effects of nicotine were operating to promote a higher degree of activity during these last two days of context training. No significant differences were discovered on the first two days of context training; therefore, sensitization was apparent in the escalation of general activity across training sessions. Despite the fact that operant-response means were much greater for the nicotine-associated context during contextual testing (in the absence of the direct effects of nicotine), these differences were not significant (t (7) =1.85, p. = 0.10) likely due to the high degree of variability across subjects for this behavioral measure (see standard errors, Fig. 5). These results would suggest that nicotine sensitization is manifested in pre-session blackout responses, both in terms of operant and head-entry responses. Some contextual control is evidenced in terms of operant (procurement) responding, but not in terms of head-entry (consumption) responding during the pre-session blackout period.
Figure 5.
The figure illustrates data obtained from the 10-min pre-session blackout periods. Mean operant and head-entry responses (and standard errors) for the experimental group, per training and testing conditions, are depicted. Significant differences, based on a paired-t test, were only evident for the last two days of training. An effect of nicotine sensitization is apparent in the comparison of the first two administrations of nicotine (i.e. Block 1) versus the last two days (Block 6) for both operant and head-entry responses. For operant responses, some contextual control was exerted by the nicotine context during the test, but there was great variability in the degree to which the context controlled operant responding during the pre-session blackout.
4. Discussion
The present data suggest that contextual cues possess the capacity to exert control over DRL-schedule performance, and this effect is a novel discovery that complements the existing literature of contextual control over appetitive behavior (Balsam, 1985). During the context-training sessions, the experimental group received explicit pairings of nicotine's effects with a particular set of contextual cues while saline was associated with a different set of contextual cues. Compared to saline administration, nicotine administration promoted poor performance accuracy on the DRL 29.5-s schedule. During context testing in which no nicotine was delivered, the nicotine-associated context engendered significantly poorer performance accuracy than did the saline context. The contextual cues (contexts A and B) were counterbalanced across subjects, so contextual control cannot be attributed to the particular characteristics of each context. Furthermore, control-group α received saline in the presence of both contexts, and their performance was not differentially altered by contexts A and B. The subjects in control-group β were exposed to the same context after both nicotine and saline delivery during the training sessions. When nicotine was no longer delivered during testing, control β's performance did not exhibit residual effects of nicotine; thus, for the experimental group, the performance perturbations caused by the nicotine-associated context during the context-testing sessions cannot be related to residual direct effect of nicotine. Taken together, these results provide strong evidence that nicotine-associated contextual cues can assume some of nicotine's power to induce response-disinhibition in the absence of the direct effects of the drug.
The two primary dependent measures of response disinhibition (proportion-correct trials and the cumulative IRT frequency distributions) revealed parallel evidence that DRL-schedule performance during testing differed between the two contexts. Other dependent variables, such as frequency of operant responses, head-entry consumption responses and adjunctive responses on the inactive manipulanda failed to demonstrate an effect of contextual control, likely due to the fact that these variables are not representative of schedule-controlled responding. The contextual control exhibited as performance-accuracy perturbations dissipated after two consecutive days of testing; therefore, although contextual control was evidenced significantly, the effect was transient as might be expected for conditioned stimuli undergoing extinction (see Fig. 2). The degree of DRL-performance perturbations evoked by the nicotine context was also less pronounced than the performance decrements created by the effects of nicotine after it had been delivered for 12 consecutive days, see Figures 2 and 3. The modest nature of these results prevent the assertion that environmental cues assume all of nicotine's capability (once sensitization had occurred) to produce performance decrements on the DRL schedule; however, the degree of response disinhibition produced by contextual cues shared some resemblance to the nicotine-induced disinhibition in the first exposure to nicotine dosing (e.g. Fig. 3). The data reported here provide evidence that nicotine-associated contextual cues can promote a magnitude of response disinhibition similar to the acute effects of nicotine.
Figure 3 provides some evidence that the experimental group's performance for the saline context was less motivated than in the nicotine context because greater than 15% of total IRT's were administered after 80 s had elapsed. Although a provocative interpretation, the data from the general performance measures do not support this hypothesis given that other indicators of motivation (head-entry and operant responses) did not differ between the two contexts. An alternative explanation of the presence of long (> 80 s) IRTs in the saline context is that these may represent an anticipatory effect; schedule-controlled performance may have slowed toward the end of the saline-administration sessions just prior to the delivery of nicotine in the second session (during training). However, an anticipatory effect such as this should also be evident in the data obtained from the β-control group because those subjects also experienced nicotine delivery in the second session during training, yet the IRT distributions between the first and second session did not differ during testing. Although the presence of long IRTs in the saline context is noteworthy, a fair percentage of long IRT's is not atypical of rats maintained on DRL schedules (e.g. see the control/saline group in Ballcells-Olivero et al. 1997). In an attempt to avoid this result, McClure and McMillan (1997) utilized a DRL schedule with a narrow time window during which responses were reinforced; in other words, IRT's greater than “X” but below “Y” resulted in a reinforcing event. Perhaps a future replication of the present investigation would involve such a schedule to limit the percentage of IRT's greater than 80 s, but also important to note is that the presence of an upper time-limit does not entirely eliminate the presence of long IRTs (McClure and McMillan, 1997). Furthermore, in the present investigation, when IRTs greater than 60-s were removed from the proportion-correct-trials analysis comparing saline and nicotine contexts during testing, significant differences in response disinhibition were still apparent.
The results from the context-training sessions replicated previous findings that nicotine administration engenders poor response-inhibition on the DRL 29.5-s schedule, and this effect becomes more pronounced over the course of consecutive once-daily injections (see Figures 2 and 3, and Kirshenbaum et al. 2008). Control group β played a role in the evaluation of how exclusively-paired contexts modulate the development of sensitization. Figure 4 illustrates that the development of sensitization to 0.3mg/kg nicotine was not dependent upon whether a single context was (experimental group) or was not (β control) exclusively paired with nicotine. This result is somewhat surprising given the existing literature showing that a reliable context speeds the induction of sensitization to psychomotor stimulants (Crombag, Badiani, Maren, and Robinson, 2000). Null results are always difficult to interpret, but a potential explanation is that the experimental context, whether exclusively or not-exclusively paired with nicotine, was still novel compared to the home cage environment and thus able to induce sensitization (Badiani, Anagnostaras, and Robbinson, 1995; Badiani, Browman, and Robinson, 1995). Other possibilities exist, for instance, the combination of different cues making up each context may not have been salient at the beginning of drug administrations during context training, and only after 12 consecutive days of nicotine exposure did the context assume any associative control.
Prior studies (e.g. Gould and Wehner, 1999) also have found that nicotine facilitates learning processes most likely through cholinergic activation, particularly in the hippocampus (Tinsley, Quinn, and Fanselow, 2004). Gould and colleagues (Gould and Higgins, 2003; Gould, Feiro, and Moore, 2004; Gould and Wehner, 1999) have demonstrated that nicotine facilitates context learning in a conditioned-immobility paradigm and this evidence supports the notion that nicotine aids neural mechanisms of associative learning. The context-dependent response disinhibition witnessed in the present experiment may have been due to the unique cholinergic activation produced by nicotine and may not be applicable to other psychomotor stimulants; this is an empirical question that will need to be explored further before context-dependent impulsivity can be scrutinized as a potential model of relapse to psychomotor-stimulant dependence.
The effects of nicotine sensitization are manifested in responding during the pre-session blackout period (see Fig 5). These results replicate those reported elsewhere in the literature that chronic dosing of nicotine leads to the sensitization of global locomotor activity (Shim et al. 2002). Furthermore, during the blackout period, the nicotine-associated context exerted some control over operant responses but not head-entry responses. The differences between the contextual control exhibited by consumption (head-entry) and procurement (operant) responses is important because it suggests that the conditioned response (CR) may involve the behaviors that coincide with procuring a food reward but not the consumption of that reward. In terms of context-induced impulsivity that might propagate drug use, such a distinction between operant and consumption behavior may also hold for drug-seeking versus drug-taking responses.
A limitation of the present investigation, and the conclusion drawn from it, is that contextual cues were paired not only with the acute delivery of nicotine, but also nicotine sensitization resulting from chronic-like dosing. Perhaps future experimentation ought to employ fewer nicotine administrations in an exclusive context to evaluate whether drug effects - in the absence of drug sensitization – will sufficiently produce contextual control over response inhibition. This limitation of the present study does not insinuate that the induction of impulsivity exerted by the nicotine-associated context is an anomaly that can only be expected to occur under the tight experimental control of a laboratory. One perspective suggests that sensitization may be a result of the synergistic effects of an anticipatory conditioned response (CR) to drug-related conditioned stimuli (CS) and the predictable biological effects of the drug (Hinson and Poulos, 1981; Pert, Post and Weiss, 1990). The Pavlovian view suggests that sensitization would not be apparent in the absence of a reliable and predictive CS. Given that drug-related CS are omnipresent in the world of a substance-dependent individual (cigarette packs, lighters, etc.) sensitization is assumed to be a necessary component of drug dependence; only in the experimental domain can CS be withheld and the process of sensitization encumbered. The results of the present study may therefore be considered a face-valid model of the natural behavioral ecology of a substance user. Sensitization to nicotine might develop during the transition from isolated use to chronic abuse, and the environmental cues accompanying sensitization might serve to elicit poor response inhibition during an attempt at cessation. Thus, CS may serve not only to promote subjective ratings of drug craving (Carter and Tiffany, 1999 for a review; Donny, Griffin, Shiffman and Sayette, 2008 for a recent study), but also hinder the ability to withhold a response, whether that response be a self-administration response (such as procuring a pack of cigarettes) or otherwise. Naturally, the next step in research on this topic of context-elicited impulsive responding would be to require rats to self-administer nicotine while operating a DRL schedule because the analogy to tobacco-use relapse is more direct.
Impulsivity is a governing factor in stimulant-dependence relapse (Dallery and Raiff, 2007; Krishnan-Sarin et al., 2007; Yoon et al. 2007), and impulsivity and cue-reactivity are correlated variables (Doran, Spring, and McChargue, 2007; 2008). Contextual cues may drive or perpetuate impulsive response tendencies, and there may be few extinction events in the daily routine of one who is dependent upon a substance because impulsivity is reinforced by its very nature. Extinction of impulsive response patterns may be a novel target for cue-exposure treatments of stimulant dependence and help to reinforce the connection between cue-reinstatement paradigms and clinical treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson K, Woolverton W. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–430. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras S, Robinson T. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology. 1995;117:443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman K, Robinson T. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674:291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Baker T, Tiffany S. Morphine tolerance as habituation. Psychol Rev. 1985;921:78–108. [PubMed] [Google Scholar]
- Balcells-Olivero M, Richards JB, Seiden LS. Sensitization to amphetamine on the differential-reinforcement-of-low-rate 72-s schedule. Psychopharmacology. 1997;133:207–213. doi: 10.1007/s002130050393. [DOI] [PubMed] [Google Scholar]
- Balsam PD. Context and Learning. Hillsdale, NJ: 1985. The function of context in learning and performance; pp. 1–21. [Google Scholar]
- Belin D, Mar A, Dalley J, Robbins T, Everitt B. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman I. Differential effects of psychomotor stimulants on attentional performance in rats: Nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Bouton M. Context and behavioral processes in extinction. Learning Memory. 2004;115:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton M. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R, Pennicott D, Sugathapala C, Robbins T, Everitt B. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carter B, Tiffany S. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter B, Tiffany S. Cross-tolerance of associative and nonassociative morphine tolerance in the rat with mu- and kappa-specific opioids. Psychopharmacology. 1996;123:289–296. doi: 10.1007/BF02246583. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Davis K, Reynoso J, Harraid J. Associative and behavioral tolerance to the analgesic effects of nicotine in rats: tail-flick and paw-lick assays. Psychopharmacology. 2005;180:224–233. doi: 10.1007/s00213-005-2151-4. [DOI] [PubMed] [Google Scholar]
- Crombag H, Badiani A, Maren S, Robinson T. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Damianopoulos E, Carey R. Conditioning, habituation and behavioral reorganization factors in chronic cocaine effects. Behav Brain Res. 1992;492:149–157. doi: 10.1016/s0166-4328(05)80159-7. [DOI] [PubMed] [Google Scholar]
- Di Pietro N, Black Y, Kantak K. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser J, Richards J. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;275:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Diller J, Saunders B, Anderson K. Effects of acute and repeated administration of caffeine on temporal discounting in rats. Pharmacol Biochem Behav. 2008;89:546–555. doi: 10.1016/j.pbb.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Donny E, Griffin K, Shiffman S, Sayette M. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10:934–942. doi: 10.1080/14622200802133681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, McChargue D, Spring B. Effect of impulsivity on cardiovascular and subjective reactivity to smoking cues. Addict Behav. 2008;33:167–172. doi: 10.1016/j.addbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology. 2007;194:279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Doughty AH, Richards JB. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. J Exp Anal Behav. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due D, Huettel S, Hall W, Rubin D. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Eagle D, Bari A, Robbins T. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle D, Robbins T. Inhibitory Control in Rats Performing a Stop-Signal Reaction-Time Task: Effects of Lesions of the Medial Striatum and d-Amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle D, Tufft M, Goodchild H, Robbins T. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Feola T, Wit H, Richards J. Effects of d-Amphetamine and ethanol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Field M, Rush M, Cole J, Goudie A. The smoking Stroop and delay discounting in smokers: effects of environmental smoking cues. Journal Of Psychopharmacology. 2007;21:603–610. doi: 10.1177/0269881106070995. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fillmore M, Rush C, Hays L. Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Fillmore M, Rush C, Marczinski C. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Wang J, Sciortino N, Harper D, Li Y. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gould T, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould T, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould T, Wehner J. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Helms C, Reeves J, Mitchell S. Impact of strain and D-amphetamine on impulsivity delay discounting in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–28. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Hinson R, Poulos C. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hutchison E, Blumstein S, Myers E. An event-related fMRI investigation of voice-onset time discrimination. Neuroimage. 2008 March 1;40:342–352. doi: 10.1016/j.neuroimage.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontosotriatal dysfunction in drug abuse: implications for the control of behavior by reward related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Katz J, Higgins S. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kilts C, Schweitzer J, Quinn C, Gross R, Faber T, Muhammad F. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Brown S, Hughes D, Doughty A. DRL schedules and nicotine administration: A systematic evaluation of dose and schedule requirement. Behav Pharmacol. 2008;19:683–697. doi: 10.1097/FBP.0b013e328315ecbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans J, ter Wal A, Quik E, Kemner C, Westenberg H, Verbaten MN, van Engeland H. Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. Eur Psychiatry. 2006;21:544–547. doi: 10.1016/j.eurpsy.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PEA, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2006;14:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- McClernon F, Kozink R, Rose J. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McClure GYH, McMillan DE. Effects of drugs on response duration differentiation. VI: differential effects under differential reinforcement of low rates of responding schedules. J Pharmacol Exp Ther. 1997;281:1368–1380. [PubMed] [Google Scholar]
- McBride D, Barrett S, Kelly J, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- MED Associates, Inc. Tatham TA. MED-PC Medstate notation. MED Associates, Inc.; East Fairfield, NH: 1991. [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6:819–828. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology. 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Morrison CF. A comparison of the effects of nicotine and amphetamine on DRL performance in the rat. Psychopharmacologia. 1968;12:176–180. doi: 10.1007/BF00401548. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharamcology. 2004a;17:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology. 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Olmstead MC. Animal models of drug addiction: Where do we go from here? Q J Exp Psychol. 2006;59:625–653. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley J, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learning Memory. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Paine T, Olmstead M. Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacology. 2004;1754:443–450. doi: 10.1007/s00213-004-1845-3. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss S. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. NIDA Res Monogr. 1990;97:208–241. [PubMed] [Google Scholar]
- Potter A, Newhouse P. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Popke JE, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav. 2000;652:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective accumbens core and shell lesions on impulsive-choice behavior and salience learning in rats. Eur J Neurosci. 2005;22:2605–2616. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- Pradhan SN, Dutta SN. Comparative effects of nicotine and amphetamine on timing behavior in rats. Neuropharmacology. 1970;9:9–15. doi: 10.1016/0028-3908(70)90043-2. [DOI] [PubMed] [Google Scholar]
- Randich A, Jacobs WJ, LoLordo VM, Sutterer JR. Conditioned suppression of DRL responding: Effect of UCS intensity, Schedule parameters, and schedule context. Q. J. Exper. Psychol. 1978;30:141–150. [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: Quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Sanabria F, Killeen P. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;84:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA. Cognitive-behavioral approaches to ADHD treatment in adulthood. J Clin Psychiatry. 2006;67:46–50. [PubMed] [Google Scholar]
- Sanger D. Effects of d-amphetamine on temporal and spatial discrimination in rats. Psychopharmacology. 1978;58:185–188. doi: 10.1007/BF00426905. [DOI] [PubMed] [Google Scholar]
- Saulsgiver K, McClure E, Wynne C. Effects of amphetamine on differential reinforcement of low rates of responding. Behav Pharmacol. 2007;18:119–133. doi: 10.1097/FBP.0b013e3280ae6caa. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Zimmerman J. Timing behavior during prolonged treatment with dl-amphetamine. J Exp Anal Behav. 1961;4:327–330. doi: 10.1901/jeab.1961.4-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. Effects of dl-amphetamine under concurrent VI DRL reinforcement. J Exp Anal Behav. 1962;51:105–112. doi: 10.1901/jeab.1962.5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman I, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shim I, Javiad JI, Wirtshafter D, Jang S, Shin K, Lee H, Chung Y, Chun B. Nicotine-induced behavioral sensitization is associated with extracellular dopamine release and expression of c-Fos in the striatum and nucleus accumbens of the rat. Behav Brain Res. 2001;121:137–147. doi: 10.1016/s0166-4328(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Siegel S, Hinson R, Krank M. The role of predrug signals in morphine analgesic tolerance: support for a Pavlovian conditioning model of tolerance. J Exp Psychol Anim Behav Process. 1978;42:188–196. doi: 10.1037//0097-7403.4.2.188. [DOI] [PubMed] [Google Scholar]
- Simon N, Mendez I, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka M, Bühler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sterling R, Dean J, Weinstein S, Murphy J, Gottheil E. Gender differences in cue exposure reactivity and 9-month outcome. J Subst Abuse Treat. 2004;27:39–44. doi: 10.1016/j.jsat.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Sensitization of the nervous system. New York, Telford press; New York: 1988. Conditioning and behavioral sensitization; pp. 206–223. [Google Scholar]
- Stoffle EC, Cunningham AK. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–94. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Tinsley M, Quinn J, Fanselow M. Learning Memory. Vol. 11. Cold Spring; Harbor, N.Y.: 2004. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance; pp. 35–42. [DOI] [PubMed] [Google Scholar]
- Ven den Broek VD, Bradshaw CM, Szabadi I. Behaviour of `impulsive' and `non-impulsive' humans in a temporal differentiation schedule of reinforcement. Personality Individual Differences. 1987;8:233–239. [Google Scholar]
- van den Bergh Filip S., Bloemarts E, Chan JSW, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- van Gaalen M, Brueggeman R, Bronius P, Schoffelmeer A, Vanderschuren L. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade T, de Wit H, Richards J. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wiley J, Compton A, Golden K. Separation of drug effects on timing and behavioral inhibition by increased stimulus control. Exp Clin Psychopharmacol. 2000;8:451–461. doi: 10.1037//1064-1297.8.4.451. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology. 2008;200:357–65. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton W, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir A, Rigbi A, Kanyas K, Pollak Y, Kahana G, Karni O, Eitan R, Kertzman S, Lerer B. Why do young women smoke? III. Attention and impulsivity as neurocognitive predisposing factors. Eur Neuropsychopharmacol. 2007;17:339–351. doi: 10.1016/j.euroneuro.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Yoon J, Higgins S, Heil S, Sugarbaker R, Thomas C, Badger G. Delay Discounting Predicts Postpartum Relapse to Cigarette Smoking Among Pregnant Women. Exp Clin Psychopharmacol. 2007;15:176–186. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]