Abstract
Background
High-sensitivity C-reactive protein (CRP) is an inflammatory marker that predicts coronary heart disease (CHD) and, in recent studies, incident heart failure (HF). Whether the association of inflammation with incident HF is explained by worse baseline left ventricular dysfunction or by underlying CHD is unknown.
Methods and results
Serum CRP was measured in a cohort of 985 outpatients with established CHD from the Heart and Soul Study. During 3 years of follow-up, 15% of the participants with elevated CRP levels (>3 mg/L) were hospitalised for HF, compared with 7% of those with CRP ≤3 mg/L. In multivariate analysis, elevated CRP was associated with HF after adjustment for traditional risk factors, baseline CHD severity and interim MI (adjusted HR 2.1, 95% CI, 1.2–3.6; p=0.009). However, elevated CRP was no longer associated with HF after further adjustment for the presence of diastolic dysfunction on echocardiography (adjusted HR 1.6, 95% CI, 0.8–3.2; p=0.1).
Conclusions
Among outpatients with stable CHD, elevated CRP levels predict hospitalisation for heart failure, independent of baseline heart failure, medication use, CHD severity, and subsequent MI events. This relationship appears to be at least partly explained by abnormal diastolic function in patients with elevated CRP levels.
Keywords: C-reactive protein, Coronary heart disease, Heart failure, Diastolic dysfunction, Inflammation, Echocardiography
1. Introduction
High-sensitivity C-reactive protein (CRP), produced by the liver in response to an inflammatory stimulus, is a risk factor for incident myocardial infarction [1–4] and portends a poor prognosis in patients with established coronary heart disease (CHD) [5–8]. More recently, CRP has also emerged as a risk factor for heart failure (HF). In a prospective cohort study of 6437 Dutch outpatients, CRP was found to predict incident HF in men, even after adjustment for traditional cardiovascular risk factors and comorbid CHD [9]. In an elderly population without prior myocardial infarction (MI) or HF, inflammatory markers including CRP were associated with incident HF [10].
Additionally, CRP predicts morbidity and mortality in patients with established HF [11–13] and progression of HF in patients after myocardial infarction [14]. Higher CRP levels have been associated with poorer New York Heart Association (NYHA) functional class [15] and greater severity of HF [16]. In the Valsartan Heart Failure Trial (Val-HeFT), CRP was an independent predictor of mortality and morbidity in patients with established HF [16]. Whether the association of inflammation with HF is explained by either underlying CHD or left ventricular (LV) dysfunction is unknown.
A recent study demonstrated that increased CRP levels were associated with elevated LV end-diastolic pressure in patients undergoing coronary angiography [17]. This suggests that greater baseline LV filling pressure may contribute to the association of CRP with HF. We sought to examine the association between CRP, CHD, and HF in a prospective cohort of outpatients with established CHD. We hypothesized that CRP would predict HF independent of baseline CHD severity or interim MI, and that this association would be explained by elevated filling pressures.
2. Methods
2.1. Study population
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients who have stable coronary heart disease (CHD). Details regarding the study population and recruitment procedures have been previously described [18]. In summary, outpatients who had documented CHD were recruited from two Veterans Affairs medical centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System, both in California), the University of California at San Francisco Medical Center, and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had a history of either: myocardial infarction, angiographic evidence of ≥50% stenosis in one or more coronary arteries, evidence of exercise-induced ischaemia by treadmill or nuclear testing, or coronary revascularization. Patients were excluded if they were unable to walk one block or were planning to relocate within 3 years. All subjects gave informed consent and approval of the research protocol was granted by the appropriate institutional review boards.
2.2. Data collection
Between September 2000 and December 2002, 1024 subjects were enrolled and completed a day-long study appointment at the San Francisco VA Medical Center. Subjects completed a comprehensive health interview and questionnaire regarding past medical history and medication use. In addition, subjects were screened with a number of questions regarding depressive symptoms and health-related quality of life, the results of which have been reported in prior studies [18–21]. A total of 39 participants without serum samples were excluded due to a lack of CRP measurements, leaving 985 participants for this prospective study.
2.3. Heart failure
Study participants (or their proxies) were contacted by telephone for interviews annually during an average of 3 years of follow-up (range 2 to 4 years) to inquire about CHD events, including hospital admissions for heart failure. For any reported event, medical records, electrocardiograms (ECGs), death certificates, and coroner’s reports were retrieved and reviewed by two independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, they conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary.
HF was defined as hospitalisation for a clinical syndrome involving at least two of the following: paroxysmal nocturnal dyspnoea, orthopnoea, elevated jugular venous pressure, pulmonary rales, third heart sound, and either cardiomegaly or pulmonary oedema on chest radiography [22]. These clinical signs and symptoms must have represented a clear change from the normal clinical state of the patient, and must have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary oedema requiring intravenous diuretics, inotropes, or vasodilators.
2.4. C-reactive protein
Details of the measurement of CRP have been previously described [23,24]. Participants were instructed to fast for 12 h (except for medication, which they were to take with water), not to take aspirin for 1 week, and not to smoke for 5 h. Fasting venous blood samples were obtained, and serum was frozen at −70 °C until the time of the CRP assay. We used the Roche Integra high-sensitivity assay (Roche, Indianapolis, Indiana) to measure CRP levels in approximately 25% of participants and (due to a change at the laboratory) the Beckman Extended Range high-sensitivity CRP assay to measure CRP in the remaining 75% of participants. Results from these assays were highly correlated (r=0.99 in a sample of 185 participants).
2.5. Cardiac measurements
Transthoracic echocardiographic data were obtained in all study participants using an Acuson Sequoia ultrasound system (Mountain View, California) with a 3.5-MHz transducer. We obtained standard two-dimensional parasternal short-axis and apical 2- and 4-chamber views during held inspiration and performed planimetry with a computerized digitisation system to determine end-diastolic and end-systolic left ventricular volumes and left ventricular ejection fractions. Images were stored on magneto-optical disks and parameters of systolic and diastolic function were measured by an experienced echocardiographer blinded to the patients’ clinical history, CRP measurements, and all other study data. LV mass index, LV end-systolic volume index, LV end-diastolic volume index, and left atrial volume index were calculated by dividing these measurements by body surface area (m2). We used Doppler measurements of pulmonary venous and mitral valve inflow to assess diastolic function, which was categorized as normal (systolic dominant with 0.75<E/A<1.5), impaired relaxation (systolic dominant with E/A≤0.75), pseudonormal (diastolic dominant with 0.75<E/A<1.5) or restrictive (diastolic dominant with E/A≥1.5) according to previously described and validated criteria [22]. Of note, the vast majority of subjects were in sinus rhythm at the time of their visit (92%). A small percentage had atrial fibrillation or flutter (4%), or a paced rhythm (3%), which precluded an assessment of diastolic function.
To estimate the correlation between CRP and LV end-diastolic pressure, we attempted to measure the end-diastolic pulmonic regurgitation (EDPR) gradient in all subjects as described previously [25]. The EDPR gradient has been shown to correlate with pulmonary artery diastolic pressure, a surrogate of LV end-diastolic pressure [26,27]. Of the 985 subjects, 577 had a measurable EDPR gradient.
All participants also underwent full exercise treadmill testing according to a standard Bruce protocol with continuous 12-lead ECG monitoring. Echocardiography was repeated immediately after exercise, and inducible ischaemia was defined as the presence of one or more new wall motion abnormalities at peak exercise [25,28].
2.6. Other characteristics
Self-reported age, gender, ethnicity, medical history, physical activity, and smoking status were determined by questionnaire. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. We measured weight and height, and calculated body mass index (kg/m2). Levels of total cholesterol (mg/dL), high-density lipoprotein (HDL) cholesterol (mg/dL), low-density lipoprotein (LDL) cholesterol (mg/dL), and triglycerides were measured from sera after the overnight fast. We calculated creatinine clearance from 24-h urine collections.
2.7. Statistical analysis
All data were analyzed with the Statistical Analysis Systems version 8 software (SAS Institute, Cary, North Carolina). CRP levels were categorized as normal (CRP≤3 mg/L) or elevated (CRP>3 mg/L) based on clinical guidelines [29–31]. Differences in baseline characteristics and in echocardiographic parameters between subjects by CRP category were compared with 2-tailed Student’s t tests for continuous variables that were normally distributed, Wilcoxon’s tests for continuous variables that were not normally distributed, and chi-square tests for dichotomous variables.
To assess the association between CRP and hospitalisation for HF, proportional hazards models were used to calculate the adjusted hazard ratios (HR) and 95% confidence intervals (CI). Potential confounding variables, including traditional cardiovascular risk factors, measures of baseline CHD severity, 24-h urinary creatinine clearance, and use of preventive medications, were included in these models. Hazard ratios were further adjusted for the presence of inducible ischaemia on exercise stress echocardiography and for interim myocardial infarction during the follow-up period (model 2). Adjustment was then made for left ventricular (LV) mass index and resting LV ejection fraction (model 3). Finally, hazard ratios were adjusted for the presence of diastolic dysfunction, defined as impaired relaxation, pseudonormal or restrictive filling (model 4). In our multivariable analysis, additional testing was done to confirm goodness-of-fit and all covariates were tested for multicollinearity.
Since diastolic dysfunction appeared to influence the association of CRP and hospitalisation for HF, we performed additional univariate and multivariate logistic regression to explore the association of CRP and diastolic dysfunction. In our multivariate analysis, we adjusted for age and all variables in Tables 1 and 2 that were associated with CRP at p<0.05.
Table 1.
Baseline characteristics of the cohort according to CRP category
| CRP≤3 mg/L |
CRP>3 mg/L |
P value | |
|---|---|---|---|
| N=595 | N=390 | ||
| Demographic variables | |||
| Age — years* | 67±11 | 66±11 | 0.1 |
| Male — no. (%) | 507 (85%) | 295 (76%) | 0.0002 |
| White ethnicity — no. (%) | 359 (60%) | 236 (61%) | 1.0 |
| Annual income <$20,000 — no. (%) | 275 (47%) | 199 (61%) | 0.1838 |
| Smoking — no. (%) | 88 (15%) | 107 (27%) | <0.0001 |
| Regular alcohol use — no. (%) | 177 (30%) | 108 (28%) | 0.5 |
| Physically active — no. (%) | 419 (71%) | 203 (52%) | <0.0001 |
| Body mass index — kg/m2 | 28±5 | 30±6 | <0.0001 |
| Medical conditions | |||
| Myocardial infarction — no. (%) | 324 (55%) | 203 (52%) | 0.4 |
| Heart failure — no. (%) | 86 (15%) | 87 (22%) | 0.002 |
| Hypertension — no. (%) | 410 (69%) | 284 (73%) | 0.2 |
| Diabetes — no. (%) | 150 (26%) | 110 (28%) | 0.3 |
| Revascularization — no. (%) | 366 (62%) | 211 (54%) | 0.02 |
| Medication use | |||
| Statin — no. (%) | 407 (68%) | 226 (58%) | 0.0008 |
| ACE/ARB — no. (%) | 316 (53%) | 189 (48%) | 0.2 |
| Beta blocker — no. (%) | 351 (59%) | 214 (55%) | 0.2 |
| Aspirin — no. (%) | 473 (80%) | 288 (74%) | 0.04 |
| Oral diabetes medication — no. (%) | 118 (20%) | 87 (22%) | 0.3 |
| Lab measurements | |||
| Creatinine clearance — mL/min | 83±28 | 78±29 | 0.005 |
| HbA1C — g/dL | 5.9±1.1 | 6.0±1.3 | 0.2 |
| Total Cholesterol — mg/dL | 174±40 | 183±46 | 0.001 |
| LDL — mg/dL | 101±31 | 110±38 | 0.0001 |
| HDL — mg/dL | 46.6±14.4 | 44.1±13.3 | 0.007 |
| Triglycerides — mg/dL | 137±138 | 147±114 | 0.2 |
| Median CRP — mg/L | 1.12 | 6.30 | |
Plus–minus values are means±standard deviations. P values are for differences between subjects with normal vs. elevated CRP.
Table 2.
Echocardiographic characteristics of the Heart and Soul cohort according to CRP category
| CRP≤3 mg/L |
CRP>3 mg/L |
P value | |
|---|---|---|---|
| N=595 | N=390 | ||
| LV parameters | |||
| LV mass index — g/m2 | 96.3±24.1 | 100.9±29.0 | 0.008 |
| LV end-systolic volume index — mL/m2 | 21.8±17.6 | 20.9±13.9 | 0.4 |
| LV end-diastolic volume index — mL/m2 | 52.2±18.1 | 50.3±18.0 | 0.1 |
| LV ejection fraction | 0.62±0.09 | 0.61±0.10 | 0.3 |
| Inducible ischaemia — no. (%) | 133 (24%) | 84 (24%) | 0.9 |
| Diastolic function — no. (%) | |||
| – Normal | 337 (63%) | 198 (58%) | 0.1 |
| – Impaired relaxation | 126 (24%) | 106 (31%) | 0.02 |
| – Pseudonormal or restrictive | 69 (13%) | 37 (11%) | 0.3 |
| Left atrial volume index — mL/m2 | 32.7±11.3 | 33.1±12.5 | 0.7 |
| Isovolumic relaxation time — ms | 118.4±25.8 | 116.5±26.3 | 0.3 |
| Early LV diastolic filling velocity: E wave velocity — m/s | 0.76±0.21 | 0.79±0.25 | 0.02 |
| Late LV diastolic filling velocity: A wave velocity — m/s | 0.76±0.24 | 0.83±0.27 | 0.0002 |
| Early/atrial LV filling ratio: E/A ratio | 1.1±0.5 | 1.0±0.5 | 0.1 |
| Early mitral inflow (E) deceleration time — ms | 242.9±64.2 | 241.1±64.0 | 0.7 |
| PR end-diastolic gradient — mmHg | 3.4±2.6 | 4.2±3.0 | 0.0009 |
Plus–minus values are means±standard deviations. LV = left ventricle; E = early mitral inflow; A = late (atrial) mitral inflow; PR = pulmonic regurgitation.
3. Results
3.1. Patient characteristics
The median value of baseline CRP in the study cohort of 985 patients (all of whom had underlying CHD) was 2.26 mg/L, which is higher than that measured in the general population [32]. Characteristics of the study population at baseline are shown in Table 1, stratified by CRP group. The interquartile values (25–75% tile) for the normal CRP group were 0.46–1.92 mg/L, and for the high CRP group were 4.04–10.07 mg/L. Of the participating subjects, 390 (39.6%) had elevated CRP (greater than 3 mg/L). Compared with participants who had CRP levels ≤3 mg/L, those with elevated CRP were more likely to be female and smoke, and less likely to be physically active, have a history of revascularization, or be using aspirin or statins. Participants with elevated CRP also had higher body mass index, higher total and LDL cholesterol levels, lower HDL cholesterol, and lower creatinine clearance.
3.2. Echocardiography
Subjects with elevated CRP had significantly higher left ventricular mass, but there was no difference in mean LV ejection fraction or inducible ischaemia (Table 2). Diastolic function was assessed in 89% of all subjects. Patients with elevated CRP were more likely to have diastolic dysfunction, based on an increased prevalence of impaired relaxation in the high CRP group. In the 577 patients with an identifiable pulmonic regurgitation jet, increases in CRP were associated with a stepwise increase in EDPR gradient (Fig. 1; p value for trend <0.0001).
Fig. 1.
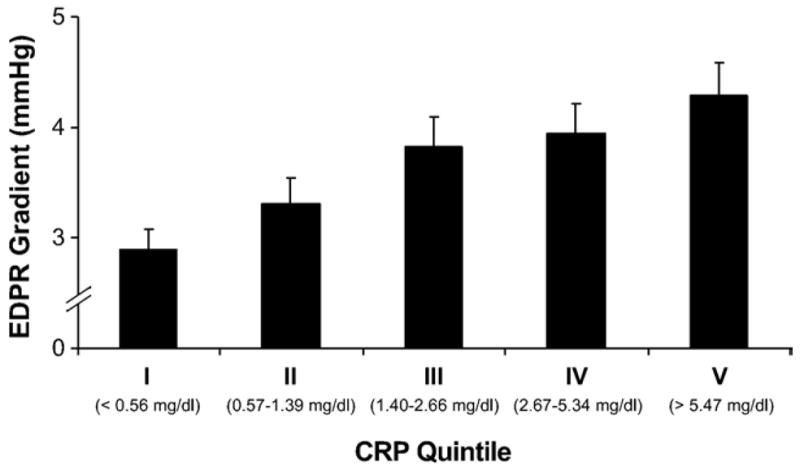
End-diastolic pulmonic regurgitation gradient by quintile of CRP. *p<0.0001 for the trend. EDPR = end-diastolic pulmonic regurgitation; CRP = high-sensitivity C-reactive protein.
3.3. Association of CRP with diastolic dysfunction
On univariate logistic regression, CRP independently predicted diastolic dysfunction, which was defined as impaired relaxation, pseudonormal, or restrictive filling patterns on Doppler echocardiography (odds ratio 1.21, 95% CI 1.06–1.38, p=0.005 for each standard deviation increase in log CRP). On multivariate analysis, we found that CRP was still independently associated with diastolic dysfunction (Table 3). Even when we defined diastolic dysfunction as only pseudonormal and restrictive patterns, log CRP was still independently associated with diastolic dysfunction (odds ratio 1.36, 95% CI 1.08–1.73, p=0.01 for each standard deviation increase).
Table 3.
Independent predictors of diastolic dysfunction
| Predictor | Odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Log C-reactive proteina | 1.22 | 1.02–1.46 | 0.028 |
| Ageb | 1.86 | 1.53–2.27 | <0.0001 |
| Ejection fractionc | 0.79 | 0.64–0.97 | 0.025 |
| Inducible ischaemia | 1.79 | 1.24–1.60 | 0.002 |
Adjusted for age, sex, body mass index, physical activity, history of heart failure or revascularization, statin or aspirin use, creatinine clearance, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, myocardial infarction events, inducible ischaemia, left ventricular mass index, and left ventricular end-diastolic volume index.
Per standard deviation increase in high-sensitivity C-reactive protein.
Per decade increase in age.
Per 10% decrease in ejection fraction.
3.4. Association of CRP with heart failure
A total of 99 subjects (10%) were hospitalised for heart failure during the follow-up period. Of the 390 participants with elevated CRP levels, 15% (56/390) were hospitalised for HF, compared with 7% (43/595) of those with normal CRP [hazard ratio (HR) 2.3, 95% confidence interval (CI): 1.5–3.3, p<0.0001]. The association between elevated CRP and HF remained significant after adjusting for traditional cardiovascular risk factors (Table 4). Further adjustment for both inducible ischaemia at baseline and subsequent myocardial infarction during the follow-up period did not eliminate this association, nor did adjustment for ejection fraction or LV mass index (models 2 and 3). However, the association between CRP and HF was no longer significant after adjustment for the presence of diastolic dysfunction (model 4a) or EDPR gradient (model 4b).
Table 4.
Risk of heart failure hospitalisation associated with elevated CRP>3 mg/L
| Model | Hazard ratio (95% CI) | P value |
|---|---|---|
| Unadjusted | 2.3 (1.5–3.3) | <.0001 |
| 1 | 1.7 (1.1–2.7) | 0.01 |
| 2 | 2.1 (1.3–3.5) | 0.004 |
| 3 | 2.1 (1.2–3.6) | 0.009 |
| 4a | 1.6 (0.8–3.2) | 0.1 |
| 4b | 1.0 (0.4–2.1) | 0.9 |
Model 1: adjusted for male sex, smoking history, physical activity, body mass index, history of HF, history of revascularization, statin use, aspirin use, creatinine clearance, LDL, and HDL.
Model 2: adjusted for Model 1 variables plus myocardial infarction events and inducible ischaemia.
Model 3: adjusted for Model 2 variables plus LV mass index, resting LVEF and LV end-diastolic volume index.
Model 4a: adjusted for Model 3 variables plus diastolic dysfunction (defined as impaired relaxation, pseudonormal or restrictive filling on echocardiography).
Model 4b: adjusted for Model 3 variables plus end-diastolic pulmonic regurgitation gradient.
4. Discussion
In our cohort of elderly patients with coronary heart disease, elevated CRP at baseline was found to be a risk factor for incident heart failure, a finding similar to that reported in three recent studies [8–10]. The results of our study further indicate that CRP predicts hospitalisation for HF independent of traditional risk factors, baseline CHD severity (as assessed by stress echocardiography) and interim myocardial infarction. These findings suggest that the association between CRP and HF is not mediated by a greater degree of CHD. However, increasing levels of CRP were associated with increases in EDPR gradient, a surrogate of LV filling pressures, and adjustment for either diastolic dysfunction or EDPR markedly attenuated the association between CRP and HF. Finally, we found that CRP is independently associated with diastolic dysfunction. These findings imply that abnormal diastolic function and/or elevated filling pressures may explain the risk of HF associated with CRP.
The relationship of HF to elevated CRP levels was first reported decades ago [33]. Since then, several mechanisms have been postulated to explain the association between inflammatory markers and HF. Interleukin-6 (IL-6) is a powerful stimulus of CRP production in hepatocytes, and is produced by a number of cell types, including cardiomyocytes, in response to hypoxic stress [34]. Left ventricular dysfunction, low cardiac output, hypoperfusion and venous congestion have all been proposed as potential contributors to IL-6, and hence CRP, secretion. Indeed, IL-6 has been shown to be elevated in patients with asymptomatic systolic dysfunction [35], and elevated IL-6 and CRP levels have been demonstrated to predict incident HF in an asymptomatic elderly population [10].
The cytokine hypothesis of HF posits that the progression of HF is stimulated by the activation of inflammatory mediators and their resultant adverse effects on the cardiovascular system. It has been reported, for example, that proinflammatory markers such as tumour necrosis factor (TNF) alpha and IL-6 are activated in HF at an earlier stage (NYHA class II) than the classic neurohormones [36,37]. Infusion of recombinant TNF-α and IL-6 cause myocardial dysfunction in canine [38] and rodent [39] models, suggesting that inflammation may play a causal role in the pathogenesis of HF as well. Curiously, therapies targeted toward inhibiting cytokines have failed to improve the clinical course of patients with chronic HF [40,41].
In our study, elevated filling pressures at least partly explained the increased risk of heart failure associated with elevated CRP. These findings extend those of a study demonstrating that CRP was associated with left ventricular dysfunction in 98 patients undergoing cardiac catheterization. In those patients, CRP was closely associated with left ventricular end-diastolic pressure [17]. In a recent study of outpatients with HF and systolic dysfunction, elevated CRP levels correlated with more advanced NYHA class and a poorer prognosis, but not with the LV ejection fraction [42]. Another study identified CRP as an independent predictor of mortality and rehospitalisation in 214 patients treated for acute HF [43]. In this study, it was noted that increases in CRP were most prominent in patients with HF and preserved ejection fraction. The results of these studies concur with our finding that elevated CRP was associated with diastolic dysfunction but not with the degree of systolic dysfunction. Of note, prior studies on CRP in both acute and chronic HF have not shown a correlation between CRP and ejection fraction [12,14,15].
Elevated LV filling pressures have been recognized as an important treatment target in patients with acute HF, as they predict an increased risk of fatal decompensation and sudden death [44]. Our finding that the association of CRP with incident HF appears to be explained by abnormal diastolic function and increased LV filling pressure, underscores the importance of elevated LV filling pressures as a potential treatment target in patients at risk for HF, such as outpatients with CHD. Regardless of the precise sequence of events by which inflammation and increased filling pressures contribute to HF, CRP appears to identify a group of CHD patients with an increased risk of developing HF and therefore a group likely to benefit from interventions to prevent or delay its onset.
Our study has several limitations. First, as with any observational study, the association of CRP with diastolic dysfunction and heart failure does not infer a causal relationship. Our multivariate analyses sought to minimize the possibility of confounding by taking several known cardiovascular risk factors into account. For example, though body mass index was higher on average in the elevated CRP group, it was not shown to be an independent predictor of diastolic dysfunction, nor did it alter the relationship between CRP and HF in our study.
CRP is a nonspecific marker of systemic inflammation, and we cannot exclude the possibility that the role of inflammation in HF is an epiphenomenon or a byproduct of more biologically relevant pathologic processes. Also, as CRP is an acute phase reactant, it often becomes difficult to interpret in study participants with concurrent infection, such as pneumonia.
Finally, our participants were mostly men, so our results may not generalize to other populations. Notably, epidemiological studies have shown that women are at higher risk of developing HF in the setting of pressure overload states, such as hypertension and aortic stenosis. Although it appears that age-related changes in systolic and diastolic function in these women are more pronounced on echocardiography [45], gender was not independently associated with diastolic dysfunction in our study. It is possible, however, that women with diastolic dysfunction due to CHD represent a distinct phenotype from hypertensive women, and that they are in fact similar to their male counterparts. Indeed, adjusting for gender did not significantly change the association between CRP and HF hospitalisation in our study.
5. Conclusions
In summary, elevated CRP levels are a risk factor for future hospitalisation for heart failure in outpatients with stable CHD. This association is independent of concurrent CHD severity, and appears to be at least partly explained by abnormal diastolic function and increased filling pressures. Further studies on the potential role of elevated filling pressure and diastolic dysfunction in triggering systemic inflammation are warranted.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, and the Nancy Kirwan Heart Research Fund, San Francisco, CA. Dr. Shah is supported by a Heart Failure Society of America Research Fellowship Award.
Abbreviations
- CHD
coronary heart disease
- CRP
high-sensitivity C-reactive protein
- ECG
electrocardiogram
- EDPR
end-diastolic pulmonic regurgitation
- EF
ejection fraction
- HDL
high-density lipoprotein
- HF
heart failure
- IL-6
interleukin-6
- LDL
low-density lipoprotein
- LV
left ventricular
- LVEDP
left ventricular end-diastolic pressure
- NYHA
New York Heart Association
- TNF
tumor necrosis factor
References
- 1.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 5.Tommasi S, Carluccio E, Bentivoglio M, et al. C-reactive protein as a marker for cardiac ischemic events in the year after a first, uncomplicated myocardial infarction. Am J Cardiol. 1999;83:1595–9. doi: 10.1016/s0002-9149(99)00162-9. [DOI] [PubMed] [Google Scholar]
- 6.Tomoda H, Aoki N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. Am Heart J. 2000;140:324–8. doi: 10.1067/mhj.2000.108244. [DOI] [PubMed] [Google Scholar]
- 7.Ferreiros ER, Boissonnet CP, Pizarro R, et al. Independent prognostic value of elevated C-reactive protein in unstable angina. Circulation. 1999;100:1958–63. doi: 10.1161/01.cir.100.19.1958. [DOI] [PubMed] [Google Scholar]
- 8.Sabatine MS, Morrow DA, Jablonski KA, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–36. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 9.Kardys I, Knetsch AM, Bleumink GS, et al. C-reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–20. doi: 10.1016/j.ahj.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 11.Lamblin N, Mouquet F, Hennache B, et al. High-sensitivity C-reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005;26:2245–50. doi: 10.1093/eurheartj/ehi501. [DOI] [PubMed] [Google Scholar]
- 12.Yin WH, Chen JW, Jen HL, et al. Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am Heart J. 2004;147:931–8. doi: 10.1016/j.ahj.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Chirinos JA, Zambrano JP, Chakko S, et al. Usefulness of C-reactive protein as an independent predictor of death in patients with ischemic cardiomyopathy. Am J Cardiol. 2005;95:88–90. doi: 10.1016/j.amjcard.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 14.Berton G, Cordiano R, Palmieri R, Pianca S, Pagliara V, Palatini P. C-reactive protein in acute myocardial infarction: association with heart failure. Am Heart J. 2003;145:1094–101. doi: 10.1016/S0002-8703(03)00098-X. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Martinez JL, Llorente-Diez B, Echegaray-Agara M, Olaz-Preciado F, Urbieta-Echezarreta M, Gonzalez-Arencibia C. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4:331–6. doi: 10.1016/s1388-9842(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 16.Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–34. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 17.Shah SJ, Marcus GM, Gerber IL, et al. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12:61–5. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. Jama. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005;62:661–6. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruo B, Rumsfeld JS, Pipkin S, Whooley MA. Relation between depressive symptoms and treadmill exercise capacity in the Heart and Soul Study. Am J Cardiol. 2004;94:96–9. doi: 10.1016/j.amjcard.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–20. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 23.Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–50. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubbock LA, Goh A, Ali S, Ritchie J, Whooley MA. Relation of low socioeconomic status to C-reactive protein in patients with coronary heart disease (from the heart and soul study) Am J Cardiol. 2005;96:1506–11. doi: 10.1016/j.amjcard.2005.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ristow B, Ahmed S, Wang L, et al. Pulmonary regurgitation end-diastolic gradient is a Doppler marker of cardiac status: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2005;18:885–91. doi: 10.1016/j.echo.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge Z, Zhang Y, Ji X, Fan D, Duran CM. Pulmonary artery diastolic pressure: a simultaneous Doppler echocardiography and catheterization study. Clin Cardiol. 1992;15:818–24. doi: 10.1002/clc.4960151106. [DOI] [PubMed] [Google Scholar]
- 27.Lee RT, Lord CP, Plappert T, Sutton MS. Prospective Doppler echocardiographic evaluation of pulmonary artery diastolic pressure in the medical intensive care unit. Am J Cardiol. 1989;64:1366–70. doi: 10.1016/0002-9149(89)90583-3. [DOI] [PubMed] [Google Scholar]
- 28.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108:2987–92. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 30.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107:370–1. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- 31.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 32.Ajani UA, Ford ES, Mokdad AH. Prevalence of high C-reactive protein in persons with serum lipid concentrations within recommended values. Clin Chem. 2004;50:1618–22. doi: 10.1373/clinchem.2004.036004. [DOI] [PubMed] [Google Scholar]
- 33.Elster SK, Braunwald E, Wood HF. A study of C-reactive protein in the serum of patients with congestive heart failure. Am Heart J. 1956;51:533–41. doi: 10.1016/0002-8703(56)90099-0. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–4. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- 35.Raymond RJ, Dehmer GJ, Theoharides TC, Deliargyris EN. Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction. Am Heart J. 2001;141:435–8. doi: 10.1067/mhj.2001.113078. [DOI] [PubMed] [Google Scholar]
- 36.Mann DL. Targeted anticytokine therapy and the failing heart. Am J Cardiol. 2005;95:9C–16C. doi: 10.1016/j.amjcard.2005.03.007. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 37.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 38.Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest. 1992;90:389–98. doi: 10.1172/JCI115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 40.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 41.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 42.Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153:1048–55. doi: 10.1016/j.ahj.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca HP, Perruchoud AP. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006;151:845–50. doi: 10.1016/j.ahj.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 44.Fonarow GC. The treatment targets in acute decompensated heart failure. Rev Cardiovasc Med. 2001;2(Suppl 2):S7–S12. [PubMed] [Google Scholar]
- 45.Lim JG, Shapiro EP, Vaidya D, et al. Sex differences in left ventricular function in older persons with mild hypertension. Am Heart J. 2005;150:934–40. doi: 10.1016/j.ahj.2005.01.013. [DOI] [PubMed] [Google Scholar]


