Abstract
The predictive value of left atrial (LA) dilatation in ambulatory adults with coronary artery disease is not known. It was hypothesized that echocardiographic LA volume index (LAVI) predicts heart failure (HF) hospitalization and mortality with similar statistical power as left ventricular ejection fraction (LVEF) in ambulatory adults with coronary artery disease. We measured LAVI in 935 adults without atrial fibrillation, atrial flutter, or significant mitral valve disease in the Heart and Soul Study. LAVI was calculated using the biplane method of disks. Outcomes included HF hospitalization and mortality. Logistic regression odds ratios (ORs) were calculated and adjusted for age, demographics, medical history, left ventricular mass, diastolic function, and LVEF. Mean LAVI was 32 ± 11 ml/m2, and mean LVEF was 62 ± 10%. Sixty-six patients (7%) had LAVI >50 ml/m2. There were 108 HF hospitalizations and 180 deaths at 4.3 years of follow-up. C statistics calculated as the area under the receiver-operator characteristic curve were the same (0.60) for LAVI and LVEF in predicting mortality. The unadjusted OR for HF hospitalization was 4.4 for LAVI > 50 ml/m2 and 5.3 for LVEF <45% (p <0.001). In those with normal LVEF, the ORs for LAVI >50 ml/m2 were 5.2 for HF hospitalization (p <0.0001) and 2.5 for mortality (p = 0.006). After multivariate adjustment, LAVI > 50 ml/m2 was predictive of HF hospitalization (OR 2.4, p = 0.02), and LAVI >40 ml/m2 was predictive of mortality (OR 1.9, p = 0.005). In conclusion, LAVI had similar predictability as LVEF for HF hospitalization and mortality in ambulatory adults with coronary artery disease.
Left atrial (LA) dilatation occurs in the setting of both systolic and diastolic dysfunction.1–3 The American Society of Echocardiography recommended LA volume index (LAVI), the value of LA volume divided by body surface area, to measure LA size.4 Increased LAVI was shown to predict mortality after acute myocardial infarction (MI),5,6 but the predictive power of degrees of LAVI dilatation has not been established in the frequently encountered clinical setting of ambulatory adults with coronary artery disease. We hypothesized that LAVI predicts heart failure (HF) hospitalization and mortality with similar statistical power as left ventricular ejection fraction (LVEF) in ambulatory adults with coronary artery disease.
Methods
The Heart and Soul Study was a prospective cohort study of psychosocial factors and health outcomes in patients with coronary disease. Methods and objectives have been described previously.7 Criteria for enrollment were (1) history of MI, (2) angiographic evidence of ≥50% diameter stenosis in ≥1 coronary vessel, (3) evidence of exercise-induced ischemia using treadmill electrocardiogram or stress nuclear perfusion imaging, or (4) history of coronary revascularization. Patients were excluded if they deemed themselves unable to walk 1 block, were within 6 months of an acute coronary syndrome, or were planning to move out of the local area within 3 years. All study subjects provided informed consent for baseline echocardiographic testing and review of medical records. The institutional review board at each of the enrolling centers approved the study protocol. Between September 2000 and December 2002, a total of 1,024 subjects were enrolled. Of these, 49 subjects were excluded for atrial fibrillation, atrial flutter, mitral stenosis, or greater than mild mitral regurgitation, categorized using reference criteria from the American Society of Echocardiography.4
Each subject completed a detailed interview and questionnaire detailing age, gender, race, medical history, medications, current smoking, and level of alcohol consumption. Echocardiographic studies were performed in the standard left lateral recumbent and supine positions using a commercially available ultrasound system with harmonic imaging (Acuson Sequoia; Siemens Corp., Mountain View, California). By protocol, LA size was maximized in both 2- and 4-chamber views. A single cardiologist (NBS) blinded to clinical and laboratory information evaluated each echocardiogram at rest. Our reader’s reproducibility was previously established.8
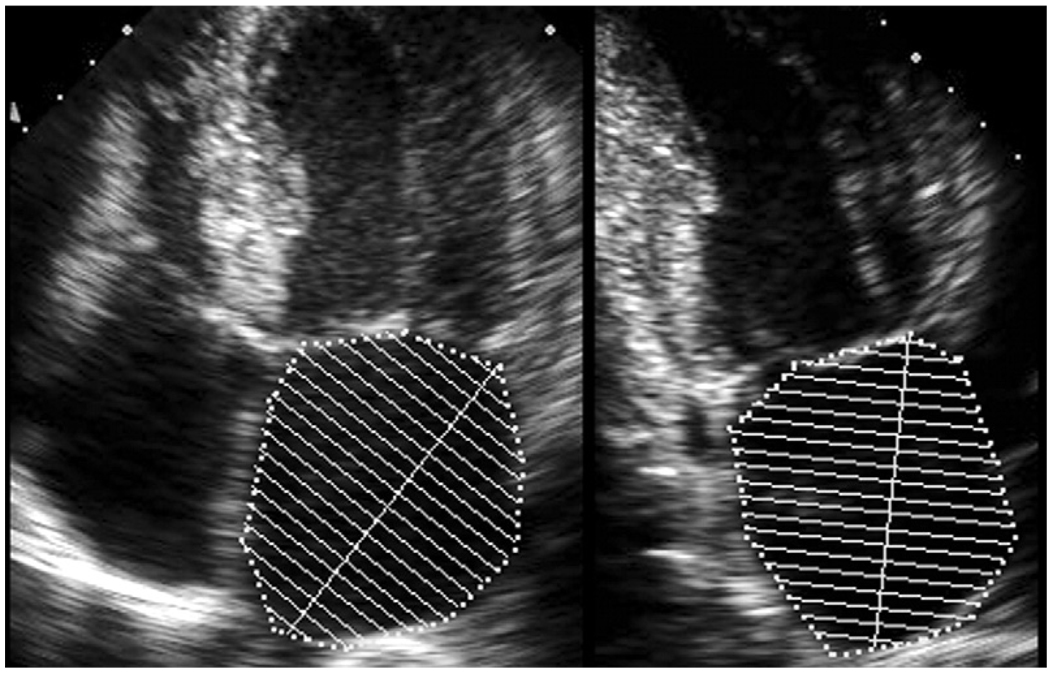
LA volume was measured from standard apical 2- and 4-chamber views at end-systole. LA borders were traced using planimetry. The borders consisted of the walls of the left atrium and a line drawn across the mitral annulus (Figure 1). Attention was given to bridge the ostia of pulmonary veins (when visualized) to not include the veins in the measurement. If seen, the LA appendage was excluded from measurement. The biplane method of disks was used to calculate LA volume,4 and measurements were rounded to the nearest integer. LAVI was calculated by dividing LA volume by body surface area. The American Society of Echocardiography criterion for severe LA dilatation (LAVI >40 ml/m2) was used to compare baseline characteristics of subjects.
Figure 1.
Measurement of LA volume using the biplane method of disks in apical 4- and 2-chamber views.
LVEF was calculated using the biplane method of disks from apical 4- and 2-chamber views. Diastolic dysfunction was categorized according to the classification by Khouri et al9; the presence of pseudonormal or restrictive diastolic dysfunction was defined as diastolic dominant pulmonary vein flow and mitral diastolic early/late velocity flow ratio >0.75. Diastolic function was not able to be categorized in 55 subjects because of the absence of measurable or discordance between mitral inflow and pulmonary venous flow parameters. Inducible myocardial ischemia was defined as a new wall abnormality not present on treadmill stress echocardiography images at rest.
We conducted telephone follow-up interviews and questioned subjects or their proxies regarding recent emergency department visits, hospitalizations, or death. For any reported event, medical records, death certificates, and coroner’s reports were retrieved. Two blinded adjudicators reviewed each event, and if there was agreement, the outcome classification was binding. If there was disagreement, a third blinded adjudicator reviewed the event and determined the outcome classification. Outcome adjudications were available for all except 5 subjects.
Mortality adjudications were based on hospital records, death certificates, and autopsy results. Hospitalization for HF was defined as a clinical syndrome requiring a minimum 1-night hospital stay with ≥2 of paroxysmal nocturnal dyspnea, orthopnea, increased jugular venous pressure, pulmonary rales, a third heart sound, cardiomegaly on chest radiograph, or pulmonary edema on chest radiograph.10 These clinical signs and symptoms must have represented a clear change from the previous clinical state of the patient. Stroke was considered an acute neurologic deficit of ischemic or hemorrhagic origin requiring supportive clinical and imaging documentation.
The outcome of MI was determined using standard diagnostic criteria developed by the American Heart Association.11 Death was considered caused by heart disease if (1) the subject died during the same hospitalization in which an acute MI was documented or (2) the subject experienced sudden death, defined as unexpected otherwise unexplained death within 1 hour of the onset of terminal symptoms.
Baseline characteristics were reported as mean ± SD for continuous variables and percentage for categorical variables. Differences in baseline characteristics between groups were determined using Student’s t test for continuous variables and chi-square test for dichotomous variables. The cutoff of 40 ml/m2 was used based on American Society of Echocardiography criteria for severe LAVI dilatation.4 Differences between categories of LA dilatation by reference standards for LAVI and LVEF were determined using chi-square test. We used logistic regression to examine the association of LAVI with HF, stroke, and mortality.
We report odds ratios (ORs) with 95% confidence intervals. Multivariate adjustments, when applicable, were made for all baseline characteristics listed in Table 1. We calculated c statistics (the numerical equivalent of area under the receiver-operator characteristic curve) for LAVI and LVEF. Analyses were performed using SAS software (version 8; SAS Institute Inc., Cary, North Carolina). Outcome events were counted once for each subject (e.g., recurrent HF hospitalizations in the same subject were not counted). Predefined end points were HF hospitalization, stroke, MI, cardiovascular mortality, and all-cause mortality.
Table 1.
Baseline characteristics by left atrial volume index (LAVI)
| Variable | LAVI (ml/m2) |
p Value | |
|---|---|---|---|
| <40 (n = 749; 80%) |
≥40 (n = 186; 20%) |
||
| Age (yrs) | 66 ± 11 | 69 ± 11 | 0.0004 |
| Men | 610 (81%) | 152 (82%) | 0.77 |
| Systolic blood pressure (mm Hg) | 132 ± 20 | 137 ± 24 | 0.01 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 | 74 ± 12 | 0.53 |
| Body mass index (kg/m2) | 29 ± 5 | 28 ± 5 | 0.07 |
| Body surface area (m2) | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.02 |
| Race | |||
| White | 433 (58%) | 120 (65%) | 0.10 |
| Black | 128 (17%) | 31 (17%) | 0.88 |
| Asian | 91 (12%) | 14 (8%) | 0.07 |
| Other | 96 (13%) | 21 (11%) | 0.57 |
| History of: | |||
| Hypertension | 578 (69%) | 144 (78%) | 0.02 |
| MI | 400 (54%) | 105 (56%) | 0.52 |
| Stroke | 105 (14%) | 25 (13%) | 0.82 |
| Diabetes mellitus | 200 (27%) | 45 (24%) | 0.47 |
| HF | 106 (14%) | 57 (31%) | <0.0001 |
| Revascularization (surgical or percutaneous) | 428 (57%) | 119 (64%) | 0.10 |
| Medications: | |||
| Aspirin | 599 (80%) | 145 (78%) | 0.54 |
| β Blocker | 428 (57%) | 112 (60%) | 0.45 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker |
359 (48%) | 111 (60%) | 0.004 |
| Diuretic | 201 (27%) | 64 (34%) | 0.04 |
| Antiarrhythmic drug | 32 (4%) | 27 (15%) | <0.0001 |
| Statin | 482 (64%) | 118 (63%) | 0.82 |
| Current smoker | 158 (21%) | 30 (16%) | 0.12 |
| Regular alcohol use | 215 (29%) | 53 (29%) | 0.93 |
| Inducible ischemia present | 149 (22%) | 54 (33%) | 0.002 |
| Left ventricular mass index (ml/m2) | 94.5 ± 25 | 110.1 ± 27.3 | <0.0001 |
| Left ventricular diastolic dysfunction (pseudonormal or restrictive) |
33 (4%) | 24 (13%) | <0.0001 |
| LVEF | 62.7 ± 9.4 | 58.2 ± 10.2 | <0.0001 |
Results
After exclusion of 49 patients with atrial fibrillation, atrial flutter, mitral stenosis, or greater than moderate mitral regurgitation, 970 subjects were suitable for analysis; 935 had measurable LA volume and 945 had measurable LVEF. Mean LA volume was 63 ± 21 ml, mean LAVI was 32 ± 11 ml/m2, and mean LVEF was 62 ± 10%. Baseline characteristics of LAVI separated by the cutoff of 40 ml/m2 are listed in Table 1.
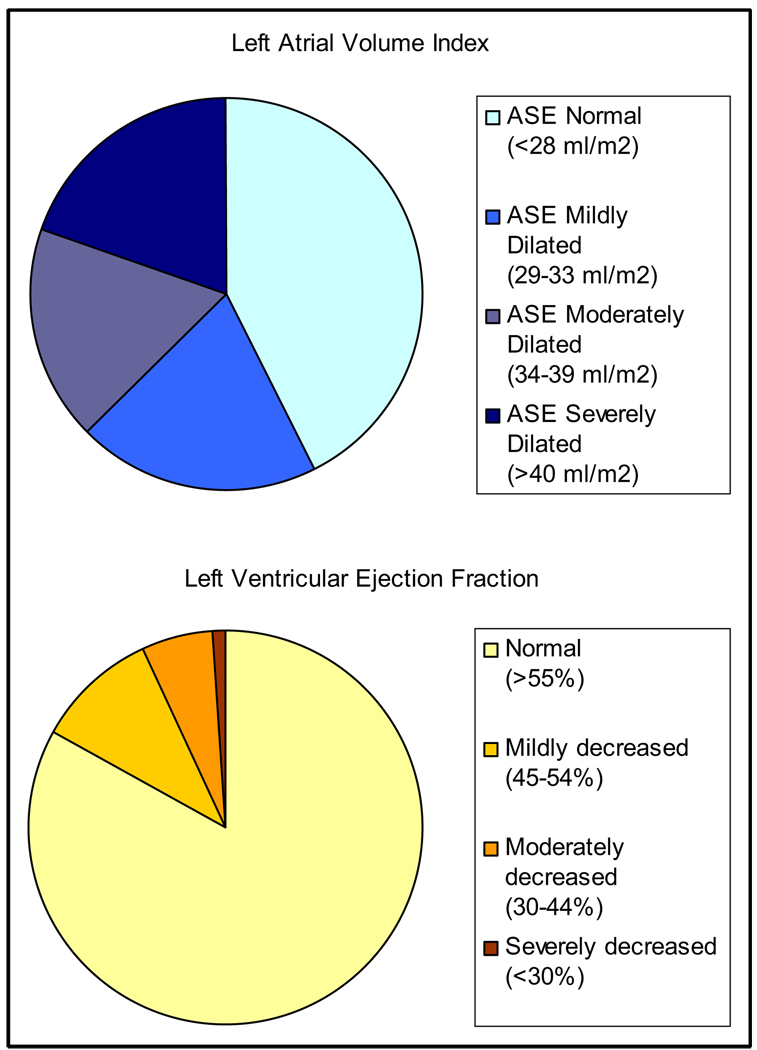
Categories of normal and abnormal LAVI and LVEF are shown in Figure 2, based on the reference standards of the American Society of Echocardiography.4 According to the reference limits, 43% of subjects had normal LAVI (<28 ml/m2) and 83% had normal LVEF (>55%). The difference in distribution between LAVI and LVEF was statistically significant (chi-square = 0.003).
Figure 2.
Distribution of LAVI and LVEF categorized using American Society of Echocardiography (ASE) standard reference limits (chi-square for difference in distribution p = 0.003).
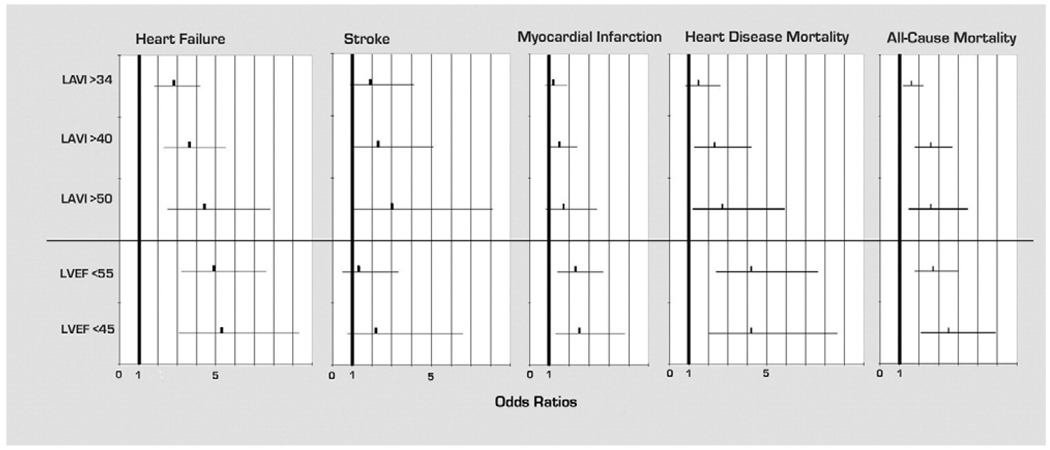
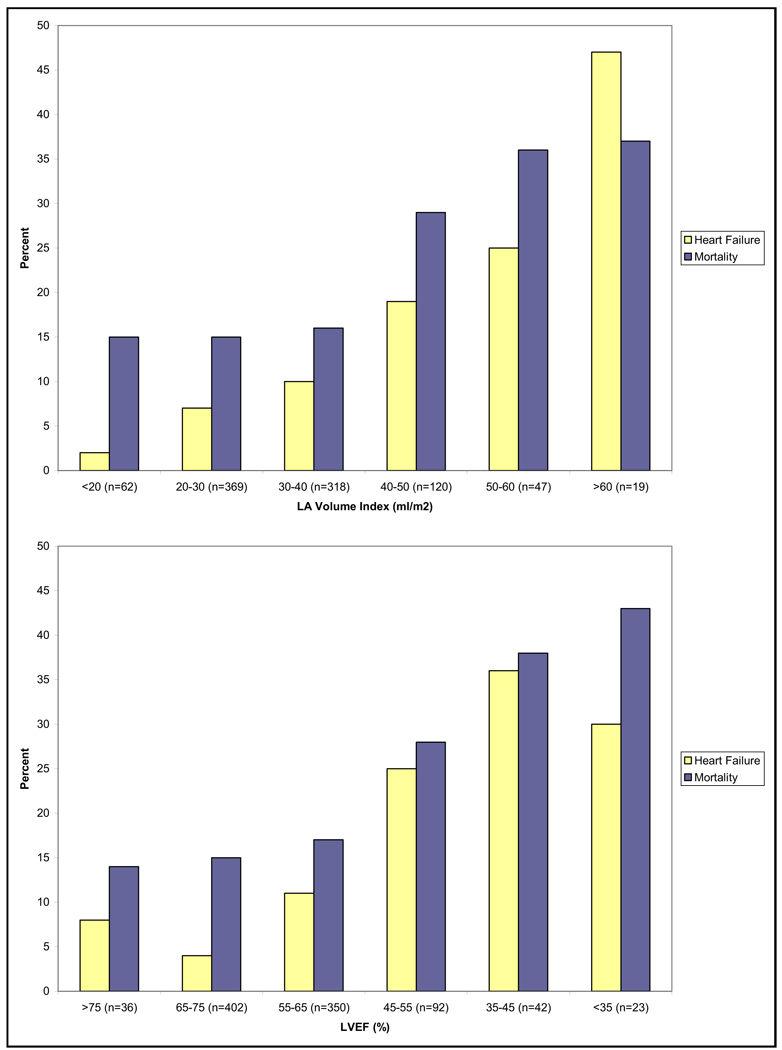
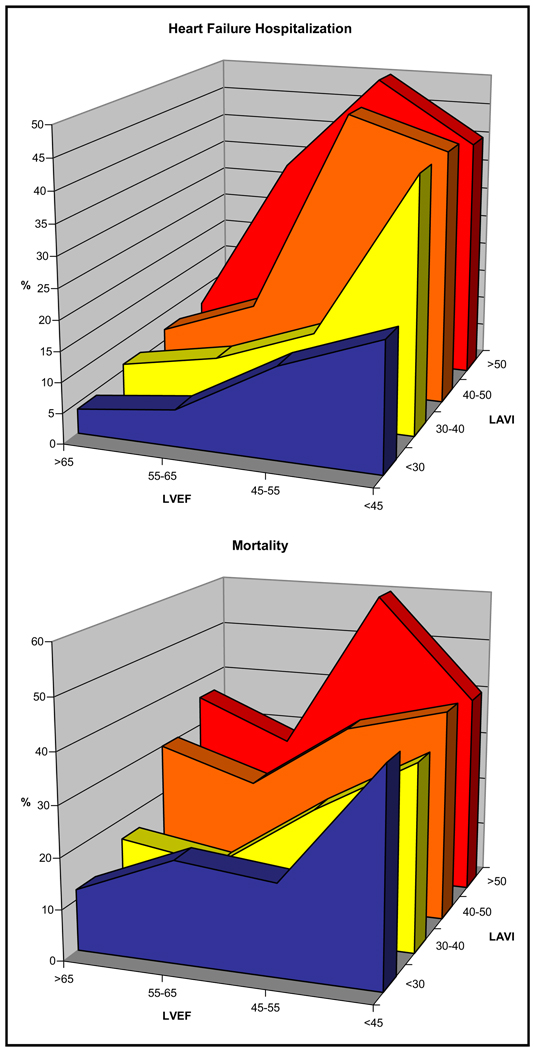
At an average of 4.3 years of follow-up, there were 108 subjects hospitalized for HF, 29 strokes, 94 MIs, 54 heart disease deaths, and 180 all-cause deaths. When LAVI and LVEF were analyzed as continuous variables, c statistics (area under the receiver-operator characteristic curve) for LAVI were 0.68, 0.63, and 0.60 for HF hospitalization, stroke, and mortality. C statistics for LVEF were 0.72, 0.61, and 0.60 for HF hospitalization, stroke, and mortality. Unadjusted ORs for categories of LAVI and LVEF are shown in Figure 3. For example, LAVI >40 ml/m2 was predictive of HF hospitalization (OR 3.6, p <0.0001), stroke (OR 2.3, p = 0.04), heart disease mortality (OR 2.3, p = 0.006), and all-cause mortality (OR 2.6, p <0.0001). Percentages of outcomes based on categories of LAVI and LVEF are shown in Figure 4. Percentages of adverse events increased with increasing LAVI, becoming evident as LAVI increased to >40 ml/m2 and most pronounced at >50 ml/m2.
Figure 3.
Unadjusted ORs for LAVI and LVEF in predicting outcomes of HF hospitalization, stroke, MI, heart disease mortality, and all-cause mortality.
Figure 4.
Percentages of subjects with HF hospitalization or mortality by distribution of LAVI and LVEF.
For subjects with normal LVEF (>55%), LAVI was predictive of HF hospitalization and mortality (Table 2). The prognostic value of relative changes in LAVI and LVEF in relation to HF and mortality is shown in Figure 5. Subjects with either increased LAVI or decreased LVEF had higher proportions of events.
Table 2.
Odds ratios (ORs) for left atrial volume index (LAVI) predicting heart failure (HF) hospitalization or mortality in those with normal left ventricular ejection fraction (LVEF; ≥55%)
| Variable | HF Hospitalization |
Mortality |
||
|---|---|---|---|---|
| OR | p Value | OR | p Value | |
| LAVI ≥34 ml/m2 | 2.3 (1.3–3.9) | 0.003 | 1.6 (1.1–2.3) | 0.02 |
| LAVI ≥40 ml/m2 | 3 (1.7–5.3) | 0.0002 | 2.6 (1.7–4.1) | <0.0001 |
| LAVI ≥50 ml/m2 | 5.2 (2.5–10.7) | <0.0001 | 2.5 (1.3–4.9) | 0.006 |
Figure 5.
Percentages of subjects with HF hospitalization or mortality stratified by both LAVI and LVEF.
There were 66 subjects with LAVI >50 ml/m2 and 66 subjects with LVEF <45%, providing a similar number of subjects in each group. There were 23 subjects with HF hospitalization in the low-LVEF group and 21 subjects with HF hospitalization in the high-LAVI group. Similarly, there were 27 deaths in the 66 subjects with decreased LVEF and 24 deaths in the 66 subjects with LAVI >50 ml/m2.
To account for the effects of age, gender, blood pressure, race, medical history, tobacco use, alcohol use, left ventricular mass index, diastolic dysfunction, and LVEF, multivariate adjusted ORs were calculated, as listed in Table 3. ORs for LVEF were adjusted for LAVI.
Table 3.
Multivariate-adjusted odds ratios (ORs)
| Variable | HF Hospitalization | p Value | All-Cause Death | p Value |
|---|---|---|---|---|
| LAVI ≥34 ml/m2 | 1.7 (1.014–2.8) | 0.047 | 1.1 (0.76–1.7) | 0.56 |
| LAVI ≥40 ml/m2 | 1.9 (1.1–3.2) | 0.02 | 1.9 (1.2–2.9) | 0.005 |
| LAVI ≥50 ml/m2 | 2.4 (1.2–4.8) | 0.02 | 1.8 (0.97–3.3) | 0.06 |
| LVEF <55% | 3 (1.6–5.4) | 0.0005 | 1.5 (0.87–2.4) | 0.15 |
| LVEF <45% | 2.6 (1.2–5.7) | 0.02 | 1.7 (0.84–3.3) | 0.15 |
ORs adjusted for age, sex, systolic and diastolic blood pressure, body mass index, race, medical history, smoking, alcohol use, LVMI, and diastolic dysfunction. LAVI adjusted for LVEF, and LVEF adjusted for LAVI.
Discussion
Our findings support our hypothesis that LAVI and LVEF are similar predictors of HF hospitalization and mortality. After multivariate adjustment for LVEF, diastolic dysfunction, left ventricular mass, and medical history, LAVI >50 ml/m2 predicted HF to a similar degree as LVEF <45% in ambulatory adults with coronary artery disease. LA dilatation provided prognostic information even in subjects with normal LVEF.
LA dilatation has been shown to predict mortality, atrial fibrillation,12–15 HF,16,17 and stroke.18,19 LA dilatation was evaluated in patient subgroups, including those with no previous heart disease,20 the Framingham cohort,21 and those with ischemic,22 dilated,23,24 or hypertrophic cardiomyopathy.25,26 LAVI <28 ml/m2 at rest was predictive of a normal stress echocardiogram,27 and LAVI >32 ml/m2 predicted mortality in patients with acute MI.5,6 However, the prognostic significance of LAVI was not previously reported in ambulatory adults with coronary artery disease.
The distribution of LA dilatation in this study merits consideration. Degrees of LA dilatation >40 or >50 ml/m2 are frequently encountered in ambulatory adults with coronary artery disease. Although the American Society of Echocardiography recommends >40 ml/m2 as the cutoff for “severe” LA dilatation, a higher cutoff provides more prognostic information. Table 2 and Table 3 show higher ORs for LAVI >50 ml/m2 (compared with >40 ml/m2) in predicting HF hospitalization.
The current American Society of Echocardiography reference guidelines for describing LAVI dilatation are based on a number of studies involving healthy subjects.4,28 The reference guidelines do not address the prognostic cut-off values for predicting adverse events in patients with coronary artery disease. We suggest that the descriptive category severe dilatation be reserved for LAVI >50 ml/m2 in ambulatory adults with coronary artery disease.
In our study population, the prevalence of both decreased LVEF and increased LAVI would be expected to be higher than in the general population. However, the relative prevalence of severely enlarged LAVI using published American Society of Echocardiography reference criteria considerably exceeded the prevalence of severely decreased LVEF (p = 0.003), as shown in Figure 2. With a cutoff of 50 ml/m2 for LAVI, 6% of subjects in our population would be described as having severe dilatation, which still remains higher than for subjects in the study population with severely decreased LVEF (1%). These findings provide consideration for using a higher cutoff, such as 50 ml/m2, for severe LA dilatation, at least in the commonly encountered population of patients with ambulatory coronary artery disease.
Several limitations need to be considered in interpretation of the study findings. Patients in the study population were predominantly men with known coronary artery disease and a high prevalence of co-morbidities, including hypertension. The outcome implications in young subjects, women, and those without coronary artery disease were not addressed. Second, we used mitral inflow and pulmonary vein flow patterns to evaluate diastolic function.9 We did not record Doppler tissue imaging velocities, which may have provided further characterization of diastolic function. Third, the presence of atrial fibrillation or atrial flutter was evaluated on a single baseline electrocardiogram. Subjects with paroxysmal atrial fibrillation or flutter would not have been identified if they were in sinus rhythm at the time of the electrocardiogram. Interim monitoring for atrial fibrillation was not performed in this study. Atrial fibrillation was shown to increase the risk of stroke,29 HF hospitalization, and mortality.30 However, even if the possible rhythm disturbance of atrial fibrillation were to cause additional adverse events, the adverse prognostic implications of LA dilatation remain.
Acknowledgments
This work was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Foundation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Nancy Kirwan Heart Research Fund, and an equipment loan from Siemens Corp., Mountain View, California.
References
- 1.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 2.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982. doi: 10.1016/0735-1097(93)90787-2. [DOI] [PubMed] [Google Scholar]
- 3.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 6.Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H, Matetzky S, Behar S, Eldar M, Feinberg MS. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44:327–334. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kircher B, Abbott JA, Pau S, Gould RG, Himelman RB, Higgins CB, Lipton MJ, Schiller NB. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine-computed tomography. Am Heart J. 1991;121:864–871. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 9.Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–297. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, et al. AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 12.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 14.Flaker GC, Fletcher KA, Rothbart RM, Halperin JL, Hart RG. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Am J Cardiol. 1995;76:355–358. doi: 10.1016/s0002-9149(99)80100-3. [DOI] [PubMed] [Google Scholar]
- 15.Bolca O, Akdemir O, Eren M, Dagdeviren B, Yildirim A, Tezel T. Left atrial maximum volume is a recurrence predictor in lone atrial fibrillation: an acoustic quantification study. Jpn Heart J. 2002;43:241–248. doi: 10.1536/jhj.43.241. [DOI] [PubMed] [Google Scholar]
- 16.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 19.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 20.Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 22.Sabharwal N, Cemin R, Rajan K, Hickman M, Lahiri A, Senior R. Usefulness of left atrial volume as a predictor of mortality in patients with ischemic cardiomyopathy. Am J Cardiol. 2004;94:760–763. doi: 10.1016/j.amjcard.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 23.Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, Enriquez-Sarano M. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425–1430. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 24.Dini FL, Cortigiani L, Baldini U, Boni A, Nuti R, Barsotti L, Micheli G. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol. 2002;89:518–523. doi: 10.1016/s0002-9149(01)02290-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle ED, Rakowski H. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr. 2005;18:1074–1082. doi: 10.1016/j.echo.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Kjaergaard J, Johnson BD, Pellikka PA, Cha SS, Oh JK, Ommen SR. Left atrial index is a predictor of exercise capacity in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2005;18:1373–1380. doi: 10.1016/j.echo.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Alsaileek AA, Osranek M, Fatema K, McCully RB, Tsang TS, Seward JB. Predictive value of normal left atrial volume in stress echocardiography. J Am Coll Cardiol. 2006;47:1024–1028. doi: 10.1016/j.jacc.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 28.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, et al. American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 30.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]