Abstract
Adenosine-induced renovasodilation in Dahl rats is mediated via activation of adenosine 2A receptors (A2AR) and stimulation of epoxyeicosatrienoic acid (EET) synthesis. Unlike Dahl salt-resistant (SR) rats, salt-sensitive rats exhibit an inability to upregulate the A2AR-EET pathway with salt-loading; therefore, we examined the effect of in vivo inhibition of the A2AR-EET pathway on blood pressure (BP) and the natriuretic response to salt-loading in Dahl SR rats. MS-PPOH (20 mg/Kg/day), an epoxygenase inhibitor, or ZM241385 (ZM; 5 mg/Kg/day), an A2AR antagonist, was given daily as an i.v. bolus dose for 3 days before and after placing rats on high salt (HS) intake (2% saline). After 3 days of HS, systolic (S) BP/24 h increased from 108 ± 2 mmHg to 136 ± 5 mmHg and 140 ± 4 mmHg, when treated with MS-PPOH or ZM, respectively (p<0.001). Plasma levels of EETs and dihydroxyeicosatrienoic acids (DHTs) during salt-loading and MS-PPOH (29.3 ± 1.8 ng/mL) or ZM treatment (9.8 ± 0.5 ng/mL) did not increase to the same extent as in vehicle-treated rats (59.4 ± 1.7 ng/mL; p<0.001) and renal levels of EETs+DHTs were 2-fold lower with MS-PPOH or ZM treatment. On Day 3 of HS intake, MS-PPOH- and ZM-treated rats exhibited a positive Na+ balance, and plasma Na+ levels were significantly increased (163.3 ± 1.2 and 158.1 ± 4.5 mEq/L, respectively) compared with vehicle-treated rats (142.1 ± 1 mEq/L), reflecting a diminished natriuretic capacity. These data support a role for the A2AR-EET pathway in the adaptive natriuretic response to modulate BP during salt-loading.
Keywords: Salt-sensitive hypertension, adenosine, EETs
Introduction
The kidney plays an integral role in the maintenance of extracellular fluid volume and electrolyte balance and thus, contributes to the long-term control of arterial pressure. It has been recognized for many years that sodium chloride intake is one of the main environmental factors responsible for the development of hypertension 1. Increased sodium chloride intake results in increased renal sodium chloride excretion. This adaptive process prevents progressive salt retention and volume expansion, with elevation of blood pressure (BP); thus, an impaired ability of the kidney to excrete sodium requires an increase in BP to increase natriuresis and correct the sodium balance, resulting in hypertension 2,3.
Epoxyeicosatrienoic acids (EETs), cytochrome P450 (CYP) epoxygenase metabolites of arachidonic acid (AA), have long been recognized as antipressor compounds; their vasodilator and natriuretic properties have been extensively documented 4-6. Indeed, inhibition of the epoxygenase pathway with a selective epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH), has been reported to increase BP in pregnant rats 7. Moreover, single nucleotide polymorphisms in the CYP2J2 epoxygenase gene have been associated with a hypertensive phenotype in humans 8. The inability to upregulate CYP2C epoxygenases in response to salt-loading has been associated with the development of salt-sensitive hypertension 9,10. The Dahl salt-sensitive (SS) rat is a genetic model of salt-dependent hypertension 11, that exhibits a rightward shift in the pressure-natriuresis curve 12, the hallmark of salt-sensitive hypertension. In addition to deficient renal cortical production of EETs, Dahl SS rats exhibit enhanced reabsorption of Na+ in the thick ascending limb of the loop of Henle (TALH) 13, which has been attributed to a deficient production of 20-hydroxyeicosatetraenoic acid (20-HETE), an inhibitor of the Na+/K+/2Cl- co-transporter in the TALH 14,15.
Adenosine-induced renovascular dilation in Sprague Dawley (SD) and Dahl salt-resistant (SR) rats is mediated via activation of adenosine 2A receptors (A2AR), and subsequent stimulation of EET synthesis 16,17. It has been reported that treatment of Wistar rats with 1,3-dipropyl-8-sulphophenylxanthine (DPSPX), a non-selective adenosine receptor antagonist, results in hypertension 18. Furthermore, BP is elevated in transgenic A2AR knockout mice on a normal salt (NS) diet 19, suggesting that A2AR activation can serve in mechanisms that contribute to the basal regulation of blood pressure. We have shown that in isolated, perfused kidneys from normotensive SD 16 and Dahl SR rats, but not hypertensive Dahl SS rats 17, high salt (HS) intake augments vascular responses to a stable analog of adenosine, 2-chloroadenosine, increases the production of natriuretic EETs, and upregulates the renal cortical and medullary protein expression of A2AR, as well as CYP2C23 and CYP2C11, salt-inducible epoxygenases. As wild-type mice, but not A2A knockout mice, respond to a selective A2AR agonist by a 30 mm Hg drop in BP 19, it is evident that A2AR activation can exert an EET-dependent antipressor action.
As the A2AR-epoxygenase pathway promotes vasodilation and subsequently diuresis-natriuresis, the inability of Dahl SS rats to upregulate this pathway may contribute to the development of salt-sensitive hypertension. Therefore, we examined the effect of in vivo inhibition of the A2AR-epoxygenase pathway on the adaptive natriuretic response to salt-loading in Dahl SR rats, and report that either epoxygenase inhibition or A2AR antagonism results in a delay in the natriuretic response and hypertension.
Methods
Male Dahl SR rats weighing 150-180 g (6- to 7-weeks old, Harlan Sprague Dawley, Inc., Chicago, IL) were fed Purina Lab diet 5001 and were used in accordance with National Institutes of Health guidelines for the care and use of animals. The New York Medical College Institutional Animal Care and Use Committee approved all experimental protocols.
Measurement of blood pressure
Blood pressure [systolic (SBP), diastolic (DBP) and mean (MBP)] and heart rate (HR) were monitored by radiotelemetry. Transoma (Data Sciences International; DSI, St. Paul, MN) radiotelemetry probes for small animals (TA11PA-C40) were implanted into Dahl SR rats at 6- to 7-weeks of age, using sterile surgical techniques as described 20. Animals were allowed 7 days to recover from the surgery and acclimate to the experimental environment.
Administration of drugs in vivo
One week after implantation with radiotelemetry probes, Dahl SR rats were anesthetized with Rompun (xylazine, 10 mg/Kg) and Ketaset (ketamine, 60 mg/Kg) i.m. The right jugular vein was exposed through a sterile incision and cannulated with a catheter (Braintree Scientific, Inc., Braintree, MA), which was exteriorized between the scapulae. The patency of the catheter was maintained by daily flushing with 0.1 ml of a heparinized saline solution. MS-PPOH (20 mg/Kg/day; i.v.) 7, a selective epoxygenase inhibitor, or the selective A2AR antagonist, ZM 241385 (5 mg/Kg/day; i.v.) 21, were administered as a bolus dose through the catheter in 0.1 ml 45% 2-hydroxypropyl-β cyclodextrin solution (Sigma-Aldrich, St. Louis, MO). The inhibitors were dissolved by 4-5 cycles of sonication (5 min), followed by shaking at 37°C for 15 min. In the first part of the protocol, MS-PPOH was given daily for 3 days prior to placing rats on a HS (2% saline drinking water) intake. After 3 days of HS intake with MS-PPOH treatment, MS-PPOH was withdrawn while rats remained on HS intake for 3 more days. Rats were then switched to water and NS (0.4% NaCl) diet for 7 days. After 7 days of NS diet, the selective A2AR antagonist, ZM 241385, was administered daily for 3 days prior to switching rats back to a HS intake. ZM 241385 treatment continued during 3 days of HS intake. A separate group of control, vehicle-treated Dahl SR rats was also placed on HS intake for 3 days. Rats were housed in metabolic cages for quantitative collection of urine and measurement of food and water intake during 24-h periods to monitor electrolyte balance. Urinary sodium and potassium concentrations were measured using an IL-943 flame photometer (Instrumentation Laboratories, Lexington, MA).
Analysis of CYP-AA metabolites
Blood was obtained from rats anesthetized with isoflurane via retro-orbital bleeding during NS diet, 3 days of HS intake, 3 days of HS intake with MS-PPOH treatment, and 3 days of HS intake with ZM 241385 treatment. After centrifugation at 3000 rpm, internal standards (1 ng of D2 20-HETE, 1 ng each of D8 8, 9-EET, 11, 12-EET and 14, 15-EET, and 1 ng each of D8 8, 9-DHT, 11, 12-DHT and 14, 15-DHT; Biomol, Plymouth Meeting, PA) and 200 μl of dH20 were added to 200 μl plasma. Total plasma lipids were extracted twice with 2 ml chloroform:methanol (2:1) and the extract subjected to alkaline hydrolysis to release esterified CYP-AA metabolites 22. Samples were acidified to pH 4.0, extracted with ethyl acetate, evaporated and reconstituted in 20 μl methanol for HPLC purification and subsequent GC-MS analysis 16.
Kidneys were obtained from an additional age-matched group of rats which were placed on HS and treated i.v. with either vehicle, MS-PPOH or ZM 241385, as described above. After 3 days of treatment, the rats were anesthetized, the kidneys excised and cortical and medullary tissues frozen in liquid nitrogen. The frozen renal tissues were homogenized in ethyl acetate, containing internal standards, and CYP-AA metabolites quantified with a Q-trap 3200 linear ion trap quadrupole LC/MS/MS equipped with a Turbo V ion source operated in negative electrospray mode (Applied Biosystems, Foster City, CA). Extracted samples were suspended in 10 μl of methanol and injected into the HPLC via a 1200 series autosampler equipped with a thermostat set at 4°C. The HPLC component consisted of an 1100 series binary gradient pump equipped with an Eclipse plus C18 (50 × 4.6 mm, 1.8 μm) column (Agilent Technologies, Santa Clara, CA). The column was eluted at a flow rate of 0.5 ml/min with 100% mobile phase A (methanol/water/acetic acid (60:40:0.01, v/v/v) from 0-2 min and a gradient increasing to 100% B (100% methanol) at 13 min. Synthetic standards were used to obtain standard curves (5- 500 pg) for each eicosanoid (linear regression R2 values > 0.99) and internal standards.
Analysis of data
All data are expressed as means ± S.E.M. One-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test was used when multiple comparisons were made. BP measurements were analyzed using repeated measures ANOVA followed by the Bonferroni post-hoc test. Unpaired t-test was used for all other data. A P value of <0.05 was considered statistically significant.
Results
Effect of in vivo epoxygenase inhibition or A2AR antagonism on SBP in Dahl SR rats
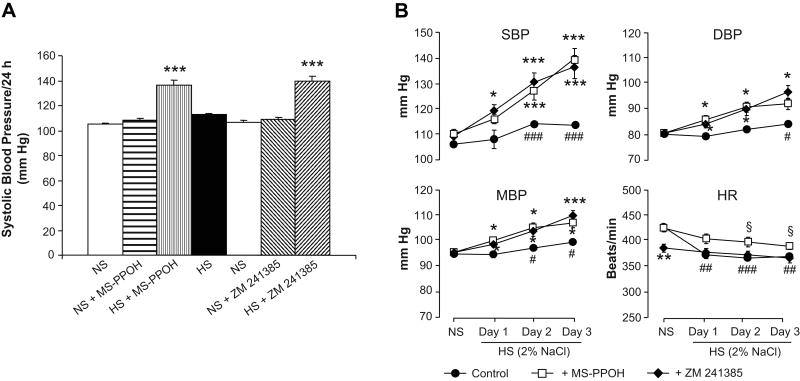
Figure 1 displays the results of in vivo epoxygenase inhibition or A2AR antagonism with MS-PPOH or ZM 241385, respectively, on SBP, DBP, MBP and HR in Dahl SR rats. As seen in Figure 1A, MS-PPOH treatment had negligible effects on SBP during NS (0.4% NaCl) diet; after administration of MS-PPOH for 3 days, SBP showed little change (105 ± 1 vs 108 ± 2 mm Hg). After 3 days of MS-PPOH treatment on NS diet, Dahl SR rats were then switched to a HS (2% saline drinking water) intake. SBP, DBP and MBP increased incrementally from the first through the third day of HS intake (Figure 1B); by the third day of HS intake with MS-PPOH treatment, SBP increased to 136 ± 5 mm Hg, indicating that EETs contribute to the regulation of BP during increased dietary sodium intake. MS-PPOH treatment was then withdrawn while rats remained on HS intake for another 3 days, during which time SBP came down to levels not different than those during NS diet; SBP decreased to 113 ± 1 mm Hg. Rats were then switched from HS to NS diet for 7 days, after which SBP further decreased to 107 ± 1 mm Hg (Figure 1A).
Figure 1. Effect of epoxygenase inhibition or A2AR antagonism on systolic blood pressure (BP) of Dahl SR rats after 3 days treatment (A) and on systolic, diastolic and mean BP and heart rate on Days 1-3 of HS intake (B).
Dahl SR rats on NS diet were pretreated with the epoxygenase inhibitor, MS-PPOH (20 mg/Kg/day), for 3 days prior to switching rats to a HS (2% saline drinking water) intake. After 3 days of HS intake with MS-PPOH treatment, MS-PPOH was withdrawn while rats remained on HS intake for 3 more days. Rats were then switched to NS diet for 7 days. The selective A2AR antagonist, ZM 241385 (5 mg/Kg/day), was then given for 3 days prior to switching rats back to HS intake. ZM 241385 treatment continued during 3 days of HS intake. Data are expressed as means ± SEM; n = 6-7; * p<0.05, ** p<0.005, *** p<0.001 vs. NS; # p<0.05, ## p<0.005, ### p<0.001 vs. control on each day. Note scale differences.
After 1 week of recovery on NS diet, we then assessed the role of A2AR in the regulation of BP during salt-loading by administering the selective A2AR antagonist, ZM 241385, for 3 days prior to switching Dahl SR rats back to a HS intake. On a NS diet, administration of ZM 241385 for 3 days also had negligible effects on SBP, DBP and MBP; after 3 days of ZM 241385 treatment, SBP showed little change (107 ± 1 vs 109 ± 2 mm Hg). After 3 days of ZM 241385 treatment on NS diet, Dahl SR rats were switched to a HS intake. Similar to the results obtained with epoxygenase inhibition, SBP, DBP and MBP increased incrementally from the first through the third day of HS intake (Figure 1B); by the third day of HS intake with ZM 241385 treatment, SBP increased to 140 ± 4 mm Hg, indicating that A2AR also contribute to the regulation of BP during increased dietary salt intake in Dahl SR rats. BP of control, vehicle-treated Dahl SR rats was significantly increased by day 2 of HS intake (Figure 1B). Unlike MS-PPOH-treated rats on NS intake, treatment of rats with ZM 241385 on NS intake exhibited a lower HR than control rats, data which are consistent with a previous report that A2AR agonism may mediate increases in HR 23.
Effect of in vivo epoxygenase inhibition or A2AR antagonism on the natriuretic response to salt-loading in Dahl SR rats
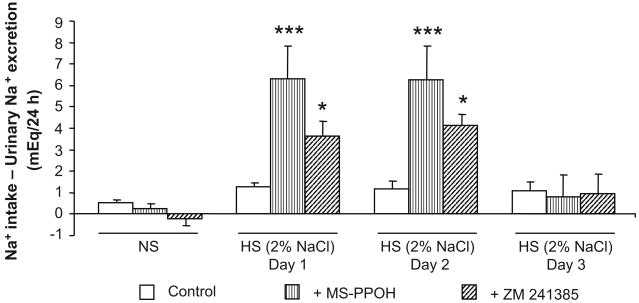
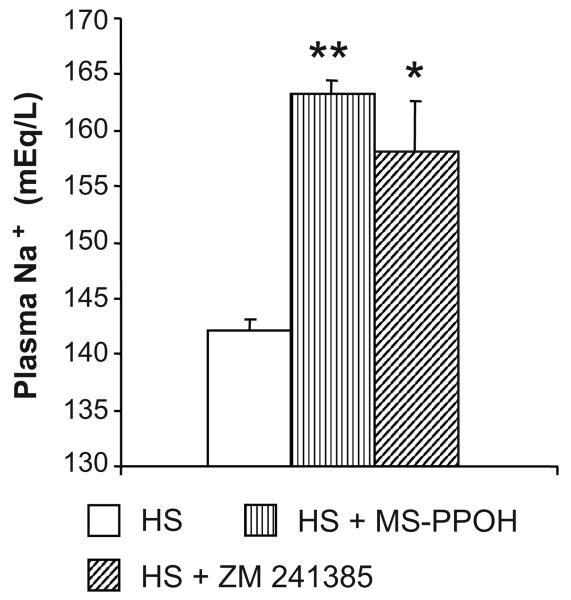
To assess the effect of in vivo epoxygenase inhibition or A2AR antagonism on the adaptive natriuretic response to salt-loading in Dahl SR rats, we calculated the daily difference between sodium intake (based on food consumption and fluid intake) and urinary sodium excretion over 3 days of HS intake (Figure 2). The fluid and sodium intake and output data for days 1 and 2 are shown in the Table. Fluid intake and urine volume were greater with MS-PPOH or ZM 241385 treatment, whereas, urinary sodium balance was significantly increased with MS-PPOH or ZM 241385 treatment compared to control rats on HS intake (Figure 2). During NS diet, in vivo epoxygenase inhibition or A2AR antagonism with MS-PPOH and ZM 241385, respectively, had negligible effects on the sodium excretion of Dahl SR rats. When switched to a HS intake, vehicle-treated Dahl SR rats were able to maintain their adaptive natriuretic capability; however, when treated with either MS-PPOH or ZM 241385, this adaptive natriuretic capability was altered when switched to a HS intake, as reflected by the more positive difference between sodium intake and urinary sodium excretion (Figure 2). The difference between sodium intake and urinary sodium excretion was 6.3 ± 1.5 and 3.6 ± 0.7 mEq/24 h for Dahl SR rats treated with MS-PPOH and ZM 241385, respectively, compared with 1.3 ± 0.2 mEq/24 h for vehicle-treated Dahl SR rats, during the first 24 h of salt-loading. During the second day of HS intake, the positive difference between sodium intake and urinary sodium excretion remained (6.3 ± 1.6 and 4.2 ± 0.8 mEq/24 h for Dahl SR rats treated with MS-PPOH and ZM 241385, respectively, compared with 1.2 ± 0.4 mEq/24 h for vehicle-treated Dahl SR rats). By the third day of salt-loading, the more positive difference between sodium intake and urinary sodium excretion observed in MS-PPOH- and ZM 241385-treated Dahl SR rats compared with vehicle-treated Dahl SR rats was no longer evident. As shown in Figure 3, the increased plasma levels of Na+ in MS-PPOH- and ZM 241385-treated rats indicate that these rats were retaining sodium compared with vehicle-treated Dahl SR rats during HS intake (142.1 ± 1.0 after 3 days of HS intake compared with 163.3 ± 1.2 and 158.1 ± 4.5 mEq/L when treated with HS+MS-PPOH and HS+ZM 241385, respectively).
Figure 2. Effect of epoxygenase inhibition or A2AR antagonism on the natriuretic response to salt-loading in Dahl SR rats.
The daily difference between sodium intake and urinary sodium excretion was compared between Dahl SR rats placed on NS (0.4% NaCl) diet, HS (2% saline drinking water) intake for 3 days, and HS intake for 3 days with either epoxygenase inhibition (HS + MS-PPOH; 20 mg/Kg/day) or A2AR antagonism (HS + ZM 241385; 5 mg/Kg/day). Data are expressed as means ± SEM; n = 6-9; * p<0.05, *** p<0.001 vs. control.
Table. Daily Fluid and Sodium Intake and output.
| Treatment Group | Fluid intake (mL) |
Urine Volume (mL) |
Fluid balance (mL) |
Na+ intake (mEq) |
Na+ excretion (mEq) |
|---|---|---|---|---|---|
| NS Control | 28.2 ± 3.4 | 6.4 ± 0.6 | 21.7 ± 3.3 | 1.2 ± 0.1 | 0.8 ± 0.1 |
| HS Control: Day 1 | 31.8 ± 2.7 | 23.7 ± 2.7‡ | 8.2 ± 0.6 ‡ | 11.8 ± 0.9‡† | 10.5 ± 1.0‡ |
| HS Control: Day 2 | 42.7 ± 4.7* | 29.9 ± 4.7‡ | 12.8 ± 0.8 * | 15.6 ± 1.6‡ | 14.4 ± 1.7‡ |
| NS+MS-PPOH | 33.0 ± 3.8 | 15.0 ± 1.5§ | 18.0 ± 1.8 | 1.3 ± 0.1 | 1.0 ± 0.2 |
| HS+MS-PPOH: Day 1 | 44.3 ± 3.8*† | 33.2 ± 3.5‡† | 11.2 ± 3.5 | 16.1 ± 1.3‡† | 9.8 ± 1.4‡ |
| HS+MS-PPOH: Day 2 | 82.0 ± 22.3*† | 59.3 ± 16.4‡† | 22.8 ± 7.5 | 29.0 ± 7.5‡† | 22.7 ± 6.5‡† |
| NS+ZM 241385 | 31.0 ± 4.1 | 16.4 ± 2.3§ | 12.8 ± 2.6 | 1.2 ± 0.1 | 1.4 ± 0.3 |
| HS+ZM 241385: Day 1 | 40.2 ± 3.0 | 29.9 ± 3.5* | 10.3 ± 2.6 | 14.8 ± 1.0‡† | 11.2 ± 1.2‡ |
| HS+ZM 241385: Day 2 | 81.5 ± 9.7‡† | 61.2 ± 10.1‡† | 20.3 ± 2.6† | 28.7 ± 3.2‡† | 24.5 ± 2.7‡† |
Data are expressed as means ± SEM; n = 6-7;
p<0.05 NS treated vs. NS control,
p<0.05 HS treated vs. HS control on Day 1 or Day 2,
p<0.001 HS vs. NS;
p<0.001 NS treated vs. NS control
Figure 3. Effect of epoxygenase inhibition or A2AR antagonism on plasma levels of Na+ after 3 days of HS intake in Dahl SR rats.
Plasma levels of Na+ were measured after 3 days of HS (2% saline drinking water) intake or 3 days of HS intake with either epoxygenase inhibition (HS + MS-PPOH; 20 mg/Kg/day) or A2AR antagonism (HS + ZM 241385; 5 mg/Kg/day). Data are expressed as means ± SEM; n = 4-6; * p<0.01, ** p<0.001 vs. HS.
Under both NS and HS conditions, Dahl SR rats treated with the epoxygenase inhibitor MS-PPOH showed a tendency to retain K+, as reflected by a positive difference between K+ intake and urinary K+ excretion (data not shown). The difference between K+ intake and urinary K+ excretion was not different between ZM 241385-treated and vehicle-treated Dahl SR rats, irrespective of salt diet.
Effect of in vivo epoxygenase inhibition or A2AR antagonism on plasma and renal levels of CYP-AA metabolites in Dahl SR rats
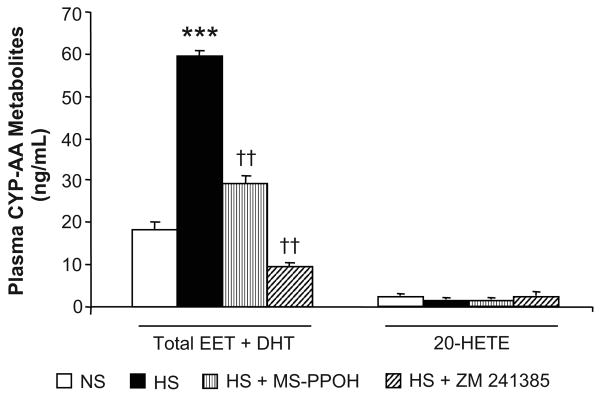
Plasma levels of CYP-AA metabolites were measured in Dahl SR rats during NS diet, and after 3 days of HS intake or 3 days of HS intake with either epoxygenase inhibition or A2AR antagonism (Figure 4). Plasma levels of EETs and DHTs increased nearly 3-fold from 18.4 ± 1.9 to 59.4 ± 1.7 ng/ml during NS compared with HS intake, respectively. When treated with MS-PPOH, the increase in plasma EET and DHT levels with salt-loading was reduced to 29.3 ± 1.8 ng/ml. During treatment with ZM 241385, plasma levels of EETs and DHTs declined with HS intake to levels below those during NS diet (9.8 ± 0.5 ng/ml). Plasma levels of 20-HETE were neither affected by salt-loading, nor epoxygenase inhibition nor A2AR antagonism.
Figure 4. Effect of epoxygenase inhibition or A2AR antagonism on plasma levels of CYP-AA metabolites in Dahl SR rats.
Plasma levels of CYP-AA metabolites were measured rats during NS (0.4% NaCl) diet, and after 3 days of HS (2% saline drinking water) intake or 3 days of HS intake with either epoxygenase inhibition (HS + MS-PPOH; 20 mg/Kg/day) or A2AR antagonism (HS + ZM 241385; 5 mg/Kg/day). Data are expressed as means ± SEM; n = 4-6; *** p<0.001 vs. NS, †† p<0.001 vs. HS.
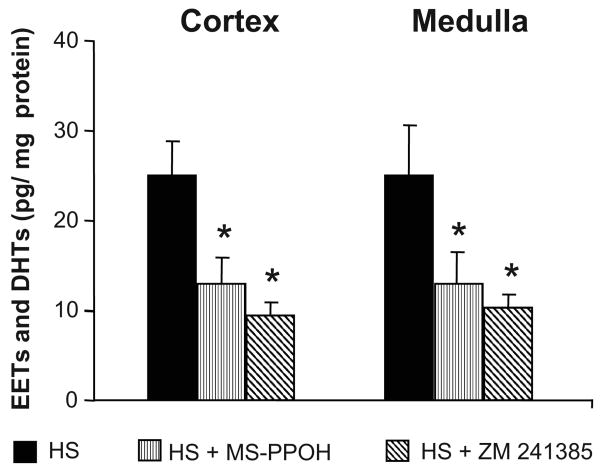
The levels of EETs and DHTs were comparable in renal cortical and medullary homogenates obtained from vehicle-treated rats. Treatment with MS-PPOH or ZM 241385 for 3 days on HS intake significantly decreased EETs and DHTs levels by approximately 2-fold (Figure 5). Renal 20-HETE levels were unaltered by epoxygenase inhibition or A2AR antagonism (data not shown).
Figure 5. Effect of epoxygenase inhibition or A2AR antagonism on renal levels of CYP-AA metabolites in Dahl SR rats.
Renal levels of epoxyeicosatrienoic acid (EET) and dihydroxyeicosatrienoic acid (DHTs) were measured rats after 3 days of HS (2% saline drinking water) intake or 3 days of HS intake with either epoxygenase inhibition (HS + MS-PPOH; 20 mg/Kg/day) or A2AR antagonism (HS + ZM 241385; 5 mg/Kg/day). Data are expressed as means ± SEM; n = 4; * p<0.05, ** p<0.01 vs. HS.
Discussion
Although adenosine has traditionally been implicated in the renal functional responses to pathological events such as ischemia and inflammation 24,25, its role in the adaptation of the kidney to enhance salt excretion has only recently become appreciated 16,26. We have shown that the A2AR-EET pathway is upregulated with salt-loading in normotensive SD 16 and Dahl SR rats, but not in hypertensive Dahl SS rats 17, and may thus contribute to the development of salt-sensitive hypertension in Dahl SS rats. In Dahl SR rats, salt-loading augmented renovascular responses to an adenosine analog, an effect associated with upregulation of the renal protein expression of the epoxygenases CYP2C23 and CYP2C11, as well as A2AR, changes that were not observed in Dahl SS rats 17.
In the present study, we examined the effect of in vivo inhibition of the A2AR-epoxygenase pathway on the adaptive natriuretic response to salt-loading in Dahl SR rats, both at the level of epoxygenase inhibition and A2AR antagonism. In agreement with Makita et al. 9, we saw an increase in BP in salt-loaded Dahl SR rats treated with an epoxygenase inhibitor. In the study by Makita et al., clotrimazole, a non-selective inhibitor of CYP epoxygenases was used, whereas we used MS-PPOH, an inhibitor selective for CYP epoxygenases. In addition, in our study we examined the first 3 days of HS intake (i.e. the early, adaptive natriuretic response to salt-loading) after epoxygenase inhibition, whereas Makita et al. examined the effect of epoxygenase inhibition after rats were maintained for 6 weeks on a HS diet. We observed a similar increase in BP in salt-loaded Dahl SR rats treated with a selective A2AR antagonist, ZM 241385. In vivo administration of ZM 241385 (10 mg/Kg, p.o.) to spontaneously hypertensive rats (SHR) attenuated the hypotensive response produced by exogenous adenosine 21. Moreover, oral administration of a selective A2AR adenosine agonist elicited a sustained hypotensive response in SHR 27. To our knowledge, our observations provide the first evidence for a role of the A2AR-EET pathway in the early, adaptive natriuretic response to salt-loading, as we have been able to render Dahl SR rats salt-sensitive, by either in vivo epoxygenase inhibition or A2AR antagonism.
The increase in BP in response to salt-loading seen in both MS-PPOH- and ZM 241385-treated Dahl SR rats was associated with a more positive sodium balance (as assessed by the daily difference between sodium intake and urinary sodium excretion), compared with vehicle-treated Dahl SR rats, during the first two days of salt-loading. By the third day of HS intake, no differences in sodium balance were detected among the three groups, indicating that either in vivo epoxygenase inhibition or antagonism of A2AR results in a delay in the natriuretic response to salt-loading. Plasma levels of sodium were higher in Dahl SR rats treated with either MS-PPOH or ZM 241385 during HS intake, compared with control, indicating that these treated rats were retaining sodium during salt-loading. Such increases in plasma Na+ concentration are not without precedent, as previous studies have shown similar changes in rats rendered salt-sensitive by uninephrectomy and DOCA 28. Increases in plasma Na+ concentration may lead to increases in sympathetic output and circulating vasopressin and these factors have been reported to relate to increases in arterial pressure of Dahl SS rats 29. Interestingly, studies by Huang et al., have shown that cerebrospinal fluid Na+ concentration is increased by HS intake in Dahl SS rats 30 and that intraventricular infusion of a mineralocorticoid receptor antagonist can prevent the subsequent hypertension.
Compared with vehicle-treated Dahl SR rats, urinary K+ excretion in Dahl SR rats treated with MS-PPOH showed a tendency to be reduced, as reflected by the positive difference between K+ intake and urinary K+ excretion, irrespective of salt diet. These results are in agreement with those of Sun et. al. 31, who showed that EETs activate large-conductance calcium-activated K+ (BKCa) channels and flow-stimulated K+ secretion in the cortical collecting duct, thereby regulating K+ secretion. Moreover, in that study, epoxygenase inhibition abolished K+ secretion mediated by BKCa and renal outer medullary K+ channels.
Dahl SR rats treated with either a CYP epoxygenase inhibitor or antagonist of A2AR were unable to increase plasma levels of EETs and DHTs to the same extent as vehicle-treated Dahl SR rats, in response to salt-loading. In fact, in Dahl SR rats treated with an A2AR antagonist, the concentration of plasma EETs and DHTs declined to levels below the basal levels on NS diet. Renal levels of EETs and DHTs were also significantly lower with either CYP epoxygenase inhibition or A2AR antagonism, thus further supporting a role for the A2AR-EET pathway in the adaptive natriuretic response to salt-loading in normotensive, Dahl SR rats.
Perspectives
An increase in the production of natriuretic EETs is one of the significant components of the adaptive response to prevent elevation of BP in response to salt-loading. Indeed, the antipressor actions of EETs have not been underscored. In this regard, inhibitors of soluble epoxide hydrolase, the enzyme responsible for the major route of rendering EETs biologically inactive, have been developed and have been shown to ameliorate the development of hypertension in several different animal models 32-35. Salt-sensitivity is an important feature of essential hypertension, and thus, identification of potential targets for the management of salt-sensitive hypertension may be of therapeutic benefit. We suggest that the A2AR-EET pathway may be an important therapeutic target for managing salt-sensitive hypertension.
Supplementary Material
Acknowledgments
We thank Katherine Gotlinger for performing the LC/MS study for us.
Sources of Funding
Research support was provided by grants from the National Institutes of Health DK69687, HL-25394 and GM31278 and, from the Pharmaceutical Research and Manufacturers of America Foundation.
Footnotes
Disclosures
None.
References
- 1.Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol. 2006;290:R514–R523. doi: 10.1152/ajpregu.00819.2005. [DOI] [PubMed] [Google Scholar]
- 2.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 3.Aperia AC, Broberger CG, Soderlund S. Relationship between renal artery perfusion pressure and tubular sodium reabsorption. Am J Physiol. 1971;220:1205–1212. doi: 10.1152/ajplegacy.1971.220.5.1205. [DOI] [PubMed] [Google Scholar]
- 4.McGiff JC, Ferreri NR. Eicosanoids and the Kidney. In: Robert Alpern, Steven Hebert., editors. The Kidney: Physiology and Pathophysiology. New York, NY: Elsevier Academic Press; 2008. pp. 359–384. [Google Scholar]
- 5.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 6.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Chang HH, Xu Y, Reddy DS, Du J, Zhou Y, Dong Z, Falck JR, Wang MH. Epoxyeicosatrienoic Acid inhibition alters renal hemodynamics during pregnancy. Exp Biol Med (Maywood) 2006;231:1744–1752. doi: 10.1177/153537020623101112. [DOI] [PubMed] [Google Scholar]
- 8.King LM, Gainer JV, David GL, Dai D, Goldstein JA, Brown NJ, Zeldin DC. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet Genomics. 2005;15:7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003;41:709–714. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- 11.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- 12.Roman RJ, Kaldunski M. Pressure natriuresis and cortical and papillary blood flow in inbred Dahl rats. Am J Physiol. 1991;261:R595–R602. doi: 10.1152/ajpregu.1991.261.3.R595. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension. 1991;17:1018–1024. doi: 10.1161/01.hyp.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 14.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 15.Carroll MA, Sala A, Dunn CE, McGiff JC, Murphy RC. Structural identification of cytochrome P450-dependent arachidonate metabolites formed by rabbit medullary thick ascending limb cells. J Biol Chem. 1991;266:12306–12312. [PubMed] [Google Scholar]
- 16.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol. 2005;289:F386–F392. doi: 10.1152/ajprenal.00421.2004. [DOI] [PubMed] [Google Scholar]
- 17.Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine 2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2008;295:F1696–F1704. doi: 10.1152/ajprenal.90502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimaraes S, Morato M, Sousa T, Albino-Teixeira A. Hypertension due to blockade of adenosine receptors. Pharmacol Toxicol. 2003;92:160–162. doi: 10.1034/j.1600-0773.2003.920404.x. [DOI] [PubMed] [Google Scholar]
- 19.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 20.Stier CT, Jr, Adler LA, Levine S, Chander PN. Stroke prevention by losartan in stroke-prone spontaneously hypertensive rats. J Hypertens Suppl. 1993;11:S37–S42. [PubMed] [Google Scholar]
- 21.Poucher SM, Keddie JR, Brooks R, Shaw GR, McKillop D. Pharmacodynamics of ZM 241385, a potent A2a adenosine receptor antagonist, after enteric administration in rat, cat and dog. J Pharm Pharmacol. 1996;48:601–606. doi: 10.1111/j.2042-7158.1996.tb05981.x. [DOI] [PubMed] [Google Scholar]
- 22.Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- 23.Dhalla AK, Wong MY, Wang WQ, Biaggioni I, Belardinelli L. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J Pharmacol Exp Ther. 2006;316:695–702. doi: 10.1124/jpet.105.095323. [DOI] [PubMed] [Google Scholar]
- 24.Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978;43:390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- 25.Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol. 2002;282:F10–F18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz JN, Weihprecht H, He XR, Skott O, Briggs JP, Schnermann J. Effects of adenosine and angiotensin on macula densa-stimulated renin secretion. Am J Physiol. 1993;265:F187–F194. doi: 10.1152/ajprenal.1993.265.2.F187. [DOI] [PubMed] [Google Scholar]
- 27.Yagil Y, Miyamoto M. The hypotensive effect of an oral adenosine analog with selectivity for the A2 receptor in the spontaneously hypertensive rat. Am J Hypertens. 1995;8:509–515. doi: 10.1016/0895-7061(95)00020-p. [DOI] [PubMed] [Google Scholar]
- 28.Kunes J, Zicha J, Jelínek J. The role of chloride in deoxycorticosterone hypertension: selective sodium loading by diet or drinking fluid. Physiol Res. 2004;53:149–154. [PubMed] [Google Scholar]
- 29.Bayorh MA, Ogbolu EC, Williams E, Thierry-Palmer M, Sanford G, Emmett N, Harris-Hooker S, Socci RR, Chu TC, Chenault VM. Possible mechanisms of salt-induced hypertension in Dahl salt-sensitive rats. Physiol Behav. 1998;65:563–568. doi: 10.1016/s0031-9384(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 30.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol. 2001;281:H1881–H1889. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 31.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J Am Soc Nephrol. 2009;20:513–523. doi: 10.1681/ASN.2008040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008;13:3480–3487. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 34.Loch D, Hoey A, Morisseau C, Hammock BO, Brown L. Prevention of hypertension in DOCA-salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys. 2007;47:87–98. doi: 10.1385/cbb:47:1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.