SUMMARY
The establishment of pregnancy requires an intimate physical interaction and a molecular dialogue between the conceptus and the maternal reproductive tract that commences at implantation and continues until the placenta is formed and fully functional. Failure of the regulatory processes that ensure the fidelity of this relationship can precipitate a catastrophic pregnancy loss. One of the earliest identified molecular mediators of blastocyst implantation is heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF), which signals between the endometrium and implanting trophoblast cells to synchronize their corresponding developmental programs. HBEGF expression by trophoblast cells of the developing placenta appears to regulate extravillous differentiation and provide cytoprotection in a sometimes-hostile environment. This versatile member of the EGF signaling system will be examined in light of its associations with key events during early pregnancy.
INTRODUCTION
Each month, the uterus prepares to receive a viable blastocyst under the direction of estrogen (E2) and progesterone (P4). The sex steroid hormones induce many changes in the endometrium that enhance its receptivity to an implanting blastocyst (Dey et al., 2004). Among these changes is the expression of secreted and membrane-bound signaling factors that influence embryo adhesion necessary for attachment to the luminal lining and later for invasion into the decidualized stroma. The embryo is also a source of several important factors, some of which are produced to augment the preparation of the endometrium. The concurrent developmental programs of the embryo and endometrium influence each other to synchronize their progression and produce an optimal environment for implantation. One critical factor that appears around the time of implantation is heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF). Evidence supporting a central role for HBEGF during implantation in mice is abundant and was recently reviewed (Lim and Dey, 2009). The cellular processes and regulatory factors that mediate the developmental program during blastocyst implantation have also been reviewed previously (Armant et al., 2000; Armant, 2005). The focus of this article is on the many timely influences of HBEGF and the EGF signaling system upon trophoblast cells in the peri- and post-implantation environment as pregnancy is established.
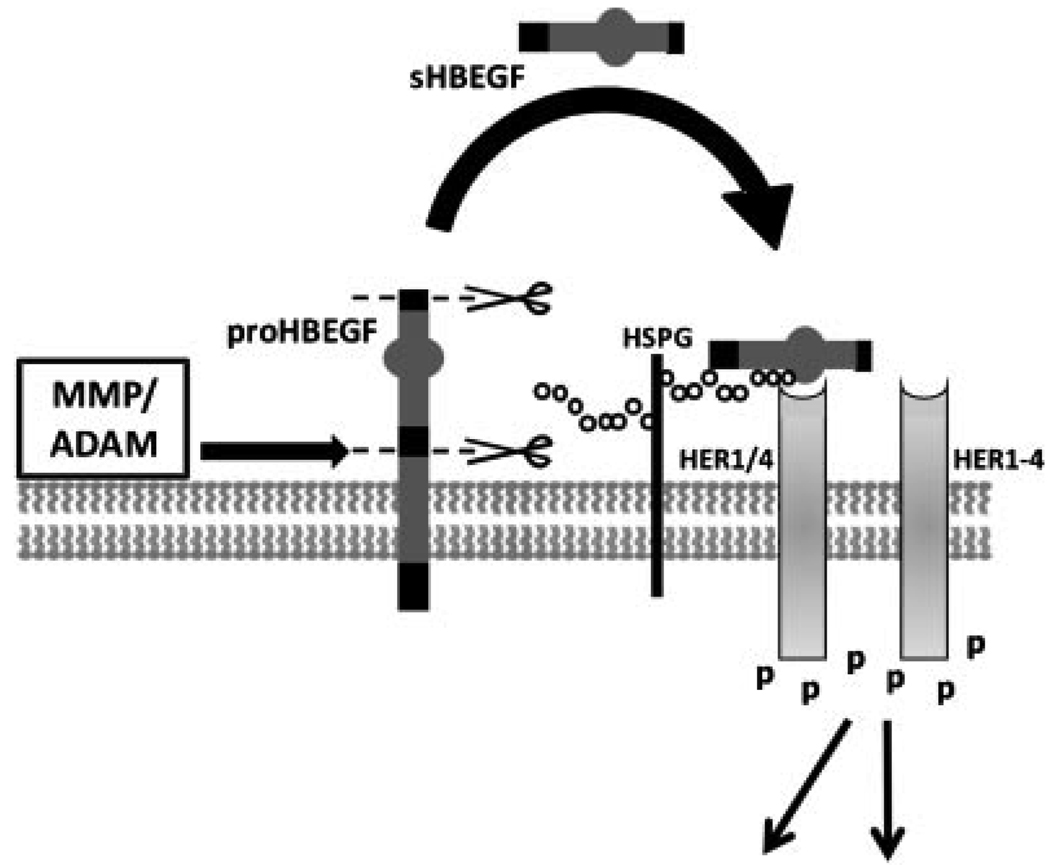
HBEGF and other members of the EGF family are synthesized as transmembrane proteins (e.g., proHBEGF) that can signal to their receptors on adjacent cells (juxtacrine) or become secreted through the shedding activity of metallo-proteinases that cleave the extracellular domain (Riese and Stern, 1998). The secreted form (e.g., sHBEGF) is free to bind receptors on the same cell (autocrine) or distant cells (paracrine). Processing of proHBEGF to sHBEGF and subsequent signaling through its receptors is illustrated in Figure 1. EGF family growth factors, operating through four HER/ErbB receptor tyrosine kinases, induce receptor dimerization and autophosphorylation, leading to downstream signaling that involves an extensive array of pathways (Holbro and Hynes, 2004). HBEGF requires heparan sulfate proteoglycan (HSPG) as a cofactor for binding to its receptors, HER1 (a.k.a. EGF receptor) and HER4. Although HBEGF does not bind directly to HER2 or HER3, they can participate in downstream signaling through heterodimerization with other ligated HER proteins.
Figure 1.
HBEGF processing and signaling. In its transmembrane form, proHBEGF is proteolytically cleaved by metalloproteinases at the cell surface to remove (scissors) its pro-domain and secrete the extracellular domain (sHBEGF shedding). sHBEGF is then free to diffuse to nearby receptor tyrosine kinases where it can bind either HER1 (EGF receptor) or HER4. Receptor binding requires heparin sulfate proteoglycan (HSPG) as cofactor, and induces HER homo- or heterodimerization. Receptor autophosphorylation initiates down-stream intracellular signaling. Heparin sulfate proteoglycan (HSPG); human epidermal growth factor receptor family member (HER); matrix metalloproteinase (MMP); a disintegrin and metalloproteinase (ADAM).
HBEGF performs a variety of functions in different cell systems (Raab and Klagsbrun, 1997). In its transmembrane form, proHBEGF appears to bind HER1 orHER4 of adjacent cells, participating in cell attachment or eliciting further signaling (Higashiyama et al., 1995; Raab et al., 1996). HBEGF induces chemotaxis and mitosis of NIH 3T3 cells through HER1 signaling (Elenius et al., 1997). Using a variety of carcinoma cell lines, it was shown that p53 expression induced by DNA damage subsequently upregulates HBEGF protein, which utilizes both MEK/ERK and PI3K/Akt pathways to inhibit cellular apoptosis (Fang et al., 2001). It also inhibits apoptosis in ovarian cancer, breast cancer, gastric cancer, melanoma, and glioblastoma cells by activating HER1 and downstream ERK (Yotsumoto et al., 2008). Many of the diverse cellular activities regulated by HBEGF transpire in the course of embryonic development as the placental tissues emerge.
EARLY CYCLICAL REGULATION OF HBEGF IN THE ENDOMETRIUM
Rodents
It was first determined that HBEGF plays a central role in the establishment of pregnancy in experiments conducted using mice and rats. HBEGF expression is regulated in the mouse endometrium by leukemia inhibitory factor (LIF) (Song et al., 2000), a cytokine required for implantation (Stewart et al., 1992), as well as by P4 and E2 (Wang et al., 1994). LIF is necessary for the expression of HBEGF, amphiregulin, and epiregulin mRNA in the luminal epithelium of the mouse uterus surrounding a newly implanted blastocyst, as evaluated in LIF−/− mice (Song et al., 2000). In both mice and rats, E2 upregulates HBEGF mRNA and protein levels in the luminal epithelium, whereas P4 and E2 together upregulate its expression in the stroma (Wang et al., 1994; Zhang et al., 1994a,b). This indicates that HBEGF mRNA is transcribed in vivo under conditions that normally stimulate cellular proliferation in the endometrium (Zhang et al., 1994b). It is important to note, however, that this hormonal regulation of HBEGF in the nonpregnant uterus does not necessarily trigger its expression during pregnancy, but may prepare the endometrium to respond to additional signals that induce HBEGF expression in specific cell populations.
The regulation of HBEGF in stromal cells suggests that it has a unique role in preparing the uterus for decidualization, in which stromal cells accumulate lipid and glycogen vacuoles and increase their secretion of a fibrous matrix. Near the implanting blastocyst, there is an increase in vascular permeability and the expression of genes such as Bmp-2, Fgf-2, and Wnt-4 (Farrar and Carson, 1992; Abrahamsohn and Zorn, 1993; Paria et al., 2001). Beads coated with HBEGF can induce local increases in vascular permeability and upregulate expression of Bmp-2, suggesting that HBEGF participates in preparing the uterus to receive an implanting blastocyst (Paria et al., 2001). In cultured mouse stromal cells, HBEGF induces decidualization-like changes, including the upregulation of cyclin D3 and the induction of stromal cell polyploidy (Tan et al., 2004).
Humans and Nonhuman Primates
HBEGF appears to regulate the endometrial cycle in other mammals, including humans and nonhuman primates. In contrast to rodents, in which sex steroids prepare the uterus for eventual HBEGF expression, E2 and P4 stimulate HBEGF expression in human endometrium prior to the appearance of a blastocyst (Lessey et al., 2002), and, in baboons, it is under the control of P4 (Leach et al., 2001). As a result of its regulation by steroids, HBEGF accumulates in a cyclical fashion. During the late proliferative phase until the early secretory phase of the menstrual cycle in humans, HBEGF is predominantly localized in the stromal compartment of the endometrium (Yoo et al., 1997; Leach et al., 1999; Chobotova et al., 2002a). Although transcript is present in the luminal and glandular epithelia, protein levels do not appreciably accumulate at this time (Yoo et al., 1997; Leach et al., 1999). These findings are consistent with evidence from baboons where protein and message levels rise in the stroma during the proliferative phase and decline by day 5 post-ovulation (Leach et al., 2001). The correlation of HBEGF with E2 levels is strengthened by ex vivo evidence demonstrating that HBEGF transcription is induced by E2 in stromal cells. Proliferative phase human stromal cells, when treated with either E2 or P4, upregulate transcription of HBEGF, though if treated with both steroids together, expression will appear in both stroma and the luminal epithelial cells (Lessey et al., 2002). When interpreted in light of the menstrual cycle, these data suggest that the high E2 levels prior to ovulation induce HBEGF expression in stromal cells.
In the early secretory phase, HBEGF expression is induced in the luminal epithelium by the combination of E2 and P4 present at that time. Consistent with this idea, HBEGF expression decreases in the stromal compartment during the early secretory phase, coincident with decreasing E2 levels, but increases in both luminal and glandular epithelial cells (Yoo et al., 1997; Leach et al., 1999). HBEGF mRNA levels peak in the luminal and glandular epithelia during the mid-secretory phase, just prior to the “window of implantation” (Yoo et al., 1997). Protein levels follow shortly thereafter, rising in the early secretory phase and peaking during the window of implantation (Days 19–22) when the uterus is optimally receptive to a blastocyst (Leach et al., 1999). Baboons display a similar pattern in which HBEGF protein levels are highest 5–10 days post-ovulation (Leach et al., 2001). In rhesus monkeys, HBEGF increases during the proliferative phase, peaks during the window of implantation, and declines thereafter (Yue et al., 2000). Its maximal expression during the mid-secretory phase supports the view that HBEGF is a key regulator of blastocyst implantation. Indeed, HBEGF is localized on the apical surface of human luminal epithelial cells and on the surface of pino-podes when it is at maximal levels in the human endometrium (Yoo et al., 1997; Stavreus-Evers et al., 2002).
The decidualization reaction in humans is completely under the control of the sex steroids (Kodaman and Taylor, 2004), as opposed to rodents that have an estrous cycle that depends upon cues from the embryo (Dey et al., 2004). The human menstrual cycle prepares the uterus for implantation prior to arrival of an embryo, beginning in the early secretory phase, and reaching prominence by the window of implantation. In humans, several stimuli that induce stromal cell decidualization appear to do so through upregulation of HBEGF. If stromal cells are treated with 8-Br-cAMP, which is known to artificially induce decidualization, the soluble form of HBEGF is upregulated, as are its receptors, HER1 and HER4 (Chobotova et al., 2005). HBEGF then induces stromal cell production of prolactin and insulin-like growth factor binding protein-1, which naturally induce decidualization (Chobotova et al., 2005). Important for decidualization, HBEGF stimulates stromal cell growth via HER1, though not through HER4 (Chobotova et al., 2002a). The proliferative activity of HBEGF could be the result of its upregulation of IL-11 secretion (Karpovich et al., 2003). When stromal cells are treated with transforming growth factor-β or tumor necrosis factor-α, HBEGF expression is induced, which subsequently protects against apoptosis (Chobotova et al., 2005). Both the transmembrane and soluble forms of HBEGF are induced by these treatments, suggesting that HBEGF could be acting in an autocrine, paracrine or juxtacrine fashion as a survival factor (Chobotova et al., 2005). In addition, tumor necrosis factor-α synergizes with HBEGF to stimulate cell growth by upregulating EGFR expression in stromal cell membranes (Chobotova et al., 2002a). More work is needed to determine which pathways are involved downstream of HBEGF signaling in stromal cells.
Decidualization and cytoprotection are only two of the important roles for HBEGF in the endometrium. HBEGF or conditioned medium from E2- and P4-treated stromal cells upregulates integrin β3, LIF and HOXA10 mRNA in primary human luminal epithelial cells (Liu et al., 2007) and β3 integrin mRNA in Ishikawa cells (Lessey et al., 2002). Each of the corresponding proteins contributes to a uterine luminal epithelial surface that is receptive to an implanting blastocyst. Integrin αvβ3 serves as an attachment factor for osteopontin (secreted phosphoprotein 1, SPP1). SPP1 and αvβ3 are localized to both the glandular and luminal epithelia during the mid- to late-secretory phase, presumably to mediate embryo attachment (Brown et al., 1992; Apparao et al., 2001; von Wolff et al., 2001; Carson et al., 2002; Kao et al., 2002; Nardo et al., 2002). LIF stimulates human embryo development to the blastocyst stage (Sargent et al., 1998). In mice, LIF expression in the uterus is essential for embryo implantation (Stewart et al., 1992), as is uterine expression of HOXA10 (Satokata et al., 1995). HOXA10 message and protein are both found in the human endometrium most abundantly during the mid-secretory phase (Taylor et al., 1998; Gui et al., 1999; Cermik et al., 2001; Li et al., 2002). It has been suggested that HOXA10 induction by P4 during the window of implantation leads to a block in the stromal cell cycle, facilitating decidualization (Qian et al., 2005). Thus, the stimulatory effects of HBEGF upon stromal cells appear to be important not only in decidualization, but also in the preparation of the uterine luminal epithelium for an attaching blastocyst.
THE ROLE OF HBEGF AT IMPLANTATION
Rodents
Expression of HBEGF in the pregnant mouse uterus is necessary for the timely and successful implantation of a blastocyst (Xie et al., 2007). Evidence suggests that HBEGF expression during pregnancy is not induced by sex steroids, as in the nonpregnant uterus (Wang et al., 1994), but by the presence of a blastocyst (Das et al., 1994b). HBEGF mRNA is expressed in the uterine epithelium exclusively at the site of implantation 6–7 hr before attachment occurs, followed by an accumulation of the protein during attachment (Das et al., 1994b). Physiological interactions between the endometrium and blastocyst can be examined by experimentally induced delayed implantation in ovariectomized mice treated with P4 (Yoshinaga and Adams, 1966). HBEGF expression does not occur during delayed implantation until the blastocyst is activated by administration of E2 (Das et al., 1994b), suggesting that the blastocyst induces HBEGF expression in the uterine luminal epithelium. Evidence from the hamster shows that HBEGF mRNA is expressed throughout the apical surface of the luminal epithelium prior to blastocyst hatching and implantation (Mishra and Seshagiri, 2000), but becomes localized to the epithelium surrounding an implanted blastocyst late on day 4 of gestation (Wang et al., 2002).
Several lines of investigation suggest that embryoderived HBEGF is at least one of the signals that direct peri-implantation expression of HBEGF in the uterus. Indeed, mouse and hamster blastocysts express HBEGF (Wang et al., 2002; Hamatani et al., 2004; Liu and Armant, 2004). Further confirmation comes from an experiment using beads coated with HBEGF that were placed in the uterus of pseudopregnant mice mated with vasectomized males (Paria et al., 2001). In this experiment, the HBEGF specifically induced endogenous uterine HBEGF gene expression, along with a local increase in vascular permeability and decidualization, comparable to changes arising from the presence of a blastocyst (Paria et al., 2001). Experiments using HBEGF-coated beads suggest that HBEGF produced by embryos induces the molecular and physiological changes in the uterus. These findings support the hypothesis that cross talk mediated temporally by HBEGF occurs between embryonic and maternal tissues in rodents. Its expression in the early preimplantation blastocyst (Wang et al., 2002; Liu and Armant, 2004) implies that trophectoderm-derived HBEGF could initially induce uterine HBEGF expression, along with other decidualization-related changes. Subsequently, uterine HBEGF secretion could advance trophoblast differentiation to an invasive phenotype and augment implantation. Additional work is required to distinguish the contribution of HBEGF from both of these sources and substantiate this hypothesis.
Evidence that HBEGF can advance the developmental program of the blastocyst is abundant and suggests that reciprocal signaling could exist between the embryo and uterus. Exogenous application of recombinant HBEGF matures rodent blastocysts in vitro, augmenting their ability to hatch from the zona pellucida, and accelerating trophoblast differentiation and adhesion competency (Das et al., 1994b; Wang et al., 2000). HBEGF increases the rate of hamster blastocyst hatching, adhesion competency, and trophoblast outgrowth (Mishra and Seshagiri, 2000; Seshagiri et al., 2002) and augments development of 8-cell rat embryos to the blastocyst stage (Tamada et al., 1999). Intraluminal injection of HBEGF induces implantation of rat embryos in a delayed implantation model (Tamada et al., 1999). Moreover, exposure of mouse embryos to HBEGF during culture before transferring blastocysts to the uterus significantly increases the number of implantation sites that eventually form (Lim et al., 2006).
In mice, HER4 trafficks to the trophectoderm surface late on day 4 and is a prerequisite for HBEGF signaling that accelerates trophoblast development (Wang et al., 2000). HBEGF can then bind HER1 and HER4 (preferentially to HER4) on the surface of mouse blastocysts in an HSPG-dependent fashion, leading to HER autophosphorylation (Das et al., 1994a; Paria et al., 1999; Wang et al., 2000; Lim et al., 2006). Lysophosphatidic acid also accelerates trophoblast outgrowth, and does so by transactivating HER1 and HER4 through Ca2+-dependent HBEGF trafficking and shedding (Liu and Armant, 2004). Cross-talk with other signaling pathways that advance blastocyst differentiation could similarly target HBEGF shedding to transactivate HER kinases. HBEGF signaling accelerates mouse blastocyst differentiation to an adhesive phenotype via Ca2+ influx through N-type voltage-gated channels and activation of protein kinase C and calmodulin (Wang et al., 2000). This signaling pathway induces trafficking of the integrin α5β1 subunit to the surface of trophectoderm cells where it mediates strong adhesion to fibronectin (Wang et al., 2000; Armant, 2005).
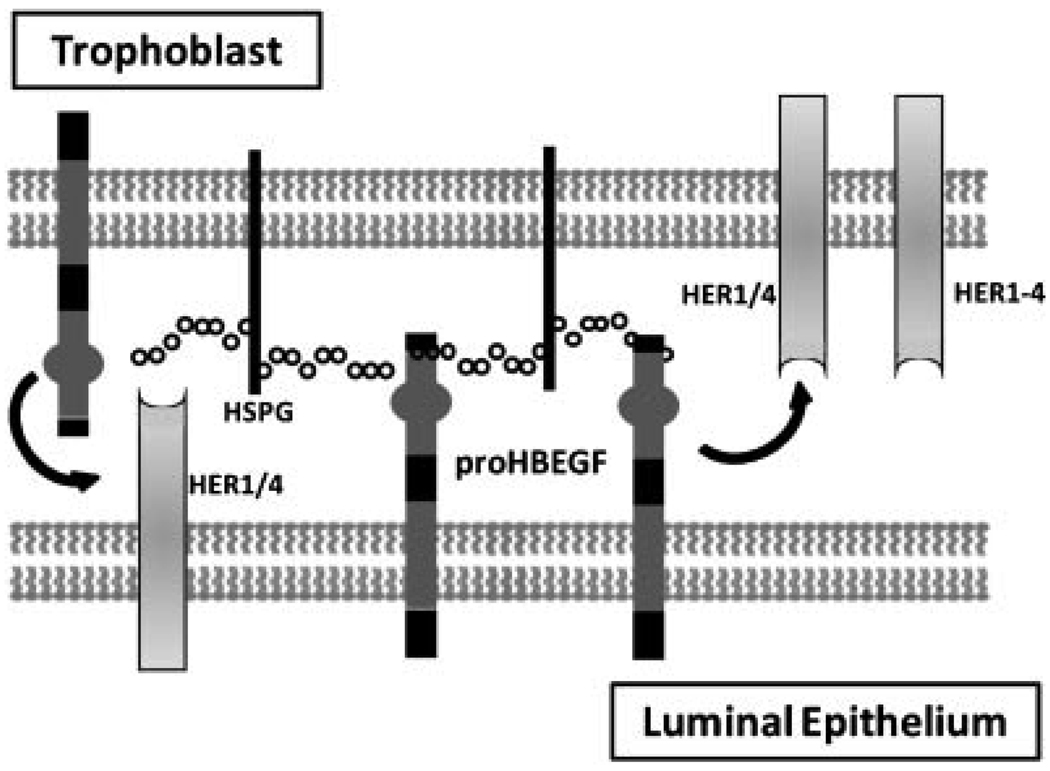
In addition to its ability to advance hatching and trophoblast differentiation, proHBEGF could directly mediate attachment of the trophectoderm to the luminal surface of the endometrium (Fig. 2). Cells engineered to express proHBEGF bind to day 4 mouse blastocysts, but not to delayed blastocysts (Raab et al., 1996), suggesting that proHBEGF, by binding to its receptors, is capable of supporting the attachment reaction during implantation. Binding between these cells and normal day 4 blastocysts is blocked by treating the blastocysts with heparinase or a synthetic peptide corresponding to the HSPG-binding domain of HBEGF (Raab et al., 1996). Furthermore, its potential binding partners HSPG, HER1 and HER4 are downregulated during delayed implantation, and are coordinately upregulated with HBEGF during activation with E2 (Paria et al., 1993, 1999; Smith et al., 1997). These reports provide intriguing information about the elusive mechanisms underlying diapause.
Figure 2.
Juxtacrine Functions of HBEGF. Transmembrane proHBEGF is depicted mediating attachment between the trophoblast cell of a blastocyst and the surface of the uterine luminal epithelium. HBEGF can potentially bind HSPG, HER1, or HER4, to both mediate cell adhesion and elicit juxtacrine signaling downstream of HER kinases.
Humans
In contrast to the rodent endometrium, which may require stimulation from an embryo for HBEGF expression (Paria et al., 2001), HBEGF expression in the human endometrium precedes the appearance of the blastocyst (Leach et al., 1999). As in other species, HBEGF can simultaneously function during human implantation as an attachment factor and a growth factor. In humans, HBEGF, HER1, and HER4 are present in peri-implantation blastocysts with HER4 more prominent in the trophectoderm (Chobotova et al., 2002b). This suggests that HBEGF present on the endometrium might bind its receptors on an apposed blastocyst, and vice versa. Indeed, mature human blastocysts adhere to immobilized proHBEGF, an interaction that is competitively inhibited by addition of sHBEGF (Chobotova et al., 2002b). Similar results were obtained when human blastocysts were applied to a fixed monolayer of CHO cells overexpressing proHBEGF. As a growth factor, HBEGF accelerates development of human embryos to the blastocyst stage and their subsequent hatching from the zona pellucida (Martin et al., 1998; Sargent et al., 1998). While LIF can only induce development of embryos up to the blastocyst stage, HBEGF influences their maturation at least through the hatching stage (Sargent et al., 1998). Although these studies are instructive, it remains to be demonstrated whether these diverse functions of HBEGF are imperative in vivo.
THE ROLE OF HBEGF IN HUMAN PLACENTATION
HBEGF is present throughout the course of human gestation. It is abundant in 1st trimester decidua, and in placental tissue during all three trimesters (Birdsall et al., 1996), though its presence in the syncytiotrophoblast (STB) layer is weak in first trimester placentas (Yoo et al., 1997). HBEGF is also expressed in villous and extravillous cytotrophoblast (CTB) from weeks 14–35 in normal placentas and those delivered preterm (Leach et al., 1999, 2002). Several members of the EGF family and HER tyrosine kinases are expressed in placental trophoblast populations (Hofmann et al., 1992; Tanimura et al., 2004), indicating that the EGF signaling system is active during placental development.
Structure of the Early Placenta
As trophoblasts begin to invade into the endometrium, they differentiate along two paths (Morrish et al., 1998; Hunkapiller and Fisher, 2008). CTBs are highly proliferative, mononuclear cells that can fuse to form multinucleated STB cells. The STB initially invades into the endometrium. At the periphery of the conceptus, the chorionic villi emerge with a vascularized mesenchymal core and a stratified epithelium composed of CTB and an outer layer of STB. The STB transports nutrients, gases, and waste products between the maternal blood and fetal blood in capillaries of the chorionic villi. A few weeks after implantation, anchoring villi appear at the surface of the endometrium (decidua basalis) as CTBs penetrate through the STB layer and assemble into dense cellular columns. The most distal CTBs differentiate to an extravillous phenotype that invades interstitially. Upon reaching uterine blood vessels, the CTBs invade and remodel them, converting the spiral arteries into highly dilated vessels (Pijnenborg et al., 1983). Endovascular trophoblast cells produce proteins characteristic of endothelial cells in a process known as pseudovasculogenesis (Blankenship and Enders, 1997; Zhou et al., 1997a,b). Invasive CTBs remodel the maternal spiral arteries into permanently dilated structures as far as the first third of the myometrium (Pijnenborg et al., 1983). Maternal blood is thus directed to the inter-villous space at increased flow rates (Hunkapiller and Fisher, 2008). As trophoblasts differentiate to extravillous and endovascular phenotypes, they undergo a process known as integrin-switching in which differentiation is accompanied by changes in expression of adhesion molecules (Damsky et al., 1994; Zhou et al., 1997a,b). Different integrins mediate attachment, invasion, and vascular remodeling. For example, α6β4 is expressed by nonmotile villous CTBs, while invasive interstitial trophoblast cells express α1β1. CTBs of the columns in anchoring villi express α5β1 with differing levels of the other two integrins, depending on whether they have a proximal or distal position within the anchoring villi (Damsky et al., 1994). Primary CTB cultures from 1st trimester placentas undergo integrin switching during extravillous differentiation on Matrigel basement membrane, providing a useful experimental model.
Challenges of the Maternal Environment
Blastocyst implantation takes place in a uterine environment low in oxygen (~18 mm Hg or 2%), a condition that appreciably changes (~60 mm Hg or 8%) only after the 10th week of gestation (Rodesch et al., 1992; Burton et al., 1999; Jauniaux et al., 2001; Burton and Jauniaux, 2004). Prior to the 10th week, invading endovascular trophoblasts occlude maternal blood vessels, leaving the intervillous space relatively hypoxic. Human trophoblast cells are programmed to function under these conditions, and, in fact, proliferate at higher rates when cultured at 2% O2 (Genbacev et al., 1996, 1997). Furthermore, localized areas within the placenta are temporarily exposed to oxygenated maternal blood during this early developmental period, but they are able to survive these reoxygenation episodes (Hung and Burton, 2006). Perhaps more profound is the extensive introduction of fully oxygenated maternal blood that occurs around week 10 as trophoblastic plugs become dislodged. The placental unit normally survives this insult and trophoblast invasion continues into the second trimester (Norwitz et al., 2001). Elevation of oxygen leads to free radical production, and evidence suggests that excessive oxidative stress may precipitate placental pathologies such as preeclampsia (Hung and Burton, 2006). Free radicals are normally deactivated in the placenta by xanthine dehydrogenase/xanthine oxidase, which is elevated in placentas during labor, when there is an abundance of local oxygen fluctuations (Many and Roberts, 1997). Clearly, mechanisms exist that protect trophoblasts from erratic oxygen levels.
HBEGF Mediates Trophoblast Motility and Survival
HBEGF stimulates trophoblast invasion from first trimester chorionic villous explants cultured on Matrigel basement membrane, but has little effect on CTB cell proliferation (Leach et al., 2004). As gestation progresses, there is a decrease in trophoblast capacity to differentiate along the extravillous pathway (Librach et al., 1991; Damsky et al., 1994). EGF augments the invasiveness of cultured first trimester CTBs, but with less effectiveness when CTBs are obtained later in gestation (Bass et al., 1994). In HT-H cells (human embryonal carcinoma that spontaneously differentiate into trophoblasts), induction of HER1 phosphorylation by EGF or HBEGF requires derepression of the receptor by trophinin (Sugihara et al., 2007), a transmembrane protein expressed in both human trophoblasts and the uterine epithelium that is capable of mediating cell adhesion through homophilic binding (Suzuki et al., 1999). When ligated, trophinin interaction with the cytoplasmic protein bystin is disrupted, releasing bystin inhibition of HER autophosphorylation (Sugihara et al., 2007). Therefore, HBEGF can activate HER downstream signaling only when opposing trophinin proteins are engaged by opposing cells. The relationship between the trophinin and EGF signaling systems could be particularly important for trophoblast invasive differentiation during blastocyst implantation.
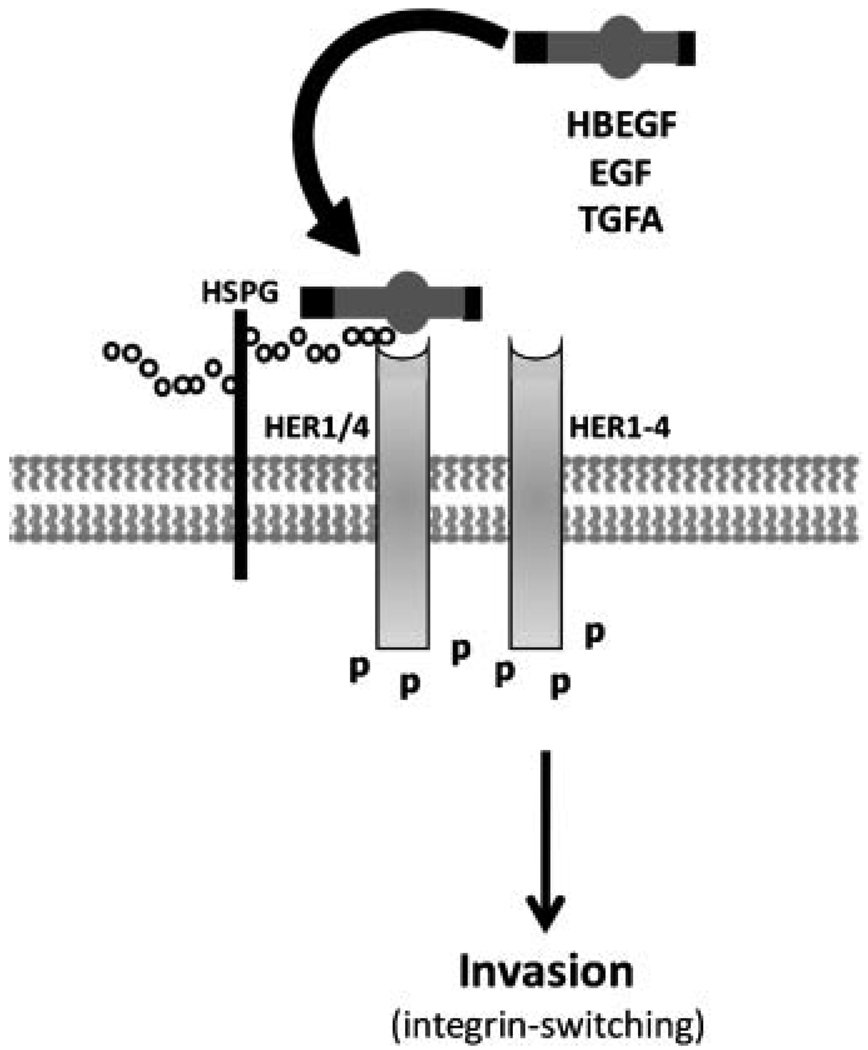
Cell lines generated from immortalized human cytotrophoblasts have proven to be highly useful for experimental investigation of implantation and early placentation. HTR-8/SVneo cells are an immortalized human CTB cell line originating from first trimester villous explants that characteristically secrete chorionic gonadotropin and become invasive in conjunction with appropriate integrin switching and upregulation of HLA-G (Graham et al., 1993; Kilburn et al., 2000). Therefore, HTR-8/SVneo cells closely resemble the primary CTB cells from which they are derived, providing a robust experimental model. HBEGF, EGF, and TGFA each induce integrin-switching in HTR-8/SVneo cells by signaling through HER1 or HER4 to increase the α1 integrin subunit and decrease α6 (Leach et al., 2004). As a result, there is an increase in trophoblast cell migration and invasive activity (Fig. 3). The extensive expression of HBEGF in trophoblasts, particularly within extravillous populations (Leach et al., 2002), could be vital for their invasive activities during the establishment of pregnancy and physiological conversion of spiral arteries. As gestation continues and trophoblasts loose their capacity for extravillous differentiation (Damsky et al., 1994; Librach et al., 1991), the involvement of HBEGF and the EGF signaling system in trophoblast invasion wanes (Bass et al., 1994). However, other roles for HBEGF could come into play late in gestation.
Figure 3.
HBEGF stimulation of trophoblast invasion. HBEGF activates HER kinases to enhance cytotrophoblast (CTB) motility and invasion. Other members of the EGF family, epidermal growth factor (EGF) and transforming growth factor alpha (TGFA) have similar capacities. Little is known about the responsible downstream signaling pathways, but there are associated changes in the expression of integrins α6β4 and α1β1 (integrin switching) by CTBs. Ligation of either HER1 or HER4 can induce integrin switching.
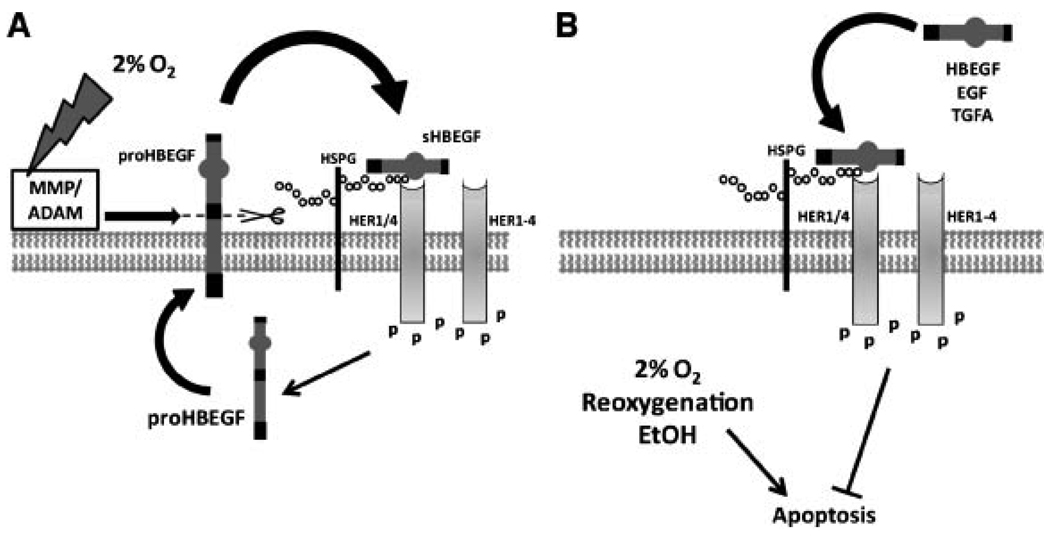
In vivo, placentation initiates in a relatively low oxygen environment. Although CTBs proliferate faster at low O2 concentrations, invasion is more aggressive at higher O2 (Genbacev et al., 1996, 1997). Therefore, pseudovasculogenesis is initially moderate, and rapid CTB growth leads to occlusion of the spiral arteries. It has not been clear how trophoblast tissues survive during this hypoxic phase. We have observed that HBEGF, but not other EGF family members, is upregulated in the HTR-8/SVneo CTB cell line when exposed to2% O2 (Armant et al., 2006). Using specific antagonists of HBEGF signaling, it can be demonstrated that HBEGF inhibits apoptosis due to hypoxia, but has no effect on proliferation rates enhanced by low O2. This study also established that cytoprotection requires metalloproteinase shedding of HBEGF and binding to either HER1 or HER4 (Fig. 4A). Quantification of mRNA and protein levels indicated that HBEGF increases 100-fold through a post-transcriptional mechanism. Antagonizing HBEGF signaling prevents its upregulation, suggesting that low levels of resident proHBEGF are cleaved through activation of metalloproteinases during hypoxia to initiate its synthesis downstream of HER kinases. A recent study suggests that ADAM-17 may be responsible for initiating this signaling event in vivo (Hung et al., 2008). Further studies are needed to determine which metalloproteinases are directly responsible for HBEGF shedding in trophoblast cells. In contrast to 1st trimester CTBs, trophoblasts in villous explants from term placentas do not elevate HBEGF levels in response to low oxygen and their survival is compromised by hypoxia (Imudia et al., 2008). However, addition of HBEGF to term villous explants cultured at 2% O2 inhibits trophoblast apoptosis, demonstrating its persistent role as a survival factor. These findings highlight the importance of HBEGF in the success of early placentation and in protecting trophoblast cells from the damaging effects of hypoxia encountered throughout gestation.
Figure 4.
HBEGF and changing oxygen levels in the placenta. In (A), HBEGF is secreted by first trimester CTBs cultured at low O2. Evidence suggests that low O2 activates metalloproteinases that cleave proHBEGF, releasing sHBEGF to signal through its receptors, HER1 or HER4. Downstream signaling activates a post-transcriptional mechanism to increase HBEGF synthesis. In (B), the cytoprotective activity of HBEGF in CTBs is depicted. Stress due to low O2, reoxygenation injury, or ethanol exposure increases CTB apoptosis. Accumulation of endogenous sHBEGF at low O2 (as in A), or induction of the EGF signaling system by exogenous HBEGF, EGF or TGFA activates signaling downstream of HER kinases that inhibits apoptosis.
Using placental villous explants, Hung et al. demonstrated that reactive oxygen species are generated in an in vitro reoxygenation injury model (Hung et al., 2001), leading to apoptosis of the trophoblasts (Hung et al., 2002). HBEGF can prevent apoptosis induced by reoxygenation injury in HTR-8/SVneo cells (Fig. 4B) by signaling through its receptors, HER1 and HER4 (Leach et al., 2008). Although HBEGF is downregulated within 30 min of elevating the O2 concentration from2% to 20%, accumulated sHBEGF generated by trophoblasts at low oxygen may protect against sudden exposure to oxygenated maternal blood in the uterine environment. Ethanol, a teratogenic substance with oxidative qualities, also induces apoptosis in HTR-8/SVneo cells (Wolff et al., 2007). When HTR-8/SVneo cells are exposed to ethanol and HBEGF together, apoptosis is inhibited in a HER1- or HER4-dependent fashion. During both reoxygenation injury and ethanol exposure, other members of the EGF family are cytoprotective to varying degrees (Wolff et al., 2007; Leach et al., 2008). In contrast, only HBEGF inhibits apoptosis in CTBs cultured at low oxygen (Armant et al., 2006). It has been proposed that insults such as reoxygenation injury are not always tolerated and can lead to placental pathologies (Hung and Burton, 2006). The activity of the EGF signaling system could be a critical variable that keeps oxidative stress in check.
Clinical Considerations
Heparin has long been used to treat pregnant women with certain thrombophilias (Greer, 2003), but its impact on trophoblasts has only recently been investigated. Since heparan sulfate is an obligate cofactor for HBEGF, clinical application of heparin during pregnancy could influence the activity of this growth factor. Indeed, unfractionated heparin inhibits apoptosis induced by diverse signals in 1st trimester cytotrophoblast cells (Hills et al., 2006). The same down-stream signaling pathways activated by heparin were also induced by treating the cells with HBEGF. It could be speculated that addition of its cofactor activates HBEGF in trophoblasts to enhance survival. On the other hand, fractionated heparin, also used as an anticoagulant, suppresses the invasive capacity of primary extravillous trophoblasts (Ganapathy et al., 2007). This suggests that there could be clinical advantages to using unfractionated heparin over fractionated heparin.
A pathological reduction of HBEGF expression is observed in placentas of patients with the hypertensive syndrome preeclampsia (Leach et al., 2002), which is associated with decreased trophoblast invasion (Brosens et al., 1972) and increased levels of trophoblast apoptosis (DiFederico et al., 1999; Allaire et al., 2000). This finding led us to hypothesize that dysregulation of HBEGF and the resulting deficiencies in trophoblast function could contribute to preeclampsia, although it remains to be determined when during the course of gestation HBEGF levels are first reduced in preeclamptic pregnancies. Trophoblast invasion is shallow in preeclampsia possibly due to a lack of HBEGF-induced cell migration and a rise in apoptosis, exacerbated by reduced cytoprotection. The increased oxidative damage to trophoblast cells (Redman and Sargent, 2005) and the paucity of HBEGF in preeclamptic placentas is consistent with the hypothesis that HBEGF performs an important role as a survival factor throughout gestation. Patients delivering preterm without hypertensive disorder produce placentas with normal levels of HBEGF (Leach et al., 2002). However, there was an intermediate reduction of HBEGF in extravillous trophoblasts for those women who delivered small for gestational age infants. Intrauterine growth restriction, like preeclampsia, has been linked to aberrant trophoblast invasion (Khong et al., 1986) and elevated apoptosis (Ishihara et al., 2002), suggesting shared elements in the etiology of both disorders. Premature placental perfusion in preeclamptic pregnancies (Jauniaux et al., 2003) could induce apoptosis by stressing trophoblast cells before HBEGF has sufficiently accumulated during the early hypoxic period. Because there are varying levels of severity of placental reoxygenation, it has been hypothesized that preeclampsia may be the result of sub-lethal levels of reoxygenation, leading to abnormal placentation, whereas more pronounced reoxygenation will terminate the pregnancy (Leach et al., 2008). More work is needed to determine if the etiology of this disease involves a lack of HBEGF production by trophoblasts or the activity of factors that suppress HBEGF signaling.
LOOKING AHEAD
HBEGF performs numerous functions during pregnancy that are conserved across mammalian species with divergent reproductive physiologies. In addition to preparing both the preimplantation embryo and uterus for their mutual interaction, HBEGF appears to directly facilitate the process of implantation. As development proceeds, HBEGF provides a stimulus for trophoblast invasion and serves as a critical survival factor in an environment subject to wide swings in oxygenation. Due to its roles in trophoblast cell differentiation and survival, dysregulation of HBEGF could have profound consequences, from complete pregnancy failure to the onset of obstetrical disorders. The association of its deficiency with preeclampsia is consistent with a critical role for HBEGF in human placentation. New insights into the contribution of HBEGF to placentation could be obtained with the mouse model, which has not been exploited. HBEGF-deficient transgenic embryos develop to term with heart defects (Iwamoto et al., 2003; Jackson et al., 2003), but this system has not been examined in depth for errors in placentation due to the absence of HBEGF. Additionally, the immune system undergoes unique changes during pregnancy that include tolerance of the fetal–maternal allograft and the recruitment of uterine natural killer cells, macrophages and dendritic cells to the implantation site. It is currently thought that these cells ensure proper implantation and that trophoblast cells help to orchestrate their immune function (Mor, 2008). However, the role of HBEGF in the trophoblast–immune relationship during pregnancy remains unexplored.
Tissue-specific differences in HBEGF function could stem from the microenvironment near targeted cells. Expression of its cofactor, HSPG, varies among cells and with differentiation. Other growth factors, such as LIF, can profoundly affect HBEGF expression, as seen in the peri-implantation uterus. HBEGF forms complexes with other transmembrane proteins, including CD9 and integrin α3β1 (Raab and Klagsbrun, 1997), but the relevance of these interactions for implantation and placentation has not been investigated. There is a need for better understanding of the molecular interactions that regulate HBEGF expression, as well as the genes induced by HBEGF in reproductive tissues.
The ability of HBEGF to regulate diverse outcomes is not well understood. Autocrine regulation of HBEGF is post-transcriptional in CTBs cultured at reduced O2 levels (Armant et al., 2006). One possibility is that HBEGF signaling releases its own message from translational repression. Maintenance of a dormant, stable HBEGF mRNA pool could provide a reserve for rapid mobilization during episodes of hypoxia without a high cost in energy. Although the exact nature of this post-transcriptional regulation is not yet understood, it would be useful to know whether HBEGF signaling similarly targets other proteins for synthesis. Finally, an examination of the intracellular circuitry downstream of the HER kinases activated by HBEGF will be important for clarifying the molecular basis of its varied functions.
ACKNOWLEDGMENTS
This study was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, DHHS and NIH Grant U54HD040093.
REFERENCES
- Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266:603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, Lessey BA. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab. 2001;86:4991–5000. doi: 10.1210/jcem.86.10.7906. [DOI] [PubMed] [Google Scholar]
- Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280:260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant DR, Wang J, Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal-embryonic dialogue. Semin Reprod Med. 2000;18:273–287. doi: 10.1055/s-2000-12565. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development. 2006;133:751–759. doi: 10.1242/dev.02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: Evidence that paracrine factors modify this process. Dev Biol. 1994;164:550–561. doi: 10.1006/dbio.1994.1223. [DOI] [PubMed] [Google Scholar]
- Birdsall MA, Hopkisson JF, Grant KE, Barlow DH, Mardon HJ. Expression of heparin-binding epidermal growth factor messenger RNA in the human endometrium. Mol Hum Reprod. 1996;2:31–34. doi: 10.1093/molehr/2.1.31. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Enders AC. Expression of platelet-endothelial cell adhesion molecule-1 (PECAM) by macaque trophoblast cells during invasion of the spiral arteries. Anat Rec. 1997;247:413–419. doi: 10.1002/(SICI)1097-0185(199703)247:3<413::AID-AR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3:1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: The Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Cermik D, Karaca M, Taylor HS. HOXA10 expression is repressed by progesterone in the myometrium: Differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab. 2001;86:3387–3392. doi: 10.1210/jcem.86.7.7675. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002a;87:5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev. 2002b;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Karpovich N, Carver J, Manek S, Gullick WJ, Barlow DH, Mardon HJ. Heparin-binding epidermal growth factor and its receptors mediate decidualization and potentiate survival of human endometrial stromal cells. J Clin Endocrinol Metab. 2005;90:913–919. doi: 10.1210/jc.2004-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- Das SK, Tsukamura H, Paria BC, Andrews GK, Dey SK. Differential expression of epidermal growth factor receptor (EGFR) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology. 1994a;134:971–981. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: A possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994b;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Li G, Liu G, Lee SW, Aaronson SA. p53 induction of heparin-binding EGF-like growth factor counteracts p53 growth suppression through activation of MAPK and PI3K/Akt signaling cascades. EMBO J. 2001;20:1931–1939. doi: 10.1093/emboj/20.8.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JD, Carson DD. Differential temporal and spatial expression of mRNA encoding extracellular matrix components in decidua during the peri-implantation period. Biol Reprod. 1992;46:1095–1108. doi: 10.1095/biolreprod46.6.1095. [DOI] [PubMed] [Google Scholar]
- Ganapathy R, Whitley GS, Cartwright JE, Dash PR, Thilaganathan B. Effect of heparin and fractionated heparin on trophoblast invasion. Hum Reprod. 2007;22:2523–2527. doi: 10.1093/humrep/dem201. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Greer IA. Thrombophilia: Implications for pregnancy outcome. Thromb Res. 2003;109:73–81. doi: 10.1016/s0049-3848(03)00095-1. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–873. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci USA. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills FA, Abrahams VM, Gonzalez-Timon B, Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M, Lam EW, Brosens JJ. Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod. 2006;12:237–243. doi: 10.1093/molehr/gal026. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Drews MR, Scott RT, Jr, Navot D, Heller D, Deligdisch L. Epidermal growth factor and its receptor in human implantation trophoblast: Immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab. 1992;74:981–988. doi: 10.1210/jcem.74.5.1569175. [DOI] [PubMed] [Google Scholar]
- Holbro T, Hynes NE. ErbB receptors: Directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- Hung TH, Burton GJ. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol. 2006;45:189–200. doi: 10.1016/S1028-4559(09)60224-2. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in pre-eclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- Hung TH, Chen SF, Hsieh CC, Hsu JJ, Li MJ, Yeh YL, Hsieh TT. Tumor necrosis factor-alpha converting enzyme in the human placenta throughout gestation. Reprod Sci. 2008;15:195–209. doi: 10.1177/1933719107310709. [DOI] [PubMed] [Google Scholar]
- Hunkapiller NM, Fisher SJ. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. doi: 10.1016/S0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imudia AN, Kilburn BA, Petkova A, Edwin SS, Romero R, Armant DR. Expression of heparin-binding EGF-like growth factor in term chorionic villous explants and its role in trophoblast survival. Placenta. 2008;29:784–789. doi: 10.1016/j.placenta.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Karpovich N, Chobotova K, Carver J, Heath JK, Barlow DH, Mardon HJ. Expression and function of interleukin-11 and its receptor alpha in the human endometrium. Mol Hum Reprod. 2003;9:75–80. doi: 10.1093/molehr/gag012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am. 2004;31:745–766. doi: 10.1016/j.ogc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Armant DR, Brudney A, Das SK, Dey SK, Fazleabas AT. Heparin-binding EGF-like growth factor modulation by antiprogestin and CG in the baboon (Papio anubis) J Clin Endocrinol Metab. 2001;86:4520–4528. doi: 10.1210/jcem.86.9.7835. [DOI] [PubMed] [Google Scholar]
- Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360:1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266:223–237. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Leach RE, Kilburn BA, Petkova A, Romero R, Armant DR. Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol. 2008;198:471.e1–471.e8. doi: 10.1016/j.ajog.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: A potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- Li H, Chen S, Xing F. Expression of HOXA10 gene in human endometrium and its relationship with unexplained infertility. Zhonghua Fu Chan Ke Za Zhi. 2002;37:30–32. [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Dey SK. HB-EGF: A unique mediator of embryouterine interactions during implantation. Exp Cell Res. 2009;315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, Kim MK. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in periimplantation mouse embryos. J Assist Reprod Genet. 2006;23:111–119. doi: 10.1007/s10815-006-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhu G, Zhong G. Regulatory effect of estrogen, progestin and HB-EGF on the expression of HOXA10 gene in Ishikawa cells. J Huazhong Univ Sci Technol Med Sci. 2007;27:464–467. doi: 10.1007/s11596-007-0430-5. [DOI] [PubMed] [Google Scholar]
- Many A, Roberts JM. Increased xanthine oxidase during labour—Implications for oxidative stress. Placenta. 1997;18:725–726. doi: 10.1016/s0143-4004(97)90015-1. [DOI] [PubMed] [Google Scholar]
- Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- Mishra A, Seshagiri PB. Heparin binding-epidermal growth factor improves blastocyst hatching and trophoblast outgrowth in the golden hamster. Reprod Biomed Online. 2000;1:87–95. doi: 10.1016/s1472-6483(10)61945-1. [DOI] [PubMed] [Google Scholar]
- Mor G. Inflammation and pregnancy: The role of toll-like receptors in trophoblast-immune interaction. Ann NY Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39:179–195. doi: 10.1016/s0165-0378(98)00021-7. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Bartoloni G, Di Mercurio S, Nardo F. Expression of alpha(v)beta3 and alpha4beta1 integrins throughout the putative window of implantation in a cohort of healthy fertile women. Acta Obstet Gynecol Scand. 2002;81:753–758. [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1408–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Paria BC, Das SK, Andrews GK, Dey SK. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci USA. 1993;90:55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: A possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Qian K, Chen H, Wei Y, Hu J, Zhu G. Differentiation of endometrial stromal cells in vitro: Down-regulation of suppression of the cell cycle inhibitor p57 by HOXA10? Mol Hum Reprod. 2005;11:245–251. doi: 10.1093/molehr/gah147. [DOI] [PubMed] [Google Scholar]
- Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Sargent IL, Martin KL, Barlow DH. The use of recombinant growth factors to promote human embryo development in serumfree medium. Hum Reprod. 1998;13:239–248. doi: 10.1093/humrep/13.suppl_4.239. [DOI] [PubMed] [Google Scholar]
- Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Seshagiri PB, Mishra A, Ramesh G, Rao RP. Regulation of peri-attachment embryo development in the golden hamster: Role of growth factors. J Reprod Immunol. 2002;53:203–213. doi: 10.1016/s0165-0378(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Smith SE, French MM, Julian J, Paria BC, Dey SK, Carson DD. Expression of heparan sulfate proteoglycan (perlecan) in the mouse blastocyst is regulated during normal and delayed implantation. Dev Biol. 1997;184:38–47. doi: 10.1006/dbio.1997.8521. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Sugiyama D, Byrne J, Wolf DP, Lowitz KP, Kobayashi Y, Kabir-Salmani M, Nadano D, Aoki D, Nozawa S, Nakayama J, Mustelin T, Ruoslahti E, Yamaguchi N, Fukuda MN. Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci USA. 2007;104:3799–3804. doi: 10.1073/pnas.0611516104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Nakayama J, Shih IM, Aoki D, Nozawa S, Fukuda MN. Expression of trophinin, tastin, and bystin by trophoblast and endometrial cells in human placenta. Biol Reprod. 1999;60:621–627. doi: 10.1095/biolreprod60.3.621. [DOI] [PubMed] [Google Scholar]
- Tamada H, Higashiyama C, Takano H, Kawate N, Inaba T, Sawada T. The effects of heparin-binding epidermal growth factor-like growth factor on preimplantation-embryo development and implantation in the rat. Life Sci. 1999;64:1967–1973. doi: 10.1016/s0024-3205(99)00128-9. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura K, Nakago S, Murakoshi H, Takekida S, Moriyama T, Matsuo H, Hashimoto K, Maruo T. Changes in the expression and cytological localization of betacellulin and its receptors (ErbB-1 and ErbB-4) in the trophoblasts in human placenta over the course of pregnancy. Eur J Endocrinol. 2004;151:93–101. doi: 10.1530/eje.0.1510093. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wolff M, Strowitzki T, Becker V, Zepf C, Tabibzadeh S, Thaler CJ. Endometrial osteopontin, a ligand of beta3-integrin, is maximally expressed around the time of the “implantation window”. Fertil Steril. 2001;76:775–781. doi: 10.1016/s0015-0282(01)02015-5. [DOI] [PubMed] [Google Scholar]
- Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC. Dual source and target of heparin-binding EGF-like growth factor during the onset of implantation in the hamster. Development. 2002;129:4125–4134. doi: 10.1242/dev.129.17.4125. [DOI] [PubMed] [Google Scholar]
- Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR. Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod. 2007;77:53–60. doi: 10.1095/biolreprod.106.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: A possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, Sonoda K, Kawarabayashi T, Mekada E, Miyamoto S. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Yue ZP, Yang ZM, Li SJ, Wang HB, Harper MJ. Epidermal growth factor family in rhesus monkey uterus during the menstrual cycle and early pregnancy. Mol Reprod Dev. 2000;55:164–174. doi: 10.1002/(SICI)1098-2795(200002)55:2<164::AID-MRD5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Funk C, Glasser SR, Mulholland J. Progesterone regulation of heparin-binding epidermal growth factor-like growth factor gene expression during sensitization and decidualization in the rat uterus: Effects of the antiprogestin, ZK 98.299. Endocrinology. 1994a;135:1256–1263. doi: 10.1210/endo.135.3.8070371. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Funk C, Roy D, Glasser S, Mulholland J. Heparin-binding epidermal growth factor-like growth factor is differentially regulated by progesterone and estradiol in rat uterine epithelial and stromal cells. Endocrinology. 1994b;134:1089–1094. doi: 10.1210/endo.134.3.8119147. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997a;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997b;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]