Abstract
The interaction between Leishmania and sand flies has been demonstrated in many Old and New World species. Besides the morphological differentiation from procyclic to infective metacyclic promastigotes, the parasite undergoes biochemical transformations in its major surface lipophosphoglycan (LPG). An upregulation of β-glucose residues was previously shown in the LPG repeat units from procyclic to metacyclic phase in Leishmania (Viannia) braziliensis, which has not been reported in any Leishmania species. LPG has been implicated as an adhesion molecule that mediates the interaction with the midgut epithelium of the sand fly in the Subgenus Leishmania. These adaptations were explored for the first time in a species from the Subgenus Viannia, L. (V.) braziliensis with its natural vectors Lutzomyia (Nyssomyia) intermedia and Lutzomyia (Nyssomyia) whitmani. Using two in vitro binding techniques, phosphoglycans (PGs) derived from procyclic and metacyclic parasites were able to bind to the insect midgut and inhibit L. braziliensis attachment. Interestingly, L. braziliensis procyclic parasite attachment was ∼11-fold greater in the midgut of L. whitmani than in L. intermedia. The epidemiological relevance of L. whitmani as a vector of American Cutaneous Leishmaniasis (ACL) in Brazil is discussed.
1. Introduction
Leishmania (Viannia) braziliensis, the etiological agent of American Cutaneous Leishmaniasis (ACL), has the widest geographic distribution in the Americas and can be transmitted by the sand flies Lutzomyia (Psychodopygus) wellcomei, Lutzomyia (P.) complexa, Lutzomyia migonei, Lutzomyia (Nyssomyia) whitmani, Lutzomyia (N.) intermedia [1, 2], and Lutzomyia (N.) neivai [3]. From those, L. (N.) whitmani is considered of the highest epidemiological importance [4].
Leishmania parasites in their sand fly vectors spend their life cycle as flagellated promastigotes within the gut, where they must confront several challenges to survive including the activity of digestive enzymes, the need to escape from the peritrophic matrix, elimination of unattached parasites from the sand fly gut with the digested blood products, and the need to develop infective forms which can be transmitted to the vertebrate host [5–8].
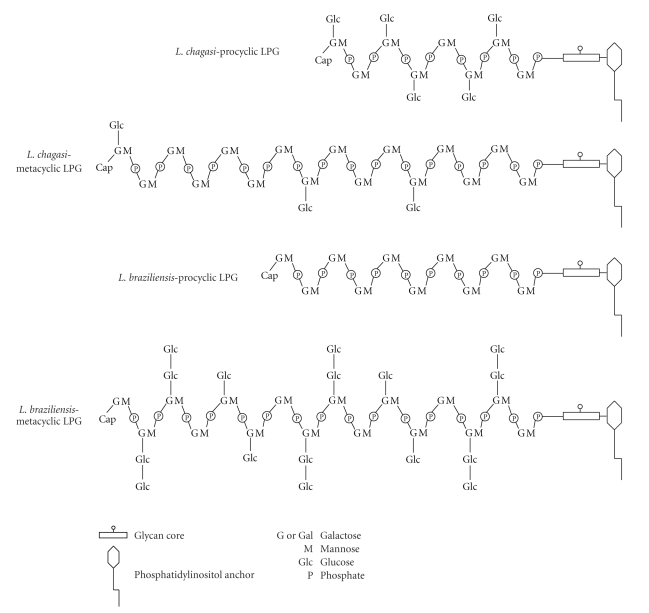
Many studies have demonstrated that the promastigotes' dominant surface lipophosphoglycan (LPG) protects the parasites against those adverse conditions preventing loss of the parasite in the gut for many species of the subgenus Leishmania. LPG has been biochemically characterized and implicated in the Leishmania specificity to different vectors [9, 11–17]. All LPGs have a conserved glycan core region of Gal(α1,6)Gal(α1,3)Galf(β1,3)[Glc(α1)-PO4]Man(α1,3)Man(α1,4)GlcN(α1) linked to a 1-O-alkyl-2-lyso-phosphatidylinositol anchor. LPG intra- and interespecific polymorphisms are in the size and variability of side-chains attached to the repeat unit Gal(β1,4)Man(α1)-PO4 backbone and in the cap [18]. In L. braziliensis LPG, a novel mechanism in the carbohydrate regulation in the LPG side-chains was observed. The LPG from the procyclic form is devoid of side chains, while in the metacyclic phase it contains one or two β(1,3)glucose residues as side chains [10]. In other species such as the Indian strain of L. donovani and L. infantum, both belonging to the subgenus Leishmania, the opposite occurs, resulting in downregulation of β(1,3)glucose residues during metacyclogenesis (Figure 1). The consequence is a loss of attachment by metacyclic LPG and midgut detachment of these Leishmania species [9, 15].
Figure 1.
Opposite mechanisms in the glucose regulation in the LPGs from procyclic and metacyclic L. chagasi (syn. L. infantum) and L. braziliensis [9, 10]. The structure of the glycan core is Gal(α1,6)Gal(α1,3) Galf(α1,3)[Glc(α1-PO4)-6]-Man(α1,3)Man(α1,4)GlcN(α1,6) linked to 1-O-alkyl-2-lyso-phosphatidylinositol anchor. The repeat units are 6-Gal(β1,4)Man(α1)-PO4.
The developmental patterns of Leishmania within the sand fly gut do not depend on the vector but rather on the inherent behavior of the parasite species involved. Those behavior patterns were used to determine the currently accepted classification of Leishmania into the subgenera Viannia and Leishmania for species that show peripylarian and suprapylarian patterns, respectively [19]. Development of Leishmania (Leishmania) spp is restricted to anterior regions of the pylorus, beginning in the thoracic and abdominal midgut [20]. However, Leishmania (Viannia) spp involves an obligatory phase in the posterior gut (mainly in the pylorus) prior to anterior migration and establishment of the parasites in the abdominal and thoracic midgut with following colonization of the foregut and mouth parts [21]. In this manuscript, we report for the first time the interaction of LPG with L. whitmani and L. intermedia midguts, vectors of ACL in Brazil.
2. Materials and Methods
2.1. Parasites
Leishmania braziliensis World Health Organization reference strain (MHOM/BR/75/M2903) was used. Starter cultures of promastigotes were grown in Medium 199 supplemented with 10% heat-inactivated FBS, penicillin (100 units/mL), streptomycin (50 μg/mL), 12.5 mM glutamine, 0.1 M adenine, 0.0005% hemin, and 40 mM Hepes, pH 7.4 at 25°C. Cells were grown at 26°C to a density of 1–1.2 × 107 cells/mL [10].
2.2. Purification of Metacyclic Cells
Parasites from stationary phase were harvested and resuspended in Medium 199 containing peanut agglutinin (PNA) from Arachis hypogaea (35 μg/mL). After 30 minutes incubation at room temperature, procyclic parasites that were agglutinated by the lectin (PNA+) were removed by low-speed centrifugation (150 g, 5 minutes, 4°C). Non-agglutinated metacyclic cells remaining in the supernatant (PNA−) were washed 2 times by centrifugation with phosphate-buffered saline (PBS) (2100 g, 15 minutes, 4°C) [10]. The yield of PNA+ and PNA − parasites was approximately 1.0 × 1011 and 5.0 × 109 cells, respectively.
2.3. Extraction and Purification of LPG from L. braziliensis
LPGs from procyclics (PNA+) and metacyclics (PNA−) parasites were extracted in solvent E (H2O/ethanol/diethyl ether/pyridine/NH4OH; 15:15:5:1:0.017), dried by N2 evaporation, and resuspended in 0.1 N acetic acid/0.1 M NaCl. Then, they were applied to a column of phenyl-Sepharose (2 mL) equilibrated in the same buffer and LPG was eluted using solvent E. For binding studies, purified LPG was treated with PI-specific phospholipase C from Bacillus cereus (16 hours, 37°C). The dilapidated PG was separated from the cleaved lipid anchor by passage through a column of phenyl-Sepharose (2 mL) [22].
2.4. Midgut Binding Studies
Lutzomyia whitmani and L. intermedia sand flies were captured in Corte de Pedra, Bahia state, Brazil (13°26′23′′S, 39°39′3′′W). Taxonomical identification was performed prior to dissecting the midguts using the taxonomic key of Young and Duncan [23]. Binding of promastigotes was quantified by an in vitro technique [12]. Blood-unfed females maintained on 30% sucrose were dissected in PBS. Midguts (6–13 per group) were opened along the length of the abdominal segment with a fine needle, placed in concave wells of a microscope chamber slide, and incubated for 30 minutes with procyclic and metacyclic promastigotes (2 × 107 cells/mL, 50 μl). Then, guts were washed in successive drops of PBS and the number of attached parasites is determined with a Neubauer-counting chamber. In a second experiment, the midguts were incubated for 20 minutes with PGs (10 μg/mL) derived from procyclic and metacyclic promastigotes, washed with PBS, and then incubated with procyclic promastigotes (2.0 × 107 cells/mL) for 20 minutes at room temperature. Controls were dissected guts incubated only with procyclic promastigotes. The guts were then individually washed and counted as described above.
For binding of purified PG to midguts in vitro, opened, dissected midguts were fixed with 2% formaldehyde in PBS (4°C, 20 minutes). After several washes in PBS, the guts were incubated for 20 minutes with PG (10 μg/mL) from procyclic or metacyclic parasites. After several washes, the guts were incubated in a 1:400 dilution of ascites containing the anti-LPG antibody CA7AE followed by incubation with fluorescein antimouse IgG (FITC) (1:1000). SCfluorescence microscope [9].
2.5. Data Analysis
The D'Agostino-Pearson omnibus test was made to test the null hypothesis—that the data are sampled from a Gaussian distribution—P value (P < .01) shows that the data deviate from Gaussian distribution. For this reason, nonparametric Kruskal-Wallis was performed to test equality of population medians among groups and independent samples. Data were analyzed by GraphPad Prism 4.0 software and P < .05 was considered significant.
3. Results and Discussion
Leishmania parasites have to face adverse conditions to accomplish their life cycle either in the invertebrate or in the vertebrate hosts [24]. For that purpose, the parasites developed a range of molecules represented by the secreted acid phosphatases (sAPs), glycoinositolphospholipids (GIPLs), filamentous proteophosphoglycans (fPPGs), lipophosphoglycans (LPGs), and lectins [25–27]. From these molecules, LPG is the most studied molecule and considered a multivirulent factor in Leishmania [28]. During the life cycle of L. braziliensis and other species of the subgenus Viannia, a crucial step for parasite survival is attachment to different parts of midgut. In this article, we focused on the interaction of this species with the midgut epithelium, where the parasites have to attach prior to arrival in the foregut and mouth parts. In the vector midgut, the ingested amastigotes need to transform into promastigotes. After metacyclogenesis, procyclic promastigotes differentiate in metacyclics, the infective forms to be passed to a new vertebrate host [20]. The relationship between stage differentiation and midgut adhesion has been previously reported for L. major, L. infantum, and L. donovani (India and Sudan). In those species, procyclic promastigotes attach to the midgut using the LPG, while metacyclic forms detach. In the case of L. major, the side chains comprising the LPG repeat units from procyclic parasites are often terminated with β(1,3)galactose, while metacyclic LPG side chains are capped with α(1,2)arabinose. In L. donovani from Sudan, procyclic LPG is devoid of side-chains, but metacyclic LPG increases in size, resulting in masking of the cap. The procyclic LPG of L. infantum and L. donovani (India) has β-glucose residues that are downregulated in the metacyclic phase [9, 12, 14, 15].
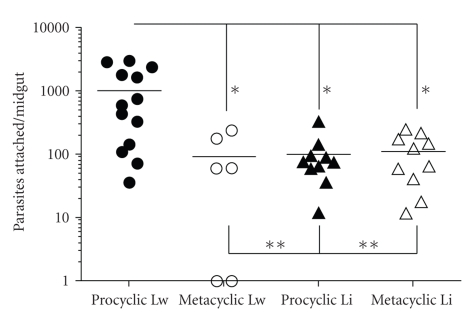
Using a similar quantitative in vitro assay for the attachment of living L. braziliensis promastigotes, dissected midguts from L. intermedia and L. whitmani were analyzed. A differential pattern of attachment was observed in procyclic and metacyclic L. braziliensis. In L. whitmani, the average number of attached procyclics was ~11-fold compared to the metacyclics (1027.71 ± 299.30 versus 90.00 ± 40.25, P = .0014). For L. intermedia, the attachment of procyclics (101.40 ± 16.10) and metacyclics (112.80 ± 27.10) was very low and not statistically different (P = .70) (Figure 2). While comparing procyclic attachment between the two sand fly species, in L. whitmani the number of parasites that attached was ~10-fold higher than in L. intermedia (1027.71 ± 299.30 versus 101.40 ± 16.10, P = .001). Those data suggest that procyclic L. braziliensis were able to attach to the midgut with a mechanism different from hemidesmosomes seen in the pyloric and anterior regions [21], since they were able to easily detach and being counted in our in vitro system.
Figure 2.
Differential attachment of L. braziliensis (procyclic and metacyclic) to L. whitmani (Lw) and L. intermedia (Li) midguts in vitro. *P < .01; **P > .05. Data are the representation of two experiments.
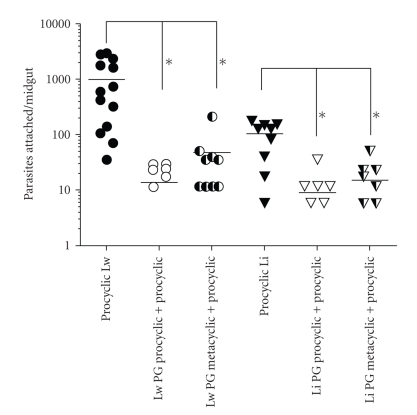
The number of metacyclic promastigotes that attach was very low in both L. whitmani and in L. intermedia (P = .71) (Figure 2) and therefore, only procyclics were used for the subsequent inhibition experiments. To test if the parasite attachment could be intermediate by LPG molecules, a competitive binding experiment was developed, where midguts were previously incubated with PGs derived from procyclic and metacyclic forms. Both PGs strongly blocked (~10-fold) the attachment of procyclic L. braziliensis in L. whitmani and L. intermedia midguts (Figure 3) (P < .0001). Interesting observation arose regarding the unexpected binding of metacyclic PG (Figure 4) to the midguts and its inhibition of parasite attachment (Figure 3). The possible explanation is that the attachment of L. braziliensis metacyclics to the midguts of L. whitmani and L. intermedia was very low in numbers. Furthermore, L. braziliensis has 10–20 less LPG molecules expressed on its cell surface than species from the subgenus Leishmania [10] and thus much less ligands for midgut attachment. These data also suggest the existence of an LPG-ligand in the sand fly midguts, which is necessary for L. braziliensis to establish and maintain the infection with further passage of the parasites towards the mouth parts. A galectin receptor for L. major LPG (subgenus Leishmania) in P. papatasi midgut was recently reported [29].
Figure 3.
Inhibition of attachment of procyclic L. braziliensis to the midgut of L. whitmani (Lw) and L. intermedia (Li) in the presence of phosphoglycans (PGs) (10 μg/mL). Midguts were incubated with PGs from procyclic and metacyclic L. braziliensis and further incubated with procyclic promastigotes. Control midguts were incubated only with procyclic promastigotes. PG procyclic: PG derived from procyclics; PG metacyclic: PG derived from metacyclics. *P < .0001. Data are the representation of two experiments.
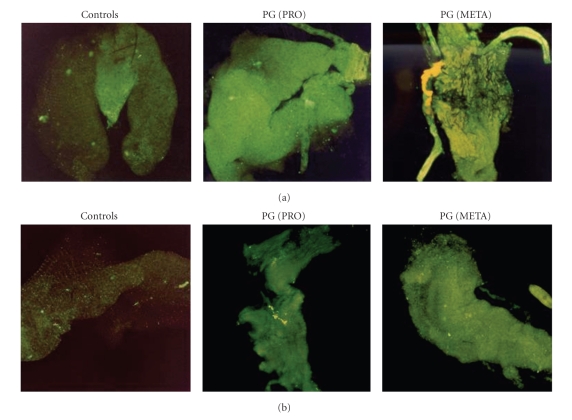
Figure 4.
Fluorescent staining of L. intermedia (a) and L. whitmani (b) midguts incubated with PGs from procyclic (PRO) and metacyclic (META) L. braziliensis, probed with CA7AE antibody (1:400) and developed with FITC (1:1000). Control midguts were incubated with primary and secondary antibodies only.
To confirm if PGs from procyclic and metacyclic promastigotes were recognizing any ligand in the midguts, the molecules were incubated with the dissected organs and revealed by immunocytochemical fluorescent staining with a specific antibody CA7AE, which recognizes PGs from procyclic and metacyclic L. braziliensis [10] (Figure 4). Consistent with our observations using live parasites, a similar pattern of stage-specific bindings was observed, where procyclic and metacyclic PGs were able to attach to opened L. whitmani and L. intermedia midguts (Figure 4(a) and (b)). The attachment of both types of PGs in this model may be attributed to the presence of β(1,3)glucose residues in the procyclic promastigote cap and in the repeat units of metacyclics [10]. The role of β(1,3)glucose residues in attachment was also demonstrated in L. donovani (India) [15] and L. infantum (Brazil) [9]. Those data suggest that upregulation of glucoses is necessary for L. braziliensis development. By adding glucoses in its LPG, perypilariam metacyclics might have to traverse midgut following their early development in the hindgut. Given that the term metacyclic normally refers to the detached infective stage available for transmission in the subgenus Leishmania, in the subgenus Viannia, this term should perhaps be renamed as suggested elsewhere [26]. However, how those parasites would detach from the midgut is still a missing step, which cannot be demonstrated in our in vitro system. A possibility is that a “second metacyclic-like” stage, if any, could detach from midgut prior to migration to foregut.
Although both studied sand flies are known to be successful vectors, the adhesion of the parasites in L. whitmani was more pronounced (~11-fold) than in L. intermedia. Another interesting result is that procyclics were able to attach more than the metacyclics in L. whitmani, while in L. intermedia this attachment was low for both forms (Figure 2). This finding has a major epidemiological relevance, once L. whitmani is known to be the most important and widely distributed ACL vector [2, 4, 30–32] mainly in the Northeast of Brazil. On the other hand, L. intermedia is more concentrated in Southeast, especially in the State of Rio de Janeiro where is the main vector of L. braziliensis [2, 33–36]. However, in Corte de Pedra, State of Bahia, the border state between Northeast and Southeast, where the insects were captured for this study, both species occur sympatrically being able to transmit ACL all over the year [37].
Recently, it was shown that L. longipalpis was more efficient vector of L. infantum than Lutzomyia evansi [38]. Infection success was dependent on the establishment of the parasite in the midgut, which was very irregular in L. evansi. Consequently, those results explain the irregularity in the Visceral Leishmaniasis transmission where L. evansi occurs. To be considered a good vector, many conditions have to be followed: the distribution of the sand fly vector has to be coincident with the human disease; the insect must be found infected in the peridomestic or domestic areas and it has to feed avidly on man and many hosts [39]. However, if differences in Leishmania attachment have an impact on the efficacy of disease transmission by L. whitmani and L. intermedia still awaits further investigation.
4. Conclusions
In the Subgenus Leishmania, there are strong biochemical and genetic evidences that LPG is a critical molecule for the attachment process to sand fly midguts. However, for L. braziliensis, which makes less LPG [10], the role of this molecule seems to be necessary at least during the transient parasite passage through the midgut. The term metacyclics refers to the vertebrate infective parasite stage able to detach from the midgut as it was observed in the species from the subgenus Leishmania. Nonetheless, in the subgenus Viannia, we found two patterns of metacyclic attachment: (1) similar to the Subgenus Leishmania as observed for L. whitmani, a very competent vector and (2) very low as observed in L. intermedia, where LPG seems to have a less important role. We thus conclude that the unusual pattern of attachment of L. braziliensis in the midgut may be a result of its perypilarian behavior related with the vector susceptibility/specificity. Yet to be elucidated is how other glycoconjugates may be critical on the anterior migration of the parasite to the mouth part in the sand fly digestive tract. In the species of the Subgenus Viannia, the pattern of membrane glycoconjugates is different from those of the Subgenus Leishmania, which results in higher expression of GIPLs in L. braziliensis and L. panamensis [40]. However, the current study reinforces our understanding that LPG may still play a key role in the interaction with those vectors.
Acknowledgments
R. P. Soares is a research fellow supported by CNPq (National Council for the Development of Research of Brazil, process no. 305008/2007-2) and Special Programme for Research and Training in Tropical Diseases (TDR ID A50880); C. Margonari is supported by CNPq (processes PDJ 155064/2006-1 and 476381/2007-0), P. F. Pimenta is a research fellow supported by CNPq and PRONEX, and S. J. Turco is supported by the National Institutes of Health Grant AI20941. The authors thank Dr. Ana Paula Madureira for help with statistical analysis.
References
- 1.Lainson R, Shaw JJ. New world leishmaniasis. In: Cox FEG, Wakelin D, Gillespie SH, Despommier DD, editors. Topley & Wilson' s Microbiology & Microbial Infections, Parasitology. 10th edition. London, UK: ASM Press; 2005. pp. 313–349. [Google Scholar]
- 2.Rangel EF, Lainson R. Ecologia das Leishmanioses: transmissores de leishmaniose tegumentar americana. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro, Brazil: FIOCRUZ; 2003. pp. 291–310. [Google Scholar]
- 3.Marcondes CB, Bittencourt IA, Stoco PH, Eger I, Grisard EC, Steindel M. Natural infection of Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae, Phlebotominae) by Leishmania (Viannia) spp. in Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(11):1093–1097. doi: 10.1016/j.trstmh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.da Costa SM, Cechinel M, Bandeira V, Zannuncio JC, Lainson R, Rangel EF. Lutzomyia (Nyssomyia) whitmani s.l . (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae): geographical distribution and the epidemiology of American cutaneous leishmaniasis in Brazil—mini-review. Memórias do Instituto Oswaldo Cruz. 2007;102:149–153. doi: 10.1590/s0074-02762007005000016. [DOI] [PubMed] [Google Scholar]
- 5.Borovsky D, Schlein Y. Trypsin and chymotrypsin-like enzymes of the sandfly Phlebotomus papatasi infected with Leishmania and their possible role in vector competence. Medical and Veterinary Entomology. 1987;1(3):235–242. doi: 10.1111/j.1365-2915.1987.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 6.Schlein Y, Jacobson RL, Shlomai J. Chitinase secreted by Leishmania functions in the sandfly vector. Proceedings of the Royal Society B. 1991;245(1313):121–126. doi: 10.1098/rspb.1991.0097. [DOI] [PubMed] [Google Scholar]
- 7.Schlein Y, Jacobson RL, Messer G. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(20):9944–9948. doi: 10.1073/pnas.89.20.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimenta PFP, Modi GB, Pereira ST, Shahabuddin M, Sacks D. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sandfly midgut. Parasitology. 1997;115:359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- 9.Soares RPP, Macedo ME, Ropert C, et al. Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sandfly vector Lutzomyia longipalpis. Molecular & Biochemical Parasitology. 2002;121:213–224. doi: 10.1016/s0166-6851(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 10.Soares RPP, Cardoso TL, Barron T, Araujo MSS, Pimenta PFP, Turco SJ. Leishmania braziliensis: a novel mechanism in the lipophosphoglycan regulation during metacyclogenesis. International Journal for Parasitology. 2005;35(3):245–253. doi: 10.1016/j.ijpara.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 11.McConville MJ, Turco SJ, Ferguson MAJ, Sacks DL. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO Journal. 1992;11(10):3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta PFP, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- 13.Pimenta PFP, Saraiva EM, Rowton E, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(19):9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks DL, Pimenta PFP, McConville MJ, Schneider P, Turco SJ. Stage-specific binding of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. Journal of Experimental Medicine. 1995;181(2):685–697. doi: 10.1084/jem.181.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry. 1999;38(31):9813–9823. doi: 10.1021/bi990741g. [DOI] [PubMed] [Google Scholar]
- 16.Kamhawi S, Modi GB, Pimenta PFP, Rowton E, Sacks DL. The vectorial competence of Phlebotomus sergenti is specific for Leishmania tropica and is controlled by species-specific, lipophosphoglycan-mediated midgut attachment. Parasitology. 2000;121(1):25–33. doi: 10.1017/s0031182099006125. [DOI] [PubMed] [Google Scholar]
- 17.Soares RPP, Barron T, McCoy-Simandle K, Svobodova M, Warburg A, Turco SJ. Leishmania tropica: intraspecific polymorphisms in lipophosphoglycan correlate with transmission by different Phlebotomus species. Experimental Parasitology. 2004;107(1-2):105–114. doi: 10.1016/j.exppara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annual Review of Microbiology. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 19.Lainson R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The Leishmaniasis in Biology and Medicine. Vol. 1. London, UK: Academic Press; 1987. pp. 1–120. [Google Scholar]
- 20.Walters LL, Modi GB, Chaplin GL, Tesh RB. Ultrastructural development of Leishmania chagasi in its vector, Lutzomyia longipalpis (Diptera: Psychodidae) American Journal of Tropical Medicine and Hygiene. 1989;41:295–317. [PubMed] [Google Scholar]
- 21.Walters LL, Chaplin GL, Modi GB, Tesh RB. Ultrastructural biology of Leishmania (Viannia) panamensis (=Leishmania braziliensis panamensis) in Lutzomyia gomezi (Diptera: Psychodidae): a natural host-parasite association. American Journal of Tropical Medicine and Hygiene. 1989;40:19–39. doi: 10.4269/ajtmh.1989.40.19. [DOI] [PubMed] [Google Scholar]
- 22.Orlandi PA, Jr., Turco SJ. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. Journal of Biological Chemistry. 1987;262(21):10384–10391. [PubMed] [Google Scholar]
- 23.Young DG, Duncan MA. Guide to the identification and geographical distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Memoirs of the American Entomological Institute. 1994;54:1–881. [Google Scholar]
- 24.Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in Leishmaniasis. Annual Review of Microbiology. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- 25.Rogers ME, Ilg T, Nikolaev AV, Ferguson MAJ, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430(6998):463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends in Parasitology. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends in Parasitology. 2007;23(3):91–92. doi: 10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Descoteaux A, Turco SJ. Glycoconjugates in Leishmania infectivity. Biochimica et Biophysica Acta. 1999;1455(2-3):341–352. doi: 10.1016/s0925-4439(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Kamhawi S, Ramalho-Ortigão M, Pham VM, et al. A role for insect galectins in parasite survival. Cell. 2004;119(3):329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Hoch A, Ryan L, Vexanat JA, Cassia A, Rosa AC, Barreto AC. Isolation of Leishmania leishmania braziliensis and other trypanosomatids from Phlebotominae in a mucocutaneous leishmaniasis endemic area, Bahia, Brazil. Memórias do Instituto Oswaldo Cruz. 1986;81(supplement):p. 62. [Google Scholar]
- 31.Azevedo ACR, Rangel EFR, Costa EM, David J, Vasconcelos AW, Lopes UG. Natural infection of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) by Leishmania of the braziliensis complex in Baturité, Ceará state, Northeast Brazil. Memórias do Instituto Oswaldo Cruz. 1990;85:p. 251. doi: 10.1590/s0074-02761990000200021. [DOI] [PubMed] [Google Scholar]
- 32.de Queiroz RG, Vasconcelos IAB, Vasconcelos AW, Pessoa FAC, Dousa RN, David JR. Cutaneous leishmaniases in Ceará state in Northeastern Brazil: incrimination of L. whitmani (Diptera: Psychodidae) as a vector of Leishmania braziliensis in Baturité municipality. American Journal of Tropical Medicine and Hygiene. 1994;50:693–698. doi: 10.4269/ajtmh.1994.50.693. [DOI] [PubMed] [Google Scholar]
- 33.de Brito M, Casanova C, Mascarini LM, Wanderley DM, Corrêa FM. Phlebotominae (Diptera: Psychodidae) in area of transmission of american tegumentar leishmaniasis in the north coast of the State of São Paulo, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2002;35:431–437. [PubMed] [Google Scholar]
- 34.Rangel EF, Souza NA, Wermelinger ED, Barboza AF. Infecção natural de Lutzomyia intermedia (Lutz & Neiva, 1912) em área endêmica de leishmaniose tegumentar do Estado do Rio de Janeiro. Memórias do Instituto Oswaldo Cruz. 1984;79:395–396. doi: 10.1590/s0074-02761984000300020. [DOI] [PubMed] [Google Scholar]
- 35.Rangel EF, Azevedo AC, Andrade CA, Souza NA, Wermelinger ED. Studies on sand fly fauna (Diptera: Psychodidae) in a focus of cutaneous leishmaniasis in Mesquita, Rio de Janeiro state, Brazil. Memórias do Instituto Oswaldo Cruz. 1990;85:39–45. doi: 10.1590/s0074-02761990000100006. [DOI] [PubMed] [Google Scholar]
- 36.Rangel EF, Barbosa AF, Andrade CA, Sousa NA, Wermelinger ED. Development of Leishmania (Viannia) braziliensis Vianna, 1911 in Lutzomyia intermedia (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) under experimental conditions. Memórias do Instituto Oswaldo Cruz. 1992;87:235–238. doi: 10.1590/s0074-02761992000200011. [DOI] [PubMed] [Google Scholar]
- 37.Miranda JC, Reis E, Schriefer A, et al. Frequency of infection of Lutzomyia phlebotomines with Leishmania braziliensis in a Brazilian endemic area as assessed by pinpoint capture and polymerase chain reaction. Memórias do Instituto Oswaldo Cruz. 2002;97(2):185–188. doi: 10.1590/s0074-02762002000200006. [DOI] [PubMed] [Google Scholar]
- 38.Montoya-Lerma J, Cadena H, Oviedo M, et al. Comparative vectorial efficiency of Lutzomyia evansi and Lu. longipalpis for transmitting Leishmania chagasi. Acta Tropica. 2003;85(1):19–29. doi: 10.1016/s0001-706x(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 39.Killick-Kendrick R. The biology and control of Phlebotomine sand flies. Clinics in Dermatology. 1999;17(3):279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 40.Zawadzki J, Scholz C, Currie G, Coombs GH, McConville MJ. The glycoinositolphospholipids from Leishmania panamensis contain unusual glycan and lipid moieties. Journal of Molecular Biology. 1998;282(2):287–299. doi: 10.1006/jmbi.1998.2014. [DOI] [PubMed] [Google Scholar]