Abstract
Objective. Salivary gland secretion is dependent on cholinergic stimulation via autonomic nerves and calcium signalling in acinar cells. Secretory dysfunction associated with SS may be partly caused by the damaging effects of increased glandular concentrations of nitric oxide (NO) derived from up-regulation of inducible NO synthase (iNOS) that accompanies glandular inflammation. The present study examines the effects of increased iNOS expression on salivary gland secretory function.
Methods. The inflammogen lipopolysaccharide (LPS) was introduced intraductally into rat submandibular glands, and glandular responsiveness to cholinergic stimulation was determined.
Results. LPS provoked a rapid, long-lasting inflammation, increasing gland weight (by almost 20%) and inflammatory cell infiltration at 3 and 24 h. Immunoblotting of glandular homogenates indicated that iNOS expression was increased ∼4-fold, and immunohistochemistry of frozen tissue sections showed increased iNOS expression in acinar cells. Salivary secretion from inflamed glands was significantly increased in response to low doses of methacholine and accompanied by increased acinar cell calcium signalling in vitro. Prior administration of the iNOS inhibitors, aminoguanidine or l-NIL [l-N6-(1-iminoethyl)-lysine dihydrochloride] abolished increased secretion and acinar cell calcium signalling.
Conclusions. Up-regulation of glandular iNOS expression can increase cholinergically evoked salivary secretion and appears to offset any secretory hypofunction linked with glandular inflammation. It seems unlikely that increased glandular levels of NO are responsible for the secretory hypofunction that accompanies SS.
Keywords: Sjögren's syndrome, Salivary gland, Secretory hypofunction, Nitric oxide, Inducible nitric oxide synthase, Cholinergic, Rat submandibular, Lipopolysaccharide
Introduction
Fluid secretion from many exocrine glands including salivary, lacrimal and submucosal glands is dependent on signalling from parasympathetic autonomic nerves evoking increases in acinar cell calcium concentration mediated by muscarinic receptors and, to a lesser extent, other non-cholinergic receptors [1]. SS is a chronic autoimmune inflammatory disease characterized by loss of exocrine gland secretory function causing dryness of mucosal surfaces and accompanying glandular tissue destruction [2]. Recent research by different investigators indicates that exocrine gland hypofunction is not necessarily correlated with the degree of secretory tissue destruction and that other non-destructive mechanisms, for example anti-M3 muscarinic receptor antibodies or reduced acetylcholine release from parasympathetic nerves, may play a significant role (see recent review by Dawson et al. [3]). A potentially significant factor in altering salivary secretion in SS is nitric oxide (NO), since nitrite is present at increased concentrations in saliva from SS patients and inducible NO synthase (iNOS) expression is increased in acinar and other cells in labial salivary glands of patients [4]. Prolonged exposure of mice to IL-12 and -18 induced widespread salivary and lacrimal cell apoptosis, which was absent in iNOS knockout mice [5]. NO generated from the constitutive NOS enzymes, neural NOS (nNOS) and endothelial NOS (eNOS), is also an important signalling molecule in mediating salivary secretion, since it modifies acinar cell calcium signalling in response to autonomic stimulation [6, 7].
Given the potential importance of NO in mediating the exocrine hypofunction associated with SS syndromes, we have studied a model in which iNOS is up-regulated in the submandibular (SM) gland. Bacterial lipopolysaccharide (LPS) or endotoxin is frequently used as an inflammogen in studies of cell and tissue function during inflammation [8]. LPS requires the active response of host cells resulting in the production of pro-inflammatory cytokines such as IL-1β and TNF-α and leading to increased expression of iNOS [9]. Previous studies of the effects of LPS on salivary secretion have usually delivered the inflammogen systemically resulting in endotoxaemia [10, 11]. In the present model, LPS was directly instilled into the rat SM gland by retrograde injection into the main excretory duct thus reducing the systemic effects.
Materials and methods
Intraductal injection of LPS
All procedures on rats were performed with approval from the local Animal Ethics and Welfare Committee and under a Home Office project licence according to Home Office regulations (guidance on the operation on animals was from the Scientific Procedures Act 1986).
Young adult male Wistar strain rats (Harlan Labs, Loughborough, UK), weighing 222–345 g, were housed under standard conditions (12 h light/dark cycle at 22–25°C) with a chow pellet diet and water ad libitum. The animals were anaesthetized with ketamine (75 mg/kg) and xylazine (15 mg/kg) i.p. Under anaesthesia, 50 μl of LPS (Escherichia coli serotype O111:B4, 0.5 mg/ml, Sigma-Aldrich Company Ltd, Gillingham, Dorset, UK) in saline were injected in the right SM gland duct from an intra-oral approach, with the help of a dissecting microscope. In three rats, the right SM gland received the same volume of saline only. The contralateral, left gland was left untreated. After 3 or 24 h, saliva collection was performed, as described below.
In three rats, dexamethasone intramuscular (3 mg/kg) was administered 30 min before the intraductal injection of LPS, in order to reach the peak plasma level by the time LPS was given. Then, 1 h and 30 min after the LPS injection (i.e. 2 h after the previous dexamethasone dose), a booster of dexamethasone intramuscular (3 mg/kg) was given, since this drug has an elimination half life of 2.3 h in the rat [12].
Stimulation of salivary secretion
Salivary secretion was stimulated at 3 or 24 h after the ductal infusion of LPS. Rats were anaesthetized with pentobarbitone (50 mg/kg) intraperitoneal A cannula was introduced into the femoral vein and chloralose (80 mg/kg) (Sigma-Aldrich Company Ltd, Gillingham, Dorset, UK) was delivered intravenous to maintain long-term anaesthesia and additional pentobarbitone was given if necessary. The trachea was cannulated providing a clear airway during infusion of methacholine, and body temperature was maintained at 38°C. Saline was given i.p. to maintain fluid levels.
For collection of saliva, the SM ducts were exposed, from an extra-oral, ventral approach, and cannulated proximal to the gland. Salivation was stimulated with methacholine (acetyl-β-methylcholine chloride; Sigma-Aldrich Company Ltd, Gillingham, Dorset, UK) diluted in saline to 48 or 144 μg/ml (0.25 or 0.74 mM). A calibrated syringe pump was adjusted to deliver 4 μg/min/kg (low dose) or 12 μg/min/kg (high dose) as previously described [13]. Saliva was collected from both ducts. In some experiments, the specific iNOS inhibitors l-NIL [l-N6-(1-iminoethyl)-lysine dihydrochloride; Acros Organics, Geel, Belgium] or aminoguanidine (AG; aminoguanidine hydrochloride, 98+%, Sigma) were given i.v. at doses of 10 or 100 mg/kg [14], respectively, to ensure complete inhibition of iNOS enzyme. After 30 min, to allow the iNOS inhibitors to reach maximum plasma concentration, doses of methacholine were given and saliva was collected as above.
Immediately following the final salivary collection period, each SM gland was removed, separated from the sublingual gland, and weighed. The animals were killed with an overdose of pentobarbitone.
After removal, each SM gland was divided into five pieces. For biochemical analyses, tissue pieces were immediately frozen in liquid nitrogen. For morphological–immunohistochemical studies, tissues were placed in optimal cutting temperature embedding medium (Thermo Fischer Scientific, Runcorn, Cheshire, UK), then frozen in a vessel of isopentane cooled in liquid nitrogen. For conventional histochemistry, tissues were immersion-fixed in formol sucrose (4% w/v formaldehyde, 7.5% w/v sucrose and 0.08 M cacodylate buffer, pH 7.2).
Salivary cell calcium imaging in vitro
Twenty-four hours following an infusion of LPS, SM glands were removed from anaesthetized rats, minced, and digested by collagenase (5 mg/20 ml buffer) as previously described [15]. After ∼2 h, cells from collagenase-digested glands were loaded with the calcium-sensitive dye Fluo-4 AM (4 μM; Invitrogen, Paisley, UK) and stimulated with increasing doses of methacholine (10−8 and 10−7 M), then ionomycin (13 μM) and finally manganese chloride (2 mM) to calibrate levels of dye within each cell [16]. Some cell preparations were additionally incubated with the iNOS inhibitor AG (100 mg/ml) for a period of 30 min before addition of Fluo-4 AM. These cells were subsequently stimulated in the absence and then in the presence of AG, which was included in the cell media. The experiments were completed by 3–4 h following removal of tissue from rats. Fluorescence levels were assessed in cells with an acinar morphology using a confocal microscope (TCS SP2; Leica Microsystems, Milton Keynes, UK) and relative fluorescence levels were expressed relative to the maximum and minimum values.
Analysis of protein composition of glandular extracts
SM gland pieces were homogenized in 10 vol of 0.1 M phosphate buffer, pH 6, containing 2 mM ethylenediaminetetraacetic acid (EDTA), 1.6 mM Triton X-100 and a protease inhibitor cocktail (CN Biosciences Ltd, Beeston, UK) using a blade homogenizer (Ultra-Turrax; Janke & Kunkel, Lewes, UK) at a speed of 20 000 r.p.m. for three cycles of 10 s on ice. Aliquots of the homogenate were stored at −20°C, whereas the remaining homogenate was then centrifuged for 5 min at 11 000 g. at 4°C and the supernatant (tissue extract) was removed and stored at −20°C.
SDS–PAGE and western blotting
Proteins in 10 μl of 10% tissue extracts of SM glands were separated by SDS–PAGE under reducing conditions in 4–12% Bis–Tris gels (Invitrogen) according to the manufacturer's instructions. After electrophoresis, proteins separated by SDS–PAGE were transferred onto nitrocellulose membranes (PROTRAN; Schleicher & Schuell UK Ltd, London, UK) that were then stained with FITC (Sigma-Aldrich Company Ltd, Gillingham, Dorset, UK) to confirm the extent of protein transfer. Nitrocellulose membranes were treated with Protoblock (National Diagnostics, Hessle, East Riding, UK) for 45 min in order to block non-specific protein binding sites, followed by incubation with rabbit anti-iNOS polyclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany) at a dilution of 1/1000 in tris-buffered saline (TBS) containing 0.1% Tween 20 (TTBS) for 45 min. After washing with TTBS, blots were incubated with a goat anti-rabbit HRP-conjugated secondary antibody (1/2000, Dako, Ely, UK) for 45 min and positive bands detected using chemiluminescence substrate consisting of a solution of 250 mM luminol, 90 mM coumaric acid and 30% hydrogen peroxide in TBS.
Assay of glandular MPO activity
Following centrifugation, the tissue pellets from glandular homogenates, prepared as above, were resuspended in phosphate buffer containing 13.7 mM hexadecyltrimethylammonium bromide (Sigma) and 10 mM EDTA. The samples were diluted and loaded onto a 96-well microplate and placed in a water bath at 37°C. Hydrogen peroxide, phosphate buffer and TMB (3,3′,5,5′-tetramethyl-benzidine) were added sequentially. The substrate reaction was stopped after 4 min by 2 M sulphuric acid. The absorbance of the sample reaction was read at 450 nm (Microplate reader; Labsystems iEMS Reader MF). Different dilutions of human MPO enzyme of known concentration were used to obtain the standard curve (Sigma). MPO activity was expressed as milliunits per gram tissue. Dapsone (diaminodiphenylsulfone; Sigma), a selective inhibitor of peroxidases, other than MPO, was used to confirm the specificity of the assay [17].
Morphological analysis of salivary glands
Formol sucrose-fixed tissue was processed to paraffin wax embedding and 5-μm sections were cut, mounted on slides and stained with Ehrlich haematoxylin and 1% eosin (H&E).
Frozen tissue sections of 6 μm were fixed with 100% methanol for 10 min and then allowed to dry at room temperature for 30 min. Endogenous peroxidase was blocked with 0.3% H2O2 for 5 min before rehydration in phosphate-buffered saline (PBS) and H2O. Slides were then treated with goat serum (Dako) followed by overnight incubation at 4°C with a 1/50 dilution of primary anti-iNOS rabbit polyclonal antibody IgG (Universal Biologicals Ltd, Papworth St Agnes, UK) diluted in PBS. The slides were then washed twice in PBS and incubated at room temperature for 1 h with the secondary antibody (1/200), HRP-conjugated goat anti-rabbit IgG (Dako). Sections were washed and then developed with diaminobenzidine substrate (Merck Serono Ltd, Feltham, UK). Sections were then stained with Mayer's haematoxylin, dehydrated in a gradient of alcohol, cleared in xylene and covered and viewed under a light microscope. Liver sections were included as positive controls following the method above. Secondary antibody controls were performed using liver and SM gland sections. Separate sections were also incubated with PBS alone.
Assay of total protein in saliva
Total salivary protein was assayed spectrophotometrically by diluting saliva samples in the ratio 1/100 with double distilled water and reading absorbance at 215 nm using a spectrophotometer (Ultrospec 4050; Biochrom Ltd, Cambridge, UK). Double-distilled water was used as a blank and bovine serum albumin (Sigma) was used to construct a standard curve.
Statistical analysis
Data were analysed using paired Student's t-test when comparing contralateral glands from the same group and unpaired Student's t-test for comparisons between glands in the dexamethasone-treated and -untreated series. For comparisons of intracellular calcium levels in cells in vitro, an unpaired t-test was used with the Holm–Bonferonni correction for multiple comparisons. Results are expressed as mean (s.e.m.) and P < 0.05 was considered as statistically significant.
Results
SM gland inflammation
In a preliminary time-course study, salivary gland weight was found to be increased at 1.5, 3, 6 and 24 h but returned to normal at 72 h following introduction of LPS. Larger numbers of rats were studied at 3 h (n = 10) and 24 h (n = 38) following LPS, and mean gland weights were significantly increased at both time points by 17 and 19%, respectively (Fig. 1), whereas the mean weight of the sublingual gland, present in the same connective tissue capsule but not injected with LPS, was unaffected [e.g. at 24 h after LPS treatment controls weighed 0.03 (0.001) g and treated glands 0.04 (0.004) g].
Fig. 1.
Submandibular gland inflammation 3 or 24 h following an intraductal infusion of lipopolysaccharide (LPS). (a) Mean submandibular (SM) gland weight was increased by 17% at 3 h (n = 10) following LPS. At 24 h (n = 38) following LPS the increase in SM gland weight was 19%. (b) Myeloperoxidase (MPO) activity per gram wet weight of submandibular glands at 24 h after LPS treatment (n = 6) was increased compared to contralateral control glands but at 3 h following LPS treatment (n = 7) the increase did not reach statistical significance. Results are expressed as means ± (s.e.m.). Differences between values in paired glands were compared using a Student's paired t-test and P < 0.05 was considered statistically significant. *P = 0.006, ***P < 0.0001 and **P = 0.002 compared to contralateral control glands.
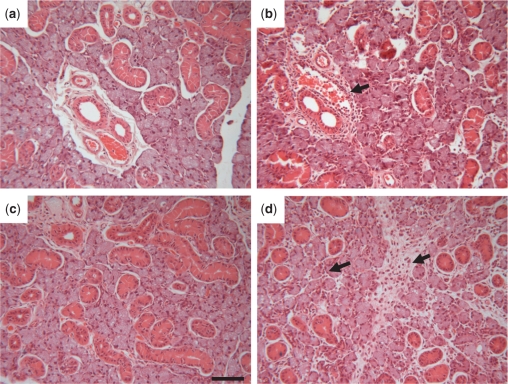
Gland enlargement was accompanied by inflammatory cell infiltration, although the extent of the latter differed at the two time points being an extensive infiltration of parenchymal tissue at 24 h but more restricted to peri-ductal connective tissue at 3 h (Fig. 2). Histological examination of tissue at 72 and 96 h following LPS administration showed that inflammatory cells persisted in periductal connective tissue (data not shown).
Fig. 2.
Submandibular gland morphology following infusion of LPS. (a)–(d) Sections of submandibular (SM) gland stained with Ehrlich Haematoxylin and 1% Eosin. (a) & (b) Sections of contralateral control (a) and treated glands (b) 3h following infusion of LPS. (c) & (d) Sections of control (c) and treated (d) glands 24h following infusion of LPS. Arrows indicate accumulations of inflammatory cells focussed around blood vessels in connective tissue at 3h but distributed more widely in parenchyma at 24h. All glands were examined morphologically and showed similar patterns of inflammatory cell infiltration. Magnification ×125, scale bar = 20 µm.
In experiments where rats received dexamethasone intramuscular 30 min before intraductal infusion of LPS, there was no increase in SM gland weight compared with the contralateral control side at 3 h [0.2 (0.01) g LPS treated; 0.2 (0.016) g control gland, n = 4], nor was there any evidence of inflammatory cell infiltration. MPO activity was used as a quantitative index of gland inflammatory cell infiltration and was elevated in LPS injected glands after 24 h (Fig. 1b). Retrograde ductal injection of the same volume of saline (the vehicle for LPS) did not result in gland enlargement compared with the contralateral gland, nor was there inflammatory cell infiltration or increased MPO activity (data not shown).
Expression of iNOS in SM glands
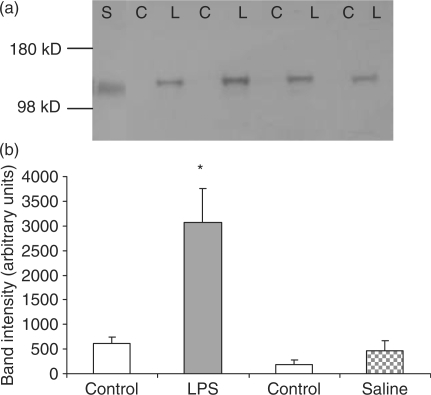
Western blots of SM gland homogenates probed with an antibody to iNOS showed an immunoreactive band of similar mobility (∼130 kDa) to a standard preparation of iNOS from LPS-activated mouse macrophages (Fig. 3). Expression of iNOS was already increased at 3 h after infusion of LPS, compared with the contralateral control gland that showed no or little expression of iNOS (data not shown). Twenty-four hours following LPS infusion, SM gland extracts showed an intense immunoreactive band of iNOS (Fig. 3a). When the primary antibody was absent, no immunostaining of gland extracts or standard iNOS was seen (data not shown). On some occasions, a faint immunoreactive iNOS band was seen in contralateral control homogenates 24 h following LPS infusion. Expression of iNOS in western blots was quantified by digital scanning and measurement of band intensity; 24 h following LPS, iNOS expression was increased by ∼6-fold (Fig. 3b). Samples (n = 2) of LPS-infused and control glands, which received dexamethasone systemically, were also assessed for the expression of iNOS. Under these conditions, there was no immunoreactivity for a band of the molecular weight of iNOS (data not shown). Likewise, there was no expression of iNOS in saline-treated and -untreated control glands (Fig. 3b).
Fig. 3.
Expression of inducible nitric oxide synthase (iNOS) 24 h following LPS treatment of submandibular glands. (a) The figure shows extracts from four sets of paired LPS-treated (L) and contralateral control (C) submandibular glands. An immunoreactive band of the predicted molecular weight for iNOS, (130 kDa) was observed in extracts of LPS treated glands but undetectable in contralateral control glands. The immunoreactive band in gland extracts was compared with standard iNOS (S) from an extract of stimulated mouse macrophages. (b) Band intensities in digital images of two iNOS Western blots were determined and are presented as arbitrary units. iNOS expression in extracts of five LPS treated glands was increased by approximately 6-fold compared to control glands. Saline treated gland extracts (n = 5) show no increase in iNOS expression. *P = 0.002 vs control glands and saline-treated glands. Results are expressed as means ± (s.e.m.). Differences between values in paired glands were compared using a Student's paired t-test and P < 0.05 was considered statistically significant.
In order to localize the expression of iNOS in the SM gland, immunohistochemistry was performed on frozen tissue sections. Strong iNOS immunoreactivity was detected in acini 24 h following LPS treatment (Fig. 4b). Incubation without primary antibody showed no binding of the secondary antibody to the tissue section and there was little or no expression in contralateral control glands (Fig. 4a).
Fig. 4.
Immunohistochemical distribution of inducible nitric oxide synthase (iNOS) 24h following an infusion of LPS. Control submandibular glands (a) showed little expression of iNOS whilst 24h following an infusion of LPS (b) iNOS was widely distributed in acini (arrow) and inflammatory cells (arrow-heads). Such increased iNOS expression was observed in 3 LPS treated glands that were examined. Magnification x125.
SM gland secretion
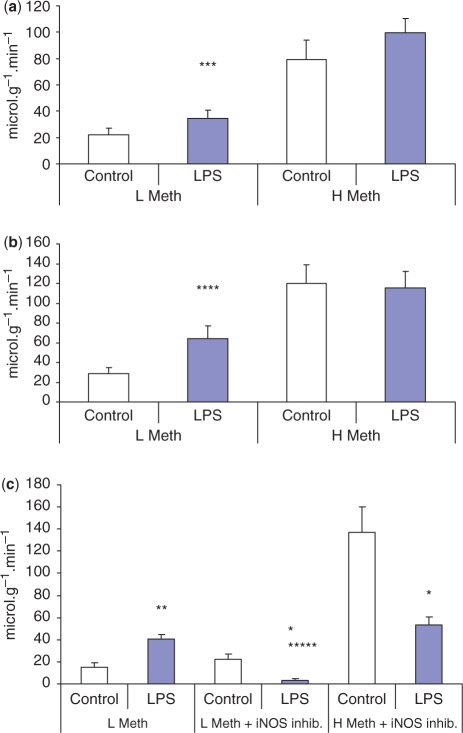
In order to evaluate secretory function in inflamed SM glands, the muscarinic cholinergic agonist methacholine was infused intravenously at two different doses. At the lower dose of methacholine, salivary flow from inflamed glands was 58% greater at 3 h following an infusion of LPS (Fig. 5a). Salivary flow from infused glands at 24 h following LPS gland was increased by 128% compared with contralateral control glands (Fig. 5b). The higher dose of methacholine did not evoke increased mean salivary flow from inflamed glands compared with control glands at 3 or 24 h following LPS (Fig. 5a).
Fig. 5.
Submandibular gland secretion of saliva following intraductal infusion of LPS. (a) At 3h (n = 7) and (b) at 24 h (n = 12) following a single intraductal infusion of LPS, secretion of submandiblar saliva was increased in response to a low dose (4 μg min−1kg−1, i.v.) of methacholine (L Meth). Saliva secretion was similar between LPS-treated and contralateral control glands in response to a high dose (12 μg min−1kg−1, i.v.) of methacholine (H Meth). (c) At 24 h (n = 4) following LPS treatment saliva secretion was again increased in response to a low dose (4 μg min−1kg−1, i.v.) of methacholine (L Meth). Following the specific iNOS inhibitor aminoguanidine (100 mg ml−1, i.v.) there was a greatly reduced secretion response to a low (L Meth) and high doses (H Meth) of methacholine. Results are expressed as means ± (s.e.m.) per gram wet weight of gland. Differences between values in paired glands were compared using a Student's paired t-test and P < 0.05 was considered statistically significant. ***P = 0.003, ****P = 0.001, **P = 0.01 and *P = 0.03 vs control gland at the same dose. *****P = 0.0007 vs LPS treated gland before iNOS inhibitor.
Total protein secretion into saliva showed a similar trend to fluid secretion. The lower dose of methacholine evoked greater total protein secretion from inflamed glands at 3 h compared with control glands [50.5 (6.7) vs 24.2 (4.2) μg/min/g gland wet weight; n = 4; P = 0.009]. At 24 h, protein secretion from inflamed glands remained elevated [56.4 (17.6) vs 25.7 (6.3) μg/min/g gland wet weight; n = 8; P = 0.040]. A higher dose of methacholine evoked no significant difference in protein secretion from inflamed compared with control glands.
In a further series of experiments, the effect of iNOS inhibitors (AG or l-NIL) on salivary secretion was examined. Following intravenous infusion of either inhibitor, salivary flow rate evoked from inflamed glands by a low dose of methacholine was only 10% of the flow rate before infusion of inhibitor. Secretion from contralateral control glands was unaffected by the iNOS inhibitor, and consequently the salivary flow rate from inflamed glands was 84% less than the flow from the control glands. In response to high-dose methacholine, secretion from inflamed glands was ∼38% of the controls following inhibition of iNOS (Fig. 5b).
Calcium signalling in SM cells
In vitro experiments were conducted using clusters of collagenase-digested acini from SM glands, which were then loaded with the calcium-sensitive dye Fluo-4. When stimulated with two doses of methacholine, acinar clusters viewed under the confocal microscope showed typical biphasic responses consisting of an initial peak followed by a plateau of intracellular calcium concentration returning to baseline on cessation of the stimulus. Salivary acinar cells taken from inflamed glands showed higher peaks and plateaux of intracellular calcium concentration in response to methacholine (Fig. 6). In order to express the data quantitatively, maximal attainable calcium-induced fluorescence was determined in response to ionomycin, a calcium ionophore. When the plateaux values for stimulated intracellular calcium obtained from several experiments were combined and expressed as mean values, the differences showed statistical significance (Fig. 6c). Intracellular calcium levels in SM acini were unaffected by an acute application of LPS to acinar clusters in vitro (data not shown).
Fig. 6.

Intracellular calcium signalling in acinar cells from LPS-treated glands following cholinergic stimulation. Typical traces showing intracellular calcium levels (as relative fluorescence) in acinar cells loaded with the calcium sensitive dye Fluo 4-AM and monitored by confocal microscope. Twenty four hours following LPS treatment acinar cells prepared from (a) LPS-treated and (b) -untreated contralateral control glands were stimulated with two doses of 10 and 100 nM methacholine followed by ionomycin (Iono) in order to gain the maximum fluorescence signal. (c) Quantification (mean ± (s.e.m.) of fluorescence intensities in acinar cells prepared from three pairs of submandibular glands. Resting calcium level in these cells was 0.12 ± 0.02. All values are a fraction of maximum fluorescence generated during ionophore incubation (set to 1). Acinar cells from LPS treated glands showed higher intracellular calcium levels in response to methacholine, particularly the lower dose (10 nM). When cells from LPS treated and control glands were pre-incubated with the iNOS inhibitor aminoguanidine (AG) for 30 min prior to stimulation intracellular calcium levels in response to methacholine were similar in cells from LPS treated glands compared to control glands. Prior incubation with AG reduced the calcium response in cells from LPS treated glands. ***Corrected P-value = 0.001 and *corrected P-value = 0.039 compared to same methacholine dose in control cells from an untreated gland. **Corrected P-value = 0.004 compared to same methacholine dose in cells from LPS treated glands without prior incubation with AG.
In a further set of in vitro experiments, intracellular calcium was determined in acinar cells from glands following in vitro incubation with the iNOS inhibitor AG. Whereas methacholine-evoked calcium levels in acinar cells from control glands remained unaffected by prior incubation with AG, fluorescence levels in cells from inflamed glands were no longer statistically greater than AG-treated control cells (Fig. 6d).
Discussion
The present study is relevant to our understanding of the mechanism of secretory hypofunction observed in SS, since we have examined secretory function in an animal model in which there is a sustained increase in iNOS expression accompanying salivary gland inflammation.
Increased iNOS expression and salivary levels of NO-derived nitrite are features of SS [4]. Up-regulation of iNOS expression can have detrimental effects on cell and tissue function, principally because NO reacts with superoxide to produce peroxynitrite, an oxidation product that at high concentrations can alter the function of proteins, nucleic acids and lipids [18]. Thus, it can be hypothesized that up-regulation of iNOS expression may lead to secretory hypofunction in SS [4, 3], possibly by interfering with important protein signalling molecules [18].
LPS infused intraductally into the rat SM gland proved to be a very effective inflammogen, since there was a rapid increase in gland weight at 3 h. Similar results were obtained previously in rat parotid and SM glands at 3 and 6 h following intraductal administration of LPS [19]. It has been shown in mouse SM glands that LPS-induced inflammation is dependent upon expression of TLR-4 [20]. The downstream intracellular signalling pathway following LPS binding to TLR-4 involves activation of the nuclear factor-κB pathway and up-regulation of the expression of inflammatory cytokines, iNOS and cyclooxygenase-2 (COX-2) [21]. We found that there was a sustained up-regulation of iNOS expression in inflamed glands between 3 and 24 h following LPS infusion as indicated by immunocytochemistry and western blots. However, despite the chronic exposure of acinar cells to increased NO derived from up-regulation of iNOS expression, salivary secretion evoked by a low dose of methacholine remained elevated at 24 h as it was at 3 h following LPS infusion. No increased responsiveness was seen in LPS-treated glands treated with the high-dose methacholine, most likely because the high dose produces a supramaximal secretory response that is not to be enhanced by increased levels of NO. Inducible NOS expression was not examined at longer time points following LPS. If expression remained elevated, it may be that there were further changes in salivary secretory responses. An isolated experiment at 96 h following LPS showed similar secretory responses for both treated and contralateral control glands (data not shown).
Hypersecretion from inflamed glands was abolished when rats were given the NOS inhibitors l-NLL or AG. Since both of these inhibitors show a high selectivity for iNOS over nNOS or eNOS [14], the results indicate that NO derived from iNOS plays a key role in mediating glandular hypersecretion. The mechanism leading to hypersecretion may involve acinar cell calcium signalling, since methacholine-evoked intracellular calcium concentrations were greater in cells from inflamed glands but returned to normal values when cells were incubated with iNOS inhibitor. NO can modulate salivary secretion through a pathway involving guanylate cyclase/cyclic guanidine monophosphate and adenosine diphosphate ribosyl cyclase/cyclic adenosine diphosphate ribose [7]. The latter mediator activates ryanodine-sensitive calcium channels leading to release of calcium from rough endoplasmic reticulum [6, 7].
The details of how NO influences salivary secretion vary depending on species, gland and study. However, there is a body of evidence indicating that NO derived from acinar cell nNOS, nNOS in autonomic nerves or endothelial cell eNOS can influence salivary secretion [7, 10, 15, 22–25], since NO is a freely permeable small molecule that rapidly signals over short distances [17]. NO alone produces only modest increases in intracellular calcium as indicated by in vitro studies using NO donors such as SNAP (S-nitroso-N-acetyl-penicillamine) [7]. Likewise, we found that basal levels of intracellular calcium were not elevated in cells from LPS-treated glands although iNOS expression was increased.
Inhibition of iNOS by AG or l-NLL revealed a secretory hypofunction in LPS-treated glands in response to cholinergic stimulation at both doses of methacholine. Such hypofunction may be due to the action of other inflammatory mediators, possibly through inducible COX-2 and prostaglandin signalling as previously suggested [10]. However, the in vitro studies suggest that hypofunction following inhibition of iNOS activity is not due to reduced cholinergically stimulated levels of intracellular calcium.
In conclusion, the results obtained using the present model of LPS-induced inflammation suggest that raised NO derived from increased iNOS expression enhances intracellular calcium signalling and increases fluid secretion.

Acknowledgement
Funding: Project grant funding by the Wellcome Trust. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M, Font J. Primary Sjögren's syndrome: current and emergent aetiopathogenic concepts. Rheumatology. 2005;44:1354–67. doi: 10.1093/rheumatology/keh714. [DOI] [PubMed] [Google Scholar]
- 3.Dawson LJ, Fox PC, Smith PM. Sjögrens syndrome - the non-apoptotic model of glandular hypofunction. Rheumatology. 2006;45:792–8. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 4.Konttinen YT, Platts LAM, Tuominen S, Eklund KK, Santavirta N, Tornwall J, et al. Role of nitric oxide in Sjögren's syndrome. Arthritis Rheum. 1997;40:875–83. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- 5.Kimura-Shimmyo A, Kashiwamura SI, Ueda H, Ikeda T, Kanno S, Akira S, et al. Cytokine-induced injury of the lacrimal and salivary glands. J Immunother. 2002;25:S42–51. doi: 10.1097/00002371-200203001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Harmer AR, Gallacher DV, Smith PM. Role of Ins(1,4,5)P-3, cADP-ribose and nicotinic acid-adenine dinucleotide phosphate in Ca2+ signalling in mouse submandibular acinar cells. Biochem J. 2001;353:555–60. doi: 10.1042/0264-6021:3530555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looms DK, Tritsaris K, Nauntofte B, Dissing S. Nitric oxide and cGMP activate Ca2+-release processes in rat parotid acinar cells. Biochem J. 2001;355:87–95. doi: 10.1042/0264-6021:3550087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosshart H, Heinzelmann M. Targeting bacterial endotoxin - two sides of a coin. Signal Transduct Pathways. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:321S–9S. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 10.Lomniczi A, Mohn C, Faletti A, Franchi A, Mccann SM, Rettori V, et al. Inhibition of salivary secretion by lipopolysaccharide: possible role of prostaglandins. Am J Physiol Endocrinol Metab. 2001;281:E405–11. doi: 10.1152/ajpendo.2001.281.2.E405. [DOI] [PubMed] [Google Scholar]
- 11.Kawabata A, Kuroda R, Morimoto N, Kawao N, Masuko T, Kakehi K, et al. Lipopolysaccharide-induced subsensitivity of protease-activated receptor-2 in the mouse salivary glands in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:281–4. doi: 10.1007/s002100100449. [DOI] [PubMed] [Google Scholar]
- 12.Suitthimeathegorn O, Turton JA, Mizuuchi H, Florence AT. Intramuscular absorption and biodistribution of dexamethasone from non-aqueous emulsions in the rat. Int J Pharm. 2007;331:204–10. doi: 10.1016/j.ijpharm.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Osailan SM, Proctor GB, Carpenter GH, Paterson KL, McGurk M. Recovery of rat submandibular salivary gland function following removal of obstruction: a sialometrical and sialochemical study. Int J Exp Pathol. 2006;87:411–23. doi: 10.1111/j.1365-2613.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proskuryakov SY, Konoplyannikov AG, Skvortsov VG, Mandrugin AA, Fedoseev VM. Structure and activity of NO synthase inhibitors specific to the l-arginine binding site. Biochemistry. 2005;70:8–23. [PubMed] [Google Scholar]
- 15.Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signalling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996;491:647–62. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter GH, Osailan SM, Correia P, Paterson KP, Proctor GB. Rat salivary gland ligation causes reversible secretory hypofunction. Acta Physiol. 2007;189:241–9. doi: 10.1111/j.1365-201X.2006.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas EL, Jefferson MM, Joyner RE, Cook GS, King CC. Leukocyte Myeloperoxidase and Salivary Lactoperoxidase - Identification and Quantitation in Human Mixed Saliva. J Dent Res. 1994;73:544–55. doi: 10.1177/00220345940730021001. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darnell M, Aras HC, Magnusson B, Ekstrom J. Lipopolysaccharide induced-in vivo increases in beta-defensins of the rat parotid gland. Arch Oral Biol. 2006;51:769–74. doi: 10.1016/j.archoralbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Yao CJ, Li XF, Murdiastuti K, Kosugi-Tanaka C, Akamatsu T, Kanamori N, et al. Lipopolysaccharide-induced elevation and secretion of interleukin-1 beta in the submandibular gland of male mice. Immunology. 2005;116:213–22. doi: 10.1111/j.1365-2567.2005.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonizzi G, Karin M. The two NF-kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Takai N, Uchihashi K, Higuchi K, Yoshida Y, Yamaguchi M. Localization of neuronal-constitutive nitric oxide synthase and secretory regulation by nitric oxide in the rat submandibular and sublingual glands. Arch Oral Biol. 1999;44:745–50. doi: 10.1016/s0003-9969(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 23.Sayardoust S, Ekstrom J. Nitric oxide-dependent in vitro secretion of amylase from innervated or chronically denervated parotid glands of the rat in response to isoprenaline and vasoactive intestinal peptide. Exp Physiol. 2003;88:381–7. doi: 10.1113/eph8802543. [DOI] [PubMed] [Google Scholar]
- 24.Sakai T, Michikawa H, Furuyama S, Sugiya H. Methacholine-induced cGMP production is regulated by nitric oxide generation in rabbit submandibular gland cells. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:801–9. doi: 10.1016/s1096-4959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 25.Sugiya H, Mitsui Y, Michikawa H, Fujita-Yoshigaki J, Hara-Yokoyama M, Hashimoto S, et al. Ca2+-regulated nitric oxide generation in rabbit parotid acinar cells. Cell Calcium. 2001;30:107–16. doi: 10.1054/ceca.2001.0218. [DOI] [PubMed] [Google Scholar]