Abstract
Objective. To compare the efficacy and safety of mycophenolate mofetil (MMF) and intravenous cyclophosphamide (IVC) as induction treatment for lupus nephritis (LN), by race, ethnicity and geographical region.
Methods. A total of 370 patients with active Class III–V LN received MMF (target dose 3.0 g/day) or IVC (0.5–1.0 g/m2/month), plus tapered prednisone, for 24 weeks. Renal function, global disease activity, immunological complement (C3 and C4) and anti-dsDNA levels are the outcomes that were assessed in this study.
Results. MMF was not superior to IVC as induction treatment (primary objective). There were important pre-specified interactions between treatment and race (P = 0.047) and treatment and region (P = 0.069) (primary endpoint). MMF and IVC response rates were similar for Asians (53.2 vs 63.9%; P = 0.24) and Whites (56.0 vs 54.2%; P = 0.83), but differed in the combined Other and Black group (60.4 vs 38.5%; P = 0.03). Fewer patients in the Black (40 vs 53.9%; P = 0.39) and Hispanic (38.8 vs 60.9%; P = 0.011) groups responded to IVC. Latin American patients had lower response to IVC (32 vs 60.7%; P = 0.003). Baseline disease characteristics were not predictive of response. The incidence of adverse events (AEs) was similar across groups. Serious AEs were slightly more prevalent among Asians.
Conclusions. MMF and IVC have similar efficacy overall to short-term induction therapy for LN. However, race, ethnicity and geographical region may affect treatment response; more Black and Hispanic patients responded to MMF than IVC. As these factors are inter-related, it is difficult to draw firm conclusions about their importance.
Trial registration. National Institutes of Health, www.clinicaltrials.gov, registration number NCT00377637.
Keywords: Cyclophosphamide, Lupus nephritis, Mycophenolate mofetil, Race, Randomized clinical trial
Introduction
SLE is a genetically complex, highly heterogeneous, autoimmune disease with an incidence, prevalence, disease activity and prognosis that have been shown to differ with race and ethnicity [1–6].
Up to 60% of patients with SLE develop lupus nephritis (LN), a manifestation that is associated with a worse overall prognosis [7, 8]. As with SLE, there may also be racial, ethnic or regional variations in the incidence, prevalence and prognosis of LN. Studies have reported greater risks of LN among African and Hispanic Americans compared with European Americans [9, 10], with further observations that Black and Hispanic patients with LN are more likely to have a worse prognosis and an increased risk of renal disease and mortality compared with other racial/ethnic groups [10–14].
One current debate is whether the treatment of severe LN with intravenous cyclophosphamide (IVC) plus corticosteroids may be less effective in patients of African or Hispanic descent [14–16]. Samples from previous trials have been limited in the extent of geographical or racial diversity and thus have not allowed rigorous comparisons by race/ethnicity and geographical area. The Aspreva Lupus Management Study (ALMS), however, offers a unique opportunity to compare different immunosuppressive treatments among different racial or ethnic groups from distinct geographical locations. ALMS was established to examine the efficacy and safety of mycophenolate mofetil (MMF) compared with IVC when administered with corticosteroids as induction therapy for patients who were from a diverse range of racial and ethnic groups and locations worldwide, with active Class III–V LN [17, 18]. In the prospectively planned primary efficacy analysis, statistically significant interactions between treatment group and race or geographical region were observed, suggesting that race and region may play a role in response to therapy, as has been suggested in smaller studies [14–16]. We, therefore, undertook additional analyses of the efficacy and safety data with respect to race, ethnicity and geographical region.
Methods
Study design
ALMS [protocol WX17801, National Institutes of Health (NIH) registration number NCT00377637; registered at www.clinicaltrials.gov] was a prospective, randomized, open-label, parallel-group, multicentre clinical trial. Detailed methodology of the study has been published elsewhere [17]. The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. The institutional review boards at all participating centres approved the protocol and all patients provided written, informed consent.
Patients
In brief, patients aged 12–75 years, who fulfilled ACR criteria [19], with a diagnosis of SLE and histologically confirmed LN (Classes III–V with active or active/chronic lesions, International Society of Nephrology/Renal Pathology Society classification [20]) were enrolled at 88 hospital clinics in 20 countries in Asia, Australia, Europe, Latin America, USA and Canada between July 2005 and October 2006. The exclusion criteria have previously been reported elsewhere [18].
Patients were categorized according to their self-reported racial (Asian, Black, White and Other) and ethnic (Hispanic or non-Hispanic) groups and according to geographical region (Asia, Latin America, USA/Canada and Rest of World).
Interventions
Patients underwent randomization (1:1, stratified by race and biopsy class) to receive oral MMF twice a day, titrated from 1 g/day in Week 1 and 2 g/day in Week 2 to a target dose of 3 g/day in Week 3, or IVC (monthly pulses of 0.5–1.0 g/m2) according to the modified NIH protocol [21] for a total of 24 weeks (induction phase). If a patient demonstrated consistent intolerance of MMF doses of 3 g/day, but could tolerate 2–2.5 g/day, or if the patient weighed ⩽50 kg, the patient could remain in the study at the dose of 2–2.5 g/day. No therapeutic drug monitoring was performed during the study, although pill counts were monitored at every visit, and blood samples were taken at random during the study for population pharmacokinetic analysis.
A 25% reduction in IVC for patients aged >60 years or a 25% reduction in serum creatinine level >300 µmol/l (3.4 mg/dl) was permitted. Temporary dose stoppage of MMF or IVC was allowed for no more than 7 days in total during the study. All patients received prednisone, using a defined taper from a maximum starting dose of 60 mg/day. Prednisone dose was decreased by 10 mg/day every 2 weeks until a dose of 40 mg/day was reached, and then by a further 5 mg/day every 2 weeks until a dose of 10 mg/day was reached [17]. Dose reduction of <10 mg/day was allowed after 4 weeks of stable response [17]. The protocol was designed to standardize the management of LN across all geographical regions.
Assessments
The primary efficacy parameter was the proportion of patients responding to treatment at the end of the 24-week induction phase of the study. Response was defined as a decrease in urine protein/creatinine ratio (P/Cr), measured over 24 h, to <3 in patients with baseline nephrotic range P/Cr (⩾3 at baseline), or by ⩾50% in patients with sub-nephrotic baseline P/Cr (<3) and stabilization (±25%) or improvement in serum creatinine levels.
Secondary efficacy variables included the BILAG index, the Safety of Exogenous Estrogens in Lupus Erythematosus National Assessment–SLEDAI (SELENA–SLEDAI) scale [22–24], the immunological complement concentration (C3 and C4) and anti-dsDNA autoantibody binding levels. Safety was assessed throughout the study by monitoring adverse events (AEs), vital signs, and clinical laboratory parameters, and performing physical examinations.
Statistical analysis
The primary endpoint analysis was performed on the intent-to-treat population (all randomized patients). In the initial prospectively planned, primary efficacy analysis, interactions between treatment and the covariates of race, disease class (V or other) and region were assessed at the 0.1 level. If the P-value of the interaction term was ⩽0.10, the interaction for that term was explored. Odds ratios (ORs) were calculated using logistic regression models for response, which included a term for treatment group and the covariates defined above.
At randomization, patients were stratified by race into three groups—Asian, White and a combined Black and Other group. The combined Black and Other group was split, and analysis of the effects of the variable Black (self-reported racial group) on response to treatment was performed post hoc using the chi-square test. The additional post hoc analysis of the variable Black and subsequent terminology reported herein differs from that documented previously [18]. To assess the effects of ethnicity (Hispanic vs non-Hispanic), the primary efficacy endpoint data were analysed post hoc using logistic regression models that included the covariates of treatment and disease class.
Baseline disease characteristics and secondary efficacy variables by race and region were summarized post hoc using descriptive statistics. No formal statistical tests were performed. To identify potential risk factors for death, serious AEs (SAEs) and infection, post hoc analyses using logistic regression were performed. Factors included demographic variables and baseline disease characteristics. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC, USA).
Results
Demographics
A total of 370 patients were randomly assigned to treatment with MMF or IVC. Of these, 147 (39.7%) patients reported their race as White, 123 (33.2%) as Asian (mostly in China), 46 (12.4%) as Black and 54 (14.6%) as Other. Of the 54 patients classified as Other, 28 (7.6%; all from Latin America) reported their race as Mexican–Mestizo, 9 (2.4%) as mixed race, 3 (0.8%) as Hispanic and 14 (3.8%) were unclassifiable. Across all racial groups, 131 (35.4%) patients, mostly from Latin America, stated their ethnicity as Hispanic. Demographic and baseline characteristics by race and region are presented in Table 1 and Supplementary Table 1, respectively.
Table 1.
Baseline disease characteristics of patients, by race and treatment group
| Race |
||||||||
|---|---|---|---|---|---|---|---|---|
| Asian |
White |
Other |
Black |
|||||
| Characteristic | MMF (n = 62) | IVC (n = 61) | MMF (n = 75) | IVC (n = 72) | MMF (n = 22) | IVC (n = 32) | MMF (n = 26) | IVC (n = 20) |
| Age at diagnosis of SLE, years | 27.9 (8.64) | 26.9 (9.31) | 27.2 (11.01) | 26.6 (10.58) | 27.2 (9.58) | 25.8 (8.74) | 27.5 (11.92) | 28.6 (8.70) |
| Time since diagnosis of SLE, years | 1.0 (1–23) | 1.0 (1–11) | 3.0 (1–24) | 4.0 (1–27) | 6.0 (1–25) | 3.0 (1–30) | 2.0 (1–38) | 4.0 (1–12) |
| Age at diagnosis of LN, years | 28.8 (8.53) | 27.3 (9.44) | 30.5 (11.55) | 29.4 (10.80) | 32.1 (10.86) | 28.8 (10.69) | 31.3 (14.19) | 30.9 (9.31) |
| Time since diagnosis of LN, years | 1.0 (1–21) | 1.0 (1–11) | 1.0 (1–19) | 1.0 (1–23) | 1.5 (1–15) | 1.0 (1–21) | 1.0 (1–8) | 1.0 (1–12) |
| Renal biopsy class | ||||||||
| Class III | 5 (8.1) | 4 (6.6) | 7 (9.3) | 6 (8.3) | 4 (18.2) | 2 (6.3) | 5 (19.2) | 2 (10.0) |
| Class III/III + V | 7 (11.3) | 9 (14.8) | 12 (16.0) | 10 (13.9) | 7 (31.8) | 3 (9.4) | 6 (23.1) | 4 (20.0) |
| Class IV/IV + V | 47 (75.8) | 45 (73.8) | 53 (70.7) | 53 (73.6) | 12 (54.5) | 21 (65.6) | 12 (46.2) | 9 (45.0) |
| Class IV only | 44 (71.0) | 41 (67.2) | 50 (66.7) | 48 (66.7) | 10 (45.5) | 15 (46.9) | 10 (38.5) | 7 (35.0) |
| Class V only | 8 (12.9) | 7 (11.5) | 10 (13.3) | 9 (12.5) | 3 (13.6) | 8 (25.0) | 8 (30.8) | 7 (35.0) |
| Scarring on renal biopsy | 13 (21.0) | 14 (23.0) | 30 (40.0) | 24 (33.3)a | 10 (45.5) | 10 (31.3) | 13 (50.0) | 8 (40.0) |
| Hypertension | 33 (53.2) | 24 (39.3) | 36 (48.0) | 39 (54.2) | 17 (77.3) | 18 (56.25) | 19 (73.1) | 16 (80.0) |
| Urine P/Cr | 4.7 (5.62)b | 4.1 (2.75)b | 3.7 (3.21)c | 3.8 (3.32)d | 4.3 (3.55) | 5.0 (3.78)e | 3.8 (2.85) | 3.8 (2.85)f |
| Estimated GFR, ml/min/1.73 m2 | 86.4 (42.58) | 103.4 (47.68) | 83.1 (44.82) | 81.7 (38.10) | 66.0 (34.88) | 91.9 (43.05) | 103.8 (44.46) | 97.1 (55.99) |
| GFR <30 mL/min/1.73 m2 | 7.0 (11.3) | 2.0 (3.4) | 9.0 (12.0) | 7.0 (9.7) | 4.0 (18.1) | 2.0 (2.3) | 0 | 1 (5.0) |
| Serum albumin concentration, g/l | 31.6 (6.96)g | 29.3 (6.66) | 30.6 (7.21) | 29.6 (7.13) | 28.7 (5.70) | 27.5 (7.18) | 29.0 (6.54) | 25.15 (6.20)h |
| Patients with negative anti-dsDNA antibody binding level at baseline | 16 (25.8) | 11 (18.0) | 6 (8.3) | 8 (11.1) | 0 (0) | 2 (6.25) | 10 (38.5) | 2 (10.0) |
| Geometric anti-dsDNA antibody binding level | 77.2 (3.64) | 99.5 (5.84) | 109.2 (2.21) | 106.9 (2.52)i | 98.0 (1.98)j | 116.0 (2.28)e | 77.8 (1.66) | 63.2 (1.65)f |
| Patients with low (<90 mg/dl) C3 concentrations at baseline | 47 (75.8) | 48 (78.6) | 54 (72.0) | 51 (70.8) | 13 (59.1) | 25 (78.1) | 11 (42.3) | 15 (75.0) |
| Geometric C3 concentration, mg/dl | 63.8 (1.57)e | 55.9 (1.69) | 67.7 (1.44) | 62.8 (1.59)k | 69.1 (1.58)j | 58.2 (1.58) | 77.81 (1.66) | 63.22 (1.65)f |
| Patients with low (<16 mg/dl) C4 concentrations at baseline | 30 (48.4) | 40 (65.6) | 48 (64.0) | 49 (68.1) | 11 (50.0) | 23 (71.8) | 15 (57.7) | 13 (65.0) |
| Geometric C4 concentration, mg/dl | 13.2 (2.05)e | 10.4 (2.01) | 9.7 (2.20) | 9.6 (2.05)k | 11.9 (2.01)j | 8.3 (2.13)e | 12.86 (2.32) | 10.47 (2.35) |
| SELENA–SLEDAI total score | 14.9 (5.47) | 15.7 (6.28) | 15.3 (6.91) | 16.3 (7.28) | 14.4 (8.72) | 16.1 (7.71) | 13.1 (6.67) | 14.2 (5.83) |
Values are given as mean (s.d.) or median (range) for continuous variables, and n (%) for categorical values. aData missing for one patient; bn = 60; cn = 72; dn = 71; en = 31; fn = 19; gn = 59; hn = 18; in = 68; jn = 21; kn = 69.
Baseline disease characteristics by race and region
At baseline, age at onset of SLE was comparable across racial and treatment groups. Asian patients had the shortest median time since the diagnosis of SLE when assessed by race (Table 1) and region (Supplementary Table 1).
Compared with their counterparts assigned to IVC, patients in the Asian and Other race groups who were assigned to MMF appeared to have worse renal function with respect to mean glomerular filtration rate (GFR) and were also more likely to have an estimated GFR of <30 ml/min/1.73 m2 (Table 1). However, within racial groups, no statistically significant treatment differences in the proportion of patients with estimated GFR <30 ml/min/1.73 m2 were observed (Asian: 95% CI −1.2, 17.0; P = 0.093; White: 95% CI −7.7, 12.3; P = 0.658; Black: 95% CI −14.5, 4.6; P = 0.249; Other: 95% CI −6.4, 30.0; P = 0.170). Similarly, patients receiving MMF had a lower mean GFR, notably those from Asia and, to a lesser extent, those from Latin America (Supplementary Table 1). Asian and Other race groups had slightly higher mean P/Cr ratios than White or Black groups (Table 1). However, the regional groups appeared similar for this parameter (Supplementary Table 1). USA and Canada had the highest percentage of Class V patients with LN, whereas the Rest of World had the lowest percentage of Class V patients (Supplementary Table 1). A slightly higher percentage of patients in the Black group had Class V LN in the MMF and IVC groups compared with those in the other racial groups. A slightly higher percentage of patients in the Other group receiving MMF compared with IVC had combined Class III/III + V disease and renal scarring on biopsy (Table 1). A higher proportion of patients in the Black group (76%) and patients from USA/Canada (76%) and Rest of World (82%) had a medical history of hypertension at baseline compared with other races (46–65%) and regions (46–50%). Similar proportions of patients were taking an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) in the MMF and IVC groups: 73.9% in both groups. Overall, there were no striking differences between racial or regional groups for either treatment group in any other baseline parameters (Table 1 and Supplementary Table 1).
More patients withdrew from the MMF than IVC group in the Asian [19/62 (30.6%) vs 7/61 (11.5%); P = 0.009; 95% CI 5.1, 33.1] and White [11/75 (14.7%) vs 6/72 (8.3%); P = 0.230; 95% CI −3.8, 16.6] groups. In contrast, there were fewer withdrawals from the MMF than IVC group among patients in the Black [3/26 (11.5%) vs 11/20 (55.0%); P = 0.002; 95% CI −68.5, −18.5] and Other race group [2/22 (9.1%) vs 5/32 (15.6%); P = 0.482; 95% CI −23.9, 10.9]. When withdrawal rates were examined by region, more patients in USA/Canada withdrew vs those receiving IVC in other regions. Conversely, a higher number of patients from Asia withdrew from the MMF group compared with those from other regions.
Drug exposure
Exposure to treatment was assessed in the safety population. The median dosage was calculated for 170 patients in the MMF group as 2.6 g/day [18]. The races were well balanced in terms of the average daily dose of MMF received: each group received between 2.4 and 2.8 g/day. In general, most patients [168/184 (91.3%)] tolerated MMF of 2.5–3.0 g/day in all regional groups. The duration of MMF exposure was slightly lower in the Asian group (mean exposure of 146.2 vs 159.2–165.8 days for other racial groups). The median MMF dose was also lower among patients from the Asian group (2.4 g) compared with other groups (2.6–2.8 g). Due to flat dosing in the MMF arm, MMF exposure by body weight was examined. At Weeks 20–24, median daily MMF dose was similar across the racial groups: 0.042 g/kg in White, 0.044 g/kg in Black, 0.049 g/kg in Asian and 0.05 g/kg in Other. At Weeks 20–24, patients in USA/Canada had a lower median daily MMF dose (0.036 g/kg) compared with other regions where median daily dose ranged from 0.043 to 0.051 g/kg. The results of the population pharmacokinetic analysis of patients receiving MMF suggested that race did not influence the pharmacokinetics of this drug.
The median total dosage per infusion of IVC was 0.75 g/m2 [18]. The median total dosage per infusion was slightly higher in the Other and Black racial groups (0.840 and 0.875 g/m2, respectively) compared with the Asian and White groups (0.785 and 0.750 g/m2, respectively) [18]. However, the mean duration and number of doses of IVC were less for patients in the Black group (138 days and 4.83 infusions, respectively) compared with 160.5–167.04 days and 5.65–5.80 infusions for other groups. Only 50.0% of the patients in the Black group received treatment for 24 weeks compared with the entire IVC safety population (71.7%). There were observed differences in exposure by region: patients from USA/Canada who were receiving IVC had fewer infusions compared with patients from other regions.
Efficacy
Primary efficacy endpoint
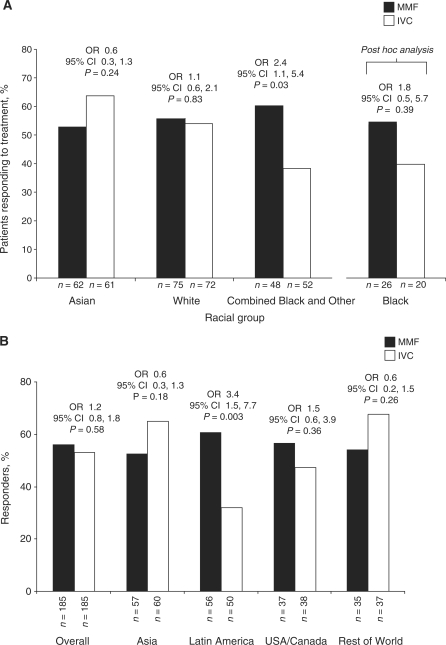
The primary efficacy endpoint was achieved in 104 (56.2%) patients receiving MMF compared with 98 (53.0%) patients receiving IVC (P = 0.58; OR 1.2; 95% CI 0.8, 1.8) [18]. As previously reported, there were important pre-specified interactions between treatment group and race (P = 0.047) and between treatment group and geographical region (P = 0.069) [18]. The number of patients achieving the primary efficacy endpoint was not significantly different between the treatment groups, irrespective of adjustment for covariates. Although there were racial and regional differences in the incidence of LN classes between the groups (Table 1 and Supplementary Table 1), this did not appear to contribute to differences in outcome as Class V patients showed a similar response to non-Class V patients. The pre-specified interaction between treatment group and LN class was not significant (P > 0.10). Use of non-immunosuppressive co-medications and blood pressure control had no impact on response. Response rates with MMF and IVC were similar for Asian (53.2 and 63.9%, respectively; P = 0.24; OR 0.6; 95% CI 0.3, 1.3) and White (56.0 and 54.2%, respectively; P = 0.83; OR 1.1; 95% CI 0.6, 2.1) groups. However, more patients in the combined Other and Black group responded to MMF (60.4 and 38.5%; P = 0.03; OR 2.4; 95% CI 1.1, 5.4) compared with IVC. Among patients in the Black group, 53.9% responded to MMF and 40.0% to IVC (OR 1.75; 95% CI 0.54, 5.70; P = 0.39) (Fig. 1A). Response rates among Hispanic patients also differed: 60.9% for MMF vs 38.8% for IVC (OR 2.5; 95% CI 1.2, 5.1; P = 0.011). Consistent with these findings, patients from Latin America were more likely to have responded to MMF than IVC (60.7 vs 32.0%; P = 0.003; OR 3.4; 95% CI 1.5, 7.7) (Fig. 1B). There was no significant difference in response rates between the treatments in the remaining regions, and rates for MMF were comparable across all regions (Fig. 1B). However, the response rate for IVC was lower in Latin America (32%) than in the other regions (47–68%).
Fig. 1.
Percentage of patients achieving the primary efficacy endpoint, by race and treatment group (A), where Black is a subset of the Other racial group; and percentage of patients achieving the primary efficacy endpoint, by region and treatment group (B) (intent-to-treat populations).
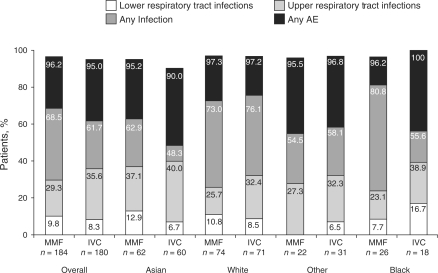
Fig. 2.
Percentage of patients reporting AEs, by race and treatment group. Patients could experience more than one AE. Any infection includes lower and upper respiratory tract plus any other infections. Any AE includes infections plus other AEs. Groups of AEs are not exclusive. Values on the y-axis represent a cumulative percentage; for example, the value for any AE represents the total percentage of patients with lower and upper respiratory tract infection, any other infection plus any other AE.
Asian, Other and Black racial groups had greater improvements with MMF than IVC in analyses of the median change from baseline in urine P/Cr, whereas the opposite was observed for patients in the White group (Table 2). In Asia and Latin America, improvements in P/Cr for MMF were greater than those for IVC (Table 3). However, in USA/Canada, greater improvements were observed for IVC compared with MMF.
Table 2.
Endpoint/changes from baseline to endpoint measurements in patients, by race and treatment group
| Race |
||||||||
|---|---|---|---|---|---|---|---|---|
| Asian |
White |
Other |
Blacka |
|||||
| Characteristic | MMF (n = 62) | IVC (n = 61) | MMF (n = 75) | IVC (n = 72) | MMF (n = 22) | IVC (n = 32) | MMF (n = 26) | IVC (n = 20) |
| Change from baseline in urine P/Cr | −2.8 (4.58)b | −2.0 (2.82) | −1.9 (2.81)c | −2.3 (3.67)d | −2.8 (3.29)e | −1.0 (4.57)f | −2.3 (3.25) | −1.7 (1.61)g |
| Time to 50% reduction in 24-h urine protein, days | 56.0 (55.0, 83.0) | 83.0 (56.0, 111.0) | 84.0 (56.0, 112.0) | 91.0 (84.0, 138.0) | 56.0 (54.0, 84.0) | 112.0 (60.0, 113.0) | 113.0 (56.0, 162.0) | 60.0 (28.0, 111.0) |
| Change from baseline serum albumin concentration, g/l | 7.1 (6.92)h | 9.3 (6.82)i | 6.2 (6.76)j | 8.4 (7.72)c | 7.4 (4.98)e | 6.6 (6.79)k | 6.6 (5.36) | 6.5 (6.01)l |
| Patients with negative anti-dsDNA antibody binding level at endpoint | 27 (47.4) | 18 (31.0) | 23 (33.3) | 13 (19.7) | 7 (35.0) | 3 (10.0) | 11 (42.3) | 4 (23.5) |
| Patients with low (<90 mg/dl) C3 concentrations at endpoint | 25 (40.3) | 34 (55.7) | 31 (41.3) | 30 (41.2) | 8 (36.4) | 16 (50.0) | 6 (23.1) | 10 (50.0) |
| Patients with low (<16 mg/dl) C4 concentrations at endpoint | 9 (14.5) | 23 (37.7) | 29 (38.7) | 23 (31.9) | 4 (18.2) | 17 (53.1) | 5 (19.2) | 9 (45.0) |
| Change from baseline to endpoint in total SLEDAI score | −5.82 (7.87)h | −6.19 (6.93)m | −6.26 (12.45)j | −7.66 (7.58)c | −6.24 (10.44)e | −6.00 (9.89)k | −7.12 (6.92) | −5.33 (9.29)l |
Values are given as median (s.d.) for change in urine P/Cr, median (95% CI) for time to 50% reduction in 24-h urine protein, mean (s.d.) for change in serum albumin concentration and for change in SLEDAI score and n (%) for categorical values. Primary endpoint was the number and percentage of patients showing treatment response at 24 weeks. Response was defined as decrease in proteinuria or a decrease in urine P/Cr ratio by ≥50% in patients with sub-nephrotic proteinuria and stabilization or improvement of serum creatinine level. Secondary endpoints included change from baseline in serum creatinine, urine protein and serum albumin; immunological parameters (C3, C4 anti-dsDNA); and total SELENA–SLEDAI score. aBlack patients analysed separately here, rather than as part of the Other group; bn = 57; cn = 70; dn = 68; en = 21; fn = 30; gn = 17; hn = 60; in = 58; jn = 72; kn = 31; ln = 18; mn = 59.
Table 3.
Endpoint/changes from baseline to endpoint measurements in patients by geographical region and treatment
| Region |
||||||||
|---|---|---|---|---|---|---|---|---|
| Asia |
Latin America |
USA/Canada |
Rest of World |
|||||
| MMF (n = 57) | IVC (n = 60) | MMF (n = 56) | IVC (n = 50) | MMF (n = 37) | IVC (n = 38) | MMF (n = 35) | IVC (n = 37) | |
| Change in urine P/Cr | −2.9 (4.72)a | −2.0 (2.84)b | −2.0 (3.28)a | −1.1 (4.02)c | −1.9 (2.98)d | −2.5 (3.55)e | −2.4 (2.40) | −2.50 (3.23)f |
| Time to 50% reduction in 24-h urine protein, days | 56.0 (55.0, 83.0) | 83.0 (56.0, 111.0) | 84.0 (56.0, 114.0) | 113.0 (83.0, unknown) | 96.5 (56.0, 145.0) | 61.0 (55.0, 88.0) | 83.0 (54.0, 109.0) | 90.0 (79.0, 118.0) |
| Serum albumin change from baseline concentration, g/l | 6.8 (6.66)g | 9.4 (6.88)h | 5.8 (5.96)g | 4.6 (6.14) | 7.0 (7.03)e | 7.3 (6.62)d | 7.6 (6.18) | 12.2 (7.02)i |
| Patients with negative anti-dsDNA antibody binding level at endpoint | 26 (50) | 18 (31.6) | 14 (26.4) | 11 (22.4) | 15 (42.9) | 6 (17.6) | 13 (38.2) | 3 (9.7) |
| Patients with low (<90 mg/dl) C3 concentrations at endpoint | 23 (44.2) | 34 (59.6) | 25 (46.3) | 21 (43.8) | 9 (25.7) | 18 (52.9) | 13 (37.1) | 17 (48.6) |
| Patients with low (<16 mg/dl) C4 concentrations at endpoint | 8 (15.4) | 23 (40.4) | 23 (42.6) | 19 (40.4) | 7 (20.0) | 17 (50.0) | 13 (37.1) | 13 (37.1) |
| Change from baseline to endpoint in total SLEDAI score | −5.84 (7.80)g | −6.14 (6.98)j | −3.85 (12.91)a | −4.48 (7.32) | −7.97 (7.61)i | −9.26 (8.46)d | −8.69 (10.08) | −8.00 (9.20)i |
Values are given as median (s.d.) for change in urine P/Cr, median (95% CI) for time to 50% reduction in 24-h urine protein, mean (s.d.) for change in serum albumin concentration and for change in SLEDAI score and n (%) for categorical values. Primary endpoint was the number and percentage of patients showing treatment response at 24 weeks. Response was defined as decrease in proteinuria or a decrease in urine P/Cr ratio by ≥50% in patients with sub-nephrotic proteinuria and stabilization or improvement of serum creatinine level. Secondary endpoints included change from baseline in serum creatinine, urine protein and serum albumin, immunological parameters (C3, C4 and anti-dsDNA) and total SELENA–SLEDAI score. an = 53; bn = 57; cn = 49; dn = 34; en = 35; fn = 32; gn = 55; hn = 57; in = 36; jn = 58.
There was no difference between the two treatment groups with respect to median time to 50% reduction in proteinuria (86 days for IVC vs 81 days for MMF). In all racial groups, with the exception of the Black group, the median time to a 50% reduction in 24-h urine protein was shorter for MMF than IVC (Table 2), although these findings were not statistically significant. Similarly, the time to a 50% reduction in 24-h urine protein was shorter for MMF than IVC across all regions except USA/Canada (Table 3).
Secondary efficacy endpoints
Mean changes from baseline to endpoint in the BILAG subscales and total SLEDAI score (Table 2) did not differ between treatment groups or between racial groups. However, when comparing regions, patients from USA/Canada and the Rest of World showed greater improvements in total SLEDAI score compared with other regions, regardless of treatment group (Table 3).
Additional analyses
There appeared to be no significant differences in the change between the groups in C3 and C4 or anti-dsDNA levels at endpoint compared with baseline in racial (Table 2) and regional (Table 3) groups. Any differences observed were also apparent at baseline; therefore, any change in a measured parameter was relative to the pattern observed at baseline and deemed to be of no clinical relevance. In addition, duration of disease, baseline anti-dsDNA antibody levels and mean BMI did not appear to influence response to treatment.
Safety
Overall, the incidence of AEs was comparable between the two treatment groups; the majority of patients in all racial groups reported at least one AE (Table 4).
Table 4.
Most commonly reported treatment-emergent AEs, by race and treatment group (safety population)
| Race |
||||||||
|---|---|---|---|---|---|---|---|---|
| Asian |
White |
Other |
Black |
|||||
| Patients who experienced at least one AE | MMF (n = 62) | IVC (n = 60) | MMF (n = 74) | IVC (n = 71) | MMF (n = 22) | IVC (n = 31) | MMF (n = 26) | IVC (n = 18) |
| All AEs | 59 (95.2) | 54 (90.0) | 72 (97.3) | 69 (97.2) | 46 (95.8) | 48 (98.0) | 25 (96.2) | 18 (100) |
| Diarrhoea | 10 (16.1) | 6 (10) | 27 (36.5) | 9 (12.7) | 6 (27.3) | 5 (16.1) | 9 (34.6) | 3 (16.7) |
| Nausea and vomiting | 13 (21.0) | 32 (53.3) | 20 (27.0) | 40 (56.3) | 3 (13.6) | 17 (54.8) | 6 (23.1) | 9 (50.0) |
| Headache | 6 (9.7) | 3 (5.0) | 18 (24.3) | 24 (33.8) | 1 (4.5) | 14 (45.2) | 6 (23.1) | 9 (50.0) |
| Joint-related signs and symptoms (arthralgia) | 6 (9.7) | 8 (13.3) | 18 (24.3) | 24 (33.8) | 0 (0) | 8 (25.8) | 7 (26.9) | 3 (16.7) |
| Gastrointestinal and abdominal pain | 5 (8.1) | 4 (6.7) | 19 (25.7) | 11 (15.5) | 4 (18.2) | 9 (29.0) | 6 (23.1) | 6 (33.3) |
| Musculoskeletal and connective tissue signs and symptoms (back pain) | 4 (6.5) | 3 (5.0) | 15 (20.3) | 14 (19.7) | 4 (18.2) | 6 (19.4) | 8 (30.8) | 5 (27.8) |
| Oedema | 13 (21.0) | 12 (20.0) | 18 (24.3) | 22 (31.0) | 4 (18.2) | 10 (32.3) | 7 (26.9) | 7 (38.9) |
| Upper respiratory tract infections | 23 (37.1) | 24 (40) | 3 (4.1) | 10 (14.1) | 6 (27.3) | 10 (32.3) | 6 (23.1) | 7 (38.9) |
| Alopecia | 7 (11.3) | 17 (28.3) | 10 (13.5) | 31 (43.7) | 4 (18.2) | 9 (29.0) | 2 (7.7) | 4 (22.2) |
| Coughing | 12 (19.4) | 4 (6.7) | 10 (13.5) | 10 (14.1) | 4 (18.2) | 6 (19.4) | 6 (23.1) | 6 (33.3) |
| Hypertension | 6 (9.7) | 1 (1.7) | 11 (14.9) | 12 (16.9) | 5 (22.7) | 9 (29.0) | 4 (15.4) | 3 (16.7) |
| Anaemia | 19 (16.1) | 0 (0) | 6 (8.1) | 4 (5.6) | 3 (13.6) | 8 (25.8) | 4 (15.4) | 1 (5.6) |
| Rashes, eruptions and exanthema | 6 (9.7) | 5 (8.3) | 8 (10.8) | 12 (16.9) | 0 (0) | 3 (9.7) | 5 (19.2) | 6 (33.3) |
| Asthenic conditions (fatigue) | 7 (11.3) | 4 (6.7) | 13 (17.6) | 18 (25.4) | 0 (0) | 4 (12.9) | 6 (23.1) | 5 (27.8) |
| Febrile disorders | 5 (8.1) | 15 (25.0) | 7 (9.5) | 12 (16.9) | 0 (0) | 2 (6.5) | 0 (0) | 1 (5.6) |
| Muscle-related signs and symptoms (muscle spasm) | 3 (4.8) | 1 (1.7) | 9 (12.2) | 10 (14.1) | 1 (4.5) | 5 (16.1) | 6 (23.1) | 3 (16.7) |
| Urinary tract infections | 0 (0) | 0 (0) | 10 (13.5) | 7 (9.9) | 2 (9.1) | 5 (16.1) | 4 (15.4) | 2 (11.1) |
| Leucopenias | 5 (8.1) | 8 (13.3) | 2 (2.7) | 17 (23.9) | 4 (18.2) | 12 (38.7) | 1 (3.8) | 5 (27.8) |
| Potassium imbalance (hypokalaemia) | 0 (0) | 0 (0) | 2 (2.7) | 2 (2.8) | 2 (9.1) | 3 (9.7) | 6 (23.1) | 1 (5.6) |
| Menstruation with decreased bleeding (amenorrhoea) | 0 (0) | 1 (1.7) | 0 (0) | 2 (2.8) | 0 (0) | 5 (16.1) | 1 (3.8) | 2 (11.1) |
| All SAEs | 21 (33.9) | 17 (28.3) | 18 (24.3) | 16 (22.5) | 5 (22.7) | 5 (16.1) | 7 (26.9) | 3 (16.7) |
| All infections | 39 (62.9) | 29 (48.3) | 54 (73.0) | 54 (76.1) | 33 (68.8) | 28 (57.1) | 21 (80.8) | 10 (55.6) |
Values are given as n (%).
In the MMF group, fewer Asian patients reported diarrhoea than all other patients. Anaemia, a known adverse effect of MMF, was reported least by the White group (Table 4). More patients in the Rest of World category reported anaemia than in any other region, irrespective of treatment. Alopecia was more commonly reported with IVC than MMF.
A higher incidence of hypertension expressed as an AE was reported in patients from the Other group compared with the Asian, White and Black groups (Table 4). Similar proportions of patients in the MMF and IVC Other group reported this AE (22.7 vs 29.0%). Fewer patients in the Asian racial (61%) and regional (62%) groups were receiving ACEIs/ARBs at any time during the study compared with those from other racial (81–89%) and regional (81–85%) groups. Infection was reported less frequently among Asian patients (Fig. 2). Sites from Asia also reported the lowest rate of infections.
More patients in the White, Asian and combined Other and Black groups who received MMF withdrew due to AEs (8.1, 22.6 and 9.1%, respectively) compared with the IVC group (2.8, 5.0 and 3.2%, respectively). In contrast, more patients in the Black group receiving IVC withdrew due to AEs (38.9%) compared with those receiving MMF (7.7%).
Among patients from Asia, those receiving IVC had a lower rate of withdrawal due to AEs (5.1%) than MMF (22.8%). Differences in withdrawal due to AEs between the treatment groups did not appear to be influenced by variations between the groups with respect to BMI or overexposure to the drug (dose per body weight).
In the MMF group, there were two deaths in Argentina (both due to septic shock), six deaths in China (interstitial lung disease = one, lung infection = one, pneumonia = two, respiratory tract infection = one and unknown = one) and one death in Malaysia (due to septicaemia). In the IVC group, there were two deaths in the USA (LN = one and unknown = one), two in China (serious mixed infection = one and cerebrovascular accident = one) and one death in the UK (due to subacute endocarditis).
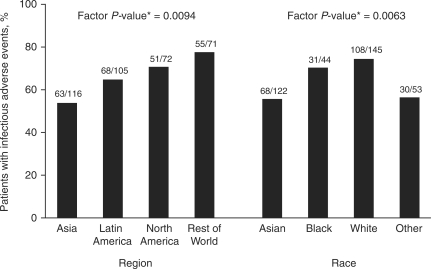
Post hoc analysis of treatment differences for deaths, SAEs and infections did not show statistical significance between MMF and IVC (4.9 vs 2.8%; P = 0.29; 27.7 vs 22.8%; P = 0.28; and 68.5 vs 61.7%; P = 0.17, respectively). The analysis also revealed no significant treatment-by-factor interactions for race, ethnicity, region, body surface area or weight on adverse outcome (deaths, SAEs or infectious AEs). Independent of treatment, however, race and region were associated with the incidence of infectious AEs (P = 0.0072 and 0.0095, respectively) (Fig. 3).
Fig. 3.
Effects of race and region on the incidence of infectious AEs. The asterisk indicates P-value for factor, obtained from a logistic regression analysis of all patients modelling infectious AEs with a main effect for factor.
Discussion
Observations suggest that the mechanisms of LN may differ among racial and ethnic subsets; hence, the effectiveness of therapeutic interventions may also vary. For instance, Black and Hispanic patients have an increased risk of aggressive disease [12, 13], and a greater prevalence of renal failure has also been reported among Black patients [25], with genetic rather than socio-economic factors believed to be a more likely cause.
Previous trials have found MMF to be at least as effective as IVC as induction treatment for LN; but analyses were limited to patients in single countries and the trial sizes did not allow for comparisons between racial groups [26–30]. ALMS, however, provides the most extensive dataset to date that allows variations in response to the two most commonly used treatments for LN to be addressed directly.
The results from this global study suggest that race and geographical region do influence response to therapy. Possible reasons that may have contributed to this observation include differences between subgroups with respect to disease characteristics at baseline, differences in how subgroups metabolize the respective drugs, variations in treatment tolerability and regional differences in patient management/socio-economic factors. The greater number of responders with MMF than IVC in the Black and Latin American groups suggests a difference in efficacy between the two drugs in these populations. This difference in efficacy observed in Black and Latin American groups is supported by the reduction in mean IVC dose among patients in the Black group compared with other racial groups.
Further, more patients in the Black group receiving IVC were likely to withdraw prematurely from the study due to AEs than other racial groups, resulting in differences in exposure across racial groups. Variation in exposure to either of the treatments was also seen across regional groups; exposure to IVC was lower in the USA and Canada than other regions because of differences in the proportions of patients completing the full 24 weeks of treatment (withdrawal rates were higher in USA/Canada). Lower exposure was driven by fewer infusions due to premature withdrawal rather than patients requiring a reduced dose due to renal failure. Conversely, patients in the Asian group demonstrated a reduced tolerability to MMF, exhibiting a higher withdrawal rate due to AEs, compared with other racial groups.
However, it is difficult to draw firm conclusions from drug exposure according to patient weight, because dosing of both drugs was frequently reduced due to AEs, which confounds the apparent outcome in patients taking lower doses. Despite differences in withdrawal due to AEs in patients from Asia, there was no effect on overall efficacy MMF and IVC compared with other regions. Indeed, the overall efficacies of MMF and IVC were comparable, but for certain racial and ethnic groups, MMF may offer better efficacy and tolerability as induction treatment. ALMS provides the most compelling data currently available that certain patient populations are less likely to respond to IVC, supporting the anecdotal evidence from clinical practice that the efficacy of IVC varies between racial and ethnic groups.
Baseline disease characteristics did not appear to have any noticeable predictive value for response to therapy. There were differences in baseline factors observed between racial/regional groups and within some racial groups. More patients in the Black group and from USA/Canada had Class V LN, and these patients were also more likely to be hypertensive and taking immunosuppressive co-medications. However, there is no indication that these parameters had an impact on treatment response. Overall, patients from Asia reported the fewest infections, but those infections were more likely to be severe, resulting in hospitalization or death. Notably, these events were largely localized to one region within Asia. Analysis of the potential impact of possible predictor variables on adverse outcomes did not explain the numerical differences between treatment groups in the numbers of deaths, SAEs and infections.
Firm conclusions cannot be drawn from this study as a number of the assessments described here were exploratory analyses performed on a post hoc basis subsequent to the initial, prospectively planned, primary efficacy analysis, and thus must be interpreted as such. Furthermore, the trial was not designed to be powered to detect an effect of a specific region, race or ethnicity; the small numbers of patients in each subgroup do not allow these findings to be generalized to the larger population of patients with LN.
Also, due to the complex nature of the relationship between race, ethnicity and geographical region, we cannot distinguish between these factors in terms of importance. Designations of race and ethnicity are often arbitrary (and in this study, self-reported) and heterogeneous [31], and there can be notable differences in clinical, prognostic and socio-economic features, education and access to medical care within a geographical region [32]. Further, these categories are not discrete, and the overlap of races and ethnicities may have masked differences in response among these groups. In spite of the attempt to standardize the management of LN across regions by strict trial monitoring, differences in treatment response were observed in Latin America compared with the other regions. Although the role of some socio-economic factors can be reduced in a clinical study, they cannot be removed entirely, and their differentiation from genetic factors remains a challenge.
Other factors that were uncontrolled in this study may have been a source of bias, such as regional differences in prior immunosuppressive drug use. For example, patients who had received previous IVC therapy might have been less likely to respond to IVC in ALMS. As details on prior therapy were not collected, it is not clear whether this potential source of bias contributed, for example, to the difference in IVC response seen across regions.
Another limitation may have been the 24-week induction period, which may have been too short to differentiate between the treatments. The duration of this induction phase was comparable with that of previous studies, however [15, 28, 30, 33, 34], and further data will be collected during the ongoing maintenance phase.
In conclusion, exploratory findings from this large, international study indicate that although MMF and IVC are of similar efficacy, race, ethnicity and geographical region may be important factors in response to treatment among patients with LN. More patients from the Black and Hispanic groups appeared to respond to MMF than IVC, and more patients from the Asian group withdrew from MMF than IVC treatment. However, due to the complex nature of the relationship between race, ethnicity and geographical region, we cannot distinguish between these factors in terms of importance. Nonetheless, the ALMS data provide some valuable insights regarding the interaction of these factors.

Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
Acknowledgements
The members of the ALMS Group take full responsibility for the contents of this paper. We thank Steven Nettler, MPH, Aspreva Pharmaceuticals, Inc., Basking Ridge, NJ, USA, for the statistical analysis, and Phillippa Curran and Nicola West of Caudex Medical Ltd, Oxford, UK (supported by Aspreva Pharmaceuticals, Inc.), for editorial assistance with the preparation of the manuscript.
Funding: This study was sponsored by F Hoffman-La Roche Ltd/Inc/AG as part of the Aspreva Pharmaceuticals Corporation Rare Disease Program. Funding to pay the Open Access publication charges for this article was provided by Vifor Pharma Ltd (formerly Aspreva Pharmaceuticals).
Disclosure statement: N.S. is an employee of Aspreva (now called Vifor). G.B.A. has received consultant fees, research/grant support and speakers’ honoraria from Aspreva, Vifor and Genentech. D.I. has consulted for Merck-Serono, Teva, Human Genome Sciences and Bristol-Myers Squibb. He does not accept personal remuneration from these companies and asks that the honoraria are paid to a local arthritis charity. E.M.G. has received honoraria and grants from Aspreva. D.J., in the last 12 months, has received financial support for investigator-initiated clinical trials from Hoffman-La Roche and Vifor (Aspreva). G.C. has received honoraria as speaker for conferences sponsored by Aspreva and Roche. M.A.D. has been a member of the advisory board for Aspreva and has been site PI for clinical trials of lupus therapies for Aspreva, UCB, Bristol-Myers Squibb, Human Genome Sciences, Genentech and Amgen. All other authors have declared no conflicts of interest.
References
- 1.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–18. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon GS, McGwin G, Jr, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001;45:191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11:95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 5.Alarcon GS, McGwin G, Jr, Roseman JM, et al. Systemic lupus erythematosus in three ethnic groups. XIX. Natural history of the accrual of the American College of Rheumatology criteria prior to the occurrence of criteria diagnosis. Arthritis Rheum. 2004;51:609–15. doi: 10.1002/art.20548. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle MR, James JA, Dennis GJ, et al. Rapid clinical progression to diagnosis among African-American men with systemic lupus erythematosus. Lupus. 2003;12:99–106. doi: 10.1191/0961203303lu334oa. [DOI] [PubMed] [Google Scholar]
- 7.Font J, Ramos-Casals M, Cervera R, et al. Cardiovascular risk factors and the long-term outcome of lupus nephritis. Q J Med. 2001;94:19–26. doi: 10.1093/qjmed/94.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Waldman M, Appel GB. Update on the treatment of lupus nephritis. Kidney Int. 2006;70:1403–12. doi: 10.1038/sj.ki.5001777. [DOI] [PubMed] [Google Scholar]
- 9.Seligman VA, Lum RF, Olson JL, Li H, Criswell LA. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med. 2002;112:726–9. doi: 10.1016/s0002-9343(02)01118-x. [DOI] [PubMed] [Google Scholar]
- 10.Austin HA, III, Boumpas DT, Vaughan EM, Balow JE. High-risk features of lupus nephritis: importance of race and clinical and histological factors in 166 patients. Nephrol Dial Transplant. 1995;10:1620–8. [PubMed] [Google Scholar]
- 11.Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18:244–54. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 12.Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039–46. doi: 10.1093/ndt/gfg345. [DOI] [PubMed] [Google Scholar]
- 13.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 14.Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51:1188–95. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 15.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971–80. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 16.Illei GG, Takada K, Parkin D, et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 2002;46:995–1002. doi: 10.1002/art.10142. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair A, Appel G, Dooley MA, et al. Mycophenolate mofetil as induction and maintenance therapy for lupus nephritis: rationale and protocol for the randomized, controlled Aspreva Lupus Management Study (ALMS) Lupus. 2007;16:972–80. doi: 10.1177/0961203307084712. [DOI] [PubMed] [Google Scholar]
- 18.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 21.Gourley MF, Austin HA, III, Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549–57. doi: 10.7326/0003-4819-125-7-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Hay EM, Bacon PA, Gordon C, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 24.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus. 1999;8:685–91. doi: 10.1191/096120399680411281. [DOI] [PubMed] [Google Scholar]
- 25.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology. 2006;45:1144–7. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 26.Ong LM, Hooi LS, Lim TO, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology. 2005;10:504–10. doi: 10.1111/j.1440-1797.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156–62. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 28.Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005;16:1076–84. doi: 10.1681/ASN.2004080686. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Liu Z, Chen H, et al. Mycophenolate mofetil vs cyclophosphamide therapy for patients with diffuse proliferative lupus nephritis. Chin Med J. 2002;115:705–9. [PubMed] [Google Scholar]
- 30.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–28. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon GS. Of ethnicity, race and lupus. Lupus. 2001;10:594–6. doi: 10.1191/096120301682430159. [DOI] [PubMed] [Google Scholar]
- 32.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine. 2004;83:1–17. doi: 10.1097/01.md.0000104742.42401.e2. [DOI] [PubMed] [Google Scholar]
- 33.Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–31. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 34.Houssiau FA, Vasconcelos C, D’Cruz D, et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum. 2004;50:3934–40. doi: 10.1002/art.20666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.