Abstract
Neuroticism is a personality trait associated with negative mood states, sensitivity to negative information, negative appraisal and vulnerability to psychopathology. Previous studies have associated the sustained processing of negative information (words) with individual differences such as rumination and depression but not with personality. In the current study, we aimed to investigate the relationship between neuroticism and changes in sustained patterns of activity within a brain region implicated in emotional self-evaluation and appraisal, the Medial Prefrontal Cortex (MedPFC), when responding to emotional facial expressions (happy, fearful, and sad). We tested whether higher scores of neuroticism are associated with greater sustained patterns of brain activity in the MedPFC when responding to blocks of negative facial expressions. We found that higher scores of neuroticism were associated with greater sustained MedPFC activity throughout blocks of sad facial expressions, but not fearful or happy facial expressions. Based on the relationship between neuroticism and sensitivity to negative information, the current finding identifies a sustained temporal mechanism to this relationship.
Keywords: Neuroticism, Personality, Sustained, Medial prefrontal cortex, Depression, Rumination, fMRI
Introduction
Neuroticism is a fundamental personality trait that involves, in large part, enhanced emotional and cognitive processing of negative affective stimuli (Gunthert et al., 1999; Suls and Martin, 2005). Therefore, one strategy to elucidate the neural substrate of neuroticism has been to study brain responses to negative affective (Canli, 2004) or conflicting (Eisenberger et al., 2005; Haas et al., 2007b) stimuli with noninvasive neuroimaging techniques. This work has begun to identify brain regions in which the amplitude of activation in response to negative (relative to neutral or positive) stimuli correlates significantly with neuroticism.
However, the brain is also a highly dynamic system, in which a significant amount of information is coded in the temporal domain (Henson et al., 2002; Seifritz et al., 2002). Yet to date, nothing is known about how the neural substrate of neuroticism is represented in the temporal dynamics of brain response to emotional stimuli. Some clues can be gleaned from studies that reveal individual differences in the temporal dynamics of brain response to negative stimuli in depressed patients and in individuals who report high levels of rumination, because depression and rumination both are associated with neuroticism (Nolan et al., 1998; Roberts et al., 1998). For example, Siegle et al. (2003) reported greater sustained autonomic arousal (as measured by pupil dilation) following the presentation of negative word stimuli in depressed patients (relative to controls), which correlated with patients’ rumination scores. This group also identified a neural correlate to this process, showing sustained amygdala activity in response to negative stimuli in depressed patients, compared to controls (Siegle et al., 2002). Thus, we hypothesized that higher scores of neuroticism correlate positively with greater sustained amygdala activation in response to negative stimuli.
Neuroticism is associated with increased automatic negative self-evaluation and appraisal (Robinson and Meier, 2005; Stöber, 2003). One brain region that has been specifically linked to this type of process is the Medial Prefontal Cortex (MedPFC) (Fossati et al., 2003; Ochsner et al., 2004b). In particular, through a series of neuroimaging studies, Ochsner and colleagues (2004a; 2004b) have identified that MedPFC activity increases when individuals self-reference emotional information relative to when they count the number of syllables in a given adjective (Ochsner et al., 2005). Based on the tendency of those scoring high in neuroticism to negatively self-appraise when faced with negative information (Hemenover, 2001; Suls and Martin, 2005), we predicted that higher scores of neuroticism would be associated with more sustained MedPFC activity when processing negative stimuli.
We tested these predictions by investigating the temporal characteristics of participants’ brain response to emotional (happy, fearful and sad) facial expressions in twenty-nine healthy individuals scored on trait neuroticism. We utilized a modeling approach to compare the relative fit of temporal response functions to BOLD (blood oxygenation-level dependent) signal obtained during the processing of facial expressions, as a function of participants’ neuroticism scores. This analysis approach enabled us to test whether neuroticism is associated with sustained amygdala and MedPFC response to fearful and sad facial expressions.
Materials and methods
Participants
Twenty-nine healthy right-handed subjects (14 females) were recruited from either Yale or Stony Brook University. The subjects’ mean age was 22.4 years (SD=2.8; range: 18–29). Exclusion criteria (as assessed by self-report) included personal history of diagnosed psychopathology, current use of mood-altering medication, substance abuse during the 6 months prior to scan, history of severe head trauma, neurosurgery, or neurological condition, pregnancy or any standard MRI counter indications. This study was conducted with the approval from the Institutional Review Boards of both Stony Brook University and Yale University. Informed consent was obtained from all participants.
Prior to scanning, all participants completed the NEO PI-R (Costa and McCrae, 1992). Personality data were scored to represent T values, with the population mean defined as T= 50 and one standard deviation of T= 10. The sample scores for neuroticism (M = 52.45, SD = 11.07, range 31–75), were well within the range of the normal non-clinical population. There were no significant positive inter-correlations between neuroticism and any of the other four personality traits (extraversion, openness, agreeableness, conscientiousness). Additionally, there were no significant differences as a function of gender (F =.80; p =.38) or site (Yale versus Stony Brook) (F =.13; p =.72) for trait neuroticism. Prior to scanning, each participant also completed the Profile of Mood States (POMS) (McNair et al., 1971). Neuroticism was moderately positively correlated (r =.40, p =.03) with negative mood.
Experimental design
Subjects were placed into the scanner, while they made gender discriminations of emotional facial expressions selected from a standardized series (Ekman and Friesen, 1976). Face stimuli were presented in 18-second blocks of fearful, happy, sad and neutral emotional facial expressions (Fig. 1B). Within each block, an equal number of male and female faces (3) were randomly presented. The duration of each trial was 3 s, consisting of a 1-second fixation cross followed by 2 s of stimulus presentation. Trials for each condition were presented in 4 blocks of 6 trials each (18-second duration). The order of block presentation was pseudo-randomized, such that within a series of 4 blocks, each block type was selected once at random and within the entire experiment no condition was repeated. This non-repeating design should therefore reduce carryover effects by randomizing the probability of a particular block type preceding another. Throughout the entire experiment, no trials were repeated (all were novel). Each run began and ended with a 30-second fixation period.
Fig. 1.
Schematic representation of experimental design and analysis procedure. Subjects responded to 4 blocks of each condition (Sad, Fear, Happy and Neutral). Each block (B) consisted of the presentation of six face stimuli. Each trial began with a 1000 ms fixation followed by 2000 ms of stimulus presentation. Throughout the entire experiment, no trials or blocks were ever repeated. Sustained BOLD response within particular block types was assessed by applying the sustained temporal response function (blue; sTRF) (A). This function characterizes signal change (relative to neutral) that begins at the onset of a particular block type and remains consistently active throughout the entire block. Onset BOLD response within particular block types was assessed by applying the onset temporal response function (red; oTRF) (C). This function characterizes signal changes (relative to neutral) that begins at the onset a particular block type and then decays shortly thereafter. Parametric maps were created for each subject that displayed the extent to how well a given voxel fit the sTRF (A) and the oTRF (C) separately. The oTRF map was subtracted from the sTRF map for each subject, thus creating maps that represent the extent to how well a given voxel fits the sTRF more than the oTRF. Each of the resulting maps were entered into a regression analysis with higher scores of neuroticism predicting greater fit of the sTRF relative to the oTRF. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Image acquisition
Whole-brain imaging data were acquired on a 3-Tesla Siemens Trio Scanner. For structural whole-brain images, a three-dimensional high resolution spoiled gradient scan (SPGR) and a T1 scan (24 slices, 5 mm thickness; oriented parallel to the line between the anterior and posterior commissure) were conducted. Functional images were acquired using a gradient echo T2*-weighted echo-planar imaging (EPI) scan and were conducted with a flip angle of 80°, repetition time (TR)=1.5 s, echo time (TE)=30 ms, and a field of view (FOV)=220×220 mm matrix.
Functional data were preprocessed and statistically analyzed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). The images were temporally realigned to the middle slice, spatially realigned to the first in the time series, coregistered to the T1 volume image, which was segmented and normalized to the gray matter template. Spatial transformations derived from normalizing to the segmented gray matter were then applied to all functional volumes, which were then spatially smoothed with an 8 mm full width-half maximum isotropic Gaussian filter.
Temporal characterization of brain activity
In order to localize brain regions on the basis of temporal characteristics of BOLD response, a modeling approach implementing temporal response functions (TRFs) was utilized. This analysis approach applies regressors which are based on the canonical hemodynamic response function to fMRI data collected when using a blocked design. By comparing the relative fit of these functions, spatiotemporal dissociations can be made between regions that respond in different temporal patterns throughout a given block type. In this study, we aimed to investigate individual differences in sustained response to emotional facial expressions presented in a blocked design. We therefore modeled a function to be maximally sensitive to the sustained temporal component of a block and compared this function with another that was modeled to be maximally sensitive to the beginning (onset) of a block. In particular, the sustained TRF (sTRF) was modeled to be reflective of signal that was consistently active throughout the entire block (6 presentations, 18 s) (Fig. 1A). The latency and shape of the sTRF was based on research identifying BOLD signal response to stimuli presented for a similar duration (Boynton et al., 1996). On the other hand, the onset TRF (oTRF) was modeled to be reflective of signal primarily driven by the presentation of the first trial (1 presentation, 3 s) within a given block. In particular, the peak of the oTRF was modeled to occur approximately 6 s following onset of the initial stimulus within a block (Fig. 1C). This time-course of signal is consistent with event-related research demonstrating that the peak of the HRF occurs roughly between 4 and 8 s following single stimulus presentation (Buckner, 2003; Buckner et al., 1996). For each subject, parametric maps were created in order to identify how well a given voxel fit a TRF (sustained or onset) for each emotional condition (sad, fearful and happy) relative to neutral.
In order to differentiate patterns of brain activity displaying a sustained pattern from an onset pattern, the oTRF parametric map was subtracted from the sTRF parametric map for each subject. Thus, the resulting map contains values that are representative of how well a given voxel fits the sustained TRF more than the onset TRF. In order to investigate the relationship between neuroticism and sustained brain activity, each of the above described contrast maps (sustained–onset) for each emotional condition (relative to neutral) were entered into a regression analysis with higher scores of neuroticism predicting greater fit of the sustained relative to the onset TRF. We applied a significance threshold of p<.005 (uncorrected) and one-hundred contiguous voxel spatial extent, yielding a per-pixel false-discovery rate of approximately p<.000001 (Forman et al., 1995) for this analysis.
Time-course and slope-characterization
We next aimed to further characterize and validate the pattern of activation in the regions identified above. This was conducted by performing a linear regression analysis on the time-course of activation in these regions. This analysis was based on another study that associated slope values of physiological responses with personality (Larowe et al., 2006). In our analysis, we extracted the time-course of percent signal change (relative to neutral) for each region/cluster, for each subject. This time-course was then averaged across blocks such that one representative time-course was created for each emotional condition (eg., sad), for each subject. A linear regression analysis was then performed on each representative time-course with scan number (within an average block) predicting change of signal (relative to neutral), yielding standardized beta-values.
The resulting standardized beta-values were thus representative of the slope of decay throughout each average block, for each subject. The standardized beta-values for all subjects were entered into a regression analysis with higher scores neuroticism predicting greater (more sustained) slope values throughout an average block. This analysis was conducted using a standard statistical software package.
Results
Neuroticism and sustained processing of emotional facial expressions
We investigated the relationship between neuroticism and BOLD temporal dynamics (sustained versus onset response) during each of the emotional conditions, relative to the neutral control condition. For a priori regions of interest, we found a positive correlation between neuroticism and sustained activation during presentation of sad facial expressions in the MedPFC (MNI: −8, 36, 38; t(1, 27)=4.03; p<.001, effect size: R2=.38) (Fig. 2), but not amygdala. A whole-brain analysis using the same threshold identified no other clusters that displayed this relationship in response to sad facial expressions. Additionally, we verified that this effect was specific to the MedPFC by identifying that no voxels within this cluster were localized in a neighboring structure, the anterior cingulate cortex, defined by an anatomically based masking method (Maldjian et al., 2003). We confirmed that the observed sustained activation was specific to sad facial expressions. Using a whole-brain analysis, no other clusters were identified to display greater sustained relative to onset response to any of the other emotional conditions (happy or fear).
Fig. 2.
Changes in sustained left medial prefrontal cortex activity associated with neuroticism in response to sad facial expressions. Areas of significant sustained activation (yellow) are overlaid on a sagital slice (x=−8) of a standardized template brain. The cluster of activation encompassed 137 contiguous voxels. Data are plotted from this cluster, with the x-axis representing variation in neuroticism and the y-axis representing contrast values (extracted from SPM) reflective of greater fit of the sTRF relative to the oTRF. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We obtained regression coefficients between neuroticism and the sustained MedPFC response during each of the emotional face conditions. Neuroticism accounted for a significant proportion of variance of sustained response during the sad condition (R2 =.38; p<.001) but not the fear (R2 =.13; p>.05) or happy (R2 =.00; p>.05) conditions. Neuroticism explained a greater proportion of variance during the sad condition relative to the happy condition (F=9.83; p=.005) though the comparison between sad versus fear and happy versus fear did not reach statistical significance. The relationship between neuroticism and sustained MedPFC response during the sad condition was significant for both males and females independently. Additionally, the relationship between neuroticism and sustained MedPFC response during the sad condition remained significant when subjects scoring greater than one standard deviation (±11.07) from the mean (52.45) in neuroticism (remaining subjects: N=19) were excluded.
Facets of neuroticism, negative mood and sustained MedPFC response
We investigated the relationship between facets of neuroticism (anxiety, angry hostility, depression, self-consciousness, impulsiveness, and vulnerability) and the observed pattern of activity by entering all of the facets simultaneously into a regression predicting sustained MedPFC response. As expected, the overall model with all facets simultaneously predicting MedPFC response was significant (R2=.43, p<.05). Within this model, no individual facet of neuroticism (while controlling for all other 5 facets) explained a significant proportion of variance while controlling for the others. This is likely explained by the relatively high inter-correlations between each of the facets. However, independently, each facet was significantly positively correlated with greater sustained MedPFC response (anxiety: R2=.25, p<.01; angry hostility: R2=.31, p<.005; depression: R2=.23, p<.01; self-consciousness: R2=.23, p<.01; impulsiveness: R2=.15, p<.05; vulnerability: R2=.15, p<.05). This effect was not driven only by either of the anxiety (N1) or angry hostility (N3) subscales. Neuroticism remained significantly positively correlated with sustained MedPFC response when controlling for the N1 anxiety facet (p=.002) and controlling for the N2 angry hostility facet (p=.022).
The observed pattern of sustained MedPFC activity was not driven by individual differences in negative mood. Neuroticism remained significantly (p<.005; 100 voxel extent threshold) positively correlated with sustained MedPFC activity in response to sad facial expressions when controlling for negative mood (McNair et al., 1971). Using a whole-brain analysis, no activity was identified to be associated with negative mood when controlling for neuroticism.
In addition, we investigated the relationship between two other personality traits (extraversion and agreeableness) that are characteristically negatively correlated with neuroticism with sustained activity in the MedPFC. This analysis did not identify any sustained MedPFC activity that was significantly negatively correlated with either extraversion or agreeableness during any of the emotional facial expression conditions (happy, fear or sad).
Neuroticism and temporal characterization of MedPFC response
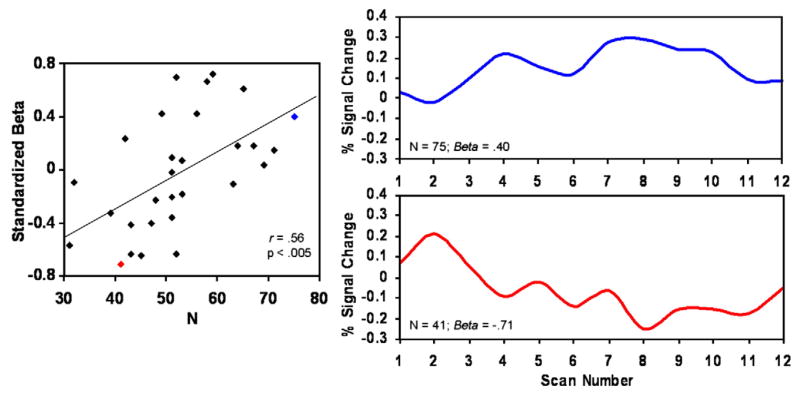
The pattern of signal change within the MedPFC cluster described above was further characterized for each participant. This was conducted by investigating the relationship between neuroticism and the slope of signal change throughout averaged blocks of sad facial expressions. The raw time-course from the peak voxel within the cluster identified in the previous analysis was extracted and converted into percent signal change. Each time point was calculated to represent the signal change difference between the sad and the neutral face conditions. The time-course for each block (4) was averaged to create a single representative time-course for each subject. A regression analysis was then performed on this time-course for each subject yielding a standardized-beta. These standardized beta-values were entered into an additional regression analysis with neuroticism predicting change of slope (see Materials and methods). Higher scores of neuroticism correlated positively with higher standardized beta-values (r=.56; t=3.51; p<.005) (Fig. 3). The right side of Fig. 3 displays the percent signal change throughout an average block of sad facial expressions for a representative low neurotic (Neuroticism score=41) and high neurotic (Neuroticism score=71). As can be seen, the representative high neurotic (blue) exhibits more sustained MedPFC activity throughout average sad blocks compared to the low neurotic (red).
Fig. 3.
Relationship between neuroticism (N) and change of linear slopes of MedPFC activity within blocks of sad facial expressions. Data were extracted and converted to percent signal change (relative to neutral) from the MedPFC. Slope (Standardized Beta) values were calculated for each subject during average blocks of sad facial expressions and entered into a regression analysis with higher scores of neuroticism predicting more positive (sustained) slope values (left). Right side: extracted average time-course of percent signal change and Beta values are presented for an individual scoring high on neuroticism (N=75; blue) and low on neuroticism (N=41; red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Discussion
We hypothesized that sustained activation in response to negative stimuli may serve as a neural substrate of neuroticism. We now find evidence for such a mechanism in the Medial Prefrontal Cortex (MedPFC), a brain region that has previously been associated with negative self-evaluation (Fossati et al., 2003; Ochsner et al., 2004b). Our finding is consistent with studies that reported greater sensitivity to negative stimuli as a function of neuroticism (Derryberry, 1994), and greater sustained processing of negative information in patients diagnosed with depression and/or scoring higher on trait rumination (Deldin et al., 2001; Siegle et al., 2001, 2003, 2002).
The studies cited above used word stimuli that were categorized as either personally relevant negative, positive or neutral. It is therefore unknown whether sustained processing associated with neuroticism, depression or rumination is characteristic of all negatively valenced stimuli, or possibly specific to discrete emotion categories. The use of discrete emotional stimuli in the current study made it possible to address this question. Our use of discrete emotional facial expressions provided the opportunity to dissociate sustained processes between two forms of negatively valenced stimuli (sad and fearful). The data we obtained suggest that the sustained processing of negative stimuli associated with neuroticism is specific to sad facial expressions, and not happy or fearful facial expressions. This observation is consistent with a previous study that reported a significant correlation between neuroticism and the experience of sadness (Stewart et al., 2005). In the current study neuroticism and negative mood were positively associated with one another.
Neuroticism is also associated with both trait and clinical anxiety (Bienvenu et al., 2004), both of which correspond to greater reactivity to fearful emotional stimuli (Bishop, 2007). In the current study we did not observe a significant relationship between neuroticism and sustained response to fearful facial expressions. This may be due to our choice of personality/trait measurement tools. Future research may elucidate the relationship between anxiety and sustained fear processing by using either the Spielberger State-Trait Anxiety Inventory or Beck Anxiety Inventory. Additionally, our analysis technique was targeted at dissociating patterns of brain activation as a function of temporal dynamics (sustained processing). This pattern of brain function has been shown in depressed individuals (Siegle et al., 2003, 2002) but not anxious individuals. Our finding that neuroticism is associated with sustained processing of sad but not fearful stimuli may suggest that this neural substrate is a better predictor of vulnerability to developing depression versus anxiety.
Our observation of sustained response to sad stimuli was unique to the MedPFC. The location of sustained activation is consistent with previous studies that reported a significant correlation between MedPFC activation and rumination (Ray et al., 2005) and MedPFC activation and mindfulness (Creswell et al., 2007). A whole-brain analysis utilizing the same threshold did not identify any other regions of the brain to display this pattern. Only at much reduced statistical thresholds (p=.01, uncorrected; not commonly used in functional neuroimaging analyses of whole-brain data) did we identify two additional regions, the left inferior parietal and precuneus, that exhibited a similar pattern.
Given the data by Siegle et al. who reported sustained amygdala activation to negative stimuli in depressed patients (2002), we also conducted a region-of-interest analysis there (data not shown). We did not find any evidence that neuroticism was associated with sustained amygdala activation to sad faces even at much reduced thresholds (p<.05, uncorrected). We also did not find any evidence that neuroticism was associated with sustained amygdala activation to sad faces in either men (N=15) or women (N=14) independently. It is possible that differences between sample groups and experimental paradigms could explain this difference. In particular, sustained amygdala activation may represent a consequence of depression, as opposed to a preceding condition that is observable in healthy samples such as ours. Finally, there are differences with regard to stimulus choice (words versus faces) and attentional focus (attention to emotion versus gender) between Siegle’s study and ours.
The fact that the association between neuroticism and sustained brain activation to sad faces was localized to the MedPFC is intriguing. Although we did not obtain any individual difference scores on ruminative tendencies, the location of sustained MedPFC activity observed here is consistent with previous neuroimaging studies on rumination. For example, Ray et al. (2005) noted that participants in their study were not constrained by task instructions during viewing of negative stimuli, and speculated that greater MedPFC activation in highly ruminative individuals could represent more self-reflective processing. Indeed, recent fMRI studies have observed greater MedPFC activity when participants were asked to attribute emotions to one’s self (Fossati et al., 2003; Ochsner et al., 2005, 2004a). Similarly in our study, the task was very easy (determining the gender of a displayed face), and may have left participants with sufficient cognitive resources to engage in self-reflective processing, particularly when viewing sad faces. Because we did not explicitly manipulate or measure the level of self-reflection or rumination in this study, we offer this interpretation of the data with caution. A future study needs to address a priori whether sustained activation in the MedPFC, as a function of neuroticism, represents individual differences in self-reflective processes or other effortful social cognitive processes (Lieberman, 2007). This is likely going to be a promising area for future work, particularly with regard to the clinical implications of affective appraisal mechanisms in relation to mood disorders.
The location of the medial prefrontal cortex activation reported here is within the dorsal division of this structure. We did not observe any sustained response within the ventral MedPFC. Recently, it has been suggested that the ventral and dorsal regions of the MedPFC subserve different functions (Mitchell et al., 2006). The dorsal region has been shown to be active while forming impressions of other people (Mitchell et al., 2005), mentalizing about others that are dissimilar (Mitchell et al., 2006) and during self-referential processing (Northoff et al., 2006). Our finding that individual differences in neuroticism were associated with sustained response in the dorsal MedPFC during sad facial expressions may indicate that those high in neuroticism are self-referencing and mentalizing about the sad information to a greater extent compared to those lower in neuroticism. Future studies using explicit self and other referential task instructions and personality measurement tools may further elucidate this relationship.
The specificity of the relationship between neuroticism and sustained processing of sad stimuli may provide insights to the fundamental characteristics of this trait. Specifically, this relationship may reflect that those scoring high on neuroticism are self-referencing the characteristics of sadness to a greater extent relative to characteristics of fearfulness. Previous studies have linked neuroticism with the negative self-appraisal (Robinson and Meier, 2005; Stöber, 2003) and with the experience of sadness (Stewart et al., 2005). In addition, previous fMRI studies have identified dissociable neural correlates to the processing of fearful and sad facial expressions (Blair et al., 1999; Kesler-West et al., 2001; Phillips et al., 2004; Whalen, 1998). Future studies may benefit from the recruitment of a sample scoring independently on the Depression and Anxiety facets of neuroticism. In our sample the two facets were highly correlated (R2 =.38, p<.01). Given the relationship between anxiety and the processing of fearful stimuli (Etkin et al., 2004; Rauch et al., 2003), a similar analysis using a sample of highly anxious and low depressed (as measured by neuroticism facets) individuals may yield insights into the possible relationship between neuroticism and sustained processing of fearful stimuli.
In this study, we investigated individual differences in sustained patterns of brain activity with the personality trait neuroticism. This trait has been identified to be associated with the vulnerability to developing psychopathology such as depression or anxiety disorders (Bienvenu et al., 2001; Durrett and Trull, 2005; Haas et al., 2007b; Trull and Sher, 1994). In our sample, participants reported no personal history of psychopathology though they were not given structured clinical interviews, so past (or familial) histories of psychiatric disorders may not have been identified. The observed sustained MedPFC response may represent a biological vulnerability marker, as opposed to a consequence of prior illness. However, in the absence of longitudinal follow-up data, and without the administration of other surveys specific to depression or anxiety disorders, this remains an intriguing speculation.
The vulnerability to psychopathology conferred by neuroticism is modulated by several other factors such as genes and environmental variables (which we did not measure in this study), or other personality traits (Keightley et al., 2003). With respect to personality traits, there were no specific facets of neuroticism (see Results section) that explained a significant proportion of variance while controlling for the others. Future longitudinal studies of healthy participants using a multitude of psychological and biological measures at baseline and throughout the duration of the study will be able to make stronger links between the neural substrate of neuroticism and the biological mechanisms that confer vulnerability for psychopathology.
Our experimental design and analysis technique was limited in that changes in temporal dynamics of BOLD activity could only be investigated on a time scale of approximately 18 s. An inclusion of varied block lengths and response functions would allow for an investigation of parametric modulation of sustained or onset activity by either individual differences or activity in other regions. The time-course and order of block presentations in the current task also limited our analysis by only being able to use neutral as a baseline condition. We have previously reported on individual differences in resting (fixation) brain activity (Canli et al., 2005, 2006). In the current series of analysis, we were interested in identifying temporal changes in activity and therefore chose neutral as a baseline based on the comparable time-course of this condition compared to each of the emotional conditions (4 18-second blocks). The fixation condition was presented during the beginning and end of the experimental run (2 30-second blocks). In addition, we recruited a sample from a non-clinical population. Given our results, it would be of interest to apply this analysis technique using a task with varying negative stimuli to a sample of depressed individuals who score high on trait rumination. One prediction derived from the current study would be that similar patterns of greater sustained response to sad stimuli would be observed in those diagnosed with depression relative to controls. Additionally, it would also be of interest to investigate patients with anxiety disorder, to asses whether these individuals would show sustained activation in response to fearful facial expressions.
In conclusion, the results presented here demonstrate that neuroticism is associated with temporal changes in brain activity when responding to negative stimuli. We observed that this pattern was localized within the MedPFC in response to sad facial expressions, suggesting that individual differences in automatic self-evaluation and appraisal mechanisms may be driving this effect. Given the relationship between personality structure and a wide range of cognitive and emotional functions (Haas and Canli, 2008; Haas et al., 2007a), this line of research will is likely continue to evolve and contribute to our understanding of brain function and psychology.
Acknowledgments
The authors wish to thank A. Aron (Stony Brook University) for helpful comments contributing to the development of this manuscript.
Footnotes
Support. This material is based upon work supported by the National Science Foundation under Grant No. 0224221 to T.C. and by the Retiree’s Dissertation Fellowship awarded to B.W.H.
References
- Bienvenu OJ, Nestadt G, Samuels JF, Costa PT, Howard WT, Eaton WW. Phobic, panic, and major depressive disorders and the five-factor model of personality. J of Nerv Ment Dis. 2001;189 (3):154–161. doi: 10.1097/00005053-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Costa PT, Reti IM, Eaton WW, Nestadt G. Anxiety and depressive disorders and the five-factor model of personality: a higher- and lower-order personality trait investigation in a community sample. Depress Anxiety. 2004;20 (2):92–97. doi: 10.1002/da.20026. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11 (7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122 (Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16 (13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The hemodynamic inverse problem: making inferences about neural activity from measured MRI signals. Proc Natl Acad Sci U S A. 2003;100 (5):2177–2179. doi: 10.1073/pnas.0630492100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Bandettini PA, O’Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Natl A cad Sci U S A. 1996;93 (25):14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. J Pers. 2004;72 (6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;103 (34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qui M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103 (43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Professional Manual of the Revised NEO Personality Inventory and NEO Five-Factor Inventory. Odessa, Fl: PAR Inc; 1992. [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69 (6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Deveney CM, Kim AS, Casas BR, Best JL. A slow wave investigation of working memory biases in mood disorders. J Abnorm Psychol. 2001;110 (2):267–281. doi: 10.1037//0021-843x.110.2.267. [DOI] [PubMed] [Google Scholar]
- Derryberry D. Temperament and attention: orienting towards and a way from positive and negative signals. J Pers Soc Psychol. 1994;66:1128–1139. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Durrett C, Trull TJ. An evaluation of evaluative personality terms: a comparison of the Big Seven and Five-factor model in predicting psychopathology. Psychol Assess. 2005;17 (3):359–368. doi: 10.1037/1040-3590.17.3.359. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci. 2005;5 (2):169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44 (6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33 (5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160 (11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S. The role of neuroticism in daily stress and coping. J Pers Soc Psychol. 1999;77 (5):1087–1100. doi: 10.1037//0022-3514.77.5.1087. [DOI] [PubMed] [Google Scholar]
- Haas BW, Canli T. Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Res Rev. 2008 doi: 10.1016/j.brainresrev.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Is automatic emotion regulation associated with agreeableness? A perspective using a social neuroscience approach. Psychol Sci. 2007a;18 (2):130–132. doi: 10.1111/j.1467-9280.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007b;121 (2):249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15 (1):83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hemenover SH. Self-reported processing bias a nd naturally occurring mood: mediators between personality and stress appraisals. Pers Soc Psychol Bull. 2001;27 (4):387–394. [Google Scholar]
- Keightley ML, Seminowicz DA, Bagby RM, Costa PT, Fossati P, Mayberg HS. Personality influences limbic-cortical interactions during sad mood induction. Neuroimage. 2003;20 (4):2031–2039. doi: 10.1016/j.neuroimage.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, Blonder LX. Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res. 2001;11 (2):213–226. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- Larowe SD, Patrick CJ, Curtin JJ, Kline JP. Personality correlates of startle habituation. Biol Psychol. 2006;72 (3):257–264. doi: 10.1016/j.biopsycho.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19 (3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McNair ML, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17 (8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50 (4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Nolan SA, Roberts JE, Gotlib IH. Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cogn Ther Res. 1998;22 (5):445–455. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31 (1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004a;16 (10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004b;23 (2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28 (4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21 (4):1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann NY Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci. 2005;5 (2):156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Gilboa E, Gotlib IH. Ruminative response style and vulnerability to episodes of dysphoria: gender, neuroticism, and episode duration. Cogn Ther Res. 1998;22 (4):401–423. [Google Scholar]
- Robinson MD, Meier BP. Rotten to the core: neuroticism and implicit evaluations of the self. Self and Identity. 2005;4 (4):361–372. [Google Scholar]
- Seifritz E, Esposito F, Hennel F, Mustovic H, Neuhoff JG, Bilecen D, et al. Spatiotemporal pattern of neural processing in the human auditory cortex. Science. 2002;297 (5587):1706–1708. doi: 10.1126/science.1074355. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry. 2001;49 (7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51 (9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cogn Ther Res. 2003;27 (3):365–382. [Google Scholar]
- Stewart ME, Ebmeier KP, Deary IJ. Personality correlates of happiness and sadness: EPQ-R and TPQ compared. Pers Individ Differ. 2005;38 (5):1085–1096. [Google Scholar]
- Stöber J. Self-pity: exploring the links to personality, control beliefs, and anger. J Person. 2003;71 (2):183–220. doi: 10.1111/1467-6494.7102004. [DOI] [PubMed] [Google Scholar]
- Suls J, Martin R. The daily life of the garden-variety neurotic: reactivity, stressor exposure, mood spillover, and maladaptive coping. J Person. 2005;73 (6):1–25. doi: 10.1111/j.1467-6494.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ. Relationship between the five-factor model of personality and Axis I disorders in a nonclinical sample. J Abnorm Psychol. 1994;103 (2):350–360. doi: 10.1037//0021-843x.103.2.350. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7 (6):177–188. [Google Scholar]