Abstract
The present study investigated the potential efficacy of buspirone for treating marijuana dependence. Participants received either buspirone (maximum 60 mg/day) (n=23) or matching placebo (n=27) for 12 weeks, each in conjunction with motivational interviewing. In the modified intention-to-treat analysis, the percentage of negative UDS results in the buspirone-treatment group was 18 percentage points higher than the placebo-treatment group (95% CI: −2% to 37%, p=0.071). On self-report, participants receiving buspirone reported not using marijuana 45.2% of days and participants receiving placebo reported not using 51.4% of days (p=0.55). An analysis of participants that completed the 12-week trial showed a significant difference in the percentage negative UDS (95% CI: 7% to 63%, p=0.014) and a trend for participants randomized to the buspirone-treatment group who completed treatment to achieve the first negative UDS result sooner than those participants treated with placebo (p=0.054). Further study with buspirone in this population may be warranted; however, strategies to enhance study retention and improve outcome measurement should be considered in future trials.
Keywords: marijuana, buspirone, placebo, motivational interviewing
1. INTRODUCTION
Marijuana is the most commonly used drug in the world, with an estimated 166 million users globally (UNODC, 2008). The 2006 National Survey on Drug Use and Health reports that 97.8 million (39.8%) of Americans 12 years of age or older have tried marijuana at least once in their lifetime and 25.4 million (10.3%) used marijuana in the past year (SAMHSA, 2007). Lifetime prevalence rates of marijuana abuse and dependence among adults in the United States have been estimated at 7.2 and 1.3%, respectively (Stinson et al., 2006).
Results from the Epidemiological Catchment Area Survey demonstrated that marijuana abuse is significantly associated with affective disorders (Regier et al., 1990) with the odds of having an affective disorder being 3.8 times higher in individuals with a marijuana use disorder diagnosis. Anxiety disorders, in particular, may be related to continued marijuana use (Gruber et al., 1997), as users of marijuana report that its use relieves unpleasant feeling states such as anxiety. Data from the National Comorbidity Study indicate approximately a two-fold risk of generalized anxiety disorder in individuals with lifetime marijuana dependence (Agosti et al., 2002). Another study documented a 28.8% lifetime prevalence of anxiety disorders among those with marijuana use disorders (Conway et al., 2006).
The relationship between anxiety and marijuana withdrawal has also been explored. The concept of a marijuana withdrawal syndrome, characterized by irritability, anxiety, and insomnia, has recently been recognized (Kouri and Pope, 2000; Budney et al., 2004; Budney and Hughes, 2006; Copersino and Boyd, 2006). Budney and colleagues reported that high anxiety scores were correlated with increased withdrawal scores in marijuana-dependent adults (Budney et al., 1999). It is possible, therefore, that individuals continue to use marijuana to alleviate or avoid these withdrawal symptoms. The use of an anxiolytic agent may possibly improve substance use outcome measures in individuals attempting to reduce or stop marijuana use. However, limited research has focused on pharmacological agents in the treatment of marijuana use disorders (McRae et al., 2003; Levin et al., 2004; Nordstrom and Levin, 2007; Tirado et al., 2008). Buspirone is a non-benzodiazepine anxiolytic with little or no abuse potential (Lader, 1991). An open-label study of buspirone was shown to improve marijuana use outcomes (McRae et al., 2006), and the purpose of this study was to further explore these promising preliminary findings in a larger, placebo-controlled trial.
2. METHODS
The study was a 12-week, double-blind, placebo-controlled trial of a flexible dose of buspirone (up to 60 mg/day) in marijuana-dependent individuals, conducted between April 2004 and September 2007. Patients were recruited primarily through newspaper advertisements and fliers. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received approval from the Medical University of South Carolina Institutional Review Board. All participants gave written, informed consent prior to study participation.
To be eligible for participation, individuals had to be between 18 and 65 years of age and meet DSM-IV criteria for current marijuana dependence. Exclusion criteria included dependence on any other substance (with the exception of caffeine or nicotine); history of psychotic disorder; current major depression or eating disorder; current treatment with a psychoactive medication; major medical illnesses; and pregnancy, nursing, or inadequate birth control.
The Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 1994) was used to assess for psychiatric exclusions. Marijuana use for the 90 days prior to study entry was estimated using Time-Line Follow-Back (TLFB) procedures (Sobell and Sobell, 1978), and TLFB data were collected weekly throughout the study. Weekly assessments included scales to assess marijuana craving (Marijuana Craving Questionnaire (MCQ); Heishman et al., 2001), and marijuana withdrawal symptoms (Marijuana Withdrawal Checklist; Budney et al., 1999). The Hamilton Anxiety Scale (HAM-A) (Hamilton, 1959) was administered at baseline, weeks 1–4, and weeks 6, 8, and 12. Semi-quantitative urine drug screens (UDSs) for cannabinoids were performed at baseline and weekly throughout the study. To note, initially twice weekly UDSs were collected; however the protocol was amended due to poor participant compliance and the concern that the requirement of multiple weekly clinic visits was affecting retention. UDSs were performed using the AXSYM® system from Abbott Laboratories.
Both groups received adjunctive motivational interviewing (MI) for the first four weeks of the treatment period. This intervention was modeled after the Drinker’s Check-Up (Miller et al., 1993). Participants completed a series of worksheets as part of the intake process (Marijuana Use Summary Sheet, Self-Efficacy Questionnaire, Marijuana Problem Scale, Reasons for Quitting Questionnaire; Steinberg et al., 2005). This information was then used to prepare personalized feedback reports which were used as a framework to discuss the participant’s frequency of marijuana use, problems related to use, reasons for quitting, and high-risk situations for use. Initially, participants completed two sessions; however, to improve treatment retention a third session was added. The first session occurred prior to medication initiation, and a second session occurred approximately one week later. A third session occurred at week 4 to follow-up on action plans (if any) and formation of short- and long-term goals.
Urn randomization (Stout et al., 1994) was used to determine treatment assignment. Urn variables used were age (less than 35 years of age or older), gender, and HAM-A score (less than 18 or 18 and above). Buspirone and placebo tablets were packaged in identical opaque gelatin capsules with cornstarch. Medication dosage was initiated at 5 mg buspirone or placebo twice daily and increased by 5–10 mg every three to four days as tolerated to a target dose of 60 mg/day. Medication side effects were evaluated weekly by a clinician by asking the participant open-ended questions such as “Have you had any problems or side effects since we saw you last (such as cold, flu, headache, nausea, or any other problem)?” The type of adverse event, severity of the adverse event, and the relationship to the study medication was recorded. Compliance was assessed by patient report and pill count. Subjects received nominal monetary reimbursement ($10) for time and travel associated with study visits; this compensation was added after approximately 30 participants were enrolled.
2.1 STATISTICAL CONSIDERATIONS
2.1.1 Sample Size Considerations
The sample size calculations for this study were based on the prediction that 20% of the placebo-treated participants would report a reduction in marijuana use and that 60% of the participants receiving buspirone would have a reduction in marijuana use. At the alpha=0.05 level of significance and 80% power, a sample size of 28 per group was necessary, but this number was inflated to account for anticipated attrition. In total, at least 40 participants per group were determined to be the minimum sample size. It is worth noting that while the estimated effect size (i.e., odds ratio of 6.0) was viewed at the time of planning as being clinically relevant, it was believed that should a smaller effect size be observed, the data and experience generated from this study would still be important.
2.1.2 Outcome measures
The primary outcome for this study was based on semi-quantitative urine drug screen for cannabinoids, simply denoted as a UDS result for the remainder of the manuscript. Submitted UDSs were deemed negative if the specimen contained less than 50ng/ml of cannabinoids. The difference in the percentage of negative UDS results between treatment groups served as the primary efficacy measure. In addition, secondary outcomes included: 1) time to the first negative UDS result (all subjects enrolled had a positive UDS at baseline), 2) summary measures of self-reported use (including percent days abstinent and mean amount used per using day), and 3) measures of marijuana craving (as measured by the MCQ), marijuana withdrawal symptoms, and anxiety as quantified by the HAM-A.
2.1.3 Statistical Methods
Two analytical approaches were used to test for between group differences in the primary endpoint. The first method was the traditional summary of percent negative UDS that is obtained by first by calculating the percent of negative UDS within participant then testing for between group differences on the calculated percentages. Since each participant’s dependent variable was a percentage (and inherently not normally distributed), between group differences were tested using the Wilcoxon Rank Sum test, the non-parametric equivalent to the two group student’s t test. For this analytical approach, two within-participant percentages of negative UDSs calculations were calculated. The first was the intention-to-treat approach that considered any weekly UDS to be positive if it were missing (i.e., the denominator for each participant was 12, the number of the study weeks, and the numerator was the observed number of negative urine drug screens). The second method assumed any missing UDSs were missing completely at random so that the within-participant percentage of negative UDS was simply the observed fraction of negative UDSs.
The second analytical method for testing for between group difference in percentage of negative UDS results (i.e., risk difference) was estimated using a generalized linear model (GLM) framework (McCullagh and Nelder, 1989). A GLM, as the name implies, is a robust modeling framework that allows for non-normally distributed dependent variables and alternative ways of “linking” the dependent variable to the independent variables of interest. To estimate the risk difference directly, the GLM was configured with the identity link (the same link function used in ordinary least squares regression), a binomial distribution for the dependent variable, and a systematic component (i.e., independent variables) of an intercept and an indicator variable for the main effect of the randomized treatment group. Configured in this manner, the beta-coefficient associated with the main effect of the treatment group was the primary parameter of interest. That is, a test of this beta-coefficient equal to zero is a test of the null hypothesis that there was no difference in the percentage of negative UDS results between the two groups. Generalized estimating equations (GEE) (Liang and Zeger, 1986) were used to account for the clustering of UDS results within a participant.
Secondary analyses consisted of measuring the difference in self-reported use, assessing the effect of the behavioral intervention, and assessing the potential mediating effects of changes in anxiety. The same GLM/GEE framework used for the primary outcome was used in these analyses. To test for the potential of effect modification due to the behavioral sessions (quantified as either the per protocol number of sessions or the actual number received) on the primary analysis, the systematic component of the GLM/GEE framework was extended to include the main effect of the number of behavioral sessions and its interaction with the treatment group. For this analysis, the link in the GLM framework was changed from the identity link to the logit link to ensure the model-based estimates of the probability for a negative UDS were between 0 and 1; the inclusion of continuous covariates necessitated this change.
For secondary outcomes including anxiety, marijuana craving and withdrawal, between group differences on the change from baseline scores were tested using the Wilcoxon Rank Sum Procedure. Last observation carried forward (LOCF) was used to estimate the change from baseline to Week 12.
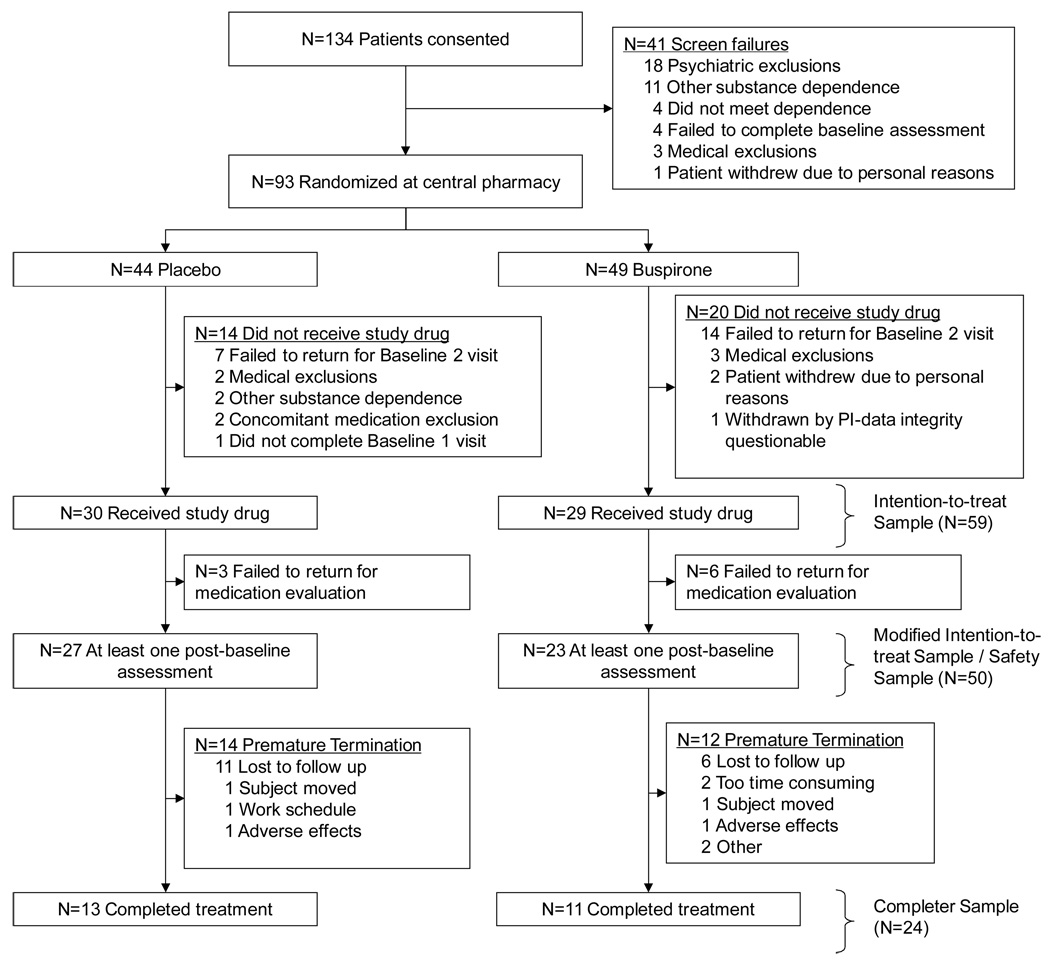
All primary analyses were conducted on the modified intention-to-treat (ITT) sample. This analysis set included all participants that were randomized and provided at least one post-randomization UDS assessment. For comparative purposes, demographic summaries of the full ITT sample (Figure 1, n=59) were computed; however, since nine of these subjects failed to provide any on-study UDS assessments, the results of the full ITT would be identical to the results of the modified ITT results unless the missing data were imputed. Sensitivity analyses were conducted on the subset of participants that completed the study. All analyses were conducted using the SAS System (version 9.1.3, Cary, NC). The type I error rate was established a priori at 0.05, and no corrections for multiple comparisons have been applied to reported p-values.
Figure 1.
Progress of patients in the study.
3. RESULTS
3.1 Sample Description
The participant disposition through the study is present as Figure 1. A total of 134 participants were consented, and 93 met initial eligibility and were randomized. Randomization occurred prior to comprehensive medical evaluation, and five randomized participants were deemed ineligible upon further examination. A significant number of participants (n=21) failed to return for the second baseline visit and were lost to follow up before distribution of study medication. The remaining eight participants were excluded for a variety of reasons including presence of other substance dependence (n=2), concomitant medications exclusions (n=2), personal reasons (n=2), and failure to complete necessary baseline examinations (n=1). One additional participant was excluded by the principal investigator when the data were determined to be unreliable. This participant received study medication, but there were significant concerns regarding treatment non-compliance confirmed by discussion with the participant; furthermore, the participant also belatedly reported use of a concomitant medication that would exclude study participation. The participant was discontinued from the study after one week and the data were not included in any analyses. The remaining 59 randomized subjects constituted the full ITT sample; however, only 50 participants were included in the modified ITT analysis set. The nine participants that were not included in the modified ITT did not provide any on study UDS results, although one participant did provide self reported data. Overall, there were no statistically significant differences in demographic variables between the two ITT definitions (Table 1), but there was a trend for the nine excluded participants to have higher self reported use (97% days using prior to study vs. 89%, p=0.07). Within the modified ITT sample, there were no statistically significant differences between the two treatment groups with respect to baseline characteristics (Table 1). For the remainder of this report, we report outcomes on the modified ITT sample, unless stated otherwise.
Table 1.
Sample description
| Modified Intention to Treat Sample (ITT, n=50) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full ITT Sample (n=59) | Modified ITT | Buspirone ( n=23) | Placebo (n=27) | |||||||
| Domain Variable | Mean | SD | Mean | SD | p-value {a} | Mean | SD | Mean | SD | p-value {b} |
| Demographics | ||||||||||
| Age | 31.4 | 9.8 | 31.6 | 10.2 | 0.97 | 29.5 | 8.4 | 33.4 | 11.3 | 0.32 |
| Sex, N (%) male | 52 | 88% | 45 | 90% | 0.29 | 19 | 83% | 26 | 96% | 0.17 |
| Race, N (%) Caucasian | 51 | 86% | 43 | 86% | 1.00 | 19 | 83% | 24 | 89% | 0.69 |
| Psychiatric Measures | ||||||||||
| Hamilton Anxiety Rating Scale | 9.1 | 6.6 | 9.1 | 6.9 | 0.67 | 10.3 | 7.1 | 8.1 | 6.7 | 0.24 |
| Substance Use Measures | ||||||||||
| Self Efficacy Score | 3.2 | 1.2 | 3.3 | 1.2 | 0.82 | 3.2 | 1.2 | 3.3 | 1.2 | 0.55 |
| Reason for Quiting Score | 10.9 | 6.2 | 11.0 | 6.6 | 0.76 | 12.1 | 7.3 | 10.2 | 5.8 | 0.34 |
| Marijuana Problem Score | 8.8 | 4.4 | 9.0 | 4.5 | 0.25 | 9.1 | 4.4 | 9.0 | 4.7 | 0.91 |
| Marijuana Craving Questionnaire (MCQ) | ||||||||||
| MCQ: Compulsion | 8.4 | 4.4 | 8.3 | 4.3 | 0.95 | 8.3 | 4.6 | 8.4 | 4.1 | 0.91 |
| MCQ: Emotion | 10.5 | 4.6 | 10.2 | 4.6 | 0.26 | 9.5 | 5.5 | 10.7 | 3.8 | 0.49 |
| MCQ: Expectancy | 13.0 | 4.4 | 12.9 | 4.4 | 0.84 | 12.4 | 4.0 | 13.3 | 4.8 | 0.46 |
| MCQ: Purposefulness | 13.3 | 5.2 | 13.2 | 5.3 | 0.86 | 12.5 | 6.1 | 13.9 | 4.5 | 0.35 |
| MCQ: Total Score | 45.1 | 14.4 | 44.6 | 14.4 | 0.59 | 42.6 | 15.9 | 46.3 | 13.1 | 0.40 |
| Withdrawal checklist | 18.6 | 10.4 | 17.9 | 9.4 | 0.70 | 18.6 | 7.4 | 17.4 | 10.9 | 0.50 |
| Self-reported use based on 90 day time line follow back | ||||||||||
| % Days of TLFB with reported use | 90% | 15% | 89% | 16% | 0.07 | 87% | 20% | 91% | 11% | 0.80 |
| Amount Using Per Using Day | 4.0 | 2.9 | 3.8 | 2.5 | 0.76 | 3.3 | 2.1 | 4.2 | 2.7 | 0.28 |
For a comparison for differences in the n=9 participants included in the full ITT sample that were not included in the n=50 modified ITT sample.
For a comparison of differences between treatment groups in the modified ITT sample.
3.2 Primary Analysis
All modified ITT participants submitted at least one UDS after starting study medication. Four urine specimens were not evaluable at the laboratory, but the remaining 395 specimens were analyzable and were included in the primary analysis. Participants submitted between one and 23 urine specimens while on study medication (Mean (SD): 7.9 (5.3); Median (IQR): 8.5, (3 to 11)). The estimate of the intraclass correlation of UDS results within participant was 0.57.
Participants randomized to the placebo treatment condition had an estimated (i.e., model-based) probability of a negative UDS of 11% (95% CI: 1% to 21%) using all available UDS results (Table 2). The estimated probability of a negative UDS in the buspirone treatment group was 18 percentage points higher (95% CI: −2% to 37%, p=0.071). These results were quite similar to the traditional participant-specific calculations described in the methods and presented in Table 2; however, due to their lower power, the p-values were larger.
Table 2.
Outcome summary by treatment group
| Buspirone | Placebo | Measure of Treatment Effect | ||||
|---|---|---|---|---|---|---|
| Variable | Estimate | Estimate | Description | Estimate | Standard Error | p-value |
| Urine Drug Screens for Cannabis | ||||||
| Mean (SD) % Negative, ITT (all missed assumed positive) | 20.3% (32.5%) | 6.5% (16.2%) | Wilcoxon Rank Sum Test | N/A | N/A | 0.13 |
| Mean (SD) % Negative, only observed specimens | 27.6% (40.9%) | 11.7% (27.8%) | Wilcoxon Rank Sum Test | N/A | N/A | 0.16 |
| Model-based estiamte % negative{a} | 28.8% | 11.0% | Risk Difference | 17.8% | 9.9% | 0.07 |
| Timeline Follow-Back | ||||||
| % Days reporting no use{a} | 45.2% | 51.4% | Risk Difference | −6.2% | 10.4% | 0.55 |
| % Reduced days using relative to baseline{b} | 69.6% | 85.2% | Fisher's exact | N/A | N/A | 0.30 |
| % Reduced amount using/using day relative to baseline{b} | 91.3% | 92.6% | Fisher's exact | N/A | N/A | 1.00 |
| Hamilton Anxiety Rating Scale, mean (SD){d} | ||||||
| Total score | −6.10 (7.11) | −4.00 (5.68) | Wilcoxon Rank Sum Test | N/A | N/A | 0.40 |
| Marijuana Craving Questionnaire, mean (SD){d} | ||||||
| Compulsion subscale | −Q4.50 (4.70) | −4.00 (4.72) | Wilcoxon Rank Sum Test | N/A | N/A | 0.97 |
| Emotion subscale | −5.25 (5.20) | −3.88 (4.74) | Wilcoxon Rank Sum Test | N/A | N/A | 0.43 |
| Expectancy subscale | −5.40 (4.47) | −5.84 (5.15) | Wilcoxon Rank Sum Test | N/A | N/A | 0.88 |
| Purposefulness subscale | −7.50 (5.61) | −5.08 (5.05) | Wilcoxon Rank Sum Test | N/A | N/A | 0.17 |
| Total score | −22.65 (16.29) | −19.40 (15.75) | Wilcoxon Rank Sum Test | N/A | N/A | 0.71 |
| Withdrawal checklist, mean (SD){d} | ||||||
| Total score | −10.87 (8.99) | −10.40 (7.73) | Wilcoxon Rank Sum Test | N/A | N/A | 0.99 |
Summaries represent model-based estimates from a generalized linear model using all available data.
For the Intention-to-Treat (ITT) analysis, the denominators were n=23 for Buspirone and n=27 for Placebo. Only observed results that were negative were considered
Values are change from baseline using last observation carried forward method of imputation. One placebo subject did not have any post baseline scale-based
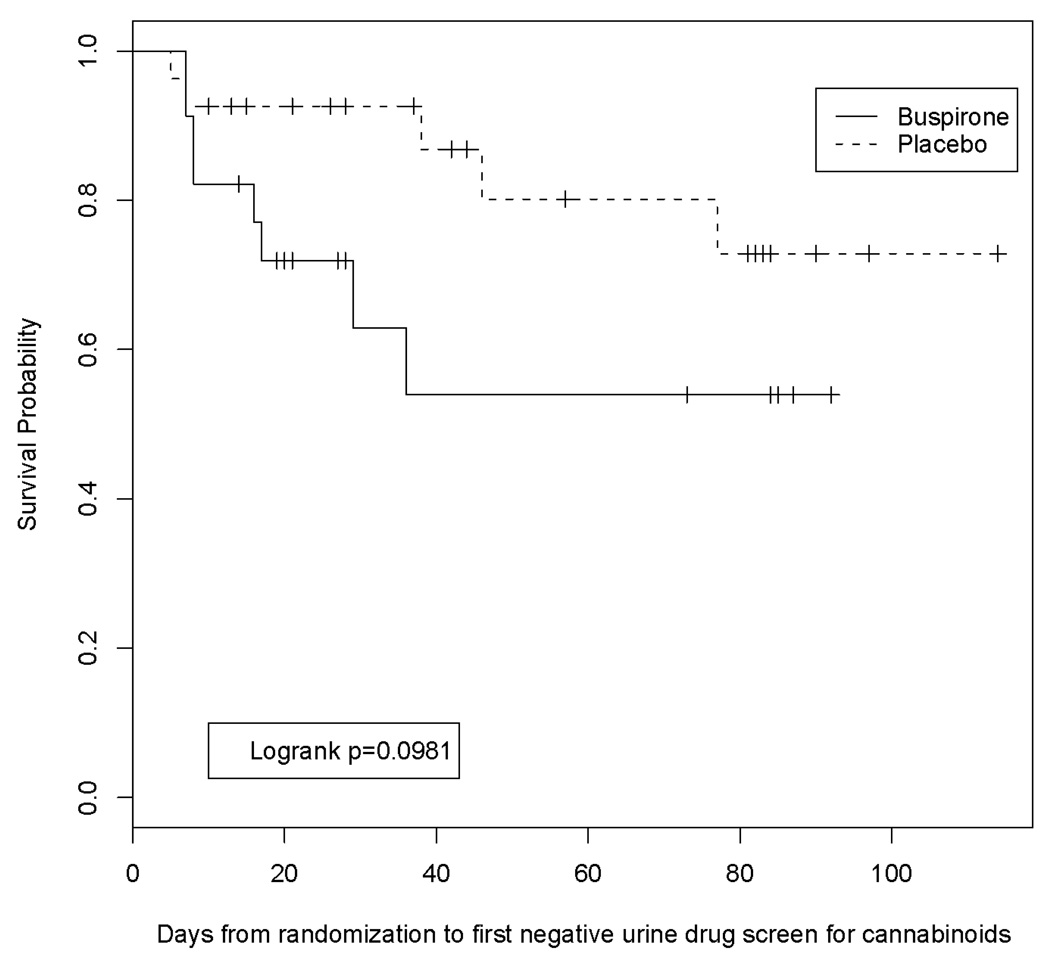
The time to first negative UDS result was consistent with these findings. There was a trend towards faster achievement of the first negative UDS in participants randomized to the buspirone treatment group compared to participants treated with placebo (Figure 2, p=0.098).
Figure 2.
Survival analysis, modified ITT sample
No additional statistically significant results were observed on the secondary outcome measures (Table 2). Overall, the vast majority of participants reduced the amount of use per using day, but no between group differences were observed (91% buspirone vs. 93% placebo; p=1.0).
3.3 Anxiolytic Effect (exploratory analysis)
As the study was designed, it was hypothesized that buspirone’s anxiolytic effects may contribute to reduced substance use through interactions with withdrawal symptoms. To explore this hypothesis, the treatment effect on the HAM-A scores as well as potential mediating effects of reductions in anxiety were examined. Participants treated with buspirone had a 6.1 (SD=7.1) point reduction in the HAM-A versus a 4.0 (SD=5.7) point reduction among those in the placebo arm, but the difference between groups did not near statistical significance (p=0.40) (Table 2). However, when a participant’s change in HAM-A scores was used as a predictor in a GLM/GEE regression model (i.e., main effect of change in HAM-A only in the model), the change in HAM-A score was associated with associated with the probability of having a negative UDS result (p=0.03). In particular, for every one point increase in the HAM-A delta score (which indicates less improvement over the course of the study), the probability of observing a negative UDS decreased by 1.6 percentage points, or simply, large improvements in the HAM-A were associated with higher probabilities of having a negative UDS. When the main effect of the randomized treatment was added to this regression model, the statistical significance of the change in HAM-A diminished (p=0.09) as did the beta coefficient (reduced from − 1.6 to −0.9 percentage points per unit change), and the main effect of buspirone reached the threshold for statistical significance (p=0.025). The point estimate for the risk difference in this adjusted analysis was 20.3 percentage points, a value comparable to that in the unadjusted analysis (Table 2).
Similar, albeit less pronounced, results were obtained using the change in withdrawal symptoms over the course of the study. No between group differences on the change in withdrawal symptoms were found (Table 2), but participants with less improvement in withdrawal symptoms tended to have lower estimated probabilities of a negative UDS (p=0.06). When treatment group and change in withdrawal were modeled jointly, the main effect of treatment group remained marginally significant (p=0.07) and the estimated treatment effect was slightly attenuated (estimated risk difference of 16.2% instead of 17.8%).
3.4 MI Intervention Effect
The GLM modeling framework was used to explore the potential effect modification due to the MI intervention that accompanied the pharmacological treatment. No significant interaction of the number of per protocol MI sessions (2 or 3 sessions) with treatment was observed (p=0.49), and after removing the interaction term from the model, the main effect of the number of MI sessions was also non-significant (p=0.44). Together, these findings suggest that while the protocol was modified to include a third MI session, this did not affect the primary study findings. Similarly, the actual number of sessions attended (range 0 to 3) was modeled as a continuous variable along with its interaction with the treatment group. Both the interaction term and main effect of number of sessions were not statistically significant (p=0.21, p=0.40; respectively).
3.5 Completers Analysis
Exploratory analyses were conducted in the subset of participants that completed the study (n=24, Figure 1). In particular, the difference in the percentage of negative UDS results between the two groups was a 35 percentage point difference (95% CI: 7% to 63%, p=0.014). The percentage negative UDS for the placebo group remained comparable to the full data set (completers: 10.0%; modified ITT sample: 11.0%), so this increase in the absolute value of the risk difference is attributable to a greater increase in the probability of a negative UDS result in the buspirone group. Similarly, the Kaplan-Meier survival curves retained the similar overall shape to the modified ITT sample, and the logrank statistic comparing the two survival curves was marginally significant (p=0.054).
3.6 Safety and Tolerability
No serious adverse events were observed in the study. All reported adverse events were mild to moderate in severity. The majority (96%) of buspirone-treated participants experienced at least one adverse event compared to 78% of placebo-treated participants (Table 3). Dizziness was reported more frequently for participants on buspirone (Relative Risk-RR=3.52, 95% CI 1.08 to 11.49). Dry mouth (RR=2.35), flushing/sweating (RR=2.93), and cold-like symptoms (RR=2.35) were also observed with greater frequency among buspirone-treated participants, but these relative risks did not reach significance. Other reported adverse events occurred in comparable frequencies in the two groups.
Table 3.
Adverse event summary for the Modified Intention-to-Treat Sample
| Buspirone (n=23) | Placebo (n=27) | Relative Risk {a} | ||||
|---|---|---|---|---|---|---|
| Adverse Event | Freq. | Percent | Freq. | Percent | Estimate | 95% CI |
| At least one AE | 22 | 96% | 21 | 78% | 1.23 | [0.99, 1.53] |
| Anxiety/depression | 0 | 0% | 1 | 4% | -- | -- |
| Dizziness | 9 | 39% | 3 | 11% | 3.52 | [1.08, 11.49] |
| Drowsiness | 7 | 30% | 7 | 26% | 1.17 | [0.48, 2.85] |
| Dry mouth | 4 | 17% | 2 | 7% | 2.35 | [0.47, 11.67] |
| Flushing/sweating | 5 | 22% | 2 | 7% | 2.93 | [0.63, 13.73] |
| Gastrointestinal | 8 | 35% | 8 | 30% | 1.17 | [0.52, 2.63] |
| Headache | 9 | 39% | 9 | 33% | 1.17 | [0.56, 2.46] |
| Insomnia | 1 | 4% | 2 | 7% | 0.59 | [0.06, 6.06] |
| Lightheaded/shakiness | 4 | 17% | 3 | 11% | 1.57 | [0.39, 6.28] |
| Metallic taste | 1 | 4% | 0 | 0% | -- | -- |
| Musculoskeletal | 2 | 9% | 2 | 7% | 1.17 | [0.18, 7.69] |
| Sexual dysfunction | 1 | 4% | 1 | 4% | 1.17 | [0.08, 17.74] |
| Sinus/allergies/flu | 10 | 43% | 5 | 19% | 2.35 | [0.94, 5.88] |
| Tingling | 2 | 9% | 0 | 0% | -- | -- |
| Unusual dreams | 2 | 9% | 2 | 7% | 1.17 | [0.18, 7.69] |
| Other | 2 | 9% | 2 | 7% | 1.17 | [0.18, 7.69] |
Relative risk, which is the ratio of the Buspirone percentage to the Placebo percentage, is not reported if one of the two percentages is zero.
4. DISCUSSION
In this small study, no significant differences between treatment groups were observed in the modified ITT analysis. However, there were trends for buspirone-treated participants to have a reduction in positive urine drug screens and to have shorter time to first negative UDS compared to placebo-treated participants. It is worth noting that the percentage of buspirone-treated participants with reduction in days of use relative to baseline was on par with sample size calculations (69.9% observed, 60% expected); however, the placebo-treated participants reduced use far above what was initially hypothesized (85% observed, 20% expected). These differences are in contrast to the urine drug screen findings which indicated a trend towards greater percentage of use among participants in the placebo-treatment condition.
These results suggest there may be some discordance between participant self-report and urine drug screen results, as there were no treatment group differences in TLFB analyses. A reduction from pre-treatment self-reported drug use was observed in both buspirone- and placebo-treated participants. It is difficult to validate self-report in marijuana using individuals due to the long excretion half-life of marijuana in urine (Eskridge and Guthrie, 1997). Creatinine normalization has been proposed as a method to differentiate new marijuana use from residual drug excretion (Huestis and Cone, 1998). However, the utility of creatinine normalization has not been well established with urine sampling conducted at weekly intervals. As noted previously, initially subjects were asked to provide two UDSs weekly. However, there was poor compliance with multiple clinic visits and concern that the increased visit burden was adversely affecting recruitment and retention, and these interim UDS results were not included in the primary analyses.
Changes in anxiety severity over the course of the study were found to be a significant predictor of urine drug screen results, and when the analysis adjusted for these changes the main effect of treatment group became significant. This latter finding, while not tested in a formal mediation setting, may suggest additional hypotheses to be tested in future studies.
The mechanism of action of buspirone has not been clearly established; however, buspirone has been shown to have high affinity for serotonin receptors (Glaser and Traber, 1983; Eison and Eison, 1984; Kastenholz and Crimson, 1984). Serotonin has been implicated in a variety of neuropsychiatric behaviors and disorders including mood, anxiety, appetite, sexual functioning, cognition, substance abuse, depression, and response to antidepressants and antipsychotics. Of relevance, a body of basic science evidence also implicates cannabinoid interactions with the serotonin system. Cannabidiol (CBD), a major component of cannabis, has been shown to be a modest affinity agonist at the 5-HT1A receptor (Russo et al., 2005). Further, cannabinoid receptor (CB1) agonists have been found to diminish 5-HT release (Nakazi et al., 2000) while antagonists have been shown to stimulate 5-HT release (Darmani et al., 2003). Additionally, Hill et al. (2005) demonstrated in an animal model that long-term administration of a synthetic cannabinoid agonist, HU-210, decreased 5-HT1A receptor activity. In vitro, buspirone has been shown to have activity at both presynaptic and postsynaptic 5-HT1A receptors (Robinson et al., 1990), leading to its classification as a partial 5-HT1A agonist. Although speculative, a possible mechanism for the improved marijuana use outcome measures observed in some buspirone-treated participants may be related to its interaction with the effects of cannabinoids at the level of the 5-HT1A receptor, such as having similar actions as cannabidiol or reversing a state of 5-HT1A downregulation caused by chronic marijuana use.
Participants retained for the entire treatment period had improved outcomes with buspirone treatment. A significant number of participants did not complete the screening period. Poor retention with this population has been reported by others (Levin et al., 2004). Strategies to improve retention may prove useful in future pharmacotherapy studies. For example, contingency management has been shown to be effective in increasing retention and improving medication compliance and treatment outcomes in adults with substance use disorders (Carroll et al., 2001; Carroll et al., 2002; Higgins et al., 1994; Preston et al., 1999).
Buspirone appeared to be well-tolerated in this population, with no serious adverse events reported and no difference in rates of adverse events between participants receiving buspirone or placebo, with the exception of dizziness. However, it should be noted that future research, with a much larger sample, is needed to further assess the safety profile of buspirone in marijuana-dependent individuals since even relatively common adverse events, with a prevalence as high as 13%, could have been unobserved in this sample. This calculation is based on the “Rule of Threes” for determining the approximate upper limit of a confidence interval on the rate of occurrence of adverse events when no events are observed (Van Belle, 2002). Thus, subsequent studies may yield additional adverse effects.
This study has several limitations. The sample size was small, and a significant number of participants did not complete the 12-week trial period. As noted above, the use of creatinine normalized urine cannabinoid levels proved difficult; therefore, it is not possible to validate the self-report marijuana use data that were collected. Also, the treatments tested reflected a combination of buspirone + MI versus MI alone, and thus are not a direct test of medication efficacy per se. The MI intervention was included here based on the primary rationale that a true placebo control group (i.e., without any treatment at all) would be unethical (Carroll et al., 2004). A limitation of the study’s efficacy findings stems from the modified ITT definition. The modification came from including only those subjects with at least one post-randomization assessment instead of including all randomized subjects. The modified definition was used due to the enrollment/randomization procedures used in the study. In particular, as urn randomization was used, it was necessary to collect baseline data prior to randomization by the central pharmacy. Thus, two baseline visits were needed—one to consent, screen, and collect randomization data and another to confirm eligibility criteria and dispense blinded medication to those that were eligible. As Figure 1 illustrates, 34 randomized participants did not receive study drug at the second baseline visit. An additional nine participants did return to the second baseline visit to receive study medication but failed to return to any subsequent visits. Under ITT, these participants should be included in the analysis, but these nine participants did not contribute to the preliminary description of safety and efficacy in this population. As such, these participants were excluded from this preliminary investigation (i.e., their data were not imputed for the primary analysis). Finally, as noted in the methods, several protocol changes were made during the course of the study in efforts to enhance retention and recruitment.
In spite of these limitations, the study results are suggestive that buspirone may have some utility in reducing marijuana use in dependent individuals, and that further study with larger sample sizes may be warranted in this challenging population. Interventions to enhance treatment retention and improvements in measurement of marijuana use outcomes should be incorporated in future trials.
Acknowledgements
We thank Ms. Kathleen White, who assisted with the preparation of this manuscript.
Role of Funding Source
Funding for this study was provided by NIDA Grants K23DA15440 (McRae), K23DA020482 (Carpenter), and K24DA00435 (Brady). NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Authors McRae and Brady have received grant support from Bristol-Myers Squibb. Author Brady has received honoraria from Bristol-Myers Squibb. All other authors declare that they have no conflict of interest.
REFERENCES
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Bachman J, Reese RT. Personality correlates of cannabis dependence. Addict Behav. 1979;4:361–371. doi: 10.1016/0306-4603(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos. 1995;23:825–831. [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1321. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan D, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville B. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976;19:300–309. doi: 10.1002/cpt1976193300. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Kumar N, Crim JL. Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol Biochem Behav. 2003;75:777–787. doi: 10.1016/s0091-3057(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Eison MS, Eison AS. Buspirone as a midbrain modulator: Anxiolysis unrelated to traditional benzodiazepine mechanisms. Drug Develop Res. 1984;4:109–119. [Google Scholar]
- Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497–519. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders – Patient Edition (SCID-I/P, vs 2.0) New York: State Psychiatric Institute, Biometrics Research Department, New York; 1995. [Google Scholar]
- Glaser T, Traber J. Buspirone: Action on serotonin receptors in calf hippocampus. Eur J Pharmacol. 1983;88:137–138. doi: 10.1016/0014-2999(83)90404-1. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, Oliva P. Very long-term users of marijuana in the United States. Substance Use Misuse. 1997;32:249–264. doi: 10.3109/10826089709055849. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Ligouri A. Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MTL, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- Hilleman DE, Mohiuddin SM, DelCore MG. Comparison of fixed-dose transdermal nicotine, tapered-dose transdermal nicotine, and buspirone in smoking cessation. J Clin Pharmacol. 1994;34:222–224. doi: 10.1002/j.1552-4604.1994.tb03989.x. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi: 10.1007/BF00428793. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Kastenholz KV, Crismon ML. Buspirone, a novel nonbenzodiazepine anxiolytic. Clin Pharm. 1984;3:600–607. [PubMed] [Google Scholar]
- Kouri EM, Pope HG. Abstinence symptoms during withdrawal from chronic marijuana use. Exper Clin Psychopharmacol. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Lader M. Can buspirone induce rebound, dependence, or abuse? Br J Psychiatry. 1991;159 Suppl:45–51. [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans S, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: A double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: A review of the literature. J Subst Abuse Treat. 2003;24:369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- McRae AL, Brady KT, Carter RE. Buspirone for treatment of marijuana dependence: A pilot study (Letter) Am J Addictions. 2006;15:404. doi: 10.1080/10550490600860635. [DOI] [PubMed] [Google Scholar]
- Miller WR, Benefield GS, Tonigan JS. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. J Consult Clin Psychol. 1993;61:455–461. doi: 10.1037//0022-006x.61.3.455. [DOI] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- Nazaki M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn-Schmeideberg’s Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: A review of the literature. Am J Addictions. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–131. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Reid MJ, Bornheim LM. Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem Pharmacol. 2001;61:1357–1367. doi: 10.1016/s0006-2952(01)00616-5. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Rickels K, Feighner J, Fabre LF, Jr, Gammans RE, Shrotriya RC, Alms DR, Andary JJ, Messina ME. Clinical effects of the 5-HT1A partial agonists in depression: A composit analysis of buspirone in the treatment of depression. J Clin Psychopharmacol. 1990;10(3 Suppl):67S–76S. doi: 10.1097/00004714-199006001-00013. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. New York: Plenum Press; 1978. [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, Kadden R, Duresky D, Stephens R. DHHS Publication No. (SMA) 05-4022. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administation; 2005. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: NSDUH Series H-34, DHHS Publication No. SMA 08-4343; 2008. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. [Google Scholar]
- Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP. Atomoxetine for treatment of marijuana dependence: A report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94:254–257. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Drug Report. Vienna, Austria: United Nations Publication Sales No. #.08.XI.1; 2008. United Nations Office on Drugs and Crime, 2008. [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]