Abstract
Only a few specific chemokines that mediate interactions between inflammatory and satellite cells in muscle regeneration have been identified. The chemokine CXCL16 differs from other chemokines because it has both a transmembrane region and active, soluble chemokine forms. Indeed, we found increased expression of CXCL16 and its receptor, CXCR6, in regenerating myofibers. Muscle regeneration in CXCL16-deficient (CXCL16KO) mice was severely impaired compared with regeneration in wild-type mice. In addition, there was decreased MyoD and myogenin expression in regenerating muscle in CXCL16KO mice, indicating impaired satellite cell proliferation and differentiation. After 1 month, new myofibers in CXCL16KO mice remained significantly smaller than those in muscle of wild-type mice. To understand how CXCL16 regulates muscle regeneration, we examined cells infiltrating injured muscle. There were more infiltrating neutrophils and fewer macrophages in injured muscle of CXCL16KO mice compared with events in wild-type mice. Moreover, absence of CXCL16 led to different expression of cytokines/chemokines in injured muscles: mRNAs of macrophage-inflammatory protein (MIP)-1α, MIP-1β, and MIP-2 were increased, whereas regulated on activation normal T cell expressed and secreted, T-cell activation-3, and monocyte chemoattractant protein-1 mRNAs were lower compared with results in muscles of wild-type mice. Impaired muscle regeneration in CXCL16KO mice also resulted in fibrosis, which was linked to transforming growth factor-β1 expression. Thus, CXCL16 expression is a critical mediator of muscle regeneration, and it suppresses the development of fibrosis.
Skeletal muscle regeneration following injury involves proliferation and differentiation of satellite cells leading to the formation of new myofibers.1 The regeneration process initially involves infiltration of inflammatory cells into injured muscle, including neutrophils, monocytes and macrophages; these accumulate in response to cytokines and chemokines.2 This is important because the types of infiltrating cells influence the severity of the injury and the regeneration processes. For example, when neutrophils were depleted by administering an antibody, muscle regeneration following lipopolysaccharide-induced muscle fiber damage was accelerated.3 Neutrophil infiltration was emphasized because these cells cause tissue damage by processes that are related to the production of reactive oxygen species.4,5,6 The respiratory bursts from infiltrating leukocytes produce oxidizing reactions that damage cells during the early inflammatory period. Indeed, neutrophils obtained from humans or rodents were shown to damage cell membranes of C2C12 myotubes.7
In contrast to the adverse influence of infiltrating neutrophils on injured muscle, infiltration of monocytes/macrophages can be beneficial.8,9,10,11,12 For example, when macrophage infiltration into injured muscle was suppressed, muscle regeneration was sharply impaired and this was associated with the development of muscle fibrosis.13,14 Macrophages not only remove necrotic myofibers by phagocytosis, they also release cytokines as well as growth factors including hepatocyte growth factor, insulin-like growth factor-1, fibroblast growth factor, and tumor necrosis factor-α.8,9,10,12,15 Release of these cytokines and growth factors stimulate satellite cells, which are closely linked to the processes of muscle regeneration.
The recruitment of neutrophils and macrophages into injured muscles is at least partially mediated by chemokines, and consequently, their influence has been examined extensively. For example, the reports of Warren et al15 and Shireman et al16 provided the critical evidence that the CC chemokine, monocyte chemoattractant protein-1 (MCP-1), and its receptor, CCR2, were critical for the regeneration processes occurring in injured muscle. Specifically, knocking out of the CCR2 receptor or blocking the action of MCP-1 significantly delayed the muscle regeneration occurring in injured tissue. There is evidence, however, that changes in the expression of cytokines besides MCP-1 contribute to muscle regeneration.17
Structurally and functionally, CXCL16 differs from MCP-1 and other chemokines.18 MCP-1 and the majority of other chemokines are small molecules secreted by inflammatory cells, whereas CXCL16 is synthesized as a transmembrane multidomain molecule consisting of a chemokine domain plus a glycosylated mucin-like stalk linked to a single transmembrane helix. There are two forms of CXCL16 resulting from cleavage at the cell surface. The soluble form of CXCL16 is composed of the extracellular stalk and the chemokine domain. It functions as chemoattractant to promote cell migration and changes in the functions of recruited cells.19 The remaining transmembrane structure of CXCL16 interacts with its receptor, CXCR6, to establish cell to cell adhesion. Indeed, CXCR6 is expressed on several types of inflammatory cells including macrophages.18,20,21,22,23,24,25,26 Previously, we found that inhibition of CXCL16 significantly reduces the infiltration of macrophages into the kidney of rats with anti-glomerular basement membrane antibody-associated glomerulonephritis.27 Given the unique features of CXCL16 and the importance of macrophages in the processes of muscle regeneration, we studied the role of CXCL16 in regulating muscle regeneration. We studied CXCL16 knockout (CXCL16KO) mice using a standard model of muscle injury and regeneration, cardiotoxin injection into tibialis anterior (TA) muscles. Our results reveal that CXCL16 is critical for recruitment of macrophages, which are essential for satellite cell proliferation and differentiation in vivo.
Materials and Methods
Reagents and Antibodies
Anti-Mac2 was obtained from Cedarlane Laboratories (Burlington, NC), anti-myeloperoxidase and anti-glyceraldehyde-3-phosphate dehydrogenase were from Abcam (Cambridge, MA), anti-Pax-7, embryonic myosin heavy chain (eMyHC) (F1.652), and myogenin were from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), antibodies of laminin, α-smooth muscle actin, and myosin-skeletal, slow and fast, were from Sigma-Aldrich (St. Louis, MO), anti-MyoD was from Santa Cruz Biotechnology (Santa Cruz, CA), and the anti-CXCL16 antibody has been described previously.27 Recombinant CXCL16 was from R&D Systems (Minneapolis, MN). 5-Bromo-2′-deoxyuridine (BrdU) labeling and detection were accomplished using Kit II from Roche Applied Science (Indianapolis, IN). Gomori trichrome staining reagent was from Fisher Scientific (Kalamazoo, MI). Cardiotoxin was from Calbiochem (La Jolla, CA).
Muscle Regeneration Model
Animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. The CXCL16KO mouse on a background of C57BL/6J was generated by Dr. S. Han. Male, KO and control, wild-type C57BL/6J mice were studied at 6 to 10 weeks old. After anesthesia with ketamine, a TA muscle was injected with 80 μl of 10 μmol/L cardiotoxin in saline; the contralateral muscle was injected with saline.
RT-PCR
Total RNA was extracted from TA muscles using TRIzol; the cDNA was synthesized using the first-strand cDNA Synthesis Kit using random primers (Invitrogen, Carlsbad, CA), and RT-PCR was performed as described previously.28 Relative expression was calculated from cycle threshold values using 18S as the internal control (Ct; relative expression = 2(sample Ct − 18S Ct)).
RNase Protection Assay
RPA assays were performed as described using glyceraldehyde-3-phosphate dehydrogenase and L-32 as controls.28
Histochemical and Immunohistochemical Analyses
Serial, transverse cryosections (8 μm thick) of the midbelly region of frozen TA muscles were stained with H&E. Standard immunohistochemical techniques were used to detect changes in muscle regeneration.28,29 Collagen in the interstitium of muscle was stained by Gomori trichrome, and the green colors of collagen were selected to calculate the percentage of fibrosis to muscle area examined using NIS-Elements Br 3.0 software (Nikon, Melville, NY). To calculate the cross-sectional area of individual myofibers, we used the NIS-Elements Br 3.0 software and myofibers that were immunostained for laminin. The distribution of fiber sizes was expressed as a percentage of myofibers examined.
Satellite Cell Isolation
These cells were isolated from leg muscles of wild-type (C57B/L6) mice as described by Morgan.30 Briefly, muscles were incubated at 37°C for 30 minutes in Dulbecco’s modified Eagle’s medium with 1% penicillin-streptomycin and 125 U/ml collagenase type IIA (Sigma-Aldrich). Unaggregated cells were passed through a 100-μm filter and subjected to density centrifugation. A total of 2 × 104 to 1 × 105 cells from the interface between 40 and 70% Percoll were plated in 35-mm Matrigel-coated plates (BD Biosciences, San Jose, CA). Culture media was F12-C supplemented with 15% horse serum and 1 nmol/L fibroblast growth factor-2. Cells were cultured at 37°C in a 5% CO2 incubator.
Cells were identified as satellite cells by immunostaining with antibodies against Pax7 and MyoD.30 Isolated satellite cells were treated with 25 μmol/L CXCL16 for 16 hours before BrdU was added to assess proliferation (BrdU was immunostained after it was incubated with cells for 30 minutes.).
Statistical Analysis
Values are presented as means + SE. Results were analyzed using Student’s t-test when results from two experimental groups were compared or using analysis of variance when data from three groups were studied; for data analyzed by analysis of variance, pairwise comparisons were made by the Student-Newman-Keuls test.
Results
Injured Muscles Express CXCL16
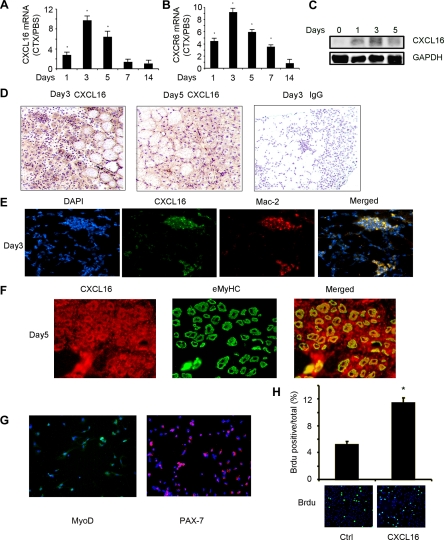
It has been shown that several cytokines/chemokines are expressed in injured skeletal muscle to recruit inflammatory cells and, hence, could impact the processes of muscle regeneration.8,15,17 To establish that CXCL16 is expressed during the regeneration, we examined injured muscle at different times to assess CXCL16 mRNA and protein. At 1 day after injury, there was a 2.5-fold increase in CXCL16 mRNA in the mixed fiber, TA muscles compared with results from uninjured, contralateral muscles (there was a minimal level of CXCL16 in uninjured muscle). By 3 days, the peak value of CXCL16 mRNA was increased ninefold over the level in uninjured, contralateral muscles. Subsequently, CXCL16 expression decreased gradually; at 14 days, CXCL16 mRNA had returned to control values (Figure 1A). The mRNA of the CXCL16 receptor, CXCR6, in injured muscle also increased in comparison with the level in the contralateral, normal muscle (Figure 1B). The protein level of CXCL16 had the same trend as mRNA (Figure 1C). Next, we examined which cells contribute to the sharp increase in CXCL16 in injured muscles. At days 3 and 5 after injury of TA muscles, CXCL16 expression increased in areas with regenerating myofibers compared with its expression in uninjured myofibers (Figure 1D). A negative control was included on the right panel consisting of normal IgG as the primary antibody instead of the CXCL16 antibody; as with other stains, a rabbit, biotin-conjugated secondary antibody was used. Double immunoflorescence staining revealed colocalization of CXCL16 with Mac-2, a marker of monocytes/macrophages (Figure 1E). In addition, CXCL16 colocalized the eMyHC, a marker of newly regenerated fibers from satellite cells (Figure 1F). Thus, in response to injury, CXCL16 is expressed in both regenerating myofibers and infiltrating macrophages.
Figure 1.
The expression of CXCL16 in injured TA muscles. A: Time sequence for CXCL16 mRNA expression in TA muscles following injury. Values are expressed as the fold change over the value in the contralateral, uninjured muscle after correction for 18S mRNA. *, Significantly (P < 0.01) greater than control value. B: CXCR6 mRNA expression was examined with the same protocol as A. C: Western blotting was used to detect CXCL16 levels in injured muscle. D: CXCL16 expression following injury of 3 and 5 days was detected by immunostaining with anti-CXCL16 antibody (brown). Nuclei were stained by hematoxylin (blue). A negative control, by replacing the CXCL16 antibody with normal IgG, is shown in the left panel. E: Injured TA muscles (3 days after injury) were immunostained with anti-CXCL16 (green) and anti-Mac-2 (red) antibodies. Merged yellow color indicates macrophages that express CXCL16. F: Anti-CXCL16 (red) and anti-eMyHC (green) were used in immunostaining of muscle at 5 days after injury. In the merged panel, the yellow color indicates newly generated myofibers that express CXCL16. G: Isolated satellite cells were identified by immunostaining with PAX-7 and MyoD. H: The increase in satellite cell proliferation in response to CXCL16 stimulation was examined by BrdU, indicating that CXCL16 directly stimulates satellite cell proliferation. Lower panel: BrdU incorporation was identified by immunostaining (green color). In the upper panel, the percentage of BrdU-positive cells is increased by CXCL16.
We also isolated satellite cells from wild-type mice and treated them with 25 μmol/L CXCL16 for 16 hours. Satellite cells were identified by immunostaining with PAX-7 and MyoD (Figure 1G). The increase in satellite cell proliferation in response to CXCL16 stimulation was examined by BrdU, indicating that CXCL16 directly stimulates satellite cell proliferation (Figure 1H). These findings indicate that CXCL16 is expressed at a high level early in the regeneration process and in a cell-specific fashion. Our results are consistent with the hypothesis that this chemokine is integral to muscle regeneration processes.
Knockout of CXCL16 Impairs Muscle Regeneration
To explore whether CXCL16 affects the capacity for muscle regeneration, we studied CXCL16KO mice. KO of CXCL16 does not affect their growth rates, muscle development, or the number of slow (type 1) and fast (type II) fibers compared with values in wild-type mice (data not shown). Thus, the absence of CXCL16 permits normal development and growth of mice.
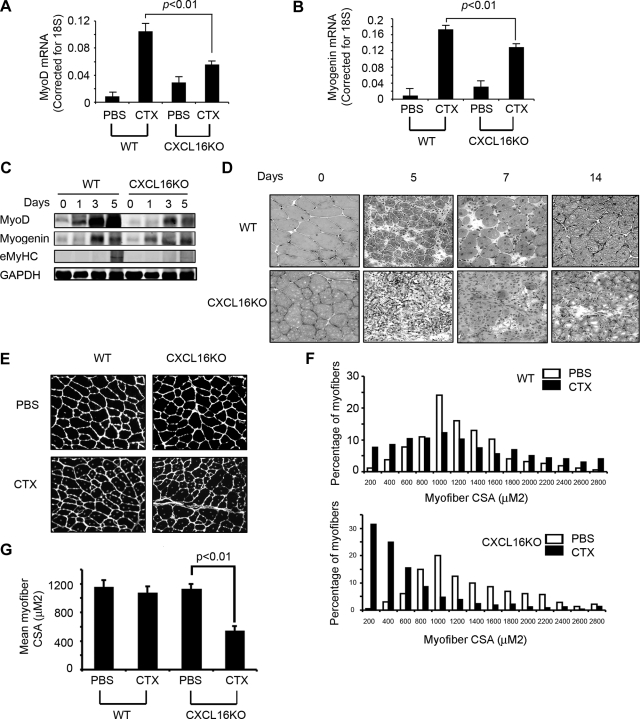
We injured TA muscles of CXCL16KO and wild-type mice and compared the expressions of MyoD and myogenin, markers of satellite cells proliferation and differentiation.10,31 At 3 days after injury, regenerating muscle in CXCL16KO mice had significantly lower levels of MyoD (Figure 2A) and myogenin mRNAs (Figure 2B) compared with values in control mice. Protein levels corresponded to lower mRNA levels. By Western blotting, we found a lower expression of the eMyHC protein. This protein is expressed in newly formed myofibers, which appears 5 days after injury. The major point, however, is that the eMyHC protein in injured muscle of CXCL16KO mice was substantially lower than in wild-type mice, consistent with reduced formation of new myofibers in CXCL16KO mice (Figure 2C).
Figure 2.
Knockout of CXCL16 impairs regeneration of injured muscle. A and B: At 3 days after injury, expression of MyoD (A) or myogenin (B) mRNA was assessed (n = 5 in each group) after normalizing to 18S mRNA. C: The protein levels of MyoD, myogenin, and eMyHC from muscles of control and CXCL16KO mice were assessed by Western blotting at days 1, 3, and 5 after injury (n = 5 in each group). D: At days 5, 7, and 14 after injury, H&E-stained muscles from CXCL16KO and wild-type (WT) mice were examined (n = 5 in each group). E: At 1 month after injury, muscles from WT and CXCL16KO mice were immunostained with laminin (nuclei were stained with 4′,6-diamidino-2-phenylindole; n = 3 in each group). F: The distribution of myofiber sizes from E was calculated from the cross-sectional area (CSA) of 250 myofibers in each sample. G: The average myofiber sizes in injured muscles of control and CXCL16KO mice at 1 month after injury (*P < 0.01 compared with contralateral, uninjured muscles).
At 5 days after injury, H&E-stained muscle sections from CXCL16KO and control mice revealed that CXCL16KO muscles had fewer and smaller newly formed myofibers (indicated by central nuclei) compared with control values. At 7 and 14 days after injury, CXCL16KO muscles still had fewer myofibers (ie, exhibiting central nuclei), which were smaller and more disorganized with more interstitial space (Figure 2D). These differences had long-term consequences. After 1 month, the size distribution of myofibers in control mice had virtually returned to levels present in uninjured muscles. In contrast, in CXCL16KO mice, the size distribution of regenerated myofibers was much smaller and less uniform (Figure 2, E–G). These results indicate that CXCL16 is required for satellite cell proliferation, differentiation, and muscle regeneration.
Knockout of CXCL16 Favors Recruitment of Neutrophils over Monocytes/Macrophages
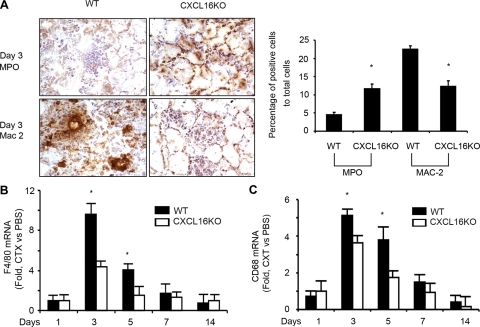
Injured muscle produces cytokines/chemokines that attract neutrophils and macrophages into injured tissue, and these cells contribute to the processes of muscle regeneration.32,33,34,35 To explore if CXCL16 regulates neutrophils and monocytes infiltration into injured muscle, we assessed the types of infiltrating cells by immunostaining the cell surface markers, myeloperoxidase and Mac-2 (myeloperoxidase is a peroxidase abundantly present in neutrophils, and Mac-2 is a ∼30 kDa carbohydrate-binding protein expressed on the surface of macrophages.). In CXCL16KO mice, compared with wild-type mice, there was a greater infiltration of neutrophils cells and fewer Mac-2-positive macrophages at 3 days after injury (Figure 3A). Quantification of these differences is in the right panel of Figure 3A. We also evaluated macrophage-specific genes, CD68 and F4/80, by RT-PCR at different days after injury. CXCL16KO reduced macrophage infiltration in muscle compared with control values (Figure 3, B and C).
Figure 3.
Knockout of CXCL16 increases the recruitment of neutrophils and decreases recruitment of macrophages. A: Infiltration of neutrophils and macrophages was detected by immunohistochemical staining with antimyeloperoxidase (MPO) or Mac-2. The percentage of positive cells per 2000 cells is indicated on the right (*P < 0.01 different from control). B and C: The mRNA expression of macrophage markers, F4/80 and CD68, from injured muscles of wild-type (WT) and CXCL16KO mice was determined by RT-PCR (values normalized to 18S mRNA; *P < 0.01 versus CXCL16KO values; n = 5 mice in each group).
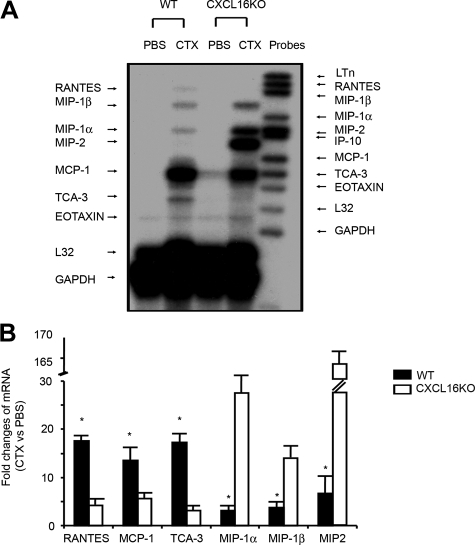
We assessed the expression of certain chemokines/cytokines expressed by neutrophils or macrophages using RNase protection assay and RT-PCR analyses. At 3 days after injury, chemokines expressed by macrophages, regulated on activation normal T cell expressed and secreted, macrophage-inflammatory protein (MIP)-1β, MIP-1α, MCP-1, and T-cell activation-3, were dramatically increased in muscle of wild-type mice compared with results from the uninjured, contralateral muscle (Figure 4A). Comparing results from injured muscles of CXCL16KO mice with those from wild-type mice, we found reduced expression of regulated on activation normal T cell expressed and secreted, MCP-1, and T-cell activation-3 but higher levels of chemokines associated with increased neutrophil infiltration, MIP-1α, MIP-1β, and MIP-2 (Figure 4A). These results from a RNase protection assay were consistent with those from the more quantitative RT-PCR analysis (Figure 4B).
Figure 4.
The expression of chemokines/cytokines in injured muscle of wild-type (WT) mice differs from the pattern in muscles of CXCL16KO mice. A: At 3 days after injury, mRNAs of regulated on activation normal T cell expressed and secreted (RANTES), MIP-1α, MIP-1β, MIP-2, MCP-1, T-cell activation-3, and eotaxin were assessed by RNase protection assay; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and L32 were used as loading controls. Left side indicates genes expressed while probes used are labeled on the right side. B: mRNAs from A were examined using RT-PCR. The fold change present in injured muscle compared with contralateral uninjured muscles are indicated (*P < 0.01 significantly different from muscle of CXCL16KO mice; n = 3 mice in each group).
Interstitial Fibrosis Develops in Injured Muscle of CXCL16KO Mice
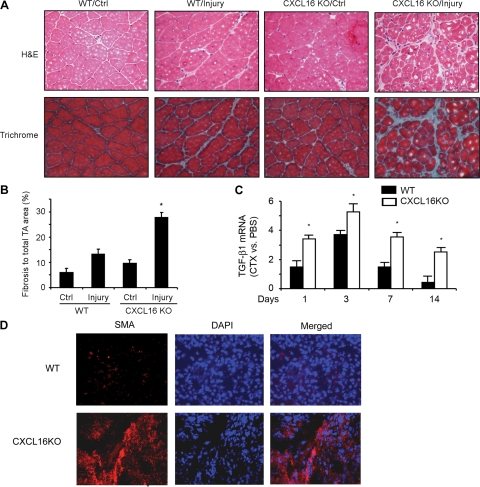
Suppression of macrophage infiltration can lead to increased expression of fibrosis-related genes with the development of fibrosis in injured muscles.13 Since we found that the absence of CXCL16 was associated with decreased macrophage infiltration into injured muscles, we examined if there is more fibrosis in regenerating muscle of CXCL16KO mice. At 1 month after injury, collagen deposition was detected by Gomori trichrome histochemical staining. There was more fibrosis in injured muscles of CXCL16KO mice compared with results from control mice (Figure 5A, lower panel, and B), and H&E staining showed that the fibrosis occurred in areas with regenerating myofibers (indicated by central nucei) (Figure 5A, upper panel). In uninjured, contralateral muscles of both strains of mice, we found no fibrosis. The level of transforming growth factor-β1 (TGF-β1) mRNA was significantly increased in injured muscles from CXCL16KO mice at days 1 to 14 (Figure 5C).
Figure 5.
Interstitial fibrosis develops in injured muscles of CXCL16 KO mice. A: At 1 month after injury, muscles from wild-type (WT) and CXCL16KO mice were histochemically stained with Gomori trichrome or H&E to identify fibrous tissue and nuclei. Muscle fibers (red color) collagen (green) and nuclei (dark purple) are shown. B: TA Muscle sections were divided into 10 areas and photographed. The average area of containing collagen from each of three WT and three CXCL16KO mice was calculated used the NIS-Elements Br 3.0 software and analyzed statistically between the two groups of mice (*P < 0.05 versus values in injured TA muscle of WT mice). C: At different days after injury, mRNA of TGF-β1 (normalized for 18S) was analyzed in injured muscles of WT and CXCL16 KO mice. The fold change in injured muscle over values from control muscles was calculated (*P < 0.01; vs values in WT mice; n = 3 mice in each group). D: Injured muscles at 5 days after injury were immunostained with anti-α-smooth muscle actin (SMA) antibody.
We also immunostained injured muscles for α-smooth muscle actin, a marker for myofibroblasts at day 5 after injury (Figure 5D). There were abundant α-smooth muscle actin-positive cells in injured muscle of CXCL16KO versus control mice.
Discussion
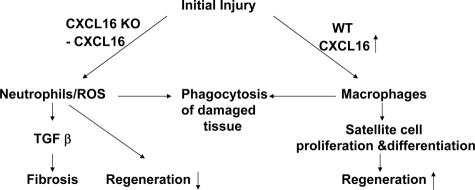
Regeneration of injured muscle involves interactions between inflammatory cells recruited into the injured tissue and satellite cells. Specific chemokines required for the recruitment of inflammatory cells and the successful regeneration of injured muscle are not fully understood. CXCL16, a chemokine with unique properties, is a chemoattractant that recruits cells into inflamed organs, but its role in muscle regeneration is unknown.19 We have found that CXCL16 plays a critical role in muscle regeneration after injury. In injured muscles of CXCL16KO mice, there was decreased infiltration of macrophages and increased neutrophil infiltration versus values in wild-type mice leading not only to impaired muscle regeneration but also to the development of fibrous tissue (Figure 6). Despite the clear demonstration of an important role for CXCL16 in regenerating muscle, we recognize that the processes of regeneration could differ in other models of muscle injury because cardiotoxin induces a severe injury and therefore could change circulating hormones and growth factors.
Figure 6.
A model of the influence of the chemokine, CXCL16, on the processes that regenerate injured muscle.
Cytokines/chemokines can be expressed by different types of cells during muscle regeneration.17 We found that CXCL16 is expressed both in regenerating muscle and in infiltrating, Mac-2 positive macrophages (Figure 1). It is known that the receptor for CXCL16, CXCR6, is present in inflammatory cells,36,37 and we found its expression is increased in regenerating muscle. These results are compatible with our hypothesis that CXCL16 influences the infiltration of inflammatory cells into injured muscle (Figure 1).
Our results point to another critical role for CXCL16 in muscle regeneration; in injured muscles of CXCL16KO mice, the expression of MyoD and myogenin was significantly lower than in muscle of wild-type mice, indicating that CXCL16 influences satellite cell function (Figure 2, B and C). This is important because stimulation of satellite cells is necessary for muscle regeneration.38 Consistent with these conclusions, we found that muscle regeneration was impaired in CXCL16KO mice; even after 1 month, regenerating myofibers were smaller and nonuniform compared with results from wild-type mice (Figure 2).
How does CXCL16 determine muscle regeneration? Previously, we found that CXCL16 functions as a potent chemoattractant for macrophages.27 In control mice, increased CXCL16 expression was associated with macrophage infiltration into injured muscle, but in injured muscle of CXCL16KO mice, there was sharply decreased macrophage recruitment (Figure 3). The function of CXCL16 to regulate macrophage recruitment is relevant because several investigators have demonstrated that macrophages secrete cytokines and growth factors, which can stimulate satellite cells, increasing their proliferation and differentiation into myofibers.8,9,10,11,12 Indeed, infiltration of macrophages is essential for successful regeneration of injured muscle; in the absence of macrophages, muscle regeneration is sharply impaired.13,14 In addition, our results obtained from isolated satellite cells indicate that CXCL16 directly stimulates their proliferation (Figure 1G).
CXCL16 also can influence neutrophil accumulation since regenerating muscle of CXCL16KO mice had a significant increase in the accumulation of neutrophils (Figure 3). An increase in neutrophil infiltration is relevant because Dumont et al3 reported that blocking the infiltration of neutrophils into injured muscles improves processes of regeneration. Therefore, an underlying mechanism for CXCL16-induced improvement in regeneration could be related to reduced infiltration of neutrophils because neutrophil can induce cytotoxic reactive oxygen species-mediated damage to myofibers.3,5,6
In addition to establishing a critical role of CXCL16 in recruiting different inflammatory cells into injured muscle, our results indicate that CXCL16 influences the variation in the pattern of cytokine/chemokine expressed in injured muscles. This is of interest because Pelosi et al17 recently reported that muscle overexpression insulin-like growth factor-1 enhances regeneration through a mechanism associated with decreased expression of MIP-1α and MIP-1β. Our results are consistent with this report because we found an increase in MIP-1α, MIP-1β, and MIP-2 and impaired regeneration in injured muscle of CXCL16KO mice compared with events in control mice (Figure 4). Because it has been reported that MIP expression is associated with neutrophil infiltration into injured tissues, an increase in CXCL16 expression in injured muscle would be associated with reduced both MIPs expression and the infiltration of neutrophils.17,27
A novel finding about the consequences of the absence of CXCL16 was the increase in fibrosis in injured muscle along with an increase in TGF-β1 mRNA expression (Figure 5). CXCL16 is not the only chemokine that is associated with muscle fibrosis as Warren et al15 reported that mice with CCR2 receptor KO exhibited impaired regeneration and increased fibrosis in a muscle injury caused by freezing. Since TGF-β1 is associated with the development of fibrosis in other organs, it seems likely that the increase in TGF-β1 in injured muscle of CXCL16KO mice played a role in the development of fibrosis. An increase in TGF-β1 could also inhibit myogenic cell proliferation and differentiation by silencing the transcriptional activation of the MyoD family of transcription factors.39 The increase in TGF-β1 therefore could be another mechanism by which the absence of CXCL16 suppresses muscle regeneration after injury.
In summary, we have identified critical roles for CXCL16 in mediating muscle regeneration (Figure 6). Injury to muscle stimulates the expression of CXCL16 in muscle and in inflammatory cells leading to increased macrophage infiltration plus reduced neutrophil infiltration. These events repair of injured muscle and prevent fibrosis from developing in injured muscles.
Footnotes
Address reprint requests to Jie Du, Ph.D, Nephrology Division, M/S: BCM 285, One Baylor Plaza, Alkek N-520, Baylor College of Medicine, Houston, TX 77030. E-mail: jdu@bcm.edu.
Supported by a grant from Satellite Healthcare to L.Z. and National Institutes of Health grants R37 DK37175 and P50 DK64233 to W.E.M., R01 DK62828 to J.D., and AI055554 to S.H.
References
- Grefte S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Skeletal muscle development and regeneration. Stem Cells Dev. 2007;16:857–868. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont N, Bouchard P, Frenette J. Neutrophil-induced skeletal muscle damage: a calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1831–R1838. doi: 10.1152/ajpregu.90318.2008. [DOI] [PubMed] [Google Scholar]
- McLoughlin TJ, Mylona E, Hornberger TA, Esser KA, Pizza FX. Inflammatory cells in rat skeletal muscle are elevated after electrically stimulated contractions. J Appl Physiol. 2003;94:876–882. doi: 10.1152/japplphysiol.00766.2002. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37:284–286. doi: 10.1136/bjsm.37.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza FX, McLoughlin TJ, McGregor SJ, Calomeni EP, Gunning WT. Neutrophils injure cultured skeletal myotubes. Am J Physiol Cell Physiol. 2001;281:C335–C341. doi: 10.1152/ajpcell.2001.281.1.C335. [DOI] [PubMed] [Google Scholar]
- St. Pierre BA, Tidball JG. Macrophage activation and muscle remodeling at regenerative responses in ischemic muscle. Am J Pathol. 1994;145:1463–1471. [PMC free article] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J. 2002;16:1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- Winn N, Paul A, Musaro A, Rosenthal N. Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease. Cold Spring Harb Symp Quant Biol. 2002;67:507–518. doi: 10.1101/sqb.2002.67.507. [DOI] [PubMed] [Google Scholar]
- Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A:822–832. [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, Yoshie O, Yonehara S. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75:267–274. doi: 10.1189/jlb.1003465. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. Bonzo/CXCR6 expression defines type 1-polarized T cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Katakai T, Lee JH, Nambu Y, Nakajima-Nagata N, Gonda H, Sugai M, Shimizu A. A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion. Int Immunol. 2006;18:301–311. doi: 10.1093/intimm/dxh369. [DOI] [PubMed] [Google Scholar]
- Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, Kawano K, Ohshima S, Chihara R, Itoh K, Yonehara S, Ohno H. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol. 2006;176:43–51. doi: 10.4049/jimmunol.176.1.43. [DOI] [PubMed] [Google Scholar]
- van d V, van Lieshout AW, Toonen LW, Sloetjes AW, Van Den Berg WB, Figdor CG, Radstake TR, Adema GJ. Elevated CXCL16 expression by synovial macrophages recruits memory T cells into rheumatoid joints. Arthritis Rheum. 2005;52:1381–1391. doi: 10.1002/art.21004. [DOI] [PubMed] [Google Scholar]
- Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005;174:277–283. doi: 10.4049/jimmunol.174.1.277. [DOI] [PubMed] [Google Scholar]
- Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Truong LD, Li P, Zhang P, Johnson RJ, Wilson CB, Feng L. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Lee IH, Wang X, Shang H, Zhang L, Du J, Mitch WE. PTEN expression contributes to the regulation of muscle protein degradation in diabetes. Diabetes. 2007;56:2449–2456. doi: 10.2337/db06-1731. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Myogenicity in vitro and in vivo of mouse muscle cells separated on discontinuous Percoll gradients. J Neurol Sci. 1988;85:197–207. doi: 10.1016/0022-510x(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol. 2001;91:1669–1678. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- Peterson JM, Trappe TA, Mylona E, White F, Lambert CP, Evans WJ, Pizza FX. Ibuprofen and acetaminophen: effect on muscle inflammation after eccentric exercise. Med Sci Sports Exerc. 2003;35:892–896. doi: 10.1249/01.MSS.0000069917.51742.98. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Sorichter S, Mair J, Berg A, McKenzie DC. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol. 2001;84:180–186. doi: 10.1007/s004210170002. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U, Orthenblad N, Sjodin B, Richter EA. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol. 1997;498(Pt 1):239–248. doi: 10.1113/jphysiol.1997.sp021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaida MM, Gunther F, Wagner C, Friess H, Giese NA, Schmidt J, Hansch GM, Wente MN. Expression of the CXCR6 on polymorphonuclear neutrophils in pancreatic carcinoma and in acute, localized bacterial infections. Clin Exp Immunol. 2008;154:216–223. doi: 10.1111/j.1365-2249.2008.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. CXCR6 promotes atherosclerosis by supporting T cell homing, interferon-γ production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474. [DOI] [PubMed] [Google Scholar]
- Gnocchi VF, Ellis JA, Zammit PS. Does satellite cell dysfunction contribute to disease progression in Emery-Dreifuss muscular dystrophy? Biochem Soc Trans. 2008;36:1344–1349. doi: 10.1042/BST0361344. [DOI] [PubMed] [Google Scholar]
- Martin JF, Li L, Olson EN. Repression of myogenin function by TGF-β1 is targeted at the basic helix-loop-helix motif and is indpendent of E2A products. J Biol Chem. 1992;267:10956–10960. [PubMed] [Google Scholar]