Abstract
Perinatal white matter injury, or periventricular leukomalacia (PVL), is the most common cause of brain injury in premature infants and is the leading cause of cerebral palsy. Despite increasing numbers of surviving extreme premature infants and associated long-term neurological morbidity, our understanding and treatment of PVL remains incomplete. Inflammation- or ischemia/hypoxia-based rodent models, although immensely valuable, are largely restricted to reproducing short-term features of up to 3 weeks after injury. Given the long-term sequelae of PVL, there is a need for subchronic models that will enable testing of putative neuroprotective therapies. Here, we report long term characterization of a neonatal inflammation-induced rat model of PVL. We show bilateral ventriculomegaly, inflammation, reactive astrogliosis, injury to pre-oligodendrocytes, and neuronal loss 8 weeks after injury. We demonstrate neuroprotective effects of oligodendrocyte precursor cell transplantation. Our findings present a subchronic model of PVL and highlight the tissue protective effects of oligodendrocyte precursor cell transplants that demonstrate the potential of cell-based therapy for PVL.
Premature, low-birth weight infants are commonly diagnosed with perinatal white matter damage, which leads to long-term neurological deficits including cerebral palsy.1,2,3,4 Congenital encephalomyelitis was described almost 150 years ago, a disease in newborn children characterized by pale softened zones of degeneration within the deep white matter surrounding lateral ventricles and is now often referred to as periventricular leukomalacia (PVL).5 The white matter damage that occurs in preterm infants has more recently been shown to be also accompanied by significant cerebral-cortex and deep-gray matter abnormalities leading to neurodegeneration and altered neurobehavioral performance.6
PVL is characterized by selective oligodendrocyte precursor cell (OPC) loss resulting in delayed or disrupted myelination, white matter atrophy and ventriculomegaly, neuronal loss, and cyst and scar formation. Extreme premature birth (approximately 23 to 32 weeks) accompanied by inflammation or infection corresponds to the period when immature dividing and differentiating oligodendrocytes predominate in the cerebral white matter.7,8,9,10 PVL has a complex etiology. The two most important determinants are cerebral hypoperfusion and maternal intrauterine infection. Periventricular white matter is susceptible to hypoperfusion due to the comparative immaturity of the periventricular vasculature of the preterm infant.11,12 Early oligodendrocyte lineage cells are vulnerable to the consequences of hypoperfusion and subsequent microglial and astrocytic activation on account of amplified glutamate receptor-mediated responses and lack of efficient antioxidant protection.13,14,15,16,17,18 Maternal infection, recently implicated as a causative factor in the pathogenesis of PVL,19,20,21,22,23 is believed to initiate an inflammatory/cytokine cascade that results in the release of early-response pro-inflammatory cytokines such as tumor necrosis factor–α (TNF-α), interleukin−1 β (IL-1β) and interleukin−6 (IL-6), and causes damage to immature oligodendrocytes.24 TNF-α appears to have a particularly important role in PVL pathogenesis with in vitro evidence suggesting direct damage to OPCs,25,26 while IL-1β and IL-6 modulate injury indirectly.27,28
Although cells of the oligodendrocyte lineage are regarded as the primary target in the pathogenesis of PVL, there is increasing evidence that neonatal white matter damage is accompanied by gray matter abnormalities, including neuronal loss, impaired axonal guidance, and altered synaptogenesis.6,29 Premature newborns affected by PVL often have smaller cerebral cortex and deep gray matter volumes, reduced cortical neurons, and alterations in the orientation of central white matter fiber tracts.6,30,31,32 Together, these data suggest that developing neurons, like immature oligodendrocyte lineage cells, are also vulnerable to injury caused by inflammatory response or hypoxia.
Aspects of PVL have been modeled by ischemia/hypoxia and inflammation-mediated rodent models. Animal models of hypoxia-ischemia have clearly shown that following brain injury there is reduced myelination, enlarged ventricles, loss of neurons and damage to axons and dendrites and altered neurobehavioral performances.33,34,35,36,37,38,39 Experimentally induced inflammation has been used in a number of studies to model PVL pathology and test potential treatments.23,40,41,42 Administration of the endotoxin lipopolysaccharide (LPS), a potent inducer of innate immune response and inflammation,43,44 either intracerebrally during early neonatal period, intrauterine or peritoneally to a pregnant mother results in inflammation and hypomyelination.20,22,23,38 Moreover, in animal models of LPS-induced PVL neuronal loss and a reduction in neurite length in the parietal cortex has been observed.42,45 In vitro evidence suggests LPS is not directly toxic to OPCs, but causes injury through the activation of Toll-like receptor 4-positive microglia, which is a source of pro-inflammatory cytokines, nitric oxide, and free radicals.44,45,46 Furthermore, it has been shown that LPS-induced inflammation increases the susceptibility of white matter to injury in response to otherwise “harmless” subthreshold hypoxic-ischemic insult.47 Current models of PVL are typically short term, with studies using either hypoxia-ischemia or inflammation rarely extending analysis beyond 14 days. There is therefore a need to evaluate the longer-term, subchronic consequences of LPS injury and specifically address whether hypomyelination and neuronal injury are self-limiting or indeed spontaneously repair.
Cellular therapeutic strategies are predicated on cell replacement and/or tissue protection independent of specific cellular differentiation. Progenitor cells including OPCs have previously been used for cellular replacement of damaged or lost cells in a variety of CNS injury models where damage to myelin and neuronal loss occur.48,49,50,51,52,53 Furthermore, there is accumulating evidence that implicates progenitor cell mediated neuroprotection through a variety of mechanisms including graft derived neurotrophic support independent of directed differentiation.54,55 Moreover, there is evidence to suggest that OPCs secrete factors that are capable of supporting neuronal survival,56,57,58,59,60,61 thus suggesting they may be a potential therapeutic source for replacing lost or damaged cells and protecting healthy tissue following neonatal brain injury. In this study we have examined the subchronic effects of LPS induced injury and then examined the putative neuroprotective effects of OPC transplantation.
Materials and Methods
Intracerebral LPS Lesions
Intracerebral injections of LPS (1 mg/kg, n = 25) or saline (n = 14) to 5-day-old (P5) rats were performed as previously described.23 Briefly, postnatal day 5 (P5) Sprague Dawley rats (Harlan, UK) were anesthetized by placing them on ice, before being placed in full body mold to prevent movement during the injection. Bregma was identified using white light lamp. A needle was then used to puncture the skin and skull at the following coordinates from bregma: anteroposterior (AP) −1 mm, medial-lateral (ML) −1 mm, dorsal-ventral (DV) −2 mm.22 The dose of LPS was chosen based on the results of previous studies demonstrating white and gray matter injury.22,23 Using a 10-μl precise Hamilton micropipette, a 1-μl solution of LPS (10 mg/kg) was infused over 3 minutes into the periventricular area of the neonatal rat brain (see Supplemental Figure 1 at http://ajp.amjpathol.org). The needle was left in situ for a further 3 minutes before slowly being withdrawn. The P5 rats were then placed on a heated blanket before being return to the mother. On weaning the rats were given food and water ad libitum.
Tissue Dissection
All cell culture reagents were obtained from Invitrogen/Gibco (UK) unless otherwise specified. Experimental animals in this study were used under the United Kingdom Animals (Scientific Procedures) Act 1986. Green fluorescent protein (GFP) neonatal rats [gift from Dr. Okabe, postnatal day (P)0-2] were terminated by lethal injection of Euthatal (sodium pentobarbital, 0.05 ml). Following removal of meninges, cortex was isolated and manually minced before enzymatic digestion with 0.1% trypsin/EDTA, followed by 0.001% DNase (Sigma, UK) and centrifugation at 1500 rpm for 5 minutes. The resulting cell pellet was resuspended in triturating solution (containing 0.05% w/v trypsin inhibitor, 1% w/v bovine serum albumin, and 0.002% w/v DNase in Hanks balanced salt solution) and triturated using a flamed glass pipette to obtain a single cell suspension.
Mixed Glial Preparation
To obtain OPC populations for transplantation neonatal cerebral cortex suspensions were seeded onto flasks (Poly-D-Lysine-coated, approximately 1.5 × 106 cells seeded/T75 flasks) and maintained in serum buffer [Dulbecco’s modified Eagle’s medium (4.5 g/L glucose) supplemented with 1% penicillin-streptomycin-fungizone, and 10% fetal calf serum, v/v] for 7 days with complete media changes 24 hours, 3 days, and 6 days, as previously described.62
Transplantation of OPCs into Rat Cortex
One day before transplantation experiments, GFP-mixed glial preparations (7 days) were shaken in an incubated chamber for 1 hour to remove top dwelling microglia. Media was removed and fresh Dulbecco’s modified Eagle’s medium (with 1% penicillin-streptomycin-fungizone, 10% fetal calf serum) was then added and the mixed glial preparations returned to incubator for at least 1 hour. Flasks were then shaken overnight (16 to 18 hours) to remove the OPCs ready for transplantation. OPCs were maintained as a single cell suspension at 8.0 × 104 cells/μl in Hanks balanced salt solution on ice.
The care and treatment of the animals was in accordance with United Kingdom Animals (Scientific Procedures) Act 1986. For the transplantation experiments, Sprague-Dawley postnatal day 11 (P11) neonatal animals previously lesioned at P5 were anesthetized using isoflurane (see Supplemental Figure 1 at http://ajp.amjpathol.org). GFP-OPCs were implanted 1 week after injury. Animals were divided into three groups: LPS-lesioned followed by GFP-OPC transplant (n = 8); LPS-lesioned, followed by vehicle control transplant (n = 8); and control-lesioned, no transplant (saline, n = 6). Animals were placed into a neonatal adaptor to prevent movement during the injection. Coordinates were taken from bregma: AP −1 mm and ML −1 mm. GFP-OPCs (8.0 × 104 cells/μl) or saline control was then drawn into a Hamilton syringe and 1 μl injected unilaterally above the ventricle at a depth of DV −2 mm over 3 minutes. The syringe was left in situ for a further 3 minutes before withdrawing slowly. The pup was then placed on a heated blanket before being returned to the mother.
Immunohistochemistry
Animal groups (n = 6) were terminated at 8 weeks (when the rats reached young adulthood, see Supplemental Figure 1 at http://ajp.amjpathol.org) following LPS lesioning at P5, using an overdose of sodium pentobarbital (80 mg/kg i.p.), and perfused transcardially with 0.1 M/L PBS followed by 4% paraformaldehyde in 0.1 M/L PBS (pH 7.4). The brains were then removed and post-fixed for 4 hours before being immersed in 30% sucrose solution. Immunohistochemistry was performed using standard procedures as previously described.62 Fixed brains were sectioned coronally at 40 μm using a freezing microtome (Leica, UK). A one-in-twelve series of sections was processed for each stain unless otherwise stated. Tissue was washed in Tris-buffered saline (TBS) and subsequently blocked in TBS/0.2% Triton X-100/5% normal donkey serum for 2 hours before overnight incubation at 4°C with primary antibody. Tissue sections were subsequently washed and appropriate secondary antibodies (1:500; Molecular Probes, Paisley, UK) were applied. Finally, the sections were washed three times for 5 minutes each in Tris-buffered non-saline before being mounted on gelatinized slides and coverslipped using Flurosave (Calbiochem, Nottingham, UK). The following antibodies were used: anti-GFP (1:500, rabbit & goat, Molecular Probes); neuronal and neurofilament markers; neuronal nuclei (NeuN) (1:400, mouse, Chemicon); doublecortin (1:500, goat, Santa Cruz Biotechnology, Heidelberg, Germany); neurofilament nonphosphorylated antibody SMI 32 (1:500, Abcam, Cambridge, UK); astrocyte markers; glial fibrillary acidic protein (GFAP, 1:500, mouse CY3 conjugated, Sigma, Dorset, UK); glial cell marker S100 (1:400, rabbit, DAKO, Ely, UK); oligodendrocyte markers; chondroitin sulfate proteoglycan, NG2 (1:200, kind gift of W. Stallcup); oligodendrocyte lineage transcription factor 2 (Olig2, 1:500, rabbit, Millipore, Watford, UK); oligodendrocyte marker RIP recognizing 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) (1:1000, Millipore, Watford, UK); oligodendrocyte marker O4 (1:1000, Chemicon); and inflammatory marker microglial cell marker OX-42 (1:400, cd11b, mouse, Chemicon). For confocal analysis, stacks of images were captured on a Leica TCS-NT-UV confocal laser scanning microscope using a 63 × 1.25 N.A. Leica lens. Typical stacks were composed of 10 to 20 optical sections of 1 μm thickness taken at 0.8 μm intervals. Sequential acquisitions were performed in the different channels to avoid any misinterpretation of the results due to signal cross talk.
Real-Time PCR
Total cellular RNA was extracted from snap frozen LPS-lesioned (n = 4) and saline control-lesioned (n = 4) animals using the RNeasy Mini isolation kit (Qiagen). RNase-free-DNase (New England Biolabs)-treated RNA samples (2 μg) were reverse transcribed in 100 μl with random hexamers using MMLV RT (Invitrogen Ltd, UK) according to the manufacturer’s protocol. PCR was conducted in 25 μl reaction volume using 2 μl cDNA synthesized as described above and forward and reverse primers IL-1 β forward: 5′-GCTTCCTTGTGCAAGTGTCTG-3′, reverse: 5′-CTGTCCATTGAGGTGGAGAGC-3′; TNF-α forward: 5′-CCACGTCGTAGCAAACCACCAAGTG-3′, reverse: 5′-CACAGAGCAATGACTCCAAAG-3′; and hypoxanthine phosphoribosyl-transferase (HPRT) forward: 5′-AGCTACTGTAATGATCAGTCAACG-3′, HPRT reverse: 5′-GAGGTCCTTTTCACCAGCA-3′), with BioTaq polymerase (BioLine Ltd, UK). To compensate for variable RNA and cDNA yields, the expression of HPRT was used as a control for which the optimal number of PCR cycles for linear amplification was determined. The amplification products were analyzed by agarose gel electrophoresis.
Preparation of Semithin Resin Sections
Animals for resin embedding (n = 5 per group) were fixed using 4% glutaraldehyde in PBS. The brain was dissected out as previously described and sections post-fixed overnight in 4% paraformaldehyde. The brain was then sectioned so that a piece of tissue including the cortex, corpus callosum, and periventricular region was transferred to 4% glutaraldehyde for 48 hours before embedding. Sections were washed in PBS, before being placed in a 2% osmium tetraoxide solution (Oxkem Limited, Reading, UK) overnight at 4°C. The next day the tissue was dehydrated in a graded series of ethanol washes (70% ethanol for 15 minutes; 95% ethanol for 15 minutes; 100% ethanol for 10 minutes, repeated twice). Resin (TAAB Labs, Aldermaston, UK) impregnation was performed by washing with propylene oxide (twice for 15 minutes) and then with propylene oxide/resin (50:50, for 3 hours). Following two immersions in 100% resin (minimum of 6 hours each), sections were embedded individually in beam capsules and hardened at 60°C over 24 hours. Semithin sections were cut using 6-mm glass knives on a Leica RM2065 microtome. These were placed on Polysine slides (VWR international, Lutterworth, UK) in a droplet of distilled water and flattened by placing and manually rotating the slide on a heat plate. Once sections were dry, the slide was flooded with toluidine blue (5% in a Borax solution) and heated again until vapors evolved. The stain was removed in a stream of hot water and sections dried on a hot plate. Finally slides were cleared in xylene, before being mounted using distyrene, platicizer, xylene (D.P.X.) mountant solution. Semithin sections (n = 5, a minimum of three sections per animal) were analyzed by counting toluidine blue stained normal myelin, thinly myelinated axons (denoting hypomyelination and potential remyelination), and preserved demyelinated axons (with a total of five counts per section taken).
Ventricle Ratio
For ventricle ratio analysis, brain sections were mounted onto gelatin-coated microscope slides and allowed to dry overnight. Slides were then dehydrated through increasing alcohol before being immersed in H&E (1 minute). Slides were then washed and excess staining was removed before dehydrating in alcohol and finally in xylene before using distyrene, platicizer, xylene (D.P.X.) mountant mounting medium to mount glass coverslips. Slides were allowed to dry overnight before being analyzed. Ventricle surface areas and whole brain surface areas were measured with stereology microscope (Olympus with CAST-Grid analysis software). By dividing the ventricle sizes by the whole brain sizes, ventricle ratios were calculated.
NeuN Counts
For NeuN analysis, brain sections were washed in TBS and subsequently blocked in TBS/0.2% Triton X-100/5% normal donkey serum for 2 hours before overnight incubation at 4°C with biotinylated NeuN (Millipore, Watford, UK). Subsequently sections were washed in TBS, and thereafter incubated with ABC kit (Vector, Peterborough, UK 1:200) at room temperature for 2 hours. After two washes with TBS and one with Tris-buffered non-saline, the sections were briefly incubated with Sigma Fast 3,3′-diaminobenzidine (DAB, Sigma, Dorset, UK) and the reaction stopped with Tris-buffered non-saline. After three washes in Tris-buffered non-saline the sections were mounted. Following mounting sections were dehydrated through alcohols into xylene before using distyrene, platicizer, xylene (D.P.X.) mountant to mount glass coverslips. Following overnight drying the slides were used to analyze neuronal nuclei in the parietal cortex of both lesioned and transplanted groups.
Data Analysis
Images were captured using Lucia or Leica software (Nikon, UK) via a digital camera. SigmaScan Pro (SPSS, Chicago, IL) was used for subsequent quantitative measurements. Immunopositive cell density measurements were made on threshold overlays of brain segments surrounding the corpus callosum and including ventricles and periventricular areas (in which all pixels overlying immunopositive cells had a greyscale value of 68, and all other pixels had a value of 0), such that average intensity could be converted to density measurements by dividing the output by 68. This analysis used an automated thresholding procedure.63,64 GFAP and OX-42 densitometric measurements were made on the resulting combined threshold overlays as a function of distance from the dorsal surface and through the corpus callosum respectively. This method of quantification measures the density of immunopositive objects independent of their individual intensities. For NG2, Olig-2, and S100 analyses, this technique was modified such that within the area of interest, for each antibody stain, irrespective of the density reading it was possible to define how much of the area measured contained a positive signal, with raw images being thresholded before analysis to normalize the results. A percentage of the brain segment containing a positive antibody signal could be generated by dividing the number of positive readings by the total number of potential readings, multiplied by 100. A minimum of four sections per animal were analyzed and for each section readings were taken at similar positions through the cortex, corpus callosum, ventricle, and periventricular areas from which a mean percentage area of the dorsal funiculus containing a positive signal could be calculated, allowing comparisons between LPS-lesioned and vehicle control animals. Averaged GFAP and OX-42 cell density measurements were plotted as line graphs of density of signal against depth and percentage changes as bar graphs (Sigma Plot, SPSS, Chicago, IL). Statistical analysis was performed using one way analysis of variance (with the Holm-Sidak posthoc analysis) or t-test and significance was only assumed if P < 0.01 (SigmaStat 3.0). All error bars represent SE.
Results
Unilateral LPS Injection Leads to Bilateral Ventricle Dilatation
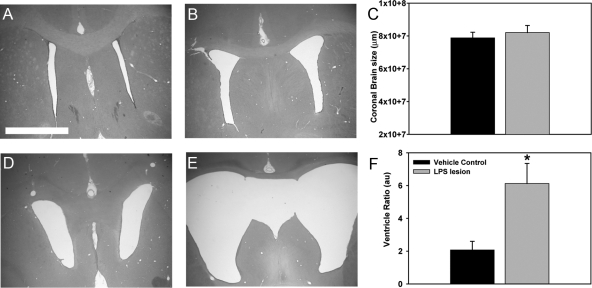
The subchronic consequences of LPS-induced injury in neonatal rats were first characterized. Animals were examined 8 weeks after unilateral injections of vehicle saline (n = 6) or LPS (n = 8) (10 mg/kg). Stereological analysis of H&E-stained brain section undertaken to compare whole brain and ventricle sizes revealed no significant differences in coronal brain size between lesion or control groups at 8 weeks (Figure 1C). However, an average increase in ventricle size of 187% was observed (Figure 1F). Unilateral injection of LPS resulted in significant bilateral ventricle enlargement while saline did not alter ventricle size (Figure 1, A–B, D–E). This enlargement of ventricles led to a significant increase in ventricle ratio (ventricle size/whole brain) with an average of threefold increase in ventricle ratio in the LPS-injected group compared with vehicle control (Figure 1F, P < 0.01).
Figure 1.
Unilateral LPS injury results in bilateral ventricle dilation. Tissue sections from LPS-lesioned (n = 8) and vehicle control (n = 8) animals were stained using H&E and stereology software was used to measure whole brain sizes and ventricle ratios. H & E analysis revealed that compared with vehicle control (A–B), unilateral LPS lesioning resulted in bilateral enlargement of the ventricles (D–E). The average coronal brain size did not differ between groups (C). However, a significant increase in ventricle ratio of almost threefold was observed in LPS-lesioned animals at 8 weeks post-lesioning (F, * P < 0.01). Scale bar = 500 μm.
Pathological Characterization
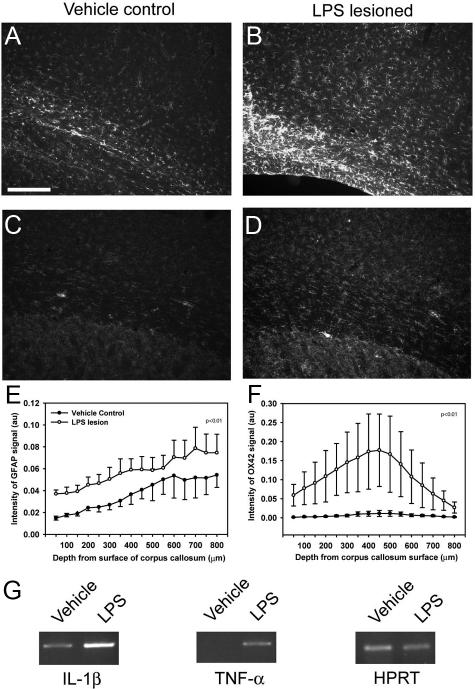
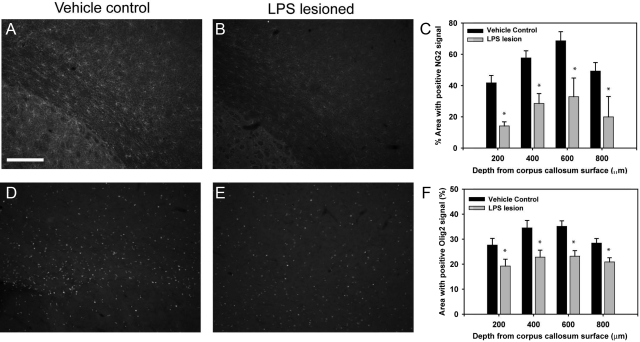
Having established ventriculomegaly we next undertook detailed histology at 8 weeks. Astrogliosis was measured using GFAP immunoreactivity. A significant increase in GFAP immunoreactivity was observed at 8 weeks following LPS lesioning (Figure 2A–B, E, P < 0.01). Additionally, the LPS-injected group showed a higher percentage area with S100-positive signal, as compared with vehicle control group, further confirming increased reactive astrocytosis (data not shown). Inflammation, as measured by the activated microglial marker OX-42, was significantly elevated in LPS-lesioned animals compared with vehicle control (Figure 2, C, D, F, P < 0.01). Semiquantitative PCR analysis also revealed that regions of the brain surrounding the epicenter of the LPS injection sites had higher levels of mRNA for IL-1β and TNFα compared with corresponding areas in the vehicle control groups (Figure 2G). NG2 and Olig2 immunoreactivity, studied to quantify oligodendrocyte lineage cell loss, was significantly lower in LPS-lesioned animals than in vehicle control at 8 weeks in areas around the periventricular white matter (Figure 3, A–C, D–F respectively, P < 0.01). Semithin analysis of toluidine blue-stained sections revealed significantly thinner hypomyelinated axons in LPS-lesioned animals, as compared with the vehicle control (see Supplemental Figure 2, P < 0.05, at http://ajp.amjpathol.org) and a decrease in the total number of surviving axons in LPS-lesioned animals (93.9 ± 0.7% and 96.4 ± 0.5% respectively, P < 0.05).
Figure 2.
LPS injury results in astrogliosis and inflammation. Astrogliosis was observed using GFAP immunoreactivity. LPS-lesioned animals had significantly more immunoreactivity using imaging based-threshold analysis than vehicle control animals (A–B, E, P < 0.01). Inflammation, as measured by the activated microglial marker OX-42 had a significantly higher immunoreactive signal in LPS-lesioned animals, as compared with vehicle control 8 weeks following injury at all depths from the corpus callosum surface (C–D, F, P < 0.01). Semiquantitative PCR analysis of brain regions surrounding the epicenter of lesioning revealed significant increase of inflammatory cytokines Il-1β and TNF-α in the LPS lesioned animals compared with the vehicle control (G). Scale bar = 200 μm.
Figure 3.
LPS injury results in loss of oligodendrocyte precursors. Tissue sections were labeled for NG2 and Olig2 as markers of oligodendrocyte precursor cells 8 weeks following lesioning, and threshold analysis was performed. Analysis revealed that there was a significant loss in NG2 positive immunolabeling (A–C) and Olig2-positive immunolabeling (D–F) in the LPS-lesioned animals, as compared with vehicle control. Scale bar = 200 μm, *P < 0.01.
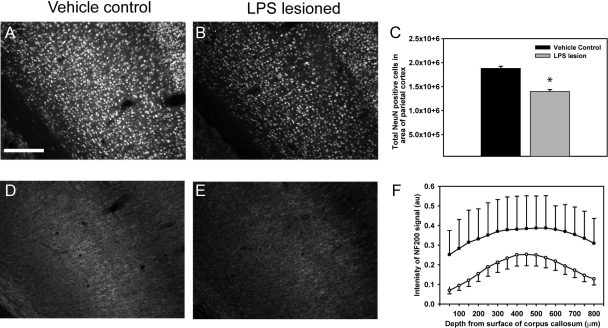
To determine neuronal pathology we undertook stereological NeuN analysis and quantitative neurofilament immunohistochemistry. Significant cortical neuronal loss (1.40 × 106), as compared with vehicle control animals (1.88 × 106) was evident at 8 weeks following LPS lesioning (Figure 4, A–C, P < 0.01). This was accompanied by a significant reduction in cortical neurofilament (NF200) staining, which was not observed in the vehicle control animals (Figure 4, D–F, P < 0.01).
Figure 4.
LPS injury results in cortical neuronal cell loss and reduced neurofilament. Eight weeks following injection with vehicle or LPS, brain sections were labeled with NeuN before stereology-based analysis. LPS lesioning resulted in a significant reduction in cortical neuronal cells, as compared with vehicle control at 8 weeks (A–C). This neuronal cell loss was accompanied by a reduction in immunoreactivity of NF200 following threshold analysis in the LPS-lesioned animals, as compared with the vehicle control animals (D–F). Scale bar = 200 μm, *P < 0.01.
Together these findings suggest that 8 weeks after unilateral LPS lesion, key chronic histological aspects of PVL persist including bilateral ventriculomegaly, astrogliosis, inflammation, oligodendrocyte loss, and neuronal loss.
Oligodendrocyte Precursor Cell Isolation and Characterization
To test whether OPC transplantation may influence the longer-term, subchronic effects of LPS-mediated injury, we next isolated enriched OPCs. Before transplantation paired aliquots of freshly isolated minimally manipulated GFP-OPCs derived from standard neonatal mixed glial preparations62 were characterized. Following plating for 24 hours, the majority of cells expressed the antigens NG2 (96.0 ± 2.63%) and Olig2 and A2B5 (93.2 ± 5.37%), consistent with an OPC phenotype (see Supplemental Figure 3, A–C, F, at http://ajp.amjpathol.org). GFAP+ cells made up <1% of the population in vitro (0.65 ± 0.51%). The remainder of cells were identified as differentiating oligodendrocyte lineage cells, expressing O4 (5.70 ± 2.90%, see Supplemental Figure 3D at http://ajp.amjpathol.org). No neuronal differentiation was observed. All subsequent experiments used the highly enriched minimally manipulated OPC population without further in vitro propagation or ‘priming’ treatment steps before transplantation.
Graft-Derived GFP Cells Survive, Integrate and Retain a Glial Fate Following Transplantation into LPS-Lesioned Neonatal Rat Brain
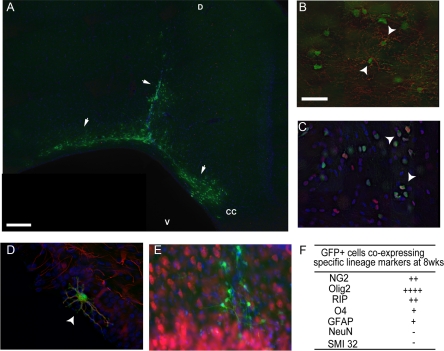
One week following LPS lesioning at P5, animals received unilateral delivery of either vehicle control (n = 8) or GFP-cell transplantation (n = 8) adjacent to the LPS injection. Animals were sacrificed 7 weeks after transplantation (8 weeks post-lesioning). A substantial number of engrafted GFP+ cells were observed in transplanted animals (Figure 5A). GFP+ cells were primarily identified within the periventricular white matter and were also observed to be migrating along the white matter tract of the cingulum around the periphery of enlarged ventricles confirming engraftment and integration into the host neonate. The overwhelming majority of GFP+ (green) cells co-expressed NG2 (Figure 5, B and F) and Olig2 (Figure 5, C and F). Co-staining was confirmed by confocal microscopy (Figure 5, B–C) GFP+ cells were also identified co-expressing further markers of the oligodendrocyte lineage (RIP and O4, Supp. Figure 4 at http://ajp.amjpathol.org). GFP+ cells were also identified by confocal analysis in close proximity to astrocytes and indeed a small number co-localized with the astrocytic marker GFAP (Figure 5, D and F, Supplemental Figure 3 at http://ajp.amjpathol.org). Importantly, GFP+ cells were negative for the neuronal markers doublecortin, NF200, SMI 32, and NeuN (Figure 5, E and F, Supplemental Figure 4 at http://ajp.amjpathol.org).
Figure 5.
GFP-OPCs survive transplantation for at least 8 weeks and commit to glial progeny. GFP cells were clearly visible in both gray and white matter at 8 weeks post-transplantation in lesioned animals (arrows, A n = 8, where D: dorsal surface, V: ventral and CC: Corpus callosum). Using confocal imaging GFP-positive (+) cells (green) were identified to express phenotypic markers for NG2-positive cells (arrowhead, red, B), Olig2-positive cells (arrowhead, red, C) and GFAP positive astrocytes (arrowhead, red, D). No NeuN co-labeled cells were observed at 8 weeks (E). Semiquantitative analysis revealed the majority of GFP-positive cells co-labeled with Olig2 and NG2, while none were NeuN co-labeled (F). In all images, Hoechst-positive nuclei were labeled in blue. (−:<1%, +:<25%, ++:25 to 50%, +++:50 to 75%, ++++:>75% of GFP+ cells). Scale bar = 200 μm (A), 100 μm (B, C, E) and 50 μm (D).
OPC-Transplantation Reduces Endogenous Neuronal and Oligodendrocyte Cell Loss Following LPS Lesion
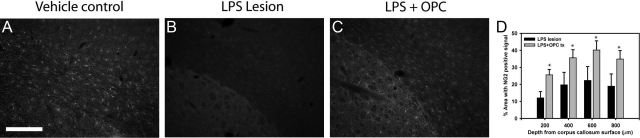
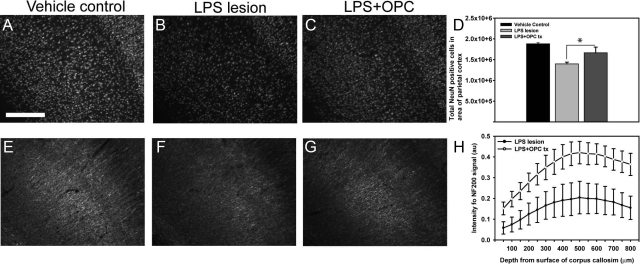
Transplantation with GFP-OPCs did not reduce the ventricle ratio following LPS lesion (see Supplemental Figure 5, A–C at http://ajp.amjpathol.org). Analysis of GFP+ cell-transplanted animals and the saline-treated control group revealed elevated astrogliosis 8 weeks after LPS lesioning (data not shown). We examined whether OPC transplantation prevented the loss of immature oligodendrocytes following LPS lesioning. Following transplantation there was a significant increase in NG2-positive immunoreactivity, as compared with the control-treated animals in regions adjacent and but not containing GFP-OPC+ cells (Figure 6, A–D, P < 0.01). Having confirmed grafted GFP+ cells do not differentiate into neurons we next investigated the neuroprotective potential of transplanted GFP-OPCs by stereology and image-based threshold analysis. Stereology counts of parietal cortex of LPS-lesioned animals with and without GFP-OPC transplants revealed that GFP-OPC treated animals had significantly reduced loss of neurons compared with LPS lesion alone animals in regions adjacent to the cellular transplant (Figure 7, A–D, P < 0.01) and an increase in NF200 positive signal compared with LPS alone was also observed (Figure 7, E–H, P < 0.01). Together, these findings are consistent with a neuroprotective effect of transplanted OPCs independent of directed neuronal differentiation.
Figure 6.
GFP-OPC transplantation increases density of NG2-positive OPCs following LPS-lesion. Following LPS lesioning and transplantation, sacrificed animals were analyzed for NG2 oligodendrocyte precursor cell loss at 8 weeks. Threshold analysis revealed a significant increase in NG2-positive immunolabeling, at every depth from corpus callosum surface measured, (A–D, D, *P < 0.01) in the LPS-lesioned animals (B, n = 10) that received the GFP+ cell transplant, as compared with saline treated control (C, n = 10) animals. Scale bar = 200 μm.
Figure 7.
GFP-OPC transplantation reduces neuronal loss. Following LPS lesioning and transplantation, sacrificed animals were analyzed for neuronal cell loss using NeuN and NF200. GFP-OPC transplantation was observed to significantly reduce cortical neuronal loss, as compared with saline-treated lesioned animals (A–D). Using stereology software, NeuN-positive cell counts were significantly higher in GFP-OPC transplanted groups than LPS alone (A–D, *P < 0.01). Neuroprotection of neuronal filaments from transplanted GFP-OPCs was also observed with an increase in neurofilament (NF200) immunoreactivity, as compared with LPS lesion alone (E–H). Scale bar = 200 μm.
Discussion
We report longer term, subchronic characterization of a neonatal LPS-induced rat model of PVL and show that bilateral ventriculomegaly, inflammation, reactive astrogliosis, injury to immature oligodendrocytes, and neuronal loss persists 8 weeks after lesioning. We demonstrate unilateral transplantation of OPCs results in neuronal protection, an effect not due to cell replacement. These findings demonstrate a neuroprotective effect of OPC transplantation.
Periventricular leukomalacia is a major contributor to cerebral palsy with approximately 50% of cerebral palsy cases associated with PVL.2 Although PVL is a chronic disorder, current animal models of white matter damage generally model short-term injury typically focused on early time points in the course of disease progression from 3 days to 3 weeks post-lesion.15,22,23,36,38,44,65 PVL leads to chronic disease with long-term clinical implications and thus it is important to evaluate later time points. In the current study we have extended the temporal analysis of the consequence of LPS mediated injury to 8 weeks. We demonstrated that intracerebral injections of LPS increased the ventricle size independent of any alteration in whole brain size at 8 weeks, as confirmed by significant increases in bilateral ventricle ratios. These findings are in agreement with those of previous studies examining earlier time points in this model,20,23,40,44 where ventriculomegaly was observed and ratios were up to 10-fold greater than control.44,66 This ventricle dilation may be the result of impaired myelination and white matter rarefaction, which alters the tensile strength of the ventricular wall. LPS-induced inflammation also alters fluid transport.11,12
The acute LPS-induced model of neonatal damage leads to significant decreases in immature oligodendrocytes23 loss of neurons and axons42 and induction of pro-inflammatory cytokines, TNFα, IL-1β, and IL-6.20 We observed in the subchronic model, a persistent decrease in NG2 and Olig2 signal, markers of immature oligodendrocyte cells indicating death or delayed development of these cells that continues for at least 8 weeks after LPS injury. This decrease is similar to that observed by others using the LPS injury as an acute model. We also observed that LPS-lesioned brains had a decrease in myelin surrounding axons by semithin analysis. Similarly, we observed a sustained level of astrogliosis for up to 8 weeks following LPS lesioning. This was accompanied by significant inflammation in the LPS-injected but not control brains as shown by increased activated microglial signal and elevated TNFα and IL-1β, which have previously been reported in the lesioned neonates at the acute time points.20,23,44,45,66 Previous studies have identified that the inflammatory environment caused by LPS leads to white matter damage,40 but also neuronal and axonal damage that correlates to the impaired physical development and neurobehavior.42,66 We also report persistent neuronal loss that extends to 8 weeks following LPS injury. Together our pathological findings reveal that astrogliosis, inflammation, oligodendrocyte, and neuronal loss persist at 8 weeks and thus allow more accurate evaluation of putative neuroprotective therapies such as cell transplantation. Longer term analysis into aged adults would be of interest to determine whether neuronal loss is progressive.
Previous studies using the acute LPS lesion have observed beneficial effects with antioxidants and anti-inflammatory agents, such as α-phenyl-n-tert-butyl-nitrone, minocycline, and N-acetyl cysteine.1,40,66,67,68 However, to the best of our knowledge, no cellular therapy to replace or protect oligodendrocytes and neurons lost following LPS-mediated injury has been described. In the current study we examined whether OPC transplantation had any therapeutic benefit. Previously the effects of both endogenous and exogenous OPCs following demyelination and CNS lesioning have been observed.49,50,51,52,53 Cell therapy has typically been used to replace lost and damaged cells through targeted cellular differentiation. However, accumulating evidence now reveals that exogenously transplanted cells or endogenous cells stimulated in situ are capable of a ‘bystander’ effect implicating progenitor cell-mediated neuroprotection through mechanisms independent of targeted cell differentiation.54 OPCs secrete soluble factors that support neuronal survival.56,57,58
Transplanted highly enriched GFP+ predominantly OPCs freshly isolated from mixed glial preparations survived and integrated into the LPS injured rat brain surviving for at least 8 weeks consistent with previous studies.51 Following transplantation many of the GFP-OPCs retained an immature glial phenotype with no evidence of co-localization with the neuronal markers doublecortin, NeuN and NF200 or more mature oligodendrocytes. In vitro inflammatory cytokines TNFα and interferon-γ have previously been shown to limit maturation of OPC cultures,69 and thus may inhibit mature differentiation in vivo. Furthermore, we have previously reported limited maturation of OPCs on transplantation into the intact neonatal and adult rat brain.62 Following transplantation we observed no change in GFAP+ immunoreactivity compared with saline treated controls although a significantly higher density of NG2 immunoreactivity was observed 8 weeks post-lesioning. Previous studies have reported endogenous and exogenous OPC recruitment to regions of damage following delivery of a therapy and promote repair.52,53,70,71
Importantly, in the current study we observed significant protection from neuronal (NeuN) and axonal (NF200) loss following transplantation of GFP-OPCs into the LPS-lesioned rat brain. The failure to identify any GFP-OPCs co-labeled with NeuN, doublecortin or NF200 suggests that the effect on protecting/reducing the neuronal deficits following transplantation are not due to cell replacement cells but due to indirect tissue protection mediated most likely by amelioration of the local environments. This phenomenon is known as a bystander effect. These attributes have previously been observed following OPC transplantation into both demyelinating lesions where OPCs and myelin are specifically lost49 and in CNS injuries where neuronal populations are targeted.50
Although transplantation did not reduce the ventricle ratio of LPS-lesioned animals, we conclude that OPC transplantation can protect the immature oligodendrocyte and neuronal deficits in a long term model of neonatal white matter damage. Furthermore, to enhance the beneficial effects, combination of therapies using antioxidants and/or anti-inflammatory agents could help reduce the ventriculomegaly while the cellular therapy reduces OPC loss and neuronal damage.
To conclude, we have demonstrated that the LPS-induced rodent model of PVL can be extended to ‘chronic’ stages with continued oligodendrocyte and neuronal loss. Moreover, we have shown that OPC-transplantations have a protective effect on neurons and oligodendrocytes. These findings open the way to study therapeutic strategies for PVL-like white and gray matter injury on a long-term rodent model and have implications for the development of cell-based treatment strategies for PVL.
Supplementary Material
Footnotes
Address reprint requests to Siddharthan Chandran, Euan MacDonald Centre, University of Edinburgh, Chancellors Building, Little France Crescent, Edinburgh, EH16 4SB. E-mail: sc222@cam.ac.uk.
Supported by grants from the MS Society (S.C. and D.J.W.), National Institute for Health Research [Cambridge Comprehensive Biomedical Research Centre] (S.C), Erasmus grant and Netherlands Brain Foundation (M.v.B.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
D.J.W. and M.V.B. contributed equally to this work.
References
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Drougia A, Giapros V, Krallis N, Theocharis P, Nikaki A, Tzoufi M, Andronikou S. Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15-year review. Early Hum Dev. 2007;83:541–547. doi: 10.1016/j.earlhumdev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Resch B, Vollaard E, Maurer U, Haas J, Rosegger H, Muller W. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leucomalacia. Eur J Pediatr. 2000;159:663–670. doi: 10.1007/pl00008403. [DOI] [PubMed] [Google Scholar]
- Pierrat V, Duquennoy C, van HI, Ernst M, Guilley N, de Vries LS. Ultrasound diagnosis and neurodevelopmental outcome of localised and extensive cystic periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed. 2001;84:F151–F156. doi: 10.1136/fn.84.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Blumenthal I. Periventricular leucomalacia: a review. Eur J Pediatr. 2004;163:435–442. doi: 10.1007/s00431-004-1477-y. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Leviton A, Gilles F. Ventriculomegaly, delayed myelination, white matter hypoplasia, and “periventricular” leukomalacia: how are they related? Pediatr Neurol. 1996;15:127–136. doi: 10.1016/0887-8994(96)00157-9. [DOI] [PubMed] [Google Scholar]
- Inage YW, Itoh M, Takashima S. Correlation between cerebrovascular maturity and periventricular leukomalacia. Pediatr Neurol. 2000;22:204–208. doi: 10.1016/s0887-8994(99)00153-8. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Okudera T, Hashimoto T. Vascular architecture in white matter of neonates: its relationship to periventricular leukomalacia. J Neuropathol Exp Neurol. 1994;53:582–589. doi: 10.1097/00005072-199411000-00005. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Greco A, Levi G, Minghetti L. Differential lipid peroxidation, Mn superoxide, and bcl-2 expression contribute to the maturation-dependent vulnerability of oligodendrocytes to oxidative stress. J Neuropathol Exp Neurol. 2003;62:509–519. doi: 10.1093/jnen/62.5.509. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 2001;23:203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Boggess KA. Pathophysiology of preterm birth: emerging concepts of maternal infection. Clin Perinatol. 2005;32:561–569. doi: 10.1016/j.clp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, Hiatt M, Sanocka U, Shahrivar F, Abiri M, Disalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Rosenfeld D, Schonfeld S, Share J, Collins M, Genest D, Shen-Schwarz S. Developmental Epidemiology Network Investigators. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr Res. 1999;46:566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, Verellen G, De PC, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cammer W. Effects of TNFalpha on immature and mature oligodendrocytes and their progenitors in vitro. Brain Res. 2000;864:213–219. doi: 10.1016/s0006-8993(00)02178-8. [DOI] [PubMed] [Google Scholar]
- Pang Y, Fan LW, Zheng B, Cai Z, Rhodes PG. Role of interleukin-6 in lipopolysaccharide-induced brain injury and behavioral dysfunction in neonatal rats. Neuroscience. 2006;141:745–755. doi: 10.1016/j.neuroscience.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci. 2006;24:341–350. doi: 10.1111/j.1460-9568.2006.04918.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11:343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Cai Z, Lin S, Rhodes PG. Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur J Pharmacol. 2002;437:139–145. doi: 10.1016/s0014-2999(02)01289-x. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Mitchell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eur J Neurosci. 2008;27:1475–1484. doi: 10.1111/j.1460-9568.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Effects of lipopolysaccharide on oligodendrocyte progenitor cells are mediated by astrocytes and microglia. J Neurosci Res. 2000;62:510–520. doi: 10.1002/1097-4547(20001115)62:4<510::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Sharp J, Keirstead HS. Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells. Curr Opin Biotechnol. 2007;18:434–440. doi: 10.1016/j.copbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Zhao C, Zawadzka M, Roulois AJ, Bruce CC, Franklin RJ. Promoting remyelination in multiple sclerosis by endogenous adult neural stem/precursor cells: defining cellular targets. J Neurol Sci. 2008;265:12–16. doi: 10.1016/j.jns.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Furlan R, Martino G. Cell-based remyelinating therapies in multiple sclerosis: evidence from experimental studies. Curr Opin Neurol. 2004;17:247–255. doi: 10.1097/00019052-200406000-00003. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Compston A. Trophic factors attenuate nitric oxide mediated neuronal and axonal injury in vitro: roles and interactions of mitogen-activated protein kinase signalling pathways. J Neurochem. 2005;92:1487–1496. doi: 10.1111/j.1471-4159.2004.02981.x. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Chandran S, Compston A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia. 2001;36:48–57. doi: 10.1002/glia.1094. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Schneider A, Barrie JA, Klugmann M, McCulloch MC, Kirkham D, Kyriakides E, Nave KA, Griffiths IR. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. J Comp Neurol. 1998;394:506–519. doi: 10.1002/(sici)1096-9861(19980518)394:4<506::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Webber DJ, Compston A, Chandran S. Minimally manipulated oligodendrocyte precursor cells retain exclusive commitment to the oligodendrocyte lineage following transplantation into intact and injured hippocampus. Eur J Neurosci. 2007;26:1791–1800. doi: 10.1111/j.1460-9568.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Steeves JD, Fawcett JW, Ramer MS. Spinally upregulated noggin suppresses axonal and dendritic plasticity following dorsal rhizotomy. Exp Neurol. 2007;204:366–379. doi: 10.1016/j.expneurol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- MacDermid VE, McPhail LT, Tsang B, Rosenthal A, Davies A, Ramer MS. A soluble Nogo receptor differentially affects plasticity of spinally projecting axons. Eur J Neurosci. 2004;20:2567–2579. doi: 10.1111/j.1460-9568.2004.03715.x. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–576. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–361. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- Feldhaus B, Dietzel ID, Heumann R, Berger R. Effects of interferon-gamma and tumor necrosis factor-alpha on survival and differentiation of oligodendrocyte progenitors. J Soc Gynecol Investig. 2004;1:89–96. doi: 10.1016/j.jsgi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. doi: 10.1007/978-3-540-73677-6_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.