Abstract
The nuclear bile acid receptor, farnesoid X receptor (FXR), may play a pivotal role in liver fibrosis. We tested the impact of genetic FXR ablation in four different mouse models. Hepatic fibrosis was induced in wild-type and FXR knock-out mice (FXR−/−) by CCl4 intoxication, 3,5-diethoxycarbonyl-1,4-dihydrocollidine feeding, common bile duct ligation, or Schistosoma mansoni (S.m.)-infection. In addition, we determined nuclear receptor expression levels (FXR, pregnane X receptor (PXR), vitamin D receptor, constitutive androstane receptor (CAR), small heterodimer partner (SHP)) in mouse hepatic stellate cells (HSCs), portal myofibroblasts (MFBs), and human HSCs. Cell type-specific FXR protein expression was determined by immunohistochemistry in five mouse models and prototypic human fibrotic liver diseases. Expression of nuclear receptors was much lower in mouse and human HSCs/MFBs compared with total liver expression with the exception of vitamin D receptor. FXR protein was undetectable in mouse and human HSCs and MFBs. FXR loss had no effect in CCl4-intoxicated and S.m.-infected mice, but significantly decreased liver fibrosis of the biliary type (common bile duct ligation, 3,5-diethoxycarbonyl-1,4-dihydrocollidine). These data suggest that FXR loss significantly reduces fibrosis of the biliary type, but has no impact on non-cholestatic liver fibrosis. Since there is no FXR expression in HSCs and MFBs in liver fibrosis, our data indicate that these cells may not represent direct therapeutic targets for FXR ligands.
The farnesoid X receptor (FXR;NR1H4) is a key regulator of hepatic bile acid homeostasis, lipoprotein and glucose metabolism, bacterial colonization of the small intestine, the inflammatory response, and liver regeneration.1,2,3 Hereditary and acquired FXR defects may contribute to cholestasis and gallstone formation in humans.4,5,6,7 Defects in its target genes (eg, bile salt export pump/ABCB11; multidrug resistance gene 3/ABCB4 (a phosphatidylcholine floppase); multidrug related protein 2/ABCC2) cause well-characterized clinical syndromes.8,9,10,11 Moreover, FXR knockout mice (FXR−/−) have impaired resistance to bile acid feeding,12,13 and show substantial differences in the cholestatic phenotype in response to common bile duct ligation,14,15,16 have increased susceptibility for diet-induced gallstone disease,17,18 and impaired liver regeneration following partial hepatectomy.19 FXR may also directly or indirectly (eg, by the interaction with other members of the nuclear receptor family such as PXR/NR1I2 and VDR/NR1I1) regulate the metabolism and hepatic clearance of xenobiotics.20,21,22
Recent studies also reported mRNA expression of FXR in hepatic stellate cells and FXR protein in renal proximal tubules23,24,25 suggesting that FXR could represent a therapeutic target for the treatment of liver fibrosis and diabetic nephropathy.23,24,25,26 Moreover, FXR ligands were claimed to repress collagen expression in HSCs in vitro via a postulated FXR/SHP-dependent mechanism.23 It is also attractive to hypothesis that genetic FXR variants may predispose patients suffering from various forms of liver diseases to liver fibrosis as a kind of genetic disease modifier.7,27 Taken together its pleiotrophic functions (eg, central regulator of bile acid homoeostasis, glucose and lipid metabolism, inflammation) make FXR an extremely attractive candidate for therapeutic targeting in cholestatic liver diseases and nonalcoholic fatty liver disease including their major sequel liver fibrosis.28,30 However, little is known on hepatic cell-type FXR expression in human liver fibrosis.
The aims of this study were threefold. First, we aimed to determine the impact of genetic FXR ablation on the degree of liver fibrosis in untreated mice and four different well established mouse models including CCl4-intoxicated mice, 3,5 -diethoxycarbonyl-1,4-dihydrocollidine (DDC)-intoxicated mice and common bile duct-ligated (CBDL) mice for biliary fibrosis, and infection with Schistosoma mansoni (S.m.), which has been shown to induce “pipe-stem” fibrosis and granuloma formation.31,32 Comparison of cholestatic (DDC, CBDL) and non-cholestatic (CCl4, S.m.) mouse models for liver fibrosis should provide differentiated knowledge on the role of FXR in various types and etiologies of liver fibrosis. Based on previous studies reporting that pharmacological activation of FXR is antifibrotic in liver but also kidney23,25 we hypothesized that FXR−/− mice spontaneously develop liver fibrosis and are more susceptible to experimentally induced liver fibrosis due to the lack of a postulated FXR/SHP-dependent down-regulation of collagen mRNA expression in profibrotic states.23,24 We therefore compared the extent of fibrosis in FXR−/− mice and wild-type controls in a longitudinal study under baseline conditions and in response to cholestatic and non-cholestatic fibrogenic injury. Second, we aimed to determine the expression of genes involved in bile acid transport/metabolism and their regulatory nuclear receptors (including FXR, PXR, CAR/NR1I3, VDR, and SHP/NR0B2) in isolated profibrogenic rodent cells [ie, periductal myofibroblasts (MFBs), and quiescent as well as activated hepatic stellate cells (HSCs)] and to test the effects of FXR ligands on FXR target genes in vitro. Cell type-specific FXR protein expression was determined in five different in vivo models for liver fibrosis. Finally, we cross-validated these findings in isolated human HSCs and histological sections from human prototypic fibrotic liver diseases [eg, primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), and alcoholic steatohepatitis (ASH)].
Materials and Methods
Animals
For longitudinal comparison experiments were performed with 2, 4, and 17-month-old male FXR−/− mice (C57/BL6; obtained from Frank J. Gonzalez, National Cancer Institute, National Institutes of Health, Bethesda, MD) and corresponding wild-type controls. Shp knock-out mice (Shp−/−) were kindly provided by David Moore (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX). Animals were housed with a 12:12 hour light:dark cycle and permitted ad libitum consumption of water. Experimental fibrosis was induced in 2-month-old male FXR−/− and wild-type controls weighing 25 to 30 g treated according to following four protocols: Mice were fed a 0.1% DDC-supplemented diet for 1, 4, and 8 weeks. Mice were challenged with CCl4 (10% v/v in corn oil, 5 μl/g body weight i.p. twice a week) for 4 and 12 weeks. Common bile duct ligation for 8 weeks was performed as described.33,34 For S.m.-induced liver fibrosis, each mouse was infected with 50 cercariae percutaneously. In addition, cell type-specific FXR expression was determined including two additional models for sclerosing cholangitis and liver fibrosis of the biliary type (ie, 8 weeks-old Abcb4/Mdr2 knock-out mice and Swiss albino mice fed a lithocholic acid [1%]-supplemented diet for 7 days). The experimental protocols where approved by the local animal Care and Use Committees according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences, as published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Human Liver Tissue
Tissue samples were obtained from the Biobank of the Medical University Graz after approval by the local ethics committee and analyzed in an anonymized fashion.
Serum biochemical analysis, liver histology, determination of bile flow and composition, preparation of total liver protein, and analysis for determination K19 protein and bile duct morphometry were performed as described.33,35,36
Determination of Biliary Porphyrin Concentration
Bile was collected from mice fed a 0.1% DDC-supplemented diet and total porphyrins in bile were measured fluorometrically (using a F-2500 Fluorescence Spectrophotometer, Hitachi, Tokyo, Japan). For this purpose, bile was diluted 50-fold in 0.9 mol/L HClO4: ethanol (1:1, v:v) and fluorescence was determined with excitation at 400 nm and emission wave length of 602 nm.37
Measurement of Hepatic Hydroxyproline Content
To quantify liver fibrosis, hepatic hydroxyproline content was determined as described.16
Immunohistochemistry for FXR
Immunohistochemistry for FXR was performed on proteinase K digested (1:1000 in PBS for 10 minutes, Dako, Glostrup, Denmark) and microwave-treated (0.01 mmol/L citrate buffer, pH6.0) paraffin sections (4 μm thick) using the monoclonal mouse anti-FXR antibody (dilution 1:100; Persues Proteomics, Tokyo, Japan). Binding of the antibody was detected using the ABC system (Dako, Glostrup, Denmark) and DAB as substrate.
Isolation of Periductal MFBs
To determine nuclear receptors, as well as ABC transporter expression in periductal MFBs, those cells were isolated from 7-day bile duct-ligated mice using standard protocols.38 To test the effects of FXR ligands on FXR target genes Shp/SHP and Ostβ/OSTβ in vitro MFBs and HepG2 cells (as positive controls) in Dulbecco’s Modified Medium (DMEM) 2% fetal bovine serum were either treated for 24 hours with the natural FXR ligand cholic acid (250 μmol/L and 500 μmol/L) or the synthetic FXR agonist GW 4064 (5 μmol/L, obtained from Tocris Bioscience, Cat. # 2473). Experiments were performed in triplicate.
Isolation of HSCs and Primary Human Hepatocytes
Human HSCs were isolated from liver tissue unsuitable for transplantation (n = 6) as described.39 Primary human hepatocytes (PHHs) and whole human liver tissue were analyzed for comparison.
Real-Time and Semiquantitative Reverse Transcription-PCR
Quantification of mRNA levels in human HSCs and PHHs was performed using quantitative PCR. Mouse HSCs and MFBs were analyzed by semi-quantitative PCR. Briefly, RNA was extracted and reverse transcribed into cDNA. PCR reaction (20 μl) contained 12.5 ng cDNA, 330 nmol/L of each primer, and 10.5 μl of SYBR Green Master mix (Applied Biosystems). Thirty cycles were performed and 10 μl loaded on a 3% agarose gel to determine the size and specificity of the PCR product. In addition, mRNA levels in mouse MFBs and human HSCs, as well as mRNA levels of hepatic porphyrin metabolism enzymes and transporters in mice were performed using quantitative PCR. Human primers are summarized in Table 1 and mouse primers used in Table 2.
Table 1.
Primers Used for Analysis of mRNA Expression Levels in Mouse Livers
| Gene | Forward primer | Reverse primer | Access No. |
|---|---|---|---|
| 18sRNA | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ | NR_003278 |
| Alas1 | 5′-GATGCCAGGCTGTGAAATTTACT-3′ | 5′-CTGTTGCGAATCCCTTGGAT-3′ | NM_020559 |
| Alad | 5′-GGAGAGTTTGCCATGTTGTG-3′ | 5′-TCCTTCAGCCACTTCAACAG-3′ | NM_008525 |
| Uros III | 5′-AGCAGTGAAGCTGTGTTTGG-3′ | 5′-CACAGACTTGGCATTCCATC-3′ | NM_009479 |
| Urod | 5′-GCTGCTTGGCATACTCACTG-3′ | 5′-GGACTCAAAGAGCTGCAATG-3′ | NM_009478 |
| Cpox | 5′-TTGCCATTTACTGCTATGGG-3′ | 5′-ACCACCAGTGTGTGTTACCG-3′ | NM_007757 |
| Ppox | 5′-TGGTCCATCTACACAAGAACTGTAT-3′ | 5′-AGGAATTGCATAGCTGAGTCTAGTT-3′ | NM_008911 |
| Fech | 5′-AGGTAGGAGCCACTGTCCAC-3′ | 5′-AAAGCCCTTTGATAGCCTCA-3′ | NM_007998 |
| Hmox1 | 5′-CACTTCGTCAGAGGCCTGCTA-3′ | 5′-GTCTGGGATGAGCTAGTGCTGAT-3′ | NM_010442 |
| Blvra | 5′-TATTTCTGCCACCATGGAAA-3′ | 5′-CAGGCCCTTTCTCTTCAATC-3′ | NM_026678 |
| Abcb6 | 5′-ACATTTGCAGCCGAGAACTT-3′ | 5′-GCCCTCCAGAGGTCACATAA-3′ | NM_023732 |
| Bcrp | 5′-CCGGAAAACAGTTGAGAAAGAAATA-3′ | 5′-CTAAGACATCTAGCAACGAAGAC-3′ | NM_011920 |
| Pgc-1α | 5′-GACTCAGTGTCACCACCGAAA-3′ | 5′-TGAACGAGAGCGCATCCTT-3′ | NM_008904 |
| Col1a2 | 5′-GCAGGGTTCCAACGATGTTG-3′ | 5′-GCAGCCATCGACTAGGACAGA-3′ | NM_007743 |
| Shp | 5′-AAGGGCACGATCCTCTTCAA-3′ | 5′-GTACCAGGGCTCCAAGACT-3′ | NM_011850 |
| Ost-β | 5′-GACAAGCATGTTCCTCCTGAG-3′ | 5′-GATGCAGGTCTTCTGGTGTTTC-3′ | NM_178933 |
| TNF-α | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | 5′-TGGGAGTAGACAAGGTACAACCC-3′ | NM_013693 |
Real-time polymerase chain reaction using the SYBR GREEN assay was performed
Table 2.
Primers Used for Analysis of mRNA Expression Levels in Human Tissues and Cells
| Gene | Forward primer | Reverse primer | Access no. |
|---|---|---|---|
| 28S rRNA | 5′-CGGCTCTTCCTATCATTGTG-3′ | 5′-CCTGTCTCACGACGGTCTAA-3′ | NR_003279 |
| NTCP | 5′-GGAGGGAACCTGTCCAATGTC-3′ | 5′-CATGCCAAGGGCACAGAAG-3′ | NM_003049 |
| OATP1B1 | 5′-CATATAGAACGGAGATTTGAG-3′ | 5′-CTTTGGTCTATGTAGTTTGGAT-3′ | NM_006446 |
| BSEP | 5′-GACATGCTTGCGAGGACCTT-3′ | 5′-GGTTCGTGCACCAGGTAAGAA-3′ | NM_003742 |
| MRP2 | 5′-TCCTTGCGTTTGTGATGAAT-3′ | 5′-TGCTGGATAGTCGGTGCTAT-3′ | NM_000392 |
| MRP3 | 5′-GCCACAGTCCTTCTTTGACA-3′ | 5′-ATTGAGCAGCATGAGGATGA-3′ | NM_020037 |
| MRP4 | 5′-CTTATTCTCCTAAACACTGCAGCTC-3′ | 5′-ATCTAGCTTCTCGGTTACATTTCCT-3′ | NM_005845 |
| UGT1A1 | 5′-TCTGGCTGTTTAGAAGTGACTTT-3′ | 5′-CATTAATGTAGGCTTCAAATTCCT-3′ | NM_000463 |
| UGT2B4 | 5′-TTCAATTTCCTCACCCACTCTTA-3′ | 5′-AAACTCTTCCATTTCCTTCGGTA-3′ | NM_021139 |
| UGT2B7 | 5′-CATCCACTCTTACCAAATGTTGA-3′ | 5′-GTCATGTTACTGACCATTGACC-3′ | NM_001074 |
| CYP3A4 | 5′-GATGAAGAATGGAAGAGATTACGAT-3′ | 5′-CCTCAGATTTCTCACCAACACA-3′ | NM_017460 |
| FXR | 5′-CTCATTGAACATTCCCATTTACCTAC-3′ | 5′-GGACCTGCCACTTGTTCTGTTA-3′ | NM_005123 |
| SHP | 5′-GCTTCAATGCTGTCTGGAGTC-3′ | 5′-CTTGGAGGCCTGGCACATC-3′ | NM_021969 |
| VDR | 5′-TGCCGCATCACCAAGGACAAC-3′ | 5′-TGCTCCTCAGACAGCTTGGG-3′ | NM_009504 |
| CAR | 5′-CTGCCTCTGGTCACACACTT-3′ | 5′-CCTTGAGAAGGGAGATCTGG-3′ | NM_001338 |
| PXR | 5′-TCCCCAAATCTGCCGTGTAT-3′ | 5′-AGCCCTTGCATCCTTCACAT-3′ | NM_003889 |
| LXR α | 5′-CTTGCTCATTGCTATCA GCATCTT-3′ | 5′-ACATATGTGTGCTGCAGCCTC T-3′ | NM_005693 |
| LXR β | 5′-TCACCTACAGCAAGGACGAC-3′ | 5′-AGAAGATGTTGATGGCGATG-3′ | NM_007121 |
NOTE. Real-time polymerase chain reaction using the SYBR GREEN assay was performed.
Statistical Analysis
Data for mouse experiments are reported as arithmetic means ± SD of five animals in each group. Statistical analysis was performed using Student’s t-test when appropriate or analysis of variance with Bonferroni post-testing, when three or more groups were compared using the Sigmastat statistics (Jandel Scientific, San Rafael, CA). A P value <0.05 was considered significant. Morphometric data for K19 expressing cholangiocytes represented a hierarchical structure, where portal fields (level-one observation units) were nested within mice (level-two observation units). A linear regression model was fitted to calculate means and confidence intervals for K19-positive areas in each of the four study groups, as well as to test the significance of differences between them.
Results
Are FXR−/− Mice Prone to Liver Fibrosis?
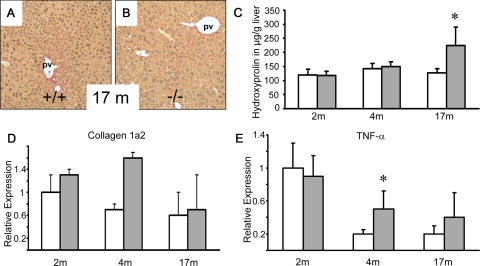
Since we hypothesized that FXR−/−mice may be prone to development of spontaneous liver fibrosis as a result of lacking FXR-mediated repression of hepatic inflammation and fibrosis,40 we first conducted a comparative long-term study between FXR−/−mice and wild-type controls in regard to hepatic hydroxyproline content, collagen 1a2 mRNA, and tumor necrosis factor (TNF)-α mRNA expression levels at 2, 4, and 17 months of age (Figure 1). The time points of 2 and 4 months were selected since experimental fibrosis (eg, via common bile duct ligation) is usually induced in 2- to 4-month-old mice. Collagen 1a2 mRNA expression levels were comparable at 2 and 17 months of age and elevated in 4-month-old FXR−/−mice (without reaching statistical significance due to multiple comparison analysis and the large variation in 17-month-old mice) (Figure 1D). Hepatic hydroxyproline levels revealed no significant differences at 2 and 4 months of age, but were significantly increased in 17-month-old naïve FXR−/− mice (Figure 1C). Sirius red-stained liver sections of 2- and 4-month-old FXR−/− mice were not distinguishable from wild-type controls (not shown). The observed differences in hepatic hydroxyproline levels in 17-month-old mice were hardly detectable on Sirius red-stained liver sections (Figure 1, A and B). Since FXR may also play a key role in the regulation of the hepatic inflammatory response40 and increased hepatic inflammation may trigger fibrosis in aging FXR −/− mice, we next determined hepatic TNF-α mRNA expression levels. TNF-α mRNA levels were significantly elevated in 4-month-old FXR−/− mice (Figure 1E) while the differences did not reach statistical significance in other groups. Collectively these data suggest that FXR−/−mice have no signs of liver fibrosis at 2 and 4 months of age, but are prone to probably inflammation-driven liver fibrosis with increasing age. Therefore we hypothesized that FXR−/− mice may also show an increased fibrotic response in different models for liver fibrosis.
Figure 1.
Longitudinal comparison between FXR−/−mice and wild-type controls. Sirius-red stain for collagen staining in naïve 17-month-old wild-type (+/+) (A) and FXR knock-out (−/−) mice (B). C: Quantification of liver fibrosis by determination of hepatic hydroxyproline content shows equal levels in both genotypes at 2 and 4 months of age and significantly increased levels in 17-month-old FXR−/− mice (gray bars). D: Equal collagen 1a2 mRNA expression at 2 and 17 months of age, and elevated levels in 4-month-old FXR−/−mice. E: Equal hepatic TNF-α mRNA expression levels in both genotypes at 2 and 17 months of age and significantly elevated levels in 4-month-old FXR−/−mice. *P < 0.05 (wild-type versus FXR−/− mice). Open bars, wild-type mice; gray bars, FXR knock-out mice. pv, portal vein. Original magnification for A, B, ×10.
Does Absence of FXR Impact on the Degree of Liver Fibrosis in CCl4-Intoxicated Mice?
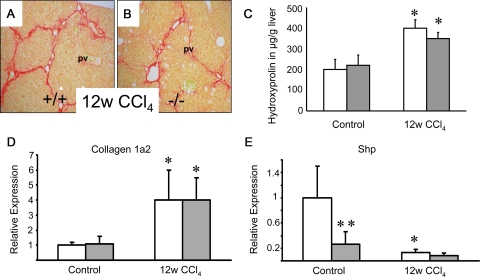
CCl4-intoxication for up to 12 weeks resulted in even more severe liver injury in FXR−/− mice, as compared with wild-type controls, as indicated by significantly increased alanine aminotransferase (ALT) levels suggesting increased susceptibility of FXR−/− mice to CCl4 intoxication (Table 3). However, in contrast to increased hepatocyte injury in these animals, no significant differences in regard to liver fibrosis were observed (Figure 2). Sirius red staining and hepatic hydroxyproline levels in CCl4-intoxicated FXR−/− mice and wild-type controls were similar (Figure 2, A–C), which was also the case for collagen 1a2 mRNA expression (Figure 2D). SHP expression was significantly repressed in wild-type mice in response to CCl4-treatment comparable with the levels in CCl4-intoxicated FXR−/− mice (Figure 2E). An FXR-dependent collagen 1a2 regulatory pathway may therefore not be critical in CCl4-intoxicated mice since the observed Shp repression was FXR-independent. Taken together these results clearly demonstrate that FXR loss has no negative impact on the degree of liver fibrosis in CCl4-intoxicated mice.
Table 3.
Serum Liver Tests in Different Models and Genotypes
| FXR+/+
|
FXR−/−
|
|||||
|---|---|---|---|---|---|---|
| ALT (U/L)l | AP (U/L) | Total bilirubin (mg/dL) | ALT (U/L) | AP (U/L) | Total bilirubin (mg/dL) | |
| Naïve | 81 ± 14 | 69 ± 12 | 0.38 ± 0.1 | 92 ± 21 | 71 ± 15 | 0.12 ± 0.06 |
| 4w DDC | 1356 ± 387 | 403 ± 123 | 1.3 ± 0.8 | 1185 ± 879 | 411 ± 65 | 12 ± 4.8* |
| 8w DDC | 1753 ± 424 | 343 ± 75 | 1.75 ± 0.7 | 840 ± 392* | 415 ± 85 | 8.68 ± 1.8* |
| SOP | 36 ± 8 | 52 ± 6 | 0.29 ± 0.02 | 333 ± 186* | 77 ± 6.3 | 0.3 ± 0.04 |
| 8w CBDL | 204 ± 21 | 1847 ± 613 | 19 ± 10 | 534 ± 215* | 591 ± 128* | 12.4 ± 3 |
| S.m. | 278 ± 82 | 54 ± 12 | 0.2 ± 0.03 | 271 ± 132 | 109 ± 51* | 0.45 ± 0.2 |
| 12w CCl4 | 386 ± 68 | n.d. | n.d. | 579 ± 72* | n.d. | n.d. |
CBDL, common bile duct ligation; CCl4, carbontetrachloride; DDC, diethoxycarbonyl-1,4-dihydrocollidine; n.d., not determined; S.m., Schistosoma mansoni-infected mice; SOP, sham-operated controls. n = 5 animals in each group.
P < 0.05: FXR+/+ versus FXR−/−
Figure 2.
Loss of FXR has no effect on liver fibrosis in CCl4-intoxicated mice. Sirius-red staining for collagen in 12 weeks CCl4-intoxicated wild-type (+/+) (A) and FXR knock-out (−/−) (B). A similar degree of Sirius red staining was observed in both wild-type (A) and FXR−/− (B) after CCl4-intoxicated. C: Quantification of liver fibrosis by determination of hepatic hydroxyproline content shows similar levels between genotypes. D: Significantly increased collagen 1a2 mRNA expression in 12w CCl4-intoxicated wild-type mice and FXR−/−mice. E: Significantly lower Shp mRNA expression levels in naïve FXR−/−mice and CCl4-intoxicated wild-type mice. Open bars, wild-type mice; gray bars, FXR knock-out mice. *P < 0.05 (naïve versus CCl4-intoxicated wild-type/FXR−/−), **P < 0.05 (WT vs. FXR−/−). pv, portal vein. Original magnification for A, B, ×10.
Does Absence of FXR Impact on the Degree of Liver Fibrosis of the Biliary Type in DDC-Intoxicated Mice?
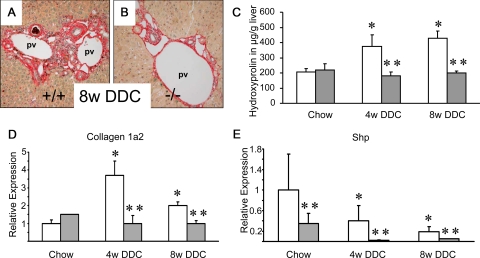
We used DDC feeding as a model system for xenobiotic-induced cholangiopathies with cholangitis and partial biliary obstruction leading to cholestasis and liver fibrosis of the biliary type.37 After 4 weeks of DDC feeding livers of wild-type mice showed a pronounced ductular reaction (see Supplemental Figure S1 at http://ajp.amjpathol.org), also observed at week 8 of DDC intoxication (see Supplemental Figure S1 at http://ajp.amjpathol.org). In addition, the ductular reaction was confirmed by significantly increased K19 protein levels (see Supplemental Figure S2 at http://ajp.amjpathol.org) and morphometry of K19-positive cells representing a significantly increased ductular area (see Supplemental Figure S3 at http://ajp.amjpathol.org). At 4 weeks of intoxication, ductules and small bile ducts in wild-type mice frequently contained pigment plugs (see Supplemental Figure S1 at http://ajp.amjpathol.org), which significantly increased over time (see Supplemental Figure S1 at http://ajp.amjpathol.org). DDC-fed wild-type mice developed sclerosing cholangitis of the onion skin-type (see Supplemental Figure S1 at http://ajp.amjpathol.org) in line with findings previously reported in detail.36 These morphological alterations where accompanied by a consistent increase in serum ALT levels as an indicator of hepatocyte injury (Table 3). In contrast, cholangitis was significantly abrogated in FXR−/− mice receiving DDC (see Supplemental Figure S1 at http://ajp.amjpathol.org). ALT levels were comparable after 4 weeks, but significantly lower after 8 weeks of DDC intoxication in FXR−/− mice compared with wild-type controls (Table 3) indicating less pronounced toxicity in FXR−/− mice at least after 8 weeks intoxication. In parallel, the ductular reaction was attenuated in FXR−/− mice as demonstrated by significantly reduced hepatic K19 protein expression (see Supplemental Figure S2 at http://ajp.amjpathol.org) and significantly lower bile duct mass (see Supplemental Figure S3 at http://ajp.amjpathol.org). In regard to the degree of liver fibrosis we found significantly smaller Sirius red-positive areas in DDC-fed FXR−/− animals (Figure 3B) compared with wild-type controls (Figure 3A). The reduced biliary fibrosis was further confirmed biochemically by significantly lower hepatic hydroxyproline levels in FXR−/− mice (Figure 3C). Moreover, collagen 1a2 mRNA expression in FXR−/− mice was significantly lower despite significantly lower Shp expression compared with wild-type controls (Figure 3, D and E). Taken together these findings clearly demonstrate that the degree of cholangitis and ductular reaction is significantly lower in DDC-challenged FXR−/− mice compared with corresponding wild-type controls. Genetic FXR ablation even protected mice from DDC-induced biliary fibrosis.
Figure 3.
Loss of FXR significantly reduces liver fibrosis in 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-fed mice. Sirius-red stain for collagen staining in mice fed 0.1% (w/w) DDC-supplemented diet for 8 weeks (A,B). Significantly increased Sirius-red staining in DDC-fed wild-type (+/+) mouse (A). However, this increase is not observed in DDC-fed FXR−/− mouse (B). C: In addition, quantification of liver fibrosis by determination of hepatic hydroxyproline content shows significant increased levels only in DDC-fed wild-type mice (open bars), as compared with FXR−/− mice (gray bars) showing unchanged hepatic hydroxyproline levels under this treatment. D: Significantly increased collagen 1a2 mRNA expression in 4-week and 8-week DDC-fed wild-type mice and unchanged levels in DDC-fed FXR−/−mice. E: Significantly lower Shp mRNA expression levels in DDC-fed FXR−/−mice. *P < 0.05 (chow-fed versus DDC-fed wild-type). Open bars, wild-type mice; gray bars, FXR knock-out mice. **P < 0.05 (DDC-fed wild-type versus DDC-fed FXR−/− mice). pv, portal vein. Original magnification for A, B, ×10.
FXR−/− Mice Show Significantly Lower Hepatic Protoporphyrin IX Content, Equal Serum Bile Acid Levels and Increased Serum Bilirubin Levels in Response to DDC Intoxication Compared with Wild-Type Controls
Since DDC treatment of FXR−/− mice resulted in substantially fewer porphyrin plugs within the liver in H&E stained liver sections (see Supplemental Figure S1 at http://ajp.amjpathol.org), we next compared the levels of protoporphyrin IX in serum, bile, urine, and liver in FXR−/− and wild-type mice.36 Hepatic protoporphyrin IX content was significantly lower in DDC-intoxicated FXR−/− mouse livers compared with wild-type (see Supplemental Figure S4 at http://ajp.amjpathol.org), but no differences in serum and urine were observed (not shown). To rule out increased biliary elimination of protoporphyrin IX in FXR−/− mice we determined the biliary protoporphyrin IX output in both genotypes. The data demonstrate a significantly reduced bile flow (see Supplemental Figure S5 at http://ajp.amjpathol.org) and biliary excretion of protoporphyrin IX in DDC-fed FXR−/− mice (see Supplemental Figure S5 at http://ajp.amjpathol.org). Serum bilirubin levels were significantly higher in FXR−/− mice (Table 3). Serum bile acid levels representing a major parameter for the degree of cholestasis did not show significant differences (152 ± 57 μmol/L in DDC-fed FXR−/− mice versus 197 ± 75 μmol/L in DDC-fed wild-type mice). These findings were contrasted by the morphological evidence of less severe cholestatic liver disease in DDC-fed FXR−/− mice. We therefore compared mRNA enzyme levels of the porphyrin synthesis pathway between normal chow-, and DDC-fed FXR−/− mice and wild-type controls (see Supplemental Table S1 at http://ajp.amjpathol.org). However, no significant differences in the mRNA levels of the key porphyrin synthesis enzymes were observed. In addition, the recently identified mitochondrial porphyrin carrying ABC transporter Abcb6 was equally expressed in both genotypes.41 Thus, these data therefore do not provide a sufficient explanation for the observed differences in the hepatic protoporphyrin IX levels between FXR−/− mice and wild-type controls.
Does Absence of FXR Impact on the Degree of Liver Fibrosis of the Biliary Type in CBDL Mice?
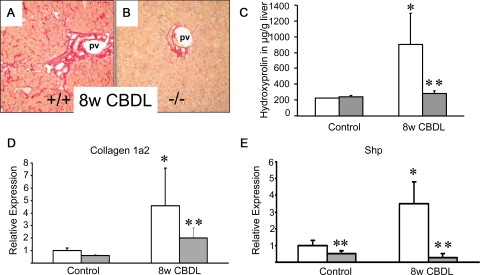
We next compared the degree of liver fibrosis between FXR−/− and wild-type mice in response to long-term common bile duct ligation. This condition represents the classical cholestatic model for biliary fibrosis with probably the highest intrahepatic bile acid levels and the highest degree of FXR activation in rodents. Significantly increased serum ALT levels in CBDL FXR−/− mice compared with wild-type controls (Table 3) pointed toward an increased hepatocyte susceptibility in this genotype confirmed by a higher degree of disseminated liver cell necrosis on H&E-stained liver sections (not shown) comparable with previous findings by us and others in response to short-term common bile duct ligation in these mice.14,15 Serum bilirubin levels did not differ between genotypes (Table 3). Common bile duct ligation for 8 weeks led to pronounced liver fibrosis in wild-type animals (Figure 4A) with significantly increased hepatic hydroxyproline content (Figure 4C). Of interest, long-term common bile duct ligation in wild-type mice led to porto-portal bridging but also pericellular fibrosis (Figure 4A). In contrast, common bile duct ligation in FXR−/− did not induce liver fibrosis (Figure 4, B and C) despite the knockout mice having a higher degree of liver cell injury and pronounced cholestasis as suggested by robust increased serum ALT and bilirubin levels (Table 3). Again, collagen 1a2 mRNA levels in CBDL FXR−/− were significantly lower despite very low Shp expression (Figure 4, D and E). TNF-α mRNA expression levels were elevated in both genotypes, but did not differ significantly (not shown). Induction of hepatic K 19 protein expression in response to long-term common bile duct ligation was pronounced in wild-type mice and again lower in FXR−/− mice (see Supplemental Figure S6 at http://ajp.amjpathol.org). However this difference was not detectable immunohistochemically (see Supplemental Figure S6 at http://ajp.amjpathol.org) suggesting that ductular reaction in response to common bile duct ligation declines over time. Since we have previously observed significantly lower serum bile acid levels, increased urinary bile acid excretion (mainly polyhydroxylated bile acids) together with induced hepatocellular basolateral export systems (Mrp3 and Mrp4) in short-term CBDL FXR−/− mice compared with controls14,16 we hypothesized that such adaptive changes may also contribute to the differences in their fibrotic response to long-term common bile duct ligation. Serum bile acid levels were indeed significantly lower in long-term CBDL FXR−/− mice (110 ± 21 μmol/L vs. 2401 ± 1380 μmol/L in CBDL wild-type; P < 0.05). Taken together these findings suggest that genetic loss of FXR protects mice against common bile duct ligation-induced liver fibrosis despite increased hepatocyte injury. These findings also clearly demonstrate that differences in the cholestatic phenotype between genotypes (ie, the degree of ductular reaction, bile infarcts versus disseminated liver cell necrosis, differences in bile acid hydroxylation and activation of alternative export routs)14,15,16 are critically related to the degree of liver fibrosis at least of the biliary type.
Figure 4.
Loss of FXR significantly reduces liver fibrosis in common bile duct-ligated (CBDL) mice. Sirius-red stain for collagen staining in 8-week CBDL wild-type (+/+) (A) and FXR knock-out (−/−) (B). In contrast to pronounced increased Sirius-red-positive areas in CBDL wild-type mice (A), the staining pattern in CBDL FXR−/− remains normal (B). C: In addition, quantification of liver fibrosis by determination of hepatic hydroxyproline content shows significantly increased levels only in CBDL wild-type mice (open bars). In contrast, CBDL FXR−/− mice (gray bars) show unchanged hepatic hydroxyproline levels. D: Significantly increased collagen 1a2 mRNA expression in CBDL wild-type mice and significantly lower levels in CBDL FXR−/−mice. E: Significantly induced Shp mRNA expression levels in CBDL wild-type mice. Significantly lower Shp mRNA expression levels in CBDL FXR−/−mice. Open bars, wild-type mice; gray bars, FXR knock-out mice. **P < 0.05 (CBDL wild-type versus CBDL FXR−/− mice). pv, portal vein. Original magnification for A, B, ×10.
Does Absence of Shp Influence the Levels of Hepatic Collagen1a2 mRNA Expression in Common Bile Duct-Ligated Mice?
To specifically determine the impact of the FXR downstream target Shp on hepatic collagen 1a2 mRNA expression we compared 10-week-old Shp−/− mice and wild-type controls after common bile duct ligation for 2 weeks, since this model is associated with the highest serum bile acid levels (with probably the highest FXR activation) and degree of liver fibrosis (ie, the highest hepatic hydroxyproline levels). However, we found no differences in hepatic collagen 1a2 mRNA expression levels between CBDL Shp−/− mice and corresponding wild-type controls (data not shown). Therefore these data do not suggest a major role of Shp in the regulation of hepatic collagen 1a2 mRNA expression in response to common bile duct ligation.
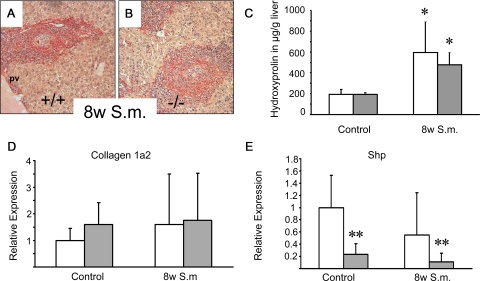
Does Absence of FXR Impact on the Degree of Inflammation-Triggered Liver Fibrosis in S.m.-Infected Mice?
FXR−/− mice may be prone to development of infection-induced liver fibrosis as a result of lacking FXR-mediated repression of hepatic inflammation.40 We therefore compared the fibrotic response of S.m.-infected FXR−/− mice and wild-type controls. However, infection with S.m. did not show any differences in the fibrotic response of both genotypes, as reflected by comparable Sirius red staining and even more important similar hepatic hydroxyproline levels in the two groups of animals (Figure 5, A−C). Collagen 1a2 and Shp mRNA levels did not differ between genotypes (Figure 5, D and E). These results clearly demonstrate that FXR loss has no negative impact on S.m.-induced liver fibrosis in mice.
Figure 5.
Loss of FXR has no effect on liver fibrosis in Schistosoma mansoni (S.m.)-infected mice. Sirius-red staining for collagen in 8 weeks S.m.-infected wild-type (+/+) (A) and FXR knock-out (−/−) (B). A similar degree of Sirius red staining was observed in both wild-type (A) and FXR−/− (B) after S.m.-infected. C: In addition, quantification of liver fibrosis by determination of hepatic hydroxyproline content shows similar levels between genotypes. D: Equal collagen 1a2 mRNA levels in S.m.-infected wild-type and FXR knock-out mice. Open bars, wild-type mice; gray bars, FXR knock-out mice. *P < 0.05 (naïve versus in S.m.-infected wild-type/FXR−/−). E: Significant lower Shp mRNA levels in FXR Knock-out mice.**P < 0.05 (wild-type versus FXR-/- mice). pv, portal vein. Original magnification for A, B, ×10.
Taken together, the findings in four etiologically different mouse models for liver fibrosis demonstrate that loss of FXR does not increase the degree of liver fibrosis in mice and may even be protective for fibrosis of the biliary type.
Are There Differences in the Expression Pattern of NHRs between Mouse Periductal Myofibroblasts and Stellate Cells?
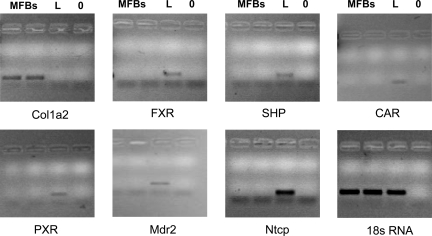
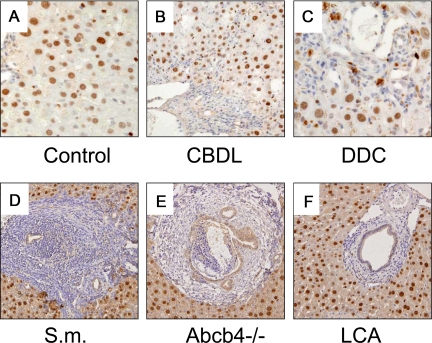
The cellular sources of the extracellular matrix (eg, HSCs, activated MFBs) may substantially differ between the herein used models of liver fibrosis. Those cells may also significantly differ with respect to their gene expression profiles, which could, at least in part, explain the observed differences in the fibrotic response between the genotypes. We therefore determined FXR and SHP expression in isolated periductal MFBs from common bile duct-ligated mice and compared these with isolated HSCs as well as whole liver tissue. Compared with liver tissue we found no significant mRNA expression levels for FXR, SHP, CAR, and PXR in isolated MFBs whereas collagen 1a2 expression was significantly higher expressed when compared with whole liver tissue (Figure 6, for quantitative PCR data, please see Supplemental Table S2 at http://ajp.amjpathol.org). In addition, the main bile acid uptake system Ntcp was not expressed in MFBs. These findings therefore make it very unlikely that a bile acid-activated FXR/SHP-dependent pathway is biologically relevant in MFBs. To confirm these findings we compared these findings with the expression levels in freshly-isolated but also activated HSCs (Table 4). Again we found only very low or even undetectable levels of FXR, SHP, and Ntcp mRNA. These findings were independent of the activation of HSCs as demonstrated by significantly induced collagen 1a2 levels in activated HSCs. In contrast, HSC activation was paralleled by a robust induction of VDR receptor expression clearly demonstrating the ability of the experimental system to respond to the tested stimuli (Table 4). We next determined cell type-specific FXR expression in vivo by immunohistochemistry using a specific antibody on terminal ileum, kidney, and liver tissue for positive controls and in five mouse models for liver fibrosis (ie, CBDL, DDC-fed, S.m.-infected, Abcb4−/−, and lithocholic acid–fed mice). Hepatocyte nuclei, renal tubular epithelial cells, and terminal ileum epithelial cells stained clearly positive (see Supplemental Figure S7 at http://ajp.amjpathol.org). In contrast, MFBs and HSCs showed no reactivity in all of experimental situations tested (Figure 7, A−F). For further functional characterization isolated MFBs and HepG2 cells were treated either with cholic acid (natural FXR ligand) or GW4064 (synthetic FXR agonist) and mRNA expression levels of FXR target genes Shp/SHP and Ostβ/OSTβ were determined (see Supplemental Figures S8−S10 at http://ajp.amjpathol.org). In contrast to the pronounced induction of SHP and OSTβ mRNA expression in HepG2 cells the corresponding gene expression levels did not change in MFBs. Taken together these findings indicate very low mRNA expression levels for nuclear hormone receptors (NHRs), especially FXR, in mouse MFBs and HSCs (except VDR) and make a pivotal role of FXR-dependent gene regulation in mouse MFBs and HSCs rather unlikely.
Figure 6.
mRNA expression of collagen1a2, FXR, SHP, CAR, PXR, Mdr2, Ntcp in isolated mouse periductal myofibroblasts (MFBs) compared with liver tissue. RNA was isolated and PCR (for 30 cycles) performed as described in the Materials and Methods. Note that there is no mRNA expression of FXR, SHP, CAR, PXR, Mdr2, and Ntcp in MFBs. MFB = 2 samples of periductal MFBs, L = liver; 0 = blank.
Table 4.
Expression of Ntcp, FXR, SHP, VDR, and Collagen1a2 mRNA in Quiescent and Activated Mouse Hepatic Stellate Cells Compared to Whole Mouse Liver Tissue
| mHSC
|
LT | |||||
|---|---|---|---|---|---|---|
| Gene | Freshly isolated | 5 days culture | 10 days culture | P1 SFIF | 10% FBS | |
| Col 1a2 | 0.22 | 0.23 | 7.80 | 11.26 | 17.09 | 0.05 |
| FXR | 0.01 | n.d. | n.d. | n.d. | n.d. | 11.90 |
| SHP | 0.005 | 0.026 | 0.019 | n.d. | n.d. | 5.906 |
| VDR | n.d. | n.d. | 11.07 | 17.54 | 30.65 | n.d. |
| Ntcp | 0.01 | n.d. | 0.001 | n.d. | 0.002 | 53.30 |
n.d., not detectable; LT, liver tissue; mHSCs, mouse hepatic stellate cells.
Data are presented as ratio to 18S rRNA expression.
Figure 7.
FXR is expressed in hepatocytes and bile duct epithelial cells in mouse liver. Immunohistochemical staining for FXR in chow-fed control (A), CBDL (B), DDC-fed (C), S.m-treated (D), Abcb4 knock-out (E), and lithocholic acid (LCA)-fed mice (F). Please note that hepatocytes and bile duct epithelia cells show a positive nuclear FXR staining pattern while MFBs and HSCs stain negative. Please note also that some macrophages and hepatocytes in DDC-fed mice (C) are porphyrin-loaded (ie, dark brown pigment localized in the cytoplasm). Data indicate undetectable FXR protein expression in MFBs and HSCs in mouse models for liver fibrosis. Original magnification for A−C ×40, D−F ×20.
What Is the Expression Pattern of NHRs in Human Hepatic Stellate Cells?
NHR expression pattern in human HSCs is crucial to know in regard to the feasibility of FXR-targeted deactivation of HSCs. We therefore extended our experiments with mouse MFBs and HSCs to human tissues and determined mRNA expression levels of FXR, SHP, VDR, CAR, and PXR, as well as NTCP, OATP1B1, and bile salt export pump (key bile acid transporters) in human HSCs, PHHs, and whole liver tissue. In addition, expression of selected phase I and II bile acid detoxification systems CYP3A4 (hydroxylation), UGT2B7 and UGT2B4 (glucuronidation), was compared (summarized in Table 5). FXR and PXR mRNA levels were not detectable by q-PCR in 1 and 3 of 6 HSC preparations, respectively, while CAR mRNA was absent in all samples. SHP expression was found in all HSC preparations. However, FXR, SHP and PXR mRNA levels in HSCs were only 8%, 2% and 6% of PHHs, respectively, questioning their biological significance. In contrast, we observed a robust expression for VDR in HSCs (Table 5) as reported previously.42 NTCP, OATP1B1 and bile salt export pump mRNA could not be detected in HSCs but were present in PHHs. Members of the MRP family were expressed in all HSC samples: ABCC2, 3, and 4 mRNA levels were 1%, 32%, and 2400%, as compared with PHHs. Expression of phase I and II bile acid detoxification systems CYP3A4 and UGT2B7 was low (4% and 2% of PHHs, respectively) and UGT2B4 mRNA was absent in HSCs. Taken together these findings suggest that expression of the NHRs FXR, SHP, and PXR is far lower in HSCs as compared with levels in PHHs. Bile acid transport systems were undetectable and expression of bile acid detoxification systems was low in HSCs. However, MRP4 was highly expressed and could function to protect HSCs from endogenous and exogenous toxins (eg, unconjugated bile acids). These findings demonstrate very low expression levels of NHRs in human HSCs with the exception of VDR.
Table 5.
Expression of Bile Acid Transport and Detoxification Systems and Their Regulatory Nuclear Receptors in Human Hepatic Stellate Cells, Compared to Primary Human Hepatocytes and Whole Human Liver Tissue
| Gene | hHSCs (n = 6) | PHH (n = 3) | LT (n = 3) |
|---|---|---|---|
| NTCP | n.d. | 5.6 ± 3.1 | 12.6 ± 8.0 |
| OATP1B1 | n.d. | 2.5 ± 1.7* | 10.5 ± 4.2 |
| BSEP | n.d. | 1.1.±0.5* | 4.2 ± 0.3 |
| MRP2 | 0.05 ± 0.06*† | 3.5 ± 3.4 | 6.5 ± 3.0 |
| MRP3 | 0.4 ± 0.3* | 1.3 ± 0.9* | 3.0 + 0.8 |
| MRP4 | 1.0 ± 1.0 | 0.04 ± 0.02† | 0.07 ± 0.06 |
| UGT1A1 | n.d. | 0.7 ± 0.7 | 0.7 + 0.2 |
| UGT2B4 | n.d. | 1.4 ± 0.7* | 4.6 ± 0.1 |
| UGT2B7 | 0.02 ± 0.04*† | 1.0 ± 0.2* | 7.3 ± 2.0 |
| CYP3A4 | 0.3 ± 0.4*† | 5.8 ± 6.6 | 374 ± 208 |
| FXR | 0.06 ± 0.01*† | 0.8 + 0.3 | 2.5 ± 0.3 |
| SHP | 0.03 ± 0.01*† | 1.5 ± 1.0 | 3.9 ± 0.3 |
| VDR | 16.1 ± 8.3*† | 0.7 ± 0.6 | 1.3 ± 0.1 |
| CAR | n.d. | 1.1 ± 0.6 | 10.7 ± 1.6 |
| PXR | 0.5 ± 0.4*† | 8.4 ± 3.8 | 30.0 ± 11.1 |
| LXRα | 0.3 ± 0.07*† | 0.8 ± 0.3* | 2.5 ± 0.3 |
| LXRβ | 4.8 ± 1.9* | 1.4 ± 1.0 | 1.0 ± 1.0 |
Data are presented as ratio to 28S rRNA expression.
hHSCs, human hepatic stellate cells (n = 6); PHH, primary human hepatocytes (n = 3); LT, liver tissue (n = 3).
P < 0.05: HSCs or PHH versus LT.
P < 0.05: HSCs versus PHH.
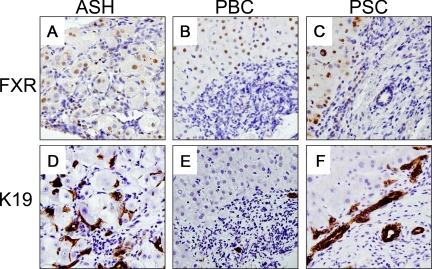
Cell-Type Specific FXR Expression in Human Fibrotic Liver Diseases
For further cross-validation of our rodent findings we next determined the cell type-specific hepatic FXR tissue localization and cellular distribution in prototypic cholestatic (eg, PSC, PBC) and metabolic liver diseases (eg, ASH) with pronounced liver fibrosis or cirrhosis. In normal liver, marked FXR expression was observed in hepatocytes while expression in BECs was weak (see Supplemental Figure S11 at http://ajp.amjpathol.org). Hepatocellular FXR expression was preserved in hepatocytes and low in BECs of ASH, PBC, and PSC livers (Figure 8, A−F). In contrast, HSCs and MFBs showed no reactivity. For better orientation and overview (larger images and different magnification levels) please also see Supplemental Figures S12−S14 (at http://ajp.amjpathol.org). FXR expression was also detectable in reactive ductules in obstructive cholestasis (see Supplemental Figure S14 at http://ajp.amjpathol.org). These findings suggest that FXR protein expression is undetectable in human HSCs and MFBs in fibrotic livers.
Figure 8.
FXR tissue localization in prototypic human fibrotic liver diseases. Immunohistochemical staining for FXR (A–C) and keratin 19 (K19, D–F) in alcoholic steatohepatitis (ASH), primary sclerosing cholangitis (PSC), and primary biliary cirrhosis (PBC). Please note that hepatocytes show a positive FXR staining pattern while bile ducts epithelial positivity is weaker (for better orientation also see lower panel using the cholangiocyte specific marker K 19) whereas MFBs and HSCs stain negative. Original magnification for A−F ×40.
Discussion
The current study shows that genetic ablation of FXR may predispose aging mice to liver fibrosis, protects against hepatic fibrosis in two mouse models for the biliary type of liver fibrosis (ie, CBDL and DDC feeding), and does not influence fibrogenesis in two other classical models (ie, CCl4- and S.m.-treated mice). The presented results further show that, with the exception of VDR, investigated NHR expression levels, as well as bile acid transporter mRNA levels, are very low in rodents and even more importantly also in human HSCs and MFBs. In addition, we provide for the first time data on FXR tissue localization and cellular distribution in prototypic cholestatic (eg, PSC, PBC, obstructive cholestasis) and metabolic liver diseases (eg, ASH) with pronounced liver fibrosis or cirrhosis showing that FXR expression is extremely low in human MFBs and HSCs.
Our study shows that several aspects have to been taken into account when FXR−/− mice are used to study liver fibrosis, and the results obtained require careful interpretation. As such FXR−/− mice show significantly lower biliary pressure in response to common bile duct ligation (and probably other models for liver fibrosis of the biliary type), which may explain reduced ductular reaction,14 as probably the major pathological hallmark and trigger for liver fibrosis, at least of the biliary type. Therefore, lower biliary pressure with resulting reduced ductular reaction after common bile duct ligation14 could, at least in part, explain decreased liver fibrosis in FXR−/− mice as observed in the current study. Differences in ductular reaction as determined by hepatic K19 expression were most pronounced between genotypes at early time points after common bile duct ligation but decreased with time suggesting that ductular reaction in response to common bile duct ligation declines with time and consequently may probably be critical for the initiation but not pivotal for the perpetuation of fibrogenesis in this specific model. However, it has to be taken into account that despite changes in the cholestatic phenotype (ie, decreased ductular reaction and biliary fibrosis) FXR−/− mice are not resistant to common bile duct ligation-induced cholestatic liver injury as previously demonstrated by significantly increased ALT levels in short-term CBDL FXR−/−14,15 and long-term CBDL mice in the current paper. Increased ALT levels in CBDL FXR−/− mice are primarily related to a significant increase in single cell necroses.14,15 In other words, FXR−/− mice are not resistant to cholestasis-induced hepatocyte injury but to liver fibrosis of the biliary type in response to common bile duct ligation.
We extended our study to additional cholestatic (DDC) and non-cholestatic (CCl4-intoxication, S.m.-infection) experimental models of liver fibrosis. In DDC-fed mice serving as model for xenobiotic-induced cholangiopathy with fibrosis of the biliary type36 all parameters of the fibrogenic response including hepatic hydroxyproline levels were significantly reduced in FXR−/− when compared with wild-type controls. An unexpected finding of these studies was, however, the significantly reduced hepatic porphyrin content in DDC-fed FXR−/− mice, despite the absence of significant mRNA changes of enzymes so far known involved in porphyrin synthesis including the recently described porphyrin transport protein Abcb6.41 The decreased hepatic porphyrin content may indicate a previously unrecognized but probably important effect of FXR in porphyrin and/or DDC metabolism, which deserves further studies. Liver injury as quantified by serum ALT levels was comparable between groups (at least at 4 weeks of DDC-feeding) and cholestasis was even more pronounced as suggested by significantly higher bilirubin levels, comparable serum bile acid levels in both genotypes, and even significantly lower bile flow in DDC-fed FXR−/− mice. However, collagen 1a2 mRNA expression levels were significantly lower in DDC-fed FXR−/− mice compared with wild-type controls despite high serum bile acid levels questioning the biological relevance of a bile acid-activated, FXR/SHP-dependent regulatory pathway for hepatic collagen 1a2 in this model. We also determined the fibrotic response of FXR−/− mice in the CCl4-intoxicated mouse model serving as a classical non-cholestatic model for liver fibrosis and observed no differences in the fibrotic response and collagen expression levels between genotypes. In addition, we studied S.m.-infected mice to determine a potential role for FXR in infection-induced liver fibrosis, and again observed no differences in the fibrotic response between genotypes in this model. These findings demonstrate that loss of FXR has no impact on the degree of liver fibrosis in two non-cholestatic mouse models. Taken together our findings obtained in four different mouse models of liver fibrosis suggest a critical role for FXR in determining the fibrotic response in liver fibrosis of the biliary type but do not support a general major role of an FXR-/SHP-dependent mechanism in the regulation of hepatic collagen expression and consequently the degree of liver fibrosis in mice. However, our experimental findings do not rule out general antifibrotic properties of FXR ligands.
Expression levels of mRNA for most regulatory proteins and nuclear receptors, especially FXR, were very low and probably biologically negligible in mouse MFBs and HSCs and most important in human HSCs. However, we observed a robust expression of VDR in line with findings of others.42 In addition, the observed low expression levels for conjugated bile acid uptake systems suggests that conjugated bile acids (representing FXR ligands) should not be taken up by these cells in sufficient amounts. This is however less relevant in case potent and promising FXR ligands such as chenodeoxycholic acid and its derivatives are applied, which undergo less effective taurine conjugation and therefore do not require active transport for their uptake. Since rodent MFBs were isolated from CBDL mice and mouse HSCs were studied after activation in vivo as well as before and after activation in cell culture it also seems to be very unlikely that the findings of the current study are flawed by the lack of HSC activation. In addition, we also show undetectable FXR protein expression in HSCs and MFBs in five in vivo mouse models for liver fibrosis of different etiology and unchanged Shp mRNA expression in FXR ligand-treated MFBs. The relevance of these findings obtained in vitro and in animal models is further underlined by the lack of FXR protein expression in HSCs and MFBs in human liver tissue samples with pronounced liver fibrosis due to various causes. Although FXR mRNA was detectable at extremely low levels, the FXR ligand GW4064 did not stimulate expression of classic FXR downstream target genes (eg, Shp), indicating such low expression levels (with undetectable protein) are also functionally irrelevant. Therefore it seems to be rather unlikely that HSCs and MFBs can be directly targeted by FXR ligands. However, whether the absence of bile acid uptake systems and—even more importantly—low expression of bile acid-activated nuclear receptors such as FXR have an impact on therapeutic bile acid or synthetic FXR ligand effects on HSC function in humans remains to be determined in future studies.
Taken together the findings of the current study suggest that genetic loss of FXR does not increase the degree of fibrosis in four different mouse models of liver fibrosis but even attenuates liver fibrosis in two models for the biliary type of liver fibrosis. The presented results also indicate that an FXR/SHP-dependent regulatory cascade of collagen expression in HSCs/MFBs cannot account for differences in the fibrotic response between the studied genotypes under these experimental situations. In addition, expression levels of bile acid uptake systems as well as NHRs seem to be extremely low in mouse MFBs and HSCs with the exception of VDR. Importantly, these findings have been confirmed in isolated human HSCs and prototypic human fibrotic liver diseases. Although FXR ligands may have miscellaneous direct or indirect antifibrotic effects (eg, modulation of bile composition and flow, activation of detoxification pathways, beneficial metabolic effects), data of the present study specifically suggest that FXR in mouse and human HSCs may not represent a first line direct therapeutic target in liver fibrosis due to its extremely low expression in these cells. Future research will have to unravel the previously observed antifibrotic mechanisms of FXR ligands in more detail.
Supplementary Material
Acknowledgments
We thank Dr. Wolfgang Erwa (Graz) and colleagues for performing liver tests, Judith Gumhold, Dagmar Silbert, and Katharina Leski for excellent technical assistance, and Dr. Alan F. Hofmann for fruitful discussion of the manuscript.
Footnotes
Address reprint requests to Michael Trauner, M.D., Laboratory of Experimental and Molecular Hepatology, Division of Gastroenterology and Hepatology, Department of Medicine, Medical University Graz, Auenbruggerplatz 15; A-8036 Graz; Austria. E-mail: michael.trauner@meduni-graz.at.
Supported by grant P-18613-B05 (to M.T.) from the Austrian Science Foundation and a GEN-AU project grant from the Austrian Ministry for Science (to M.T.), and grant from the Fondo Nacional de Desarrollo Cieintifico y Tecnológico, FONDECYT 1080170 (to M.A.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett. 2006;580:5492–5499. doi: 10.1016/j.febslet.2006.07.082. [DOI] [PubMed] [Google Scholar]
- Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, Nervi F, Mendez-Sanchez N, Uribe M, Bock HH, Schirin-Sokhan R, Stumvoll M, Mossner J, Lammert F, Wittenburg H. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–124. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Silbert D, Gumhold J, Zatloukal K, Denk H, Trauner M. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27:920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- Wang L, Dong H, Soroka CJ, Wei N, Boyer JL, Hochstrasser M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology. 2008;48:1558–1569. doi: 10.1002/hep.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Nemeth A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- Thompson R, Strautnieks S. BSEP: function and role in progressive familial intrahepatic cholestasis. Semin Liver Dis. 2001;21:545–550. doi: 10.1055/s-2001-19038. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, Trauner M. Role of nuclear bile acid receptor. FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney, and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjovall J, Trauner M. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- van Erpecum KJ, Wang DQ, Moschetta A, Ferri D, Svelto M, Portincasa P, Hendrickx JJ, Schipper M, Calamita G. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. 2006;47:32–41. doi: 10.1194/jlr.M500180-JLR200. [DOI] [PubMed] [Google Scholar]
- Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Sasaki S, Kobayashi Y, Misawa H, Nakamura H. 1,25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. J Endocrinol. 2006;188:635–643. doi: 10.1677/joe.1.06105. [DOI] [PubMed] [Google Scholar]
- Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, Yamazoe Y. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J BiolChem. 2003;278:17838–17844. doi: 10.1074/jbc.M210634200. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta JJ, Kullak-Ublick GA. The role of FXR in disorders of bile acid homeostasis. Physiology (Bethesda) 2008;23:286–295. doi: 10.1152/physiol.00020.2008. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Huang W. FXR, a target for different diseases. Histol Histopathol. 2008;23:621–627. doi: 10.14670/HH-23.621. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile Acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Rojkind M, Warren KS, Hait PK, Rifas L, Seifter S. Liver collagen synthesis in murine schistosomiasis. J Clin Invest. 1977;59:666–674. doi: 10.1172/JCI108685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler DJ, Wahl SM, Wahl LM. Hepatic fibrosis in schistosomiasis: egg granulomas secrete fibroblast stimulating factor in vitro. Science. 1978;202:438–440. doi: 10.1126/science.705337. [DOI] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H. Bile acid-induced Mallory body formation in drug-primed mouse liver. Am J Pathol. 2002;161:2019–2026. doi: 10.1016/S0002-9440(10)64480-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. The rat canalicular conjugate export pump (ABCC2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- Wagner M, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Gujral JS, Zatloukal K, Denk H, Jaeschke H, Trauner M. Hepatobiliary transporter expression in intercellular adhesion molecule 1 knockout and Fas receptor-deficient mice after common bile duct ligation is independent of the degree of inflammation and oxidative stress. Drug Metab Dispos. 2007;35:1694–1699. doi: 10.1124/dmd.107.015610. [DOI] [PubMed] [Google Scholar]
- Fickert P, Stoger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, Trauner M. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchio K, Tuchweber B, Manabe N, Gabbiani G, Rosenbaum J, Desmouliere A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619–628. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034–1042. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.