Abstract
γ-Glutamyl transferase (GGT) regulates glutathione metabolism and cysteine supply. GGT inactivation in GGTenu1 mice limits cysteine availability causing cellular glutathione deficiency. In lung, the resultant oxidant burden is associated with increased nitric oxide (NO) production, yet GGTenu1 mice still exhibit higher mortality in hyperoxia. We hypothesized that NO metabolism is altered under severe oxidant stress and contributes to lung cellular injury and death. We compared lung injury, NO synthase (NOS) expression, nitrate/nitrite production, nitroso product formation, peroxynitrite accumulation, and cell death in wild-type and GGTenu1 mice in normoxia and hyperoxia. The role of NOS activity in cell death was determined by NOS inhibition. Exposure of wild-type mice to hyperoxia caused increased lung injury, altered NO metabolism, and induction of cell death compared with normoxia, which was attenuated by NOS inhibition. Each of these lung injury indices were magnified in hyperoxia-exposed GGTenu1 mice except nitrosation, which showed a diminished decrease compared with wild-type mice. NOS inhibition attenuated cell death only slightly, likely due to further exacerbation of oxidant stress. Taken together, these data suggest that apoptosis in hyperoxia is partially NO-dependent and reiterate the importance of cellular glutathione in lung antioxidant defense. Therefore, reduced denitrosylation of proteins, possibly resulting in impaired cellular repair, and excessive apoptotic cell death likely contribute to increased lung injury and mortality of GGTenu1 mice in hyperoxia.
Glutathione (γ-glutamyl-cysteinyl-glycine; GSH) is the most abundant intracellular non-protein thiol, and is an antioxidant by virtue of its sulfhydryl group.1 Component amino acids, including glutamate, cysteine and glycine, serve as substrates for de novo synthesis of cellular glutathione, but this pathway is insufficient to cope with increased GSH demand under heightened oxidative stress. Extracellular glutathione metabolism by the ectoenzyme γ-glutamyl transferase (GGT) is a crucial prerequisite for cellular GSH homeostasis, as inefficient transfer of this molecule across the cell membrane prevents direct uptake of glutathione from the blood. GGT enzyme activity enhances cellular glutathione synthesis by increasing the availability of component amino acids, specifically cysteine. This amino acid supply role was confirmed in mouse models of GGT deficiency,2 including the GGTenu1 mouse.3 In this mouse model, GGT protein synthesis is prematurely terminated by an ethylnitrosourea-induced point mutation within the protein coding domain of the GGT gene, and as such, the enzyme is inactive.4 Loss of glutathione metabolism causes a deficiency of precursor amino acids and translates into substantially decreased intracellular GSH levels. Cellular glutathione deficiency, in turn, alters the redox balance and predisposes to oxidant stress in tissues of the GGTenu1 mouse even under normoxia.4,5 Within the lung, increased oxidant burden is reflected by increased levels of glutathione disulfide, increased expression of 3-nitrotyrosine, a marker of peroxynitrite (ONOO−) formation, as well as heightened levels of oxidative and nitrative stress. This stress is particularly evident in non-ciliated bronchiolar epithelial (Clara) cells, as GGT is normally expressed as a membrane-bound enzyme on the apical surface of these cells; in alveolar macrophages, as soluble GGT is present within surfactant; and in vascular cells, as GGT circulates normally as a plasma protein in the blood.5 Compared with normal mouse lung, these cells in GGTenu1 mice are much more susceptible to injury and the lung develops edema and hemorrhage more rapidly on exposure to hyperoxia.5
Hyperoxia induces lung injury via multiple mechanisms, including the production of reactive oxygen species. GSH is a critical antioxidant known to protect the lung from injury in hyperoxia.6,7 Additionally, GSH possesses diverse biological functions involved in cell proliferation and apoptosis, redox signaling, and nitric oxide (NO) metabolism,8 and acts as a scavenger of reactive nitrogen oxide species.9 Thus, changes in intracellular GSH availability can have major effects on cellular oxidative/nitrosative balance.
NO-mediated nitrosation reactions can serve as a mechanism to impact cellular apoptosis by altering the post-translational modification of proteins, including caspases. NO is a unique biological molecule with antioxidant and pro-oxidant properties, depending on its concentration and the milieu in which it is produced.9 In the presence of oxygen and reactive oxygen species, NO is converted into reactive nitrogen oxide species such as ONOO−, which can modify a variety of biological molecules via oxidation, nitrosation, and nitration reactions.9 Our previous studies have demonstrated enhanced ONOO− formation in lung cells of the GGTenu1 mice at baseline, suggesting that GSH deficiency produces an excess of reactive oxygen species even under normoxia. Since exposure to hyperoxia is associated with excessive glutathione depletion in GGTenu1 lung,5 we hypothesized that the exacerbated oxidant burden is likely to play a key role in tissue injury. Therefore, we sought to examine the role of oxidative/nitrosative stress in this process and hypothesized that a change in ONOO− and other reactive nitrogen oxide species formation may contribute to the diffuse lung injury observed in these mice under hyperoxia. To gain a better understanding of the interplay between glutathione and NO-mediated cell injury, we compared glutathione deficient GGTenu1 mice with wild-type mice in normoxia and hyperoxia (95% O2) for NO synthase (NOS) expression, nitrate/nitrite production, nitroso product formation, peroxynitrite accumulation, and evidence of cell death.
Materials and Methods
Animal Models and Exposure to Hyperoxia
GGTenu1 and wild-type mice (C57Bl6 background) were bred and housed in the Laboratory and Animal Science Center at Boston University School of Medicine. Animals were fed Purina mouse chow and allowed access to water ad libitum. Hemizygous pairs were mated and offspring were genotyped at 4 weeks of age using tail-derived DNA and direct sequencing of PCR products as described.4 Wild-type and GGTenu1 mice were exposed to an atmosphere of >95% O2/5% N2 as described,5 while continuously monitoring O2 concentrations by oximetry. Mice were checked every 6 hours for viability and sacrificed after 48, 72, or 96 hours in hyperoxia according to guidelines approved by the local Institutional Animal Care and Utilization Committee in protocol number AN-14284.
Measurement of Lung Injury
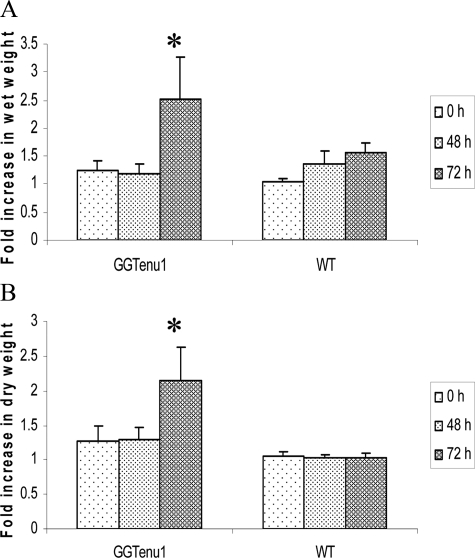
Since our previous study showed excessive lung edema and hemorrhage following exposure to hyperoxia,5 lung injury was assessed through measurement of wet weight for edema formation and dry weight for accumulation of hemorrhage. After exposure to hyperoxia for either 48 or 72 hours, lung was harvested, weighed (wet weight), and placed at 42°C. The weights were assessed every 24 hours until the weight stabilized over two successive measures (dry weight). Lungs were harvested at baseline to serve as a control for these experiments.10 Results are reported as fold increase compared with the respective lung genotype at baseline.
NOS Protein Expression
NOS protein expression was assessed by Western analysis and immunohistochemistry for cellular localization.
Western Analysis
In separate experiments, the lung was excised from mice at baseline (normoxia) or after exposure to 48, 72, or 96 hours of hyperoxia, and placed immediately into liquid nitrogen for subsequent extraction of RNA and protein. Total lung NOS expression (NOSI, II, and III) was analyzed by Western analysis. Protein was isolated using radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology) with 30 μl 100 mmol/L phenylmethanesulfonyl fluoride and 30 μl aprotinin (Sigma Chemicals), separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose as described.11,12 NOSI expression was assayed using a commercially available rabbit generated polyclonal antibody (Assay Design Laboratories) at a concentration of 1:250 followed by a donkey anti-rabbit horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology) at 1:5000 in the same buffer. NOSII and NOSIII expression were assayed using commercially available mouse-generated monoclonal antibodies (Transduction Laboratories) at a concentration of 1:1000 (NOSII) or 1:2500 (NOSIII) in Tris-buffered saline/Tween 20 with 5% nonfat dry milk and a goat-anti-mouse horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology) at 1:5000 in the same buffer. Antibody binding was assessed by enhanced chemiluminescence (PerkinElmer Life Sciences). Tubulin expression was assessed to ensure equal protein loading.
Immunohistochemistry
After de-paraffinization and quenching of endogenous peroxidases with 3% H2O2 in PBS, immunohistochemistry for endothelial (e)NOS expression was performed using a commercially available mouse monoclonal antibody (Transduction Laboratories) (1:250) and the protocols included in the mouse-on-mouse kit from Vector Laboratories. Labeled antibody was stained using 3-3′diaminobenzidine.
Measurement of NO-Related Metabolites
To complement our assessment of NOS expression, several NO-related metabolites were quantified as a measure of actual NOS activity. Lung homogenates of GGTenu1 and wild-type mice at baseline (normoxia) and after exposure for 72 hours to hyperoxia were analyzed for endogenous nitrite and nitrate as final end-products of NO oxidation (NOx) using ion chromatography.13 Additionally, total nitroso species (RXNO), encompassing products of protein nitrosation at thiol and amine residues, were measured as markers of nitrosative stress using gas-phase chemiluminescence.13
Measurement of Peroxynitrite Formation
GGTenu1 and wild-type mouse lungs were excised at baseline or after exposure to 96 hours of hyperoxia, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for immunohistochemistry, as described.11,12 Lung peroxynitrite accumulation was assayed by immunohistochemistry for 3-nitrotyrosine as described using a commercially available antibody (Upstate Biotechnology, Lake Placid, NY).5
Analysis of Cell Death
Cell death was assessed via use of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay and caspase-3 expression and activity with the goal of increasing specificity for apoptosis.14
TUNEL Assay
DNA fragmentation was assessed on tissue sections using a commercially available TUNEL assay kit (Serological Corporation). Following de-paraffinization and quenching of endogenous peroxidases, DNA fragmentation was assessed after application of active TdT enzyme. Apoptotic cells were labeled with diaminobenzidine (Vector Laboratories). All nuclei were counterstained with methyl green (Vector Laboratories). To quantify apoptosis, TUNEL-positive cells were counted on 10 randomly selected high-power fields per slide. Data were analyzed as TUNEL-positive cells per 500 nuclei.
Measurement of Caspase-3 Expression and Activity
As an early marker of activation of the caspase and apoptotic pathway, caspase-3 protein expression and enzymatic activity were measured. Caspase-3 protein expression was assessed by Western analysis using a rabbit polyclonal antibody (Santa Cruz Biotechnology), directed against full-length caspase 3, at a concentration of 1:100 in Tris-buffered saline/Tween 20 + 5% milk, followed by a goat anti-rabbit horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology) at 1:5000 in the same buffer. Caspase-3 enzymatic activity was assayed from total lung homogenates using a commercially available kit according to manufacturer protocol (Promega Corporation).
Assessment of the Effect of NOS Inhibition on Cell Death
To examine the effects of NOS activity on apoptosis, GGTenu1 and wild-type mice were given the NOS inhibitor, N-ω-nitro-l-arginine methyl ester (L-NAME), or its inactive isomer, N-ω-nitro-d-arginine methyl ester (D-NAME), at 100 μg/ml in their drinking water for 12 days before exposure to hyperoxia.15 Groups of three from the pretreated GGTenu1 and wild-type mice were placed in hyperoxia for 72 hours and the lungs were harvested as described above. Groups of three from the pretreated wild-type mice and one group of three GGTenu1 mice treated with L-NAME were maintained in normoxia to serve as controls.
Image Processing
All histology preparations were visualized with a ×10 and ×40 lens on a Leitz orthoplan microscope. Photographs were obtained using the Improvision Open-Lab Users Software program (Quincy, MA).
Statistical Analysis
All numerical data are presented as means ± SEM. Data obtained from GGTenu1 and wild-type mice in normoxia and in hyperoxia were compared with analysis of variance followed by a posthoc Tukey or Bonferroni (NOx/RXNO assays) test; P values ≤0.05 were considered significant.
Results
Hyperoxia Exposure Accentuates Injury in GGTenu1 Mouse Lung
GGTenu1 mice (n = 3 to 5) exposed to hyperoxia exhibited an abrupt and significant rise in both lung wet weight (2.5-fold over baseline, P < 0.05) and dry weight (2.1-fold, P < 0.05) at 72 hours consistent with formation of edema and hemorrhage, respectively. Wild-type mice (n = 3 to 5) exposed to hyperoxia exhibited an insignificant increase in lung wet weight by 1.2-fold at 48 hours and 1.5-fold at 72 hours, but no increase in lung dry weight for up to 72 hours consistent with the accumulation of edema alone (Figure 1, A–B).
Figure 1.
Assessment of lung edema and hemorrhage. Edema and hemorrhage were assessed by measuring the wet (A) and dry (B) weights of lung as described in Materials and Methods. Neither GGTenu1 nor wild-type (WT) mice showed a significant change in lung weight after 48 hours of exposure to hyperoxia. After 72 hours of hyperoxia, the GGTenu1 mice exhibited significant increases in lung wet (A) and dry (B) weights, whereas the wild-type mice exhibited a change only in lung wet weight. *P < 0.05.
Hyperoxia Is Associated with Enhanced eNOS Expression, NO Production, Protein Nitration, and Nitrosative Stress in GGTenu1 Mice
Increased eNOS Expression
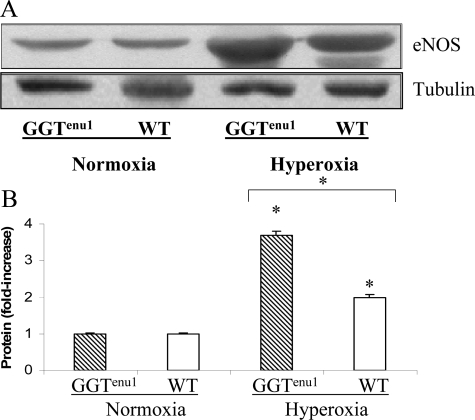
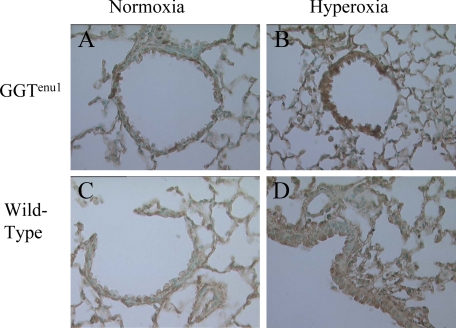
RNA expression of NOS I and II remained unchanged in hyperoxia in either strain of mice (data not shown). By Western analysis, both strains revealed a twofold induction of NOSI expression after 72 hours of exposure to hyperoxia. NOSII expression did not change in wild-type mice and actually decreased in GGTenu1 mice exposed to hyperoxia (see Supplemental Figure S1 at http://ajp.amjpathol.org). Because of these findings, subsequent experiments focused on NOSIII (eNOS). NOS III protein expression was similar in GGTenu1 and wild-type mice in normoxia. However, exposure of GGTenu1 mice to hyperoxia for 96 hours induced eNOS protein expression fourfold over baseline (P < 0.05) and twofold above levels in wild-type mice exposed to hyperoxia (P < 0.05, n = 3) (Figure 2, A–B). Protein induction was associated with an increase in eNOS mRNA as determined by real-time PCR (see Supplemental Figure S2 at http://ajp.amjpathol.org). Exposure of wild-type mice to hyperoxia for 96 hours induced only a twofold increase in lung eNOS protein and mRNA levels with normoxia (P < 0.05). Immunohistochemistry revealed that eNOS expression was localized to vascular endothelium of GGTenu1 and wild-type mice in normoxia, and to occasional airway epithelial cells in GGTenu1 but not wild-type mice. After exposure to hyperoxia for 96 hours, eNOS expression was induced diffusely throughout the airway epithelium and cells of the alveolar wall of GGTenu1 mice (Figure 3, A–D).
Figure 2.
eNOS protein expression. A: Western analysis for eNOS protein was performed on extracts of lung tissue from GGTenu1 and wild-type (WT) mice in normoxia and hyperoxia as described in Materials and Methods. The level of lung eNOS protein was significantly affected by hyperoxia exposure in both mouse strains. Tubulin controlled for loading. B: eNOS protein increased four fold in the GGTenu1 mouse lung and twofold in the wild-type mouse lung compared to the respective normoxic controls, *P < 0.05. The twofold higher level of eNOS protein induction in the GGTenu1 mouse lung in hyperoxia was significantly greater than that of the wild-type mouse lung in hyperoxia, *P < 0.05.
Figure 3.
Immunohistochemistry for eNOS. Immunolocalization was performed as described in Materials and Methods. Lung sections of GGTenu1 (A, B) and wild-type (WT) (C, D) mice are shown in normoxia (A, C), and after exposure to hyperoxia for 96 hours (B, D). After exposure to hyperoxia for 96 hours, eNOS expression was induced throughout the airway epithelium and cells of the alveolar wall of GGTenu1 mice only. All photomicrographs are at ×400 magnification.
Alterations in NO Oxidation and Nitrosation Products
The concentration of nitroso species (RXNO) as well as the NO oxidation products, nitrite (NO2−) and nitrate (NO3−) were quantified in lung, liver, and plasma to assess overall NO production and extent of nitrosative stress. Lung and liver RXNO concentrations were similar in wild-type and GGTenu1 mice under normoxia while plasma RXNO was significantly elevated in GGTenu1 mice (see Table 1). NOx (nitrite + nitrate) concentrations were similar in normoxia at all three sites. Exposure to hyperoxia for 72 hours did not increase NOx concentrations in lung, liver, or plasma of wild-type mice, but did so mildly in GGTenu1 mice, though only significantly in the plasma (see Table 1). The latter was exclusively attributable to an increase in nitrate concentration; nitrite concentrations remained almost identical during hyperoxia in wild-type and GGTenu1 mice (not shown). Unexpectedly, the increase in NO production triggered by hyperoxia did not translate into an increase, but rather, a decrease in the concentration of nitrosation products in lung of wild-type (P = 0.27) and GGTenu1 (P = 0.05) mice. Nevertheless, the hyperoxia steady-state concentrations of lung (and liver) RXNO remained significantly higher in GGTenu1 mice compared with their wild-type counterparts (see Table 1). RXNO content decreased in wild-type liver but increased in GGTenu1 liver with hyperoxia. Plasma RXNO content did not change with hyperoxia in either strain. These data suggest that the enhanced NO production in the GGTenu1 mice could potentially lead to more pronounced alterations in tissue protein structure and function. Alternatively, GGTenu1 mice may be less efficient at removing the NO+ group from nitrosated moieties, which may translate into persistent enzyme inhibition and tissue damage.
Table 1.
Hyperoxia-Induced Alterations in NO-Related Products in Tissues of Glutathione Deficient GGTenu1 and Wild-type Animals
| RXNO (pmol/g wet weight)
|
NOx (nmol/g wet weight)
|
|||||
|---|---|---|---|---|---|---|
| Lung | Liver | Plasma | Lung | Liver | Plasma | |
| Wild-type normoxia | 89 ± 31 | 146 ± 25 | 5.9 ± 0.8 | 5.8 ± 1.5 | 4.3 ± 0.8 | 5.9 ± 0.7 |
| GGTenu1 normoxia | 79 ± 7 | 167 ± 10 | 9.7 ± 0.9∗ | 7.4 ± 0.7 | 4.3 ± 1.6 | 7.2 ± 1.7 |
| Wild-type hyperoxia | 36 ± 3 | 119 ± 07 | 6.5 ± 1.0 | 7.3 ± 0.5 | 3.7 ± 1.6 | 6.1 ± 1.7 |
| GGTenu1 hyperoxia | 59 ± 5† | 258 ± 38‡ | 9.3 ± 0.9 | 9.6 ± 1.2 | 7.9 ± 0.4 | 10.4 ± 0.4§ |
Mean ± SEM from n = 3 to 4 animals/group.
RXNO, sum of S- and N-nitroso species; NOx, sum of nitrite and nitrate.
P = 0.03 for plasma RXNO elevation GGTenu1 versus wild-type mice in normoxia.
P = 0.047 for higher lung RXNO content of GGTenu1 versus wild-type in hyperoxia.
P = 0.012 for liver RXNO content of GGTenu1 versus wild-type in hyperoxia.
P = 0.01 for plasma NOx elevation in GGTenu1 in normoxia versus hyperoxia.
Enhanced Nitrative Stress after Hyperoxia in GGTenu1 Mice
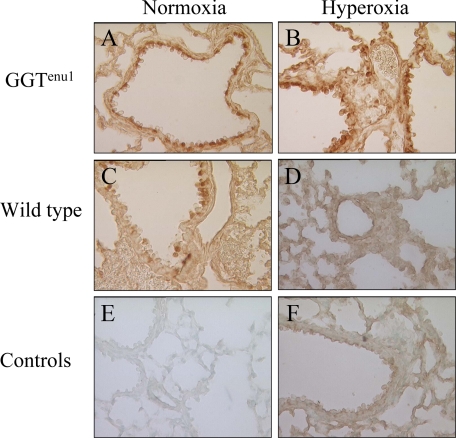
Peroxynitrite is a short-lived reactive nitrogen oxide species (10 ms in vivo) that has the potential to cause oxidative and nitrative damage.16 We probed for changes in 3-nitrotyrosine related immunoreactivity, a long-lived protein modification and a marker of cellular nitrative stress, as a proxy for ONOO− production. These experiments were performed in triplicate and 10 representative photos from each slide were obtained. In normoxia, 3-nitrotyrosine immunoreactivity was detected in almost all airway epithelial cells and many alveolar macrophages in GGTenu1 lung (Figure 4A), but only few, scattered airway epithelial cells and no macrophages in wild-type lung (Figure 4, C and E) in agreement with our previous results.5 In hyperoxia, positive immunoreactivity was evident in most airway epithelium and cells of the alveolar wall of GGTenu1 lungs (Figure 4B), but no NO2Tyr-positive signal was detected in cells from wild-type lung (Figure 4, D and F). In agreement with earlier findings5 the immunoreactive signal was eliminated by competition with 10 mmol/L nitrotyrosine and induced diffusely in lung tissue pretreated with peroxynitrite as a positive control (data not shown).
Figure 4.
Immunohistochemistry for 3-nitrotyrosine. Immunolocalization was performed as described in Materials and Methods. Lung sections of GGTenu1 and wild-type (WT) mice are shown in normoxia (A, C), and after exposure to hyperoxia for 96 hours (B, D). Control stains are shown in (E, F). All photomicrographs are at ×400 magnification.
Hyperoxia Accentuates Cell Death in GGTenu1 Mouse Lung
Apoptosis was studied in our model by two different techniques, TUNEL assay to assess DNA fragmentation and measurement of expression and activity of caspase-3, a key enzyme of the caspase apoptotic pathway.17
TUNEL Reactivity
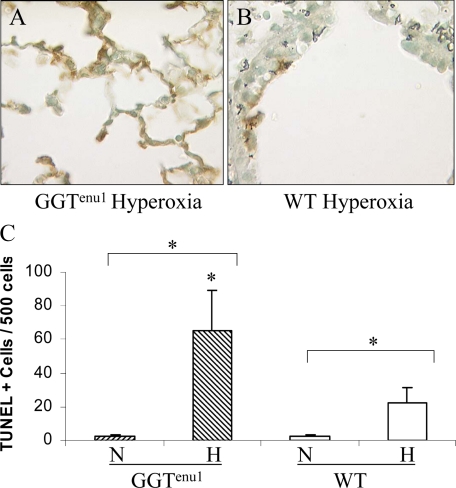
In normoxia, the baseline level of TUNEL reactivity was similar in lung cell nuclei from GGTenu1 (2.2 ± 1.0 cells/500 nuclei) and wild-type mice (2.2 ± 1.5 cells/500 nuclei). Exposure for 96 hours to hyperoxia increased the number of TUNEL-positive cells 30-fold in GGTenu1 (65.2 ± 24.3 cells/500 nuclei, P < 0.05, Figure 5A and 5C) but only 10-fold in wild-type lung (22.3 ± 8.8 cells/500 nuclei, P < 0.05, Figure 5 B, C). Hence, the number of TUNEL positive cells in hyperoxia was three-fold higher in GGTenu1 mice compared with wild-type mice in hyperoxia suggesting a greater extent of DNA fragmentation.
Figure 5.
TUNEL assay of GGTenu1 and wild-type (WT) Lungs. Lung sections from GGTenu1 (A) and wild-type (B) mice were assayed for DNA fragmentation after exposure to hyperoxia for 96 hours as described in Materials and Methods, and TUNEL positive cells/500 nuclei were quantified (C). Exposure to hyperoxia (H) increased the number of TUNEL positive cells in the lungs of GGTenu1 and wild-type mice. The number increased by 30-fold over the normoxia (N) baseline for GGTenu1 mouse lung and this was almost threefold higher than that observed in the hyperoxia-exposed wild-type mouse lung. TUNEL-positive cells/500 nuclei increased almost tenfold in the wild-type mouse lung in hyperoxia compared with normoxia (WT-N). *P < 0.05.
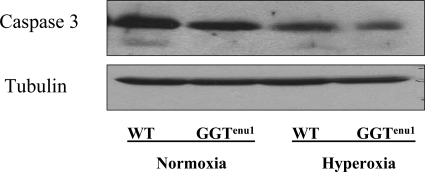
Caspase-3 Expression
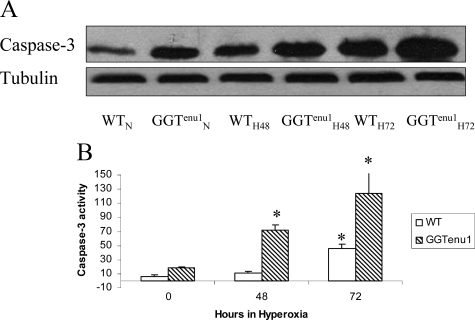
In normoxia, GGTenu1 mouse lung showed a higher expression of caspase-3 protein compared with wild-type mouse lung (Figure 6A). In hyperoxia, the temporal pattern and the magnitude of caspase-3 protein induction was accentuated in the GGTenu1 lung compared with wild-type lung. Compared with normoxia, caspase-3 expression in GGTenu1 mice increased twofold at 48 hours (GGTenu1H48) and threefold at 72 hours (GGTenu1H72, n = 4, P < 0.05). By contrast, wild-type lung exhibited a twofold induction of caspase-3 protein expression in hyperoxia only after 72 hours (WTH72, n = 4, P < 0.05).
Figure 6.
Caspase 3 protein expression and enzymatic activity. Caspase-3 was assayed in lung extracts from wild-type (WT) and GGTenu1 mice in normoxia (N) and hyperoxia (H) as described in Materials and Methods. A: Caspase-3 protein expression by Western analysis in GGTenu1 mouse lung showed a twofold induction at 48 hours (GGTenu1H48) and a threefold induction at 72 hours (GGTenu1H72), as compared with the normoxia control (GGTenu1N). In contrast, the wild-type mouse lung showed a twofold induction of caspase 3 protein expression at 72 hours of hyperoxia (WTH72) compared with the wild-type normoxia control (WTN). B: Caspase 3 activity was increased at baseline in GGTenu1 mouse lung compared with wild-type mouse lung. In hyperoxia, caspase 3 enzymatic activity in GGTenu1 mouse lung increased fourfold at 48 hours and 11-fold at 72 hours compared with baseline. In the wild-type mouse lung, induction of caspase 3 activity was only significant at 72 hours as compared with baseline and was sixfold less at 48 hours, and threefold less at 72 hours as compared with the GGTenu1 mouse lung. *P < 0.05 compared with baseline.
Caspase-3 Activity
Baseline enzymatic activity of caspase-3 under normoxia was considerably higher in GGTenu1 mouse lung (19.20 ± 1.04 μmol/L) compared with wild-type mouse lung (5.84 ± 2.63 μmol/L) (Figure 6B). In hyperoxia, the temporal pattern and magnitude of induction was further accentuated in the GGTenu1 lung. In hyperoxic GGTenu1 lung, caspase-3 activity increased nearly fourfold at 48 hours (72.18 ± 7.05 μmol/L, P < 0.05, n = 3) and 6.5-fold at 72 hours (124.59 ± 32.3 μmol/L), as compared with baseline. In hyperoxic wild-type lung, caspase-3 activity was only significantly increased at 72 hours compared with baseline (*). Compared with GGTenu1 lung, the induction of caspase-3 activity in wild-type lung was nearly sixfold less at 48 hours (11.56 ± 1.78 μmol/L, P < 0.05) and nearly threefold less at 72 hours (46.01 ± 6.25 μmol/L).
NOS Inhibition Decreases Hyperoxia-Induced Cell Death in Wild-Type and GGTenu1 Mice
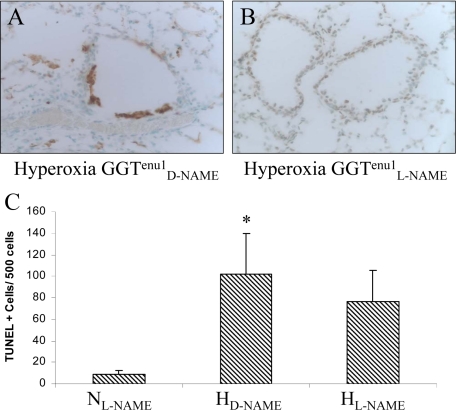
To examine the role of NOS-derived NO in hyperoxia-induced apoptosis in the GGTenu1 and wild-type mice, both strains were pretreated with the NOS inhibitor L-NAME or its inactive isomer D-NAME for 12 days before experimentation. L-NAME treatment had no effect on the number of TUNEL-positive staining under normoxia in either mouse strain (wild-type, 5.3 ± 0.5 cells/500 nuclei; GGTenu1, 8.8 ± 3.4 cells/500 nuclei). For GGTenu1 mice in hyperoxia (Figure 7, A–C), TUNEL-positive lung cells increased 11-fold in the D-NAME pre-treated group (HD-NAME) (101.9 ± 39.2 cells/500 nuclei) (P < 0.05), but only eightfold in the L-NAME pre-treated group (76.4 ± 29.6 cells/500 nuclei) (HL-NAME) (P = 0.06), as compared with normoxia (NL-NAME), representing a trend toward decreased apoptosis on NOS inhibition (P = 0.55).
Figure 7.
TUNEL assay in GGTenu1 lung after treatment with L-NAME or D-NAME. GGTenu1 mice were treated with D-NAME (A) or L-NAME (B) and exposed to hyperoxia (H) as described in the Materials and Methods. C: Compared with the L-NAME pre-treated GGTenu1 mice in normoxia as control, there was 11-fold increase in lung TUNEL positive cells/500 nuclei in D-NAME treated GGTenu1 mice after exposure to hyperoxia (*P < 0.05), but only an eightfold increase L-NAME treated GGTenu1 mice exposed to hyperoxia. The 1.4-fold decrease in the number of lung TUNEL-positive cells/500 nuclei between the D-NAME and L-NAME treated mice exposed to hyperoxia was not statistically significant.
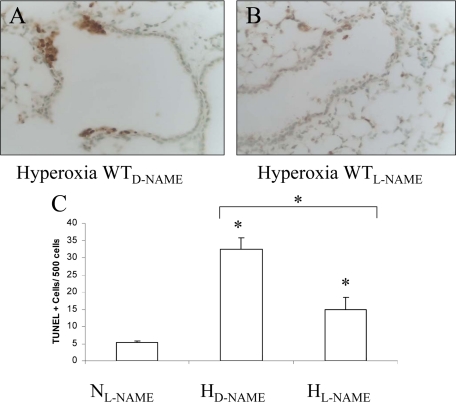
In a similar fashion, L-NAME pretreatment of wild-type mice was able to abrogate some of the hyperoxia-induced apoptosis (Figure 8, A–C).Compared with normoxia (NL-NAME), TUNEL-positive cells in the hyperoxia-exposed, D-NAME pre-treated group (HD-NAME) increased sixfold (32.4 ± 3.5 cells/500 nuclei) (P < 0.05), but only threefold in the L-NAME pretreated (15.0 ± 3.5 cells/500 nuclei) (HL-NAME) group (P < 0.05). The 2.2-fold decrease in the L-NAME pretreated group was statistically significant compared with the D-NAME pre-treated group (P < 0.05) Thus, NOS-dependent NO production appears to contribute, in part, to apoptotic lung cell injury in hyperoxia.
Figure 8.
TUNEL assay in wild-type (WT) lung after treatment with L-NAME or D-NAME. Wild-type mice were treated with D-NAME (A) or L-NAME (B) and exposed to hyperoxia (H) as in Figure 7. C: Compared with the L-NAME pretreated wild-type mice in normoxia as control (NL-NAME), there was a sixfold increase in TUNEL positive cells/500 nuclei in the D-NAME treated wild-type lung (HD-NAME) after exposure to hyperoxia, but only a threefold increase in the L-NAME treated wild-type lung (HL-NAME) after exposure to hyperoxia. The 2.2-fold decrease in the number of lung TUNEL positive cells/500 nuclei in L-NAME treated mice was significant compared with wild-type mice treated with D-NAME in hyperoxia. *P < 0.05.
Pretreatment with L-NAME had no effect on lung caspase-3 protein expression in either wild-type or GGTenu1 mice (Figure 9). After 48 hours in hyperoxia, L-NAME pretreatment decreased caspase-3 expression to a similar extent in lungs from GGTenu1 and wild-type mice (67% and 50%, as compared with baseline).
Figure 9.
Analysis of lung caspase 3 expression after treatment with L-NAME. Wild-type (WT) and GGTenu1 mice were pre-treated with L-NAME before exposure to hyperoxia for 48 hours as described in Materials and Methods. L-NAME treatment was associated with a 67% decrease in caspase 3 protein expression by Western blot in GGTenu1 lung exposed to hyperoxia compared with normoxia, and a 50% decline in wild-type lung exposed to hyperoxia compared with normoxia. Tubulin expression was assessed as a loading control.
Discussion
Glutathione, the most abundant non-protein thiol, is an essential component of the cellular antioxidant response. It acts directly as an antioxidant and as a substrate for the enzyme glutathione peroxidase, which catalyzes the reduction of H2O2 and lipid hydroperoxides. Glutathione is synthesized from glutamate, cysteine and glycine via reactions catalyzed by the cytosolic enzymes, γ-glutamylcysteine ligase and GSH synthetase. The intracellular GSH concentration is determined by both the rate of GSH synthesis and the combined rate of GSH consumption and efflux. Under conditions of increased oxidant burden, GSH is consumed, necessitating an increase in de novo synthesis for repletion.18 A limiting factor in de novo GSH synthesis is cysteine availability.2,3,19 GGT plays a critical role in initiating extracellular glutathione metabolism to provide cysteine for cellular GSH synthesis and thereby modulates the cellular response to oxidant stress.20,21,22 Absence of GGT decreases cysteine bioavailability and leads to cellular glutathione deficiency. As a result, the GGTenu1 mouse is characterized by an inability to appropriately respond to an increased cellular oxidant burden.5,23 Alterations in the intracellular redox environment occur in the GGTenu1 mouse lung at baseline, as reflected by increased heme oxygenase-1 expression in vascular cells and accumulation of 3-nitrotyrosine, suggestive of ONOO− formation, in epithelial cells and macrophages.5 These data suggest an environment in which the availability of free NO is shunted toward enhanced formation of reactive nitrogen oxide metabolites (RNOS) such as peroxynitrite, nitrogen dioxide, dinitrogen trioxide, and others.9 In addition, GGT deficiency is expected to enhance the lifetime of S-nitrosoglutathione (GSNO), a major reaction product of glutathione with RNOS. This results from increased extracellular glutathione concentrations (presumably leading to higher circulating GSNO levels), and decreased intracellular GSNO breakdown (GGT is the major GSNO metabolizing enzyme).24 Possible localization of GGT to the alveolar type I cells may make this cell type a more likely target of hyperoxic injury.11,25,26 Taken together, GGT deficiency would seem to lead to enhanced nitrosative stress and oxidative post-translational protein modification. Although not specifically investigated in this study, it is conceivable that such alterations will include proteins involved in the regulation of apoptosis such as caspases. In addition to the changes in protein expression, which we have observed in the present study, caspase activity is known to be modulated by S-nitrosation/denitrosylation.27 Consistent with this notion, we here demonstrate that the enhanced oxidant burden of hyperoxia is associated with higher levels of protein nitrosation in lung tissue and with increased cellular apoptosis in GGTenu1 compared with wild-type mice.
Alterations in the production and fate of NO in response to hyperoxia in GGTenu1 mice are characterized by an eNOS-dependent increase in NO formation combined with a preferential shunting of NO to RNOS resulting in oxidation, nitrosation, and nitration reactions with other biological targets. It is possible that NOS-derived RNOS act as mediators of caspase-dependent cell death in hyperoxia in both wild-type and GGTenu1 mouse lung. However, the impaired ability of GGTenu1 mice to synthesize glutathione in the setting of an increased oxidant burden5 makes them exquisitely more sensitive to RNOS-related toxicity compared with the wild-type lung, where glutathione metabolism is intact.
The steady-state concentration of protein nitrosation products in tissues is a function of formation (via nitrosative chemistry) and degradation (via enzymatic and nonenzymatic denitrosylating processes). Thiol nitrosation may serve a protective role in that it prevents critical protein thiols from irreversible oxidation. In most cases, however, this post-translational modification is associated with an inhibition of enzyme activity. Thus, rapid denitrosylation is essential to restore enzyme function/activity. While hyperoxia was found to result in a decrease in tissue nitrosation products in either mouse strain, the remaining steady-state levels of nitroso species were higher in GGTenu1 mice compared with wild-type mice. No attempts were made to identify the nature of the nitrosated proteins, but if one or more of the enzymes affected was involved in cell repair and/or ATP/NAD(P)H generation a higher degree of nitrosation (and thus inhibition) may translate into increased tissue damage.
NO participates in a wide range of biochemical reactions. It is synthesized via a five-electron oxidation from l-arginine in a reaction catalyzed by a family of enzymes termed nitric oxide synthases.28,29 The NOS isoforms include neuronal NOS (nNOS, NOSI), inducible NOS (iNOS, NOSII), and endothelial NOS (eNOS, NOSIII) and they share approximately 55% homology. The two constitutive isoforms, nNOS and eNOS, are calcium-dependent and responsible for the majority of basal NO synthesis in most tissues. The relatively low concentrations of NO they produce serves to regulate physiological functions, including vasodilation and inhibition of fibroblast and smooth muscle cell migration. During inflammation, significantly higher levels of NO are produced, leading to NO-mediated cytotoxic killing of pathogens, tumor cells, and nonpathogenic host cells.30 Traditionally, it has been thought that iNOS was the key mediator of NO-related cellular death.31 More recently, a growing body of evidence supports the notion that eNOS can also be induced under certain conditions including hypoxia, hyperoxia, and shear stress.32 In the current study, we demonstrate that hyperoxia specifically induces eNOS expression in both the GGTenu1 and wild-type mice with no induction of iNOS expression. In the setting of increased eNOS expression and oxidative stress (as observed in hyperoxia), enhanced ONOO− formation can occur due, in part, to NOS uncoupling.33 One possible downstream effect of this is an increase in apoptotic cell death. Cell death occurred in the lung of both wild-type and GGTenu1 mice, however, the effects on lung tissue from GGTenu1 mice were much more profound. There was a thirtyfold increase in TUNEL positive cells in the GGTenu1 lungs in hyperoxia compared with baseline and a threefold increase compared with the wild-type mouse under similar hyperoxic conditions. These findings are in line with the increased eNOS expression, NO and ONOO− formation observed in the GGTenu1 mouse lung. Even so, NOS inhibition with L-NAME had only a minor effect on cell death suggesting that this process is only in part NO-dependent and likely more complex than originally suspected.
NO induces cellular death via two different mechanisms, apoptosis and necrosis. Mechanisms of NO-mediated apoptosis include inhibition of mitochondrial respiration, oxidation of mitochondrial phospholipids and induction of mitochondrial permeability by NO-related metabolites such as ONOO−.30,34,35 NO inhibits mitochondrial respiration directly via inhibition of cytochrome oxidase and indirectly through the production of RNOS-mediated nitrosation of other components of the electron transport chain.36 NO-related oxidants can additionally produce necrosis in the setting of glutathione depletion.30,37 One of the key features of NO-mediated apoptosis is caspase activation, resulting in downstream events such as DNA fragmentation and phosphatidylserine externalization.30 In our study, hyperoxia induced caspase-3 protein expression and enzymatic activity in the GGTenu1 lung at an earlier time and to a greater extent than in the wild-type lung. However, NOS inhibition diminished caspase expression in both strains correlating with lower numbers of TUNEL-positive cells, particularly in the wild-type, after pretreatment with L-NAME. These data suggest that caspase activation is related, at least in part, to NOS activity and an important mediator of apoptosis in both GGTenu1 and wild-type mice. It is possible that the large differences in TUNEL-positive cells between the two strains of mice reflect apoptosis via a caspase-independent mechanism or more likely, represent NO-mediated necrosis. Future studies will be required to elucidate the relative roles of these mechanisms in GGTenu1 lung cell death in hyperoxia.
Our study is limited by the fact that it does not address the exact mechanism by which eNOS affects cellular survival. Although we surmise the involvement of an NO-dependent mechanism, an alternative possibility exists. Key to NOS function is the availability of the amino acid substrate, l-arginine and its enzyme co-factors, particularly tetrahydrobiopterin. In the setting of decreased arginine or tetrahydrobiopterin availability, the eNOS tetramer uncouples into dimers and generates O2− instead of NO.38,39 In the GGTenu1 mouse lung, it is possible that eNOS plays some role in generating NO, but also participates in the formation of reactive oxygen species through O2− generation. Another study limitation relates to the lack of information about to the interaction between GSNO and cellular targets of nitrosation at the molecular level,13 the role of glutathione in transnitrosation (the transfer of NO equivalents from one molecule to another) and its impact on caspase activity. Future studies are necessary to address these issues.
In conclusion, we have demonstrated that, in the GGTenu1 mouse lung, perturbations in glutathione metabolism compromise the ability of this organ to respond appropriately to oxidative stress. We have observed a marked up-regulation of lung eNOS and NO production that were accompanied by distinct alterations in nitrosative and nitrative stress and NOS-dependent caspase activation and apoptosis in these mice. Taken together, this study demonstrates the importance of cellular glutathione in the lung antioxidant defense system and begins to elucidate the mechanisms by which cell death occurs in hyperoxia.
Supplementary Material
Footnotes
Address reprint requests to Martin Joyce-Brady, M.D., Associate Professor of Medicine, Boston University School of Medicine, The Pulmonary Center, R-304, 715 Albany Street, Boston, MA 02118. E-mail: mjbrady@bu.edu.
Supported by the National Institutes of Health (grant PO1HL47049 to M.J.B.), the National Heart, Lung, and Blood Institute (grant K23 HL079003-01 to E.S.K.), and American Heart Association Beginning Grant-In-Aid (also to E.S.K.).
Supplemental material for this article can be found at http://ajp.amjpathol.org.
Current address of B.O.F. and M.F.: Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, United Kingdom.
References
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci USA. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CO, Williams P, Wagner E, Chang DS, Wild K, Colwell RE, Wolff JA. Mice with genetic gamma-glutamyl transpeptidase deficiency exhibit glutathionuria, severe growth failure, reduced life spans, and infertility. J Biol Chem. 1997;272:12560–12567. doi: 10.1074/jbc.272.19.12560. [DOI] [PubMed] [Google Scholar]
- Jean JC, Harding CO, Oakes SM, Yu Q, Held PK, Joyce-Brady M. Gamma-glutamyl transferase (GGT) deficiency in the GGTenu1 mouse results from a single point mutation that leads to a stop codon in the first coding exon of GGT mRNA. Mutagenesis. 1999;14:31–36. doi: 10.1093/mutage/14.1.31. [DOI] [PubMed] [Google Scholar]
- Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L766–L776. doi: 10.1152/ajplung.00250.2000. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Rahman I, Antonicelli F, MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. J Biol Chem. 1999;274:5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- Nardell EA, Brody JS. Determinants of mechanical properties of rat lung during postnatal development. J Appl Physiol. 1982;53:140–148. doi: 10.1152/jappl.1982.53.1.140. [DOI] [PubMed] [Google Scholar]
- Joyce-Brady M, Takahashi Y, Oakes SM, Rishi AK, Levine RA, Kinlough CL, Hughey RP. Synthesis and release of amphipathic gamma-glutamyl transferase by the pulmonary alveolar type 2 cell. Its redistribution throughout the gas exchange portion of the lung indicates a new role for surfactant. J Biol Chem. 1994;269:14219–14226. [PubMed] [Google Scholar]
- Oakes SM, Takahashi Y, Williams MC, Joyce-Brady M. Ontogeny of gamma-glutamyltransferase in the rat lung. Am J Physiol. 1997;272:L739–L744. doi: 10.1152/ajplung.1997.272.4.L739. [DOI] [PubMed] [Google Scholar]
- Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007;139:143–156. doi: 10.1016/j.jss.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Strohmeier GR, Walsh JH, Klings ES, Farber HW, Cruikshank WW, Center DM, Fenton MJ. Lipopolysaccharide binding protein potentiates airway reactivity in a murine model of allergic asthma. J Immunol. 2001;166:2063–2070. doi: 10.4049/jimmunol.166.3.2063. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Bregere C, Gallaher TK, Sohal RS. Detection and characterization of peroxynitrite-induced modifications of tyrosine, tryptophan, and methionine residues by tandem mass spectrometry. Methods Enzymol. 2008;441:283–294. doi: 10.1016/S0076-6879(08)01215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechle FL, Zhang X. Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta. 2002;326:27–45. doi: 10.1016/s0009-8981(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5:137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- Huseby NE, Asare N, Wetting S, Mikkelsen IM, Mortensen B, Sveinbjornsson B, Wellman M. Nitric oxide exposure of CC531 rat colon carcinoma cells induces gamma-glutamyltransferase which may counteract glutathione depletion and cell death. Free Radic Res. 2003;37:99–107. doi: 10.1080/1071576021000036434. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci USA. 1979;76:5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- Barrios R, Shi ZZ, Kala SV, Wiseman AL, Welty SE, Kala G, Bahler AA, Ou CN, Lieberman MW. Oxygen-induced pulmonary injury in gamma-glutamyl transpeptidase-deficient mice. Lung. 2001;179:319–330. doi: 10.1007/s004080000071. [DOI] [PubMed] [Google Scholar]
- Henson SE, Nichols TC, Holers VM, Karp DR. The ectoenzyme gamma-glutamyl transpeptidase regulates antiproliferative effects of S-nitrosoglutathione on human T and B lymphocytes. J Immunol. 1999;163:1845–1852. [PubMed] [Google Scholar]
- Ingbar DH, Hepler K, Dowin R, Jacobsen E, Dunitz JM, Nici L, Jamieson JD. Gamma-glutamyl transpeptidase is a polarized alveolar epithelial membrane protein. Am J Physiol. 1995;269:L261–L271. doi: 10.1152/ajplung.1995.269.2.L261. [DOI] [PubMed] [Google Scholar]
- Knickelbein RG, Ingbar DH, Seres T, Snow K, Johnston RB, Jr, Fayemi O, Gumkowski F, Jamieson JD, Warshaw JB. Hyperoxia enhances expression of gamma-glutamyl transpeptidase and increases protein S-glutathiolation in rat lung. Am J Physiol. 1996;270:L115–L122. doi: 10.1152/ajplung.1996.270.1.L115. [DOI] [PubMed] [Google Scholar]
- Na HJ, Chung HT, Ha KS, Lee H, Kwon YG, Billiar TR, Kim YM. Detection and measurement for the modification and inactivation of caspase by nitrosative stress in vitro and in vivo. Methods Enzymol. 2008;441:317–327. doi: 10.1016/S0076-6879(08)01217-2. [DOI] [PubMed] [Google Scholar]
- Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetsch B, Jin RC, Loscalzo J. Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol. 2004;122:353–367. doi: 10.1007/s00418-004-0675-z. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Brown G. What else has to happen for nitric oxide to induce cell death? Biochem Soc Trans. 2005;33:1394–1396. doi: 10.1042/BST0331394. [DOI] [PubMed] [Google Scholar]
- Li CQ, Wright TL, Dong M, Dommels YE, Trudel LJ, Dedon PC, Tannenbaum SR, Wogan GN. Biological role of glutathione in nitric oxide-induced toxicity in cell culture and animal models. Free Radic Biol Med. 2005;39:1489–1498. doi: 10.1016/j.freeradbiomed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol. 2004;24:405–412. doi: 10.1161/01.ATV.0000109171.50229.33. [DOI] [PubMed] [Google Scholar]
- Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387:1521–1533. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- Lee VY, McClintock DS, Santore MT, Budinger GR, Chandel NS. Hypoxia sensitizes cells to nitric oxide-induced apoptosis. J Biol Chem. 2002;277:16067–16074. doi: 10.1074/jbc.M111177200. [DOI] [PubMed] [Google Scholar]
- Walford GA, Moussignac RL, Scribner AW, Loscalzo J, Leopold JA. Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways. J Biol Chem. 2004;279:4425–4432. doi: 10.1074/jbc.M310582200. [DOI] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Ischiropoulos H. Nitric oxide chemistry and cellular signaling. J Cell Physiol. 2001;187:277–282. doi: 10.1002/jcp.1085. [DOI] [PubMed] [Google Scholar]
- Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.