Abstract
The E-cadherin receptor CD103 (αEβ7-integrin) is expressed on specific populations of pulmonary dendritic cells (DC) and T cells. However, CD103 function in the lung is not well understood. Matrilysin (MMP-7) expression is increased in lung injury and cleaves E-cadherin from injured lung epithelium. Thus, to assess matrilysin effects on CD103-E-cadherin interactions in lung injury, wild-type, CD103−/−, and Mmp7−/− mice, in which E-cadherin isn’t cleaved in the lung, were treated with bleomycin or bleomycin with nFMLP to reverse the defect in acute neutrophil influx seen in Mmp7−/− mice. Pulmonary CD103+ DC were significantly increased in injured wild-type compared with Mmp7−/− mice, and CD103+ leukocytes showed significantly enhanced interaction with E-cadherin on injured wild-type epithelium than with Mmp7−/− epithelium in vitro and in vivo. Bleomycin-treated CD103−/− mice had persistent neutrophilic inflammation, increased fibrosis, and increased mortality compared with wild-type mice, a phenotype that was partially recapitulated in bleomycin/nFMLP-treated Mmp7−/− mice. Soluble E-cadherin increased IL-12 and IL-10 and reduced IL-6 mRNA expression in wild-type bone marrow-derived DC but not in CD103−/− bone marrow-derived DC. Similar mRNA patterns were seen in lungs of bleomycin-injured wild-type, but not CD103−/− or Mmp7−/−, mice. In conclusion, matrilysin regulates pulmonary localization of DC that express CD103, and E-cadherin cleavage may activate CD103+ DC to limit inflammation and inhibit fibrosis.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are characterized by acute epithelial and endothelial damage, leakage of proteinaceous edema fluid into the alveolar space and interstitium, and a leukocytic cellular infiltrate, with polymorphonuclear neutrophils being the key inflammatory cell population in both humans and in experimental animals.1 Unfavorable outcomes in patients with ALI/acute respiratory distress syndrome are associated with an exaggerated pulmonary inflammatory response that persists unabated over time.2,3 Failure to resolve acute inflammation also contributes to chronic lung injury and pulmonary fibrosis, and the presence of extensive fibrosis may be an independent risk factor that correlates with poor outcome.4 Impaired epithelial repair contributes to fibrosis in the lung, liver, kidney, and other tissues,5,6 and epithelial cell interactions with inflammatory and mesenchymal cells are central to both physiological lung repair and pathological lung remodeling.
Important among the pulmonary responses to injury is the increased expression and activation of enzymes in the matrix metalloproteinase (MMP) family.7 MMPs are zinc-binding enzymes with activity against a wide range of extracellular proteins,8 and MMP expression is typically limited to tissue remodeling associated with development, involution, inflammation, tumor growth, and repair. Our laboratory found that matrilysin (MMP-7) is strongly induced in injured alveolar epithelium in emphysema, desquamative interstitial pneumonitis, cystic fibrosis, and acute respiratory distress syndrome.9,10 In bleomycin-induced lung injury in mice, matrilysin expression is increased in alveolar epithelium early after injury and regulates acute neutrophil influx by controlling KC chemokine release into the alveolar compartment during the first 5 days following injury.11 Beyond the acute phase of injury, matrilysin expression increases as neutrophilic inflammation subsides and fibrosis ensues,11 and thus, matrilysin has been implicated in the progression of pulmonary fibrosis.12 However, when acute neutrophil influx is restored in bleomycin-treated matrilysin-null (Mmp7−/−) mice with the neutrophil chemotactic peptide nFMLP, mortality is higher in Mmp7−/− mice than in wild-type mice.11 Thus, observations of increased fibrosis in bleomycin-treated Mmp7−/− mice likely reflect the early acute injury phenotype, and in chronic lung injury, matrilysin activity may regulate physiological functions that promote repair.
E-cadherin regulates cell-cell adhesion in most epithelia and maintains epithelial integrity, restricts migration and proliferation, and promotes differentiation.13 The proteolytic cleavage of membrane proteins from the cell surface has been described as “ectodomain shedding,”14,15,16,17 and we described a physiological role for matrilysin-dependent shedding of the E-cadherin ectodomain in airway mucosal repair.10 We also found that matrilysin cleaves E-cadherin from alveolar epithelium during the progression of bleomycin-induced pulmonary fibrosis, and Mmp7−/− mice do not shed E-cadherin in the injured lung. A few in vitro studies have evaluated the function of E-cadherin shedding in cancer cells, suggesting potential roles in regulating cancer cell migration or gene expression.18,19 However, to our knowledge, in vivo functions for E-cadherin shedding in chronic lung injury and fibrosis have not been previously assessed.
The leukocyte-specific αEβ7-integrin (CD103) is expressed on nearly all intraepithelial lymphocytes and on specific populations of dendritic cells (DC), and E-cadherin is the only known CD103 ligand.20,21 Transforming growth factor-β1 (TGF-β1) induces CD103 expression, and increased TGF-β1 in injured tissues may up-regulate CD103 on infiltrating leukocytes.22,23,24 Via interaction with E-cadherin, CD103 has been suggested to be an epithelial recognition molecule that retains CD103+ lymphocytes at epithelial surfaces, targets epithelial tumor cells for destruction by cytolytic T cells, or regulates kidney allograft rejection.23,25,26,27,28 CD103+ pulmonary DC arise from myeloid mononuclear precursors, do not express plasmacytoid DC markers,29,30 and appear to have distinct cytokine and antigen presentation capabilities compared with CD103− myeloid DC populations.31,32 However, the function of CD103 in lung injury has not been defined. Therefore, we explored the possibility that E-cadherin shedding could be a mechanism controlling interactions between leukocytes that express CD103 and epithelial cells that express its ligand E-cadherin in bleomycin-induced lung injury in mice.
Materials and Methods
Animals and Bleomycin Lung Injury Model
Mice carrying a targeted deletion of the matrilysin gene on the C57BL/6 background33 are maintained in our laboratory and are designated Mmp7−/− mice. Mice carrying a targeted deletion of the αE-integrin subunit gene (Itgae) on the C57BL/6 background34 were proved by G. Hadley (Department of Surgery, Ohio State University, Columbus, OH) and are designated CD103−/− mice. Because αE-integrin subunits partner only with β7-integrin subunits, deficiency of the αE-integrin subunit only affects the αEβ7-integrin, and expression or function of other integrins is not affected.35 Homozygous CD103 deficiency does not affect fecundity, morphogenesis, growth, or postnatal survival in mice raised in a specific pathogen free animal facility.25 Control mice were congenic C57BL/6 wild-type mice. For bleomycin instillation, age- and sex-matched wild-type and transgenic mice (8 to 10 weeks of age) were anesthetized with i.p. injection of 2.5% Avertin in 0.9% normal saline, net dose 500 mg/kg body weight (Sigma-Aldrich, St. Louis, MO), and tracheas were cannulated via endotracheal intubation by passing a blunt tip 22-gauge angiocatheter through the vocal cords using direct visualization. Bleomycin was diluted in normal saline to provide a dose of 1.25 units/kg body weight in 50-μl volume, which was administered via a bolus into the hub of the angiocatheter and aspirated into the lungs of the spontaneously breathing, anesthetized mice. Animals were allowed to recover from anesthesia and take food and water ad libitum. At specified time points after injury, animals were euthanized by carbon dioxide asphyxiation, and thoracotomy was performed. Tracheas were cannulated with a 22-gauge 1-in angiocatheter (BD Biosciences, San Diego, CA), and bronchoalveolar lavage (BAL) was collected in 2 × 0.8-ml aliquots of sterile 0.9% saline solution. Lungs were removed and either homogenized in RNeasy RLT buffer (Qiagen, Germantown, MD) for RNA isolation or 0.5 M acetic acid for collagen quantification, snap-frozen in optimal cutting temperature tissue freezing medium for immunohistochemistry, or prepared for flow cytometry analysis as described below. All experiments were performed with the approval of the University of Washington School of Medicine Institutional Animal Use and Care Committee.
Antibodies
Monoclonal antibodies against mouse antigens CD16/CD32 (Fc-block, clone 2.4G2), CD45 (30-F11), and CD103 (M290) were obtained from BD Biosciences and against CD103 (2E7), CD11c (N418), Langerin (eBioL31), and CD3e (500A2) were obtained from eBioscience (San Diego, CA). Anti-mouse E-cadherin (ECCD-2) was obtained from Invitrogen (Carlsbad, CA). Anti-mouse F4/80 (MCA497BB) was obtained from AbD Serotec (Raleigh, NC). Fluorescent-conjugated anti-Armenian hamster secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA), and anti-rat fluorescent-conjugated antibodies were from Invitrogen (Molecular Probes).
Immunohistochemistry
Five-micrometer sections were cut from blocks of frozen lung tissues, air-dried for 5 minutes, fixed in 75% acetone/25% ethanol, washed in PBS, and blocked with TBST (25 mmol/L Tris-HCL (pH 7.5), 150 mmol/L NaCl, and 0.1% Tween 20) containing 1% BSA and 2% goat serum. Fixed tissues or airway epithelial cell cultures were incubated with primary antibodies diluted in 1% BSA/PBS for 60 minutes at 37°C, washed with PBS, and incubated with appropriate fluorescent-conjugated secondary antibodies diluted in 1% BSA/PBS at the manufacturer’s recommended concentrations for 60 minutes at 37°C, followed by washing and mounting with ProLong Gold Antifade (Invitrogen). Immunofluorescence images of tissues and cells were captured with an Olympus DP70 digital camera system (Olympus America, Center Valley, PA).
Cell Culture
All cell culture media and supplements were obtained from Mediatech (Herndon, VA) unless otherwise specified. Air-liquid interface airway epithelial cultures were established from wild-type and matrilysin-null mice and maintained as described previously.36 MTC-1 cells, a mouse T-hybridoma line, were a gift from P. Kilshaw (Babraham Institute, Cambridge, UK) and were maintained in RMPI 1640 supplemented with 10% FCS and 5 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN) with CD103 expression verified by flow cytometry. Wounds were made in confluent air-liquid interface cultures grown in 6.5-mm Transwells (Corning Life Sciences, Lowell, MA) by gently scratching with a P1000 pipette tip, rinsing with sterile PBS and refeeding with fresh medium. A total of 103 MTC-1 cells were suspended in PBS and added to the apical chamber of wounded cultures and allowed to incubate overnight under standard culture conditions. The next day, cultures were vigorously washed three times with sterile PBS, fixed for immunostaining in 100% methanol, followed by washing with PBS, blocking with PBS supplemented with 1% BSA, and staining for E-cadherin and CD103. After immunostaining, membranes were removed from Transwells and mounted onto glass slides with Prolong Gold Antifade, and uniform 250-μm-wide images of the wound edges were digitally captured. The adherent MTC-1 cells per 250-μm wound edge visualized in each image were counted, and the number adherent cells per millimeter of wound was calculated.
Bone marrow-derived DC (BMDC) were cultured from femurs of wild-type and CD103−/− mice. Briefly, femurs were removed and cleaned of all excess tissue, ends were cut, and bone marrow was obtained by sterilely flushing marrow cavity with RPMI 1640 and filtering marrow through 70-μm mesh filters to remove debris. Cells were counted and resuspended at 2 × 106 cells/ml in RPMI 1640 supplemented with sodium pyruvate, nonessential amino acids, penicillin/streptomycin, l-glutamine, 10% FBS (HyClone, Logan, UT), and 20 ng/ml GM-CSF (RDI, Concord MA) and seeded in 6-well cell culture plates for 6 days before maturation with 5 ng/ml lipopolysaccharide (LPS from E. coli O111:B4; List Biological Labs, Campbell, CA) for 24 hours. Two days before LPS addition, 5 ng/ml TGF-β1 was added to induce expression of CD103 on BMDC. Flow cytometry confirmed that CD103 was highly expressed on 30 to 35% of CD11c+ BMDC compared with 5 to 6% on BMDC that did not receive TGF-β1. Soluble recombinant mouse E-cadherin/human Fc chimeric protein (R&D Biosystems) was added to BMDC cultures at a final concentration of 100 ng/ml at the time of LPS addition. The chimeric protein contains human IgG1 residues 100–330, and because the mouse Fc receptor binding site on human IgG is at residues 341–439, the Fc portion does not interact with BMDC or other mouse cells that express Fc receptors.37,38
Total Lung Collagen Assay
Total lung collagen was determined using the picrosirius red Sircol Assay (Biocolor, Carrickfergus, County Antrim, UK) per the manufacturer’s protocol. Briefly, at specified time points after bleomycin administration, mice were euthanized, and the left lung was removed and homogenized in 0.5 M acetic acid solution. A total of 200 μl of the acid homogenate was digested by adding 1 ml of pepsin solution (2 mg/ml in 0.5 M acetic acid) and incubating overnight at 4°C with continuous shaking. After digestion, samples were centrifuged, and 100 μl of the supernatant containing soluble collagen was incubated with 1 ml of Sircol dye reagent for 30 minutes at room temperature. Samples were centrifuged, the supernatant was discarded, and the precipitated pellet was resuspended in 1 ml of Sircol alkali reagent. Collagen concentration was then determined by spectrophotometric absorbance at 540 nm as compared with a standard curve.
Western Blotting
For detection of E-cadherin in culture medium from wounded ALI epithelial cell cultures, equal volumes (1 ml) of fresh medium was added to each well at the time of wounding. At 24 hours after wounding, condition media samples were collected from each well, and a 200-μl aliquot of each sample was concentrated 10-fold with Centricon spin concentrator columns (Millipore, Billerica, MA). The entire volume of concentrated sample (∼20 μl) was resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were blocked overnight with 5% dry milk in TBST, probed with ECCD-2 rat anti-mouse E-cadherin at 1/1000 dilution, and developed with Pierce Super Signal West Pico Chemiluminescence substrate (Thermo Scientific, Rockford, IL). Chemiluminescence images were captured with a digital gel documentation system (UVP Bioimaging Systems, Upland, CA).
Flow Cytometry
Mouse lungs were minced into 1-mm pieces with scissors and digested with 2 mg/ml bacterial collagenase (Roche, Indianapolis, IN) and 0.5 mg/ml DNase (Sigma-Aldrich) for 1 hour at 37°C on a rocking platform. Digested lung was filtered through a 40-μm nylon cell strainer to form a single-cell suspension. Lung digest or BAL cells were washed with flow cytometry buffer (0.5% BSA in PBS), counted, and blocked with rat IgG2b anti-mouse CD16/CD32 mAb (BD Biosciences) 1 μg per 106 cells. Cells were stained with conjugated primary antibodies for 60 minutes on ice, washed with flow cytometry buffer twice, and analyzed using the Beckman Coulter FC500 Flow Cytometer and CXP Analysis software.
Quantitative PCR
Total RNA was isolated with Qiagen RNeasy kits according to manufacturers protocols. Primers and TaqMan probes (FAM dye-labeled) for a1(I) procollagen, interleukin-12 (IL-12) (p40 subunit), interferon γ, IL-10, TGF-β1, connective tissue growth factor, and glyceraldehyde-3-phosphodehydrogenase were added to cDNA synthesized from 5 μg of total RNA with a High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA), and product amplification was measured with an ABI HT7900 Fast Real-Time PCR System. The threshold cycle (Ct) was obtained from duplicate aliquots of each sample and averaged. The ΔCt was the difference between the average Ct for the specific cDNA and the average Ct for glyceraldehyde-3-phosphodehydrogenase. The ΔΔCt, or the difference between ΔCt of the experimental and that of control condition, was calculated as the average ΔCt at a given time point minus the average ΔCt of day 0 samples. The data are expressed as fold change relative to the control condition and was calculated as 2−ΔΔCt.
Results
Matrilysin Regulates Pulmonary Influx of Leukocytes Expressing CD103
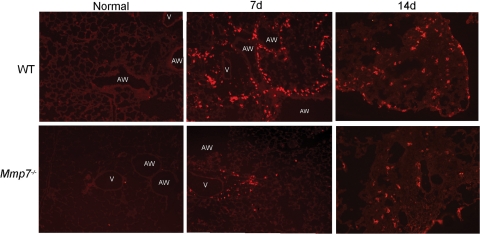
Matrilysin cleaves the CD103-ligand E-cadherin; therefore, we assessed CD103-expressing leukocytes in bleomycin/nFMLP-treated wild-type compared with Mmp7−/− mice, in which E-cadherin is not cleaved or “shed” from the cell surface.10 To evaluate matrilysin function independent of its role in regulating the early acute neutrophil influx, wild-type mice and Mmp7−/− mice were cotreated with bleomycin and 1 μmol/L nFMLP, which reverses the defect in early neutrophil influx.11 A low dose of bleomycin was used (1.25 units/kg body weight) that resulted in minimal mortality in mice of either genotype. In normal lungs of both genotypes, CD103 staining was observed on rare cells associated with large airway epithelium, consistent with localization to intraepithelial lymphocytes or resident subepithelial dendritic cells (Figure 1). At 7 days after bleomycin, CD103+ cells were prominently seen in the subepithelial compartment around airways and alveolar spaces of wild-type lungs and in areas of nascent fibrosis, whereas in Mmp7−/− lungs, CD103+ cells were fewer in number and were mainly localized around larger blood vessels. By 14 days after bleomycin, CD103+ cells were seen in areas of fibrosis in both genotypes; however, more CD103+ cells were observed in lungs from wild-type mice than in Mmp7−/− lungs. There were no differences in numbers of CD103+ cells in lungs of uninjured mice or in numbers or localization of intestinal CD103+ IEL cells (data not shown), indicating that there was no generalized defect in CD103+ expression or ability of CD103+ leukocytes to migrate into tissues in Mmp7−/− mice.
Figure 1.
Increased staining for CD103 in lungs of wild-type (WT) as compared with Mmp7−/− mice after bleomycin treatment. Frozen sections of bleomycin-injured lung tissues collected at 0, 7, and 14 days after injury were stained for CD103 with Armenian hamster anti-mouse CD103 antibody and Cy3-conjugated goat anti-hamster secondary antibody. Sections are representative of four to five mice per genotype at each time point (AW = airway, V = vessel).
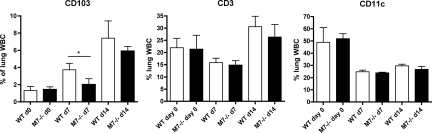
Flow cytometry was used to quantify differences in CD103+ cells in single-cell suspensions of collagenase/DNase-digested lungs after injury. In both uninjured and bleomycin/nFMLP-treated lungs, total leukocyte counts did not differ between genotypes, although predictably, the leukocyte counts in injured lungs were higher than in untreated mice (data not shown). Because of variability in the efficiency of lung digestion and leukocyte viability, comparisons were made based on the percentage of lung leukocytes expressing CD103 (indicated by positive staining for both the pan-leukocyte marker CD45 and CD103). No differences in CD45+/CD103+ leukocytes were seen in untreated mice. After bleomycin/FMLP treatment, the number of CD103+ leukocytes increased in both genotypes with a significantly greater percentage of CD103+ leukocytes seen in lungs of wild-type as compared with Mmp7−/− mice at day 7 (Figure 2). Consistent with what was observed by immunostaining, more CD103+ leukocytes were seen in lungs of wild-type as compared with Mmp7−/− mice at day 14, but differences did not reach statistical significance at the later time point (P = 0.11). Percentages of total CD11c+ or CD3+ cells did not differ between genotypes. Collectively, these data show that matrilysin regulates pulmonary influx of CD103+ leukocytes.
Figure 2.
Greater numbers of CD103+ leukocytes in lungs of wild-type (WT) mice compared with Mmp7−/− mice. Lung tissues were digested in 0.2% collagenase/0.05% DNase, and single-cell suspensions were stained for CD45, CD103, CD11c, and CD3. CD103+, CD11c+, and CD3+ cells are expressed as a mean percentages of total lung CD45+ cells from six to eight animals per group ± SD (*P < 0.05 for WT versus Mmp7−/− mice at day 7 by t-test).
Matrilysin Regulates Localization of CD103+ Leukocytes to Areas of Injured Lung Epithelium
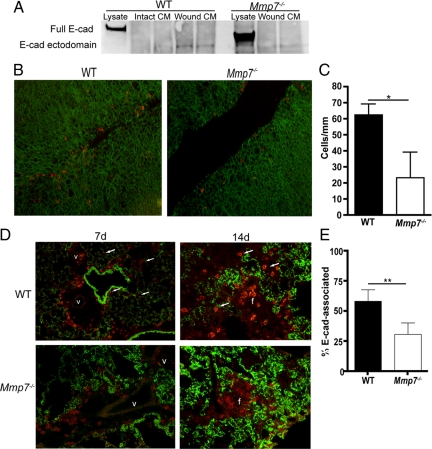
Because we observed a striking accumulation of CD103+ cells around airways in the early fibroproliferative phase of injury (7 days) that correlated with the timing of induction of matrilysin-mediated E-cadherin shedding in this injury model,10 we used a primary mouse airway ALI epithelial cell culture model to assess if matrilysin-dependent wound-repair responses regulate epithelial cell E-cadherin and CD103+ leukocyte interactions. Wounds in air-liquid interface cultures of Mmp7−/− cells close more slowly than in wild-type cultures,39 recapitulating the defects in airway re-epithelialization observed in Mmp7−/− mice in vivo.9,10 Wild-type and Mmp7−/− air-liquid interface cultures were wounded and allowed to heal for 24 hours in the presence of MTC-1 cells, a mouse T cell hybridoma cell line that constitutively expresses CD103.40,41 Wounded cultures from wild-type mice shed E-cadherin ectodomain into the culture medium whereas cells from Mmp7−/− did not (Figure 3A). At 24 hours after wounding, the association of MTC-1 cells with the CD103 ligand E-cadherin at the wound edge was quantified as the number of MTC cells per millimeter per wound, and a significantly higher number of MTC-1 cells were attached at to wound edges of wild-type cells than in Mmp7−/− cells, indicating a matrilysin-dependent enhancement of interactions between CD103+ cells and injured lung epithelium (Figure 3, B and C).
Figure 3.
Matrilysin regulates localization of CD103+ leukocytes to injured epithelium. A: Tracheal epithelium air-liquid interface cultures of wild-type (WT) mice and Mmp7−/− mice were wounded with a pipette tip, and the conditioned medium was collected at 24 hours after wounding and concentrated 10 times by spin-column centrifugation, and equal volumes of concentrated medium were loaded per lane. Soluble E-cadherin was detected by Western blotting with an antibody specific for the mouse E-cadherin extracellular domain (ECCD-2). As expected, E-cadherin is very highly expressed in cell lysates. Cells from WT mice shed soluble E-cadherin after wounding whereas those from Mmp7−/− mice do not (blot shows two wells per condition and is representative of three independent experiments). B and C: CD103+ MTC-1 cells were co-cultured with wounded ALI cultures for 24 hours, and cultures were fixed and stained for E-cadherin (green) and CD103 (orange). MTC-1 cells adherent to migrating epithelial cells at the wound edge were quantified by imaging random wound edge 250-μm-wide visual fields (n = 6–8 wounds/genotype) and enumerating CD103+ cells per millimeter of total wound length. MTC-1 cells adhered in significantly greater numbers to WT cells than to Mmp7−/− cells (mean ± SD, *P < 0.001 by t-test). D: Lung tissues of bleomycin/nFMLP-injured mice collected from WT and Mmp7−/− mice at 7 and 14 days after injury were stained for CD103 (orange) and E-cadherin (green). CD103+ cells were observed to closely localize to E-cadherin-positive epithelium in WT tissues (arrows) but remained in the perivascular space around large vessels (v) early (d7) after injury in Mmp7−/− mice and in fibrotic areas (f) at the later (d14) time point. E: CD103+ cell association with E-cadherin was quantified by imaging lung tissue sections at d14 after bleomycin treatment and counting the number of CD103+ cells in direct contact with E-cadherin as a percentage of total CD103+ cells per random medium power field (×200, three fields per section, four animals per genotype; mean ± SD, **P < 0.01 by t-test).
Consistent with the in vitro observations, we observed that in wild-type mice, CD103+ cells had moved from the perivascular space, and greater numbers were seen in close approximation to airway and alveolar epithelial cells expressing E-cadherin than in Mmp7−/− lungs, in which the CD103+ cells were largely constrained to the perivascular space (Figure 3D). CD103+ cells in wild-type lung tissues were scattered throughout the injured lung closely approximated to epithelial cells, whereas in the Mmp7−/− lungs, CD103+ cells were localized in areas of fibrotic lung more distant from E-cadherin staining. This was quantified at 14 days after bleomycin/nFMLP treatment by determining the percentages of CD103+ staining cells that were in direct contact with E-cadherin compared with total CD103+ cells in random high-power fields, and a significantly greater percentage of CD103+ cells were localized with E-cadherin in wild-type tissues compared with those from Mmp7−/− mice (Figure 3E).
CD103+ Leukocytes in Bleomycin/ nFMLP-Injured Lung Tissues Are Mainly DC
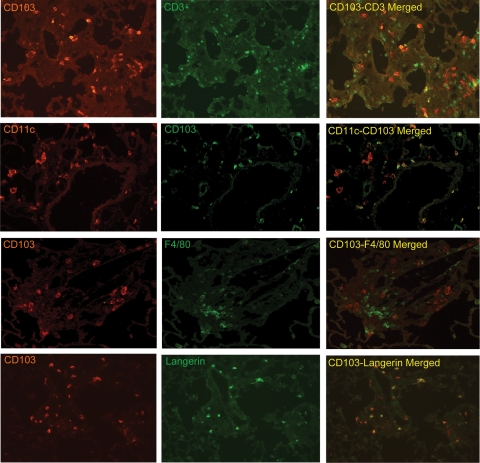
To determine the phenotypes of leukocytes expressing CD103+ in lungs of bleomycin/nFMLP-injured mice, tissue sections at 14 days after injury were stained for CD103 in relation to the T cell receptor CD3 and the DC marker CD11c. On the basis of observations made in human BAL,42 we predicted that the CD103+ cells in the bleomycin-injured lung would be T cells. However, most of the CD103+ cells in the lung tissues were negative for the T cell marker CD3, positive for CD11c, and positive for Langerin, which was reported to be expressed on pulmonary and dermal CD103+ DC.29,43 CD103+ cells were negative for F4/80, a widely used tissue macrophage marker (Figure 4). Although there is an ongoing debate as to whether CD11c+ effector cells in tissues are macrophages or DC,44 CD103 has not been found on CD11c− or plasmacytoid DC in the lung.29,30 Thus, the CD11c+/CD103+ cells in bleomycin-injured lungs are clearly mononuclear derived and are likely DC.
Figure 4.
CD103+ leukocytes in bleomycin-injured lungs are CD3+ T cells and CD11c+ dendritic cells. Frozen lung sections of bleomycin/nFMLP-injured C57BL/6 mice at 14 days after injury were stained for CD103 and the T cell receptor CD3 (ε chain), the myeloid DC marker CD11c, Langerin, or the tissue macrophage marker F4/80 and visualized by indirect immunofluorescence microscopy. Most CD103+ cells were CD11c+ and Langerin+. Fewer were CD3+. CD103+ cells were negative for F4/80−.
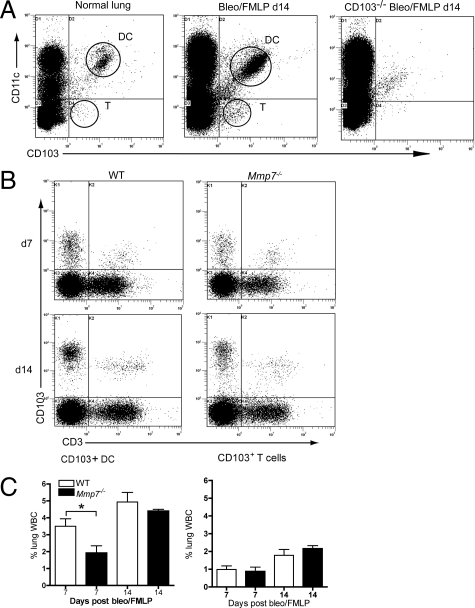
Flow cytometry was used to quantify the pulmonary CD103+ DC and T cells in collagenase/DNase-digested lung tissues and in cells pelleted from BAL of bleomycin-injured mice. Representative dot plots of lung digests show that the majority of CD103+ cells in the normal and bleomycin-injured lung are CD11c (Figure 5A), with the remainder CD3+ T cells (Figure 5B). The CD11c+/CD103− cells are populations of macrophages and other DC populations. A representative plot from a bleomycin-injured CD103−/− mouse shows the specificity of the anti-CD103 antibodies. At 7 days after bleomycin/nFMLP, a significantly higher percentage of leukocytes in the lungs of wild-type mice were CD103+/CD11c+ DC as compared with samples from Mmp7−/− mice (Figure 5C). Percentages of CD103+/CD3+ T cells in wild-type lung tissues did not differ from that observed in Mmp7−/− mice (Figure 5C).
Figure 5.
Quantitatively more CD103+ dendritic cells (DC) are present in lungs of wild-type (WT) mice than in Mmp7−/− mice. Leukocytes from collagenase/DNase-digested lung tissues from bleomycin/nFMLP-treated mice were isolated using CD45 MACS columns and labeled with fluorescent-conjugated antibodies against CD103, CD11c, and CD3. A: Representative flow cytometry dot plots showing distribution of CD103+ and CD11c+ cells in normal lung tissues and in WT mouse at 14 days after bleomycin/nFMLP treatment. A plot from a CD103−/− mouse shows specificity of CD103 labeling. B: The CD103+/CD11c− population are CD3+ T cells. C: Significantly greater numbers of CD103+/CD11c DC are in lung tissue digests of WT compared with Mmp7−/− mice at day 7 (d7). C: No differences were seen in CD103+/CD3+ T cells between WT and Mmp7−/− mice (n = 8 mice/group, mean ± SD, *P < 0.05 by t-test).
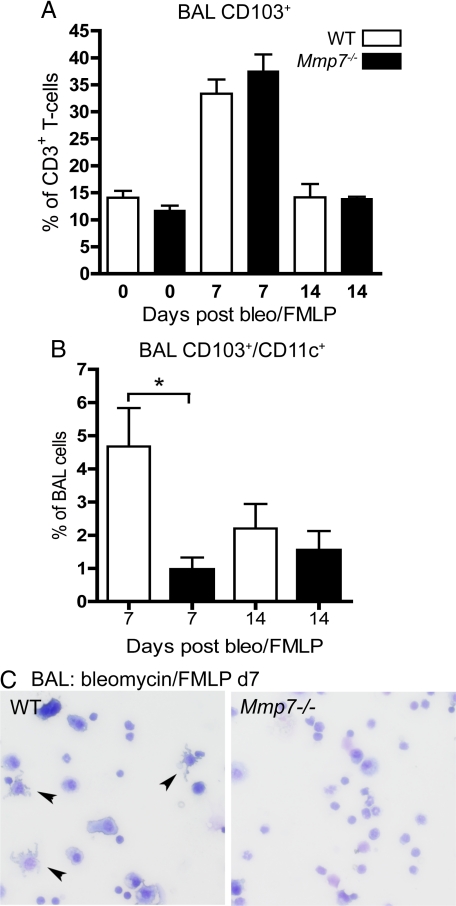
Most of the CD103+ cells in BAL were also CD3+, indicating that they were T cells, and the percentages of BAL CD103+ T cells did not differ between genotypes (Figure 6A). However, the CD103+ BAL cells that were negative for CD3 were CD11c+. CD103+/CD11c+ cells were not detected in BAL of uninjured mice. However, at 7 days after injury, a significantly higher percentage of the BAL leukocytes in wild-type were CD103+/CD11c+ cells as compared with Mmp7−/− BAL (Figure 6B), and more cells with a dendritic morphology were observed on BAL cytospins from wild-type than Mmp7−/− mice (Figure 6C). Taken together, these results indicate that the mechanisms regulating recruitment and localization of CD103+ DC and T cells in the lung are different. Specifically, T cells moved into the airspaces rather than being retained in the tissues, and their localization was not regulated by matrilysin activity. In contrast, CD103+/CD11c+ cells are recruited into the lungs in significantly higher numbers in wild-type mice and are largely retained in the lung tissues with smaller numbers moving into the airspaces.
Figure 6.
Increased numbers of CD103+ dendritic cells in bronchoalveolar lavage (BAL) of bleomycin-injured wild-type (WT) mice. A: Percentages of CD3+ cells that were also CD103+ in BAL did not differ between WT and Mmp7−/− mice. B: CD11c+/CD103+ cells were present in BAL in significantly greater numbers in WT mice as compared with BAL from Mmp7−/− mice (n = 8 mice/group, mean ± SD, *P < 0.02 by t-test). C: Cells with dendritic morphology (arrowheads) are more abundant on BAL cytospins of WT mice.
CD103 Promotes Resolution of Acute Inflammation and Inhibits Pulmonary Fibrosis
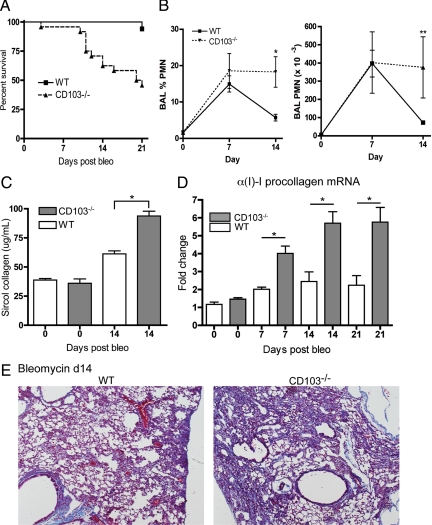
To determine the consequences of pulmonary influx of CD103+ leukocytes in lung injury, CD103−/− mice were treated with intratracheal bleomycin. At a bleomycin dose that caused little mortality (1.25 units/kg body weight) in wild-type mice, CD103−/− mice had a significantly increased susceptibility to bleomycin with a greater than 50% mortality by 21 days (Figure 7A), and the decline in survival in the CD103−/− mice correlated with the timing of pulmonary influx of CD103+ cells into wild-type mice (7 to 14 days). Analysis of BAL from CD103−/− mice showed elevated percentages of BAL neutrophils and total BAL neutrophils compared with wild-type mice at 14 days after bleomycin (Figure 7B). Sircol assay showed significantly more total lung collagen accumulation in CD103−/− mice at 14 days after bleomycin (Figure 7C). This was associated with significant increases in whole-lung αI(1)-procollagen mRNA expression in CD103−/− mice at 7 to 21 days after bleomycin (Figure 7D) and increased collagen staining in lung tissue sections (Figure 7E).
Figure 7.
Persistent inflammation and increased fibrosis in lungs of CD103−/− mice after bleomycin. C57BL/6 wild-type (WT) mice and mice with a targeted deletion of the αE-integrin gene were treated with intratracheal bleomycin (1.25 units/kg body weight) for 0–21 days. A: Survival curve indicates that CD103−/− mice were significantly more susceptible to bleomycin (n = 16/group, P < 0.001). B: Persistent elevation in bronchoalveolar lavage (BAL) neutrophils in CD103−/− compared with WT mice (total neutrophils/lavage, n = 6/group, mean ± SD, *P < 0.002, **P < 0.02 by t-test). C: Collagen accumulation as assessed by Sircol total collagen assay (n = 6–8/group, mean ± SD, *P < 0.001 by t-test) and (D) αI(I)-procollagen mRNA expression, show that CD103−/− mice have significantly more fibrosis than WT mice after bleomycin treatment (mean ± SD, *P < 0.01 by analysis of variance with Bonferroni multiple comparisons test for indicated data pairs). E: Trichrome staining of representative lung tissue sections showed increased fibrosis in lungs CD103−/− mice at day 14 (d14) after bleomycin.
Matrilysin Regulates Pulmonary Neutrophil Clearance in Chronic Lung Injury
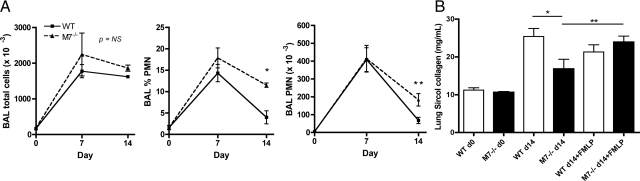
To determine whether differences in CD103-expressing DC affected lung injury in wild-type and Mmp7−/− mice, comparisons were made in bleomycin/nFMLP-treated mice. At 14 days after bleomycin/nFMLP instillation, Mmp7−/− mice had persistent elevation of absolute neutrophil counts and percentage of neutrophils in the BAL as compared with wild-type mice (Figure 8A). Total BAL cellularity was higher in Mmp7−/− mice at 14 days, but differences were not significant, likely because neutrophils accounted for ∼10% of total BAL cells at day 14, with the balance of cells being macrophage/mononuclear cells and lymphocytes being similar between genotypes. Neutrophil counts did not differ at 7 days, suggesting that matrilysin was functioning in resolution of neutrophilic inflammation. Mmp7−/− mice treated with bleomycin alone have reduced fibrosis compared with wild-type mice;11 however, when mice were cotreated with bleomycin/nFMLP, the protection against pulmonary fibrosis in Mmp7−/− mice treated with bleomycin alone was eliminated, and Mmp7−/− mice had slightly higher total lung collagen accumulation, although these differences were not statistically significant (Figure 8B).
Figure 8.
Persistent inflammation and lack of protection against fibrosis in lungs of Mmp7−/− bleomycin-induced acute lung injury when neutrophil influx defect is corrected with nFMLP. C57BL/6 wild-type (WT) mice and mice with a targeted deletion of the matrilysin gene (M7−/−) were subjected to lung injury with a single intratracheal dose of bleomycin (1.25 units/kg body weight) co-instilled with 1 μm of nFMLP in 50 μl of sterile PBS to equalize matrilysin-dependent differences in pulmonary neutrophil influx in early phase of bleomycin-induced lung injury. A: Total cells and neutrophils in bronchoalveolar lavage (BAL) were quantified and neutrophil percentages determined by cytospin (total cells or neutrophils/lavage, n = 6/group, mean ± SD, *P < 0.005, **P < 0.02). B: Total lung collagen was determined by Sircol assay (mean ± SD, *P < 0.05 for lung collagen comparisons of WT versus Mmp7−/− mice and **P < 0.05 for Mmp7−/− treated with bleomycin alone versus Mmp7−/− mice treated with bleomycin/nFMLP by analysis of variance with Bonferroni multiple comparisons posttest).
E-cadherin-CD103 Interactions Alter DC Cytokine Production
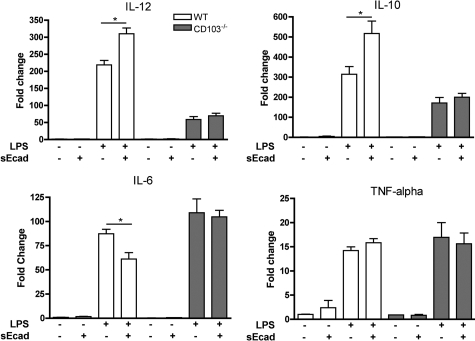
To evaluate whether E-cadherin-CD103 interactions directly affects DC function, an in vitro model of BMDC was used. TGF-β activity regulates CD103 expression;24 therefore, immature BMDC were treated with TGF-β1 (5 ng/ml) to induce CD103 in culture. Cytokine mRNA expression in wild-type or CD103−/− BMDC was low at baseline, and the addition of soluble E-cadherin to nonstimulated BMDC had no effect on cytokine gene expression (Figure 9). Consequently, LPS was used as an inflammatory stimulus to assess if E-cadherin altered cytokine expression in activated CD103+ BMDC. At 24 hours after LPS treatment, mRNA expression for IL-12, IL-6, and IL-10 were significantly increased both wild-type and CD103−/− BMDC with a higher level of mRNA induction for IL-12 and IL-10 in wild-type cells. Addition of soluble E-cadherin-Fc protein (100 ng/ml) with the LPS significantly reduced LPS-stimulated increases in expression of IL-6 and significantly increased expression of IL-10 and IL-12 above that of LPS alone (Figure 9). No effect of sE-cad on LPS-induced cytokine mRNA expression was seen in CD103−/− BMDC, which cannot interact with E-cadherin.
Figure 9.
Soluble E-cadherin alters the cytokine mRNA expression profile in wild-type (WT) BMDC but not in CD103−/− BMDC. Total RNA was isolated from WT or CD103−/− BMDC stimulated with 5 ng/ml LPS in the presence or absence of 100 ng/ml soluble E-cadherin-FC protein, and gene expression was determined by quantitative RT2-PCR. E-cadherin increased BMDC expression of IL-10 and IL-12 and reduced expression of IL-6. TNF-α expression was not affected by soluble E-cadherin. Data are average fold change compared with nontreated cells for three to six replicate wells per condition and are representative of three experiments. *P < 0.05 by t-test relative to WT.
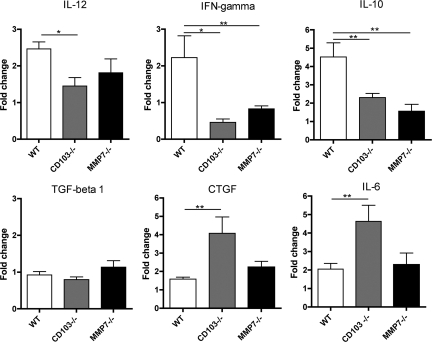
Evidence that E-cadherin-CD103 interactions could be the effector of matrilysin-dependent differences in inflammation in vivo was obtained by comparing whole-lung cytokine mRNA levels in bleomycin-injured mice. Compared with wild-type mice at 14 days after injury, Mmp7−/− and CD103−/− mice had lower whole-lung mRNA levels for IL-12, interferon γ, and IL-10 (Figure 10). A lack of increase in these genes in CD103−/− mice likely reflects a lack of CD103+ DC that induce expression of those cytokines. In the Mmp7−/− lungs, which have some CD103+ DC, the differences may be indicative of a lack of activation due to the absence of E-cadherin shedding. mRNA levels for TGF-β1 did not differ between genotypes, but mRNA for connective tissue growth factor and IL-6, which are profibrotic in this model,45,46,47,48,49 were significantly higher in CD103−/− lung tissues than in wild-type lungs, consistent with the marked increase in pulmonary fibrosis in seen CD103−/− mice. The lack of significant increases in connective tissue growth factor or IL-6 Mmp7−/− lung tissues correlates with the intermediate effect on fibrosis observed in bleomycin-nFMLP-treated Mmp7−/− mice.
Figure 10.
Patterns of inflammatory cytokine mRNA expression in bleomycin-injured Mmp7−/− and CD103−/− lung tissues are similarly altered compared with those of wild-type (WT) mice. Total lung RNA was isolated from bleomycin or bleomycin/nFMLP-injured mice and mRNA expression for specified genes determined by quantitative RT2-PCR. Data are shown as average fold change for four to six animals per condition compared with WT uninjured control (*P < 0.01, **P < 0.05 by t-test relative to WT).
Discussion
Matrilysin can contribute to tissue injury in the lung and gastrointestinal tract by stimulating excess neutrophil influx.11,50 However, matrilysin also has beneficial functions such as promoting airway re-epithelialization.9,10 Equalizing early neutrophil influx by coinstillation of nFMLP with the bleomycin eliminated the protection against bleomycin-induced fibrosis seen in Mmp7−/− mice in a prior report,11 and the data presented here indicate a lung protective role for matrilysin in chronic lung injury by regulating a population of CD103+ DC that limit acute inflammation and inhibit progression of pulmonary fibrosis.
Both CD103−/− and Mmp7−/− mice showed similar phenotypes with persistent neutrophilic inflammation, increased fibrosis, and similarly altered lung tissue IL-10 and interferon γ mRNA expression compared with wild-type mice. The reduced severity of the BAL neutrophilia and fibrosis phenotypes in Mmp7−/− mice compared with CD103−/− mice likely reflects the fact that matrilysin is not absolutely necessary for CD103 expression. Indeed, there are CD103+ cells in the lungs of Mmp7−/− mice. Whereas loss of E-cadherin from tumor cells has been suggested to be a mechanism that reduces interaction of CD103+ cytolytic T cells with target tumor cells,26 shedding of E-cadherin from injured lung epithelium localizes CD103+ DC to the alveolar and peribronchial compartments. It may seem counterintuitive that shedding of the CD103 ligand could increase association with the epithelial cells. However, only a portion of the E-cadherin present on the cell surface is shed,10 and we speculate that shed E-cadherin may be functioning as a soluble chemokine that induces movement of CD103+ DC out of the vasculature and into the alveolar and peribronchial compartments. Alternatively, matrilysin regulates gene expression in injured epithelium36 and could induce expression of chemokines that stimulate the influx of CD103+ leukocytes. One other possibility is that E-cadherin molecules engaged in homophilic binding at cell junctions are sterically hindered from binding CD103,51 and therefore, E-cadherin shedding and the resultant adherens junction disassembly could expose CD103 binding sites on remaining, cell-associated E-cadherin. Work on elucidating the specific mechanism is ongoing.
We observed that both CD103+ T cells and DC move into the injured lung. On the basis of the fact that we did not see quantitative differences in CD103+ T cells between genotypes, the localization of these cells appears to be independent of the matrilysin function. We did not assess activation of CD103+ T cells, but that the lack of soluble E-cadherin in Mmp7−/− mice could be associated with functional differences in CD103 T cells in those mice. Thus, the mechanisms that differentially regulate CD103+ DC and T cells remain to be determined. Effects on CD103+ DC were significant at the time point of transition from acute to chronic injury (7 days after bleomycin) with less of a difference in DC numbers between wild-type and Mmp7−/− mice at later time points, although the localization of those cells did differ between genotypes at 14 days. Thus, our data suggest that matrilysin has a more prominent effect on CD103+ DC function (emigration from the vasculature, localization to sites of epithelial injury, and DC cytokine gene expression) rather than on CD103 expression itself.
Observations in other systems support an anti-inflammatory function for CD103+ DC in the lung. Aging CD103−/− mice have reduced numbers of dermal DC compared with wild-type mice and are more susceptible to hyperproliferative, inflammatory skin lesions.52,53 In a T cell transfer model of colitis, CD103 function on intestinal DC was essential for immune regulatory activity as regulatory T cells could not abrogate colitis induced by transfer of disease-inducing CD4+CD45RBhigh T cells in the absence of DC CD103 in the host.54 Interestingly, CD103 expression on T cells did not appear to mediate epithelial injury in colitis or in renal transplant tubulitis.54,55,56 CD103+ DC were reported to produce lower levels of the potent fibroblast mitogens TNF-α and IL-6 than CD103− DC,57 and pulmonary CD103+ DC produced more IL-12.29 Consistent with these reports, we observed higher levels of mRNA for IL-12, interferon γ, and IL-10 in lungs of wild-type mice than in either Mmp7−/− or CD103−/− mice. Indeed, these cytokines have been shown to inhibit fibrosis in bleomycin-induced lung injury in mice,58,59,60,61,62,63,64 and therefore, this may explain the worse lung disease we observed in CD103−/− mice. Although LPS stimulation in vitro does not replicate bleomycin injury in vivo, the similarities in E-cadherin-induced changes in cytokine expression in wild-type BMDC and those observed in vivo suggest that E-cadherin interaction with DC CD103 shifts the pulmonary immune response from proinflammatory to anti-inflammatory.
In summary, matrilysin has multiple functions in lung injury, and our data support a protective role in chronic lung injury by regulating CD103+ DC. We propose a model by which mononuclear DC precursors enter the injured lung as part of the ongoing inflammatory response to injury and are induced by TGF-β1 to up regulate expression of CD103, thus enabling their interaction with soluble E-cadherin generated by proteolytic cleavage. We propose that this interaction activates a CD103+ DC-mediated pathway that limits acute inflammation and inhibits progression of fibrosis. Further determination of the effector mechanisms induced by E-cadherin-CD103 interactions in lung injury is the focus of ongoing work in our laboratory.
Acknowledgments
We thank Cliff Rims, Ben Jacobson, and Michele Lin for excellent technical assistance, Ying Wang for maintaining our mouse colony, Fred Lewis for assistance with flow cytometry, and William C. Parks, Peter Chen, and Edward Clark for helpful discussions.
Footnotes
Address reprint requests to John K. McGuire, M.D., Center for Lung Biology, University of Washington, 815 Mercer Street, Box 358052, University of Washington, Seattle, WA 98109. E-mail: mcguirej@u.washington.edu.
Supported by grants HL068780 and HL02959 from the National Institutes of Health and by the American Heart Association.
Current address of I.H.: Division of Pulmonary, Critical Care, and Sleep Medicine, Brody School of Medicine, East Carolina University, Greenville, NC.
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS: plasma IL-1β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- Ichikado K, Suga M, Muranaka H, Gushima Y, Miyakawa H, Tsubamoto M, Johkoh T, Hirata N, Yoshinaga T, Kinoshita Y, Yamashita Y, Sasaki Y. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology. 2006;238:321–329. doi: 10.1148/radiol.2373041515. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Resp Res. 2002;3:1–8. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal RG, Bitterman PB, Mossman B, Schwartz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TEJ, Leinwald LA, Loiotta L, Martin GR, Schwartz DA, Schultz GS, Wagner CR, Musson RA. Future research direction in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2002;166:236–246. doi: 10.1164/rccm.2201069. [DOI] [PubMed] [Google Scholar]
- Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JK, Li QL, Parks WC. Matrilysin mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1861–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QL, Park PY, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveal matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- Dello Sbarba P, Rovida E. Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol Chem. 2002;383:69–83. doi: 10.1515/BC.2002.007. [DOI] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- Reiss K, Saftig P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Nawrocki-Raby B, Gilles C, Pollette M, Bruyneel E, Laronze J-Y, Bonnet N, Foidart J-M, Mareel M, Birembaut P. Up-regulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer. 2003;105:790–795. doi: 10.1002/ijc.11168. [DOI] [PubMed] [Google Scholar]
- Kilshaw PJ. αEβ7. Mol Pathol. 1999;52:203–207. doi: 10.1136/mp.52.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraszka KS, Higgins JM, Tan K, Mandelbrot DA, Wang JH, Brenner MB. Molecular basis for leukocyte integrin αEβ7 adhesion to epithelial (E)-cadherin. J Exp Med. 2000;191:1555–1567. doi: 10.1084/jem.191.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley G. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KL, Dulphy N, Bahl P, Salio M, Maskell K, Piris J, Warren BF, George BD, Mortensen NJ, Cerundolo V. Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol. 2007;178:2908–2915. doi: 10.4049/jimmunol.178.5.2908. [DOI] [PubMed] [Google Scholar]
- Wang D, Yuan R, Feng Y, El-Asady R, Farber DL, Gress RE, Lucas PJ, Hadley GA. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J Immunol. 2004;172:214–221. doi: 10.4049/jimmunol.172.1.214. [DOI] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Le Floc'h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LJ, Kirby JA, Cunningham AC. Role of the mucosal integrin αE(CD103)β7 in tissue-restricted cytotoxicity. Clin Exp Immunol. 2007;149:162–170. doi: 10.1111/j.1365-2249.2007.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley G. Role of integrin CD103 in promoting destruction of renal allografts by CD8+ T cells. Am J Transplant. 2004;4:1026–1032. doi: 10.1111/j.1600-6143.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, El-Asady R, Liu K, Wang D, Drachenberg CB, Hadley GA. Critical role for CD103+CD8+ effectors in promoting tubular injury following allogeneic renal transplantation. J Immunol. 2005;175:2868–2879. doi: 10.4049/jimmunol.175.5.2868. [DOI] [PubMed] [Google Scholar]
- Kilshaw PJ, Murant SJ. Expression and regulation of β7(β p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991;21:2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Neauport-Sautes C, Ellerson JR, Fridman WH. Binding site of human IgG subclasses and their domains for Fc receptors of activated murine T cells. J Immunol. 1977;119:1077–1083. [PubMed] [Google Scholar]
- Ratcliffe A, Stanworth DR. The localization of the binding site(s) on human IgG1 for the Fc receptors on homologous monocytes and heterologous mouse macrophages. Immunology. 1983;50:93–100. [PMC free article] [PubMed] [Google Scholar]
- Chen P, McGuire JK, Hackman RC, Kim KH, Black RA, Poindexter K, Yan W, Liu P, Chen AJ, Parks WC, Madtes DK. Tissue inhibitor of metalloproteinase-1 moderates airway re-epithelialization by regulating matrilysin activity. Am J Pathol. 2008;5:1256–1270. doi: 10.2353/ajpath.2008.070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2207. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- Roberts K, Kilshaw PJ. The mucosal T cell integrin αM290β7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol. 1993;23:1630–1635. doi: 10.1002/eji.1830230735. [DOI] [PubMed] [Google Scholar]
- Braun RK, Foerster M, Grahmann PR, Haefner D, Workalernahu G, Kroegel C. Phenotypic and molecular characterization of CD103+ CD4+ T cells in bronchoalveolar lavage from patients with interstitial lung diseases. Cytometry B Clin Cytom. 2003;548:19–27. doi: 10.1002/cyto.b.10021. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self-antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is up-regulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275:L365–L371. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y, Yamada W, Shiraishi Y, Hasegawa N, Fujishima S, Takano H, Ishizaka A. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38:566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1α expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- Tabata C, Tabata R, Kadokawa Y, Hisamori S, Takahashi M, Mishima M, Nakano T, Kubo H. Thalidomide prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol. 2007;179:708–714. doi: 10.4049/jimmunol.179.1.708. [DOI] [PubMed] [Google Scholar]
- Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karecla PI, Green SJ, Bowden SJ, Coadwell J, Kilshaw PJ. Identification of a binding site for integrin αEβ7 in the N-terminal domain of E-cadherin. J Biol Chem. 1996;271:30909–30915. doi: 10.1074/jbc.271.48.30909. [DOI] [PubMed] [Google Scholar]
- Schon MP, Schon M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin αE(CD103)-deficient mice. J Invest Dermatol. 2002;119:190–193. doi: 10.1046/j.1523-1747.2002.17973.x. [DOI] [PubMed] [Google Scholar]
- Schon MP, Schon M, Warren HB, Donohue JP, Parker CM. Cutaneous inflammatory disorder in integrin αE (CD103)-deficient mice. Immunology. 2000;165:6583–6589. doi: 10.4049/jimmunol.165.11.6583. [DOI] [PubMed] [Google Scholar]
- Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithauser F, Meinhardt-Krajina T, Fink K, Wotschke B, Moller P, Reimann J. Foxp3-expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168:1898–1909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einecke G, Fairhead T, Hidalgo LG, Sis B, Turner P, Zhu LF, Bleackley RC, Hadley GA, Famulski KS, Halloran PF. Tubulitis and epithelial cell alterations in mouse kidney transplant rejection are independent of CD103, perforin, or granzymes A/B. Am J Transplant. 2006;6:2109–2120. doi: 10.1111/j.1600-6143.2006.01483.x. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol. 2001;281:L92–L97. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Zhao LH, Jain F, Kradin R. IL-12p40(−/−) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp Mol Pathol. 2002;72:1–9. doi: 10.1006/exmp.2001.2409. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim HY, Kim S, Chung JH, Park WS, Chung DH. Natural killer T (NKT) cells attenuate bleomycin-induced pulmonary fibrosis by producing interferon γ. Am J Pathol. 2005;167:1231–1241. doi: 10.1016/s0002-9440(10)61211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, Nishino K, Osaki T, Tachibana I, Kaneda Y, Hayashi S. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol. 2000;278:L914–L922. doi: 10.1152/ajplung.2000.278.5.L914. [DOI] [PubMed] [Google Scholar]
- Kradin RL, Sakamoto H, Jain F, Zhao LH, Hymowitz G, Preffer F. IL-10 inhibits inflammation but does not affect fibrosis in the pulmonary response to bleomycin. Exp Mol Pathol. 2004;76:205–211. doi: 10.1016/j.yexmp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J, Yamamoto K. In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-β in the lung. Thorax. 2006;61:886–894. doi: 10.1136/thx.2005.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]