Abstract
The signal regulatory protein-β1 (SIRPβ1) is a DAP12-associated transmembrane receptor expressed in a subset of hematopoietic cells. Recently, it was shown that peritoneal macrophages express SIRPβ1, which positively regulated phagocytosis. Here, we found that SIRPβ1 was up-regulated and acted as a phagocytic receptor on microglia in amyloid precursor protein J20 (APP/J20) transgenic mice and in Alzheimer’s disease (AD) patients. Interferon (IFN)-γ and IFN-β stimulated gene transcription of SIRPβ1 in cultured microglia. Activation of SIRPβ1 on cultured microglia by cross-linking antibodies induced reorganization of the cytoskeleton protein β-actin and suppressed lipopolysaccharide-induced gene transcription of tumor necrosis factor-α and nitric oxide synthase-2. Furthermore, activation of SIRPβ1 increased phagocytosis of microsphere beads, neural debris, and fibrillary amyloid-β (Aβ). Phagocytosis of neural cell debris and Aβ was impaired after lentiviral knockdown of SIRPβ1 in primary microglial cells. Thus, SIRPβ1 is a novel IFN-induced microglial receptor that supports clearance of neural debris and Aβ aggregates by stimulating phagocytosis.
Microglial cells are the tissue resident macrophages of the central nervous system (CNS).1 Under pathological conditions microglia become activated, migrate to the lesion site, release a wide range of soluble factors including cytokines, and clear cellular debris by phagocytosis.1,2,3 In Alzheimer’s disease (AD) microglia are either beneficial by phagocytosing amyloid-β (Aβ) deposits or harmful by secreting neurotoxins.4,5,6,7 Recently, it was shown by in vivo multiphoton microscopy in an animal model of AD that Aβ plaques appeared over 24 hours, followed by microglial activation and recruitment to the plaque within 1 to 2 days.8 Finally, dysmorphic neurites were observed over the next days to weeks.8 Although microglia migrated to the Aβ plaque, it is unclear whether and which phagocytic receptors might contribute to Aβ plaque clearance.
Recently, it was demonstrated in the fruit fly Drosophila that immunoreceptor tyrosine-based activation motif (ITAM) signaling was required for phagocytosis in the CNS.9 Draper is a fly phagocytic receptor having ITAM-containing intracellular domains and associated with Shark, a nonreceptor tyrosine kinase that is similar to mammalian Syk and Zap-70. In vivo experiments showed that Shark activity was essential for Draper-mediated signaling, including recruitment of glia to lesioned axons and phagocytosis of axonal debris and neuronal cell corpses.9 Draper ITAM-phosphorylation was necessary for the glial phagocytic activity.9 Interestingly, the Draper-ITAM signaling pathway of Drosophila is very similar to the DAP12-ITAM signaling of mammalian immunoreceptors. The mammalian DAP12 molecule is a transmembrane adaptor that contains two ITAM motifs and is expressed in microglia associated with cell membrane receptors such as triggering receptor expressed on myeloid cells 2 (TREM2).10,11 In vitro studies on TREM2 and DAP12-mediated signaling in microglia showed that TREM2 facilitates phagocytic clearance of apoptotic cell corpses without inflammation.12 Stimulation of microglial TREM2 induced reorganization of the cytoskeleton and uptake of apoptotic membranes and beads via extracellular receptor kinase (ERK) activation.12
One of the signal regulatory proteins (SIRPs) family members, SIRPβ1 also associates with DAP12.13 SIRPβ1 is a transmembrane protein that has three Ig-like domains in its extracellular region and a short cytoplasmic tail.14 The ligand of SIRPβ1 is unknown.15 Human SIRPβ1 is expressed on monocytes and granulocytes but not on lymphocytes.16 The association between SIRPβ1 and DAP12 is mediated by an ionic interaction between single amino acids of opposite charge within the transmembrane regions of both molecules.13,17,18 Ligation of SIRPβ1 results in the tyrosine phosphorylation of DAP12 and the subsequent recruitment of Syk to the SIRPβ1-DAP12 complex in rat basophilic leukaemia cell line transfectants.13 Stimulation of SIRPβ1 on murine peritoneal macrophages facilitates phagocytosis.19
Our results demonstrate that microglial cells express SIRPβ1 and expression of SIRPβ1 is up-regulated on microglia in APP/J20 transgenic mice and AD patients. Ligation of microglial SIRPβ1 induces cytoskeleton rearrangement, counterregulates proinflammatory mediators, and facilitates phagocytosis of neural debris and fibrillary amyloid-β42 (Aβ42).
Materials and Methods
Immunohistochemical Analysis of SIRPβ1 in Human Brain Tissue Sections
Formalin-fixed paraffin-embedded brain tissue sections (4 μm thick, superior temporal neocortex-gyrus temporalis superior) from six patients with histopathologically confirmed diagnosis of AD and from six age-related controls without neurological disorders were analyzed by immunohistochemistry (one section from each AD patient and age-related control for light and double-fluorescence microscopy analyses, respectively). After blocking for endogenous peroxidase activity with H2O2-methanol and for unspecific protein interactions with 10% bovine serum albumin (Sigma-Aldrich, Taufkirchen, Germany), sections were first immunostained with a purified antibody directed against SIRPβ1 (1/200; Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibody was detected with biotinylated secondary antibody, avidin-biotin complex (both from Biomeda, Foster City, CA), and diaminobenzidine-HCl (Sigma-Aldrich). Counterstaining with hematoxylin was performed afterward. Sections were mounted in Corbit-Balsam (Hecht, Hamburg, Germany). For identification of microglial cells, double-immunofluorescence staining was performed sequentially with antibodies directed against SIRPβ1 and protein ionized calcium binding adaptor molecule 1(Iba1) (polyclonal rabbit, 1/200; Wako, Osaka, Japan). The primary antibodies were detected with Cy3-labeled anti-IgG and fluorescein isothiocyanate-conjugated anti-rabbit IgG (Dianova, Hamburg, Germany) secondary fluorescence antibodies. Sections were mounted in mowiol. Positive and negative controls were performed routinely to confirm specificity of staining. For quantification, three light microscopy images of brain tissue sections of AD patients and age-related controls at magnification ×40 were randomly selected and captured, and the total number of positive stained cells per mm2 was counted by an independent observer in each image.
Immunohistochemical Analysis of SIRPβ1 in Mouse Tissue Sections
Twelve APP/J20 transgenic (line J20 backcrossed at least 10 generations in a C57BL/6 background; supplied to A.M. by Dr. L. Mucke Gladstone Institute of Neurological Disease, University of California, San Francisco) and 12 normal mice (C57BL/6; obtained from Charles River Laboratories, Sulzfeld, Germany) at 12 months of age were used. All animal and human studies have been approved by the authors’ institutional review boards and by the local government and have been conducted according to the principles expressed in the Helsinki Declaration. A perfusion fixation of the animals was performed transcardially with Tris-buffered saline plus heparin (pH 7.4), followed by the injection of 0.1 M PBS (pH 7.4) containing 4% paraformaldehyde. Subsequently, cerebral hemispheres, cerebellums, and spinal cords were removed and fixed in 4% paraformaldehyde solution at 4°C for 48 hours, passed in 15 and 30% sucrose in PBS, frozen in liquid nitrogen, and then stored at −20°C. Six sections from cerebral hemispheres, cerebellum, and spinal cord from each APP/J20 transgenic and normal mice were used for light and double-fluorescence microscopy analyses, respectively. The cerebral hemispheres, the cerebellum, the spinal cord, and the spleen were cut with a cryostat in 20-μm thick sections on glass slides and stored until use at −20°C. After blocking for endogenous peroxidase activity with H2O2-methanol and for unspecific protein interactions with 10% bovine serum albumin, frozen sections were immunostained with a purified antibody directed against SIRPβ1 (SIRPβ-84; monoclonal rat, 1/200; as described previously19). For light microscopy analysis, primary antibody in cryosections was detected with biotinylated secondary antibody, avidin-biotin complex (both from Biomeda), and diaminobenzidine-HCl (Sigma-Aldrich). Counterstaining with hematoxylin was performed afterward. Sections were mounted in Corbit-Balsam (Hecht). For fluorescence microscopy analysis, antibody directed against SIRPβ1 was detected with Carbocyanin (Cy3)-labeled anti-rat IgG secondary fluorescence antibody (Dianova). Sections were mounted in mowiol. Control stainings were performed with isotype control antibodies (BD Biosciences, Heildelberg, Germany). Immunostained tissue sections were viewed with a Leica DMBL light and fluorescence microscope (Leica, Bensheim, Germany) as well as with confocal laser scanning microscope with a ×40 or a ×60 objective (Olympus, Hamburg, Germany). To identify the cell type, double-immunofluorescence staining was performed sequentially with antibodies directed against SIRPβ1 and Iba1 (rabbit, 1/200; Wako, Japan), respectively. The primary antibodies were detected with Cy3-labeled anti-rat IgG and fluorescein isothiocyanate-conjugated anti-rabbit IgG (Dianova) secondary antibodies. Sections were mounted in mowiol and viewed with the laser scanning confocal microscope. For quantification, three light microscopy images of each tissue section of APP/J20 transgenic and normal mice were randomly selected at magnification ×40, captured and the total number of positive stained cells per mm2 was counted by an independent observer in each image.
Primary Cell Cultures
Microglial cells were prepared from the brains of C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany) on postnatal day 3 or 4 (P3 or P4) as described previously.12 In brief, meninges were removed mechanically and the cells were dissociated by trituration and cultured in basal medium (Invitrogen, Carlsbad, CA), 10% fetal calf serum (PAN Biotech, Aidenbach, Germany), 1% glucose (Sigma-Aldrich), 1% l-glutamine (Invitrogen), and 1% penicillin/streptomycin (Invitrogen) for 14 days to form a confluent glial monolayer. To collect microglial cells, the cultures were shaken on a rotary shaker (200 rpm) for 2 hours. The detached microglial cells were seeded in culture dishes for 1 hour, and then all nonadherent cells were removed and discarded. Purity of the isolated microglia was >95% as determined by flow cytometry analysis with antibody directed against CD11b (BD Biosciences). Microglial cells were cultured in basal medium as described above.
Neurons were prepared from the hippocampi of C57BL/6 mouse embryos (E15–16) as described previously.20 In brief, brain tissue was isolated and mechanically dispersed and seeded in culture dishes precoated with 0.01 mg/ml poly-l-ornithin (Sigma-Aldrich) and 10 μg/ml laminin (Sigma-Aldrich). Cells were cultured in neuronal condition medium (Invitrogen) supplemented with 2% B-27 supplement (Invitrogen), 1% glucose (Sigma-Aldrich), and 1% FCS (PAN Biotech). Cells were cultured for 5 to 10 days to obtain morphologically mature neurons.
Myeloid precursors were isolated from bone marrow cells of adult C57BL/6 mice (Charles River Laboratories) as described previously.21 Bone marrow cells were obtained from the tibia of adult mice. Removal of erythrocytes was performed by lysis with hypotonic solution. Cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) containing 10% fetal calf serum (Pan Biotech) and 10 ng/ml of granulocyte-macrophage colony-stimulating factor (R&D Systems) in 75-cm2 culture flasks (Greiner, bio-one, Frickenhausen, Germany). After 24 hours nonadherent cells were collected and reseeded in fresh 75-cm2 culture flasks. Medium was changed after 5 days, and myeloid precursor cells were collected for experiments after 10 to 11 days.
Splenocytes were obtained from the spleens of adult C57BL/6 mice after dissociation and lysis of erythrocytes by hypotonic solution.
Immunocytochemistry for SIRPβ1 in Primary Cell Cultures
Cells were fixed in 4% paraformaldehyde for 1 hour, then blocked by 1% bovine serum albumin in PBS for 2 hours and immunostained with a purified monoclonal rat antibody directed against SIRPβ1 (anti-SIRPβ1 clone 84, as described previously19) and a secondary Cy3-fluorescence-conjugated goat antibody directed against rat immunoglobulin (IgG) (1/200; Dianova). Background staining was obtained by staining of cells with a rat monoclonal isotype control antibody (BD Biosciences), followed by Cy3-conjugated goat antibody directed against rat IgG (1/200; Dianova). To identify the cell type, cells were double labeled with monoclonal mouse antibody directed against CD11b (BD Biosciences) or mouse antibody directed against glial fibrillary acidic protein or β-tubulin-III (Sigma-Aldrich), followed by a secondary fluorescein isothiocyanate-conjugated antibody directed against mouse IgG. Images were collected by confocal laser scanning microscopy with a ×40 objective (Olympus).
RT-PCR Analysis for SIRPβ1 and DAP12 in Primary Cell Cultures
Total RNA was isolated from microglia, neuronal cultures, splenocytes and bone marrow-derived myeloid precursor cells by the RNeasy Mini Kit (Qiagen, Hildesheim, Germany). Reverse transcription of RNA was performed with reverse transcriptase (SuperScript III; Invitrogen) and hexamer random primers (Roche Molecular Biochemicals). For semiquantification, all samples were normalized to 18s ribosomal RNA. The following oligonucleotides were used for RT-PCR amplification: 18s forward, 5′-ATCCATTGGAGGGCAAGTCT-3′, and reverse, 5′-CCGCGGTCCTATTCCATTAT-3′; SIRPβ1 forward, 5′-CCCGTTCACAGGAGAACATT-3′, and reverse, 5′-CCGGAGACCATAGGTGAAGA-3′; and DAP12 forward, 5′-ATGGGGGCTCTGGACCCCT-3′, and reverse, 5′-TCATCTGTAATATTGCCTCTGTGT-3′.
Real-Time PCR Analysis of Cultured Microglia and Tissue Samples
For analysis of SIRPβ1, DAP12, tumor necrosis factor-α (TNF-α), interleukin-1β, nitric oxide synthase-2 (NOS2), and transforming growth factor-β1 gene transcription in cultured microglia, total RNA of cells was isolated by the RNeasy Mini Kit (Qiagen, Hildesheim, Germany). As indicated, primary cultured microglia were stimulated by addition of TNF-α (10 ng/ml; R&D Systems), interferon γ (murine IFN-γ, 100 U/ml; HyCult Biotechnology, Uden, The Netherlands), IFN-β (103 U/ml; R&D Systems), or lipopolysaccharide (500 ng/ml; Sigma-Aldrich). For analysis of gene transcription in APP/J20 transgenic mice (line J20 backcrossed in a C57BL/6 background) and control mice (C57BL/6), animals were sacrificed, and cerebral hemispheres, cerebellums, spinal cords and spleens were collected. RNA was isolated by the RNeasy Mini Kit for Lipid Tissue (Qiagen), and reverse transcription was performed with reverse transcriptase (SuperScript III; Invitrogen) and hexamer random primers (Roche Molecular Biochemicals).
Quantitative RT-PCR with specific oligonucleotides was performed with SYBR Green PCR Master Mix (Qiagen) using the ABI 5700 Sequence Detection System (PerkinElmer) and amplification protocol for the ABI 5700 Sequence Detection System. Amplification specificity was confirmed by the analysis of the melting curves. Results were analyzed with the ABI 5700 Sequence Detection System version 1.3 after establishing the reaction efficiency for each primer pair. Oligonucleotides described above have been applied. Quantification using the δCT method was performed.
Lentiviral Vector System and Microglial Transduction
The lentiviral vector PLL3.7 of the third generation was used for transduction of microglia. The mouse SIRPβ1 gene (as described previously19) was tagged with a 3× flag sequence at the extracellular side and with green fluorescent protein (GFP) at the intracellular side (fSIRPβ1 vector). For overexpression, genes were cloned under the cytomegalovirus promoter in the PLL3.7 vector. For knockdown of SIRPβ1, a targeting short hairpin sequence and a scrambled control sequence were synthesized and cloned under the U6 promoter in the PLL3.7 backbone. Short hairpin RNA sequence (shSIRPβ1) for knockdown of SIRPβ1 (5′-TAACTGAAGACGGCAGGTATTCAAGAGATACCTGCCGTCTTCAGTTTTTTTTC-3′) and short hairpin RNA sequence for control (shControl) (5′-TAAGACTTGAGCGCAGGAATTCAAGAGATTCCTGCGCTCAAGTCTTTTTTT C-3′) were inserted into the PLL3.7 vector, respectively. The correct nature of all cloned sequences was confirmed by automated sequencing (Seqlab, Göttingen, Germany). For lentiviral transduction, PLL3.7 plasmids were purified and then cotransfected together with packaging vectors (Invitrogen) into 293FT cells (Invitrogen). Supernatant was collected after 48 hours, and viral particles in the supernatant were concentrated at 1/100 by ultracentrifugation for 90 minutes at 25,000 rpm (Sorvall Discovery 90SE Centrifuge) and recovered by suspension in PBS. Titers of viral particles ranged between 106 and 107 multiplicity of infection. Purified microglial cells were seeded at a density of 2 × 105 cells/ml into 24-well plates. Lentiviral particles and 8 μg/ml polybrene (Sigma-Aldrich) were added to the culture and centrifuged for 90 minutes at 1500 rpm. Supernatant was removed immediately after infection and replaced with basal medium (Invitrogen) containing 10% fetal calf serum and 50% glial culture supernatant obtained from the culture before transduction.
F-actin Cytoskeleton Labeling
Microglia were lentivirally transduced with the fSIRPβ1 vector. Cells were then cross-linked for 1 hour on culture dishes coated with flag-specific antibodies (10 μg/ml; Sigma- Aldrich). As control, cells were cultured on dishes coated with isotype control antibody (10 μg/ml; Sigma-Aldrich). Cells were fixed, blocked, and then stained with Alexa Fluor 546-conjugated phalloidin (Molecular Probes, Invitrogen). Serial images along the z-axis were collected by confocal laser scanning microscope with a ×40 objective (Olympus). The number of microglia cells showing F-actin staining at the bottom opposite to the culture dish were quantified under fSIRPβ1 stimulation and control conditions.
Beads Phagocytosis Assay of Primary Microglia
Primary microglia were transduced with the fSIRPβ1 vector or the GFP control vector. Cells were then cultured on flag-specific antibodies or isotype control antibodies as mentioned above. After 24 hours, red fluorescent microsphere beads (1.00 μm, Fluoresbrite Polychromatic Red Microspheres; Polysciences) were added for 1 hour to the culture. Phagocytosis of microsphere beads by microglia was detected by fluorescence microscopy. For quantification of microsphere bead phagocytosis, microglial cells were collected from the culture plates and analyzed by flow cytometry. The percentage of microglia having phagocytosed more than one bead was determined. Relative change in phagocytosis compared with the control was determined since basal phagocytosis varied between each experiment. Pharmacological inhibition of actin polymerization was performed with 1 μmol/L Cytochalasin D (Calbiochem). Inhibition of the ERK was performed by 20 μmol/L ERK inhibitor PD98059 (Calbiochem) 60 minutes before stimulation of fSIRPβ1.
Amyloid-β Phagocytosis Assay
Microglial cells were transduced with the fSIRPβ1, GFP control, ShSIRPβ1, or ShControl. After transduction, microglia were cultured for 72 hours to achieve effective expression or knockdown of SIRPβ1 by RNA interference. The fSIRPβ1 and the GFP control vector transduced cells were then cultured on flag-specific antibodies or isotype control antibodies for 24 hours as mentioned above. Cells were treated for 6 hours with biotinylated Aβ42 peptide (10 μg/ml, human amyloid β peptide 1-42 conjugated at the N terminus with biotin; Bachem, Heidelberg, Germany). The biotinylated Aβ42 peptide (1 mg/ml) was dissolved in PBS, incubated for 4 to 7 days at 37°C to obtain fibrils, and then stored by 4°C until it was used in the experiments. To demonstrate uptake of Aβ42, cells were fixed in 4% paraformaldehyde and then permeabilized with 0.2% Triton X-100. Fixed cells were incubated with Cy3-conjugated streptavidin (Amersham Biosciences). Cells were analyzed under laser scanning confocal microscope (Olympus). The percentage of microglial cells having phagocytosed Aβ42 peptide was quantified.
Phagocytosis Assay of Apoptotic Neuronal Material
Primary microglia were lentivirally transduced with fSIRPβ1, GFP control vector, shSIRPβ1 or shControl. After transduction, microglia were cultured for 72 hours to achieve effective expression or knock-down of SIRPβ1 by RNA interference. Neurons were cultured for 5 to 10 days, and then okadaic acid at a final concentration of 30 nmol/L was added for 3 hours to induce apoptosis. Apoptotic neuronal cell membranes were labeled with CellTracker CM-DiI membrane dye at the final concentration of 2 μg/μl (Molecular Probes). After incubation, apoptotic neurons were washed twice with PBS and added to the transduced microglial culture at an effector/target ratio of 1:20. At 1 hour after addition of apoptotic neurons, the number of microglia having phagocytosed neuronal membranes was quantified using a confocal fluorescence microscope (Olympus).
Results
Expression of SIRPβ1 in AD Brain Tissue
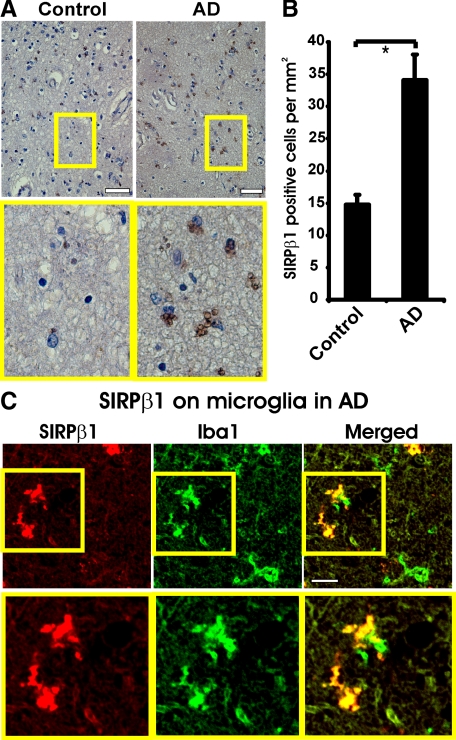
Protein expression of SIRPβ1 was analyzed in archival postmortem superior temporal neocortex tissue sections from AD and age-related control patients (Table 1). Clinical histories were analyzed by retrospective chart analysis as described previously.22 Diagnosis of AD was neuropathologically confirmed according to the National Institute on Aging-Reagan consensus criteria.23 Immunohistochemistry with specific antibody directed against SIRPβ1 was performed (Figure 1). Histopathological light microscopy analysis demonstrated small and round cells with morphologies of activated microglia in the brain tissue sections (Figure 1A). The number of SIRPβ1-immunopositive cells was higher in AD patients in comparison with control cases (Figure 1B). In detail, 34.12 ± 3.90 cells/mm2 were labeled with the SIRPβ1-specific antibody in the superior temporal neocortex of AD patients compared with 14.78 ± 1.46 cells/mm2 in the control cases. To identify the type of SIRPβ1-immunopositive cells, immunofluorescence analysis was performed. Data demonstrated expression of SIRPβ1 in brain tissue sections of AD patients on a subset of microglial cells, which were identified by antibody directed against Iba1 (Figure 1C). No other brain cell types were immunostained by the antibody directed against SIRPβ1.
Table 1.
Demographics and Clinical Data
| Characteristics/clinical diagnosis | Control subjects | AD cases |
|---|---|---|
| No. of subjects/cases | 6 | 6 |
| Female:male ratio | 3:3 | 4:2 |
| Age (years); median (± SD) | 69.2 ± 2.8 | 76.3 ± 10.6 |
| Postmortem interval (in hours) | 48 ± 30 | 44 ± 38 |
Figure 1.
Detection of SIRPβ1 in Alzheimer’s disease brain tissue. A: Immunocytochemistry with antibodies directed against SIRPβ1 of the superior temporal neocortex from a control case (control) and an Alzheimer’s disease patient (AD). Light microscopy showed expression of SIRPβ1 in cells with microglial morphology. Scale bar: 100 μm. Inset of higher magnification as indicated. B: Quantification of SIRPβ1-positive cells in AD versus control brain tissue samples. Data demonstrated an increased number of SIRPβ1-positive cells in the superior temporal neocortex of AD patients compared with control cases. Mean ± SEM of n = 6 patients/cases per group. ∗P < 0.05, unpaired t-test. C: Brain tissue sections of AD patients were double-immunolabeled with antibodies directed against SIRPβ1 and the microglial marker protein Iba1. A subset of microglial cells identified by double-labeling with Iba1 expressed SIRPβ1. Inset of higher magnification as indicated. Scale bar: 30 μm.
Increased SIRPβ1 Expression in the Brain Tissue of APP Transgenic Mice
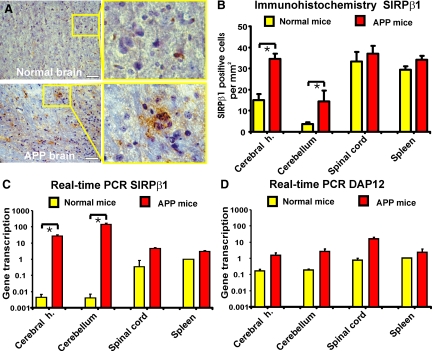
To analyze protein expression of SIRPβ1 in the brain and spinal cord of mice, tissue sections were immunostained with a rat monoclonal antibody directed against mouse SIRPβ1. In the CNS only very few cells were immunopositive for SIRPβ1 (Figure 2A). In detail, SIRPβ1 expression was detected in normal 12-month-old mice on 15.1 ± 2.8 cells/mm2 in the cerebral hemispheres, on 3.6 ± 0.9 cells/mm2 in the cerebellum and on 33.3 ± 4.6 cells/mm2 in the spinal cord (Figure 2B). However, in an animal model of AD, the number of SIRPβ1-positive cells in the cerebral hemispheres and cerebellum substantially increased. In detail, in amyloid precursor protein J20 (APP/J20) transgenic mice at the age of 12 months, SIRPβ1 expression was detected on 34.5 ± 2.4 cells/mm2 in the cerebral hemispheres, on 14.3 ± 5.3 cells/mm2 in the cerebellum and on 37.0 ± 3.8 cells/mm2 in the spinal cord (Figure 2B). Next, gene transcription of SIRPβ1 in distinct CNS regions was analyzed by real-time RT-PCR. Gene transcript levels were normalized to the values of the spleen of normal mice. Gene transcripts of SIRPβ1 were detected in the spinal cord, cerebellum, and cortex of healthy mice. SIRPβ1 expression in the cerebral hemispheres and cerebellum was very low in normal mice but was strongly increased in APP/J20 transgenic mice (Figure 2C). In detail, relative gene transcript level of SIRPβ1 was 26.7 ± 5.1 in the cerebral hemispheres of 12-month-old APP/J20 transgenic mice compared with 0.004 ± 0.002 in normal mice (Figure 2C). But no significant change in protein expression or gene transcription of SIRPβ1 was observed in the spinal cord and spleen between the 12-month-old APP/J20 transgenic and normal mice (Figure 2, B and C). Gene transcripts of DAP12 were also analyzed. However, no significant up-regulation of DAP12 transcription was detected, although there was a slight increase in all tissues analyzed in the APP/J20 transgenic mice compared with the normal control mice (Figure 2D). Thus, SIRPβ1 gene transcripts were disproportionally up-regulated in the brain of APP/J20 transgenic mice compared with DAP12 (Figure 2, C and D).
Figure 2.
Increased protein expression and gene transcription of SIRPβ1 in the cerebrum and cerebellum of APP transgenic mice. A: Immunohistochemistry with antibody directed against SIRPβ1 was performed on tissue sections of normal and APP/J20 transgenic mice at 12 months of age. Expression of SIRPβ1 was detected in a few cells with microglial morphology in the cerebral hemispheres of normal adult mice. An increased number of SIRPβ1-positive cells with microglial morphology was observed in the cerebral hemispheres of aged APP/J20 transgenic mice (APP brain). Inset of higher magnification as indicated. Scale bars: 100 μm. B: Quantification of SIRPβ1 immunostained cells in the spinal cord, cerebellum, cerebral hemisphere (cerebral h.) and spleen tissues derived from APP/J20 transgenic and normal mice at 12 months of age. The number of cells immunolabeled for SIRPβ1 was increased in the cerebral hemisphere (cerebral h.) and cerebellum of APP/J20 transgenic mice compared with normal adult mice. Data are shown as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance followed by Bonferroni’s multiple comparison test. C: Relative gene transcript levels of SIRPβ1 in the spinal cord, cerebellum, cerebral hemisphere (cerebral h.), and spleen tissues derived from APP/J20 transgenic and normal mice at 12 months of age. Gene transcript level of SIRPβ1 was increased in the cerebral hemisphere and cerebellum of APP/J20 transgenic mice compared with normal control mice. Data are shown as mean ± SEM of n = 6 independent experiments. ∗P < 0.05, analysis of variance followed by Bonferroni’s multiple comparison test. D: Relative gene transcript levels of the adaptor protein DAP12 in the spinal cord, cerebellum, cerebral hemisphere (cerebral h.), and spleen tissues derived from APP/J20 transgenic and normal mice at 12 months of age. Gene transcript levels of DAP12 were only slightly increased in all tissues analyzed in APP/J20 transgenic mice compared with normal control mice. Data are shown as mean ± SEM of n = 6 independent experiments. ∗P < 0.05, analysis of variance followed by Bonferroni’s multiple comparison test.
Expression of SIRPβ1 in Cultured Primary Microglia and Up-Regulation by IFNs
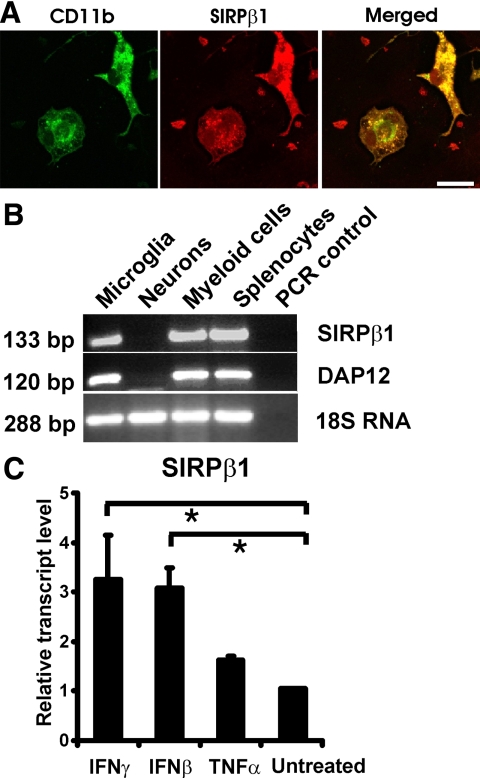
Expression of SIRPβ1 was analyzed in cultured primary brain cells by immunohistochemistry. Immunolabeling of SIRPβ1 was detected in cultured microglia by a rat monoclonal antibody directed against mouse SIRPβ1 (Figure 3A). Cells were identified as microglia by co-immunostaining with an antibody directed against CD11b (Figure 3A). In detail, 73.1 ± 4.1% (mean ± SEM) of cultured microglial cells identified by CD11b staining showed constitutive expression of SIRPβ1. No immunostaining of SIRPβ1 was detected in cultured astrocytes or neurons double-labeled with antibodies directed against glial fibrillary acidic protein or β-tubulin-III, respectively (data not shown).
Figure 3.
Protein expression and gene transcription of SIRPβ1 in cultured primary microglia. A: SIRPβ1 protein was detected in cultured primary microglia by immunofluorescence labeling with a rat monoclonal antibody directed against mouse SIRPβ1. Microglial cells were identified by double-labeling with antibodies directed against CD11b. Scale bar: 15 μm. B: RT-PCR analysis for SIRPβ1 and DAP12 gene transcripts of cultured microglia, neurons, bone marrow-derived myeloid precursor cells (myeloid cells), and splenocytes. Gene transcripts for SIRPβ1 and DAP12 were detected in microglia, bone marrow-derived myeloid cells and splenocytes but not in neurons. Amplification of 18S RNA was used as an internal control. PCR without reverse transcriptase was used as negative control (PCR control). C: Real-time RT-PCR analysis of cultured primary microglia for SIRPβ1. Cells were treated for 48 hours with IFN-γ (murine IFN-γ; 100 U/ml), IFN-β (murine IFN-β; 103 U/ml), or TNF-α (murine TNF-α; 10 ng/ml). Treatment with either IFN-γ or IFN-β up-regulated gene transcription of SIRPβ1. Data are normalized to untreated control and presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance followed by Bonferroni’s multiple comparison test.
Gene transcripts of SIRPβ1 and DAP12 were analyzed by RT-PCR in cultured primary microglial cells. Total RNA was prepared from cultures of primary microglia, neurons, bone marrow-derived myeloid cells, and splenocytes. Gene transcripts of SIRPβ1 and DAP12 were detected in microglia, bone marrow-derived myeloid cells, and splenocytes but not in cultured neurons (Figure 3B).
To study whether inflammatory mediators could induce gene transcription of SIRPβ1 in primary microglia, we treated the cells for 48 hours with IFN-γ (100 U/ml), IFN-β (103 U/ml), or tumor necrosis factor-α (TNF-α 10 ng/ml) and analyzed the respective gene transcripts by real-time RT-PCR. Treatment with IFN-γ and IFN-β up-regulated gene transcription of SIRPβ1 in primary microglia (Figure 3C). In contrast, TNF-α had no significant effect on the gene transcription of SIRPβ1 (Figure 3C).
Actin Reorganization after Cross-Linking of SIRPβ1
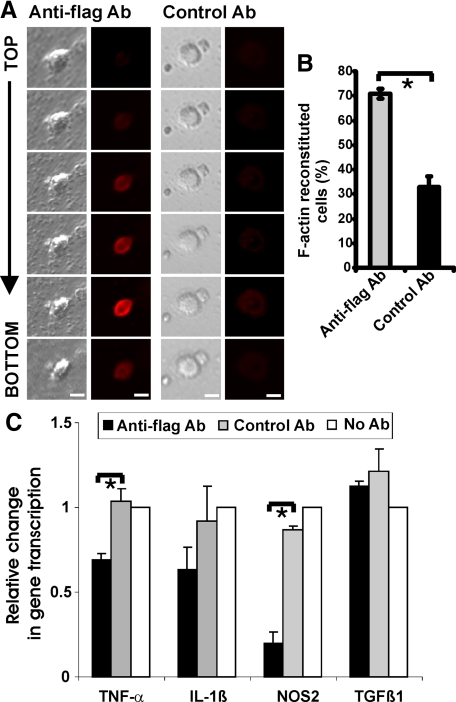
To analyze whether SIRPβ1 signals on the cytoskeleton, primary microglial cells were lentivirally transduced to overexpress mouse SIRPβ1 modified at the extracellular part with a 3× flag epitope. Transduction efficiency of microglia was reasonably high (Table 2), leading to expression of flag-tagged SIRPβ1 (SIRPβ1) in >90% of microglia. Transduced microglial cells were cultured on dishes either coated with flag-specific antibodies or isotype control antibodies. F-actin staining and confocal images along the z-axis were performed to analyze any cytoskeleton changes induced by SIRPβ1 signaling. Flag-tagged SIRPβ1-transduced microglial cells that were cultured on dishes coated with flag-specific antibodies showed increased F-actin labeling at the bottom side, where the cell was binding to the culture dish (Figure 4A). Quantification demonstrated that F-actin staining localized in microglia toward the culture dish increased to 70.9 ± 1.9% of cells after cross-linking of SIRPβ1 with flag-specific antibody compared with 32.8 ± 4.4% of cells with the control antibody (Figure 4B). Thus, stimulation of SIRPβ1 reorganized the cytoskeleton.
Table 2.
Transduction Efficiency and Cell Survival of Primary Microglia at 3 Days after Lentiviral Vector Application
| Vector type applied to microglia | No. of experiments | Percentage of cells showing expression of GFP (mean ± SEM) | Relative cell number in percentage (before vector application = 100%) |
|---|---|---|---|
| fSIRPβ1 | 3 | 91.87% (SEM ± 2.70) | 86.97% (SEM ± 4.44) |
| GFP-control | 3 | 93.10% (SEM ± 2.49) | 91.63% (SEM ± 3.35) |
| ShSIRPβ1 | 3 | 95.43% (SEM ± 1.69) | 87.47% (SEM ± 2.83) |
| ShControl | 3 | 92.03% (SEM ± 3.66) | 89.9% (SEM ± 3.53) |
| No vector | 3 | 0% | 91.73% (SEM ± 2.93) |
Figure 4.
SIRPβ1 cross-linking on microglia induced F-actin reorganization and reduced gene transcription of proinflammatory mediators. A: Phase contrast and confocal images (z-stacks from top to bottom) of flag-tagged SIRPβ1-transduced primary microglia cultured for 1 hour on dishes coated with flag-specific antibody (anti-flag Ab) or isotype control antibody (control Ab). Detection of F-actin was performed by labeling with fluorescence dye-conjugated phalloidin. Reorganization of the actin cytoskeleton (F-actin labeling) was observed in flag-tagged SIRPβ1-stimulated cells at the bottom of the cells at the region of antibody binding. Scale bar: 10 μm. B: Percentage of F-actin-labeled microglial cells transduced with flag-tagged SIRPβ1 was quantified after stimulation with flag-specific antibody (anti-flag AB) or control antibody (control Ab). Cross-linking of SIRPβ1 increased the number of microglial cells showing F-actin staining at the bottom of the cells. Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, unpaired t-test. C: Primary microglial cells were transduced with flag-tagged SIRPβ1 and treated with lipopolysaccharide for 24 hours to induce gene transcription of proinflammatory mediators. Microglial cells were cultured on dishes coated with cross-linking antibody directed against the flag epitope (anti-flag Ab). An irrelevant antibody of the same isotype (control Ab) and no antibody were used as controls. Cross-linking of SIRPβ1 reduced lipopolysaccharide-induced gene transcription of TNF-α and NOS2. Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance, followed by Bonferroni’s multiple comparison test.
Decreased Proinflammmatory Mediators after Stimulation of SIRPβ1
Primary microglial cells were lentivirally transduced to express fSIRPβ1. Transduced microglial cells were cultured on dishes either coated with flag-specific antibodies, isotype control antibodies, or nonmodified dishes. Furthermore, microglial cells were treated with lipopolysaccharide (500 ng/ml) for 24 hours. Total RNA was collected and gene transcription for TNF-α, interleukin-1β (IL 1β), nitric oxide synthase-2 (NOS2), and transforming growth factor-β1 (TGFβ1) was determined by real-time RT-PCR (Figure 4C). Stimulation of SIRPβ1 by cross-linking antibodies counterregulated the lipopolysaccharide-induced stimulation of TNF-α and NOS2. In detail, relative gene transcription of TNF-α was reduced from 1.04 ± 0.10 (mean ± SEM) in microglia cultured on isotype control antibody to 0.69 ± 0.04 in microglia cultured on fSIRPβ1 cross-linking antibody (Figure 4C). Likewise, relative gene transcription of NOS2 was reduced from 0.87 ± 0.02 in the isotype control group to 0.19 ± 0.07 after SIRPβ1 stimulation (Figure 4C).
Increased Phagocytosis of Beads, Fibrillary Aβ and Neural Debris after Stimulation of SIRPβ1
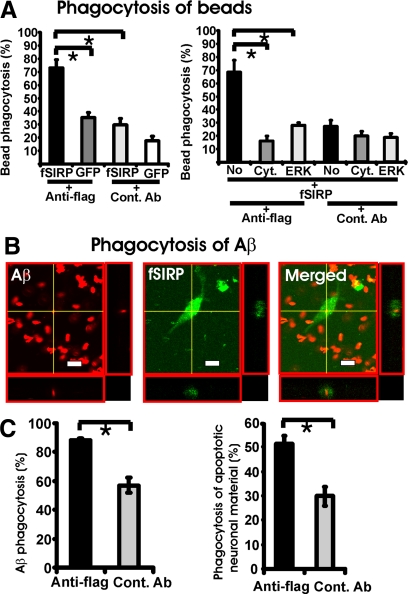
To analyze phagocytosis, we lentivirally transduced fSIRPβ1 in primary microglia and stimulated the cells with a flag-tag-specific antibody. Phagocytosis of microsphere beads was quantified by flow cytometry. Microglial cells showed increased uptake of microsphere beads after stimulation of fSIRPβ1 (Figure 5A). In detail, stimulation of fSIRPβ1 with cross-linking antibodies increased the relative level of microsphere beads phagocytosis to 72.0 ± 6.2% compared with an isotype control antibody treatment (29.7 ± 5.0% SEM) (Figure 5A). No significant effect of the flag-specific antibody on phagocytosis was observed in microglia transduced with the GFP control vector (Figure 5A). Increased uptake of beads after stimulation of fSIRPβ1 was prevented by inhibition of actin polymerization with cytochalasin D or the ERK inhibitor PD98059, demonstrating that SIRPβ1 acted via phagocytosis to increase beads uptake (Figure 5A).
Figure 5.
Increased microglial phagocytosis after stimulation of SIRPβ1. A: Increased phagocytosis of microsphere beads after stimulation of flag-tagged SIRPβ1 (fSIRPβ1). Primary microglial cells were lentivirally transduced with the flag-tagged SIRPβ1 (fSIRP) or the control GFP vector (GFP) and were cultured for 24 hours on dishes coated with flag-specific antibody (anti-flag) or control antibody (cont. Ab). The percentage of microglia having phagocytosed beads was quantified by flow cytometry. Stimulation of fSIRPβ1 on transduced primary microglia by the flag-specific antibody (anti-flag) increased the percentage of microglia having taken up microsphere beads compared with GFP-transduced microglia (GFP) and the isotype control antibody (Cont. Ab). Treatment of microglia with the actin polymerization inhibitor cytochalasin D (Cyt.) or the mitogen-activated protein kinase inhibitor PD98059 (ERK) prevented the increased phagocytosis induced by stimulation of fSIRPβ1 (no: no treatment). Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05 analysis of variance, followed by Bonferroni’s multiple comparison test. B: Phagocytosis of Aβ42 after stimulation of fSIRPβ1. Primary microglial cells were transduced with the fSIRPβ1 vector. Cells were cultured on dishes coated with flag-specific antibody and then Aβ42 was added for 6 hours before fixation. Phagocytosis of Aβ42 was analyzed by confocal microscopy. Serial sections along the z-axis were acquired and composed to images. Aβ42 was localized within the microglial cell. Scale bar: 10 μm. C: Increased phagocytosis of Aβ42 and neural debris after fSIRPβ1 stimulation. Primary microglial cells were transduced with the fSIRPβ1 vector and were cultured for 24 hours on dishes coated with flag-specific antibody (anti-flag) or isotype control antibody (Cont. Ab). Biotinylated Aβ42 or apoptotic neuronal material was added. Percentage of primary microglia having phagocytosed Aβ42 or neuronal debris was quantified by confocal microscopy. Stimulation of fSIRPβ1 increased the uptake of Aβ42 and apoptotic neuronal material. Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance.
Then analysis of fibrillary Aβ42 uptake was performed after stimulation of fSIRPβ1 in primary microglia transduced with fSIRPβ1 (Figure 5B). A biotinylated human Aβ42 in a fibrillary form was applied, which previously was shown to form fibrils very similar to unconjugated Aβ42 fibrils as determined by electron microscopy.24 Phagocytosis of fibrillary Aβ42 was visualized under confocal microscopy by demonstrating Aβ42 within the microglial cells by scanning along the z-axis (Figure 5B). Number of primary microglia having phagocytosed fibrillary Aβ42 was quantified. Microglial cells showed increased Aβ42 uptake after stimulation of fSIRPβ1 (Figure 5C). In detail, stimulation of fSIRPβ1 with cross-linking antibodies increased the relative level of fibrillary Aβ42 uptake to 88.1 ± 1.6% compared with an isotype control antibody treatment (57.1 ± 5.5%).
Furthermore, uptake of apoptotic neuronal material was analyzed after stimulation of fSIRPβ1. Cultured neurons were pretreated with okaidic acid to induce apoptosis and membranes were labeled with a red fluorescence membrane dye. Uptake of red fluorescent labeled apoptotic neuronal membranes by microglia was analyzed by confocal microscopy. Phagocytosis of apoptotic neuronal material was increased on stimulation of fSIRPβ1 on primary microglial cells (Figure 5C). In detail, stimulation of fSIRPβ1 with cross-linking antibodies increased the relative level of apoptotic neuronal material phagocytosis to 51.4 ± 3.1% compared with the isotype control antibody treatment (29.9 ± 3.9%).
Thus, stimulation of fSIRPβ1 with cross-linking antibodies increased phagocytosis of microsphere beads, fibrillary Aβ42, and apoptotic neuronal cell membranes.
Impaired Phagocytosis after Lentiviral Knockdown of SIRPβ1 in Primary Microglia
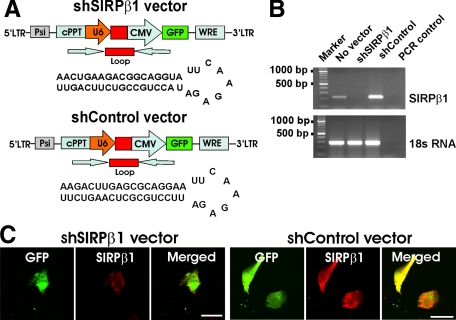
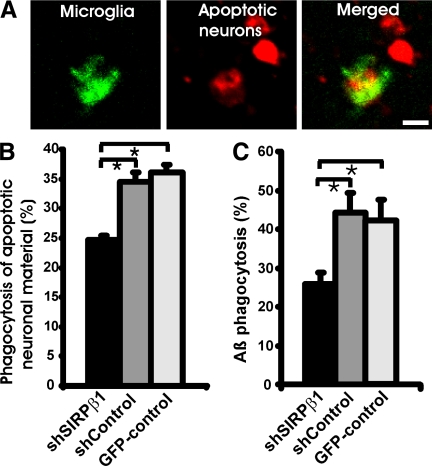
To further analyze the function of SIRPβ1 on phagocytosis in primary microglia, we applied a short hairpin lentiviral knockdown vector targeting the SIRPβ1 gene by RNA interference (shSIRPβ1) to primary microglial cells (Figure 6A). To confirm a successful knockdown of SIRPβ1 gene transcripts, microglial RNA was analyzed 72 hours after transduction with the lentiviral shSIRPβ1 RNA interference vector. No gene transcripts of SIRPβ1 were detected after 35 PCR cycles in reverse-transcribed RNA of microglia transduced with shSIRPβ1 vector (Figure 6B). By contrast, the control vector (ShControl), a scrambled RNA interference vector did not knockdown SIRPβ1 gene transcript (Figure 6B). Immunohistochemical analysis was also performed to confirm the absence of SIRPβ1 expression on microglia after knockdown. SIRPβ1 was detected in the majority of microglial cells by specific antibodies in the shControl vector-transduced cells but not in cells transduced with the shSIRPβ1 vector (Figure 6C). Transduction efficiency of microglia was relative high (Table 2), virtually leading to the loss of SIRPβ1 function in the majority of microglial cells after lentiviral transduction with the shSIRPβ1 vector. Capacity to phagocytose apoptotic neural material or Aβ was then compared between microglia transduced with the shSIRPβ1 and the control vectors. Microglia transduced with the shControl vector phagocytosed the red fluorescent-labeled apoptotic neuronal membrane fragments (Figure 7A). Phagocytosis of apoptotic membrane fragments was reduced, when the SIRPβ1 receptor was knocked down in microglia (Figure 7B). In detail, after 24 hours 24.7 ± 1.7% of microglia showed phagocytosis of neuronal material after knockdown of SIRPβ1, whereas 34.5 ± 6.1% of microglia transduced with the ShControl vector and 36.1 ± 3.2% of microglial transduced with the GFP control vector demonstrated phagocytosis of apoptotic neuronal material (Figure 7B).
Figure 6.
Lentiviral knock-down of SIRPβ1. A: Schematic drawings of the lentiviral short hairpin RNA interference vector targeting mouse SIRPβ1 (shSIRPβ1) and the scrambled short hairpin RNA control (shControl) vector. B: RT-PCR of cultured primary microglia transduced with shSIRPβ1 or shControl vector. SIRPβ1 gene transcripts were knocked down after transduction with shSIRPβ1, while SIRPβ1 gene transcripts were detected by RT-PCR without any vector (no vector) or after transduction with the shControl vector (shControl). 18S RNA: control for RNA. PCR control: RT-PCR without reverse transcription as control. C: Cultured primary microglial cells were either transduced with the shSIRPβ1 or shControl vector and immunolabeled with rat monoclonal antibodies directed against SIRPβ1. No immunostaining of SIRPβ1 was detected after knockdown of SIRPβ1, while SIRPβ1 was detected on shControl vector-transduced microglia. Scale bar: 20 μm.
Figure 7.
Reduced phagocytosis after lentiviral knockdown of SIRPβ1. A: Microglial cells were transduced with the shControl vector and co-cultured with apoptotic neural membranes labeled with a red fluorescent dye. Confocal images of the shControl vector-transduced microglia showed uptake of the red dye-labeled membranes into a GFP+ microglia. Scale bar: 10 μm. B: Quantification of microglial cells having phagocytosed apoptotic neuronal cell membranes within 24 hours. Microglial cells were lentivirally transduced with shSIRPβ1, shControl, or GFP vector. Number of microglial cells having phagocytosed apoptotic neuronal material was reduced after lentiviral knockdown of SIRPβ1. Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance, followed by Bonferroni’s multiple comparison test. C: Quantification of Aβ42 phagocytosis by primary microglia lentivirally transduced with shSIRPβ1, shControl, or GFP-control vector. Phagocytosis of Aβ42 was reduced after knockdown of SIRPβ1. Data are presented as mean ± SEM of n = 3 independent experiments. ∗P < 0.05, analysis of variance, followed by Bonferroni’s multiple comparison test.
Impaired Uptake of Aβ after Lentiviral Knockdown of SIRPβ1 in Primary Microglia
Phagocytosis of Aβ by microglia or macrophages could facilitate removal of plaque in AD lesions. Internalization of fibrillary Aβ42 by primary microglia was analyzed by fluorescence confocal microscopy. The lentivirally transduced microglial cultures were treated with biotinylated fibrillary Aβ42, fixed, and then incubated with Cy3-conjugated streptavidin to visualize uptake of fibrillary Aβ42 into the cells. Using confocal microscopy, we observed that biotinylated fibrillary Aβ42 peptide internalization in SIRPβ1-silenced microglia was reduced to 25.8 ± 2.8% compared with the ShControl vector (44.2 ± 5.1%) and the GFP control vector (42.2 ± 5.3%) -transduced cells (Figure 7C). Thus, SIRPβ1 was either directly or indirectly involved in the internalization of fibrillary Aβ42.
Discussion
Microglial cells are activated in chronic neurodegenerative diseases including AD.6,7 Activated microglia produce pro-inflammatory mediators and show increased phagocytic activity.1 The microglial clearance function is essential for tissue homeostasis and repair in neurodegenerative diseases.25,26 Interestingly, microglial cells show a dysfunction and defective Aβ clearance capacity in aging APP/PS1 transgenic mice,27 indicating that Aβ plaques partially could result from impaired microglial removal. Several microglial receptors have been implicated to contribute to clearance of Aβ plaque in AD, but it is unclear which receptors solely scavenge Aβ and which induce a microglial phagocytic signaling response.
Recently, it was shown in Drosophila that glial phagocytic activity requires ITAM and Src family kinase signaling.9 The best characterized phagocytic receptors signaling via ITAM are members of the Fc receptor (FcR) family, which recognize the Fc region of IgG bound to antigen presented on microbial pathogens or autoantigens.28,29,30 An ITAM is also present in the intracellular tail of human FcγRIIA (CD32a), and its phosphorylation induces phagocytosis in macrophages. By contrast, FcγRI (CD64) and FcγRIIIA (CD16a) need to bind to the adaptor protein Fcγ subunit, which contains the ITAM, to transmit the phagocytic signaling.30 The phosphorylated ITAM serves as a docking site for the tyrosine kinase Syk. Downstream signaling is mediated via Rho family small GTP-binding proteins then triggers phagocytosis of IgG-coated and opsonized particles or antigens.31 Microglial cells have been shown to express CD16, CD32, and CD64, and to phagocytose antigens via the corresponding IgG subtypes.32 Apart from the FcR, all molecules signaling via the adaptor DAP12 principally could activate an ITAM-src kinase signaling pathway. Expression of TREM2, signaling via the ITAM-containing adaptor protein DAP12, was observed in microglia.10,11,12 TREM2 is a phagocytic receptor with unknown ligand, which is involved in clearance of cellular apoptotic material.11,12,21 Interestingly, loss-of-function mutations of either TREM2 or DAP12 are responsible for a chronic neurodegenerative disease named Nasu-Hakola.33 Patients with mutations of TREM2 show as a major symptom pure early-onset dementia,34 whereas patients with DAP12 mutations have dementia and bone cysts.35
Here, we aimed to identify new microglial receptors that have a phagocytic ITAM signaling capacity and are involved in clearance of Aβ in AD. We demonstrated expression of SIRPβ1 ion microglia. Microglial SIRPβ1 was involved in phagocytic uptake of fibrillary Aβ42 peptide. Increased phagocytosis after stimulation of SIRPβ1 was not specific for Aβ but also detected for neural debris and microsphere beads. Furthermore, we observed that SIRPβ1 expression was increased in APP/J20 transgenic mice and AD patients. Up-regulation of SIRPβ1 expression in APP/J20 transgenic mice was detected in the cerebral hemispheres and cerebellum but not in the spinal cord. Real-time RT-PCR data of the microglial gene transcripts SIRPβ1 and DAP12 demonstrated a disproportional increase of SIRPβ1 (∼1000-fold) compared with DAP12 (∼10-fold) in the cerebral hemispheres and cerebellum of the animal model of AD, indicating that up-regulation of SIPRβ1 does not simply reflect an increase of Iba1-positive microglia. Furthermore, we analyzed amyloid deposition in the cerebellum and spinal cord of 12-month-old APP/J20 mice. No amyloid plaques were detected by immunostaining with antibodies directed against APP in the cerebellum and spinal cord (our unpublished observation). Thus, up-regulation of SIRPβ1 is not directly triggered by the amyloid plaques but by another disease-associated process (eg, increased soluble inflammatory mediators such as IFNs). Indeed, IFNs, including IFN-β and -γ, were observed to up-regulate gene transcription of SIRPβ1 in cultured microglia in our analysis. Previously, IFN-γ has been shown to increase the uptake of Aβ by microglia.36 We have no direct in vivo evidence for a direct pathophysiological relevance of SIRPβ1. We can detect SIRPβ1 also on those microglial cells, which are not directly associated with plaques. But, we can demonstrate that SIRPβ1 is a potent regulator of Aβ42 fibril clearance in vitro. Furthermore, we do not think that up-regulation of SIRPβ1 is specific for AD or APP/J20 transgenic mice, because we also observed a significant, although not so dramatic, increase of SIRPβ1 in mice afflicted by experimental autoimmune encephalomyelitis (our unpublished observation). SIRPβ1 immunostaing was mainly detected on round but not ramified microglia in the tissue of AD patients and APP/J20 trangenic mice, indicating that activated microglia possibly stimulated by IFNs express SIRPβ1. Data on the cellular density of SIRPβ1 expressing cells in APP/J20 transgenic mice and AD patients suggest that resident microglial cells up-regulate SIRPβ1; however, invading monocytes and macrophages could be another principal source of cells increasing SIRPβ1 expression in the brain.
The physiological ligand for SIRPβ1 is still unknown.15 SIRPβ1 was investigated before in peritoneal macrophages.19 It was shown that SIRPβ1 engagement by specific monoclonal antibodies induced phagocytic activity in macrophages, but no ligand was identified.19 Indirect evidence from our knockdown experiments suggests that SIRPβ1 might recognize a cellular component, an aggregated Aβ, and/or a serum component. We have no evidence for a direct binding of aggregated Aβ to SIRPβ1. SIRPα, a homolog of SIRPβ1, binds surfactant protein A and D, both of which are members of the collectin family.15,37 Collectins are soluble pattern recognition molecules, which opsonize particles to increase their phagocytic uptake.37 Interestingly, it has been suggested that aggregated Aβ is also opsonized by complement components, opening the possibility that Aβ uptake is executed by receptors binding complement components or collectins.38
Several other receptors have been reported in vitro to facilitate uptake of Aβ by microglia. Scavenger receptors39 and CD1424 promote uptake of Aβ, but CD14 alone might bind Aβ without inducing an ITAM signaling pathway. Recently, a direct role of Vav and Syk kinases in fibrillary Aβ-stimulated intracellular signaling cascades was established in vitro.40 Vav-deficient microglia showed a dramatically attenuated phagocytic response of fibrillary Aβ.40
Our data also indicate that SIRPβ1 is a receptor capable to trigger a phagocytic ITAM-Syk kinase signaling cascade toward the cytoskeleton. First, we show that SIRPβ1 cross-linking within a few minutes stimulates β-actin reorganization, which is a prerequisite for phagocytosis. Second, stimulation of SIRPβ1 leads to ingestion of microsphere beads dependent on the cytoskeleton and the mitogen-activated protein kinase ERK.
Thus, data indicate that microglia possesses several receptors, which cooperate in phagocytic clearance of Aβ in vitro. Particularly, two distinct types of innate immune receptors are involved in phagocytosis of Aβ, namely scavenger receptors, which bind pathogens or debris, and receptors leading to a full-blown phagocytosis signaling via ITAM-Src-Syk activation and cytoskeleton rearrangement.
Deposits of Aβ are considered as a pathological hallmark of AD and are associated with activated microglia and proinflammatory cytokines.6,41,42 Several molecules have been implicated in Aβ clearance in animal models of AD. Particularly, deficiency of complement 3 led to accelerated Aβ plaque deposition and neurodegeneration in APP transgenic mice.43 Inhibition of complement 3 in a mouse model of AD increased plaque formation and the level of neurodegeneration,44 but signaling via the complement cascade has not been shown to directly induce a phagocytic ITAM signaling. FcRs have been suggested to contribute to Aβ clearance in APP transgenic mice.45 The FcRs and DAP12 show a phagocytic ITAM signaling cascade. It was suggested that antibodies directed against Aβ enter the CNS and could trigger microglial cells to clear plaques through FcR-mediated phagocytosis.45 Immunization of mice with Aβ42, which induced Aβ42-specific antibodies, reduced Aβ deposition regardless of whether the mice were genetically deficient of one of the FcR domains, the FcRγ.46 Thus, FcRγ appears to be irrelevant for antibody-mediated clearance of Aβ deposits in vivo. Recently, it was shown in aged APP23 transgenic mice by combining laser microdissection with microarray analysis and quantitative RT-PCR that TREM2, which signals via DAP12, was up-regulated in amyloid plaque-associated microglia.47 Thus, microglial cells appear to up-regulate several phagocytic receptors at a very late disease stage including TREM2 and SIRPβ1. However, the mechanism about the failure of amyloid plaque removal in vivo is unclear, despite up-regulation of phagocytic receptors by microglia in APP transgenic mice and strong evidence of their functional involvement in Aβ clearance in vitro. Several factors might be responsible for this phagocytic failure in vivo. Level of up-regulation of phagocytic receptors such as SIRPβ1 in the CNS of APP/J20 transgenic mice or AD patients might be too late or insufficient. Alternatively, the putative ligands of the phagocytic receptors are lost or hidden in Aβ plaques. Possibly, microglial molecules signaling via immunoreceptor tyrosine-based inhibitory motif such as sialic acid-binding Ig-like lectins might recognize Aβ plaque-associated sialidated glycolipids, which counteract to ITAM signaling and might be responsible for dampening the phagocytic activity of microglia in vivo.48
In summary, our results identify SIRPβ1 as a DAP12-associated and IFN-inducible phagocytic receptor on microglia. Furthermore, our data demonstrate that microglial SIRPβ1 is up-regulated in AD patients and aged APP/J20 transgenic mice and involved in uptake of Aβ42. Thus, up-regulation of SIRPβ1 at early-stage AD might be a novel target for phagocytic clearance of amyloid plaques.
Acknowledgments
We thank Dr. Lennart Mucke for APP/J20 transgenic mice. We thank Dr. Hiroshi Ohnishi for preparing the rat anti-mouse SIRPβ1 antibodies. We also thank Christine Frank and Jessica Schumacher for excellent technical support of cultures and molecular biology.
Footnotes
Address reprint requests to Harald Neumann, M.D., Neural Regeneration Unit, Institute of Reconstructive Neurobiology, University Bonn, Sigmund-Freud-Str. 25, 53127 Bonn, Germany. E-mail: hneuman1@uni-bonn.de.
Supported by the Deutsche Forschungsgemeinschaft (SFB704, KFO177) and the European Union (LSHM-CT-2005-018637). The Neural Regeneration Group at the University Bonn Life and Brain Center is supported by the Hertie Foundation and Walter und Ilse Rose Foundation.
References
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal “on” and “off” signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Streit JM. Kettenmann H, Ransome B R, editors. Oxford, UK: Oxford University Press,; Microglial cells in Neuroglia second edition, 2005:pp 60–71. [Google Scholar]
- Mrak RE, Griffin WS. Common inflammatory mechanisms in Lewy body disease and Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:683–686. doi: 10.1097/nen.0b013e31812503e1. [DOI] [PubMed] [Google Scholar]
- Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, Stanley ER, Freeman MR. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello E, Cant C, Buhring HJ, Vely F, Andre P, Seiffert M, Ullrich A, Vivier E. Association of signal regulatory proteins β with KARAP/DAP-12. Eur J Immunol. 2000;30:2147–2156. doi: 10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, Kanz L, Ullrich A, Buhring HJ. Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38− hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Cella M, Seiffert M, Buhring HJ, Colonna M. Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J Immunol. 2000;164:9–12. doi: 10.4049/jimmunol.164.1.9. [DOI] [PubMed] [Google Scholar]
- Cant CA, Ullrich A. Signal regulation by family conspiracy. Cell Mol Life Sci. 2001;58:117–124. doi: 10.1007/PL00000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Ohnishi H, Okazawa H, Nakazawa S, Ikeda H, Motegi S, Aoki N, Kimura S, Mikuni M, Matozaki T. Positive regulation of phagocytosis by SIRPβ and its signaling mechanism in macrophages. J Biol Chem. 2004;279:29450–29460. doi: 10.1074/jbc.M400950200. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health-Reagan Consensus Criteria Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132(Pt. 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcγ receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Vedeler C, Antel J, Nyland H, Mork S, Matre R. Reactive microglia in multiple sclerosis lesions have an increased expression of receptors for the Fc part of IgG. J Neurol Sci. 1994;121:125–131. doi: 10.1016/0022-510x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Chouery E, Delague V, Bergougnoux A, Koussa S, Serre JL, Megarbane A. Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat. 2008;29:E194–E204. doi: 10.1002/humu.20836. [DOI] [PubMed] [Google Scholar]
- Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, Fisher Y, Owens T, Weiner HL. Aβ-induced meningoencephalitis is IFN-γ-dependent and is associated with T cell-dependent clearance of Aβ in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- Wojtera M, Sikorska B, Sobow T, Liberski PP. Microglial cells in neurodegenerative disorders. Folia Neuropathol. 2005;43:311–321. [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar β-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid-β plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid-β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ−/− knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T. TREM2 is up-regulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer’s disease. J Mol Med. 2009;87:697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]