Abstract
We have identified the nonreceptor tyrosine kinase syk as a marker of differentiation/tumor suppressor in pancreatic ductal adenocarcinoma (PDAC). Syk expression is lost in poorly differentiated PDAC cells in vitro and in situ, and stable reexpression of syk in endogenously syk-negative Panc1 (Panc1/syk) cells retarded their growth in vitro and in vivo and reduced anchorage-independent growth in vitro. Panc1/syk cells exhibited a more differentiated morphology and down-regulated cyclin D1, akt, and CD171, which are overexpressed by Panc1 cells. Loss of PDAC syk expression in culture is due to promoter methylation, and reversal of promoter methylation caused reexpression of syk and concomitant down-regulation of CD171. Moreover, suppression of syk expression in BxPC3 cells caused de novo CD171 expression, consistent with the reciprocal expression of syk and CD171 we observe in situ. Importantly, Panc1/syk cells demonstrated dramatically reduced invasion in vitro. Affymetrix analysis identified statistically significant regulation of >2000 gene products by syk in Panc1 cells. Of these, matrix metalloproteinase-2 (MMP2) and tissue inhibitor of metalloproteinase-2 were down-regulated, suggesting that the MMP2 axis might mediate Panc1/mock invasion. Accordingly, MMP2 inhibition suppressed the in vitro invasion of Panc1/mock cells without effect on Panc1/syk cells. This study demonstrates a prominent role for syk in regulating the differentiation state and invasive phenotype of PDAC cells.
Pancreatic ductal adenocarcinoma (PDAC) has one of the highest mortality rates of all cancers.1 Despite this, the biology of PDAC remains poorly understood. Studies have identified key factors in the etiology of the disease, which have been incorporated into a genetic model of PDAC development.2 Although such a timeline is significant in the definition of factors contributing to disease onset, a similar timeline has been difficult to address with regard to disease progression.
Syk is a nonreceptor tyrosine kinase central to the signaling of many hematopoietic cell types.3 Syk has also been implicated in the signaling processes of nonhematopoietic cell types,4 and syk has further been identified as a putative breast cancer (BC) suppressor in humans, based in part on the reduced expression of syk in a progression-related manner and on the fact that ectopic expression of syk in syk-negative BC cells retarded their growth in vivo, whereas suppression of endogenous syk activity reciprocally enhanced BC tumorigenicity.5 More recent studies have shown that syk is a negative regulator of BC mitosis,6 transcription,7 motility,8,9 and invasion,10 as well as anchorage-independent growth and tumorigenicity of melanoma cells.11 In patients, loss of syk correlates with poor survival and tumor metastasis in breast,12,13,14 bladder,15 liver,16 and gastrointestinal tract tumors.17,18,19 Together these data clearly implicate syk as a potential tumor suppressor in epithelial tissues. However, it is important to note that higher levels of syk expression were observed in squamous cell carcinomas of the head and neck and their lymph node metastases than normal tissue (high syk expression correlated significantly with poor survival), and further that syk promoted migration and invasion of squamous cell carcinoma of the head and neck cells in vitro.20 Indeed, the inappropriate activation of syk in breast epithelial cells contributes to cellular transformation,21 enhanced nuclear factor-κB activation, and resistance to tumor necrosis factor-induced apoptosis in mouse mammary tumor virus-mediated tumorigenesis.22 These data highlight the complex nature of syk activity in regulating processes associated with tumorigenesis, even within the same tissue (ie, breast epithelium), and thus it is not a foregone conclusion that syk will function as a tumor suppressor in all epithelial cells. In this study, we demonstrate the expression of syk in ductal epithelial cells of the normal pancreas, and the loss of syk during PDAC “dedifferentiation.” Our data demonstrate that syk regulates gene expression and a myriad of phenotypic parameters responsible for maintaining a more differentiated epithelial state, demonstrating for the first time a role for syk in regulating the phenotype of PDAC cells.
Materials and Methods
Cell Lines
AsPC1, CAPAN1, CAPAN2, CFPAC1, HPAFII, SU.86.86, BxPC3, MIAPaCa2, and Panc1 cells were originally from the American Type Culture Collection (Manassas, VA) and were cultured as recommended by the American Type Culture Collection. Leukemic HL60 cells were a generous gift of A. Raz (Detroit, MI), and cultured according to the American Type Culture Collection. COLO357 cells were generously provided by M. Korc (Irvine, CA) and cultured in Dulbecco’s modified Eagle’s medium/10% fetal bovine serum (FBS). Serum-free medium consisted of all media components except serum, as appropriate for the cell line, supplemented with 0.5% bovine serum albumin.
Antibodies and Reagents
Anti-syk-LR polyclonal antibody (pAb), 4D10 monoclonal antibody (mAb), and anti-extracellular-regulated kinase-2 (Erk2) pAb (C14) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin was from Sigma-Aldrich (St. Louis, MO). Anti-pan-Akt pAb was from Cell Signaling Technologies (Beverly, MA). Anti-CD171 mAb UJ127 was from Neomarkers/LabVision (Fremont, CA). Anti-cyclin D1 was a kind gift from M. Just (eBioscience, San Diego, CA). pEGFP-N1 and anti-green fluorescent protein (GFP) pAb were from BD Clontech (Palo Alto, CA). 5-Aza-2′-deoxycytidine (5AzaC) was from Invivogen (San Diego, CA). N-(R)-[2-(Hydroxyaminocarbonyl)methyl]-4-methylpentanoyl-l-naphthylalanyl-l-alanine, 2-aminoethyl amide (TAPI1) was from EMD (San Diego, CA). Tissue inhibitor of metalloproteinase-2 (TIMP2) was from Novus (Littleton, CO).
Expression Constructs and Transfections
pEMCV/syk23 was a generous gift of S. Shattil (University of California, San Diego [UCSD], La Jolla, CA). The wild-type human pp72syk CDS encoding sykA was excised from pEMCV/syk and ligated into a pCDNA3.1(zeo) vector into which an IRES sequence had been introduced 5′ to a hygromycin phosphotransferase gene. Panc1 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), hygromycin-selected, and assessed for protein expression. For small interfering RNA (siRNA) studies, a sense and antisense-containing 60 mer of the syk-6 siRNA sequence24 was inserted into pSuper.25 BxPC3 cells (4 × 106) were pulsed (270 V, 550 μF) with a Bio-Rad GenePulser II in basal media/10% glucose containing 20 μg of DNA (15 μg of syk-6/pSuper and 5 μg of pEGFP) and replated in full growth medium.
Immunoassays
Tissue Samples
Samples (n = 92) were obtained under the institutional review board protocol from the UCSD Department of Pathology archives. Normal tissue was from patients who died of nonpancreatic disease or trauma and are not included in the survival analysis. Patient demographics (including sex, age, and race) and tissue characterization (including tumor size, differentiation, node status, margin involvement, and perineural or vascular invasion status) were described in detail previously.26,27 Tissue differentiation grade was categorized as the highest grade present (ie, a patient whose tumor contained elements of G2 and G3 was classified as G3).
Immunohistochemistry
Samples were deparaffinized, rehydrated, and incubated with 1% H2O2. Slides were blocked with 2% horse serum/5% bovine serum albumin/PBS, pH 7.4, and renatured using DAKO Target Retrieval Solution (UJ127) or DAKO High-pH Target Retrieval Solution (4D10), before incubation with 0.5 to 2.0 μg/ml 4D10 or UJ127. Slides were washed and biotinylated-anti-mouse was applied according to the VectaStain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Sections were developed with diaminobenzidine, counterstained with hematoxylin, dehydrated, and mounted.
Immunoprecipitation
Lysates (250 μg) were incubated overnight at 4°C with 20 μl of anti-syk LR-AC pAb (agarose conjugate). Beads were washed with lysis buffer and prepared for immunoblotting.
Immunoblotting
Samples were prepared and analyzed as described previously.28 For cyclin D1, cells were harvested at subconfluence.
Cell Growth Assays
In Vitro Growth Rate
Cells (5 × 102/well) were seeded into a 48-well plate. After 24 hours (and every 72 hours thereafter), fresh growth medium was replaced, and the initial time point was fixed with 1% paraformaldehyde/PBS, pH 7.4. Additional triplicate wells were fixed at 24-hour intervals, stained with 1% crystal violet, and compared with a standard curve of cells. Dye was extracted with 10% acetic acid and quantitated at 550 nm.
Anchorage-Independent Growth Assay
A top layer containing 5 × 103 cells in 0.5% agar/Dulbecco’s modified Eagle’s medium/10% FBS was seeded onto a base layer of 0.7% agar/Dulbecco’s modified Eagle’s medium containing 10% FBS in a six-well plate. Cultures were incubated at 37°C, medium was replaced every third day, and the assay was stopped on day 10. Cultures were stained with 0.01% crystal violet. Colonies were enumerated on a Bio-Rad GelDoc XR system using QuantityOne Software (sensitivity = 8.1, average = 5).
Subcutaneous Tumor Growth
A total of 107 cells were injected into the flanks of 6-week old nu−/nu− mice, and tumors were grown for 4 weeks, at which time they were harvested, fixed in formalin, and weighed wet.
Invasion Assay
Subconfluent cells (2.5 × 105) were seeded in serum-free media into BioCoat Growth Factor-Reduced Matrigel Invasion Chambers (BD Biosciences, Bedford, MA) in wells containing serum-free media or media containing 10% FBS. Chambers were incubated at 37°C for 24 hours before fixing, staining with 1% toluidine blue, removal of uninvaded cells, and manual enumeration. TAPI1 (40 μmol/L) or TIMP2 (8 nmol/L) assays involved preincubating cells for 15 minutes before seeding into inserts seated in wells with equal concentrations of TAPI1 (or dimethyl sulfoxide control) or TIMP2 in Dulbecco’s modified Eagle’s medium/10% FBS.
Syk Promoter Methylation Analysis
DNA Methyltransferase Inhibitor Treatment
Cells were plated in serial twofold dilutions to achieve similar densities at harvest, beginning with 1.5 × 106 cells/well, and allowed to adhere for 24 hours before growth medium was replaced, and cells were treated daily with dimethyl sulfoxide or 2 μmol/L 5AzaC in full growth medium. DNA, RNA, and protein were collected at the indicated times. For the density analysis, cells were plated at 2 × 105 or 4 × 104 cells/well (to achieve maximal density for “confluent” and subconfluence for “subconfluent” cultures at 120 hours) and daily received basal media containing 10, 3, or 1% FBS with dimethyl sulfoxide or 2 μmol/L 5AzaC.
Methylation-Specific PCR
Methylation-specific PCR was performed as described by Yuan et al.16 Genomic DNA was modified using the MethylDetector Bisulfite Kit (Active Motif, Carlsbad, CA).
RT-PCR
cDNA was synthesized from 1 μg of total RNA using oligo(dT) primer. PCR was performed on 1 μl of total cDNA using the primers described in Table 1.16,29,30,31
Table 1.
PCR Primers and Sources
| Gene | Forward primer Reverse primer | Source |
|---|---|---|
| β-Actin | 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ | Stratagene, La Jolla, CA |
| 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ | ||
| GAPDH | 5′-CCACCCATGGCAAATTCCATGGCA-3′ | Stratagene, La Jolla, CA |
| 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ | ||
| MMP2 | 5′-GCTGGCTGCCTTAGAACCTTTC-3′ | Hegedus̈ et al29 |
| 5′-GAACCATCACTATGTGGGCTGAGA-3′ | ||
| MMP9 | 5′-GCACGACGTCTTCCAGTACC-3′ | Hegedus̈ et al29 |
| 5′-GCACTGCAGGATGTCATAGGT-3′ | ||
| SYK | 5′-AAAGAAGTTCGACACGCTCTGG-3′ (F20) | Yagi et al30 |
| 5′-GCAAGTTCTGGCTCATACGGA-3′ (R19) | ||
| External SYK MS-PCR | 5′-TTTAGGGAATATGTTATGTAGTG-3′ | Yuan et al16 |
| 5′-CACATAATTTCAACACTTTTACC-3′ | ||
| Internal SYK MS-PCR methylated | 5′-CGATTTCGCGGGTTTCGTTC-3′ | Yuan et al16 |
| 5′-AAAACGAACGCAACGCGAAAC-3′ | ||
| Internal SYK MS-PCR unmethylated | 5′-ATTTTGTGGGTTTTGTTTGGTG-3′ | Yuan et al16 |
| 5′-ACTTCCTTAACACACCCAAAC-3′ | ||
| TIMP1 | 5′-CCTTCTGCAATTCCGACCTCGTC-3′ | Christopoulos et al31 |
| 5′-CGGGCAGGATTCAGGCTATCTGG-3′ | ||
| TIMP2 | 5′-TGGAAACGACATTTATGGCAACC-3′ | Christopoulos et al31 |
| 5′-ACAGGAGCCGTCACTTCTCTTGAT-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SYK, spleen tyrosine kinase; MS, methylation-specific.
Affymetrix Gene Array Analysis
Analysis was performed as described previously.32
Sample Collection and Preparation of Labeled RNA
Total RNA was prepared for hybridization according to the GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). RNA was extracted (RNeasy, Qiagen, Valencia, CA) and quality-assessed on a bioanalyzer (RNA 6000 Nano Chip, Agilent Technologies, Palo Alto, CA), and 5 μg was used as a template for cDNA synthesis (SuperScript, Invitrogen Life Technologies, Gaithersburg, MD). First-strand synthesis was primed with a T7-(dT) 24-oligonucleotide primer containing a T7 RNA polymerase promoter sequence on the 5′ end. Second-strand products were cleaned (GeneChip Sample Cleanup Module, Affymetrix) and in vitro-transcripted with biotin-labeled nucleotides (Bioarray Labeling Kit, Enzo Diagnostics, Farmingdale, NY). cRNA product was cleaned and 20 μg was heated at 94°C for 35 minutes in fragmentation buffer (Affymetrix).
Microarray Hybridization
Adjusted cRNA (15 μg) was hybridized for 16 hours at 45°C to an Affymetrix U133 Plus 2.0 GeneChip (47,000 transcripts). Each array was then stained with a streptavidin-phycoerythrin conjugate (Molecular Probes/Invitrogen), washed, and visualized with a microarray scanner (Genearray Scanner, Agilent Technologies, Santa Clara, CA). Images were inspected visually for hybridization artifacts. In addition, quality assessment metrics were generated for each scanned image and evaluated based on empirical data from previous hybridizations and the signal intensity of internal standards.
Generation of Expression Values
Microarray Suite (version 5, Affymetrix), was used to generate *.cel files, and Probe Profiler (version 1.3.11, Corimbia Inc., Berkeley, CA), developed specifically for the GeneChip system, was used to convert intensity data into quantitative estimates of gene expression. A probability statistic was generated for each probe set (gene) with the null hypothesis being that the expression level is equal to zero (background). Genes not significantly expressed above background in at least two samples (P < 0.05) were considered absent.
Gene Expression Data Analysis
Statistical tests were performed using BioConductor statistical software.33 The raw data were normalized by the Robust Multichip Analysis approach implemented in the Affy package.34 The fold change was computed based on the normalized data. A significant P value was computed by a statistical test based on a probe level analysis using the affyPLM package.35 P values were further adjusted using the Benjamini and Hochberg method.36 Genes with P < 0.05 were considered as differentially expressed genes at a statistically significant level.
Gene Ontology and Pathway Analysis
Gene Ontology annotations were obtained from Affymetrix. Biological network relationships among significantly regulated genes were explored using KEGG and GenMapp pathways using AnalyzeIt Tools.
Zymography
Panc1/mock and Panc1/syk cells were plated at equal densities, grown 3 days, and serum-starved (24 hours), and supernatant was collected. Equal amounts of clarified supernatants and serum-free media (control) were processed using gelatin-embedded SDS-polyacrylamide gel electrophoresis gels as described previously.37
Image Acquisition and Manipulation
Images of ethidium bromide-stained agarose gels were captured with Quantity One software on a Bio-Rad Gel Doc XR using the appropriate filter and transmitted UV light. Chemiluminescence-exposed films and printouts of agarose gels were scanned on an Epson Perfection 4490 Photo flatbed scanner. Images were imported into Adobe Photoshop for removal of unused levels and cropping. Minimal alterations to brightness and contrast were used for a subset of images to improve the visual nature of the image. Nonlinear adjustments were not used. Immunohistochemical images were acquired as 24-bit RGB (.tif) and phase-contrast images as 8-bit gray scale (.tif) using SpotBasic with Nikon TE2000-S or TE300 microscopes, respectively, each fitted with a model 3.2.0 charge-coupled device camera and used at the Moores UCSD Cancer Center Microscopy Shared Resource. Final images were compiled in Adobe InDesign, rasterized, and converted to jpeg format at a minimum of 300 dpi.
Statistics
Survival was evaluated according to the Kaplan-Meier method using a univariate log-rank test. Variables were coded as 1 for 100% syk-positive tumors and 0 for those that evinced heterogeneity. Colony-forming and invasion were analyzed by a two-tailed Student’s t test. Tumor growth was compared using the Mann-Whitney U test.
Results
Syk Expression Correlates with PDAC Grade in Situ
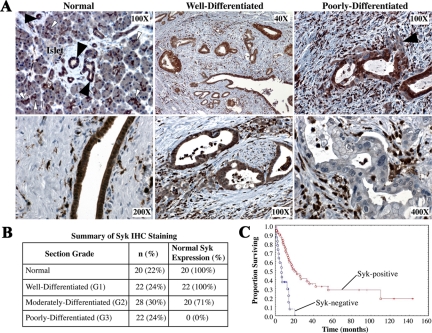
A panel of tissue samples was analyzed by immunohistochemistry with the anti-syk mAb 4D10. Uniform syk expression was observed in both the cytoplasm and nucleus of cells of normal ducts and ductules adjacent to acini (Figure 1A).26,27 Well differentiated malignant ducts also demonstrated strong uniform syk expression; however, a progressive loss of syk was noted in more pleomorphic, moderately differentiated PDAC cells. Poorly differentiated cells exhibiting distinct nuclear atypia and dramatic cellular pleomorphism were essentially devoid of syk expression. Overall, 100% of normal ducts and G1 tumors demonstrated homogeneous syk expression, whereas 71% of G2 tumors and no G3/sarcomatoid tumors retained normal syk expression (n = 92) (Figure 1B). Kaplan-Meier analysis demonstrated that syk correlates positively with overall patient survival (P < 0.001). Median survivals were 18.8 and 6.4 months for syk-positive and syk-negative patients, respectively (Figure 1C).
Figure 1.
Syk expression correlates with PDAC grade in situ. Patient samples were obtained from the UCSD Department of Pathology archives. Patient demographics and tissue characteristics were described in detail previously.26,27 A: Tissue samples of varying PDAC grade were stained with anti-syk mAb 4D10, which recognizes both isoforms of syk but not Zap70 or src family members and is not affected by tyrosine phosphorylation of syk. Antibody binding was visualized with diaminobenzidine (brown). Top left: Solid arrowheads denote examples of discrete ducts. Open arrowheads denote examples of acinus-associated ductules. Top Right: Solid arrowhead denotes a poorly differentiated group of syk-negative cells invading away from syk-positive cells that maintain ductal morphology. Very strongly syk-positive stromal cells are lymphocytes. B: Summary of tissue samples by grade. Table shows the number of normal tissue samples and PDAC samples of each grade, as well as the corresponding syk expression within each group. Tissue differentiation grade was categorized as the highest grade present (ie, a patient whose tumor contained elements of G2 and G3 was classified as G3, and so on). IHC, immunohistochemical. C: Kaplan-Meier survival curve of syk-positive versus syk-negative patient samples using the log-rank test. Variables were coded as 1 for 100% syk-positive tumors and 0 for those that evinced heterogeneity. Median survivals were 18.8 and 6.4 months for syk-positive and syk-negative samples, respectively.
Syk Expression Correlates with PDAC Grade in Vitro
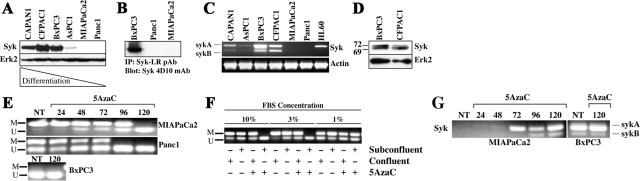
To assess the generality of our in situ findings, immunoblotting was performed on PDAC lines described in detail by Sipos et al,38 who examined ultrastructural, immunochemical, and growth/morphology characteristics, thus providing a classification of differentiation of the cell line without reference to the classification of the tumor of origin (Supplemental Table S1, see http://ajp.amjpathol.org).38 More differentiated CAPAN1 (G1), CFPAC1 (G2), BxPC3 (G2), and AsPC1 (G2) PDAC lines express significant quantities of syk, whereas the less differentiated MIAPaCa2 (G3) and Panc1 (G3) cell lines show no evidence of syk expression by immunoblotting (Figure 2A). In addition, well differentiated CAPAN2 (G1) and moderately differentiated HPAFII (G2), SU.86.86 (G2), and COLO357 (G2) cell lines proved positive for syk (not shown), further confirming the relationship between syk expression and PDAC differentiation. To rule out the possibility that Panc1 and MIAPaCa2 cells express syk at levels below the threshold of detection for immunoblotting, immunoprecipitation/immunoblotting was performed from these cells and syk-positive BxPC3 cells. Syk protein was not detectable in either Panc1 or MIAPaCa2 immunoprecipitates (Figure 2B), suggesting that they may be devoid of syk protein expression altogether.
Figure 2.
Syk expression is regulated by promoter methylation in vitro. A: Immunoblot analysis of syk expression in PDAC cell lines using the anti-syk mAb 4D10, which recognizes both isoforms of syk but not Zap70 or src family members and is not affected by tyrosine phosphorylation of syk. Erk2, control. B: Syk was immunoprecipitated from equal quantities of cell lysate with the anti-syk LR pAb, and samples were immunoblotted with the anti-syk mAb 4D10. C: RT-PCR was performed on PDAC cell lines using syk primers that amplify across the alternatively spliced sequence. HL60 cells, expression control. Actin, control. D: Extended SDS-polyacrylamide gel electrophoresis and immunoblotting of the BxPC3 and CFPAC1 lysates with anti-syk mAb 4D10 to detect syk isoforms. Erk2, control. Relative migrations of sykA (72) and sykB (69) are denoted in kDa. E: Syk-negative MIAPaCa2 and Panc1 cell lines were treated with 2 μmol/L 5AzaC for the indicated times (hours), and genomic DNA was analyzed by methylation-specific PCR with syk promoter-specific primers. BxPC3 cells, syk-expressing control. M, methylated; U, unmethylated; NT, no treatment. Part of a methylated product band in the adjacent right lane is shown to demonstrate where methylated product would be in the BxPC3 panel. F: Panc1 cells were plated to achieve confluent or subconfluent density as described in Materials and Methods and then were treated for 120 hours with 5AzaC in media containing the indicated amounts of FBS before syk promoter methylation-specific-PCR analysis. M, methylated; U, unmethylated. G: MIAPaCa2 or BxPC3 cells were treated with 5AzaC for the indicated times (hours), and then RNA was harvested and analyzed by RT-PCR with syk primers. NT, no treatment. Images are shown to achieve maximal exposure of negative lanes/bands.
Syk is expressed as two isoforms, a full-length (sykA) and a shorter form (sykB),30 which results from splicing a 69-bp exon out of the linker that connects the tandem N-terminal SH2 domains with the C-terminal kinase domain.30 Contrary to hematopoietic cells (eg, HL60), both isoforms were highly represented in syk-positive PDAC lines (Figure 2C). Importantly, MIAPaCa2 and Panc1 cells, which demonstrated no detectable syk protein, are also devoid of detectable syk mRNA. Using extended SDS-polyacrylamide gel electrophoresis/immunoblotting, we confirmed the expression of sykB at the protein level in BxPC3 and CFPAC1 cells (Figure 2D). Expression of sykB is potentially significant because it is normally quite uncommon, representing <10% of the transcript in hematopoietic samples examined39; however, the BC suppressor function of syk has been ascribed solely to sykA.10 Finally, sequencing of cDNAs from CAPAN2, BxPC3, and COLO357 cells found no coding mutations in the syk sequence (not shown). This result suggests that the intrinsic activities of syk are intact in these cells and that the loss of syk expression, not mutation, is probably responsible for alterations to this pathway during PDAC progression.
Promoter Methylation Regulates Syk Expression in PDAC Cells
Syk-negative MIAPaCa2 and Panc1 cells lack detectable syk mRNA, suggesting that the control of syk expression in these cells is transcriptional and potentially due to promoter hypermethylation as described in other systems.11,15,16,17,19,40,41 To test this possibility, syk promoter methylation-specific PCR was performed on bisulfite-converted genomic DNA from 5AzaC-treated samples of MIAPaCa2, Panc1, and BxPC3 cells. MIAPaCa2 cells exhibit a fully methylated syk promoter that becomes completely unmethylated after 120 hours of 5AzaC treatment, whereas Panc1 cells are hemimethylated and become fully unmethylated after 96 hours (Figure 2E). The BxPC3 syk promoter is unmethylated irrespective of treatment. Significantly, in Panc1 cells plated at different densities in media containing 10, 3, or 1% FBS, 5AzaC treatment inhibited syk promoter methylation only in subconfluent cultures at 10 and 3% FBS (Figure 2F). As expected syk transcript levels reflected promoter methylation status (Figure 2G), increasing in abundance as well as processing throughout the 5AzaC time course.
Syk Exhibits the Hallmarks of a Tumor Suppressor in PDAC
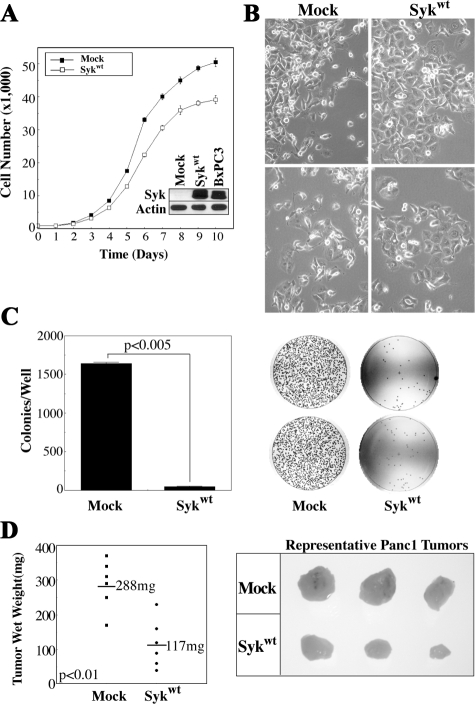
Panc1 cells stably reexpressing wild-type sykA (Panc1/syk) demonstrate syk levels comparable with endogenous levels in BxPC3 cells (Figure 3A, inset) and display a reduced growth rate in vitro and a lower density at confluence (Figure 3A); these results probably reflect the fact that Panc1/syk cells no longer pile up significantly after reaching 100% confluence, but rather demonstrate increased cell-cell interactions, resulting in more of a traditional epithelial monolayer in culture (Figure 3B). These data are consistent with those of Zhang et al,9 who demonstrated that syk promotes the formation of cell-cell contacts in BC cells. Importantly, Panc1/syk cells demonstrate a dramatically reduced ability to grow in an anchorage-independent assay, exhibiting a plating efficiency of 1%, versus 33% for Panc1/mock cells (Figure 3C). Subcutaneous growth of Panc1/syk cells was also dramatically reduced (mean tumor weight 117 mg) in comparison with Panc1/mock cells (mean weight 288 mg at the time of sacrifice, P < 0.01) (Figure 3D).
Figure 3.
Syk exhibits the hallmarks of a tumor suppressor in PDAC. Panc1 cells were stably transfected with wild-type sykA (Sykwt) or empty vector (mock). A: Growth of Panc1/mock and Panc1/syk cells was measured daily after initial plating of equal numbers of cells (5 × 102). Triplicate wells for each time point were stained with 1% crystal violet and quantitated at 550 nm after dye extraction. Inset: Expression of syk was assessed by immunoblot with the anti-syk mAb 4D10 in comparison with endogenously syk-positive BxPC3 cells. Actin, control. B: In vitro morphology of Panc1/mock and Panc1/syk cells at different densities. Note the more pronounced cell-cell contacts and lack of piling up of Panc1/syk cells, irrespective of density, in comparison with Panc1/mock cells. C: Anchorage-independent growth was determined as colonies present after 10 days of growth from 5 × 104 single cells in soft agar. Images of plates are shown with quantitation points superimposed. D: Tumor growth was assessed subcutaneously after injection of 107 cells into the flanks of nude mice. After 4 weeks, tumors were harvested, fixed in formalin, and weighed wet.
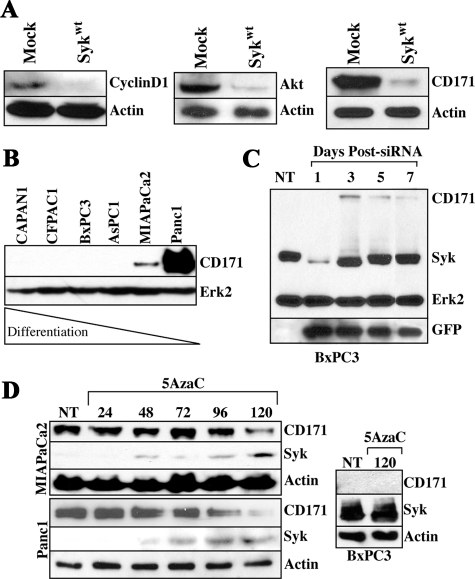
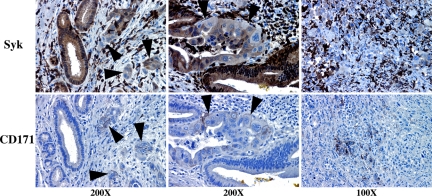
Overall, our data suggest that syk promotes a more differentiated phenotype in PDAC cells. Because Panc1 cells overexpress the β-catenin-regulated gene products akt and cyclin D1, which have been associated with tumor progression,42,43 we assessed the effect of stable syk reexpression on these molecules and also the β-catenin-regulated gene product CD171, which is expressed by poorly differentiated colorectal cancer cells44 and is highly expressed by Panc1 cells (Figure 4A). Panc1/syk cells indeed demonstrated dramatic down-regulation of cyclin D1, akt, and CD171 in comparison with Panc1/mock cells (Figure 4A). Moreover, we observed reciprocal expression of syk and CD171 in PDAC cell lines, whereby CD171 is expressed by poorly differentiated, syk-negative MIAPaCa2 and Panc1 cells but was absent from more differentiated, syk-positive cell lines (Figure 4B). Because CD171 is known to drive an invasive phenotype in other cells,28,45 syk-dependent regulation of CD171 might be a significant phenotypic event during PDAC progression. We therefore questioned whether repression of endogenous syk would be sufficient to drive CD171 expression. Transient suppression of syk with siRNA indeed results in de novo CD171 expression coincident with syk down-regulation in BxPC3 cells (Figure 4C). It should be noted that low-level expression of CD171 was present concomitant with the lowest syk expression on day1; however, this band cannot be seen in the exposure chosen to maximally demonstrate the contrasts in band intensities. Reciprocally, CD171 protein levels drop significantly concurrent with syk protein up-regulation by promoter demethylation with 5AzaC in MIAPaCa2 and Panc1 cells (Figure 4D), further supporting a regulation of CD171 expression by syk. Interestingly, in line with these in vitro findings, syk and CD171 are also inversely expressed in clinical samples (Figure 5). Both sykA and sykB are targeted by this siRNA and, as seen in Figure 4C, only residual sykB is present 24 hours after siRNA introduction. Both isoforms are evident again by 72 hours posttreatment.
Figure 4.
Syk regulates the expression of overexpressed proteins akt, cyclin D1, and CD171 in Panc1 cells. A: Immunoblot analysis of stable syk-reexpressing (Sykwt) or mock-transfected Panc1 cells with anti-cyclinD1, anti-pan-akt, and the anti-CD171 mAb UJ127. Actin, control. B: Immunoblot analysis of CD171 expression in PDAC cells. The membrane shown in Figure 2A was reprobed with the UJ127 anti-CD171 mAb. Erk2, control. C: BxPC3 cells were transiently transfected with pSUPER/syk6-siRNA and pEGFP as a reporter, and lysates were harvested on the indicated days for analysis of CD171, syk, and GFP expression by immunoblotting. Erk2, control. NT, no treatment. D: MIAPaCa2, Panc1, or BxPC3 cells were treated with 2 μmol/L 5AzaC for the indicated times (hours) and then lysates were harvested and immunoblotted with the anti-syk mAb 4D10 and the anti-CD171 mAb UJ127. Actin, control. NT, no treatment.
Figure 5.
Syk and CD171 demonstrate reciprocal expression in situ. Patient samples were obtained from the UCSD Department of Pathology archives as described in the text and legend to Figure 1. Serial sections were stained with either the anti-syk mAb 4D10 (top panels) or the anti-CD171 mAb UJ127 (bottom panels). Twenty-two patients (of 92 total) demonstrated CD171 positivity; in all cases CD171-positive cells demonstrated syk-negative nuclei and dramatically reduced or completely absent cytoplasmic syk expression. Arrowheads denote syk−/CD171+ poorly differentiated malignant cells adjacent to syk+/CD171− well differentiated, normal or reactive ducts in the left and center panels. Highly anaplastic cells are essentially devoid of syk expression but strongly express CD171 in many cases (right panels). Very strongly syk-positive stromal cells are tumor-infiltrating lymphocytes.
Matrix Metalloproteinase-2 Protease Axis Mediates Panc1 Invasion
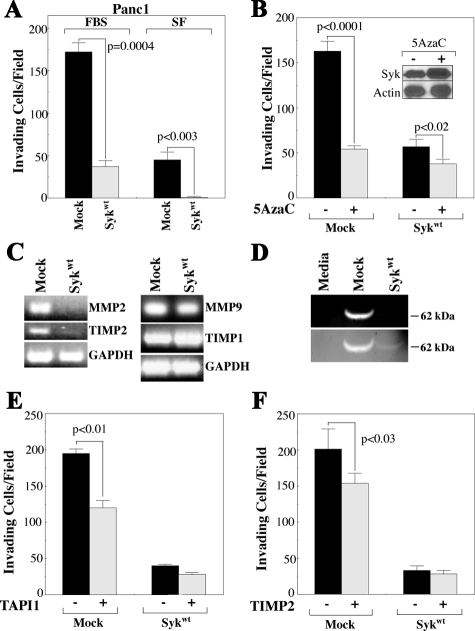
Syk inhibits BC invasion in vitro10; therefore, we questioned whether stable reexpression of syk in Panc1 cells would affect their in vitro invasion. Panc1/syk cells exhibited 78% less invasion toward FBS-containing media compared with Panc1/mock cells and virtually no invasion in the absence of FBS in the lower chamber (Figure 6A). In addition, Panc1/mock cells induced to reexpress syk by inhibition of DNA methylation with 5AzaC demonstrated dramatically reduced in vitro invasion (Figure 6B). Although 5AzaC-treated Panc1/syk cells also show a moderate reduction in invasion, this is not unexpected because they demonstrate increased syk protein, probably because of reexpression of endogenous syk (Figure 6B, inset).
Figure 6.
Syk mediates Panc1 invasion via regulation of the MMP2 axis. A and B: Cells from culture were seeded into the top chamber of BioCoat Growth Factor-Reduced Matrigel Invasion Chambers in serum-free medium and allowed to invade for 24 hours. A: Cells were provided FBS-containing (FBS) or serum-free (SF) medium in the lower chamber. B: Cells were pretreated for 120 hours with 5AzaC (or dimethyl sulfoxide) before seeding into the upper invasion chambers with FBS-containing media in the lower chamber. Inset: Immunoblot of syk expression in Panc1/syk cells at the time of application to invasion chambers. Leftover 5AzaC-treated cells were pelleted, lysed, and immunoblotted with the anti-syk mAb 4D10. Actin, control. C: RT-PCR was performed on RNA from Panc1/mock and Panc1/syk cells in normal culture using primers specific for MMP2 and its cognate partner TIMP2 or MMP9 and its cognate partner TIMP1. GAPDH control. D: Gelatin zymography was performed on equal volumes of serum-free media (media) or serum-free media conditioned by Panc1/mock or Panc1/syk cells. The relative migration of the active MMP2 species is indicated on the right. Lower panel: lighter zymogram image demonstrates the small amount of active MMP2 species in Panc1/syk conditioned media. E and F: Panc1/mock or Panc1/syk cells were pretreated for 15 minutes with 40 μmol/L TAPI1 (E) or 8 nmol/L TIMP2 (F) and then seeded into invasion chambers. Upper and lower chambers contained TAPI1 or TIMP2 for the 24-hour duration of the assay.
An Affymetrix gene array study was performed to determine pathways regulated by syk in Panc1 cells (Supplemental Table S2, see http://ajp.amjpathol.org; the full set of microarray data are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=fdaxlouissuiuva&acc=GSE18000; Gene Expression Omnibus accession number: GSE18000).
These data demonstrated a significant down-regulation of matrix metalloproteinase (MMP)-2 and its cognate partner TIMP2, which is required for MMP2 activation.46 MMP2 mediates Panc1 invasion in response to epidermal growth factor,47 suggesting that the MMP2 axis might be at least partly responsible for the high basal invasion we observed in Panc1/mock cells. RT-PCR verified the Affymetrix results of MMP2 and TIMP2 expression and further demonstrated a minimal effect of stable syk expression on the mRNA levels of the other gelatinase MMP9 and its cognate partner TIMP1 (Figure 6C). In addition, zymographic analysis demonstrated significant levels of active MMP2 in Panc1/mock but not Panc1/syk conditioned media (Figure 6D). Demonstrating the MMP dependence of Panc1/mock invasion, the MMP inhibitor TAPI1 suppressed Panc1/mock invasion by 39% (P < 0.01), without significant effect on the invasion of Panc1/syk cells (P = 0.18) (Figure 6E). Specifically implicating MMP2 as a target of TAPI1, exogenous TIMP2 reduced the invasion of Panc1/mock cells by 23% (P < 0.03), again without significant effect on Panc1/syk cells (P = 0.44) (Figure 6F). The lesser effect of TIMP2 versus TAPI in this system may represent the involvement of another MMP whose expression is not regulated at the RNA level and thus would not be identified by the Affymetrix data. In addition, MMP blockade did not suppress Panc1/mock invasion to the level of Panc1/syk cells; therefore, other syk-mediated events are probably involved in regulating the invasion of these cells.
Discussion
Syk is a putative tumor suppressor in the ductal epithelium of the breast.5 However, unlike the all-or-nothing pattern we observe in PDAC, Toyama et al12 reported 3 of 13 G1 breast tumors as having reduced syk expression and 13 of 18 G3 tumors as expressing normal levels of syk. These findings suggest potentially dramatic differences in the regulation and activity of syk in these different types of glandular epithelium. Moreover, the significance of our PDAC findings is highlighted by the fact that histological grade was the only statistically meaningful multivariate predictor of periampullary tumor patient survival at our cancer center.26 Although many factors independently correlated with survival, multivariate analyses demonstrated that grade was the only statistically significant predictor regardless of which other variables were compared. Importantly, the survival curves of patients with well and moderately differentiated tumors are strikingly similar to each other and highly dissimilar to the survival curve of patients with poorly differentiated tumors.26 Together these findings suggest either the loss of suppressors or acquisition of positive mediators of tumor aggressiveness associated with the development of G3 PDAC tumors. Because syk correlates with grade and grade correlates with survival, it is perhaps not unexpected that syk correlates with survival; however, our data suggest that syk may be one such mediator. It is important to note that grade of disease was an independent predictor of PDAC patient survival in a separate study,48 demonstrating that this pattern is not restricted to our data set.
Similar to its in situ expression, syk is absent from poorly differentiated PDAC cell lines. Consistent with this expression pattern, our data demonstrate that syk promotes a more differentiated phenotype in PDAC cells. Although ectopic expression of syk has a somewhat marginal effect on the growth of endogenously syk-negative PDAC cells in standard culture, syk has a particularly suppressive effect on growth conditions important to the tumorigenic phenotype, similar to its effects in BC5 and melanoma cells.11 Our data are also consistent with those of Zhang et al9 who demonstrated that syk promotes the formation of cell-cell contacts in BC cells. It should be noted that, although we engineered ectopic sykA expression, splicing of sykA to sykB could be performed by the cell (not assessed in this study); therefore, it is not clear which isoform is responsible for the effects observed. Immunologically, sykB is very rare and its function is unclear.39 In BC, sykB has been shown to be deficient in nuclear translocation, and this deficiency was shown to retard its suppressor functions.10 However, the nuclear targeting sequence identified in immune cells was downstream of the alternatively spliced sequence that differentiates sykA from sykB,49 suggesting that there may be separate pathways that regulate syk nuclear translocation in different cells. At this stage, we do not know whether each isoform performs specific functions or if there is redundancy. However, the relative abundance of sykB suggests it is probably functional in PDAC, because it must be spliced from sykA and thus requires active processing and energy expenditure.
Reexpression of syk in Panc1 cells significantly decreased the β-catenin-regulated gene products cyclin D1, akt, and CD171. However, unlike other adenocarcinomas (eg, colorectal), β-catenin is not considered a major regulator of the PDAC phenotype and epithelial-mesenchymal transition. Instead, a distinct pancreatic malignancy, the solid pseudopapillary tumor, has been associated with β-catenin alterations.50 In PDAC, only marginal β-catenin changes have been reported.51 Indeed, Panc1 and MIAPaCa2 cells demonstrate TOP/FOP ratios of 1.3 and 1.9, similar to those of BxPC3 cells (1.2) and actually lower than those of well differentiated CAPAN1 (2.4), CAPAN2 (2.3), and HPAFII (2.2) cells.51 No difference was observed between Panc1/mock and Panc1/syk cells by TOP/FOP analysis (not shown), demonstrating that the effect of syk is not through traditional regulation of β-catenin/Tcf-lef signaling in these cells and may instead be mediated through other transcription factors such as Snail, whose expression has been shown to correlate with reduced PDAC differentiation, being highest in the syk-negative MIAPaCa2 and Panc1 cells in comparison with more differentiated, syk-positive lines.52 On the other hand, it has been reported that syk is a transcriptional repressor through regulation of the Sp1 transcription factor in BC cells.7 Because the cyclin D153 and CD171 promoters54 contain Sp1 binding sites, it is also possible that regulation of these proteins may be due, in whole or in part, to syk-dependent regulation of Sp1 activity. However, the specific details of CD171 regulation by syk are the subject of another study.
Loss of syk expression in MIAPaCa2 and Panc1 cells is due to hypermethylation of the syk promoter, similar to melanoma,11 breast,41 liver,16 ovary,40 bladder,15 and gastrointestinal tumors.17,19 Reexpression of syk by 5AzaC led to decreased CD171 expression in these cell lines. Moreover, our data demonstrate for the first time the dependence of syk promoter methylation regulation on serum concentration and pancreatic cell density. Although the global nature of this treatment could cause off-target effects, BxPC3 cells demonstrate no effect in this assay, and these data are in line with our in vitro and in situ findings, which demonstrate that syk and CD171 are inversely expressed. Importantly, although loss of syk may be a requirement for CD171 expression in situ (ie, all CD171-positive cells noted in samples from 22 separate patients were syk-negative), it is not fully sufficient to drive this event as many syk-negative cells are also CD171-negative. Because CD171 drives an invasive phenotype in other cells,28,45 syk-dependent regulation of CD171 might be a significant event during PDAC progression.
Transient suppression of syk expression results in de novo CD171 expression coincident with syk down-regulation in BxPC3 cells. Both sykA and sykB are targeted by this siRNA, and the temporal effect of syk on CD171 in BxPC3 cells suggests that the loss of syk is required before CD171 expression, but that reexpression of syk may only affect de novo CD171 expression and not the disposition of existing protein (eg, CD171 is not immediately targeted for destruction by the proteosome). The continued expression of CD171 on days 3 to 7 (after the reexpression of syk on day 3) probably represents protein synthesized during the absence of syk, which has yet to break down. This concept is consistent with the fact that syk protein becomes reexpressed at 48 hours after 5AzaC treatment, but CD171 levels do not appreciably diminish until 96 to 120 hours after treatment, presumably due to the persistence of existing protein.
A prior publication suggested that CD171 is not expressed in PDAC in situ55; however, a subsequent report demonstrated CD171 expression in 80% of G2 and 100% of G3 PDAC tumors,56 in agreement with our current findings. That highly aggressive PDAC cells would express CD171 is not unexpected, because CD171 mediates migratory and invasive processes in neural and melanoma cells and epithelial tumors of the breast, ovary, and colon.28,45,57 More importantly, stable ectopic expression of CD171 induces the expression of invasion and metastasis-associated genes and enhances cellular migration and invasion in vitro,28,58 suggesting a potential cascade of effects centering on loss of syk in PDAC.
Prior studies demonstrated that syk inhibited the in vitro invasion of BC cells by down-regulating GRO chemokines59 and that syk suppressed the motility of BC cells by inhibition of urokinase-type plasminogen activator production.8 Our Affymetrix results did not identify these pathways as being regulated by syk in Panc1 cells; however, our data demonstrate a role for MMP2 in mediating the in vitro invasion of these cells. Stable syk reexpression significantly reduced Panc1 invasion, especially in the absence of FBS. It is not currently known whether this deficiency represents a lack of growth factor stimulation or a lack of proteolytic enzyme source. However, because Panc1 cells express significant levels of MMP2 and invade in an EGF-dependent manner,47 it is probably the former. A similar suppression of invasion was observed in Panc1 cells reexpressing syk due to 5AzaC treatment. Again, the global nature of this treatment probably affects many targets; however, these data support our prior contentions and are in agreement with those of Yuan et al,60 who demonstrated that reexpression of syk by inhibition of promoter methylation suppressed in vitro invasion of syk-negative BC cells.
In summary, we have identified syk as a normal component of the pancreatic ductal epithelium that is lost during dedifferentiation of PDAC. Contrary to what has been reported in the breast, the all-or-nothing pattern of PDAC syk expression coupled with the lack of mutations observed in syk-positive PDAC cells suggests that syk may play a more central role in pancreatic biology than in the breast. Stable reintroduction of syk into G3 Panc1 cells modulates gene expression in an antiproliferative, anti-invasive manner. Some of the observed gene expression changes may be indirect and due in part to the syk-dependent regulation of gene products (eg, CD171) that may in turn regulate the expression of other transcripts. This result suggests a potential cascade of gene expression effects centering on the loss of syk. Panc1 cells reexpressing syk demonstrate significantly reduced invasion, due at least in part to attenuation of the MMP2 protease axis. This is significant because the relationship of syk to tumor progression and clinical parameters such as dissemination has been well established in several other tumor types. As such, reduced expression of syk is associated with distant metastasis in breast12,14 and gastric cancer.17 Moreover, the 5-year survival rate is significantly higher for gastric cancer patients with nuclear syk expression, and the absence of nuclear syk correlates with lymphatic and venous invasion and lymph node metastasis.18 Likewise syk promoter hypermethylation correlates with lymph node metastasis and Dukes stage and predicts a lower 3-year survival and higher postoperative recurrence rate in patients with colorectal cancer.19 More importantly, Kunze et al15 demonstrated a loss of syk coincident with the progression of transitional cell carcinoma of the bladder to full-blown malignancy, further supporting the contention that syk is a regulator of tumor progression. Finally, we demonstrate that syk expression is a positive correlate of overall patient survival in the PDAC samples studied. Based on these data, we propose that syk is a central mediator of phenotypic changes regulating PDAC progression. Future studies will be focussed on establishing syk as a viable clinical marker to positively influence outcomes of patients with pancreatic cancer.
Supplementary Material
Acknowledgments
We thank Dr. Cynthia Behling for tissue sample procurement and analysis, Dr. Manjiri Bakre for help with syk expression studies, and Mandana Farhadieh and Mike Rasmussen for excellent technical support.
Footnotes
Address reprint requests to Steve Silletti, Ph.D. Moores UCSD Cancer Center, 3855 Health Sciences Dr. 0803, La Jolla, CA 92093-0803. E-mail: ssilletti@ucsd.edu.
S.S. is an American Cancer Society Research Scholar supported by ACS RSG-05-116-01-CSM and National Institutes of Health (NIH) grants CA130104 and CA109956. S.G. is supported by NIH grant CA108597.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of C.S.: Quest Diagnostics, Clinical Trials, Wavre, Belgium.
References
- National Cancer Institute Bethesda, MD: National Cancer Institute,; Strategic plan for addressing the recommendations of the Pancreatic Cancer Progress Review Group. 2002 [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–498. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- Coopman PJ, Do MTH, Barth M, Bowden ET, Hayes AJ, Basyuk E, Blancato JK, Vezza PR, McLeskey SW, Mangeat PH, Mueller SC. The syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- Zyss D, Montcourrier P, Vidal B, Anguille C, Merezegue F, Sahuquet A, Mangeat PH, Coopman PJ. The syk tyrosine kinase localizes to the centrosomes and negatively affects mitotic progression. Cancer Res. 2005;65:10872–10880. doi: 10.1158/0008-5472.CAN-05-1270. [DOI] [PubMed] [Google Scholar]
- Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast cancer suppression. Cancer Res. 2005;65:10289–10297. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Kundu GC. Syk, a protein-tyrosine kinase, suppresses the cell motility and nuclear factor κB-mediated secretion of urokinase type plasminogen activator by inhibiting the phosphatidylinositol 3′-kinase activity in breast cancer cells. J Biol Chem. 2003;278:6209–6221. doi: 10.1074/jbc.M208905200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shrikhande U, Alicie BM, Zhou Q, Geahlen RL. Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol Cancer Res. 2009;7:634–644. doi: 10.1158/1541-7786.MCR-08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Duke L, Zhang PS, Arlinghaus RB, Symmans WF, Sahin A, Mendez R, Dai JL. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63:4724–4730. [PubMed] [Google Scholar]
- Muthusamy V, Duraisamy S, Bradbury CM, Hobbs C, Curley DP, Nelson B, Bosenberg M. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–11193. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- Toyama T, Iwase H, Yamashita H, Hara Y, Omoto Y, Sugiura H, Zhang Z, Fujii Y. Reduced expression of the Syk gene is correlated with poor prognosis in human breast cancer. Cancer Lett. 2003;189:97–102. doi: 10.1016/s0304-3835(02)00463-9. [DOI] [PubMed] [Google Scholar]
- Repana K, Papazisis K, Foukas P, Valeri R, Kortsaris A, Deligiorgi E, Kyriakidis D. Expression of Syk in invasive breast cancer: correlation to proliferation and invasiveness. Anticancer Res. 2006;26:4949–4954. [PubMed] [Google Scholar]
- Ding YB, Wu ZY, Wang S, Fan P, Zha XM, Zheng W, Liu XA. Expression of tyrosine kinase Syk in breast cancer and their clinical significance. Zhonghua Wai Ke Za Zhi. 2004;42:137–139. [PubMed] [Google Scholar]
- Kunze E, Wendt M, Schlott T. Promoter hypermethylation of the 14-3-3σ: SYK and CAGE-1 genes is related to the various phenotypes of urinary bladder carcinomas and associated with progression of transitional cell carcinomas. Int J Mol Med. 2006;18:547–557. [PubMed] [Google Scholar]
- Yuan Y, Wang J, Li J, Wang L, Li M, Yang Z, Zhang C, Dai JL. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin Cancer Res. 2006;12:6687–6695. doi: 10.1158/1078-0432.CCR-06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ding YB, Chen GY, Xia JG, Wu ZY. Hypermethylation of Syk gene in promoter region associated with oncogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2004;10:1815–1818. doi: 10.3748/wjg.v10.i12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Natsugoe S, Ishigami S, Okumura H, Matsumoto M, Hokita S, Aikou T. Clinical significance of nuclear expression of spleen tyrosine kinase (Syk) in gastric cancer. Cancer Lett. 2006;236:89–94. doi: 10.1016/j.canlet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Yang ZL, Wang L, Kang L, Peng JS, Xiang J, Huang MJ, Wang JP. Association between hypermethylation of Syk gene and clinicopathological characteristics in colorectal cancer patients. Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11:458–461. [PubMed] [Google Scholar]
- Luangdilok S, Box C, Patterson L, Court W, Harrington K, Pitkin L, Rhys-Evans P, Ocharoenrat P, Eccles S. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck. Cancer Res. 2007;67:7907–7916. doi: 10.1158/0008-5472.CAN-07-0331. [DOI] [PubMed] [Google Scholar]
- Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, Ross SR, Monroe JG. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201:431–439. doi: 10.1084/jem.20041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Geahlen RL. The protein-tyrosine kinase syk interacts with TRAF-interacting protein TRIP in breast epithelial cells. Oncogene. 2009;28:1348–1356. doi: 10.1038/onc.2008.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller KE, MacNeil IA, Brugge JS. Protein tyrosine kinases syk and ZAP-70 display distinct requirements for src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]
- Ruschel A, Ullrich A. Protein tyrosine kinase Syk modulates EGFR signalling in human mammary epithelial cells. Cell Signal. 2004;16:1249–1261. doi: 10.1016/j.cellsig.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Bouvet M, Gamagami RA, Gilpin EA, Romeo O, Sasson A, Easter DW, Moossa AR. Factors influencing survival after resection for periampullary neoplasms. Am J Surg. 2000;180:13–17. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- Katz MHG, Bouvet M, Al-Refaie W, Gilpin EA, Moossa AR. Non-pancreatic periampullary adenocarcinomas: an explanation for favorable prognosis. Hepatogastroenterology. 2004;51:842–846. [PubMed] [Google Scholar]
- Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, Montgomery AMP. The cell adhesion molecule L1 promotes a motile and invasive phenotype via sustained MAP-kinase activation, gene transcription and induction of integrin-dependent migration. J Biol Chem. 2004;279:28880–28888. doi: 10.1074/jbc.M404075200. [DOI] [PubMed] [Google Scholar]
- Hegedüs L, Cho H, Xie X, Eliceiri GL. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. J Cell Physiol. 2008;216:480–485. doi: 10.1002/jcp.21417. [DOI] [PubMed] [Google Scholar]
- Yagi S, Suzuki K, Hasegawa A, Okumura K, Ra C. Cloning of the cDNA for the deleted syk kinase homologous to zap-70 from human basophilic leukemia cell line (KU812). Biochem Biophys Res Commun. 1994;200:28–34. doi: 10.1006/bbrc.1994.1409. [DOI] [PubMed] [Google Scholar]
- Christopoulos TA, Papageorgakopoulou N, Ravazoula P, Mastronikolis NS, Papadas TA, Theocharis DA, Vynios DH. Expression of metalloproteinases and their tissue inhibitors in squamous cell laryngeal carcinoma. Oncol Rep. 2007;18:855–860. doi: 10.3892/or.18.4.855. [DOI] [PubMed] [Google Scholar]
- Goodison S, Nakamura K, Iczkowski KA, Anai S, Boehlein SK, Rosser CJ. Exogenous mycoplasmal p37 protein alters gene expression, growth and morphology of prostate cancer cells. Cytogenet Genome Res. 2007;118:204–213. doi: 10.1159/000108302. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Collin F, Brettschneider J, Simpson K, Cope L, Irizarry RA, Speed TP. Quality assessment of Affymetrix GeneChip data. Gentleman R, Carey V, Huber W, Irizarry R, Dutoit S, editors. Heidelberg: Springer,; Bioinformatics and Computational Biology Solutions Using R and Bioconductor. 2005 [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin αvβ3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA. 2001;98:119–124. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos B, Moser S, Kalthoff H, Torok V, Lohr M, Kloppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch. 2003;442:444–452. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- Latour S, Chow LML, Veillette A. Differential intrinsic enzymatic activity of syk and zap-70 protein-tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- Dhillon VS, Young AR, Husain SA, Aslam M. Promoter hypermethylation of MGMT. CDH1, RAR-β and SYK tumour suppressor genes in granulosa cell tumours (GCTs) of ovarian origin. Br J Cancer. 2004;90:874–881. doi: 10.1038/sj.bjc.6601567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Mendez R, Sahin A, Dai JL. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res. 2001;61:5558–5561. [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann M, Arber N, Korc M. Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J Clin Invest. 1998;101:344–352. doi: 10.1172/JCI1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze'ev A. L1, a novel target of β-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Ben-Shmuel A, Raveh S, Ben-Ze'ev A. L1-CAM in cancerous tissues. Expert Opin Biol Ther. 2008;8:1749–1757. doi: 10.1517/14712598.8.11.1749. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein CE, Krieg T, Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–21062. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B, Gallinger S. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Nucleocytoplasmic trafficking of the syk protein tyrosine kinase. Mol Cell Biol. 2006;26:3478–91. doi: 10.1128/MCB.26.9.3478-3491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonello D, Gobbo S, Corbo V, Sipos B, Lemoine NR, Scarpa A. Update on the molecular pathogenesis of pancreatic tumors other than common ductal adenocarcinoma. Pancreatology. 2009;9:25–33. doi: 10.1159/000178872. [DOI] [PubMed] [Google Scholar]
- Pujal J, Capellá G, Real FX. The Wnt pathway is active in a small subset of pancreas cancer cell lines. Biochim Biophys Acta. 2006;1762:73–79. doi: 10.1016/j.bbadis.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hotz B, Arndt M, Dullat S, Bhargava S, Buhr H-J, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- Ai Z, Lu W, Ton S, Liu H, Sou T, Shen Z, Qin X. Arsenic trioxide-mediated growth inhibition in gallbladder carcinoma cells via down-regulation of cyclin D1 transcription mediated by Sp1 transcription factor. Biochem Biophys Res Commun. 2007;360:684–689. doi: 10.1016/j.bbrc.2007.06.123. [DOI] [PubMed] [Google Scholar]
- Kallunki P, Edelman GM, Jones FS. Tissue-specific expression of the L1 cell adhesion molecule is modulated by the neural restrictive silencer element. J Cell Biol. 1997;138:1343–1354. doi: 10.1083/jcb.138.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifi JT, Heidtmann S, Schurr PG, Reichelt U, Mann O, Yekebas EF, Wachowiak R, Strate T, Schachner M, Izbicki JR. Absence of L1 in pancreatic masses distinguishes adenocarcinomas from poorly differentiated neuroendocrine tumors. Anticancer Res. 2006;26:1167–1170. [PubMed] [Google Scholar]
- Sebens Müerköster S, Werbing V, Sipos B, Debus MA, Witt M, Grossmann M, Leisner D, Kotteritzsch J, Kappes H, Kloppel G, Altevogt P, Folsch UR, Schafer H. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene. 2007;26:2759–2768. doi: 10.1038/sj.onc.1210076. [DOI] [PubMed] [Google Scholar]
- Silletti S, Altevogt P, Montgomery AMP. L1 cell adhesion molecule (CD171). PROW. 2000;1:31–37. [Google Scholar]
- Gast D, Riedle S, Issa Y, Pfeifer M, Beckhove P, Sanderson MP, Arit M, Moldenhauer G, Gogel M, Kruger A, Altevogt P. The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene. 2008;27:1281–1289. doi: 10.1038/sj.onc.1210747. [DOI] [PubMed] [Google Scholar]
- Li J, Sidell N. Growth-related oncogene produced in human breast cancer cells and regulated by Syk protein-tyrosine kinase. Int J Cancer. 2005;117:14–20. doi: 10.1002/ijc.21074. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Liu H, Sahin A, Dai JL. Reactivation of SYK expression by inhibition of DNA methylation suppresses breast cancer cell invasiveness. Int J Cancer. 2005;113:654–659. doi: 10.1002/ijc.20628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.