Abstract
Recent reports suggest that commensal bacteria may play a down-regulatory role in autoimmune disease. In the present studies, we demonstrate that phosphorylated dihydroceramides, uniquely structured lipids derived from the common human oral bacterium Porphyromonas gingivalis and from bacteria commonly found in the gastrointestinal tract and other organs, are capable of enhancing autoimmunity. We have previously reported that these lipids have proinflammatory effects on human fibroblasts in vitro and, in preliminary studies, have recovered these lipids from surgically removed human carotid atheroma, suggesting that they may play a role in human inflammatory disease. To investigate whether these lipids have functional effects on autoimmunity, we administered phosphorylated dihydroceramides to mice with the murine model of multiple sclerosis, experimental allergic encephalomyelitis (EAE). We find that these lipids, and particularly the phosphoethanolamine dihydroceramide (PE DHC) fraction, significantly enhanced EAE. Mechanistically, PE DHC enhances EAE in mice lacking natural killer T cells, fails to enhance EAE in Toll-like receptor 2 (TLR2)-deficient mice and, in vitro, induces dendritic cell interleukin-6 secretion in a TLR2-dependent manner. Finally, PE DHC-treated mice with EAE demonstrate a decreased percentage of spinal cord Foxp3+ T cells, suggesting that these lipids may affect regulatory aspects of adaptive immune responses. Overall, our results suggest that phosphorylated dihydroceramides derived from common human bacteria function as TLR2 ligands and may play a previously unrecognized role in human autoimmune diseases.
Several recent reports have focused on the potential importance of commensal bacteria in down-regulating murine models of autoimmunity.1,2,3 Thus, commensal bacteria or bacteria commonly found in human tissues may play a previously unappreciated role in regulating human autoimmune disease. In the present studies, we postulated that uniquely structured lipids derived from bacteria commonly found in the gingiva, gastrointestinal (GI) tract, and vagina in humans may function in enhancing, rather than preventing, autoimmunity.
Porphyromonas gingivalis is a Gram-negative anaerobic oral bacterium commonly found in adults. We have previously characterized uniquely structured, non-lipopolysaccharide (LPS), phosphorylated sphingolipid classes, termed phosphorylated dihydroceramides, produced by this organism. We reported that these lipids promote inflammatory responses in human fibroblasts in vitro,4,5 and in recent preliminary studies (F.C. Nichols, unpublished) we have successfully recovered these lipids from surgically removed human carotid atheroma. These results suggest that these lipids gain access to the vasculature and may play a role in human inflammatory conditions. Phosphorylated dihydroceramides have unique structural components that are not seen in mammalian lipids and that are important in mediating the observed in vitro inflammatory effects.5 In addition to P. gingivalis, other periodontal organisms and at least one GI tract organism, Bacteroides fragilis, are known to produce similar lipids,6 and work in our laboratory has confirmed that B. fragilis produces the major phosphorylated dihydroceramides produced by P. gingivalis. Phylogenetically related Bacteroides organisms in the GI tract7,8 and in the vaginal area9 also likely produce these lipids.
Given that humans are potentially exposed to these bacterial phosphorylated dihydroceramides from multiple organs, that these lipids have proinflammatory effects in vitro, and that preliminary studies identify them in sites of human inflammation, we asked whether these unique lipids have functional effects on autoimmune disease. We now report that P. gingivalis phosphorylated dihydroceramides, and specifically the fraction of these lipids containing phosphoethanolamine dihydroceramide (PE DHC), significantly enhance the severity of the murine model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE). Furthermore, these lipids mediate this enhancement in a Toll-like receptor 2 (TLR2)-dependent manner. These results suggest that phosphorylated dihydroceramides may play a previously unrecognized role in initiating or exacerbating human autoimmune diseases.
Materials and Methods
Mice
Female C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). TLR2−/− mice were a generous gift of Dr. S. Akira (Osaka University, Japan).10 IL-15−/− mice and IL-15Rα−/− mice were a generous gift from Dr. Leo LeFrancois (University of Connecticut Health Center). All mice were maintained and bred in accordance with University of Connecticut Center for Laboratory Animal Care regulations.
Induction of EAE
Female mice (4–8 weeks old) were immunized with 100 to 200 μg of myelin oligodendrocyte glycoprotein peptide (35–55) (MOG) emulsified with CFA (containing 500 μg of H37RA mycobacteria) (DIFCO BD Diagnostics, Sparks, MD) via subcutaneous injection on day 0. Pertussis toxin (PTX), 200 to 250 ng (List Biologicals Laboratories, Campbell, CA), was injected intravenously on day 0 and again on day 2. In addition, mice were injected intraperitoneally on day 0 with either P. gingivalis lipid or the vehicle control, 70% ethanol (EtOH). EAE was scored as grade 1, tail paralysis; grade 2, weakness of hind limbs with an altered gait; grade 3, hind limb paralysis; grade 4, front and hind limb paralysis; grade 5, death.
Purification and Verification of P. gingivalis Lipids
P. gingivalis (ATCC no. 33277, type strain) was grown and lipids extracted and fractionated by high-performance liquid chromatography (HPLC) as previously described.4,11 HPLC fractions highly enriched for PE DHC lipids were identified via electrospray mass spectrometry (MS) using a Micromass Quattro II mass spectrometer system.4 HPLC fractions containing highly enriched PE DHC lipids were pooled and each combined fraction was verified to be of greater than 95% purity by electrospray MS.
Processing of Lipids for Administration to Animals and Addition to Tissue Culture
For treatment of mice, preweighed lipids were dissolved in 70% ethanol to achieve a final concentration of 125 ng/μl and sonicated for 2.5 minutes immediately before injection into animals. This preparation was also used for drying lipids onto tissue culture wells. For direct addition to cell cultures, the lipids were dissolved in culture medium at 125 ng/μl and sonicated for 2.5 minutes to produce a liposome preparation for administration to cells in culture.
Derivation and Stimulation of Bone Marrow DCs
Bone marrow cells from C57BL/6 and TLR2−/− mice were cultured at 2 × 105 cells/ml in RPMI containing 10% fetal calf serum, 2-ME, and 20 ng/ml recombinant murine granulocyte macrophage–colony-stimulating factor for 9 days. Bone marrow DCs (BMDCs) were harvested at day 9 and were greater than 80% CD11c+. LPS (1 μg), MMP (10 μg) (a bacterial lipoprotein and TLR-2 ligand: palmitoyl-Cys [(RS)- 2,3-di(palmitoyloxy)-propyl)-Ser-Ser-Asn-Ala-OH(pam3-Cys-Ser-Ser Asn-Ala-OH) (Bachem H-9460)],12 PE DHC (2.5 μg) or 70% EtOH, all in 20-μl volumes, were allowed to dry in the wells of a 24-well plate overnight before the addition of BMDCs. BMDCs were cultured in the ligand-bound 24-well plates at 1 × 106 cells/ml in RPMI containing granulocyte macrophage–colony-stimulating factor. After 18 hours, culture supernatants were harvested and tested for interleukin (IL)-6 via enzyme-linked immunosorbent assay.
In Vitro Generation of Th17 T Cells
CD4+CD25− T cells (Teff) were derived from wild-type mice using magnetic bead purification (Miltenyi Biotec, Auburn, CA). T cell-depleted splenocytes (Tds) were derived from wild-type or TLR2−/− mice using magnetic bead purification followed by irradiation (2600R). Teff (0.25 × 106/well) and Tds (0.75 × 106/well) were cultured in 24-well plates with anti-CD3 antibody (1 μg/ml), granulocyte macrophage–colony-stimulating factor (20 ng/ml) (Pierce Inc., Thermo Fisher Scientific, Rockford, IL), and recombinant transforming growth factor-β (2 ng/ml) (R&D). In addition, LPS (2 μg/ml), MMP (5 μg/ml), or P. gingivalis PE DHC (20 μg/ml of sonicated liposome preparations) was added to wells to stimulate the secretion of IL-6. Cultures were harvested after 5 days, stimulated in culture for 4 hours with phorbol 12-myristate 13-acetate, ionomycin brefeldin A (Sigma, St. Louis, MO) and stained for Thy1.2, intracellular interferon-γ (IFNγ), and IL-17 and analyzed by fluorescence-activated cell sorting after gating on Thy 1.2+ cells.
Derivation and Phenotypic Analysis of Spinal Cord-Derived Mononuclear Cells
Spinal cord mononuclear cells were derived as previously described13 and stained for CD4 [FITC α-CD4 (GK1.5); BD PharMingen] and Foxp3 [APC α-FoxP3 (FJK-16s; E-Bioscience)], or stimulated in culture for 4 hours with phorbol 12-myristate 13-acetate, ionomycin brefeldin A before staining for Thy1.2 (PE-Cy7 anti-CD 90.2; E-Biosciences) and intracellular IFNγ (APC αIFNγ; BD PharMingen) and IL-17 (Alexa Fluor 488 α-IL-17A; BD PharMingen).
Recovery of Bacterial Lipids from the Brains of Mice with EAE
Mice treated with phosphate-buffered saline (PBS), EtOH, or with 25 ng, 250 ng, or 2.5 μg of PE DHC were sacrificed after day 20 post-EAE immunization. The brains were removed and extracted for phospholipids according to the method of Bligh and Dyer as previously described.14 Lipid extracts were dissolved in hexane isopropanol:water (6:8:0.75, v/v/v/), and three 0.5-mg aliquots were dispensed into glass tubes supplemented with 30 ng of isobranched C20:0. Lipid samples were hydrolyzed for 4 hours in 2 N KOH, acidified and fatty acids extracted into chloroform and dried. Lipids were treated to form pentofluorobenzyl ester and trimethylsilyl ether derivatives and analyzed by negative ion chemical ionization gas chromatography-mass spectrometry.14 Fatty acid recovery was quantified by selected ion monitoring for characteristic fatty acid negative ions. The data are expressed as picograms of 3-OH isobranched (iso)C17:0 per 0.5 mg of total brain lipid extracted.
Statistical Evaluation
The cumulative disease index (CDI) was obtained by summing the daily average disease scores through day 20. A mean of these daily disease scores (±SEM) was calculated based on the 20 days of observation. The mean daily disease scores were compared using the Wilcoxon signed rank tests for two samples. Disease incidence frequencies were compared using χ2 analysis. Values for mean maximum severity of EAE were compared using the Wilcoxon signed rank test. Values for mean day of onset of EAE were compared using Student’s t-test. For analysis of spinal cord populations, percentages were compared using Student’s t-test. Bacterial fatty acid levels in brain lipid extracts for each treatment group were evaluated using least squares linear regression analysis that included calculation of correlation coefficients. For each dose of bacterial lipid administered, linear regression analysis compared the final EAE score with the mean bacterial fatty acid recovered per 0.5 mg of brain lipid extract. The mean bacterial fatty acid levels were calculated from three replicate brain lipid determinations.
Results
P. gingivalis Total Phosphorylated Dihydroceramide Lipids, and Specifically the PE DHC Fraction, Enhance EAE
Having previously demonstrated that bacterial phosphorylated dihydroceramides promote inflammatory responses in human fibroblasts in vitro,4,5 we wanted to characterize the functional effects of these lipids in autoimmunity and used the murine model of multiple sclerosis, EAE. EAE was induced in female C57BL/6 mice and these mice were also injected intraperitoneally (i.p.) on day 0 with either P. gingivalis lipid or the vehicle control, 70% EtOH. To most effectively detect effects of P. gingivalis lipids in the development of autoimmunity, we induced less severe EAE by using CFA with higher concentrations of H37RA mycobacteria (500 μg/mouse).
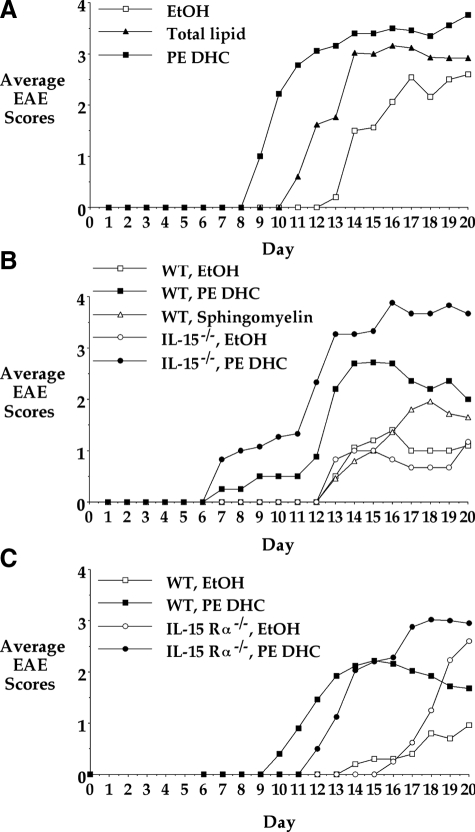
We first examined the effect of administering the P. gingivalis total phosphorylated dihydroceramide lipids (TL) on EAE in wild-type mice. A single i.p. injection of 2.5 μg of P. gingivalis TL resulted in enhanced severity of EAE (Figure 1A). To further characterize this effect, we next examined component HPLC fractions of the TL individually and found that the fraction containing greater than 95% PE DHC most consistently enhanced EAE. Administering 2.5 μg, 250 ng, and even 25 ng of PE DHC led to enhanced disease, with 250 ng being the most efficient (data not shown). A single 250-ng i.p. injection of the PE DHC fraction consistently enhanced the severity of EAE and often led to earlier onset of disease. Figure 1A shows one representative experiment of six similar studies in which 250 ng of PE DHC was administered to wild-type mice. The cumulative results from these six experiments demonstrate that PE DHC-treated mice showed essentially a doubling in CDI and mean daily disease compared with EtOH-treated mice (Table 1). In addition, wild-type PE DHC-treated mice showed a significantly earlier onset of disease when compared with wild-type EtOH-treated mice (P = 0.008). While not reaching statistical significance, PE DHC-treated mice also showed an increase in incidence of disease (Table 1). Mean maximum severity did not differ significantly between the groups. Of note, we also administered the lipids to naïve mice that were not treated with the EAE-inducing protocol and observed these mice for signs of illness. Such mice never demonstrated EAE (data not shown).
Figure 1.
P. gingivalis TL and the PE DHC lipid fraction enhance EAE in wild-type, IL-15−/−, and IL-15Rα−/− mice. EAE was induced as follows: female C57BL/6 (WT) (A), IL-15−/− (B), or IL-15Rα−/− (C) mice aged 4–8 weeks were immunized subcutaneously with MOG35–55 peptide (100–200 μg/mouse) in CFA containing 500 μg of H37Ra mycobacteria on day 0. Mice also received Ptx intravenously (150–250 ng) on days 0 and 2. On day 0, mice also received a single 20-μl i.p. injection of EtOH, P. gingivalis TL (2.5 μg), or P. gingivalis PE DHC (250 ng). In B, additional wild-type mice also received a single 20-μl i.p. injection of the control lipid, bovine sphingomyelin (250 ng). EAE was graded as follows: grade 1, tail paralysis; grade 2, abnormal gait; grade 3, hind limb paralysis; grade 4, hind and front limb paralysis; grade 5, death. Results in A, B, and C are from one representative experiment each and are depicted as the average EAE score of a given cohort of mice (n = 4–5) on each day after immunization.
Table 1.
EAE Disease Assessment
| Mouse strain* | EtOH
|
PE DHC
|
P value | n | ||
|---|---|---|---|---|---|---|
| CDI | MDD | CDI | MDD | |||
| Wild type | 10.1 | 0.5 | 19.8 | 1.0 | 0.001 | 28 |
| TLR2−/− | 7.6 | 0.4 | 8.3 | 0.4 | 0.306 | 15 |
| IL-15−/− | 9.4 | 0.5 | 24.5 | 1.2 | 0.001 | 10 |
| IL-15Rα−/− | 7.0 | 0.4 | 18.7 | 0.9 | 0.0077 | 12 |
| Mouse strain† | Mean incidence
|
Mean maximum severity
|
Mean day of onset
|
|||
| EtOH
|
PE DHC
|
EtOH
|
PE DHC
|
EtOH
|
PE DHC
|
|
| Wild type | 58.6 | 75 | 3.2 | 3.6 | 14.4 | 12.1 |
| TLR2−/− | 42.1 | 46.6 | 3.0 | 2.9 | 14.4 | 14.7 |
| IL-15−/− | 60 | 90 | 3.1 | 3.7 | 14.7 | 13.0 |
| IL-15Rα−/− | 66 | 100 | 2.7 | 2.9 | 15.1 | 13.8 |
The CDI was obtained by summing the daily average disease scores of each experimental group through day 20. A mean of these daily disease scores (MDD) was calculated based on the 20 days of observation. The MDD scores were compared using the Wilcoxon signed rank tests for two samples. n is the total number of mice studied in each experimental group.
Mean incidence of disease is represented as a percentage and was calculated by dividing the number of mice within each group that developed clinical signs of EAE by the total number of mice in that group. Disease incidence frequencies were compared using χ2 analysis. Mean maximum severity of EAE was calculated for mice that developed EAE by taking the highest score observed for each mouse in the 20-day observation period and averaging these values among mice in the same group. Statistical significance was determined using the Wilcoxon signed rank test. Mean day of onset of EAE was calculated for mice that developed EAE by using the first day of observance of signs of EAE as the value and averaging these values among mice in the same group. Statistical significance was determined using Student’s t-test.
PE DHC Enhances EAE in IL15−/− and IL-15Rα−/− Mice
The most common immune cell responding to sphingolipids is the NKT cell. Therefore, we wanted to ask whether the effects of PE DHC were mediated by NKT cells. To this end, we chose to study mice in which there is the most complete absence of NKT cells. Because the development of NKT cells is IL-15-dependent, mice deficient in either IL-15 (IL-15−/− mice) or the IL-15 receptor α (IL-15Rα−/− mice) express very few identifiable NKT cells.15,16
Wild-type mice and either IL-15−/− or IL-15Rα−/− mice (both on a C57BL/6 background) were immunized for EAE and given a single i.p. injection of EtOH or PE DHC on day 0. As in wild-type mice, PE DHC significantly enhanced EAE in both IL-15−/− and IL-15Rα−/− mice, inducing greater than a doubling of the CDI and mean daily disease compared with EtOH-treated IL-15−/− and IL-15Rα−/− mice (Table 1). Additionally, IL-15−/− and IL-15Rα−/− PE DHC-treated mice showed an earlier onset of disease and increased incidence of disease compared with EtOH-treated mice, although only incidence of disease in IL-15Rα−/− PE DHC-treated versus IL-15Rα−/− EtOH-treated mice reached statistical significance (P = 0.0285; Table 1). As with wild-type mice, mean maximum severity did not differ between the groups. Figure 1, B and C, shows representative experiments using IL-15−/− and IL-15Rα−/− mice. The finding that PE DHC enhances EAE in IL-15−/− and IL-15Rα−/− mice indicates that PE DHC does not require NKT cells to mediate its disease-enhancing effect.
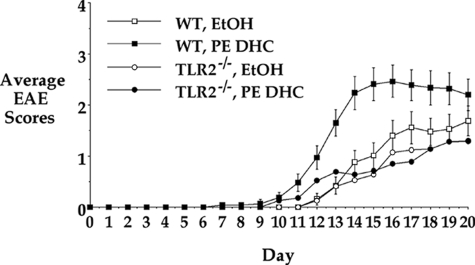
PE DHC Enhancement of EAE Is TLR2-Dependent
It has been reported that the host response to P. gingivalis involves TLR2 signaling.17 To characterize the possible TLR2 dependence of the effects of PE DHC, we next investigated whether PE DHC was capable of enhancing EAE in TLR2-deficient (TLR2−/−) mice. TLR2−/− mice were immunized with the standard EAE-inducing MOG protocol and administered a single i.p. injection of either EtOH or PE DHC on day 0. In contrast to its effect on wild-type, IL-15−/−, and IL-15Rα−/− mice, PE DHC did not mediate enhancement of CDI or mean daily disease in TLR2−/− mice. As seen in Figure 2 (a composite of four experiments; n = 15 mice) and in Table 1, PE DHC-treated TLR2−/− mice demonstrated no statistically significant enhancement of EAE CDI, mean daily disease, disease incidence, mean maximal severity, or day of onset when compared with EtOH-treated TLR2−/− mice. These results indicate that TLR2 is required for PE DHC to mediate enhancement of EAE.
Figure 2.
The PE DHC lipid fraction fails to enhance EAE in TLR2−/− mice. EAE was induced and graded as in Figure 1 using wild-type (WT) or TLR2−/− mice. On day 0, wild-type and TLR2−/− mice received a single 20-μl i.p. injection of EtOH or P. gingivalis PE DHC (250 ng). Results depicted are a composite of studies (wild-type mice, n = 28; TLR2−/− mice, n = 15) and represent the average EAE score for each group (±SEM for wild-type mice) on each day after immunization.
PE DHC Enhancement of EAE Is Not a Result of Contamination with LPS or Lipid A
Since LPS preparations have been shown to influence the development of EAE, it was critical to establish that PE DHC is not contaminated with lipid A or LPS. The Bligh and Dyer phospholipid extraction procedure that we use for recovering the P. gingivalis lipids has previously been shown to exclude LPS of P. gingivalis from the organic solvent phase containing the total bacterial lipids.14,18 Furthermore, P. gingivalis total lipids extracted by this method also did not contain lipid A species known to be produced by P. gingivalis. We have previously characterized the PE DHC lipid fraction using collisional electrospray-MS/MS studies5 and also used structural NMR studies (unpublished), both of which confirmed the structural characteristics of the lipids and lack of both carbohydrate and protein contaminants in this lipid fraction. In addition, contamination of the fraction with neutral products is extremely unlikely because the HPLC separations use a polar column and the lipid fraction in question is highly polar and therefore late eluting. All neutral lipid components elute close to the void volume and are not recovered in the lipid fractions used in these studies.
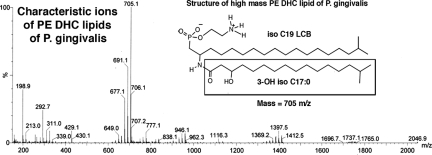
Using electrospray MS evaluation of all of the major lipid classes purified by HPLC, we confirmed that these lipid fractions are not contaminated with lipid A species of P. gingivalis LPS. Electrospray MS of the PE DHC lipid fraction of P. gingivalis demonstrated that the characteristic dominant lipid A negative ions (1195, 1435, 1449, 1690, and 1770 m/z) previously described for P. gingivalis are not recovered in this isolate19,20 (Figure 3). Thus, the approach used for preparation of the lipids and the analyses of the lipid fractions both rule out the possibility that the P. gingivalis PE DHC fraction is contaminated with lipid A or LPS.
Figure 3.
Electrospray MS of PE DHC lipids recovered from P. gingivalis. Total lipids of P. gingivalis were isolated and fractionated by HPLC as described in Material and Methods. Fractions containing the characteristic molecular ions of PE DHC lipids were pooled and repurified by HPLC. Repurified fractions demonstrating 705, 699, and 677 negative ions were pooled. The structure of the high-mass PE DHC lipid (705 m/z) is shown in the inset with the component fatty acid and long-chain base structures identified. The lower-mass PE DHC lipids indicated by 691 or 677 m/z ions contain 18 carbon or 17 carbon long-chain bases, respectively, as previously described.4 Note the absence of ions characteristic for lipid A moieties produced by P. gingivalis (1195, 1435, 1449, 1690, and 1770 m/z negative ions).
Administration of PE DHC Results in Increased Recovery of Bacterial Lipids in the Brains of Mice with EAE
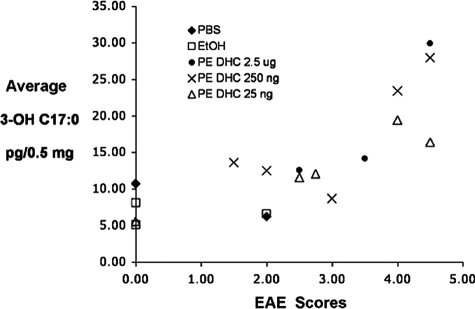
We next asked if we could detect increased levels of bacterial lipids in the brains of EAE mice treated with i.p. PE DHC. To accomplish this, we determined the level of 3-OH isobranched (iso)C17:0 fatty acid in brain specimens of mice with EAE treated with PBS, EtOH, or PE DHC. The approach of measuring 3-OH isoC17:0 fatty acid in tissues is based on the concept that mammalian tissues, unlike bacteria, have no established biochemical pathway for de novo synthesis of 3-OH isoC17:0 fatty acid. Thus, the recovery of 3-OH isoC17:0 fatty acid reflects the presence of bacterially derived products in the tissue. 3-OH isoC17:0 is a constituent fatty acid of all phosphorylated dihydroceramide lipids of P. gingivalis.4
Mice treated with PBS, with EtOH, or with 25 ng, 250 ng, or 2.5 μg of PE DHC were sacrificed after day 20 post-EAE immunization. The brains were removed, extracted for phospholipids, and fatty acid recovery was quantified by selected ion monitoring for fatty acid negative ions. Figure 4 demonstrates the average 3-OH isoC17:0 recovery (three determinations/mouse brain sample) as a function of both the final grade of EAE and the treatment received by each mouse. The data are expressed as picograms of 3-OH isoC17:0 per 0.5 mg of total brain lipid extracted. The average SEM for all determinations was ±2.2 pg/0.5 mg total lipid.
Figure 4.
Administration of PE DHC results in increased recovery of bacterial lipids in the brains of mice with EAE. PBS, EtOH, or PE DHC-injected mice (25 ng, 250 ng, or 2.5 μg) were sacrificed after day 20 post-EAE immunization. The brains of these mice were removed, extracted for phospholipids, and 3-OH isoC17:0 fatty acid quantified using negative ion chemical ionization gas chromatography-mass spectrometry as described in Materials and Methods. The average 3-OH isoC17:0 recovery (three determinations per mouse brain sample) as a function of both the treatment and final EAE score is depicted as picograms of 3-OH isoC17:0 per 0.5 mg of total brain lipid extracted. The average SEM for all brain lipid determinations was ±2.2 pg/0.5 mg total lipid.
As seen in Figure 4, lipids derived from the brains of control (PBS- or EtOH-injected) mice showed low levels of recoverable 3-OH isoC17:0 fatty acid. This most likely reflects cumulative exposure of normal mice to complex lipids and/or LPS derived from other commensal bacteria. While the presence of P. gingivalis in normal wild-type mice has not been reported, other bacteria producing 3-OH isoC17:0 fatty acid have been documented as commensal organisms in mice; for example, B. thetaiotaomicron in the GI tract.21 Significantly, our results showed higher levels of 3-OH isoC17:0 fatty acid in mice that had received PE DHC and had a disease score greater than 3.0 (Figure 4). Linear regression analysis revealed that the correlation between EAE disease score and brain 3-OH isoC17:0 fatty acid was directly associated with the dose of PE DHC injected; the strongest correlation (regression coefficient or slope) was seen with the highest dose of PE DHC (2.5 μg, y = 11.578 + 8.690x, R2 = 0.818), the next strongest with the middle dosage (250 ng, y = 2.168 + 5.014x, R2 = 0.620), and the weakest correlation with the lowest dose of PE DHC (25 ng, y = 3.789 + 3.062x, R2 = 0.808). While we have not yet precisely identified these brain-recovered bacterial lipids as PE DHC, the correlation noted between level of bacterial lipid recovered and dose of PE DHC injected suggests that the injected lipids can access the central nervous system.
PE DHC Activates Antigen Presenting Cells and Induces IL-6 Secretion in Vitro in a TLR2-Dependent Manner
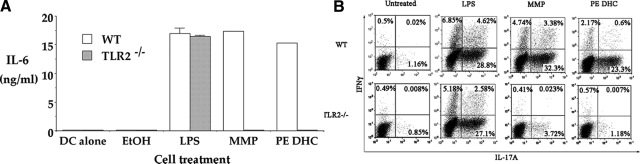
To further characterize the role of TLR2 in the function of PE DHC, we next examined the effects of PE DHC on antigen presenting cell (APC) activation in vitro. Dendritic cells (BMDCs) (>85% CD11c+) were derived from the bone marrow of wild-type or TLR2−/− mice and cultured either alone or with EtOH, LPS, MMP (a TLR2 ligand), or PE DHC. After18 hours supernatants were assayed for IL-6.
Stimulating wild-type BMDCs in the presence of LPS or MMP resulted in IL-6 secretion (Figure 5A). Culturing TLR2−/− BMDCs in the presence of LPS also resulted in IL-6 secretion, but as expected, culturing in the presence of MMP did not. Significantly, wild-type BMDCs in the presence of PE DHC demonstrated levels of IL-6 secretion that were almost equivalent to that seen with LPS (Figure 5A). However, in contrast to its effects on wild-type BMDCs, culturing PE DHC with TLR2−/− BMDCs did not result in IL-6 secretion. BMDCs were also assayed for expression of the surface activation markers B7.2 and MHC class II, and we found that PE DHC increased MHC II and B7.2 expression on wild-type but not TLR2−/− BMDCs (data not shown). These results indicate that PE DHC can activate DCs and does so in a TLR2-dependent manner.
Figure 5.
The PE DHC lipid fraction activates APCs and induces IL-6 secretion in vitro in a TLR2-dependent manner. A: Bone marrow-derived DCs from wild-type (WT) or TLR2−/− mice were cultured alone or with plate-bound EtOH, LPS (1 μg), MMP (10 μg), or PE DHC (2.5 μg). After 18 hours, culture supernatants were assayed for IL-6 via enzyme-linked immunosorbent assay. Histogram bars depict the mean ± SD (n = 4 trials). B: Naïve CD4+CD25− wild-type Teff (0.25 × 106/well) were cultured with irradiated wild-type or TLR2−/− Tds as a source of antigen presenting cells (0.75 × 106/well), anti-CD3 antibody (1 μg/ml), granulocyte macrophage–colony-stimulating factor (20 ng/ml), and transforming growth factor-β (2 ng/ml). In addition, LPS (2 μg/ml), MMP (5 μg/ml), or P. gingivalis PE DHC (20 μg/ml as a sonicated liposome preparation) were added to wells to stimulate IL-6 secretion. Cultures were harvested after 5 days, stimulated in culture for 4 hours with phorbol 12-myristate 13-acetate, ionomycin and brefeldin A and stained for Thy1.2, intracellular IFNγ, and IL-17 and analyzed by fluorescence-activated cell sorting after gating on Thy 1.2+ cells.
To further confirm the ability of PE DHC to induce IL-6 secretion, we characterized its ability to induce Th17 T cell generation from cultures of naïve CD4+CD25− T cells activated in the presence of APCs (Tds) and transforming growth factor-β.22 Adding PE DHC resulted in the generation of Th17 T cells in cultures containing wild-type but not TLR2−/− Tds (Figure 5B). These results further confirm that PE DHC can induce IL-6 secretion from APCs in a TLR2-dependent manner. When taken together, our results indicate that PE DHC mediates its in vitro and in vivo effects through TLR2-dependent mechanisms.
PE DHC Decreases the Percentage of CD4+ Foxp3+ Spinal Cord Tregs
To characterize mechanisms by which PE DHC may enhance autoimmune disease in vivo we asked whether the PE DHC-mediated enhancement of EAE is associated with alterations in T cell populations at the site of disease, ie, the spinal cord. Mice were immunized with the usual EAE-inducing protocol and treated on day 0 with EtOH or PE DHC (250 ng i.p.). Within 5 days after onset of EAE, mice were sacrificed and exsanguinated, their spinal cords were removed, and the mononuclear cells were derived from the spinal cords. These cells were analyzed directly for CD4 and Foxp3 expression by flow cytometry or were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 4 hours and then, gating on Thy1.2+ cells, analyzed for intracellular IFNγ and IL-17 by flow cytometry.
Sampling wild-type mice from three separate experiments, we found no significant difference in the total number of mononuclear cells obtained from the spinal cords of EtOH versus PE DHC-treated mice. In addition, the percentages of spinal cord-derived CD4+ T cells staining for either intracellular IFNγ or IL-17 (or cells expressing both cytokines) were not significantly different between EtOH and PE DHC-treated mice (data not shown). Notably, however, we found that the percentage of CD4+ T cells within the total mononuclear cell populations derived from the spinal cords of PE DHC-treated mice was, on average, greater than the percentage in EtOH-treated mice (Table 2). Moreover, while this increase in percentage of CD4+ T cells from PE DHC spinal cords did not reach statistical significance, we found a statistically significant decrease in the mean percentage of spinal cord CD4+ T cells that were Foxp3+, theoretically representing regulatory T cells (Tregs) in the PE DHC-treated mice (P = 0.0397) (Table 2). The mean percentage of spinal cord cells that were CD4+ was 41% in EtOH-treated mice and, on average, 6.7% of these were Foxp3+. In contrast, the mean percentage of spinal cord cells that were CD4+ T cells was 52% in PE DHC-treated mice and, on average, 4.3% of these were Foxp3+ (Table 2). It has been reported that the percentage of spinal cord CD4+ Foxp3+ T cells increases as the disease progresses.13 On average, the PE DHC-treated mice from which spinal cord cells were derived had a slightly longer duration of disease than did the EtOH-treated mice (1.5 days longer; Table 2). Thus, it is unlikely that the decrease in the percentage of Foxp3+ in PE-DHC-treated mice is related to differences in disease duration.
Table 2.
Spinal Cord T Cells
| Treatment (mouse) | Days after onset of EAE | Disease grade | CD4+ in spinal cord (%) | Foxp3+ in spinal cord (%) |
|---|---|---|---|---|
| EtOH-1 | 2 | 1.0 | 49.60 | 8.31 |
| EtOH-2 | 4 | 2.8 | 55.23 | 5.71 |
| EtOH-3 | 5 | 3.3 | 49.40 | 5.57 |
| EtOH-4 | 2 | 2.7 | 44.88 | 7.52 |
| EtOH-5 | 1 | 1.0 | 32.98 | 6.01 |
| EtOH-6 | 1 | 2.0 | 15.18 | 7.06 |
| Mean = 41.21 | Mean = 6.70 | |||
| ±14.78 | ±1.11 | |||
| PE DHC-1 | 5 | 3.3 | 72.00 | 4.53 |
| PE DHC-2 | 4 | 3.5 | 38.00 | 7.52 |
| PE DHC-3 | 5 | 2.9 | 68.86 | 2.75 |
| PE DHC-4 | 4 | 3.3 | 49.54 | 3.45 |
| PE DHC-5 | 2 | 2.9 | 40.83 | 3.21 |
| PE DHC-6 | 4 | 3.0 | 46.50 | 4.27 |
| Mean = 52.62 | Mean = 4.29 | |||
| ±14.42 | ±1.72 | |||
| P value EtOH vs. PE DHC | 0.1635 | 0.0397 |
The PE DHC fraction alters the composition of cells infiltrating the spinal cords of mice with EAE. Mice were sampled from three different experiments and sacrificed 1–5 days after onset of signs of EAE. Mononuclear cells were derived from the spinal cords, stained for CD4 and Foxp3, and evaluated by flow cytometry. Percent CD4 represents the percent CD4+T cells within the total spinal cord mononuclear cells. Percent Foxp3 represents the percent Foxp3+ T cells after gating on CD4+ T cells.
Discussion
In these studies, we chose to use EAE as our model of autoimmune disease. While it is clear that the immune system in most individuals has the potential to attack self-tissues, the “tipping” factors that initiate and propagate autoimmune diseases such as multiple sclerosis in only a subset of individuals remain unknown. Phosphorylated dihydroceramides are derived from bacteria found in multiple sites in humans (gingiva, GI tract, and vagina), and in the present studies we wanted to test the postulate that these lipids could serve as such tipping factors.
P. gingivalis is a common oral bacterium that has been extensively characterized for its ability to promote gingival tissue and alveolar bone destruction. P. gingivalis LPS has been reported to use either TLR4 or TLR2 in its signaling,19,23,24,25 and Shapira et al reported that injecting whole killed P. gingivalis bacteria into subcutaneous chambers in SJL mice resulted in enhancement of EAE.26 In contrast to these studies that used whole bacteria or P. gingivalis LPS, our studies focused on uniquely structured, non-LPS lipids produced by P. gingivalis. We have previously characterized these phosphorylated sphingolipid classes, termed phosphorylated dihydroceramides, produced by this organism and reported that these lipids promote inflammatory responses in human fibroblasts in vitro.4,5 The P. gingivalis phosphorylated dihydroceramides are integral parts of the bacterial membranes and likely released on the death or during phagocytosis/endocytosis of the organism. Phosphorylated dihydroceramides are also produced by bacteria found in the GI tract and are also likely produced by similar genera found in the vaginal tract.9 Thus, humans are potentially exposed to these bacterial lipids from multiple organs and, in preliminary studies (F.C. Nichols, unpublished), we have successfully recovered these lipids from surgically removed human carotid atheroma, suggesting that these lipids gain access to the vasculature in humans and may play a role in human inflammatory conditions.
In the present studies, we now report that the total phosphorylated dihydroceramide lipids derived from P. gingivalis enhanced the severity of EAE and that the fraction of these lipids containing 95% PE DHC was especially effective in enhancing EAE. The PE DHC-containing fraction induced almost a doubling in EAE CDI despite being administered i.p. only once and at very low concentrations. Importantly, the approach used for preparation of the lipids and the analyses of the lipid fractions both demonstrated that the PE DHC preparations do not contain LPS, the lipid A species of P. gingivalis LPS, or carbohydrate or protein contaminants. Mechanistically, this lipid enhanced EAE in both IL-15 and IL-15Rα-deficient mice. These mice are deficient in NK and NKT cells and have a defect in memory CD8+ T cell generation, suggesting that these populations are not involved in the enhancement of EAE induced by PE DHC. In contrast, both in vitro and in vivo studies using TLR2−/− dendritic cells and TLR2−/− mice demonstrated that the PE DHC fraction mediates its effects through TLR2, suggesting that P. gingivalis PE DHC represents a new class of uniquely structured lipid TLR2 ligands. The PE DHC fraction could theoretically affect numerous processes involved in the mediation of EAE, including expansion of autoreactive T cells, Treg function, or blood-brain barrier permeability, many of which may be regulated by TLR2 activation.27,28,29,30 In these studies, we found that PE DHC-treated mice with EAE showed a tendency toward an increase in frequency of spinal cord CD4+ T cells and a statistically significant decrease in percentage of spinal cord CD4+ Foxp3+ cells. These findings suggest that PE DHC may directly or indirectly affect regulatory aspects of adaptive immune responses. The specific innate and adaptive cellular populations affected by PE DHC are now a focus of investigation in our laboratory.
In the present studies, we have demonstrated that the injected PE DHC does appear to gain access to the central nervous system. However, we are not yet sure whether the lipids need to access the brain and spinal cord to mediate their effects. It is possible that they activate the peripheral immune system, which then acts on the target tissue—in this case, the central nervous system. Numerous other questions are raised by our present findings. These include how PE DHC traffics after i.p. administration, which phases of EAE induction, progression, and regulation are affected by PE DHC, and which specific structural characteristics of PE DHC are critical for binding and signaling through TLR2.
In addition, given the reports of the protective effects of factors derived from commensal/common organisms, it will be important to determine how the balance of such protective factors and disease-enhancing factors, such as those described in the present studies, play out in regulating autoimmune disease. It may be the case that protective factors and enhancing factors produced by commensal/common organisms play different roles in different anatomical sites or in different autoimmune diseases or models. Finally, given our preliminary findings of the recovery of PE DHC in human atheroma, it will be of great interest to determine whether bacterially derived phosphorylated dihydroceramides can be recovered from postmortem samples of brain tissue from patients with multiple sclerosis. These studies are presently ongoing in our laboratory.
Overall, our results represent the first description that phosphorylated dihydroceramides derived from common human bacteria are capable of enhancing autoimmune disease and do so in a TLR2-dependent manner. These lipids may thus play a previously unrecognized role in initiating or exacerbating human autoimmune diseases and may represent a new target for therapeutic intervention.
Acknowledgments
We thank Dr. Stefan Brocke for critical reading of the manuscript.
Footnotes
Address reprint requests to Dr. Robert B. Clark, Room L6032, Departments of Immunology and Medicine, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06032. E-mail: rclark@nso2.uchc.edu.
Supported by grants from the National Multiple Sclerosis Society (RG4070-A-6 to R.B.C.) NIH Training Grant (T32AI007080 to W.J.H.) and the Patterson Trust Foundation (to F.C.N.).
There is a provisional patent application pending for the use of bacterial phosphorylated dihydroceramides (F.C.N. and R.B.C.).
References
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Nichols FC, Riep B, Mun J, Morton MD, Bojarski MT, Dewhirst FE, Smith MB. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J Lipid Res. 2004;45:2317–2330. doi: 10.1194/jlr.M400278-JLR200. [DOI] [PubMed] [Google Scholar]
- Nichols FC, Riep B, Mun J, Morton MD, Kawai T, Dewhirst FE, Smith MB. Structures and biological activities of novel phosphatidylethanolamine lipids of Porphyromonas gingivalis. J Lipid Res. 2006;47:844–853. doi: 10.1194/jlr.M500542-JLR200. [DOI] [PubMed] [Google Scholar]
- Miyagawa E, Azuma R, Suto T, Yano I. Occurrence of free ceramides in Bacteroides fragilis NCTC 9343. J Biochem. 1979;86:311–320. doi: 10.1093/oxfordjournals.jbchem.a132528. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Salyers AA. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol. 1984;38:293–313. doi: 10.1146/annurev.mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- Hill GB. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Nichols FC. Novel ceramides recovered from Porphyromonas gingivalis: relationship to adult periodontitis. J Lipid Res. 1998;39:2360–2372. [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols FC. Distribution of 3-hydroxy iC17:0 in subgingival plaque and gingival tissue samples: relationship to adult periodontitis. Infect Immun. 1994;62:3753–3760. doi: 10.1128/iai.62.9.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Safavi KE, Nichols FC. Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endodont. 1993;19:76–78. doi: 10.1016/S0099-2399(06)81199-4. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reife RA, Coats SR, Al-Qutub M, Dixon DM, Braham PA, Billharz RJ, Howald WN, Darveau RP. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell Microbiol. 2006;8:857–868. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Asai Y, Makimura Y, Tamai R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front Biosci. 2007;12:3795–3812. doi: 10.2741/2353. [DOI] [PubMed] [Google Scholar]
- Ukai T, Yumoto H, Gibson FC, 3rd, Genco CA. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-alpha production. Infect Immun. 2008;76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira L, Ayalon S, Brenner T. Effects of Porphyromonas gingivalis on the central nervous system: activation of glial cells and exacerbation of experimental autoimmune encephalomyelitis. J Periodontol. 2002;73:511–516. doi: 10.1902/jop.2002.73.5.511. [DOI] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]