Abstract
Macrophages are thought to play important roles during wound healing, but definition of these roles has been hampered by our technical inability to specifically eliminate macrophages during wound repair. The purpose of this study was to test the hypothesis that specific depletion of macrophages after excisional skin wounding would detrimentally affect healing by reducing the production of growth factors important in the repair process. We used transgenic mice that express the human diphtheria toxin (DT) receptor under the control of the CD11b promoter (DTR mice) to specifically ablate macrophages during wound healing. Mice without the transgene are relatively insensitive to DT, and administration of DT to wild-type mice does not alter macrophage or other inflammatory cell accumulation after injury and does not influence wound healing. In contrast, treatment of DTR mice with DT prevented macrophage accumulation in healing wounds but did not affect the accumulation of neutrophils or monocytes. Such macrophage depletion resulted in delayed re-epithelialization, reduced collagen deposition, impaired angiogenesis, and decreased cell proliferation in the healing wounds. These adverse changes were associated with increased levels of tumor necrosis factor-α and reduced levels of transforming growth factor-β1 and vascular endothelial growth factor in the wound. In summary, macrophages seem to promote both wound closure and dermal healing, in part by regulating the cytokine environment of the healing wound.
Tissue repair requires the coordinated response of a variety of cell types and many different molecular messengers and pathways. The healing process consists of overlapping phases of inflammation, tissue formation, and remodeling, which are common to repair of most tissues.1,2,3 During the inflammatory phase of skin repair, neutrophils and macrophages infiltrate the wound and are thought to help to clear the wound of damaged tissue and produce cytokines to help regulate the repair process. In the tissue formation phase, epithelial cells proliferate and migrate to cover the wound, endothelial cells participate in angiogenesis, and fibroblasts contribute to the process of dermal healing. Finally, the remodeling phase consists of regression of capillaries and the reorganization of connective tissue into a scar.
Although the inflammatory process is thought to be important in normal wound healing, inflammatory cells are also thought to contribute to scar formation in adult animals3,4 and are associated with chronic wounds in diabetic patients.1,5 Specifically, macrophages are thought to play significant roles in each process. In their classic study, Leibovich and Ross6 demonstrated that antimacrophage serum combined with hydrocortisone reduced macrophage accumulation in healing skin wounds of adult guinea pigs, impaired removal of damaged tissue and provisional matrix, reduced fibroblast accumulation, and delayed healing. A significant limitation of this study was the use of hydrocortisone, which probably influenced other cells in addition to macrophages, leaving open the possibility that effects on other cells contributed to the impairments observed. Although other studies have provided supportive evidence that macrophages may promote both wound closure and dermal healing after skin injury,7,8,9,10 no study to date has examined the effect of macrophage depletion that is temporally selective and cell type-specific during wound healing.
In contrast to studies indicating a positive role for macrophages in wound healing, evidence also suggests that inflammatory cells may promote scar formation and that excessive and/or prolonged inflammation may impair healing. Skin wounding in embryonic mice results in a reduced inflammatory response compared with that in adult mice and subsequent healing without scar formation.4,11 In addition, mice deficient in the transcription factor PU.1 lack neutrophils and macrophages, and wounds in neonatal mice of this strain heal without scarring.12 Experiments have not been performed with adult mice of this transgenic line because PU.1-null mice die within 48 hours after birth without antibiotic treatment. Furthermore, slow-healing wounds in mice, and chronic wounds in humans are associated with prolonged inflammation, including macrophage accumulation.1,5 Whether macrophages play causal roles in scar formation and/or development of chronic wounds remains to be determined. Nonetheless, the role of macrophages in wound healing is probably multifaceted and much remains to be learned about the role of macrophages in different aspects of the healing process.
The purpose of this study was to test the hypothesis that depletion of macrophages, in a manner that is selective in time and specific with regard to cell type, would be detrimental to wound healing in adult mice by reducing the production of growth factors important in the repair process. To overcome limitations of previous studies in targeting macrophages, we used transgenic mice that express the human diphtheria toxin (DT) receptor under the control of the CD11b promoter to specifically ablate macrophages during wound healing. Previous studies used these mice to selectively and specifically ablate macrophages and study their role in other models of tissue repair and inflammation.13,14 Our data show that macrophage depletion resulted in delayed re-epithelialization, decreased collagen deposition, impaired angiogenesis, and reduced cell proliferation associated with increased production of tumor necrosis factor (TNF)-α and decreased production of transforming growth factor (TGF)-β1 and vascular endothelial growth factor (VEGF).
Materials and Methods
Mice
Transgenic mice expressing the human DT receptor (DTR) under the control of the CD11b promoter have been described previously,13,14 and these DTR mice were used in the present experiments for ablation of macrophages during wound healing. The mouse DTR binds DT poorly, and thus wild-type FVB mice are relatively insensitive to DT. In addition, in the absence of DT, DTR mice do not exhibit any overt phenotypic differences compared with their wild-type strain. DTR transgenic mice and their FVB wild-type controls were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in groups of three to five at 22 to 24°C on a 12:12-hour light-dark cycle. Food and water were provided ad libitum. All experiments were performed on 10- to 12-week-old male mice and were approved by the Animal Care Committee of the University of Illinois.
Wound Model and Tissue Preparation
DTR transgenic mice and FVB wild-type mice were anesthetized with an i.p. injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). After the dorsum of each mouse was shaved, six full-thickness excisional wounds of 3-mm diameter were made with a standard biopsy punch (Acuderm, Ft. Lauderdale, FL). DT was administered by i.p. injection (25 ng/g b.wt.) immediately before injury and at 48 hours after injury. This protocol has been used to deplete macrophages during peritoneal inflammation and liver healing.13,14 At 1, 3, 5, 7, and 10 days after injury, mice were euthanized by cervical dislocation while anesthetized, and the wound and surrounding tissue were removed from the pelt with a 5-mm biopsy punch. For analysis of cell proliferation, mice received 30 mg/kg 5-bromo-2′-deoxyuridine (BrdU) by i.p. injection 1 hour before tissue collection. Wounds were either mounted in tissue freezing medium and frozen in isopentane chilled with dry ice for histological analysis or flash-frozen in liquid nitrogen for RNA or protein analysis. For histological analysis, wounds were sectioned from one edge to well past the center, and then sections were selected from the center of the wound by microscopic assessment. Two 10-μm sections judged to be at the actual center of the wound were used for re-epithelialization measurements and adjacent 10-μm sections were used for inflammatory cell staining, trichrome staining, CD31 staining, and BrdU staining.
Re-Epithelialization
Wound re-epithelialization was measured by morphometric analysis of wound sections as described previously.15 In brief, sections taken from the center of the wound were stained with H&E. The distance between the wound edges, defined by the distance between the first hair follicle encountered at each end of the wound, and the distance that the epithelium had traversed into the wound, were measured using image analysis software. The percentage of re-epithelialization [(distance traversed by epithelium)/(distance between wound edges) × 100] was calculated for two sections per wound and was averaged over sections to provide a representative value for each wound.
Inflammatory Cell Accumulation
Immunohistochemical analysis was performed on cryosections essentially as described.16 Sections were air-dried, fixed in cold acetone, washed with PBS, quenched with 0.3% hydrogen peroxide, and washed with PBS. Sections were blocked with buffer containing 3% bovine serum albumin and then incubated with F4/80 antibody to label macrophages (1:50, Serotec, Oxford, UK), Ly6C antibody to label monocytes and neutrophils (1:400, Serotec), or Ly6G antibody to label neutrophils (1:100, BD Pharmingen, San Diego, CA). Sections were then washed with PBS and incubated with biotinylated anti-rat secondary antibody (1:200, Vector Laboratories, Burlingame, CA). After a wash with PBS, sections were incubated with avidin D-horseradish peroxidase (1:1000) and developed with a 3-amino-9-ethylcarbazole kit (Vector Laboratories). The number of labeled cells in the entire wound bed was counted at ×20 magnification (Labphot-2, Nikon, Melville, NY) and then normalized to the volume of the wound bed = section thickness × area of wound bed (SPOT software, Diagnostic Instruments, Inc., Sterling Heights, MI). The wound bed was defined as the area demarcated by the first hair follicle on each end of the wound, the superficial surface of the wound, and the muscle layer deep to the wound.
Collagen Deposition and Angiogenesis
Dermal healing was assessed using Masson’s trichrome stain for collagen deposition and immunohistochemical staining for platelet-derived endothelial cell adhesion molecule-1 (also called CD31) for angiogenesis. For trichrome analysis, staining was performed according to the manufacturer’s directions (IMEB, San Marcos, CA), and image analysis software (Scion Image, Scion, Frederick, MD) was used to quantify the percentage of blue collagen-stained area relative to the total area of the wound bed. For angiogenesis, an antibody against CD31 (BD Pharmingen) was used in conjunction with procedures identical to those for inflammatory cells, and image analysis software was used to quantify the percentage of CD31-stained area relative to the total area of the wound bed. For each assay, digital images covering the majority of the wound bed (usually two to three images at ×20 magnification) were first obtained. The percent area stained in each image was then quantified by counting the number of pixels staining above a threshold intensity and normalizing to the total number of pixels. Threshold intensity was set such that only clearly stained pixels were counted. The software allowed the observer to exclude staining identified as artifact, large vessels, and areas deemed to be outside the wound bed. For both trichrome and CD31 staining, two sections per wound were analyzed, and data were averaged over sections to provide a representative value for each wound.
Cell Proliferation
Cell proliferation was assessed by staining for BrdU, which is incorporated into newly synthesized DNA. Sections from wounds collected at 5 days postinjury were air-dried, fixed in cold acetone, washed with PBS, incubated in 2 N HCl, and washed with basic (pH 8.5) and neutral PBS (pH 7.6). Sections were then incubated in 0.1% IGEPAL and in blocking buffer containing 3% bovine serum albumin. Proliferating cells were labeled with a BrdU antibody (1:10, Roche Diagnostics, Indianapolis, IN) for 1 hour. Sections were washed with PBS and incubated with fluorescein-isothiocyanate-conjugated anti-mouse secondary antibody (1:200, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The number of labeled cells was counted in the wound bed of two sections per wound, normalized to the volume of the wound bed sampled, and then averaged over sections to provide a representative value for each wound.
RNA Analysis
Total RNA was isolated from wounds collected at 5 days postinjury using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA quantity was determined by UV absorption at 260 nm, and quality was verified by the 260/280 nm ratio and formaldehyde-agarose gel electrophoresis. RNA (2 μg) was reverse transcribed using a Thermoscript RT-PCR kit (Invitrogen, Carlsbad, CA), and semiquantitative PCR was performed for glyceraldehyde-3-phosphate dehydrogenase, TNF-α, TGF-β1, and VEGF with the primers described in Table 1. Cycling conditions were optimized to ensure that each target was within its linear range. Images of ethidium bromide-stained gels were analyzed by densitometry.
Table 1.
PCR Primers
| Gene product | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | 5′-TCCACCACCCTGTTGCTGGTA-3′ |
| TNF-α | 5′-TTCCAGATTCTTCCCTGAGGT-3′ | 5′-TAAGCAAAAGAGGAGGCAACA-3′ |
| VEGF | 5′-CTGTGCAGGCTGCTGTAACG-3′ | 5′-GTTCCCGAAACCCTGAGGAG-3′ |
| TGF-β1 | 5′-CCCCACTGATACGCCTGAGT-3′ | 5′-AGCCCTGTATTCCGTCTCCTT-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Protein Analysis
Wounds collected at 5 days postinjury were homogenized in 500 μl of cold PBS supplemented with protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride, 1 μmol/L leupeptin, and 0.3 μmol/L aprotinin) using a Dounce homogenizer. Samples were then sonicated and centrifuged at 10,000 × g, and the resulting supernatant was stored at −80°C. TNF-α, TGF-β1, and VEGF protein levels were measured using enzyme-linked immunosorbent assay kits (eBioscience, San Diego, CA; R&D Systems, Minneapolis, MN), according to the manufacturer’s directions.
Statistics
Values are reported as means ± SE. For comparisons of inflammatory cell accumulation, re-epithelialization, trichrome staining, and CD31 staining, data were compared across treatment groups and time points using two-way analysis of variance. The Student-Newman-Keuls post hoc test was used when analyses of variance demonstrated significance. For groups that did not pass tests of normality and equal variance, the nonparametric Kruskal-Wallis analysis of variance on ranks was used with Dunn’s post hoc method. Comparisons of cell proliferation, RNA, and protein levels were made between treatment groups using t-tests. Differences between groups were considered significant if P ≤ 0.05.
Results
Macrophage Ablation during Wound Healing
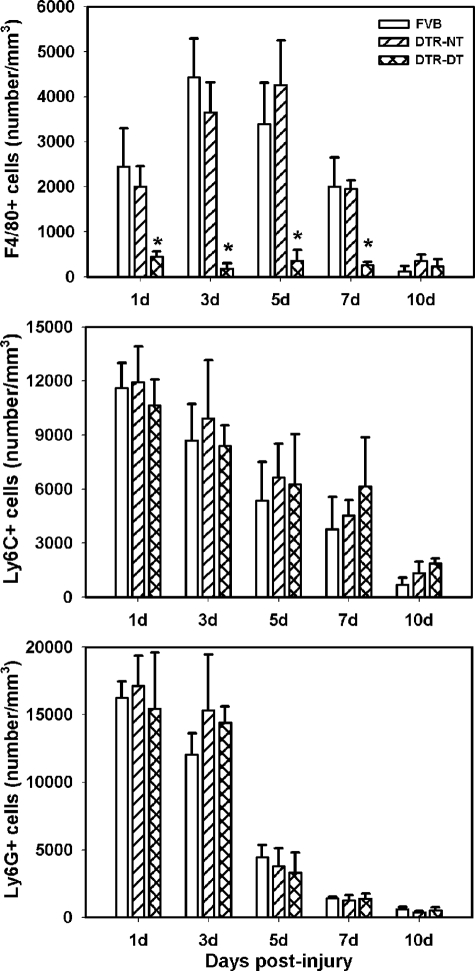
DTR transgenic mice have been used previously to study the effects of specific macrophage ablation on peritoneal inflammation and liver injury and repair.13,14 In the present study, we used these mice to determine the effects of macrophage depletion during healing of excisional skin wounds. We first assessed the accumulation of inflammatory cells in wild-type FVB and DTR transgenic mice without DT treatment, using antibodies against the Ly6G, Ly6C, and F4/80 antigens to identify neutrophils, monocytes, and mature macrophages, respectively. We did not observe any Ly6G+ or Ly6C+ cells in uninjured control skin, and very few F4/80+ cells (100 ± 80 cells/mm3). After skin wounding, immunohistochemical analysis indicated that that Ly6G+ and Ly6C+ cells accumulated in large numbers at 1 day postinjury and then progressively decreased toward control levels at 10 days postinjury (Figure 1). F4/80+ cells started to accumulate at 1 day postinjury, increased to peak at 3 to 5 days postinjury, then decreased to control levels by 10 days postinjury (Figure 1). This time course of inflammatory cell accumulation is typical of what would be expected for neutrophils, monocytes, and macrophages, respectively, and to the extent that we could determine, were nearly identical between wild-type and transgenic mice. These data indicate that the presence of the transgene did not affect the inflammatory process.
Figure 1.
Macrophage ablation during wound healing. DTR transgenic mice and FVB wild-type mice were subjected to excisional wounding and either left untreated (NT) or treated with DT to induce macrophage depletion. Cryosections of wounds collected on days 1 to 10 postinjury were stained with antibodies against F4/80 (top), Ly6C (middle), and Ly6G (bottom) as markers for macrophages, monocytes, and neutrophils, respectively. The number of labeled cells was counted using a ×20 objective and normalized to volume of the wound bed. Data are presented as means ± SE; n = 4 to 6 mice/time point. *P < 0.05 when compared to nontreated controls.
The administration of DT to DTR mice prevented macrophage accumulation without affecting the accumulation of other inflammatory cells (Figure 1). The number of F4/80+ cells was significantly reduced at each time point after injury in DTR mice treated with DT compared with wild-type FVB and untreated DTR mice. In contrast, DT administration did not alter the number of Ly6C+ or Ly6G+ cells in healing wounds (Figure 1), indicating that monocyte and neutrophil accumulation was not affected by DT administration. In addition, DT treatment of wild-type FVB mice did not influence the accumulation of F4/80+ cells (4429 ± 860 cells/mm3 untreated versus 5099 ± 505 cells/mm3 treated), Ly6C+ cells (8707 ± 1996 cells/mm3 untreated versus 7564 ± 1472 cells/mm3 treated), or Ly6G+ cells (12,050 ± 1564 cells/mm3 untreated versus 12,900 ± 2498 cells/mm3 treated) at 3 days postinjury, indicating that inflammatory cells in wild-type mice were insensitive to DT.
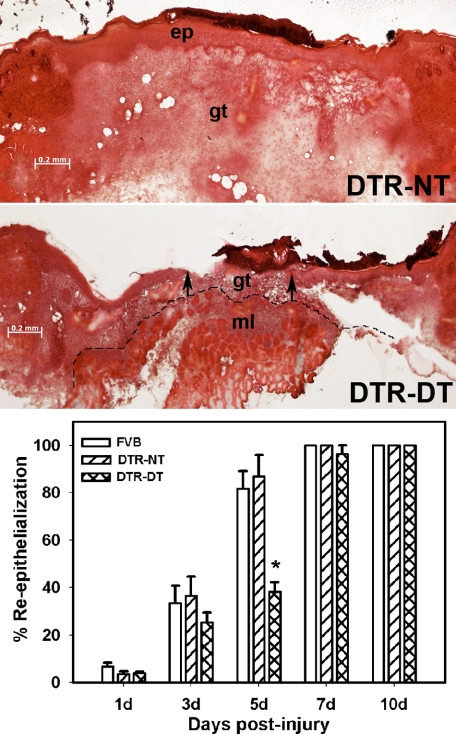
Macrophage Ablation Results in Delayed Re-Epithelialization
After excisional wounding, wild-type and untreated DTR mice showed progressive increases in percent re-epithelialization in H&E-stained sections, with most wounds closed by day 5 and all wounds closed by day 7 (Figure 2). No differences were observed between wild-type and untreated DTR mice. In contrast, DTR mice treated with DT showed significantly delayed closure with day 5 wounds showing mean re-epithelialization values that were less than one-half of those in wild-type or untreated DTR mice. At this time point, three of five wild-type and untreated DTR mice showed completely closed wounds, whereas none of the DT-treated DTR mice had completely closed wounds. DT treatment did not influence re-epithelialization in wild-type mice (33.5 ± 7.3% untreated versus 31.7 ± 5.6% treated), confirming that the wild-type mice were insensitive to DT treatment. An estimate of wound contraction was made from measurements of wound length, which was defined as the distance between the first hair follicle at either end of the wound bed. In nontreated mice, wound length was reduced from 3.1 ± 0.2 mm at 1 day postinjury to 2.0 ± 0.2 mm at 7 days postinjury, indicating that wound contraction did occur. In macrophage-depleted mice, wound length measurements were 3.0 ± 0.3 mm at 1 day postinjury and 2.0 ± 0.1 mm at 7 days postinjury and were not significantly different from the measurements in the nontreated mice. These data indicate that macrophage depletion results in delayed wound closure but does not seem to affect wound contraction.
Figure 2.
Macrophage ablation results in delayed re-epithelialization. DTR transgenic mice and FVB wild-type mice were subjected to excisional wounding and either left untreated (NT) or treated with DT. Cryosections of wounds collected on days 1 to 10 postinjury were stained with H&E. Representative sections of wounds on day 5 postinjury showing delayed re-epithelialization in DTR-DT mice (middle) compared with DTR-NT mice (top) (images obtained with a ×5 objective). Arrows indicate ends of the migrating epithelial tongues. ep, epithelium; gt, granulation tissue; ml, subcutaneous muscle layer. Dashed line indicates the border between granulation tissue and subcutaneous muscle layer. Scale bar = 0.2 mm. Bottom: the percentage of re-epithelialization [(distance traversed by epithelium)/(distance between wound edges) × 100] was measured in each section by image analysis. Data are presented as means ± SE; n = 4 to 6 mice/time point. *P < 0.05 when compared to nontreated controls.
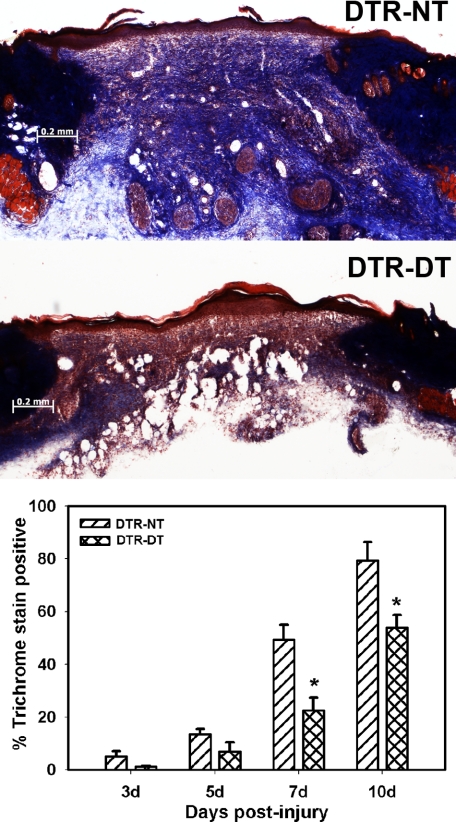
Macrophage Ablation Results in Reduced Granulation Tissue Formation and Subsequent Collagen Deposition
In H&E-stained sections, granulation tissue was abundant in wounds of untreated DTR mice, whereas granulation tissue formation was noticeably reduced in treated mice (Figure 2). This result was also evident during wound harvest, as subcutaneous tissue had to be removed with the pelt in treated mice to maintain wound integrity. Trichrome staining of wound sections revealed that, after excisional wounding in untreated DTR mice, collagen deposition increased progressively from day 3 to day 10 postinjury, such that collagen staining was near noninjured control levels on day 10 after injury (Figure 3); the dermis of noninjured control tissue was >98% positive for blue trichrome staining. On the other hand, DTR mice treated with DT showed significantly reduced collagen deposition assessed by trichrome staining on days 7 and 10 after injury. At 7 days postinjury, the area of the wound bed was 1.8 ± 0.3 mm2 in nontreated mice and 1.1 ± 0.2 mm2 in macrophage-depleted mice, indicating that wound bed area was smaller in macrophage-depleted mice. When combined with the lack of difference in wound contraction, the smaller wound bed area in the macrophage-depleted mice seemed to be due primarily to a reduction in granulation tissue formation in the wound bed.
Figure 3.
Macrophage ablation results in reduced collagen deposition. DTR transgenic mice were subjected to excisional wounding and either left untreated (NT) or treated with DT. Cryosections of wounds collected on days 3 to 10 postinjury were stained with Masson’s trichrome. Representative sections of wounds on day 7 postinjury show reduced collagen deposition, indicated by less blue staining, in DTR-DT mice (middle) compared with DTR-NT mice (top) (images obtained with a ×5 objective). Scale bar = 0.2 mm. Bottom: the area stained blue in each section was measured by image analysis and normalized to the area of the wound bed. Data are presented as means ± SE; n = 4 to 6 mice/time point. *P < 0.05 when compared to nontreated controls.
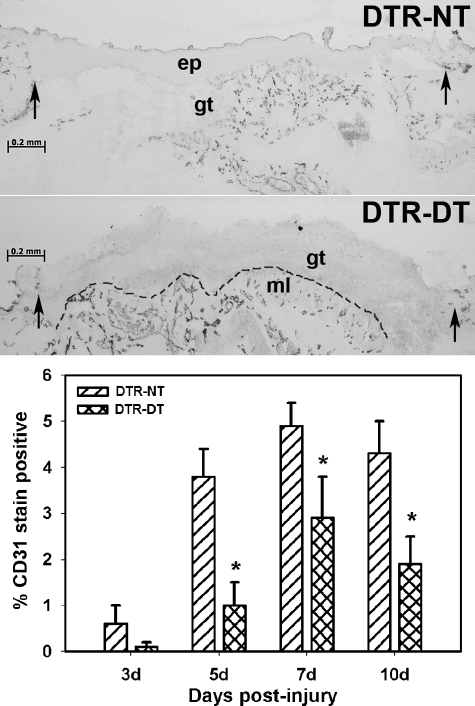
Macrophage Ablation Results in Impaired Angiogenesis
Analysis of angiogenesis in untreated DTR mice using CD31 labeling of wound sections indicated an increase in vessel density from days 3 to 7 postinjury (Figure 4). Treatment of DTR mice with DT significantly reduced vessel density on days 5, 7, and 10 postinjury. Taken together, the trichrome and CD31 data indicate that macrophage ablation results in impaired dermal healing involving reduced collagen deposition and impaired vessel formation.
Figure 4.
Macrophage ablation results in impaired angiogenesis. DTR transgenic mice were subjected to excisional wounding and either left untreated (NT) or treated with DT. Cryosections of wounds collected on days 3 to 10 postinjury were stained with an antibody against the endothelial cell marker CD31 to assess angiogenesis. Representative sections of wounds on day 5 postinjury showing impaired vessel formation in DTR-DT mice (middle) compared with DTR-NT mice (top) (images obtained with a ×5 objective). Arrows indicate the wound edges. ep, epithelium, gt, granulation tissue, ml, muscle layer. Dashed line indicates border between granulation tissue and subcutaneous muscle layer. Scale bar = 0.2 mm. Bottom: the stained area in the granulation tissue of each section was measured by image analysis and normalized to the area of the wound bed sampled. Data are presented as means ± SE; n = 4 to 6 mice/time point. *P < 0.05 when compared to nontreated controls.
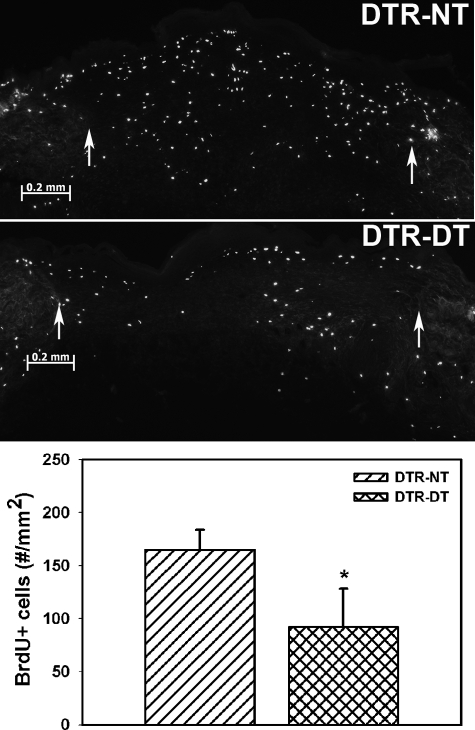
Macrophage Ablation Results in Reduced Cell Proliferation during Wound Healing
Incorporation of BrdU into newly synthesized DNA was used to assess cell proliferation on day 5 postinjury. In untreated DTR mice, a substantial number of BrdU-positive cells/mm2 were observed in the wound bed, indicating an abundance of proliferating cells (Figure 5). Treatment of DTR mice with DT significantly reduced the number of BrdU-positive cells/mm2 by ∼40%. The lower concentration of BrdU-positive cells and smaller wound bed areas in macrophage-depleted mice indicate that the number of BrdU-positive cells was smaller both in absolute numbers and in the numbers per unit area in these mice.
Figure 5.
Macrophage ablation results in reduced cell proliferation during wound healing. DTR transgenic mice were subjected to excisional wounding and either left untreated (NT) or treated with diphtheria toxin (DT). BrdU was injected into mice 1 hour before tissue collection, and cryosections of wounds collected on day 7 postinjury were stained with an antibody against BrdU. Representative sections of wounds on day 7 postinjury showing reduced proliferation in DTR-DT mice (middle) compared with DTR-NT mice (top; images obtained with a ×5 objective). Arrows indicate wound edges. Scale bar = 0.2 mm. Bottom: the number of labeled cells in each section was counted and normalized to area of the wound bed. Data are presented as means ± SE; n = 6 mice/group. *P < 0.05 when compared to nontreated controls.
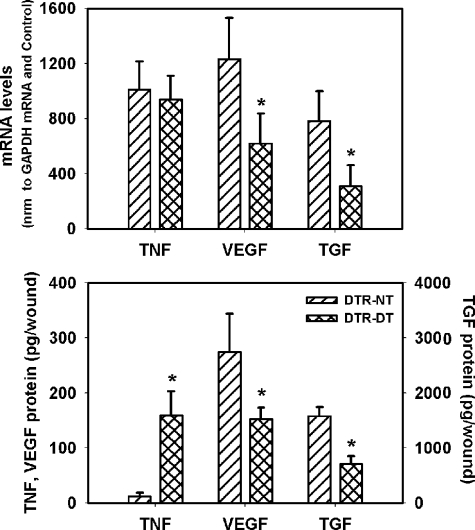
Macrophage Ablation Results in Altered Cytokine Expression
Macrophages have the capacity to produce a variety of cytokines,17 including TNF-α, VEGF, and TGF-β1. We used PCR and enzyme-linked immunosorbent assay to determine whether macrophage depletion resulted in changes of expression of these growth factors in the healing wound (Figure 6). Although TNF-α mRNA levels were not different between untreated and DTR mice treated with DT, protein levels were markedly higher in DT-treated mice at 5 days postinjury. In addition, both VEGF and TGF-β1 mRNA and protein levels were significantly reduced in DT-treated compared with untreated DTR mice. Thus, impaired healing with DT treatment was associated with an altered cytokine environment, which seems to be more pro-inflammatory and less conducive to wound cell proliferation, compared with that in nontreated mice.
Figure 6.
Macrophage ablation results in altered cytokine expression. DTR transgenic mice were subjected to excisional wounding and either left untreated (NT) or treated with DT. Wounds collected on day 5 postinjury were processed for either RNA or protein analysis and levels of TNF-α, TGF-β1, and VEGF mRNA and protein were assessed by RT-PCR and enzyme-linked immunosorbent assay, respectively. Top: densitometric values for target mRNA levels were normalized to those for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels and then to similarly normalized values for noninjured control samples. Bottom: protein levels expressed as picograms of protein in the total wound homogenate. Data are presented as means ± SE; n = 4 mice/group. *P < 0.05 when compared to nontreated controls.
Discussion
Macrophages are thought to play important roles in host defense, clearance of damaged tissue, and formation of new tissue during normal wound healing but have also been implicated in scar formation and the development of chronic wounds.1,2,3,4,5 Definition of the roles of macrophages in wound healing has been hampered by difficulties in specifically targeting these inflammatory cells. In the present study, transgenic mice expressing the human DTR under the control of the CD11b promoter were used to specifically ablate macrophages and test the hypothesis that macrophages are required for efficient skin wound healing in adult animals. The major findings of this study were that macrophage depletion resulted in delayed re-epithelialization, reduced collagen deposition, and impaired angiogenesis. These impairments in wound healing were associated with increased production of TNF-α and reduced production of VEGF and TGF-β1. Thus, macrophages regulate both wound closure and dermal healing, in part by regulating the cytokine environment of the healing wound.
DT administration to DTR transgenic mice prevented the accumulation of F4/80+ cells without influencing the accumulation of Ly6C+ or Ly6G+ cells in healing wounds. F4/80 expression is limited to cells of the macrophage lineage and is increased during differentiation of monocytes to macrophages.18,19 Thus, our data show that DT treatment prevented accumulation of mature macrophages during wound healing. In addition to macrophages, neutrophils express CD11b, and DTR transgenic mice might be expected to exhibit neutrophil depletion with DT treatment. However, our data indicate no effect of DT treatment on numbers of Ly6G+ cells, a widely used marker for neutrophils,20 suggesting that neutrophil accumulation was not influenced by DT treatment. These findings are consistent with those of previous studies showing that DT treatment depleted macrophages without affecting granulocyte or lymphocyte numbers in the circulation or during peritoneal inflammation.13,14 Finally, Ly6C is expressed on monocytes, and its expression is down-regulated during monocyte differentiation to macrophages.21,22 Ly6C can also be expressed on granulocytes and endothelial cells. However, the time course of Ly6C+ staining was substantially different from that of the widely used endothelial marker CD31; thus, the Ly6C+ cells were probably not endothelial cells. Ly6C+ cell number was similar to Ly6G+ cell number early after injury, but Ly6C+ cells decreased more slowly over time. These data indicate that Ly6C+ cells probably represent monocytes as well as neutrophils. The observation that Ly6C+ cell accumulation was not affected by DT treatment may reflect the increase in CD11b expression during monocyte/macrophage maturation,23,24 rendering mature macrophages more susceptible to DT-induced depletion. We plan to characterize the phenotype and function of the Ly6C+ cells during wound healing in future studies.
Our data showing that macrophage depletion is detrimental to wound healing are consistent with those of Leibovich and Ross.6 These investigators prevented macrophage accumulation during healing of linear incisions in adult guinea pigs using local administration of antimacrophage serum combined with systemic treatment with hydrocortisone. Their antimacrophage treatment impaired removal of fibrin and miscellaneous debris, delayed re-epithelialization, and reduced fibroblast accumulation and collagen deposition. A significant limitation of this study was the use of hydrocortisone, which probably influenced a variety of other cells involved in the healing process. Our findings are also consistent with other studies showing that interfering with selectin, integrin, or chemokine receptor binding reduces accumulation of neutrophils and macrophages and impairs wound healing.7,8,9,10 All of these studies were limited by the lack of specificity in targeting macrophages, leaving open the possibility that other cells contributed to the impairments observed. Our data demonstrate that temporally selective and cell type-specific depletion of macrophages is detrimental both to wound closure and dermal healing.
In contrast to the positive effect of macrophages on wound healing suggested by the present study, macrophages may also cause detrimental effects by promoting scar formation. Some have argued that evolution has optimized wound repair for fighting infection and rapid healing and that scar formation with its inferior structural properties is the result of such optimization.25 Mice deficient in the transcription factor PU.1 lack neutrophils and macrophages, and wounds in neonatal mice of this strain heal without scarring.12 In addition, wounds of fetal mice show reduced inflammatory cell accumulation and exhibit scarless healing, associated with reduced levels of TGF-β1.4 Macrophages are known to produce a variety of factors that affect scarring, including TGF-β1,17 and our data indicate that macrophage depletion results in reduced levels of TGF-β1 and reduced deposition of collagen. One could speculate that macrophage depletion results in delayed healing but may lead to long-term improvement in tissue structure and function; however, further experiments are needed to investigate such speculation.
Our findings that macrophage depletion resulted in impaired angiogenesis associated with reduced VEGF levels are consistent with previous studies linking macrophages to angiogenesis during wound healing. Macrophages were found to secrete proangiogenic factor(s) when cultured under hypoxic conditions,26 and inflammatory cells isolated from wounds in rabbits stimulated angiogenesis when transplanted to the cornea, an effect thought to be mediated by macrophages.27 Macrophages are known to produce VEGF, a proangiogenic factor, and this production is regulated in part by the transcription factor hypoxia-inducible factor-1α.28,29 The expression of hypoxia-inducible factor-1α, in turn, may be regulated by cytokines as well as by hypoxia during wound healing.30 However, much remains to be learned about the regulation of VEGF production by macrophages and other cells during wound healing.
Macrophage function is strongly influenced by the microenvironment in which macrophages are immersed in the healing wound, and such plasticity probably plays important roles in wound repair. Macrophages were classically viewed as having an inflammatory, pathogen-killing phenotype associated with production of high levels of inflammatory cytokines, inducible nitric oxide synthase, and reactive oxygen and nitrogen species. However, a broad spectrum of macrophage phenotypes has been described more recently, depending in part on environmental conditions.31,32,33 Our findings demonstrated that macrophage depletion resulted in decreased VEGF levels, indicating that macrophages either produced VEGF or induced other cells in the wound to produce this factor. In addition, macrophage depletion resulted in increased TNF-α levels and decreased TGF-β1 levels in the wound at 5 days postinjury. Because “alternative activation” of macrophages is known to induce an anti-inflammatory phenotype with increased TGF-β1 expression,31,32,33 these data indicate the presence of anti-inflammatory macrophages in the wound at this time point. Finally, macrophage depletion resulted in reduced cell proliferation in the wound bed. Interestingly, stimulation of cultured macrophages with interleukin-4, resulting in the alternatively activated phenotype, induced these cells to produce an unknown factor that stimulated fibroblast proliferation.34 Whether a similar process occurs in the healing wound remains to be determined. Overall, the actual macrophage phenotypes involved in wound healing and how these are regulated and contribute to healing remain to be elucidated.
While this article was in revision, Goren et al35 reported another model of macrophage depletion during wound healing, using mice that expressed the DT receptor under the control of the lysozyme M promoter. In that study, macrophage depletion resulted in delayed closure and impaired angiogenesis, associated with increased expression of inflammatory mediators and reduced expression of VEGF at 5 days postinjury; these data are consistent with those of the present study. In contrast, though, Goren et al reported a prolonged increase in neutrophil accumulation with macrophage ablation and a reduction in wound contraction, whereas we found no evidence of differences in the time course of neutrophil accumulation or in wound contraction. Differences between the studies may be explained by differences in the background strains of the transgenic mice (FVB for our study, C57BL/6 for Goren et al) or by differences in transgenic construct (CD11b-DTR transgene for our study and the Cre-lox double transgenic approach for Goren et al).
In summary, our data show that specific macrophage depletion at the time of wounding results in delayed re-epithelialization, reduced collagen deposition, impaired angiogenesis, and decreased cell proliferation. These impairments in wound healing were associated with increased expression of TNF-α and reduced expression of VEGF and TGF-β1. These data indicate that macrophages promote normal adult wound healing and regulate the cytokine environment in these wounds. In chronic wounds, the wound environment becomes dysregulated, and we speculate that impairments in macrophage function may contribute to the development of this pathological state.
Acknowledgments
We thank Traci Wilgus, Ph.D., for technical help during the early stages of this project, and Corrie Behm, Ph.D., for her critical comments of the manuscript.
Footnotes
Address reprint requests to Timothy J. Koh, Ph.D., Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 W. Taylor St., Room 650, Chicago, IL 60612. E-mail: tjkoh@uic.edu.
Supported by the National Institutes of Health (grants R01-GM50875 and P20-GM078426 to L.A.D.).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of General Medical Sciences or National Institutes of Health.
References
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525 17299434. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Mori R, Kondo T, Nishie T, Ohshima T, Asano M. Impairment of skin wound healing in β-1,4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am J Pathol. 2004;164:1303–1314. doi: 10.1016/s0002-9440(10)63217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, Wagner DD. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol. 2008;180:569–579. doi: 10.4049/jimmunol.180.1.569. [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J, Lang RA. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1α−/− and MCP-1−/− mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryer SC, Fantuzzi G, Van Rooijen N, Koh TJ. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol. 2008;180:1179–1188. doi: 10.4049/jimmunol.180.2.1179. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, Kraal G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 2005;26:506–509. doi: 10.1016/j.it.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow: RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, Melis M, Slieker WA, Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990;20:27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Miller BA, Antognetti G, Springer TA. Identification of cell surface antigens present on murine hematopoietic stem cells. J Immunol. 1985;134:3286–3290. [PubMed] [Google Scholar]
- Springer T, Galfre G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Hunt TK, Knighton DR, Thakral KK, Goodson WH, 3rd, Andrews WS. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96:48–54. [PubMed] [Google Scholar]
- Burke B, Tang N, Corke KP, Tazzyman D, Ameri K, Wells M, Lewis CE. Expression of HIF-1α by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J Pathol. 2002;196:204–212. doi: 10.1002/path.1029. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr, Reichner JS. HIF-1 expression in healing wounds: HIF-1α induction in primary inflammatory cells by TNF-α. Am J Physiol Cell Physiol. 2001;281:C1971–C1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]