Abstract
In galactosamine (GalN)-induced rat liver injury, hepatic stem/progenitor cells, small hepatocytes (SHs) and oval cells, transiently appear in the initial period of liver regeneration. To clarify the relationship between SHs and oval cells, CD44+ and Thy1+ cells were sorted from GalN-treated livers and used as candidates for SHs and oval cells, respectively. Some Thy1+ cells isolated 3 days after GalN-treatment (GalN-D3) formed CD44+ cell colonies, but those from GalN-D2 could form few. GeneChip (Affymetrix, Inc, Santa Clara, CA) analysis of the sorted cells and cultured Thy1+ cells suggested that hepatocytic differentiation progressed in the order Thy1+ (GalN-D3), Thy1+ cell colony (Thy1-C), and CD44+ (GalN-D4) cells. When Thy1+, Thy1-C, and CD44+ cells were transplanted into retrorsine/PH rat livers, they could proliferate to form hepatocytic foci. At 30 days after transplantation most cells forming the foci derived from CD44+ cells possessed C/EBPα+ nuclei, whereas only a few cells derived from Thy1-C showed this positivity. When Thy1+ (GalN-D3) cells were cultured between collagen gels in medium with hepatocyte growth factor+/dexamethasone−/dimethyl sulfoxide−, ducts/cysts consisting of biliary epithelial cells appeared, whereas with CD44+ and Thy1+ (GalN-D2) cells they did not. Taken together, these results indicate that the commitment of Thy1+ cells to differentiate into hepatocytes or biliary epithelial cells may occur between Day 2 and Day 3. Furthermore, some Thy1+ cells may differentiate into hepatocytes via CD44+ SHs.
It is well known that hepatic stem/progenitor cells are activated when the proliferation of mature hepatocytes (MHs) is inhibited by hepatotoxins.1,2,3,4,5,6 Of these hepatic stem/progenitor cells, oval cells and small hepatocytes (SHs) are well recognized. Oval cells, named for their possession of ovoid nuclei,7 are known to express markers for biliary epithelial cells (BECs), eg, cytokeratin (CK) 7 and CK19, and for hepatoblasts, eg, a-fetoprotein (AFP), and cell membrane proteins such as CD34, c-kit, and Thy-1, shared hematopoietic stem cell markers.8 Recently, the expression of delta-like protein/preadipocyte factor 1 and glypican-3 in oval cells was reported.9,10 SHs are a subpopulation of hepatocytes.11 Their size is less than half that of MHs and they possess hepatic characteristics. These cells can clonally proliferate12 and mature by interacting with hepatic nonparenchymal cells13 or as a result of treatment with Matrigel (BD Bioscience, Bedford, MA).14 The mature SHs express genes and proteins related to hepatic differentiated functions.14,15,16,17 Recently, we reported that CD44 and D6.1A were specifically expressed in cultured SHs and that the expression disappeared when SHs matured.18
In d-galactosamine (GalN)-induced rat liver injury, the appearance of oval cells and SHs is observed in a relatively short period, within 1 week.19 We observed that CD44+ SHs transiently appeared at 3 to 5 days after GalN administration and that their appearance was delayed compared with that of oval cells.18 The existence of SHs has been reported in another hepatic injury model. Paku et al20 used a 2-acetylaminofluorene (2-AAF)/partial hepatectomy (PH) model and demonstrated the appearance of SHs by analyzing their ultrastructure. In the retrorsine following PH (Ret/PH) model, small hepatocyte-like progenitor cells (SHPCs) have been reported to appear.21,22 Furthermore, Avril et al23 reported that after MHs were labeled with the β-galactosidase gene, β-galactosidase+ SHPCs were found in the liver lobules of the Ret/PH model rat. In contrast, by treating transgenic mice expressing hepatitis B surface antigen with retrorsine, Vig et al24 demonstrated that oval cells could change into SHPCs. Therefore, the differentiation of oval cells into SHs is still controversial.
In the present study we used the GalN-injury model to clarify whether oval cells could differentiate into hepatocytes or BECs. To resolve this issue, we used Thy1+ and CD44+ cells isolated from GalN-treated rat livers as oval cells and SHs, respectively. Many CD44+ cells isolated from 4 days after GalN-treatment (GalN-D4) could form CD44+ SH colonies, whereas some Thy1+ (GalN-D3) cells could form CD44+ SH colonies but those from GalN-D2 could form few. When isolated cells were transplanted into Ret/PH rat livers, both CD44+ and Thy1+ cells could proliferate to form foci. However, the size of foci was much larger for CD44+ cells than for Thy1+ cells. GeneChip (Affymetrix, Inc, Santa Clara, CA) analysis of gene expression suggested that hepatic differentiation progressed in the order Thy1+ (GalN-D3), cultured Thy1+ cells (Thy1-C), and CD44+ (GalN-D4) cells. In addition, CD44+ cells, SHs, and Thy1-C may possess very similar characteristics, but they are clearly different from MHs. When the sorted cells were cultured between collagen gels in medium with hepatocyte growth factor (HGF)+/dexamethasone (Dex)−, some Thy1+ cells could form ducts/cysts consisting of BECs and differentiate hepatocytes. In the present experiment, the commitment of Thy1+ cells to hepatocytic or biliary differentiation occurred between Day 2 and Day 3 after GalN-treatment. Some Thy1+ cells may differentiate into hepatocytes via CD44+ SHs.
Materials and Methods
Animals and Liver Injury Model
Male F344 rats (dipeptidylpeptidase IV [DPPIV]+ strain; Sankyo Lab Service Corporation, Inc, Tokyo, Japan) weighing 150 to 200 g were used. All animals received humane care and the experimental protocol was approved by the Committee of Laboratory Animals according to Sapporo Medical University guidelines. For GalN-injured livers, GalN (Acros, Geel, Belgium; 75 mg/100 g body weight dissolved in PBS) was intraperitoneally administered.18 For the transplantation experiment, female F344 rats (DPPIV− strain; Charles River, Wilmington, MA) were intraperitoneally given two injections of retrorsine (30 mg/kg body weight; Sigma Chemical Co, St. Louis, MO), 2 weeks apart.21 Four weeks after the second injection, two-thirds PH was performed. Sorted DPPIV+ cells (5 × 105 cells) and colonies (about 1 × 104 colonies) from cultured Thy1+ cells were transplanted into Ret/PH livers (DPPIV−) via the spleen (three to five rats per group).
Isolation of Cells from Liver
Rats were used to isolate hepatic cells by the collagenase perfusion method as previously described.25 After the perfusion, the cell suspension was centrifuged at 50 × g for 1 minute. The supernatant and the precipitate were used for sorting Thy1+ and CD44+ cells and preparing MHs, respectively.18
Cell Sorting and Culture
The supernatant was centrifuged at 50 × g for 1 minute again. After this procedure was repeated, the supernatant was centrifuged at 50 × g for 5 minutes. The precipitate was suspended in PBS containing 2 mmol/L EDTA and 0.5% bovine serum albumin. Antibodies used for cell sorting are listed in Table 1. First, 2 μg/ml of anti-Thy1 or 625 ng/ml of anti-CD44 antibodies was added to the cell suspension and then a microbead-labeled secondary antibody was added. The suspension volume and incubation time were followed by manufacturer’s information. Magnetic separation was done by using a MidiMACS separation unit (Miltenyi Biotec, Bergisch Gladbach, Germany). After the number of viable cells was counted, 1 × 105 viable cells were plated in a 12-well plate (Corning, Corning, NY) and cultured in the medium listed in Table 2. Some sorted Thy1+ cells were cultured for 5 days and epithelial cell colonies were collected as previously reported.17 The isolated colonies were used for the GeneChip (Affymetrix, Inc) analysis and transplantation. The selective isolation of the colonies is shown in Supplement Figure S1, see http://ajp.amjpathol.org.
Table 1.
List of Antibodies Used in the Present Experiments
| Antibodies | Company or producer | Dilution |
|---|---|---|
| Mouse anti-rat CD44 | BD Biosciences PharMingen, Franklin Lakes, NJ | 1:1000 |
| Rabbit anti-rat C/EBPα | Santa Cruz, Santa Cruz, CA | 1:400 |
| Rabbit anti-rat CK19 | Generous gift from Professor Atsushi Miyajima* | 1:1000 |
| Mouse anti-rat Thy1.1 | Serotec, Raleigh, NC | 1:500 |
| Goat anti-rat desmin | Santa Cruz | 1:200 |
| Mouse anti-rat SE1 | Immuno-Biological Lab, Takasaki, Japan | 1:200 |
| Rabbit anti-human, mouse, rat CD3 | Abcam, Tokyo, Japan | 1:500 |
| Rat anti-mouse IgG1 microbeads | Miltenyi Biotec, Bergisch Gladbach, Germany | 1:50 |
| Rat anti-mouse IgG2a+bmicrobeads | Miltenyi Biotec | 1:50 |
| Biotinylated anti-mouse IgG (H+L) | Vector Laboratories, Burlingame, CA | 1:200 |
| Alexa488-conjugated | Molecular Probes, Eugene, OR | 1:500 |
| Alexa594-conjugated | Molecular Probes | 1:500 |
From the Institute of Molecular and Cellular Biology, University of Tokyo, Tokyo, Japan.
Table 2.
Ingredients in Culture Medium
| Ingredients with final concentration added to modified DMEM* | For dish culture | For gel culture (control) | For gel culture (Dex−/HGF+) |
|---|---|---|---|
| 10 ng/ml HGF | − | − | + |
| 10−7 mol/L Dex | + | + | − |
| 1% DMSO (4 days after plating) | − | + | − |
Modified DMEM included 20 mmol/L HEPES, 30 mg/L proline, 25 mmol/L NaHCO3, 10 mmol/L nicotinamide, 1 mmol/L ascorbic acid 2-phosphate, 10 ng/ml epidermal growth factor, 0.5 mg/L insulin, and antibiotics (penicillin, streptomycin, and gentamicin).
For collagen-sandwich culture, 8.5 ml of rat tail collagen (500 μg of dried tendon/ml 0.1% acetic acid) was mixed with 2 ml of NaOH/Dulbecco’s modified Eagle’s medium (DMEM) solution (0.34 N NaOH:10×DMEM = 10:3) and 500 μl aliquots of collagen/NaOH/DMEM solution were poured into 12-well plates. To gelatinize, plates were placed at 37°C for 30 minutes. After 1 × 106 cells were plated and incubated in a CO2-incubator for 1 hour, 300 μl aliquots of the collagen/NaOH/DMEM solution were added. These cells were cultured in the control and the Dex−/HGF+ medium listed in Table 2. The medium was replaced with fresh medium three times a week.
RNA Isolation and GeneChip (Affymetrix, Inc)
Differences of the expression profiles of cells were analyzed by using an oligo microarray spotted with 31,000 probes (GeneChip Rat Genome 230 2.0 Array; Affymetrix, Inc). Total RNAs were prepared by using a GenElute-mRNA midiprep kit (Sigma). Analysis of the array data were performed with MultiExperiment Viewer (TM4, http://www.tm4.org/) by Daiichi Pure Chemical Co, Ltd (Tokai, Ibaraki, Japan).
Immunostaining
For detecting CD44+ colonies, immunocytochemistry was performed at day 10. Cells were fixed with cold absolute ethanol. After blocking the intrinsic peroxidase with 0.2% H2O2/methanol and then nonspecific staining with BlockAce (Dainippon Pharmaceuticals Co, Osaka, Japan), cells in the dish were incubated with primary antibodies at room temperature for 1 hour, followed by the avidin-biotin peroxidase complex method (Vectastain ABC Elite Kit; Vector Laboratories, Inc, Burlingame, CA). 3′-Diaminobenzidine (Tokyo Kasei Industries, Tokyo, Japan) was used as a substrate. Nuclei of the cells were counterstained with hematoxylin. The number of CD44+ colonies at day 10 was counted and the positivity was calculated. Three separate experiments were performed.
For fluorescent immunocytochemistry, livers and sandwich-cultured cells were frozen by using isopentane/liquid nitrogen and the samples were kept at −80°C until use. Seven-micrometer-thick sections were prepared and dried. To determine the characteristics of sorted cells, isolated cells were cultured on CultureSlides (Becton Dickinson Labware, Franklin Lakes, NJ) for 2 days. After blocking, the sections were incubated with primary antibodies for 1 hour at room temperature and then fluorescent-conjugated secondary antibodies were applied for 30 minutes. All antibodies used for immunostaining were listed in Table 1. The sections were embedded with 90% glycerol including 0.01% p-phenylenediamine 4,6-diamidino-2 -phenylindole (DAPI). A confocal laser microscope (Olympus, Tokyo, Japan) was used for observation and findings were analyzed by using DP Manager (Olympus). The number of the positive cells at day 2 was counted and the positivity was calculated. More than 200 cells per dish were counted and three separate experiments were performed.
Enzyme Histochemisty for DPPIV
To identify the donor cells, enzyme histochemistry for DPPIV was performed. DPPIV enzyme activity was detected as previously described.26 Briefly, sections were fixed in cold 10% buffered formalin for 5 minutes and then cold ethanol for 5 minutes and air-dried. The samples were incubated for 60 minutes in a substrate solution containing 0.5 mg/ml gly-pro-methoxy-β-naphthylamide (Sigma), 1.0 mg/ml Fast Blue BB (Sigma), and 0.1M PBS (pH 6.5). All samples were counterstained with hematoxylin and mounted in glycerol. DPPIV+ foci were photographed by using a microscope equipped with a CCD camera and the area of each focus was measured by using Image J (http://rsb.info.nih.gov/ij/index.html). After DPPIV staining, some samples were immunohistochemically stained. Primary antibodies for CD44, SE1, CK19, and CCAAT/Enhancer Binding Protein (C/EBP) α were used for clarifying the characteristics of DPPIV+ foci formed by donor cells. To detect the primary antibody, avidin-biotin complex method or fluorescent immunohistochemistry were performed as described above.
Transmission Electron Microscopy
The cells in the collagen gel were fixed in 2.5% glutaraldehyde in 0.1M cacodylate buffer (pH7.4) at room temperature for 30 minutes, postfixed in 2% osmium tetroxide in the buffer, dehydrated by graded ethanol, and embedded in situ in Epon812. The procedure was previously described in detail.13
Statistical Analysis
Statistical analysis was performed by using Student’s t-test. A P value of <0.05 was considered significant.
Results
Characterization of Sorted Cells
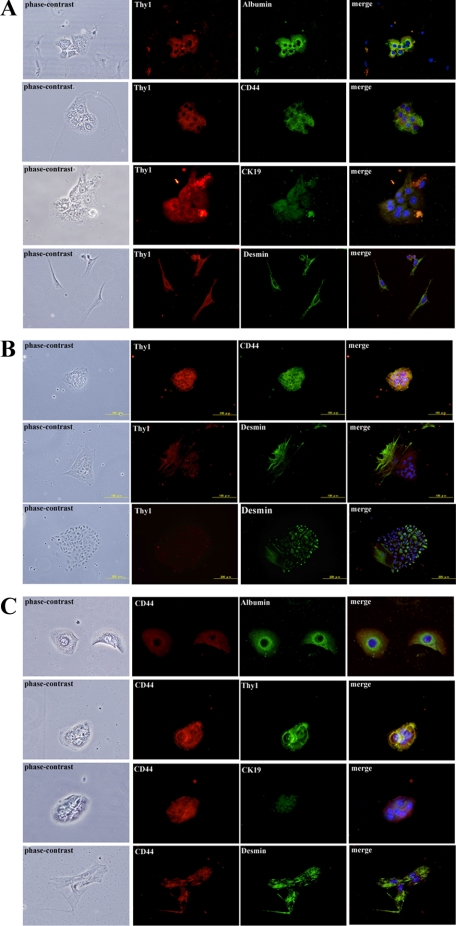
To characterize the sorted cells, we performed two experiments. The sorted cells from GalN-D3 were cultured for 2 days and immunocytochemistry for albumin, CD44, CK19, Thy1, and desmin was conducted. As shown in Figure 1A, the morphology of Thy1+ cells was roughly divided into two types, polygonal (epithelial-like) and spindle (fibroblast-like) shaped. Most of the spindle shaped cells were desmin+ and the percentage of Thy1+/desmin+ cells was about 44.2 (Table 3). On the other hand, the polygonal cells were albumin, CD44, or CK19+. The percentages of Thy1+/CK19+, Thy1+/albumin+, and Thy1+/CD44+ cells were 16.2, 30.0, and 38.3, respectively. In addition, the epithelial-like cells had a tendency to form aggregates consisting of a small number of cells at the time of sorting. The epithelial-like cells rapidly proliferated and formed a colony (Figure 1B). The morphology of cells in the colonies was divided into two types, cells with compact and granular cytoplasm, and others that were flat with few granules in their cytoplasm. The former were CD44+/desmin− and the latter CD44−/desmin+. On the other hand, most sorted CD44+ cells were polygonal and their morphology had epithelial-like features. Some cells, about 15%, were fibroblastic and positive for desmin (Figure 1C). The percentages of CD44+/CK19+, CD44+/albumin+, and CD44+/Thy1+ cells were 60.1, 62.5, and 65.0, respectively. CD44+/albumin+ cells (Figure 1C) were larger than the Thy1+/albumin+ ones (Figure 1A), whereas the size and features of CD44+/CK19+ cells (Figure 1C) were similar to those of Thy1+/CK19+ ones (Figure 1A).
Figure 1.
Immunostaining of Thy1+ and CD44+ (GalN-D3) cells and 5 day cultured Thy1+ (GalN-D3) cells with markers for hepatocytes, hepatic stem/progenitor cells, and stellate cells. A: Sorted Thy1+ (GalN-D3) cells were plated on a collagen-coated 4-well chamber plate and cultured for 2 days. B: Thy1+ (GalN-D3) cells were cultured for 5 days. C: Sorted CD44+ (GalN-D3) cells were plated on a collagen-coated 4-well chamber plate and cultured for 2 days. Cells were fixed with cold ethanol and then double-immunostaining was performed: Panels of Phase-contrast, Thy1 (red), albumin, CD44, CK19, desmin (green), and merged with DAPI were shown. Thy1+/albumin+, Thy1+/CD44+, and Thy1+/CK19+ cells are polygonal, whereas Thy1+/desmin+ cells are spindle. Scale bars: 50 μm (A and C); 100 μm (B).
Table 3.
Characterization of Sorted Cells
| Cells | Combination | Population, % |
|---|---|---|
| Sorted Thy1+ cells | ||
| Thy1+ | 71.6 ± 12.2 | |
| Thy1+ / CK19+ | 16.2 ± 4.7 | |
| Thy1+ / Desmin+ | 44.2 ± 25.1 | |
| Thy1+ / Albumin+ | 30.0 ± 12.0 | |
| Thy1+ / CD44+ | 38.3 ± 17.1 | |
| Thy1− | ||
| Thy1− / CK19+ | 3.6 ± 3.2 | |
| Thy1− / Desmin+ | 1.1 ± 1.3 | |
| Thy1− / Albumin+ | 3.9 ± 6.7 | |
| Thy1− / CD44+ | 0 | |
| Sorted CD44+ cells | ||
| CD44+ | 81.9 ± 5.8 | |
| CD44+ / CK19+ | 60.1 ± 14.1 | |
| CD44+ / Desmin+ | 14.9 ± 2.5 | |
| CD44+ / Albumin+ | 62.5 ± 6.1 | |
| CD44+/ Thy1+ | 65.0 ± 4.4 | |
| CD44− | ||
| CD44− / CK19+ | 5.4 ± 1.2 | |
| CD44− / Desmin+ | 2.3 ± 3.3 | |
| CD44− / Albumin+ | 6.0 ± 8.6 | |
| CD44− / Thy1+ | 22.1 ± 0.9 | |
Differentiation Ability of Thy1+ Cells to SHs
Sorted Thy1+ and CD44+ cells were cultured for 10 days and the formation of SH colonies was examined by CD44 immunostaining (Figure 2, A and B). CD44+ colonies were regarded as SH colonies. Although sorted Thy1+ (GalN-D2) cells were cultured for 10 days, they could rarely form CD44+ colonies, some Thy1+ (GalN-D3) cells formed morphologically typical SH colonies, all cells of which were positive for CD44 (Figure 2A). The frequency of the formation and the average area of CD44+ colonies were calculated and the data are shown in Table 4. Thy1+ (GalN-D3) cells could form SH colonies more efficiently than those from GalN-D2 and GalN-D4. Although the efficiency of the colony formation was two times greater for Thy1+ (GalN-D3) cells than for those from GalN-D4, that of Thy1+ (GalN-D3) cells had half the efficiency of CD44+ (GalN-D3) cells. Thy1+ (GalN-D4) cells could form about twofold larger colonies than those from GalN-D3. The average sizes of the colonies formed by Thy1+ (GalN-D4) cells and CD44+ (GalN-D3) cells were about the same but only about half as large as those formed by CD44+ (GalN-D4) cells. These results showed that, although most Thy1+ (GalN-D2 cells) were not committed to a hepatic lineage, some Thy1+ (GalN-D3, 4) cells may have already differentiated into CD44+ SHs.
Figure 2.
Identification of CD44+ cell colony formed by cultured sorted cells. 1 × 105 Thy1+ (A) or CD44+ (B) cells sorted from GalN-D3 rat livers were cultured in 12-well plates. Ten days after plating, cells were fixed and immunocytochemistry for CD44 was performed. The details are described in Materials and Methods. Membranes of all colony-forming cells were stained with anti-CD44 antibody. Nuclei were counterstained with hematoxylin. Scale bar = 70 μm.
Table 4.
Efficiency of SH Colony Formation
| Cells | Days after GalN | Attached cells at D1 (×104 cells) | CD44+ colonies at D10 (colonies) | Efficiency* | Average area of CD44+ colonies at D10 (× 10−3 mm2) |
|---|---|---|---|---|---|
| Thy1 | 2 | 1.6 ± 0.2 | 1 ± 0.8 | 0.6 | — |
| 3 | 2.0 ± 0.4 | 94.7 ± 10.3 | 47.4 | 263.0 ± 91.5 | |
| 4 | 1.0 ± 0.2 | 23.0 ± 3.6 | 23.9 | 448.8 ± 401.9† | |
| CD44 | 2 | 1.8 ± 0.2 | 0.7 ± 0.5 | 0.4 | — |
| 3 | 1.7 ± 0.2 | 163.0 ± 24.7 | 95.9 | 520.0 ± 477.9 | |
| 4 | 0.9 ± 0.1 | 184.0 ± 12.1 | 195.6 | 1063.6 ± 331.8‡§ |
Three independent experiments were carried out and three wells per experiments were counted.
Efficiency = (Number of colonies/Attached cells)/104.
P < 0.05 when compared to Thy1+ (GalN-D3) cells.
P < 0.05 when compared to CD44+ (GalN-D3) cells.
P < 0.05 when compared to Thy1+ (GalN-D4) cells.
Transplantation of Sorted Cells into the Retrorsine/PH Model
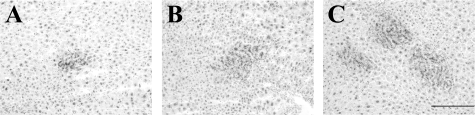
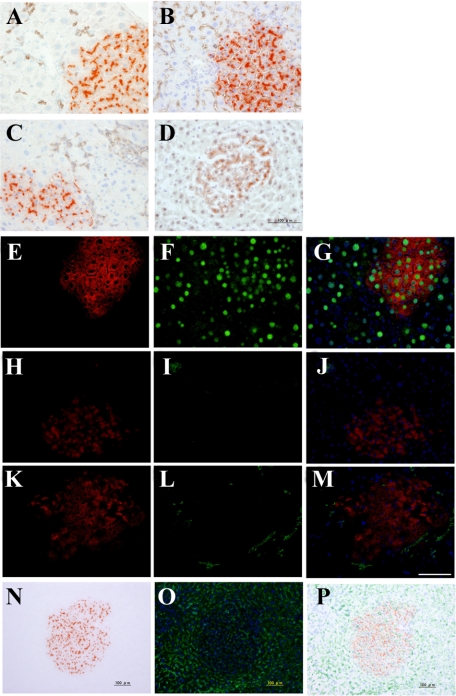
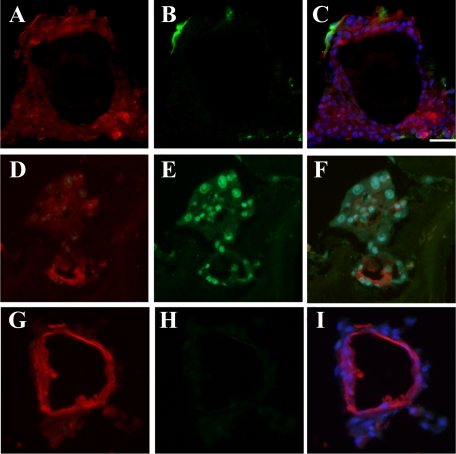
To investigate whether the sorted cells could differentiate into MHs, they were transplanted into Ret/PH-rat livers. As shown in Figure 3, A–C, DPPIV+ cells were found in recipient livers of all groups. To characterize the cells forming DPPIV+ foci, double-staining was performed. As shown in Figure 4A, CK19+ cells were observed in the portal area and some cells comprised small bile ductules. Although most DPPIV+ cells did not show CK19-positivity, some CK19+ small ductules were located in the edge of the DPPIV+ cluster. SE1+ cells were observed in sinusoids, not in DPPIV+ cells (Figure 4B). Although DPPIV+ cells did not show SE1-positivity, the number of SE1+ cells within the foci was fewer than that around the foci (Figure 4, N–P). The expression of CD44 was lost in the DPPIV+ cells at 30 days after transplantation (Figure 4C). Only BECs in ductules expressed CD44. CD3+ T cells and CK19+ BECs were not observed within DPPIV+ foci (Figure 4, H–M), while most DPPIV+ cells possessed C/EBPα+ nuclei (Figure 4, D–G). The number and the area of DPPIV+ foci were measured and the data are summarized in Table 5. Although there was no significant difference in number between Thy1+ (GalN-D2) and (GalN-D3) cells, the average area of the foci formed by Thy1+ (GalN-D3) cells was about twice that for GalN-D2. The average number and area derived from CD44+ (GalN-D4) cells were about 10-fold and twofold greater than those derived from Thy1+ (GalN-D3) cells, respectively.
Figure 3.
Transplantation of cells isolated from GalN-treated livers into Ret/PH treated livers. 5 × 105 sorted Thy1+ (GalN-D2 and -D3) cells or CD44+ (GalN-D4) cells were transplanted intrasplenically into Ret/PH treated rats. Thirty days after transplantation, each liver was perfused with PBS and some sliced pieces of the liver were frozen. Thin sections were enzymatically stained with DPPIV. DPPIV+ cells were stained in gathered colony. Nuclei were counterstained with hematoxylin. A: Thy1+ (GalN-D2) cells. B: Thy1+ (GalN-D3) cells. Some small positive foci were observed in the recipient liver. C: CD44+ (GalN-D4) cells. Relatively large foci were formed in the recipient liver. Scale bar = 500 μm.
Figure 4.
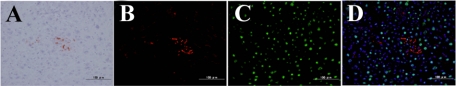
Characteristics of DPPIV+ foci in the livers after CD44+ cell transplantation. Frozen sections were obtained from livers at 30 days after transplantation of sorted CD44+ cells. Immunohistochemistry and DPPIV-enzyme histochemistry are shown: A combination of DPPIV-enzyme histochemistry and immunohistochemistry for CK19 (A); SE1 (B); CD44 (C); and C/EBPα (D). E–M: Double-fluorescent immunohistochemistry for DPPIV (E, H, K) and C/EBPα (F), CD3 (I), or CK19 (L). N–P: A combination of DPPIV-enzymatic histochemistry (N) and fluorescent immunohistochemistry for SE1 (O). Merged images are combined with DAPI-staining (G, J, M, P). Scale bar = 100 μm.
Table 5.
Number and Area of Transplanted DPPIV+ Foci
| Days after GalN | N | Cells | Average number of DPPIV+ foci per whole liver area (× 10−3 mm2) | Average of DPPIV+ area (× 10−3 mm2) |
|---|---|---|---|---|
| 2 | 5 | Thy1 | 13.5 ± 0.2 | 50.4 ± 20.2 |
| 3 | 5 | Thy1 | 11.4 ± 0.2 | 118.9 ± 54.2 |
| 4 | 5 | CD44 | 118.1 ± 0.3* | 209.5 ± 131.6 |
P < 0.05 when compared to either D2 or D3.
When Thy1+ (GalN-D3) cell-derived colonies cultured for 5 days (Thy1-C) were transplanted into Ret/PH-rat livers through the spleen, DPPIV+ foci were observed in the livers at 30 days after transplantation (Figure 5, A–D). Although such DPPIV+ cells showed the morphology of MHs and were inserted into hepatic plates, the expression C/EBPα was observed in few nuclei of the cells (Figure 5D).
Figure 5.
Characteristics of DPPIV+ foci after the transplantation of cultured Thy1+ cell colonies. Frozen sections were obtained from a liver at 30 days after transplantation of epithelial cell colonies derived from sorted Thy1+ (GalN-D3) cells. DPPIV-enzyme histochemistry (A) and double-immunohistochemistry for DPPIV (B) and C/EBPα (C) were performed. A merged image (D) is combined with DAPI-staining. Scale bar = 100 μm.
The Gene Expression Profiles in Hepatic Progenitor Cells
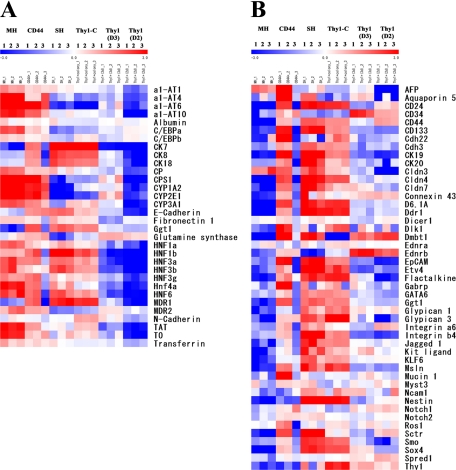
The results from the culture and transplantation of the sorted Thy1+ cells suggest that their characteristics varied depending on the time of isolation (days after GalN administration). To examine the characteristics of Thy1+ cells, we performed GeneChip (Affymetrix, Inc) analysis. The patterns of gene expression were compared among Thy1+ (GalN-D3), Thy1-C, and CD44+ (GalN-D4) cells (Figure 6). Cultured SHs from a normal liver (SH) and MHs were used as controls. Figure 6, A and B, shows genes related to hepatic functions and to hepatic stem/progenitor cells that were reported previously.27,28 As shown in Figure 6A, the gene expression pattern of CD44+ cells was the most similar to that of MHs. Although the pattern of SHs also resembled that of MHs, the genes related to the cytoskeleton and cell adhesion such as CK and cadherins, which might be amplified in cultured cells, were expressed more highly in SHs than in MHs and CD44+ cells. Thy1+ (GalN-D3) cells had some expression in the genes related to hepatic differentiated functions though the relative expression was much less than in CD44+ cells. Although the expression of some genes related to hepatic functions such as α1-antitrypsin, CYPs, and glutamine synthetase was low, Thy1-C well expressed liver-enriched transcription factors such as hepatocyte nuclear factor (HNF) and C/EBPs and CYP3A1, tyrosine aminotransferase, and tryptophan dioxygenase compared with Thy1+ (GalN-D3) cells. As shown in Figure 6B, the gene expression pattern of hepatic progenitor cell markers was similar for CD44, SH, and Thy1-C. However, it was quite different compared with Thy1(D3). This may have been due to the selection of a cell population that was committed to hepatic differentiation.
Figure 6.
Gene expression of sorted Thy1+ cells, CD44+ cells, and cultured Thy1+ cells. The gene expression pattern of sorted Thy1+ (GalN-D3) cells, Thy1+ cell-forming colonies (Thy1-C; GalN-D3), and CD44+ (GalN-D4) cells was analyzed by GeneChip (Affymetrix, Inc). Ten-day-cultured SH colonies and MHs isolated from a normal adult rat liver were used as controls. A heat map for genes that are classified into hepatic markers (A) and stem cell markers and hepatic stem/progenitor cell markers (B). Relative expression of genes in each cell type is shown in log2 scale. Increases in the mRNA level are represented as shades of red and decreases in shades of blue.
Formation of Bile Duct in Collagen Sandwich Culture
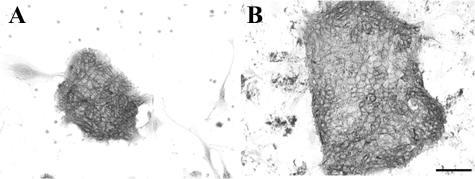
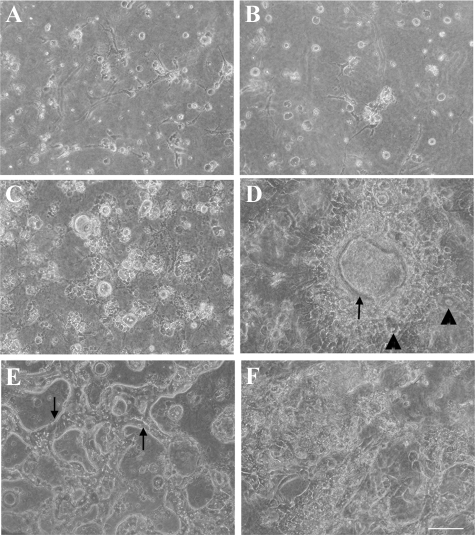
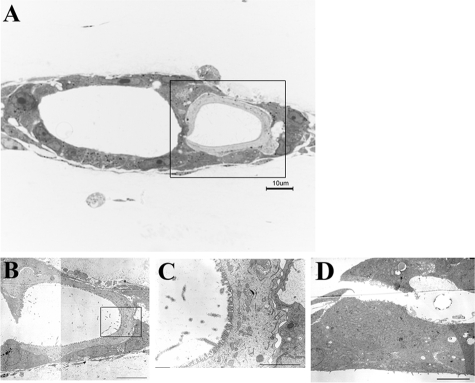
Oval cells have bipotency and can differentiate into either hepatocytes or BECs. Since Thy1+ (GalN-D3) cells were shown to possess the ability to differentiate into CD44+ SHs (Figure 2), we examined whether they also had the ability to differentiate into BECs. We cultured the sorted cells between collagen gels in control and the medium supplemented with HGF+/Dex−/dimethyl sulfoxide (DMSO)−.29 When Thy1+ (GalN-D2) cells were cultured between collagen gels, no growth of the cells was observed in the control or the modified medium (Figure 7, A and B). However, when Thy1+ (GalN-D3) cells were cultured, epithelial cells proliferated to form a colony and then cyst-like structures appeared within the colony. Some epithelial cells surrounding the structure were morphologically similar to hepatocytes (Figure 7D; arrowheads). When the cells were cultured in the control medium, epithelial cells did not appear and most cells died (Figure 7C). On the other hand, when CD44+ cells were cultured in the control medium, they gathered into a trabecular structure (Figure 7E). However, when the CD44+ cells were cultured in the medium with HGF+/Dex−/DMSO−, they could form cell clusters but neither ductules nor cysts (Figure 7F). Many cells died with time in culture. To examine the detailed structure formed by Thy1+ (GalN-D3) cells, transmission electron microscopy and immunostaining were conducted. Figure 8A shows a perpendicular section of the cyst-like structure, which consisted of two different types of cells. One type was small flattened cells possessing pale cytoplasm, and the other large cells that possessed dark cytoplasm. In addition, the cyst consisted by small flattened cells was surrounded by the large cells. The ultrastructure of the cells is shown in Figure 8, B–D. The small flattened cells possessed few organella such as small mitochondria and endoplasmic reticulum, whereas microvilli were well developed in the apical membrane (Figure 8C). Those cells were morphologically similar to BECs. On the other hand, the large cells were rich in organella such as mitochondria, rough endoplasmic reticulum, Golgi apparatus, and peroxisomes with a crystalline nucleoid, which were similar to MHs (Figure 8D). To determine whether those cells were hepatocytes or BECs, immunocytochemistry for Thy1, CK19 (a marker for BECs), and C/EBPα was conducted (Figure 9, A–I). Although the expanding cells within collagen gels were originally derived from Thy1+ (GalN-D3) cells, the cells showing the morphology of epithelial ones lost Thy1-positivity. Some cells showing fibroblastic features were Thy1+ (Figure 9B). The cyst-like structures were divided into 3 types: (1) cysts consisting of all C/EBPα+ cells (Figure 9, D–F); (2) cysts consisting of all CK19+ cells (Figure 9, G–I); and (3) cysts consisting of a mixture of CK19+ and C/EBPα+ cells (Figure 9, D–F). Most cells surrounding cyst-like structures, which are shown in Figure 7D, had C/EBPα+ nuclei, but were negative for CK19. The results showed that Thy1+ (GalN-D3) cells could differentiate into hepatocytes and BECs.
Figure 7.
Phase-contrast photos of sorted cells cultured between collagen gels. Thy1+ (GalN-D2) cells (A and B), Thy1+ (GalN-D3) cells (C and D), and CD44+ cells (E and F) were plated on collagen gel and covered with collagen gel. The cells were cultured in control medium (A, C, E) or in modified DMEM (Dex−/HGF+; B, D, F). The arrow and arrowheads in D show a cyst-like structure and cells with a large cytoplasm that are morphologically similar to MHs, respectively. E: Cells with a large cytoplasm form trabecules (arrows). Scale bar = 70 μm.
Figure 8.
Ultrastructure of cyst-like structure formed by sorted Thy1+ cells (GalN-D3) in collagen-sandwich culture at day 25. A: Semithin perpendicular sections of the structure were stained with 1% toluidine blue and examined with light microscopy. The structure consists of 2 types of cells; one is small flattened cells with pale cytoplasm and the other large cells with dark cytoplasm. B: Enlargement of the square area shown in A. Ultrastructure of the cyst-like structure formed by the two types of cells. The cells facing the lumen are morphologically similar to BECs, and the other cells surrounding BECs are similar to hepatocytes. C: The enlargement of the square area shown in B. The cells have a few organelles and well developed microvilli at their apical surface. The basal membranes of the cells are directly attached to the outer cells. D: The cells observed at other parts of the same culture are rich in organelles such as large mitochondria, rough endoplasmic reticulum, Golgi apparatus, and peroxisomes with a crystalline nucleoid, which are morphologically typical MHs. Scale bars: 2 μm (B and D); 1 μm (C).
Figure 9.
Fluorescent immunocytochemistry of the cyst-like structure formed by sorted Thy1+ cells in collagen-sandwich culture. Sorted Thy1+ (GalN-D3) cells were cultured in the medium with HGF+/Dex−/DMSO− between collagen gels. Double-fluorescent immunocytochemistry for CK19/Thy1 (A–C) and CK19/C/EBPα (D–I) was performed. CK19 (A, D, G) is stained in red, Thy1 (B) and C/EBPα (E and H) are stained in green, and nuclei are stained with DAPI (Blue). Merged photos are shown in C, F, and I. A–C: The cyst-like structure consists of CK19-positive cells. Some Thy1-positive cells morphologically similar to fibroblastic ones are observed outside of the structure. D–F: An aggregate with a small duct consisting of hepatocytes expressing C/EBPα in their nuclei. Some cells express CK19. G–I: A duct/cyst-like structure consists of CK19-positive cells. Cells showing neither CK19 nor C/EBPα, which are morphologically similar to hepatocytes, exist outside of CK19+ cells. All photos are the same magnification. Scale bar = 200 μm.
Discussion
Until we established CD44 as a marker of SHs,18 the approach used to analyze the relationship between liver stem/progenitor cells and MHs was to trace [3H]-thymidine incorporation19,30 and to follow serial changes in the morphological, enzymatic, and molecular alterations of different cell types in the hepatocyte lineage.31 Although those methods indicated that oval cells could differentiate into hepatocytes or BECs, the process of their differentiation and the origin of SHs have not been clarified. As the definition of SHs is inadequate, the pathway of oval cells to SHs has been especially hard to analyze. We recently demonstrated that CD44+ cells isolated from GalN-injured livers could proliferate to form colonies, the cells of which had characteristics quite similar to SHs.18 In GalN-injured livers, CD44+ hepatocytes appeared in the periportal regions of the lobules at 3 to 5 days after administration, whereas Thy1+ cells were observed in the area adjacent to Glisson’s sheath and then expanded inside the lobules at 2 to 4 days. In GalN-D2 livers, most Thy1+ cells were negative for CD44, and in GalN-D3 livers cells positive for both increased.18 In GalN-D4 livers, most Thy1+ cells disappeared and CD44+ cells increased in the region. Thereafter, CD44+ cells rapidly disappeared from the lobules. These results suggested that a certain number of Thy1+ cells differentiated into hepatocytes via CD44+ SHs. In this study, detailed characterization of Thy1+ and CD44+ cells was done in vitro and in vivo. The findings that for most Thy1+ (GalN-D2) cells it was hard to form SH colonies in vitro and to expand in vivo after transplantation may indicate that the cells have not acquired sufficient ability to differentiate into hepatocytes. Compared with the Thy1+ (GalN-D2) cells, the cells from GalN-D3 showed higher ability to differentiate into CD44+ SHs in vitro. Furthermore, CD44+ (GalN-D3) cells showed higher efficiency in colony formation and growth ability for CD44+ SHs than Thy1+ cells (GalN-D3). In addition, the efficiency in CD44+ (GalN-D4) cells was two times larger than that in CD44+ ones (GalN-D3). Characterization of those sorted cells from GalN-D3 showed that about 60% of CD44+ cells were albumin-positive, whereas about 30% of Thy1+ cells were albumin-positive. About 30% of the sorted Thy1+ cells were Thy1+/CD44+, whereas more than 50% of the sorted CD44+ cells were Thy1+/CD44+. These findings indicated that Thy1+/CD44+ cells temporally appeared in the hepatocytic differentiation and that the differentiation of the cells might progress from Thy1+/CD44− to Thy1−/CD44+ cells. Thus, hepatocytic differentiation steps may exist among hepatic stem/progenitor cells and rapidly progress in GalN-injured livers.
The results of the GeneChip (Affymetrix, Inc) analysis also supported this hypothesis. Thy1+ (GalN-D3) cells apparently expressed typical hepatic differentiated genes such as CYPs, CPS, and GS. The gene expression pattern of Thy1-C was similar to that of SHs. Furthermore, most liver-enriched transcription factors such as HNF3, 4, 6, and C/EBPα/β were much better expressed in CD44+ (GalN-D4) cells than in Thy1+ (GalN-D3) cells. As these transcription factors are well known to regulate highly differentiated hepatic functions,32 the expression pattern of CD44+ (GalN-D4) cells may show that hepatic functions of the cells are very close to those of MHs. Considering that cultured cells (Thy1-C and SHs) usually show less-differentiated functions than cells in vivo, Thy1-C, SHs, and CD44+ cells may possess similar characteristics. In addition, it must be emphasized that they are clearly different from MHs. Thus, the progress of hepatocytic differentiation may occur in order Thy1+ (GalN-D2), Thy1+ (GalN-D3), and CD44+ cells.
Recently, the expression of Thy1 in stellate cells/hepatic myofibroblasts was reported in rat livers in the 2-AAF/PH injury model.33,34 The question of whether Thy1 is a real oval cell marker has been raised. In the present experiment, the sorted Thy1+ cells consisted of several types of cells and at least two different cell shapes existed in culture, polygonal and fibroblastic. About 44% of the cells had the fibroblast-like appearance that possessed desmin in their cytoplasm. These cells may be the hepatic myofibroblasts that have been reported.33,34 However, cells having albumin, CD44, and/or CK19 certainly existed, and their shape was polygonal, with epithelial-like features. The epithelial-like Thy1+ cells formed CD44+ cell colonies, which were morphologically similar to SHs. Furthermore, when Thy1-C was transplanted into Ret/PH rats, they were inserted into hepatic plates (Figure 5). The finding that Thy1+ cells consist of several types of cells has been pointed out by other groups.35,36 Rat fetal livers were reported to contain a small number of Thy1+ cells that expressed hepatic lineage markers such as AFP, albumin, CK18, and CK19.35,36 Thy1+ cells expressing hepatic marker proteins could form epithelial cell colonies in vitro and, when the Thy1+ cells were transplanted into Ret/PH model rat livers, some cells could differentiate into hepatocytes and repopulate.36 However, Thy1− cells isolated from ED14 had much more ability to form epithelial cell colonies in vitro and repopulate the recipient liver than Thy1-positive ones.36 In our transplantation study, the cells forming DPPIV+ foci that were derived from donor cells were hepatocytes possessing C/EBPα+ nuclei, not SECs (SE1+ cells) or T cells (CD3+ cells). In this study, sorted Thy1+ (GalN-D3) cells included about 10% Thy1+/CK19+ cells. In addition, when Thy1+ cells were cultured between collagen gels in the medium supplemented with HGF+/Dex−/DMSO−, a population of the cells could differentiate into BECs and form cyst-like structures. In the transplantation experiment, however, we could not find any DPPIV+/CK19+ cells in bile ducts of recipient livers. Although further experiments are needed to indicate whether Thy1+ cells can differentiate into BECs in vivo, considering the present results, cells that have the ability to differentiate into either hepatocytes or BECs exist in Thy1+ cells. In addition, the commitment of Thy1+ cells to differentiate into hepatocytes or BECs may occur between Day 2 and Day 3 after GalN administration in this experimental protocol.
The origin of SHs is an important issue to understand the process of the regeneration in the injured liver. To detect SHs in livers, although we identified CD44 as a specific marker,18 CD44+ epithelial cells are not found within lobules of normal rat livers. Nevertheless, CD44+ hepatocytes transiently emerge in rat livers in the 2-AAF/PH and Ret/PH models (data not shown), as well as in this GalN-injury model. In the Ret/PH model, the appearance of SHPCs has been reported and the origin of SHs has been studied.21,22,23,24 Recently, Best and Coleman37,38 showed that SHPCs were not the progeny of oval cells, using the 2-AAF/Ret/PH model and Ret/PH model treated with 4, 4′-diaminodiphenylmethane, which produce severe bile duct damage. Avril et al23 also showed that MHs were the origin of SHPCs. They used MHs that were labeled with the β-galactosidase gene as donor cells. After transplantation of the labeled hepatocytes into the liver in the Ret/PH rats, they observed that clusters of SHPCs consisted of β-galactosidase+ cells. In the present study, it is of interest that the expression of CD44 was lost in the cells forming DPPIV+ foci even when CD44+ cells were transplanted. In vitro data also showed that proliferating SHs expressed CD44 but, when SHs differentiated into MHs, their CD44 expression decreased and, instead of it, C/EBPα expression increased.18 The present and preliminary experiments showed that transplanted CD44+-derived cells could survive and proliferate for more than 6 months, whereas, although transplanted Thy1+ cells could differentiate into hepatocytes and proliferate, the Thy1+ cell-derived hepatocytes showed low expression of C/EBPα and disappeared until 3 months after transplantation. On the other hand, Vig et al,24 using transgenic mice expressing a hepatitis B surface antigen treated with retrorsine, demonstrated that oval cells could change into SHPCs. In the liver of the 2-AAF/PH model rat, Thy1+ cells initially appeared in the periportal area soon after PH, and then CD44+ cells showing morphology quite similar to hepatocytes emerged around/near the location where the Thy1+ cells appeared (data not shown). Considering these findings, foci of CD44+ cells and SHPCs may consist of MH- or oval (Thy1+) cell-derived cells. However, the life of hepatocytes derived from Thy1+ cells may be shorter than that of the cells derived from MHs. When severe liver damage occurs, some MHs may become SHs rapidly to compensate for the loss of hepatic functions. The role of oval (Thy1+) cells may be additional and supplementary for the period of the life-threatening emergency. Therefore, CD44+ cells appear for a short period in which SHs proliferate to become a certain number of functional hepatocytes and then redifferentiate into MHs with the loss of CD44 expression.
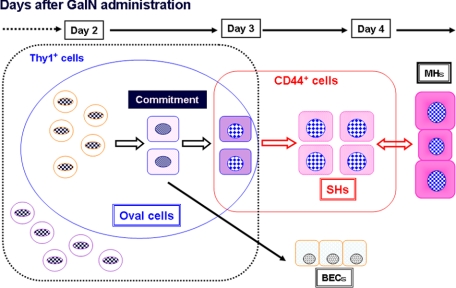
Based on the present study, we summarized the differentiation of hepatic stem/progenitor cells in Figure 10. In the GalN-injured liver, Thy1+/CD44− cells first appear and some of them differentiate into Thy1+/CD44+ cells. Thy1+/CD44+ cells differentiate into Thy1−/CD44+-SHs and then into hepatocytes (Thy1−/CD44−). Some Thy1+ cells had the ability to differentiate into BECs. At the same time, SHs originating from a subpopulation of MHs appear as CD44+ cells near the periportal region of the lobules. Thus, we think that most SHs may derive from MHs. Further detailed examinations may be necessary to clarify the issue.
Figure 10.
Schematic representation of hepatic progenitor cell differentiation in GalN-injured liver.
Supplementary Material
Acknowledgments
We thank Emeritus Professor Yohichi Mochizuki for taking electron micrographs and Dr. Yamato Kikkawa (Tokyo University of Pharmacy and Life Science, Japan) for suggestive discussions, and Ms. Minako Kuwano and Ms. Yumiko Tsukamoto for technical assistance. We also thank Mr. Kim Barrymore for help with the article.
Footnotes
Address reprint requests to Toshihiro Mitaka, M.D., Ph.D., or Junko Kon, Ph.D., Department of Pathophysiology, Cancer Research Institute, Sapporo Medical University School of Medicine, South-1, West-17, Chuo-ku, Sapporo 060-8556, Japan. E-mail: tmitaka@sapmed.ac.jp or junko.kon@ucsf.edu.
Supported by the Japan Science and Technology Agency, the Science and Technology Incubation Program in Advanced Regions (T.M.), the Ministry of Education, Culture, Sports, Science, and Technology (19790294 for J.K. and 17390353 for T.M.), Ministry of Health, Labor, and Welfare, and Health and Labor Sciences Research Grants, Research on Advanced Medical Technology (T.M.), and the Suhara Memorial Foundation (T.M.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Sell S. The role of progenitor cells in repair of liver injury and in liver transplantation. Wound Rep Reg. 2001;9:467–482. doi: 10.1046/j.1524-475x.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510–518. doi: 10.1002/path.1163. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- Knight B, Matthews VB, Olynyk JK, Yeoh GC. Jekyll and Hyde: evolving perspectives on the function and potential of the adult liver progenitor (oval) cell. BioEssays. 2005;27:1192–1202. doi: 10.1002/bies.20311. [DOI] [PubMed] [Google Scholar]
- Walkup MH, Gerber DA. Hepatic stem cells: in search of. Stem Cells. 2006;24:1833–1840. doi: 10.1634/stemcells.2006-0063. [DOI] [PubMed] [Google Scholar]
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylaminofluorene, and 3′-methy-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–149. [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Jensen CH, Jauho EI, Santoni-Rugiu E, Holmskov U, Teisner B, Tygstrup N, Bisgaard HC. Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am J Pathol. 2004;164:1347–1359. doi: 10.1016/S0002-9440(10)63221-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov PN, Yovchev MI, Dabeva MD. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest. 2006;86:1272–1284. doi: 10.1038/labinvest.3700479. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Mikami M, Sattler GL, Pitot HC, Mochizuki Y. Small cell colonies appear in the primary culture of adult rat hepatocytes in the presence of nicotinamide and epidermal growth factor. Hepatology. 1992;16:440–447. doi: 10.1002/hep.1840160224. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Kojima T, Mizuguchi T, Mochizuki Y. Growth and maturation of small hepatocytes isolated from adult liver. Biochem Biophys Res Commun. 1995;214:310–317. doi: 10.1006/bbrc.1995.2289. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- Sugimoto S, Mitaka T, Ikeda S, Harada K, Ikai I, Yamaoka Y, Mochizuki Y. Morphological changes induced by extracellular matrix are correlated with maturation of rat small hepatocytes. J Cell Biochem. 2002;1:16–28. doi: 10.1002/jcb.10274. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Hirata K, Sugimoto S, Harada K, Mitaka T. Expression of cytochrome P450 enzymes in hepatic organoid reconstructed by rat small hepatocytes. J Gastroenterol Hepatol. 2005;20:865–872. doi: 10.1111/j.1440-1746.2005.03804.x. [DOI] [PubMed] [Google Scholar]
- Oshima H, Kon J, Ooe H, Hirata K, Mitaka T. Functional expression of organic anion transporters in hepatic organoids reconstructed by rat small hepatocytes. J Cell Biochem. 2008;104:68–81. doi: 10.1002/jcb.21601. [DOI] [PubMed] [Google Scholar]
- Ooe H, Kon J, Miyamoto S, Oozone Y, Ninomiya S, Mitaka T. Cytochrome P450 expressions of cultured rat small hepatocytes after long-term cryopreservation. Drug Metabolism and Disposition. 2006;34:1667–1671. doi: 10.1124/dmd.105.008342. [DOI] [PubMed] [Google Scholar]
- Kon J, Ooe H, Oshima H, Kikkawa Y, Mitaka T. Expression of CD44 in rat hepatic progenitor cells. J Hepatol. 2006;45:90–98. doi: 10.1016/j.jhep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Shiojiri N, Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353–1361. doi: 10.1002/hep.20178. [DOI] [PubMed] [Google Scholar]
- Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best DH, Coleman WB. Cellular responses in experimental liver injury: possible cellular origins of regenerative stem-like cells. Hepatology. 2005;41:1173–1176. [Google Scholar]
- Avril A, Pichard V, Bralet M-P, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737–743. doi: 10.1016/j.jhep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, Alison MR, Forbes SJ. The source of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316–324. doi: 10.1002/hep.21018. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kon J, Ooe H, Sasaki K, Mitaka T. Selective proliferation of rat hepatocyte progenitor cells in serum-free culture. Nat Protoc. 2007;2:1197–1205. doi: 10.1038/nprot.2007.118. [DOI] [PubMed] [Google Scholar]
- Shibata C, Mizugichi T, Kikkawa Y, Nobuoka T, Oshima H, Kawasaki H, Kawamoto M, Katsuramaki T, Mitaka T, Hirata K. Liver repopulation and long-term function of rat small hepatocyte transplantation as an alternative cell source for hepatocyte transplantation. Liver Transpl. 2006;12:78–87. doi: 10.1002/lt.20558. [DOI] [PubMed] [Google Scholar]
- Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45:139–149. doi: 10.1002/hep.21448. [DOI] [PubMed] [Google Scholar]
- Germain L, Blouin MJ, Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988;48:4909–4918. [PubMed] [Google Scholar]
- Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, Yoshioka T, Enomoto K. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166:1077–1088. doi: 10.1016/S0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaoka Y. Significance of the so-called oval cell proliferation during azo-dye hepatocarcinogenesis. Gann. 1967;58:355–66. [PubMed] [Google Scholar]
- Dabeva MD, Shafritz DA. Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- Cereghini S. Liver-enriched transcriptional factors and hepatocyte differentiation. FASEB J. 1996;10:267–282. [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329:503–514. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
- Dezsõ K, Jelnes P, László V, Baghy K, Bödör C, Paku S, Tygstrup N, Bisgaard HC, Nagy P. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. Am J Pathol. 2007;171:1529–1537. doi: 10.2353/ajpath.2007.070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiegel HC, Park JJ, Lioznov MV, Martin A, Jaeschke-Melli S, Kaufmann PM, Fehse B, Zander AR, Kluth D. Characterization of cell types during rat liver development. Hepatology. 2003;37:148–154. doi: 10.1053/jhep.2003.50007. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen Y, Shafritz DA. Comparison of hepatic properties and transplantation of Thy-1+ and Thy-1− cells isolated from embryonic day14 rat fetal liver. Hepatology. 2007;46:1236–1245. doi: 10.1002/hep.21775. [DOI] [PubMed] [Google Scholar]
- Best DH, Coleman WB. Treatment with 2-AAF blocks the small hepatocyte-like progenitor cell response in retrorsine-exposed rats. J Hepatol. 2007;46:1055–1063. doi: 10.1016/j.jhep.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best DH, Coleman WB. Bile duct destruction by 4,4′-diaminodiphenylmethane does not block the small hepatocyte-like progenitor cell response in retrorsine-exposed rats. Hepatology. 2007;46:1611–1619. doi: 10.1002/hep.21876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.