Abstract
The antibody HMB45 is used to diagnose lymphangioleiomyomatosis, a hyperproliferative disorder of lung smooth muscle cells with mutations in both alleles of either TSC1 or TSC2. A subset of these tumor cells expresses the melanoma-associated antigens gp100 and melanoma antigen recognized by T cells (MART-1). To explore the feasibility of targeting tumors in lymphangioleiomyomatosis by melanoma immunotherapy, we therefore assessed melanoma target antigen expression and existing immune infiltration of affected tissue compared with normal lung and melanoma as well as the susceptibility of cultured lymphangioleiomyomatosis cells to melanoma reactive cytotoxic T lymphocytes in vitro. Tumors expressed tyrosinase-related proteins 1 and 2 but not tyrosinase, in addition to gp100 and MART-1, and were densely infiltrated by macrophages, but not dendritic cells or T cell subsets. Although CD8+ lymphocytes were sparse compared with melanoma, cells cultured from lymphangioleiomyomatosis tissue were susceptible to cytotoxic, gp100 reactive, and major histocompatibility complex class I restricted CD8+ T cells in functional assays. Responder T cells selectively clustered and secreted interferon-γ in response to HLA-matched melanocytes and cultured lymphangioleiomyomatosis cells. This reactivity exceeded that based on detectable gp100 expression; thus, tumor cells in lymphangioleiomyomatosis may process melanosomal antigens different from melanocytic cells. Therefore, boosting immune responses to gp100 in lymphangioleiomyomatosis may offer a highly desirable treatment option for this condition.
Lymphangioleiomyomatosis (LAM) is a disease that strikes primarily women of child bearing age.1 Patients with LAM develop cystic lung lesions and present with dyspnea, pneumothoraces, chylous pleural effusions, and progressive loss of lung function, often culminating in lung transplantation.2 LAM can occur in patients with hereditary tuberous sclerosis, due to loss of heterozygosity in TSC1 or TSC2 genes.3,4 The prevalence of tuberous sclerosis complex (TSC)-associated LAM in the United States is approximately 1 in 35,000, or approximately a third of patients with TSC.5 Loss of both alleles of either TSC1 or TSC2 in sporadic LAM affects approximately 1 per million individuals.6 Thus, diseased cells in the latter are generally clonal, whereas in patients with tuberous sclerosis pleiotropic effects are best explained by separate transformation events.
Following the identification of underlying mutations responsible for the symptoms incurred in LAM and intracellular signaling pathways affected by TSC1/TSC2 through the mammalian target of rapamycin complex, proposed disease treatments have been aimed at the hyperproliferative responses observed in mutant cells.7 The mammalian target of rapamycin inhibitor rapamycin has been tested in phase 1 trials with mixed results.8 As mammalian target of rapamycin inhibition affects primarily cell size and proliferation without inducing cell death in mutant cells, clinical symptoms are not permanently alleviated. Rapamycin may be particularly useful in a prophylactic setting, preventing cyst formation in patients with TSC or patients who have undergone lung transplantation for LAM to prevent recurrence.9
Patients with LAM are generally not given a diagnosis for several years after the appearance of symptoms, and a lung biopsy is sometimes required.10,11 HMB45 immunostaining of lung tissue sections has proven a definitive diagnostic marker for lymphangioleiomyomatosis.11 Thus LAM cells express an epitope recognized by antibodies to gp100, which is otherwise expressed exclusively by melanocytic cells and frequently recognized by tumor infiltrating T cells in malignant melanoma.12,13 These data raised the intriguing question of whether LAM cells have trans-differentiated to express molecules otherwise associated with pigmentation, although melanin synthesis is not commonly observed in LAM tissue.14
Melanosomes are organelles closely related to lysosomes, developing from endosomes after trafficking of melanogenic enzymes by an intricate mechanism that involves several multiprotein complexes including adaptor protein-3 and biogenesis of lysosome-related organelles complex 1 to 3.15 Tyrosinase is the rate limiting enzyme for melanogenesis, supported by tyrosinase-related proteins (TRPs) 1 and 2; TRP-2 is also known as dopachrome tautomerase and is involved in later steps of melanogenesis. The melanosomal protein MART-1, a melanoma antigen recognized by T cells, has a chaperone-like function that prepares the premelanosome for melanin synthesis, and gp100 is responsible for the striation of early stage melanosomes important for melanin deposition.16 Skin pigmentation is achieved when ripened melanosomes are transferred to neighboring keratinocytes in a process that is at least in part determined by recipient cells.17 Melanization thus precedes organelle transfer to neighboring cells.
In addition to gp100, expression of MART-1 has been reported in LAM.12 MART-1 is named for its recognition by tumor infiltrating T cells.18 Both MART-1 and gp100 are also recognized by T cells that infiltrate the skin of patients with autoimmune vitiligo, further supporting the notion that expression can prime host cells for immune destruction.19 Vaccines boosting immune responses melanoma-associated antigens have since been developed and tested in clinical trials for the treatment of malignant melanoma.20 Besides adoptive transfer of autologous T cells, identification of T-cell epitopes and reactive T cell receptor (TCR) molecules have enabled additional vaccine strategies including administration of adjuvant-supported antigen preparations or TCR transgenic T cells.21 As T cells also mediate autoimmune responses in vitiligo, boosting immune responses directed toward the gp100 and MART-1 antigens may be an effective strategy to eradicate LAM cells.19
However, expression of gp100 as well as MART-1 is observed in only a subset of LAM cells. This prompted a wider investigation of melanoma-associated antigen expression, comparing expression within LAM tissue to normal lung and metastatic melanoma, and in vitro to melanoma cells and smooth muscle cells. Tissue infiltrating leukocytes including macrophages, dendritic cells, and CD4+ and CD8+ T cells found in LAM lung tissue were also quantified and compared with normal lung tissue and melanoma. The sensitivity of cell cultures derived from LAM lung to relevant T cells was assessed in functional in vitro assays. Techniques used include single and double immunohistochemistry, light and electron microscopy, fluorescence activated cell scanning (FACS) analysis, chromium release assays, and enzyme-linked immunosorbent assay (ELISA). The data are important to evaluate the potential of melanoma immunotherapy for the treatment of LAM.
Materials and Methods
Patient Tissues
Fresh tissue was obtained from five patients with LAM through the National Disease Research Interchange tissue repository (Bethesda, MD). Control lung samples were obtained from postmortem necropsies at Loyola University Medical Center. Samples were snap frozen in part, and 8 μm cryosections were cut and fixed in cold acetone, then stored at −20°C until use. LAM diagnosis provided by the National Disease Research Interchange was confirmed by indirect HMB45 immunostaining (Dakopatts, Glostrup, Denmark). Melanoma samples from three patients were obtained as resected tissue after surgery. Frozen sections were subjected to single and double immunostaining procedures, also described below. All patient samples included in this study were obtained with prior approval from the Institutional Review Committee at Loyola University Medical Center or the University of Chicago. Strict precautions were taken to protect the patients’ identities throughout these studies.
Cell Culture
Fresh tissue samples from transplanted lung of five patients with advanced LAM disease were obtained from the National Disease Research Interchange within 24 hours. Part of the tissue was snap frozen, another part used for cell culture after over night shaking in an enzyme cocktail of 1 mg/ml collagenase type IV (Sigma, St. Louis, MO), 0.05 mg/ml thermolysin (Sigma), 0.1 mg/ml trypsin (Invitrogen, Carlsbad, CA), and 30 U/ml DNaseI (Roche, Madison, WI) followed by 70 μm cell straining (BD Falcon, Bedford, MA). Part of uncultured cells were frozen in 10% dimethyl sulfoxide (Sigma) in fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). Another part was plated in either of three media: Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) with 2 mmol/L glutamine (Invitrogen) and 10% heat-inactivated fetal bovine serum and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen); or RPMI 1640 (Invitrogen) with 10% heat-inactivated normal human AB serum (Valley Biomedical, Inc, Winchester, VA), 2 mmol/L glutamine (Invitrogen) and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen); or Ham’s F12 (Mediatech, Manassas, VA) with 10 ng/ml tetradecanoyl phorbol 13-acetate (Sigma), 0.1 mmol/L isobutyl methyl xanthine (IBMX, Sigma), 1% w/v UltroserG (Pall Life Sciences, New York, NY), 2 mmol/L glutamine (Invitrogen) and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen). To confirm LAM origin of cultured cells, cells were plated in LabTek glass chamber slides (Nunc, Rochester, NY) or cytospun (Shandon/Thermoscientific, Watham, MA), fixed in cold acetone and immunostained for smooth muscle actin (clone O.N.5, Genetex, San Antonio, TX) and estrogen receptor-α (G-20 rabbit polyclonal anti-human ERα, Santa Cruz Biotechnology, Santa Cruz, CA) as described below. Cultures with >90% cells expressing smooth muscle actin were used as LAM cells. Staining was compared with pulmonary artery smooth muscle cells (Invitrogen), maintained in Medium 231 with smooth muscle cell growth supplements (Invitrogen).
M14 melanoma cells (American Type Tissue Collection, Manassas, VA) were cultured in DMEM (Invitrogen) with 10% heat-inactivated fetal bovine serum and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen). Cells were passaged 1:4 at confluency and plated in chamber slides for immunohistochemical analysis.
TIL1520 and tyrosinase reactive TCR transduced Jurkat cells22 were maintained in RPMI 1640 (Invitrogen) with 10% heat-inactivated normal human AB serum (Valley Biomedical, Inc), glutamine (Invitrogen) and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen), glutamine (Invitrogen) and antibiotics pen/strep/amphotericin (Invitrogen), with 500 IU/ml of interleukin-2 (R&D Systems, Minneapolis, MN).
Melanocytes and fibroblasts were isolated from otherwise discarded normal human skin of anonymous donors, after separating the dermis and epidermis by incubation in 0.1% trypsin for 16 hours at room temperature. Fibroblasts emigrating from dermal tissue were maintained in medium as described for M14 melanoma cells, and melanocytes released from the epidermis were plated in Ham’s F12 (Mediatech) with 10 ng/ml tetradecanoyl phorbol 13-acetate (Sigma), isobutyl methyl xanthine (IBMX, Sigma), 1% w/v UltroserG (Pall Life Sciences), 2 mmol/L glutamine (Invitrogen), and antibiotics 100 U/ml penicillin/100 μg/ml streptomycin/250 ng/ml amphotericin (Invitrogen). Both skin cell types were passaged 1:4 on confluency.
Immunostaining Procedures
Slides were hydrated in Tris-buffered saline, and subjected to predetermined dilutions of primary antibodies in 10% normal human serum (Valley Biomedical, Inc) in Tris-buffered saline. Staining procedures were completed essentially as described.23 Briefly, specimen were subsequently subjected to species specific (for single staining procedures) or combinations of enzyme labeled isotype specific (for double staining procedures) secondary antibodies reactive with the primary antibodies. In double staining procedures, the enzyme alkaline phosphatase enzyme was detected first by dissolving 0.2 mg/ml Fast Blue BB (Sigma) in buffer containing levamisole and naphtol AS-MX phosphate (Sigma). Slides were then exposed to a solution of 0.25 mg/ml amino ethyl carbazole in dimethyl formamide added to 0.1 M NaAc pH 5.2 with 0.03% H2O2. Red staining was developed and slides were coverslipped in gelvatol (Dakopatts). For single staining, only amino ethyl carbazole staining was performed followed by hematoxilin counterstaining as indicated before gelvatol coverslipping. Antibodies used include HMB45 and NKI-Beteb to gp100 (Dakopatts and Monosan/Cell Sciences, Canton MA, respectively), M2E9 to MART-1 (Covance, Denver, PA), Ta99 and 23 to TRP-1 (Monosan/Cell Sciences and BD BioSciences, San Jose, CA, respectively), T311 to tyrosinase (NeoMarkers, Fremont, CA), goat polyclonal antiserum to TRP-2 (Santa Cruz Biotechnology), M42251 to CD3 (Fitzgerald Industries International, Inc, Concord, MA), SK3 to CD4 (BD Biosciences), 32-M4 to CD8 (Santa Cruz Biotechnology), B146 to CD11c (BD Biosciences), and KP1 to CD68 (Santa Cruz Biotechnology).
Electron Microscopy
Ultrastructural evaluation of LAM tissue and cultured cells was performed essentially as previously described.24 Cultured LAM and control melanocytes were seeded into Tissue-Tek chamber slides (Nunc, Inc) coated with 1% pig gelatin, and fixed in wells with half-strength Karnovsky’s fixative in 0.2 M sodium cacodylate buffer at pH 7.2 for 30 minutes at room temperature. Fixed cells were washed in buffer and treated with 1.0% osmium peroxide containing 1.5% potassium ferrocyanide for 30 minutes. Washed samples were stained en bloc with 0.5% uranyl acetate for 30 minutes, dehydrated, and embedded in Eponate 12. Samples were sectioned on an RMC MT 6000-XL ultramicrotome (Tucson, AZ). Ultrathin sections were stained with aqueous solutions of uranyl acetate (2%) and lead citrate (0.3%) for 15 minutes, then photographed in a JEOL JEM-100CX (Jeol, Ltd., Tokyo, Japan) transmission electron microscope. For DOPA histochemistry, cells were incubated in a 0.1% solution of l DOPA twice for 2.5 hours at 37°C before postfixation.
Cell Sorting
Tissue cells isolated by enzymatic digestion were subjected to immunostaining by incubation in primary antibody F7.2.38 to CD3 (BD Biosciences), followed by incubation in fluorescein isothiocyanate-labeled anti-mouse Ig (Southern Biotechnology Associates, Inc, Birmingham, AL). A total of 106 washed cells were subjected to FACS by using FACScanto instrumentation (BD Biosciences). The FACScanto is a benchtop flow cytometer that contains a 15 mW argon-ion laser and a red diode laser and the capability for detection of six fluorescent detection channels, plus right and forward angle scatter.
HLA-A2 Restricted T-Cell-Mediated Killing
Target cells were plated as adherent cells and labeled with 4 μCi/well of 51Cr chromium for 4 hours. Besides HLA-A2+ and HLA-A2− LAM cells, melanocytes were used as positive control, and fibroblasts as negative control targets. Cytotoxicity assays were performed essentially as described.23 Briefly, after labeling, effector cells TIL1520 (gp100 reactive) or h3T-transduced (HLA-A2 restricted tyrosinase reactive TCR-transduced) Jurkat cells were added at 2:1, 4:1, and 8:1 effector target ratios, with effector cells at constant concentrations. Cytotoxic responses were measured as chromium release in the supernatant after 16 hours, in presence of Microscint40 in a Perkin Elmer gamma plate counter. Total lysis was measured in presence of 1% Triton-X-100. All measurements were performed in duplicate and experiments were performed twice.
ELISA
Target cells were plated in three densities. Target cells included LAM cells, melanocytes, and fibroblasts. Effector and target cells were combined essentially as described for cytotoxicity experiments, omitting chromium. Effector cells were TIL1520 melanoma derived or h3T (TCR transgenic Jurkat cells). Sixteen hours after addition of effectors, supernatants were collected and stored at −20°C. Supernatants were included in an interferon (IFN)-γ ELISA (TIL1520 effectors) or an interleukin-2 ELISA (Jurkat effectors) performed according to manufacturers’ instructions. Briefly, IFN-γ or interleukin-2 standard was added to control wells for comparison and an ELISA plate precoated with 1D1K antibody to IFN-γ (MabTech, Mariemont, OH) or interleukin-2-I antibody to interleukin-2 (MabTech) hosted the supernatants to be tested. Wells were subsequently incubated with biotinylated 7-B6–1 antibody to human IFN-γ (MabTech) or biotinylated interleukin-2-II antibody to interleukin-2 (Mabtech), followed by a peroxidase-labled streptavidin step. R&D Systems peroxidase substrate reagent was added and the reaction was stopped by using 2N sulfuric acid. Absorbance of the supernatants was measured in a Polar Star Omega ELISA plate reader (BMG Labtech, Offenburg, Germany) at 450 nm. Data were analyzed by using MARS data analysis software (BMG Labtech, Cary, NC).
Results
Expression of Melanoma-Associated Antigens in LAM Tissue
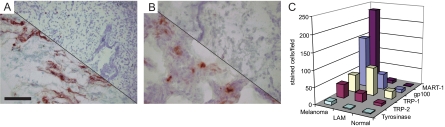
As shown in representative images displayed in Figure 1, besides gp100, TRP-1 was prominently expressed in LAM lung tissue as well as in melanoma, but not in control lung samples. No expression of tyrosinase was observed. Expression was subsequently quantified as the number of stained cells per microscopic field. It should be noted that cell densities did not significantly differ among tissue types (measured among two random samples representing each tissue type involved). Thus results represented in Figure 1 are comparable with data representing relative densities of cells expressing melanoma-associated antigens in LAM control lung and melanoma tissue. The results in Figure 1C suggest that TRP-1 is more widely expressed than gp100 in LAM, supporting the notion that not all LAM cells are detected by HMB45 staining. Expression of TRP-1 was in fact more abundant in LAM than in melanoma samples included in this study. Also, expression of TRP-2 was comparable among LAM and melanoma tissue, and increased over background detection in normal lung. Recognition of LAM cells by antibody M2–9E3 to MART-1 was marginally increased over background staining in control lung, whereas expression of gp100 expression was increased over background yet the number of stained cells was substantially less than in metastatic melanoma. The location of melanoma antigen-expressing LAM cells within affected LAM lung tissue was evaluated by comparing single-stained serial sections as well as by immuno-double staining in a comparison with melanoma tissue as shown in Figure 2. The gp100, MART-1, and TRP-2 gene products are effective targets for T cells in melanoma, whereas natural TRP-1 is known as a humoral target antigen.
Figure 1.
A: Expression of melanocyte differentiation markers in LAM tissue. Gp100 expression in lung tissue from patient sample LAM08123 detected by NKI-Beteb. Upper left: LAM lung. Lower right: control lung NL08122. Scale bar = 50 μm. B: Expression of TRP-1 detected by antibody Mel5 in patient sample LAM0841 and NL08122 control lung tissue. Upper left: LAM lung. Lower right: control lung. Magnification as in A. C: Quantification of gp100, MART-1, TRP-1, TRP-2, and tyrosinase expression in LAM lung tissues (n = 5) compared with control lung (n = 3) and metastatic melanoma specimen (n = 3). The expression of melanoma associated markers by cells in varied particularly among LAM samples. In this respect, the average number of stained cells per microscopic view ranged from 11.3 to 140.3 for gp100, from 1.7 to 30.3 per MART-1, from 2.0 to −304.4 for TRP-1, and from 0 to 55.7 for TRP-2.
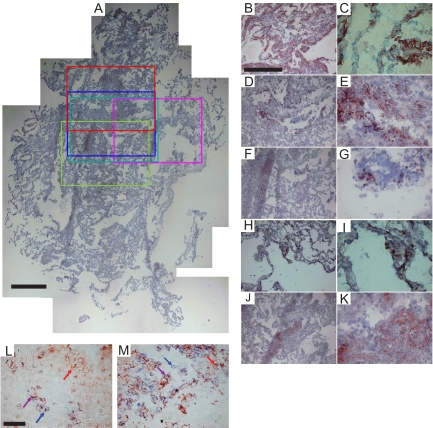
Figure 2.
Colocalization melanoma marker expression in LAM. Colocalization of melanoma-associated antigens shown by comparing single-stained serial sections of LAM0803, and immuno-double stainings of LAM08123. A: Reconstructed full section of control immunostaining (without primary antibody) and hematoxilin counterstained LAM0803 frozen tissue, with colored boxes indicating areas of interest for immunostaining with different primary antibodies selected as shown in red (α smooth muscle actin), blue (gp100), green (MART-1), pink (TRP-1), and turquoise (TRP-2). B and C: α-smooth muscle actin expression and sixfold magnification, respectively. D and E: gp100 expression and magnification. F and G: MART-1 expression and magnification. H and I: TRP-1 expression and magnification. J and K: TRP-2 expression and magnification. Subsets of lesional cells expressing either MART-1 (alkaline phosphatase label in blue) or gp100 (horseradish peroxidase label in red), or both (purple cells) were revealed in immuno-double stainings of (L) LAM specimen LAM08123 compared with (M) metastatic melanoma specimen MEL0610. Scale bars = 500 μm (A and B); 50 μm (L). Arrows identify cells stained in respective colors.
Characterization of Cultured LAM Cells
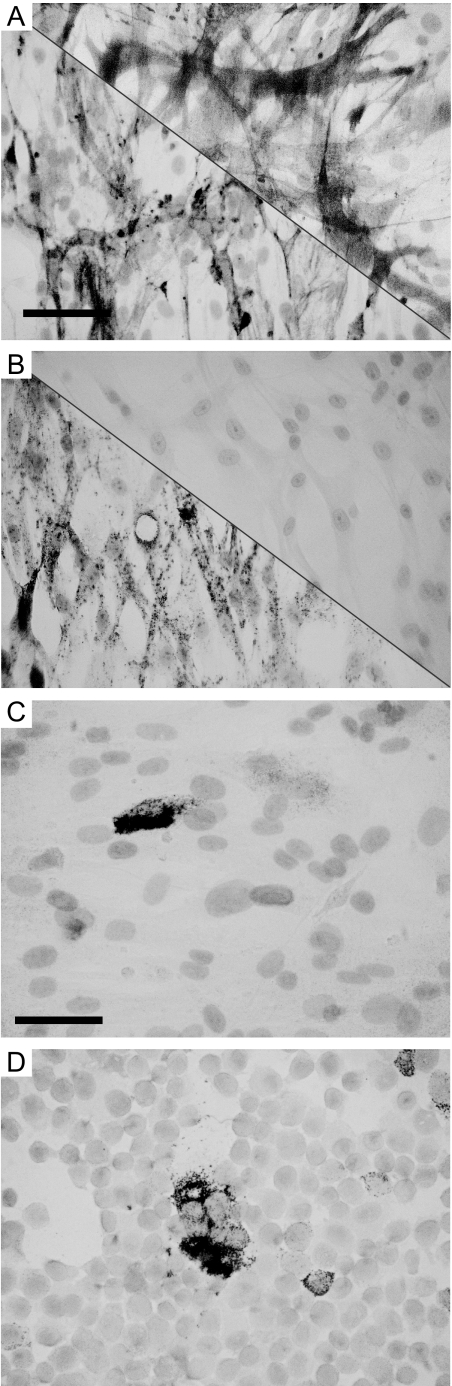
Expression of gp100 was assessed among three cell cultures initiated from LAM lung tissue and maintained in low passage (<5). Among LAM cell cultures, all abundantly expressed α-smooth muscle actin and estrogen receptor-α in comparison with pulmonary artery smooth muscle cells, expressing only actin but not estrogen receptors as demonstrated in Figure 3. This confirms a smooth muscle cell origin for the LAM-derived cultures and supports the disease association of cells. A representative example of gp100 expression is also shown in Figure 3, in comparison with similar expression in M14 melanoma cells. Analysis of cytospins for four LAM cultures maintained in DMEM with fetal bovine serum and antibiotics indicate expression of TRP-1 by 16.6% of cultured cells, gp100 by 13.4%, and MART-1 by 21.2% of cultured cells. Among culture media, serum supplemented DMEM proved the superior culture media for propagating LAM cells and maintaining melanoma-associated marker expression for four out of five cultures initiated (not shown). Tyrosinase expression was never observed. Interestingly, expression of TRP-1 was maintained even in later passages (not shown) implying that reduced expression of melanosomal markers by cultured LAM cells over time may be due to antigen loss in cultures rather than to selective advantage of non-LAM cells.
Figure 3.
Identification of cultured LAM cells. A and B: Expression of α-smooth muscle actin and estrogen receptor-α expression respectively in cultured LAM cells LAM0804P2 (lower left corners) versus primary smooth muscle cells from pulmonary artery in passage 3 (upper right corners), and an example of clearly detectable gp100 expression in LAM cells LAM0804P2 (C) versus M14 melanoma cells in red; hematoxilin counterstaining (D). Tyrosinase expression was not observed in cultured LAM cells. Scale bars: 50 μm (A and B); 33 μm (C and D).
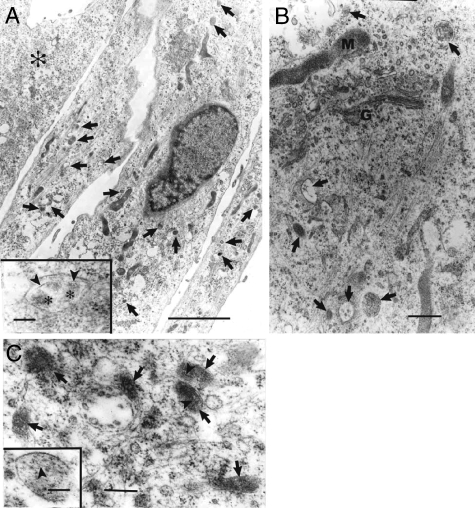
Cultured LAM cells in early passage derived from three patients with LAM demonstrated melanosome-like organelles (Figure 4). The morphology of these organelles resembled those observed in LAM lung tissue specimens (not shown) that were similar to Stage I and II melanosomes. In concordance with an absence of tyrosinase expression and detectable melanin deposition, structures resembling Stage III and IV melanosomes were not observed. In keeping with the role of gp100 and MART-1 in structural features of melanosomes, LAM cells in a later passage (passage 3) that lost expression of HMB-45 and MART-1 lacked melanosome-like organelles (not shown).
Figure 4.
Ultrastructural observation of premelanosomes in cultured LAM cells. A: Cultured cells in passage 2 demonstrating three cells with melanosome-like organelles (arrows) and one cell (asterisk) devoid of melanosome-like organelles. Inset: higher magnification of an organelle containing filaments (arrowheads) and floccular material (asterisks) resembling a Stage II melanosome. B and C: Cultured cells also in passage 2 from another two patients demonstrating melanosome-like organelles (arrows) with filaments (arrowheads). G = Golgi apparatus; M = mitochondria. Scale bars: 5 μm (A); 0.5 μm (B); 0.2 μm (C); inset = 0.1 μm (A and C).
Immune Infiltrates in LAM Lung
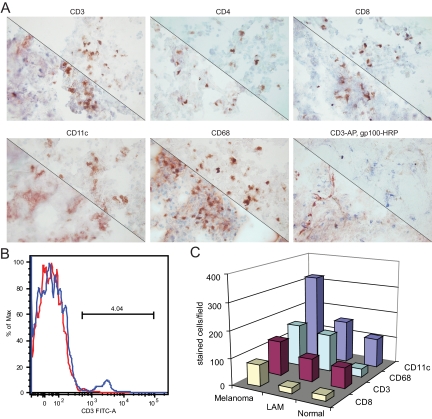
Tissues from five patients with LAM, three healthy control lungs, and three metastatic melanoma specimens were assessed for immune infiltration by measuring the relative abundance of T cells and T-cell subsets, macrophages, and dendritic cells. Representative stainings are shown in Figure 5A by a comparison with normal lung. In Figure 5B, T cell abundance in LAM lung tissue is quantified by FACS analysis, demonstrating approximately 4% T cells among tissue homogenates of LAM lung. This enables a comparison with the numbers of infiltrating cells quantified in Figure 5C, which also includes a comparison with immune cells observed in metastatic melanoma specimen. From these data it was apparent that T cell immunosurveillance was similar among normal and LAM lung tissues at approximately 4% of cells or 40 CD3+ T cells per microscopic field, doubling in melanoma tissues to approximately 80 per microscopic field. At the same time, CD11c+ dendritic cells were 50% more abundant in LAM lung compared with control lung tissue. Macrophage infiltrates were abundant in LAM lung tissue and in melanoma, at approximately 125, a fivefold increase over normal lung tissue. Thus active immune surveillance is limited in LAM tissue.
Figure 5.
Immune infiltrates in LAM lung tissue. A: Representative examples comparing the abundance of immune cells in LAM (lower left) with normal lung (upper right) specimen. Tissue specimen LAM0876 and normal lung sequentially displaying immunostaining obtained with antibodies to pan-T cell marker CD3; CD4; CD8; CD11c; and CD68; and a double staining for NKI-Beteb to gp100 (in red) and CD3 (in blue) of LAM lung specimen LAM08125 and normal lung NL08122. B: FACS analysis of CD3+ pan-T cells in LAM lung. The blue line represents fluorescein isothiocyanate labeled, CD3 expressing cells among LAM0805 tissue homogenates and the red line represents unstained cells from the same tissue (background fluorescence). C: Abundance of CD11c+ dendritic cells, CD68+ macrophages, CD3+ pan-T cells, and CD8+ cytotoxic T cells compared in melanoma, LAM lung, and control lung tissue. SDs varied from 9.3 for CD8+ cells observed in LAM as well as control lung tissue to 121.0 for CD11c+ cells in melanoma tumors, reflecting the range of infiltrating cell numbers observed among individual samples within tissue types. In this respect, taking CD68 expression as an example, average counts per optical field ranged from 28 to 32 for normal lung, 26 to 128 for LAM lung, and 115 to 183 among individual melanoma samples.
Susceptibility of LAM Cells to Gp100 Reactive T Cells
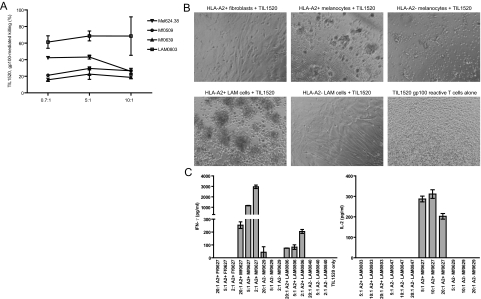
TIL1520 is an HLA-A*0201 restricted T-cell line that has been developed from a melanoma patient, and carries >95% reactivity to gp100209–217. HLA-matched cells in early passage cultured from resected lung tissue of a patient with advanced LAM disease were used as target cells. Less than 5% of cultured target cells expressed gp100 detectable by immunostaining at this stage. In cytotoxicity assays, however, TIL1520 were cytotoxic toward 62% of the cells in overnight assays as shown in Figure 6A, superseding optimal killing achieved in that time frame of HLA-matched melanocytes and melanoma cells at up to 25%, likely reflecting low effector/target ratios used to optimize detectable differences in cytotoxicity. To further confirm that cytotoxicity was HLA-A2 restricted and antigen specific, T-cell responses to target cells were compared by light microscopy and ELISA of IFN-γ production by TIL1520 in response to HLA-A2+ LAM0806 cells versus positive control, HLA-A2+ Mf0627 melanocytes and negative control, HLA-A2− Mf0629 melanocytes as well as HLA-A2+ Ff0627 fibroblasts. Data in Figure 6, B and C, demonstrate that LAM cells induce T-cell clustering and IFN-γ production by gp100-reactive T cells, increasing with increasing target cell numbers. Similarly, responses by tyrosinase responsive, HLA-A2+, tyrosinase TCR transgenic Jurkat T cells were measured in presence of HLA-matched LAM0803 and unmatched LAM0847 cells, compared with HLA-matched Mf0627 and unmatched Mf0629 melanocytes. In these experiments (Figure 6C), tyrosinase reactive T cells were not activated by LAM cells, reflective of a lack of tyrosinase expression in LAM. Thus immunotherapy not directed at tyrosinase can be a viable option for treatment of LAM.
Figure 6.
Melanoma-derived, gp100 reactive T cells respond to LAM cells. A: Graph representing cytotoxicity of TIL1520 toward LAM0803 P2 HLA-matched LAM cells, 624.38 HLA-A*0201+ melanoma cells, and Mf0509 P5 and Mf0639 P3 matched and unmatched human melanocytes, respectively. Note responses to HLA-A2+ targets only, with superior reactivity toward LAM cells. B: Light micrographs of TIL1520 combined with (sequentially) Ff0627 P2 human HLA-A2+ fibroblasts, Mf0627 P2 HLA-A2+ human melanocytes, Mf0629 P2 HLA-A2− human melanocytes, LAM0806 P3 HLA-A2+ LAM cells, LAM0840 P3 HLA-A2− LAM cells, and TIL1520 alone. Note clustering of T cells only in combinations with HLA-A2+, gp100 expressing target cells. C: Supernatants (left) from B show IFN-γ secretion by 10,000 gp100 reactive TIL1520 T cells at 20:1, 5:1, and 2:1 effector/target ratios after 3 days; note responses only to HLA-A2+, gp100 expressing target cells. Right: interleukin-2 secretion by tyrosinase TCR Tg Jurkat cells in response to HLA-matched LAM0803 P3 and Mf0627 P4, and unmatched LAM0847 P3 and Mf0629 P4; note responses to HLA-A2+, tyrosinase expressing melanocytes only.
Discussion
LAM cells have a dysregulated cell cycle, exemplified by hyperproliferative smooth muscle cells that arise from or invade the lungs.6,11 Patients can also develop abdominal tumors including renal angiomyolipomas, and recent data suggest that lung lesions may arise in response to estrogen effects on cell trafficking through lymphatic vessels.25 Slow disease progression argues against LAM as an aggressive form of cancer, yet the disease is associated with cancer features including loss of heterozygosity, a dysregulated cell cycle, unbridled cell proliferation and tumor formation, loss of contact inhibition, metastasis, and patient mortality. Rapamycin treatment holds promise for LAM yet there are concerns about the development of resistance, and this treatment primarily inhibits proliferation without resolving existing LAM lesions.7 In this regard LAM cells expressing the hallmark gp100 molecule will be particularly resistant to anti-proliferative treatment given an inverse relationship between HMB45 and PCNA expression.26 The findings described here demonstrate that cells expressing markers of melanocyte differentiation may instead be targeted by immune mediated mechanisms, complementing existing forms of treatment. HMB45 reactivity is associated with expression of gp100, which is accompanied by MART-1 primarily yet not exclusively in the same cells. Similarly, TRP-1 expression and expression of TRP-2 was consistently found in LAM lesions, with TRP-1 expression being particularly wide-spread. The data imply that antibodies to TRP-1 offer improved sensitivity for detection of LAM lesions and may thus offer superior diagnostic criteria for LAM. Of great interest to LAM treatment is the option to overcome tolerance to this self-antigen by immunizing with a vaccine that includes heteroclytic epitopes.27 Given the consistency of the expression patterns observed, promoter demethylation may contribute to expression of most genes encoding melanosomal proteins, whereas the tyrosinase gene may have additional silencing mechanism in place or require expression of additional activating transcription factors. Alternatively, there may be selective pressure toward silencing an already activated tyrosinase encoding gene.
LAM cells also demonstrated susceptibility to T-cell mediated cytotoxicity, and T cells were activated in presence of LAM cells and melanocytes, but not by mismatched target cells. After FACS sorting of gp100high versus gp100low cells, cytotoxicity analysis revealed a trend of enhanced reactivity toward LAM target cells with increased expression of gp100 (not shown). As expression of gp100 was detectable only in a subset of cultured LAM target cells, the data suggest that gp100-reactive T cells can kill LAM cells without detectable gp100 expression. LAM cells express only immature melanosomal structures, and intracellular processing, expression and maturation/degradation patterns of gp100 and other melanoma-associated antigens in LAM cells may differ from that observed in melanocytes or melanoma cells.28 It is remarkable that cytotoxicity was observed toward 62% of targeted cells, whereas expression of gp100 was observed among, on average, 13.4% of cultured LAM cells and verified for only 5% of the HLA-A2+ cells targeted in the cytotoxicity assays. Taking into account that 100% of cells within the culture expressed α-smooth muscle actin, confirming their smooth muscle cell origin; that the majority of these cells expressed the estrogen receptor not otherwise expressed by smooth muscle cells; and that TIL1520 is exclusively cytotoxic against cells expressing the unique gp100209–217 peptide processed and presented in the context of HLA-A2, it is reasonable to assume that at least 62% of cultured cells in second passage were LAM cells. A potential contribution of altered trafficking of gp100 is supported by melanocyte surface expression of gp100 detectable by antibody NKI-Beteb (but not HMB45), which was not observed on LAM cells (not shown). Effective presentation of peptides derived from such antigens in the context of HLA-A2 by LAM cells was not demonstrated previously, nor was reactivity noted to melanoma-associated antigens presented by LAM-derived tissue cells. In this light the expression, processing, and presentation of gp100 by LAM cells is significant. Reactivity to tyrosinase, an antigen frequently targeted by melanoma infiltrating T cells as well, was also assessed. Testing for susceptibility to tyrosinase-reactive T cells was completed by using Jurkat cells expressing a high affinity transgenic TCR, which respond to their cognate antigen by interleukin-2 secretion.22,29 No interleukin-2 secretion was noted in response to LAM cells, supporting absence of tyrosinase expression and melanogenesis in LAM cells.14
Since lung infiltrating T cells were no more prevalent in LAM lungs than in lung tissues from control individuals, the data imply that aberrant expression of melanoma differentiation antigens in LAM has not led to breaking of tolerance or to marked immune reactivity to these molecules. Indeed by comparing the numbers of infiltrating immune cells per area of tissue (reflecting similar numbers of cells for individual tissue types), it was clearly shown that tumor growth was not accompanied by enhanced immune infiltration. Differences in T cell numbers between LAM lung and melanoma tissue can be ascribed primarily to reduced numbers of cytotoxic T cells found in the lung. Indeed the CD4:CD8 ratios calculated from the present data are similar for control and LAM lung, and much reduced compared with melanoma (not shown). Tolerance to immunogenic melanocyte antigens remains in place despite the fact that dendritic cells are relatively more abundant in LAM. In part this discrepancy may be explained by “lesional exclusion” observed in LAM as well as in melanoma tissue, where immune cells are kept at bay, apparently incapable of infiltrating diseased tissue that can provide access to targetable antigens to initiate an immune response. These findings leave room for improvement by vaccines directed at targeting the melanosomal target antigens newly expressed in the lung. At the same time, infiltration by macrophages is markedly increased in lung tissue affected by LAM, where numbers of infiltrating macrophages are similar to those observed in melanoma. Macrophages have been both negatively and positively associated with tumor prognosis in the past, and their role in LAM remains to be elucidated.30,31
In conclusion, here the novel finding is reported that hyperproliferative cells in the lungs of patients with LAM disease are susceptible to cytotoxic T lymphocyte-mediated immune targeting directed against melanoma antigens. Existing immune responses are tempered, whereas LAM cells express a variety of highly immunogenic melanosomal proteins. It will be of great interest to explore treatment opportunities for LAM by existing anti-melanoma vaccines including but not limited to immune enhancing interleukin-2 treatment, peptide vaccines with and without adjuvants, peptide pulsed autologous dendritic cells, and TCR transgenic T cells in clinical trials at this time.32,33 The window of opportunity for treatment is higher for these slow-growing tumors compared with metastatic melanoma. Note that rapamycin is known to block interleukin-2 activation, important for T-cell activation in effective anti-tumor responses.34 Potentially negative interactions with rapamycin treatment, a known immunosuppressant, should thus be considered when treating LAM with existing melanoma vaccines.
Acknowledgments
We are indebted to patients with LAM for tissue donations and to the National Disease Research Interchange for efficient distribution of part of the included samples.
Footnotes
Address reprint requests to Caroline Le Poole, Ph.D., Loyola University Medical Center, Oncology Institute Room 203, 2160 South First Ave, Maywood, IL 60153. E-mail: ilepool@lumc.edu.
See related Commentary on page 2252
Supported by LAM Foundation pilot grants to CLP and REB, National Institutes of Health grants CA109536, CA128068, and AR054749 to CLP, and National Institutes of Health grants CA102280 and CA104947 to MIN.
References
- Tynski Z, Eisenberg R. Cytologic findings of lymphangioleiomyomatosis in pleural effusion: a case report. Acta Cytol. 2007;51:578–580. doi: 10.1159/000325799. [DOI] [PubMed] [Google Scholar]
- Reynaud-Gaubert M, Mornex JF, Mal H, Treilhaud M, Dromer C, Quétant S, Leroy-Ladurie F, Guillemain R, Philit F, Dauriat G, Grenet D, Stern M. Lung transplantation for lymphangioleyomyomatosis: the French experience. Transplantation. 2008;86:515–520. doi: 10.1097/TP.0b013e31817c15df. [DOI] [PubMed] [Google Scholar]
- Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomeyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med. 2001;163:253–258. doi: 10.1164/ajrccm.163.1.2005004. [DOI] [PubMed] [Google Scholar]
- Moss J, DeCastro R, Patronas NJ, Taveira-DaSilva A. Meningiomas in lymphangioleiomyomatosis. JAMA. 2001;286:1879–1881. doi: 10.1001/jama.286.15.1879. [DOI] [PubMed] [Google Scholar]
- Johnson SR. Lymphangioleiomyomatosis. Eur J Respir. 2006;27:1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): Progress and current challenges. J Cell Biochem. 2008;103:369–382. doi: 10.1002/jcb.21419. [DOI] [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleimyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto R, Nakao A, Yamane M, Toyooka S, Okazaki M, Aoe M, Seyama K, Date H, Oto T, Sano Y. Sirolimus amelioration of clinical symptoms of recurrent lymphangioleiomyomatosis after living-donor lobar lung transplantation. J Heart Lung Transplant. 2008;27:921–924. doi: 10.1016/j.healun.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Watz H, Oltmann A, Kimmel S, Magnussen H, Wirtz H, Kirsten D. Sporadic lymphangioleiomyomatosis: clinical and lung functional characteristics of 32 female patients. Dtsch Med Wochenschr. 2008;133:705–708. doi: 10.1055/s-2008-1067310. [DOI] [PubMed] [Google Scholar]
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- Fetsch PA, Fetsch JF, Marincola FM, Travis W, Batts KP, Abati A. Comparison of melanoma antigen recognized by T cells (MART-1) to HMB45: additional evidence to support a common lineage for angiomyomlipoma, lymphangiomyomatosis, and clear cell sugar tumor. Mod Pathol. 1998;11:699–703. [PubMed] [Google Scholar]
- Bakker AB, Schreurs MW, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svajdler M, Bohus P, Goc V, Tkácová V. Perivascular epithelioid cell tumor (PEComa) of the liver: a case report and review of the literature. Cesk Patol. 2007;43:18–22. [PubMed] [Google Scholar]
- Bonifacino JS. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann NY Acad Sci. 2004;1038:103–114. doi: 10.1196/annals.1315.018. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol. 2001;117:341–347. doi: 10.1046/j.0022-202x.2001.01411.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigens recognized by the majority of HLA-A2 restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarbide-Valencia K, van den Boorn JG, Denman CJ, Li M, Carlson JM, Hernandez C, Nishimura MI, Das PK, Luiten RM, Le Poole IC. Therapeutic implications of autoimmune vitiligo T cells. Autoimmun Rev. 2006;5:486–492. doi: 10.1016/j.autrev.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingluff CL, Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, Kittlesen D, Deacon D, Hibbitts S, Grosh WW, Petroni G, Cohen R, Wiernasz C, Patterson JW, Conway BP, Ross WG. Phase I trial of a melanoma vaccine with gp100(280-288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- Clay TM, Morse M, Lyerly HK. Redirecting cytotoxic T lymphocyte responses with T-cell receptor transgenes. Expert Opin Biol Ther. 2002;2:353–360. doi: 10.1517/14712598.2.4.353. [DOI] [PubMed] [Google Scholar]
- Roszkowski JJ, Lyons GE, Kast WM, Yee C, van Beelen K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- Boissy RE, Liu Y-Y, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97:395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, Wang C, Hernandez-Cuebas L, Seeholzer LF, Nicolas E, Hensley H, Jordan VC, Walker CL, Henske EP. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- Guevara-Patiño JA, Engelhorn ME, Turk MJ, Liu C, Duan F, Rizzuto G, Cohen AD, Merghoub T, Wolchok JD, Houghton AN. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DC, Theos AC, Herman KE, Tenza D, Raposo G, Marks MS. Premelanosome amyloid-like fibrils are composed of only golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes. J Biol Chem. 2008;283:2307–2322. doi: 10.1074/jbc.M708007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann R, Andersen E, Trussardi R, Mazer NA. Kinetics of interleukin 2 mRNA and protein produced in the human T-cell line Jurkat and effect of cyclosporin A. Biochemistry. 1989;28:1791–1797. doi: 10.1021/bi00430a055. [DOI] [PubMed] [Google Scholar]
- Spitler LE. Adjuvant therapy of melanoma. Oncology (Williston Park) 2002;16(suppl):40–48. [PubMed] [Google Scholar]
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- Duval L, Schmidt H, Kaltoft K, Fode K, Jensen JJ, Sorensen SM, Nishimura MI, von der Maase H. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin Cancer Res. 2006;12:1229–1236. doi: 10.1158/1078-0432.CCR-05-1485. [DOI] [PubMed] [Google Scholar]
- Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, Gogas HJ. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- Masri MA. The mosaic of immunosuppressive drugs. Mol Immunol. 2003;39:1073–1077. doi: 10.1016/s0161-5890(03)00075-0. [DOI] [PubMed] [Google Scholar]