Abstract
Inhibition of amyloid-β (Aβ) aggregation is an attractive therapeutic strategy for Alzheimer’s disease (AD). Certain phenolic compounds have been reported to have anti-Aβ aggregation effects in vitro. This study systematically investigated the effects of phenolic compounds on AD model transgenic mice (Tg2576). Mice were fed five phenolic compounds (curcumin, ferulic acid, myricetin, nordihydroguaiaretic acid (NDGA), and rosmarinic acid (RA)) for 10 months from the age of 5 months. Immunohistochemically, in both the NDGA- and RA-treated groups, Aβ deposition was significantly decreased in the brain (P < 0.05). In the RA-treated group, the level of Tris-buffered saline (TBS)-soluble Aβ monomers was increased (P < 0.01), whereas that of oligomers, as probed with the A11 antibody (A11-positive oligomers), was decreased (P < 0.001). However, in the NDGA-treated group, the abundance of A11-positive oligomers was increased (P < 0.05) without any change in the levels of TBS-soluble or TBS-insoluble Aβ. In the curcumin- and myricetin-treated groups, changes in the Aβ profile were similar to those in the RA-treated group, but Aβ plaque deposition was not significantly decreased. In the ferulic acid-treated group, there was no significant difference in the Aβ profile. These results showed that oral administration of phenolic compounds prevented the development of AD pathology by affecting different Aβ aggregation pathways in vivo. Clinical trials with these compounds are necessary to confirm the anti-AD effects and safety in humans.
Alzheimer’s disease (AD) is the most common form of dementia, resulting in deterioration of cognitive function and behavioral changes.1 One of the pathological hallmarks of AD is extracellular deposits of aggregated amyloid-β protein (Aβ) in the brain parenchyma (senile plaques) and cerebral blood vessels (cerebral amyloid angiopathy (CAA)).1 Deposition of high levels of fibrillar Aβ in the AD brain is associated with loss of synapses, impairment of neuronal functions, and loss of neurons.2,3,4,5 Aβ was sequenced from meningeal vessels and senile plaques of AD patients and individuals with Down’s syndrome.6,7,8 The subsequent cloning of the gene encoding the β-amyloid precursor protein and its localization to chromosome 21,9,10,11,12 coupled with the earlier recognition that trisomy 21 (Down’s syndrome) invariably leads to the neuropathology of AD,13 set the stage for the proposal that Aβ accumulation is the primary event in AD pathogenesis. In addition, certain mutations associated with familial AD have been identified within or near the Aβ region of the coding sequence of gene of the amyloid precursor proteins,14,15 presenilin-1 and presenilin-2,16 which alter amyloid precursor protein metabolism through a direct effect on γ-secretase.17,18 These findings set the stage for the proposal that Aβ aggregation is the primary event in AD pathogenesis and leading to the proposal that anti-Aβ aggregation is a strategy for AD therapy.19,20 Furthermore, there have been recent reports21,22,23,24,25 that Aβ fibrils are not the only toxic form of Aβ for developing AD, and smaller species of aggregated Aβ, Aβ oligomers, may represent the primary toxic species in AD. Therefore, it is necessary to consider the inhibition of Aβ oligomer formation as well as Aβ fibrils for the treatment of AD.26
To date, it has been reported that various compounds inhibit the formation and extension of Aβ fibrils, as well as destabilizing Aβ fibrils in vitro.19,20,27,28,29,30,31,32,33,34,35,36 Among the reported compounds, several phenolic compounds, such as wine-related polyphenols (myricetin (Myr), morin, and tannic acid, and so on), curcumin (Cur), ferulic acid (FA), nordihydroguaiaretic acid (NDGA), and rosmarinic acid (RA) had especially strong anti-Aβ aggregation effects in vitro. Furthermore, it was shown recently that a commercially available grape seed polyphenolic extract, MegaNatural-Az, inhibited fibril formation, protofibril formation, and oligomerization of Aβ.37 Moreover, MegaNatural-Az also reduced cerebral amyloid deposition as well as attenuating AD-type cognitive deterioration using transgenic mice.38 In addition to these studies by the current authors, several other researchers have reported similar effects of phenolic compounds.26,39,40,41,42,43,44 First, Cur decreased cerebral Aβ plaque burden in vivo,39,40,41,42,44 and inhibited the formation of Aβ oligomers in vitro.26,39 Second, epigallocatechin gallate efficiently inhibited fibril and oligomer formation of Aβ.43 However, a very recent in vitro study26 reported that Cur, Myr, and NDGA inhibited the formation of Aβ oligomers, but Cur and NDGA promoted the formation of Aβ fibrils. This indicated that the effects of these phenolic compounds on Aβ aggregation remain controversial. These different results may reflect different experimental conditions in these studies. To resolve this problem, a systematic in vivo study is required; however, few reports on the effects of phenolic compounds on Aβ aggregation in vivo have been published so far, except for reports about Cur.39,40,41,42,44
To elucidate the inhibitory effects of phenolic compounds on Aβ aggregation in vivo, several phenolic compounds, including Cur, FA, Myr, NDGA, and RA, were fed to AD model mice, and the cerebral plaque burden and formation of Aβ oligomers were compared systematically.
Materials and Methods
Animals
Five-month-old female Tg2576 mice45 (Taconic Farms, Germantown, NY), which express a 695-aa residue splice form of human amyloid precursor protein modified by the Swedish Familial AD double mutation K670N-M671L, were randomly assigned among one control and five treatment groups. The mice in the control group were fed a control diet (CRF-1; Oriental Yeast, Tokyo, Japan) (n = 10), and those of the five treatment groups were fed five different diets, which included 0.5% phenolic compounds, comprising Cur (Wako, Osaka, Japan) (n = 9), FA (Sigma-Aldrich, St. Louis, MO) (n = 10), Myr (Kanto Chemical, Tokyo, Japan) (n = 10), NDGA (Tokyo Chemical Industry, Tokyo, Japan) (n = 10), and RA (Sigma-Aldrich) (n = 10) (Figure 1) in CRF-1. At the age of 14 months, the mice were sacrificed. The mice were perfused before brain dissection with 0.9% normal saline, followed by HEPES buffer containing protease inhibitor mixture (Nacalai Tesque, Kyoto, Japan). Brains were harvested and hemidissected. One hemisphere was fixed in 4% paraformaldehyde for 24 hours for histological studies, and the opposite hemisphere was frozen rapidly in liquid nitrogen and stored at −80°C for biochemical studies. All animal studies were approved by the Institutional Animal Experiment Committee of Kanazawa University.
Figure 1.
Structures of Cur, FA, Myr, NDGA, and RA.
Immunohistochemistry and Morphometry of Aβ Deposits
For the assessment of brain Aβ deposition in Tg2576 mice brain, 4% paraformaldehyde-fixed, paraffin-embedded left hemi-brains were sectioned in the coronal plane using a microtome at a thickness of 5 μm. Sections were deparaffinized and hydrated in a graded series of ethanol, pretreated with 99% formic acid for 5 minutes, and immersed in 0.3% hydrogen peroxide and methanol for 30 minutes to block endogenous peroxidase before preblocking at ambient temperature with serum-free protein block (Dako, Glostrup, Denmark). Aβ immunohistochemical staining was performed using anti-human amyloid-β antibody (4G8, 1/2000; Chemicon International, Temecula, CA) in conjunction with the Liquid Diaminobenzidine Substrate Chromogen System (Dako). 4G8-positive Aβ deposits were examined under bright field using an Olympus BX-51 microscope, Olympus DP71 digital camera, and custom-designed software WinROOF (Mitani, Fukui, Japan). The percentage of 4G8-positive deposits area (Aβ plaque burden) and numbers of 4G8-positive blood vessels per 1 mm2 (CAA counts) were investigated. In total, seven coronal sections were assessed by a scientist (A.M.) who was blinded to the treatment profile of each section.
Tissue Preparation for Biochemical Studies
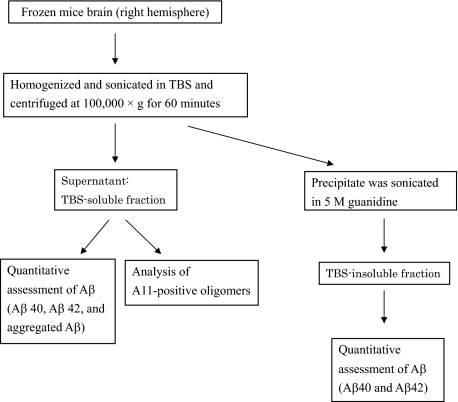
Frozen brains were homogenized in 10 volumes of Tris-buffered saline (TBS) (20 mmol/L Tris (pH 7.3) and 140 mmol/L NaCl) containing protease inhibitors mixture (Nacalai Tesque) (Figure 2). Samples were sonicated briefly (2 × 10 s) and centrifuged at 100,000 × g for 60 minutes at 4°C to generate a TBS-soluble fraction. The TBS-insoluble pellet was sonicated in 8 volumes of 5 M guanidine and 50 mmol/L Tris-HCl and solubilized by agitation at room temperature for 4 hours (TBS-insoluble fraction) (Figure 2).
Figure 2.
Schematic representation of tissue preparation for biochemical studies. Frozen brains (right hemisphere) were homogenized in TBS, sonicated briefly (2 × 10 s), and centrifuged at 100,000 × g for 60 minutes at 4°C to generate a TBS-soluble fraction. The TBS-insoluble pellet was sonicated in 5 M guanidine and solubilized by agitation at room temperature for 4 hours (TBS-insoluble fraction). TBS-soluble fractions were analyzed using Aβ40, Aβ42, and aggregated Aβ using Bio-Plex multiplex suspension array systems and analyzed for A11-positive oligomers by dot blot analysis. TBS-insoluble fractions were analyzed Aβ40 and Aβ42 using Bio-Plex multiplex suspension array system.
Quantitative Assessment of Aβ in the Brain
For quantitative assessment of Aβ in the brain, a Bio-Plex multiplex suspension array system (Bio-Rad, Hercules, CA) was used as described previously.46,47,48,49,50 This technology is based on flow cytometric separation of antibody-coated microspheres that are labeled with a specific mixture of two fluorescent dyes. After binding of a biotinylated reporter antibody, quantification was made by binding of a third fluorochrome coupled to streptavidin. In TBS-soluble fractions, Aβ1-40 (Aβ40), Aβ1-42 (Aβ42), and aggregated Aβ were analyzed using human Aβ40, Aβ42, and aggregated Aβ antibody bead kit (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions (Figure 2). TBS-insoluble fractions were diluted 1/10,000 with TBS containing 5% bovine serum albumin with protease inhibitor mixture (Nacalai Tesque) and then analyzed for Aβ40 and Aβ42 (Figure 2). Aβ40 and Aβ42 antibody bead kits recognized monomeric forms of Aβ40 and Aβ42, and they have no cross-reactivity with each other (information sheet of the kit from Invitrogen). The aggregated Aβ antibody bead kit recognized aggregated Aβ, and it had no cross-reactivity with Aβ40 but slight reactivity with Aβ42 (2.2%) (information sheet of the kit from Invitrogen).
Analysis of A11-Positive Oligomers in the Brain
To investigate TBS-soluble Aβ oligomers in the brains, dot blot assays were performed as described previously.38,51 Five micrograms of protein from the TBS-soluble fractions were applied directly to a nitrocellulose membrane, air-dried, and blocked with 5% nonfat dry milk. The membrane was probed with A11 antibody (1/1000; BioSource International, Camarillo, CA), which recognizes oligomers but not monomers or fibrils of several proteins that form amyloid, including Aβ,52,53 and immunoreactivities were quantified densitometrically using LAS-4000 mini and Multi Gauge Ver.3.X (Fujifilm, Tokyo, Japan) (Figure 2). A11-positive Aβ oligomers ranged in size from approximately tetramers to 20-mers,54 and the A11 antibody recognized a significant and important class of oligomers associated with AD.26
Statistical Analysis
All values are expressed as mean and SE. Differences between the control and each treatment groups in body weight, Aβ plaque burden, CAA counts, and concentrations of Aβ40, Aβ42, aggregated Aβ, and A11-positive oligomers were analyzed using one-way analysis of variance, followed by Dunnett’s post hoc analysis. Survival of each group was determined using Kaplan-Meier plots, and the null hypothesis on the survival experience of each group was tested using the Generalized Wilcoxon test. Significance was defined as P < 0.05. Statistical analyses were performed using SPSS 16.0 software (SPSS Japan, Tokyo, Japan).
Results
Mice Characteristics
During this experiment, one mouse in the control and Cur-, Myr-, and NDGA-treated groups, two mice in the RA-treated group, and three mice in the FA-treated group died. Survival periods were not significantly different between the groups. The numbers of mice and body weight at the age of 14 months are shown for each group in Table 1. Body weights were not significantly different between the groups.
Table 1.
Number of Mice and Body Weight at the Age of 14 Months in Each Group
| N | Body weight (g; average ± SE) | |
|---|---|---|
| Control | 9 | 31.5 ± 3.3 |
| Cur | 8 | 29.2 ± 1.3 |
| FA | 7 | 31.9 ± 3.9 |
| Myr | 9 | 30.7 ± 2.4 |
| NDGA | 9 | 29.0 ± 1.4 |
| RA | 8 | 37.2 ± 5.2 |
Aβ Plaque Burden
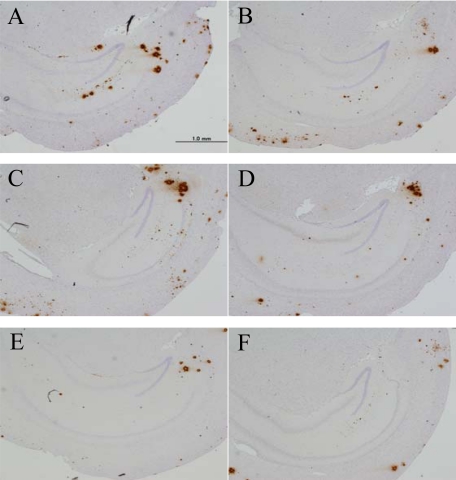
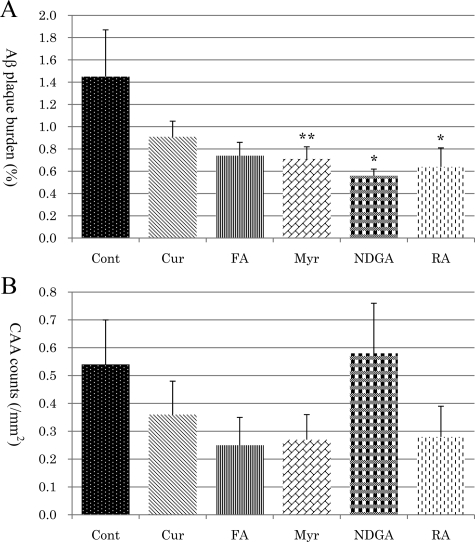
In an immunohistochemical study, significantly lower Aβ plaque burden was found in the groups treated with NDGA (Figure 3E) and RA (Figure 3F) compared with the control group (Figure 3A) (P < 0.05) (Figure 4A). In the Myr-treated group (Figure 3D), there was the tendency to attenuate Aβ plaque burden, but this did not reach a significant level (P = 0.064) (Figure 4A). There were no significant differences between the Cur- (Figure 3B) or FA-treated (Figure 3C) groups and the control group (Figure 4A). In the evaluation of CAA counts, there were no significant differences between each treatment group and the control group (Figure 4B).
Figure 3.
Amyloid β plaque burden in each treated group of mice, control (A), Cur (B), FA (C), Myr (D), NDGA (E), and RA (F).
Figure 4.
Comparison analysis of Aβ plaque burden (A) and CAA counts (B) in each group. Compared with the control group, the percentage of 4G8-positive deposit areas (Aβ plaque burden) was reduced in the NDGA- and RA-treated groups significantly (P < 0.05), and there was the tendency to attenuate Aβ plaque burden, but a significant level of attenuation was not reached in the Myr group (P = 0.064, A). The average numbers of 4G8-positive blood vessels per 1 mm2 (CAA counts) was not significantly different between the control and treatment groups (B). ∗P < 0.05; **P = 0.064.
Aβ in TBS-Soluble Fractions of the Brain
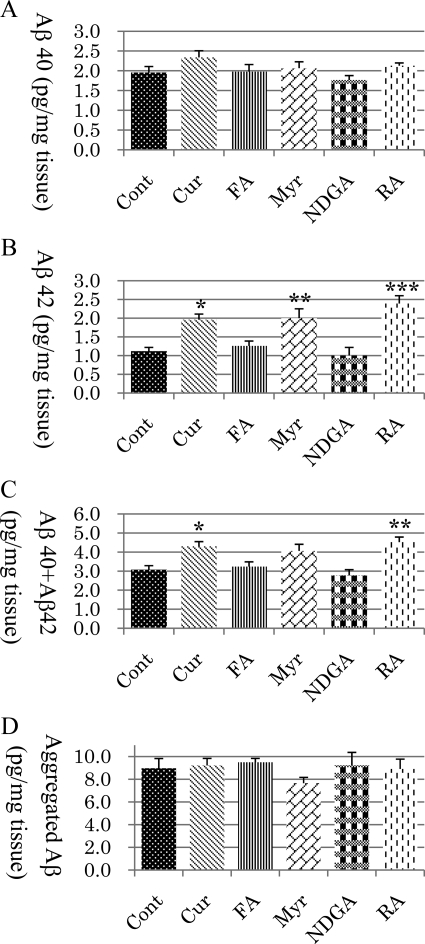
In a quantitative assessment of Aβ in the TBS-soluble fraction of brains, there was no significant differences in Aβ40 between any of the groups (Figure 5A), and Aβ42 was significantly increased in the Cur (P < 0.05), Myr (P < 0.01), and RA (P < 0.001) groups compared with the control group (Figure 5B). The Aβ42 levels in the FA and NDGA groups were not different from those in the control group. The total of Aβ40 and Aβ42 was significantly increased in the Cur (P < 0.05) and RA (P < 0.01) groups but not in the FA, Myr, or NDGA groups (Figure 5C). No significant differences were found between each treatment group and the control group in the level of aggregated Aβ (Figure 5D).
Figure 5.
Assessment of Aβ40 (A), Aβ42 (B), Aβ40 + Aβ42 (C), and aggregated Aβ (D) concentrations in the TBS-soluble fraction of mice brain tissue. There was no significant difference between the control and treatment groups in Aβ40 (A) and aggregated Aβ (D). Compared with the control, Aβ42 was significantly increased in the Cur (P < 0.05), Myr (P < 0.01), and RA (P < 0.001) groups (B), and Aβ40 + Aβ42 was also significantly increased in the Cur (P < 0.05) and RA groups (P < 0.01) (C). ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Aβ in TBS-Insoluble Fractions of the Brain
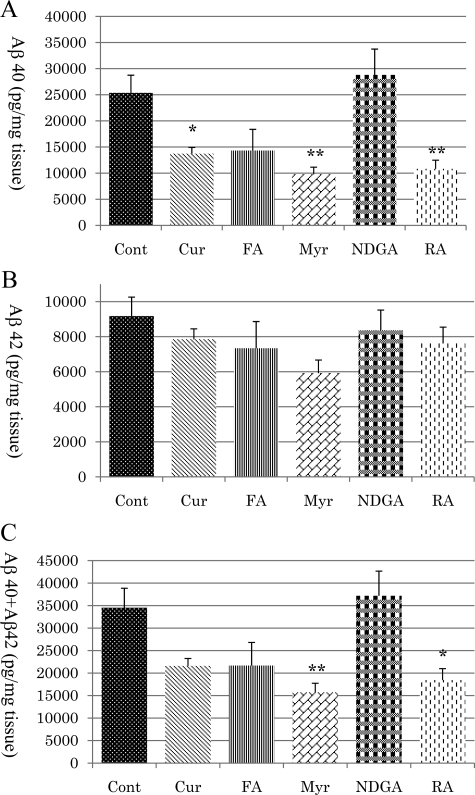
In the TBS-insoluble fractions of the brain, the level of Aβ40 was significantly decreased in the Cur (P < 0.05), Myr (P < 0.01), and RA (P < 0.01) groups but not in the FA or NDGA groups (Figure 6A). There were no significant differences between each treatment group and the control group in the level of Aβ42 (Figure 6B). The total of Aβ40 and Aβ42 was significantly decreased in the Myr (P < 0.01) and RA (P < 0.05) groups (Figure 6C).
Figure 6.
Assessment of Aβ40 (A), Aβ42 (B), and Aβ40 + Aβ42 (C) concentrations in the TBS-insoluble fraction of mice brain tissue. Compared with the control, Aβ40 was significantly reduced in the Cur (P < 0.05), Myr (P < 0.01), and RA (P < 0.01) groups (A), and Aβ40 + Aβ42 was also significantly reduced in the Myr (P < 0.01) and RA (P < 0.05) groups (C). There was no significant difference between the treatment and control groups for Aβ42 (B). ∗P < 0.05; ∗∗P < 0.01.
A11-Positive Oligomers in the TBS-Soluble Fraction of the Brain
In the analysis of A11-positive oligomers in the TBS-soluble fractions of the brain, A11-positive oligomers were found to be significantly decreased in the groups treated with Cur (P < 0.001), Myr (P < 0.001), and RA (P < 0.001) compared with the control group (Figure 7). In contrast, the level of A11-positive oligomers was significantly increased in the NDGA-treated group (P < 0.05) (Figure 7). There was no significant difference between the FA-treated and control groups (Figure 7).
Figure 7.
Assessment of TBS-soluble A11-positive oligomers in the mice brain tissue. Compared with the control, A11-positive oligomers were decreased in the Cur (P < 0.001), Myr (P < 0.001), and RA (P < 0.001) groups and increased in the NDGA (P < 0.05) group, significantly. ∗P < 0.05; ∗∗P < 0.001.
Discussion
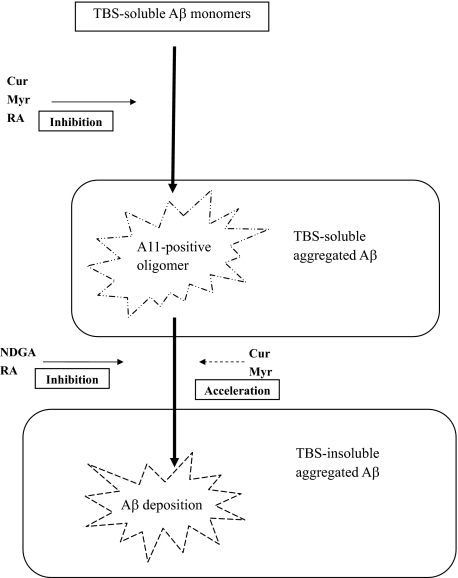
The present results showed that oral administration of NDGA and RA prevented the development of Aβ neuropathology in AD model mice. However, biochemical studies revealed different Aβ profiles in the brain between NDGA- and RA-treated mice. Treatment with RA was associated with an increase in TBS-soluble Aβ (Aβ42 and Aβ40 + Aβ42) and a decrease in A11-positive oligomers and TBS-insoluble Aβ (Aβ40 and Aβ40 + Aβ42). These results suggested that RA inhibits the Aβ aggregation pathway from Aβ monomers to A11-positive oligomers and from A11-positive oligomers to Aβ deposition (Figure 8). In the NDGA-treated group, A11-positive oligomers increased without any change in the levels of TBS-soluble or TBS-insoluble Aβ. These results suggested the possibility that NDGA might mainly inhibit the pathway from A11-positive oligomers to Aβ deposition without inhibiting the pathway from Aβ monomers to A11-positive oligomers (Figure 8). In this study, there was dissociation between Aβ plaque burden and concentration of TBS-insoluble Aβ in NDGA-treated group. The reason of this discrepancy was not determined precisely, but it was considered that Aβ dissolved in the TBS-insoluble fractions might include not only deposited aggregated Aβ but also intracellular Aβ and precipitated A11-positive oligomers recovered by 100,000 × g centrifugation. In the Cur- and Myr-treated groups, changes in the Aβ profile were similar to those in the RA-treated group, but Aβ plaque deposition was not decreased significantly. On the basis of these results, two possible hypotheses are given. One is that Cur and Myr inhibit the Aβ aggregation pathway in the same manner as RA, but the effect is weaker than RA. Another is that Cur and Myr inhibit the pathway from Aβ monomers to A11-positive oligomers, but they accelerate the pathway from A11-positive oligomers to Aβ deposition (Figure 8), which is similar to the findings of a recent in vitro study.26 The FA-treated group showed no significant difference compared with the control group. Previous studies showed that all of the phenolic compounds examined in this study inhibited Aβ fibril formation in vitro. The overall activity of these phenolic compounds was in the order of RA = Cur = NDGA = Myr > FA.28,36 The effective concentrations of RA, Cur, NDGA, and Myr for Aβ fibril formation in vitro were 0.1 to 1 μmol/L, whereas that of FA was 1 to 10 μmol/L.28,36 The discrepancy between the in vitro and in vivo studies is important for clinical application for preventing AD; various biological parameters, such as absorption into the body, passage through the blood-brain barrier, and degradation could influence their antiaggregation effects in vivo. There have been no reports on the concentrations of these phenolic compounds in the brain following long-term oral administration.
Figure 8.
Schematic representation of the in vivo effects of phenolic compounds on Aβ aggregation as suggested by this study. Cur, Myr, and RA may inhibit the pathway from Aβ monomer to A11-positive oligomers, and NDGA and RA may inhibit the pathway from A11-positive oligomers to Aβ deposition. Cur and Myr may accelerate the pathway from A11-positive oligomers to Aβ deposition. Arrows with solid lines are strongly suggested and dashed arrows indicate a possible effect on the basis of the present results.
In AD, soluble Aβ monomers undergo conformational changes and are deposited as insoluble Aβ fibrils mediated by Aβ oligomers.22,25 Previously, it was demonstrated that Aβ neurotoxicity requires insoluble fibril formation.55 However, recently, Aβ oligomers are believed to play important causal roles in AD,21,22,23,24,25 and the most efficacious therapeutic agents should target the oligomeric forms of Aβ. Some types of oligomeric assemblies of Aβ, such as protofibrils, annular assemblies, Aβ-derived diffusible ligands, Aβ*56, and secreted soluble Aβ dimers and trimers, have been reported22; however, the structures of these oligomers have not been revealed fully, and it is difficult to distinguish them precisely. At present, some conformation-dependent antibodies against oligomers, such as A11 and OC, can provide a more rational means of classifying Aβ oligomers based on their underlying structural organization.53,54 Recent studies52,54 using these antibodies have shown that there are pathways of fibril formation mediated by different types of oligomers, such as A11-positive and OC-negative oligomers or A11-negative and OC-positive oligomers. A11-positive oligomers are correlated with cognitive deficits in transgenic animal models,52 but there have been no reports on the association between cognition and OC-positive oligomers. In this study, there was no difference in the aggregated Aβ in the TBS-soluble fraction between the control and treatment groups, although the concentrations of A11-positive oligomers were significantly different. Aggregated Aβ might include not only A11-positive oligomers but also A11-negative oligomers.
RA is an ester of caffeic acid 3,4-dihydroxiphenyllactic acid, and it is commonly found in species of the Boraginaceae and subfamily Nepetoideae of the Lamiaceae.56 It has several interesting biological activities, eg, antioxidant, anti-inflammatory, antimutagen, antibacterial, and antiviral.56 For the effect on AD pathogenesis, there have been reports that RA reduces Aβ-induced neurotoxicity57,58 and protects against reactive oxygen species induced by Aβ in cell culture experiments.58 In intracerebroventricular Aβ25-35 injection mice model studies, i.p. injection of RA prevents memory impairment and Aβ-induced neurotoxicity by scavenging ONOO−.57 Taken together with the results of this study that RA reduced both Aβ deposition and A11-positive oligomers, RA is an attractive candidate for therapy or preventive strategies for AD.
NDGA is a pure compound isolated from the creosote bush, Larrea tridentate.59 It significantly reduces plasma glucose and triglyceride concentrations in rats.59,60 It also suppresses Aβ-induced accumulation of reactive oxygen species.61 In a recent study26 of Aβ fibril formation in vitro, NDGA inhibited oligomerization but did not affect fibrillization, which was contrary to the present results. The conflicting results may be related to differences in the experimental conditions. As A11-positive oligomers were increased in the brain of the NDGA-treated group in the present study, NDGA would be inappropriate for clinical application.
Cur is a potent antioxidant and an effective anti-inflammatory compound.62,63 Several studies, including the previous in vitro study, suggested that Cur could be a key molecule for the development of therapeutics for AD.26,34,39,40,41,42,44,64 Cur protected PC12 and human umbilical vein endothelial cells from Aβ insult due to its strong antioxidant properties,64 and dietary curcumin prevented Aβ-infusion induced spatial memory deficits and reduced Aβ deposits in rats.44 In an in vivo study with Tg2576 transgenic mice, a low dose (160 ppm) of Cur decreased the levels of insoluble and soluble Aβ and plaque burden, but a high dose (5000 ppm) did not change Aβ levels.41 The dose of Cur in the present study was same as the high dose (5000 ppm) of Cur in the previous study,41 and the treated mice showed an increase in TBS-soluble Aβ, a decrease in A11-positive oligomers, and no change in Aβ plaque burden. One possible explanation is that Cur has the ability to accelerate the pathway from Aβ oligomers to Aβ deposition. This explanation was supported by other in vitro findings that Cur inhibits oligomerization but does not inhibit fibrillization.26 It has been also shown that Cur inhibits the formation of Aβ oligomers and fibrils, binds plaques, and reduces plaque burden.39 Several other studies with AD model mice also reported beneficial effects of Cur.40,42 However, in a recent clinical trial of Cur for AD, 6-month administration of 1 or 4 g/day Cur had no significant effect on cognitive impairment.65 Longer and larger trials with Cur are necessary.
Myr is found in various foods, including onions, berries, and grapes, as well as red wine.66,67,68 Many studies indicated that Myr has various biological activities, such as antioxidant, anti-inflammatory, anticarcinogen, and antiviral.69 Recently, Myr was reported to act as a β-secretase inhibitor with reduced production of Aβ in a cell culture study.70 In this study, the Myr-treated mice showed a decrease in A11-positive oligomers but no change in Aβ plaque burden, as found in Cur, and this agreed with the results of an in vitro study that showed Myr inhibited oligomerization but did not affect fibrillization of Aβ.26 Therefore, Myr is also a candidate therapeutic molecule for inhibiting Aβ oligomerization.
FA is a major constituent of fruits and is well-known to be an important antioxidant.71,72 Long-term administration of FA was reported to protect mice against Aβ-induced learning and memory deficits in vivo,73,74 and FA protects neurons against Aβ-induced oxidative stress and neurotoxicity in vitro.75 In the present study, oral administration of FA did not show any significant effect on Aβ oligomers or Aβ deposition in vivo.
In the present study, body weight and survival rate were not significantly different between the treatment and control groups, suggesting that there was no adverse effect of these phenolic compounds in the concentration and duration ranges examined. In this study, each mouse was fed 1 g/kg/day of the phenolic compound where it was assumed that each mouse ate 6 g of food per day, and the body weight of a mouse was 30 g. According to the U.S. Food and Drug Administration criteria for converting drug equivalent dosages across species (www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf), ∼80 mg/kg/day of phenolic compounds was administered when converting to a human dose. It is not known whether these phenolic compounds can be administered at these doses over the long term in humans without any adverse effect, although 4 g of Cur per day (80 mg/kg/day when human body weight was assumed to be 50 kg) for 6 months did not cause any side effects in AD patients in a recent clinical trial.65 The results of this study indicate that further careful clinical studies with these compounds should be performed.
The present study did not focus on the pharmacokinetics of the phenolic compounds. There remains the possibility that the intakes of each phenolic compound were different, because the actual amount of food consumed by the mice was not monitored. In the previous study with Cur,41 Aβ deposition in the brain was decreased significantly in the mice fed on a low dose of Cur but were not changed in a high-dose group. In the previous in vitro study,28,34,36 the inhibitory effect of the Aβ aggregation increased in proportion to the concentrations of these compounds linearly, but it was not known whether these compounds had similar effects in vivo. Further studies are required in which mice are fed these compounds at different doses and over different periods to clarify whether the differences observed in the present study reflect differences in the mechanisms of antiamyloidogenic effects or possible differences in the concentrations of these compounds in the central nervous system.
In conclusion, the present study showed that the oral administration of phenolic compounds prevented the development of AD pathology by inhibiting the Aβ aggregation pathway in different ways in an AD transgenic mouse model. Among the compounds tested, RA appeared to be the most attractive molecule for preventing AD because it inhibited both Aβ oligomerization and deposition. Cur and Myr could also be candidates because they inhibited Aβ oligomerization. On the contrary, NDGA may be inappropriate because it inhibited Aβ deposition but not oligomerization. Clinical trials with these compounds are necessary to confirm their anti-AD effects and safety in humans.
Acknowledgments
We thank Drs. Kazuo Iwasa and Akiyoshi Morinaga for their valuable help and advice throughout this research. We thank Ms. Yumiko Kakuta and Ms. Yukari Yamaguchi for excellent technical support.
Footnotes
Address reprint requests to Masahito Yamada, M.D., Ph.D., Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Science, 13-1, Takara-machi, Kanazawa 920-8640, Japan. E-mail: m-yamada@med.kanazawa-u.ac.jp.
Supported in part by a Grant-in-Aid for Young Scientists (Start-up) (KAKENHI 19890083) (to T.H.), a Grant-in-Aid for Scientific Research (KAKENHI 20390242) (to M.Y.), a grant for the 21st Century Center of Excellence Program (on Innovation Brain Science for Development, Learning and Memory) (to M.Y.), a grant for Knowledge Cluster Initiative (High-Tech Sensing and Knowledge Handling Technology (Brain Technology)) (to M.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Amyloidosis Research Committee of the Ministry of Health, Labour, and Welfare of Japan (to M.Y.).
References
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Younkin SG. Evidence that Aβ42 is the real culprit in Alzheimer’s disease. Ann Neurol. 1995;37:287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Aβ1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer’s disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci USA. 1987;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid-β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Olson MI, Shaw CM. Presenile dementia and Alzheimer’s disease in mongolism. Brain. 1969;92:147–156. doi: 10.1093/brain/92.1.147. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St. George-Hyslop PH. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Ono K, Hamaguchi T, Naiki H, Yamada M. Anti-amyloidogenic effects of antioxidants: implications for the prevention and therapeutics of Alzheimer’s disease. Biochim Biophys Acta. 2006;1762:575–586. doi: 10.1016/j.bbadis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Ono K, Yamada M. Anti-amyloidogenic therapies: strategies for prevention and treatment of Alzheimer’s disease. Cell Mol Life Sci. 2006;63:1538–1552. doi: 10.1007/s00018-005-5599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid-β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid-β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Klein WL. Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid-β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Preformed β-amyloid fibrils are destabilized by coenzyme Q10 in vitro. Biochem Biophys Res Commun. 2005;330:111–116. doi: 10.1016/j.bbrc.2005.02.132. [DOI] [PubMed] [Google Scholar]
- Ono K, Hirohata M, Yamada M. Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2005;336:444–449. doi: 10.1016/j.bbrc.2005.08.148. [DOI] [PubMed] [Google Scholar]
- Ono K, Hirohata M, Yamada M. Alpha-lipoic acid exhibits anti-amyloidogenicity for β-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2006;341:1046–1052. doi: 10.1016/j.bbrc.2006.01.063. [DOI] [PubMed] [Google Scholar]
- Hirohata M, Hasegawa K, Tsutsumi-Yasuhara S, Ohhashi Y, Ookoshi T, Ono K, Yamada M, Naiki H. The anti-amyloidogenic effect is exerted against Alzheimer’s β-amyloid fibrils in vitro by preferential and reversible binding of flavonoids to the amyloid fibril structure. Biochemistry. 2007;46:1888–1899. doi: 10.1021/bi061540x. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim Biophys Acta. 2004;1690:193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Yamada M, Naiki H. Nicotine breaks down preformed Alzheimer’s β-amyloid fibrils in vitro. Biol Psychiatry. 2002;52:880–886. doi: 10.1016/s0006-3223(02)01417-8. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Yoshiike Y, Takashima A, Yamada M, Naiki H. Nordihydroguaiaretic acid potently breaks down pre-formed Alzheimer’s β-amyloid fibrils in vitro. J Neurochem. 2002;81:434–440. doi: 10.1046/j.1471-4159.2002.00904.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Anti-Parkinsonian agents have anti-amyloidogenic activity for Alzheimer’s β-amyloid fibrils in vitro. Neurochem Int. 2006;48:275–285. doi: 10.1016/j.neuint.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Condron MM, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid-β-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid-β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits: Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of β-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Kornhuber J, Vanderstichele H, Vanmechelen E, Esselmann H, Bibl M, Wolf S, Otto M, Reulbach U, Kolsch H, Jessen F, Schroder J, Schonknecht P, Hampel H, Peters O, Weimer E, Perneczky R, Jahn H, Luckhaus C, Lamla U, Supprian T, Maler JM, Wiltfang J. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: a multicenter study. Neurobiol Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Nielsen HM, Minthon L, Londos E, Blennow K, Miranda E, Perez J, Crowther DC, Lomas DA, Janciauskiene SM. Plasma and CSF serpins in Alzheimer disease and dementia with Lewy bodies. Neurology. 2007;69:1569–1579. doi: 10.1212/01.wnl.0000271077.82508.a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain β-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. β-Amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Aβ(25-35). Behav Brain Res. 2007;180:139–145. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Iuvone T, De Filippis D, Esposito G, D'Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- Luo J, Chuang T, Cheung J, Quan J, Tsai J, Sullivan C, Hector RF, Reed MJ, Meszaros K, King SR, Carlson TJ, Reaven GM. Masoprocol (nordihydroguaiaretic acid): a new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur J Pharmacol. 1998;346:77–79. doi: 10.1016/s0014-2999(98)00139-3. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Brignetti D, Luo J, Khandwala A, Reaven GM. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of type II diabetes. Diabetologia. 1999;42:102–106. doi: 10.1007/s001250051121. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Steiner MR, Steiner SM, Mattson MP. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid-β-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res. 1994;654:171–176. doi: 10.1016/0006-8993(94)91586-5. [DOI] [PubMed] [Google Scholar]
- Zhao BL, Li XJ, He RG, Cheng SJ, Xin WJ. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989;14:175–185. doi: 10.1007/BF02797132. [DOI] [PubMed] [Google Scholar]
- Sreejayan , Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA(1-42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- Sellappan S, Akoh CC. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J Agric Food Chem. 2002;50:5338–5342. doi: 10.1021/jf020333a. [DOI] [PubMed] [Google Scholar]
- Ong KC, Khoo HE. Biological effects of myricetin. Gen Pharmacol. 1997;29:121–126. doi: 10.1016/s0306-3623(96)00421-1. [DOI] [PubMed] [Google Scholar]
- Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim Biophys Acta. 2008;1780:819–825. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- Scott BC, Butler J, Halliwell B, Aruoma OI. Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic Res Commun. 1993;19:241–253. doi: 10.3109/10715769309056512. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho JY, Kim DH, Yan JJ, Lee HK, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of β-amyloid peptide (1-42) in mice. Biol Pharm Bull. 2004;27:120–121. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001;133:89–96. doi: 10.1038/sj.bjp.0704047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid-β-peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]