Abstract
Pulmonary morbidity is high following spinal cord injury and is due, in part, to impairment of airway protective behaviors. These airway protective behaviors include augmented breaths, the cough reflex, and expiration reflexes. Functional recovery of these behaviors has been reported after spinal cord injury. In humans, evidence for functional recovery is restricted to alterations in motor strategy and changes in the frequency of occurrence of these behaviors. In animal models, compensatory alterations in motor strategy have been identified. Crossed descending respiratory motor pathways at the thoracic spinal cord levels exist that are composed of crossed premotor axons, local circuit interneurons, and propriospinal neurons. These pathways can collectively form a substrate that supports maintenance and/or recovery of function, especially after asymmetric spinal cord injury. Local sprouting of premotor axons in the thoracic spinal cord also can occur following chronic spinal cord injury. These mechanisms may contribute to functional resiliency of the cough reflex that has been observed following chronic spinal cord injury in the cat.
Keywords: Spinal cord injury, Premotor, Cough, Augmented breath, Control of breathing
1. Introduction
Pulmonary complications, such as pulmonary infection and hypoventilation leading to ventilator dependence, are the leading cause of morbidity and death in patients with spinal cord injuries (Schmitt et al., 1991). This increased pulmonary morbidity is primarily due to impairment of the central regulation of respiratory muscle activity, which results in respiratory insufficiency and loss of airway protective reflexes. These reflexes, which include the cough reflex, expiration reflex, and augmented breaths, are essential for maintaining patent airways and reducing atelectasis (Korpas and Tomori, 1979). The clinical literature on respiratory function in patients with cervical spinal cord injuries clearly indicates the presence of profound deficits immediately after injury that undergo significant functional recovery within 1 year (Brown et al., 2006). Therefore, humans appear to possess compensatory mechanisms that allow recovery of airway protective function after the initial injury. Sparing of significant amounts of spinal cord tissue at the injury site can often occur (Hayes and Kakulas, 1997). In spite of functional recovery and/or tissue sparing at the injury site, the frequency of pulmonary morbidity in this patient group is high, due in part to continued impairment of pulmonary defensive reflexes (Brown et al., 2006). In essence, many of these patients recover the ability to breathe spontaneously, but have persistent reductions in the efficacy of airway protective reflexes.
Airway protective behaviors include cough, sneeze, expiration reflex, augmented breath, laryngeal adduction (alone or as a component of swallow, the diving response, or vomiting), apnea, and aspiration reflex. We believe it is more inclusive to use the term “behavior” rather than “reflex” to describe these motor tasks given that many of them can be produced voluntarily in awake humans.
These behaviors are produced by enhanced inspiratory and/or expiratory motor drive to respiratory motoneuron pools, and this increased motor drive may be as much as an order of magnitude greater than that required for normal breathing (van Lunteren et al., 1988a; Bolser, 1991; Bolser et al., 2000). Indeed, excitatory motor drive produced during airway protective behaviors may represent the most intense activation of spinal motoneuron pools that can be elicited by the respiratory motor control system (Sieck and Fournier, 1989; Bolser et al., 2000). It follows that these behaviors should be particularly sensitive to functional impairment of the respiratory motor control system by insults such as spinal cord injury.
Progress in our understanding of the mechanisms underlying functional recovery of respiratory motor function after injury has been slow due to a lack of understanding of the plasticity of respiratory motor control in models of spinal cord injury. The scope of this review will be limited to information on augmented breaths and cough. To the best of our knowledge, no information exists on the effects of spinal cord injury on the other airway protective behaviors.
2. Augmented breath
Augmented breaths (also known as sighs) are inspiratory defensive behaviors which function to prevent atelectasis in the lung and can be several-fold greater in amplitude than eupneic breaths (Glogowska et al., 1972; Cherniack et al., 1981). Augmented breaths can occur spontaneously or with a higher frequency after stimulation of vagal airway receptors (Glogowska et al., 1972; Cherniack et al., 1981). Blood gases influence the frequency of occurrence of augmented breaths, but the exact effect may be species and/or model dependent (Bell et al., 2009).
In the human, sigh frequency was not changed relative to controls in tetraplegics with injuries that were at least 2 years out from injury, but breathing frequency was elevated in these patients (Loveridge and Dubo, 1990). Sighs were defined as breaths that were greater than 2× mean tidal volume. The results of Loveridge and coworkers are also supported by data appearing in a recent review (Schilero et al., 2009). A more detailed study that included patients with shorter time intervals (3, 6 and >12 months post-injury) following injury revealed a postural effect on sigh frequency (Loveridge et al., 1992). There was no difference in sigh frequency between controls and tetraplegics in the supine position. However, sigh frequency decreased in the seated position in tetraplegics at 3 and 6 months post-injury (Loveridge et al., 1992). These investigators suggested that the increased work of breathing associated with the seated posture was responsible for the differences in sigh frequency that they observed in the injured group. While this may well be the case, their results also suggest that a sensory mechanism exists in tetraplegics that regulates sigh frequency with posture that is not manifest in healthy subjects. The identity of this proposed mechanism is unknown, but its potential importance is high.
Sighs are well known to increase pulmonary compliance in humans (Moore et al., 1997). Bronchospasm in response to inhalation of methacholine aerosol is enhanced in normal humans when deep inspirations are withheld (Skloot et al., 1995). Airway hyper-responsiveness can occur in tetraplegics (Dicpinigaitis et al., 1994; Schilero et al., 2009), as well as a reduced baseline airway caliber that is relieved after administration of anticholinergic agents (Schilero et al., 2009). The extent to which reductions in sigh frequency and/or amplitude may affect pulmonary compliance in tetraplegic patients is unknown.
All of the information available on the influence of spinal cord injury on augmented breaths in animal models was generated in the intact rat. The motor pattern of augmented breaths in the rat (Golder et al., 2005) is very similar to that reported in other species, such as the cat (Glogowska et al., 1972; Cherniack et al., 1981; van Lunteren et al., 1988b). This motor pattern consists of an increased diaphragm electromyogram (EMG) with little or no abdominal muscle activation (van Lunteren et al., 1988b). The diaphragm usually exhibits a biphasic pattern during augmented breaths. The first segment of the augmented breath, known as phase I, appears much like eupneic breaths. The second segment of the augmented breath, known as phase II, consists of a rapid rise in activity.
In the rat, augmented breath frequency is not altered at 2 weeks post spinal cord hemisection at C2, but the volume of this behavior is reduced in awake animals (Fuller et al., 2008). At longer time intervals following C2 spinal cord hemisection, augmented breath frequency was elevated along with a reduced volume (Golder et al., 2005; Fuller et al., 2008). This change in augmented breath volume was due to a reduction in both phase I and phase II volumes (Golder et al., 2005). Phase I, but not phase II, durations were reduced after spinal cord injury, indicating that this temporal component of augmented breaths was not responsible for the reduction in amplitude. Evidently, the central motor program for controlling phase II duration during augmented breaths is not sensitive to the level or type of sensory feedback present several months post spinal cord injury.
The reasons why rats and humans have different augmented breath frequencies at more chronic time points after cervical spinal cord injury are unknown. Human injuries typically result from compression or contusion of the spinal cord, whereas these studies in rats were conducted in animals after lateral spinal hemisection. There are significant differences in these two injury types with regards to functional outcomes (Mills et al., 2001). However, this is not to indicate that the spinal cord hemisection is not a relevant experimental model as it represents stab and gunshot injuries. Additionally, the lateral spinal hemisection model is relevant to Brown Sequard’s Syndrome (McKinley et al., 2007). This syndrome, which is basically a lateralized spinal cord injury, occurs in 200–400 of the 11,000 new human spinal cord injuries per year in the United States. Thus, it is very feasible that this small population (~3% of new injuries) which shows significant motor recovery is not well represented in human studies on respiratory dysfunction.
Certainly, chest wall mechanics differ between rats and humans and the lower chest wall compliance of adult humans and resultant higher work of breathing may be a primary determinant of these differences seen between species. Cervical spinal cord injuries directly affect the phrenic motoneuron pool. The phrenic motoneuron pool extends rostrocaudally from C3 to C5 in the human (Routal and Pal, 1999) and C3–C6 in the rat (Goshgarian and Rafols, 1981; Lane et al., 2008), with the maximum motoneuron density in the C4 spinal cord segment and would be most affected by an injury to the C4–C5 spinal cord segments. Even mild cervical contusion injuries can result in loss of 50% or more of cervical motoneuron populations and can result in significant loss of non-respiratory related motor functions such as extremity movements (Schrimsher and Reier, 1992; Collazos-Castro et al., 2005). The extent to which surviving phrenic motoneurons near an injury site would be hypo-functional is unknown. Indeed, given the extensive rostrocaudal dendritic fields of phrenic motoneurons (Cameron et al., 1983), it is likely that even spared motoneurons relatively distant from the areas of cavitation experience significant dendritic damage. The significant current differences between the results from current animal studies and findings in human may be resolved with further investigation of augmented breath function in animals following cervical spinal cord contusions.
3. Cough
Cough is the primary mechanism for the clearance of mucus and foreign matter from the upper respiratory tract (Basser et al., 1989). The mechanism by which cough clears the upper airway is the generation of large expiratory airflows (Korpas and Tomori, 1979). Several methods have been developed to enhance expiratory airflow during cough in patients with spinal cord injuries (Jaeger et al., 1993). However, these methods only increase expiratory airflow during cough a small amount (Jaeger et al., 1993). There are other promising approaches to enhancing the effectiveness of pulmonary defensive reflexes, such as intraspinal electrical stimulation of abdominal motoneurons (DiMarco et al., 1995). Overall advancement in this area is slow due to a lack of understanding of the physiology and plasticity of cough.
There are three primary phases to cough: inspiration, compression, and expulsion (Korpas and Tomori, 1979). The inspiratory phase of cough is generated by a large burst of activity in inspiratory muscles, such as the diaphragm, inspiratory laryngeals, and inspiratory intercostals (Korpas and Tomori, 1979; van Lunteren et al., 1989). The compressive phase of cough is produced by laryngeal closure caused by a burst in expiratory laryngeal muscles during rapidly increasing expiratory thoracic (intercostal and triangularis sterni) and abdominal muscle activity (Korpas and Tomori, 1979; van Lunteren et al., 1988a, 1989). The resulting large increase in intrapleural pressure during the compression phase produces very high airflows (up to 12 L/s in humans) (Leith et al., 1986) when the larynx opens and the expulsive phase begins. The expulsive phase is characterized by extremely large bursts of activity in expiratory thoracic and abdominal muscles (Korpas and Tomori, 1979).
Neurons that participate in the regulation of motor drive to chest wall, abdominal, and upper airway muscles during cough are located in two separate medullary regions (Shannon et al., 1998, 2004), the dorsal respiratory column (DRC) and ventral respiratory column/Bötzinger complex (VRC/Bot). The model of Shannon et al. (1998, 2004) of the cough pattern generator incorporates most of the different groups of DRC and VRC/Bot neurons that also participate in breathing. Sensory input to this cough pattern generator is mediated by relay neurons from mechano- and chemoreceptive afferents in the lower airways and larynx (Shannon et al., 1996, 1998; Sekizawa et al., 2008). Different classes of these neurons interact with one another to control inspiratory and expiratory phase durations during cough, the activation of laryngeal muscle motoneurons that determine caliber of the larynx, and the magnitude of motor drive to spinal motoneurons (Shannon et al., 1998, 2004; Baekey et al., 2001). Motor drive from the cough pattern generator to inspiratory and expiratory spinal motoneuron pools is mediated by premotor neurons in the rostral and caudal VRC that are located in and around the nucleus ambiguous and caudal nucleus retroambigualis (Feldman, 1986; Shannon et al., 1998, 2004). However, the role of a large, recently discovered population of neurons in the medial reticular formation that are premotor to inspiratory and expiratory spinal motoneuron pools in transmitting motor drive during cough is not understood at this time (Billig et al., 2003).
In the cat, the axons of expiratory premotor expiratory neurons in the caudal VRC cross the midline in the brainstem before descending the spinal cord (Feldman, 1986). The axons of most inspiratory premotor neurons do not cross in the brainstem before descending the spinal cord in the cat (Feldman, 1986). However in the rat, the axons of single inspiratory premotor neurons can decussate and descend the spinal cord bilaterally (Lipski et al., 1994). In the cervical spinal cord of the cat, descending excitatory pathways responsible for motor drive during cough are localized to the ventromedial portion of the cervical spinal cord (Davis and Plum, 1972).
Spinal cord injuries, depending on their segmental level, are likely to have complex effects on the role of the abdomen in the production of cough. The spinal locations of motoneuron pools innervating different abdominal muscles have different rostrocaudal extents (Holstege et al., 1987; Miller, 1987). The segmental extent of the external oblique and rectus abdominis motoneuron pools ranges in the cat from T4 or T6, respectively, to L3 (Holstege et al., 1987; Miller, 1987). The transversus abdominis and internal oblique motoneuron pools begin at T9 and T13, respectively, and extend also to L3 (Holstege et al., 1987; Miller, 1987). This distribution is generally consistent with the insertions and rostrocaudal extent of these muscles in the thorax and abdomen (Evans and Christensen, 1979). Therefore, low thoracic spinal cord injuries would be expected to alter regional descending motor drive during cough to the rectus abdominis and external oblique muscles, but markedly affect motor drive to almost the entire motoneuron pools innervating the transversus abdominis and internal oblique muscles. Injuries to the upper thoracic and/or cervical spinal cord would be expected to impair descending motor drive to all abdominal muscle motoneuron pools while injuries in the mid-thoracic to upper lumbar spinal cord would cause varying degrees of partial disruption.
3.1. Cough in humans with spinal cord injuries
Patients with lower cervical spinal cord injuries (C5–C8) can inspire via active control of the diaphragm and can produce weak and relatively ineffective expiratory efforts during cough (Siebens et al., 1964; De Troyer et al., 1986; Estenne and De Troyer, 1986, 1990; Estenne and Gorini, 1992). These patients have little or no contractile activity of their abdominal muscles during cough, rather, active expiration is accomplished through an active process involving contraction of upper chest muscles, such as the pectoralis, during the compressive and early expulsive phases of cough (Siebens et al., 1964; De Troyer et al., 1986; Estenne and De Troyer, 1990). The role of muscles other than pectoralis major in the production of expiratory airflow during cough in tetraplegic patients is virtually unknown, although it has been noted that the latissimus dorsi and teres major muscles can be active during cough in patients with C5–C7 spinal cord lesions (De Troyer et al., 1986). It also has been suggested, that the serratus anterior muscle may contribute to expiratory airflow during cough in tetraplegics (De Troyer et al., 1986). These studies represent the effects of complete loss of expiratory motor drive following spinal cord injury on cough. There are no studies of cough in humans with mid to low thoracic or upper lumbar spinal cord injuries in which partial control of expiratory abdominal muscles has been documented. Therefore, we have no specific information on the extent to which functional recovery of cough-related motor drive to these muscles may occur after spinal cord injury. The extent to which the cough response of these accessory muscle groups in tetraplegics represents a compensatory mechanism rather than a manifestation of motor drive to these muscles that would occur normally is unknown. Furthermore, the extent to which interruption of motor drive to abdominal muscles innervated from more caudal spinal cord segments (transversus abdominis) impairs the overall function of the abdomen in cough is unresolved.
3.2. Control of expiratory muscles during cough
The mechanisms responsible for abdominal muscle activity during cough are poorly understood. In spinal cord intact humans, only three studies have investigated the responses of abdominal muscles during normal cough (Floyd and Silver, 1950; Strohl et al., 1981; De Troyer et al., 1990). For example, Floyd and Silver (1950) concluded that the rectus abdominis was much less active during cough than other abdominal muscles, such as the external obliques but did not quantify their observations. Strohl et al. (1981) arrived at the same conclusion, but did not report quantitative data on this portion of their experiments. De Troyer et al. (1990) only concluded that the four abdominal muscles (rectus abdominis, external obliques, internal obliques, and transversus abdominis) acted as a unit during cough. In animal models, several investigators have reported vigorous increases in EMG activity of the transversus abdominis and rectus abdominis muscles and neurograms of the cranial iliohypogastric nerve (which innervates several different abdominal muscles) during cough (van Lunteren et al., 1988a; Bolser and DeGennaro, 1994; Bolser et al., 2000). However, the specific responses of the internal oblique muscles during cough has not been evaluated. Only two studies (van Lunteren et al., 1988a; Farkas et al., 1989) have correlated the EMG pattern of an abdominal muscle with cough mechanics. van Lunteren et al. (1988a) showed that transversus abdominis peak EMG activity was correlated with peak expiratory airflow during cough in 5/7 dogs. However, it was notable that transversus abdominis peak EMG activity was not correlated with peak expiratory airflowin 2 dogs, suggesting that other abdominal muscles had a greater role in the production of abdominal pressure during cough in these animals. Farkas et al. (1989) found that in the anesthetized dog, midline laparatomy had no effect on expiratory airflow or the external oblique EMG during cough but decreased transversus abdominis EMG activity by 35%. The results of these two studies are suggestive that under some conditions, transversus abdominis EMG activity is not directly related to expiratory airflow during cough.
De Troyer and coworkers (De Troyer et al., 1983; Mier et al., 1985) have investigated the influence of electrical stimulation of the abdominal muscles on chest wall mechanics and abdominal pressure in dogs and humans. According to these investigators, all four abdominal muscles increase gastric pressure when stimulated. However, unlike the internal obliques and transversus abdominis muscles, the rectus abdominis and external oblique muscles distort the lower chest wall. When individually stimulated, these muscles expand the lower chest wall in either the anteroposterior diameter or the transverse diameter (De Troyer et al., 1983; Mier et al., 1985). When simultaneously stimulated, these muscles have antagonistic effects on expansion of the lower chest wall, the net result is stiffening of this portion of the thorax (De Troyer et al., 1983; Mier et al., 1985). Theoretically, this antagonistic effect of these two muscles is appropriate for a role not only in generating increased abdominal pressures during cough, but for stiffening the lower chest wall to allow effective transmission of abdominal pressure to the thorax. This concept is supported by observations in the cat. All four anterolateral abdominal muscles are co-activated during tracheobronchial cough, consistent with their activation as a unit (Bolser et al., 2000). The extent to which the effectiveness of cough would be impaired by disruption of the relative magnitude and pattern of motor activation of these muscles following spinal cord injury is unknown.
A detailed analysis of the rectus abdominis burst pattern during cough was conducted by Tomori and Widdicombe (1969). These investigators reported that the rectus abdominis EMG had an average impulse frequency of approximately 300 Hz during the expiratory phase of tracheobronchial cough and EMG discharge of this muscle began approximately halfway into the preceding inspiratory phase. Bolser et al. (2000) observed EMG activity of the rectus abdominis in the preceding inspiratory phase. This activity occurred primarily in the last 25% of the inspiratory phase. This EMG discharge was termed pre-expiratory activity of the rectus abdominis muscle. However, other EMG studies describing the cough responses of the triangularis sterni (a chest wall expiratory muscle), transversus abdominis, and external oblique have not reported pre-expiratory activity in these muscles (van Lunteren et al., 1988a; Farkas et al., 1989). Indeed, the rise-time of the rectus abdominis EMG appears to be approximately 200 ms longer than the transversus abdominis rise-time (van Lunteren et al., 1988a), presumably because of the earlier onset of activity in this muscle. The functional importance of this pre-expiratory discharge is not well understood. One possible explanation for this discharge pattern is that pre-expiratory discharge in the rectus abdominis is necessary to stiffen the linea alba. The other abdominal muscles insert on this tendon, so that the mechanical advantage of these muscles would be maximized during cough in the presence of a stiffer insertion point. Whether this activity is due to central motor drive and/or the activation of stretch reflexes (Kondo et al., 1986) by stretch of the rectus abdominis during the inspiratory phase of cough is unknown. However, the available evidence suggests that pre-expiratory activity of abdominal muscles is unlikely to be due to stretch reflexes because these phenomena are subject to phase-dependent inhibition during inspiration (Kondo et al., 1986). Based on this argument, pre-expiratory activity of the rectus abdominis muscle during cough is likely due to a central motor program. Again, the influence of spinal cord injury on this pre-expulsive discharge is unknown as is its functional importance in optimizing the mechanical effectiveness of the behavior.
Little is known about the regulation of individual abdominal muscle motoneuron discharge. Graded increases in central motor drive to abdominal motor nuclei by increasing end-expiratory lung volume causes orderly recruitment of abdominal motoneurons consistent with Henneman’s size principle (Russell et al., 1987). However, nothing is known about the behavior of abdominal motoneurons during cough, a ballistic movement in which abdominal neurogram discharge peaks much more rapidly (150–200 ms) than during eupnea (1000–2000 ms) (Bolser, 1991). Furthermore, the EMG burst amplitude of abdominal muscles during cough is much greater than that produced during hypercapnia (van Lunteren et al., 1988a). These observations suggest greater contractile activity of abdominal muscles during cough than is produced during maximal chemical stimulation of breathing. Indeed, in humans airway pressures during cough can exceed those produced during forced expiratory efforts by 50–100% (Langlands, 1967). The extent to which this increase in contractile action of the abdominal muscles is due to rate coding and/or recruitment of motoneurons is unknown. There is precedent for the existence of a normally silent “reflex” pool of motoneurons innervating respiratory muscles. In the phrenic motor nucleus in the cat, Sieck and Fournier (1989) showed that as much as half of the phrenic motoneuron pool consisted of reflex motoneurons that could not be activated by hypercapnia or tracheal occlusion, but were activated by gag or sneeze. Sieck and Fournier (1989) speculated that this reflex pool consisted of fast twitch fatigable motor units. The presence of such a pool of motor units in abdominal muscles is not known, but these muscles consist of a high percentage of fast twitch muscle fibers (Johnson et al., 1973). Furthermore, abdominal muscle motoneurons receive much of their breathing-related motor drive from the caudal nucleus retroambigualis in the medulla (Feldman, 1986). Recordings from this area during cough in the cat indicate the presence of a large population of normally silent expiratory premotor neurons that are recruited during cough (Engelhorn and Weller, 1965; Jakus et al., 1985). Excitatory input from this recruited population of premotor neurons may be necessary to bring an abdominal reflex pool of motoneurons to firing threshold. The presence of this additional excitatory motor drive to abdominal muscles also is consistent with the fact that these muscles appear to be activated to a greater extent by cough than by voluntary expiratory efforts (Langlands, 1967).
4. Evidence for endogenous substrates for recovery
Premotor neurons in the caudal VRC play an important role in breathing-related excitation of expiratory spinal motoneurons (Cohen et al., 1985; Saywell et al., 2007). These bulbospinal expiratory premotor axons provide excitation to thoracic motoneurons via monosynaptic connections on the contralateral and ipsilateral sides via axon collaterals that can cross the spinal cord midline in thoracic segments (Kirkwood, 1995). There also are large populations of spinal interneurons that may play a role in controlling the activity of spinal expiratory motoneurons as well (Merrill and Lipski, 1987; Kirkwood et al., 1988, 1993; Schmid et al., 1993; Kirkwood, 1995). Some of these neurons can have axons confined to the segment in which they are located, whereas others can have axons that cross the spinal cord midline and extend caudally for up to five segments (Kirkwood et al., 1988). This latter population of propriospinal neurons, in addition to crossed bulbospinal premotor axons, could provide a bilateral representation of motor drive from a unilateral premotor source, allowing preservation of supraspinal synaptic input to expiratory spinal motoneurons through spared tissue after an asymmetric injury.
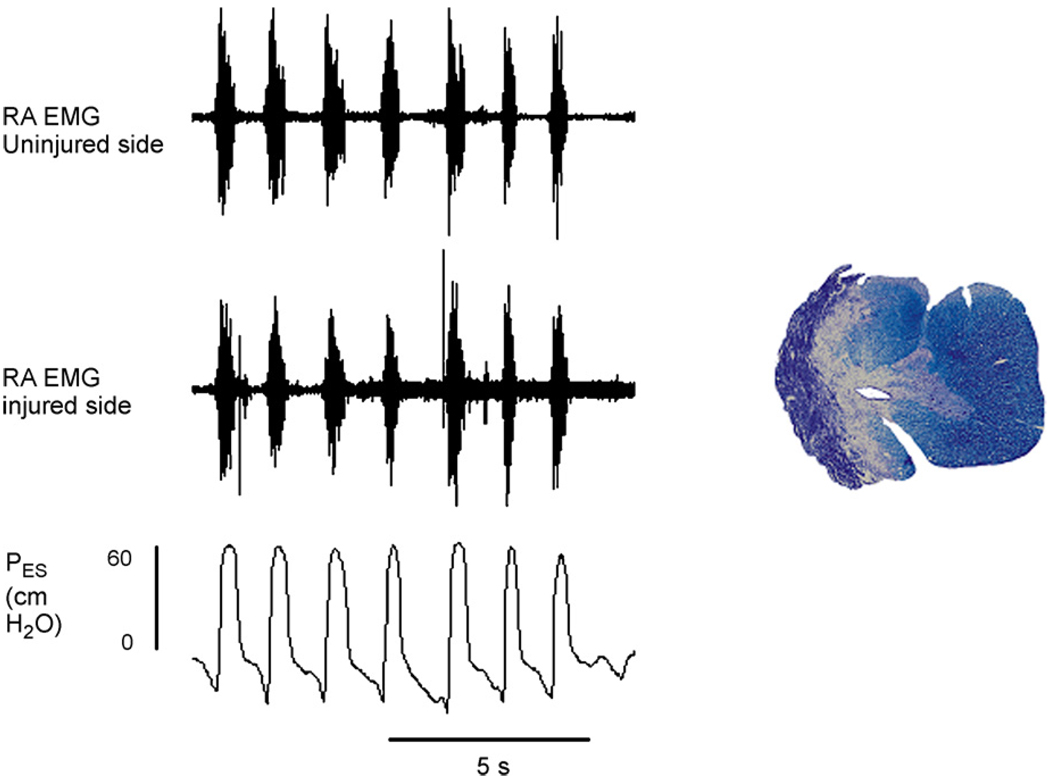
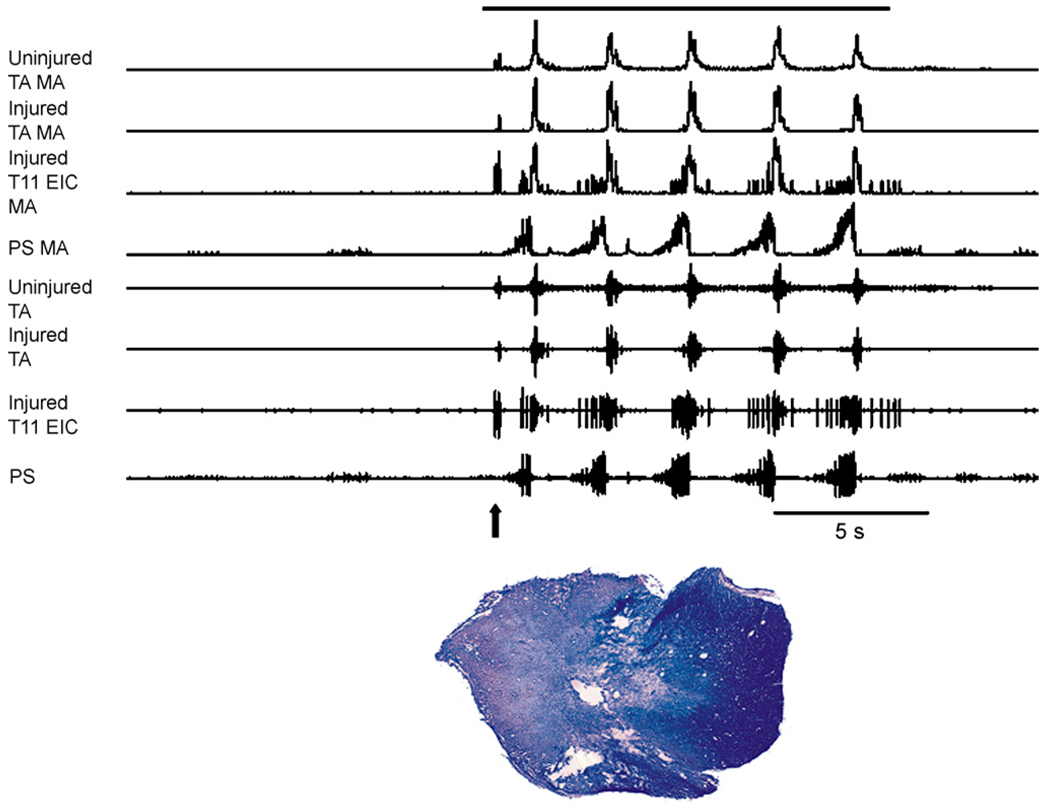
Recent preliminary results from our group support the hypothesis that cough-related motor drive to abdominal expiratory motoneurons can occur via a propriospinal relay in animals with lateral spinal cord hemisections. Cough-related electromyographic (EMG) activity from the rectus abdominis muscles at the pelvic level and esophageal pressures were recorded before and up to 6 months after spinal hemisection at T10 in survival experiments in eight cats (Jefferson et al., 2006). Cough-related rectus abdominis EMG activity was still present bilaterally after chronic spinal cord hemisection and frequently esophageal pressures during cough were 50 cm H2O or higher (Fig. 1). In the cat shown in Fig. 1, there was some sparing of ipsilateral ventromedial white matter. In other animals, the spinal cord hemisection was complete or extended into the contralateral side (Fig. 2). In the example in Fig. 2, robust EMG activity was still observed bilaterally in an acute experiment 5 months post-injury in the transversus abdominis and in the ipsilateral external intercostal muscle at T11 during cough. The results of these experiments are consistent with crossed pathways that could originate from the propriospinal pool and/or premotor axons from the contralateral side below the injury site. The functional resiliency of cough-related motor drive to abdominal motoneuron pools in the cat contrasts with the low efficacy of the crossed phrenic phenomenon in the rat (Fuller et al., 2008). The extent to which rats can express functional resiliency in thoracic spinal pathways remains to be determined. These findings also support the concept that existing spinal substrates might contribute to significant functional resiliency, especially after asymmetric injury to thoracic expiratory motor pathways.
Fig. 1.
Rectus abdominis (RA) EMGs recorded on the injured and uninjured sides and esophageal pressure (PES) during repetitive cough from an anesthetized cat 6 months following spinal cord hemisection at T10. Robust activity is present in the rectus abdominis muscles bilaterally and esophageal pressures indicate strong cough efforts. Preliminary observations indicate that vigorous cough efforts with bilateral EMGs in the rectus abdominis muscles that are similar to those recorded before injury can be observed up to many months post-injury. Rectus abdominis EMG electrode were placed percutaneously caudal to the pelvic crest approximately 1 cm lateral to the midline. Cough was elicited by mechanical stimulation of the vocal folds and epiglottis with a rigid plastic coated rod inserted orally. Some spared tissue at the lesion epicenter is present in the medial aspect of the ventral funiculus can be observed ventromedially on the injured side of this animals spinal cord.
Fig. 2.
Intercostal and abdominal EMGs recorded on the injured and uninjured sides in an anesthetized cat during repetitive cough 5 months following spinal cord hemisection at T9/T10. Arrow indicates the occurrence of an expiration reflex near the onset of stimulation. Following the expiration reflex, five coughs were produced. Robust activity was present bilaterally in the transversus abdominis muscles. The external intercostal EMG on the injured side at T11 was robust. Cough was elicited by mechanical stimulation of the intrathoracic airway with a flexible polyethylene catheter. The solid bar above the EMG records indicates the duration of mechanical stimulation. The hemisection extended across the midline both dorsally and ventrally. TA: transversus abdominis, PS: parasternal muscle, EIC: external intercostal muscle, MA: moving average 50 ms time constant.
Most human spinal cord injuries are contusions, which result in significant loss of gray matter and cavitation (Reier et al., 2002). Contusion injuries at the thoracic level would damage or eliminate interneuron pools and crossed premotor axons that contribute to functional resiliency of expiratory drive to thoracic spinal motoneuron pools. In the future, studies involving animal models of thoracic spinal contusions should clarify the importance of existing substrates in recovery and maintenance of cough function.
5. Evidence for plasticity
Plasticity can occur in spinal expiratory motor pathways after chronic spinal cord injury. Ford et al. (2000) provided evidence for sprouting of premotor expiratory axons at 4 months but not at 2 weeks post-incomplete thoracic spinal cord hemisection in the cat. These investigators used spike triggered averaging methods to evaluate focal synaptic potentials (FSPs) produced by bulbospinal caudal medullary expiratory neurons in thoracic segments just rostral to the injury site. The focal synaptic potential is a postsynaptic inward current associated with synaptic transmission (Kirkwood et al., 1991). In their study, Ford et al. (2000) showed that FSPs were selectively enhanced in frequency and magnitude in more caudal and lateral regions of the spinal cord segment in animals 4 months post-injury. This selective enhancement is consistent with a specific effect of injury on axonal sprouting (Ford et al., 2000). The temporal features of FSPs and their associated terminal potentials, conduction velocity of bulbospinal axons, and discharge frequencies of medullary premotor neurons that were recorded from injured animals were similar to those from uninjured cats. These findings suggest that bulbospinal medullary premotor expiratory neurons can survive axotomy and retain normal functioning.
6. Conclusions
There is evidence for several possible mechanisms for recovery of airway protective behaviors after spinal cord injury. In humans, spontaneous recovery of these behaviors occurs in the first year after injury. The mechanisms responsible for the recovery are not well understood, but include alterations in motor strategy such that muscles that have an accessory function under normal conditions can assume a larger role in supporting expulsive airflow. In animal models, there is evidence for significant resilience of motor drive for airway behaviors after spinal cord injury. Mechanisms for this resilience can include utilization of existing extensive crossed pathways, sprouting of premotor axons at the injury site, and presumably alterations in the balance of motor drive to chest wall muscles in which supraspinal control is relatively preserved. Most of these mechanisms have been documented to occur in the thoracic spinal cord, and future work in this area should focus on this region of the spinal cord as a model for understanding endogenous plasticity of airway protective behaviors.
Acknowledgements
This study was supported by NIH R33 HL89104 (DCB), NIH R01 HD52682 (DDF), NIH RO1 NS50699 and the Department of Veterans Affairs (DRH), and NIH NS54025 and the Anne and Oscar Lackner Chair in Medicine (PJR).
References
- Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J. Physiol. 2001;534:565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, McMahon TA, Griffith P. The mechanism of mucus clearance in cough. J. Biomech. Eng. 1989;111:288–297. doi: 10.1115/1.3168381. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Ferguson C, Kehoe V, Haouzi P. Hypocapnia increases the prevalence of hypoxia-induced augmented breaths. Am. J. Physiol. Regul. Integr.Comp. Physiol. 2009;296:R334–R344. doi: 10.1152/ajpregu.90680.2008. [DOI] [PubMed] [Google Scholar]
- Billig I, Card JP, Yates BJ. Neurochemical phenotypes of MRF neurons influencing diaphragm and rectus abdominis activity. J. Appl. Physiol. 2003;94:391–398. doi: 10.1152/japplphysiol.00282.2002. [DOI] [PubMed] [Google Scholar]
- Bolser DC. Fictive cough in the cat. J. Appl. Physiol. 1991;71:2325–2331. doi: 10.1152/jappl.1991.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC. Effect of codeine on the inspiratory and expiratory burst pattern during fictive cough in cats. Brain Res. 1994;662:25–30. doi: 10.1016/0006-8993(94)90792-7. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J. Appl. Physiol. 2000;88:1207–1214. doi: 10.1152/jappl.2000.88.4.1207. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir. Care. 2006;51:853–868. (discussion 869–870) [PMC free article] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ. Morphology of cat phrenic motoneurons as revealed by intracellular injection of horseradish peroxidase. J. Comp. Neurol. 1983;219:70–80. doi: 10.1002/cne.902190107. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, vonEuler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol. Scand. 1981;111:349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL, Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J. Physiol. 1985;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazos-Castro JE, Soto VM, Gutierrez-Davila M, Nieto-Sampedro M. Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J. Neurotrauma. 2005;22:544–558. doi: 10.1089/neu.2005.22.544. [DOI] [PubMed] [Google Scholar]
- Davis JN, Plum F. Separation of descending spinal pathways to respiratory motoneurons. Exp. Neurol. 1972;34:78–94. doi: 10.1016/0014-4886(72)90189-6. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M, Ninane V, Van Gansbeke D, Gorini M. Transversus abdominis muscle function in humans. J. Appl. Physiol. 1990;68:1010–1016. doi: 10.1152/jappl.1990.68.3.1010. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M, Vincken W. Rib cagemotion and muscle use in high tetraplegics. Am. Rev. Respir. Dis. 1986;133:1115–1119. doi: 10.1164/arrd.1986.133.6.1115. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, Kelly S. How the abdominal muscles act on the rib cage. J. Appl. Physiol. 1983;54:465–469. doi: 10.1152/jappl.1983.54.2.465. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Spungen AM, Bauman WA, Absgarten A, Almenoff PL. Bronchial hyperresponsiveness after cervical spinal cord injury. Chest. 1994;105:1073–1076. doi: 10.1378/chest.105.4.1073. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk JR, Supinski GS. Electrical activation of the expiratory muscles to restore cough. Am. J. Respir. Crit. Care Med. 1995;151:1466–1471. doi: 10.1164/ajrccm.151.5.7735601. [DOI] [PubMed] [Google Scholar]
- Engelhorn R, Weller E. Central representation of cough effecting afferent impulses in the medulla oblongata in the cat. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1965;284:224–239. [PubMed] [Google Scholar]
- Estenne M, De Troyer A. The effects of tetraplegia on chest wall statics. Am. Rev. Respir. Dis. 1986;134:121–124. doi: 10.1164/arrd.1986.134.1.121. [DOI] [PubMed] [Google Scholar]
- Estenne M, De Troyer A. Cough in tetraplegic subjects: an active process. Ann. Intern. Med. 1990;112:22–28. doi: 10.7326/0003-4819-112-1-22. [DOI] [PubMed] [Google Scholar]
- Estenne M, Gorini M. Action of the diaphragm during cough in tetraplegic subjects. J. Appl. Physiol. 1992;72:1074–1080. doi: 10.1152/jappl.1992.72.3.1074. [DOI] [PubMed] [Google Scholar]
- Evans H, Christensen GC. Miller’s Anatomy of the Dog. W.B Saunders: Philadelphia; 1979. [Google Scholar]
- Farkas GA, Schroeder MA, De Troyer A. Effect of midline laparotomy on the cough reflex in dogs. Neurosci. Lett. 1989;107:120–124. doi: 10.1016/0304-3940(89)90802-1. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Handbook of Physiology. The Nervous System. Intrinsic Regulatory Systems of the Brain. Bethesda: American Physiological Society; 1986. Neurophysiology of breathing in mammals; pp. 463–524. [Google Scholar]
- Floyd WF, Silver PH. Electromyographic study of patterns of activity of the anterior abdominal wall muscles in man. J. Anat. 1950;84:132–145. [PMC free article] [PubMed] [Google Scholar]
- Ford TW, Vaughan CW, Kirkwood PA. Changes in the distribution of synaptic potentials from bulbospinal neurones following axotomy in cat thoracic spinal cord. J. Physiol. 2000;524(Pt 1):163–178. doi: 10.1111/j.1469-7793.2000.t01-1-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp. Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir. Physiol. 1972;16:179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci. Lett. 2005;373:89–93. doi: 10.1016/j.neulet.2004.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J. Comp. Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Kakulas BA. Neuropathology of human spinal cord injury sustained in sports-related activities. J. Neurotrauma. 1997;14:235–248. doi: 10.1089/neu.1997.14.235. [DOI] [PubMed] [Google Scholar]
- Holstege G, vanNeerven J, Evertse F. Spinal cord location of the motoneurons innervating the abdominal, cutaneous maximus, latissimus dorsi and longissimus dorsi muscles in the cat. Exp. Brain Res. 1987;67:179–194. doi: 10.1007/BF00269465. [DOI] [PubMed] [Google Scholar]
- Jaeger RJ, Turba RM, Yarkony GM, Roth EJ. Cough in spinal cord injured patients: comparison of three methods to produce cough. Arch. Phys.Med. Rehabil. 1993;74:1358–1361. doi: 10.1016/0003-9993(93)90093-p. [DOI] [PubMed] [Google Scholar]
- Jakus J, Tomori Z, Stransky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol. Bohemoslov. 1985;34:127–136. [PubMed] [Google Scholar]
- Jefferson SC, Rose MJ, Tester NJ, Blum AE, Bolser DC, Howland DR. Influence of chronic T10 spinal hemisection on the cough reflex in cats: functional manifestations of crossed spinal pathways. FASEB J. 2006;20:A1213. [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurones in the cat. J. Physiol. 1995;484(Pt 1):201–225. doi: 10.1113/jphysiol.1995.sp020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J. Physiol. 1988;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Schmid K, Otto M, Sears TA. Focal blockade of single unit synaptic transmission by iontophoresis of antagonists. Neuroreport. 1991;2:185–188. doi: 10.1097/00001756-199104000-00006. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. J. Physiol. 1993;461:667–687. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Bishop B, Shaw CF. Phasic stretch reflex of the abdominal muscles. Exp. Neurol. 1986;94:120–140. doi: 10.1016/0014-4886(86)90276-1. [DOI] [PubMed] [Google Scholar]
- Korpas J, Tomori Z. Cough and Other Respiratory Reflexes. New York: S. Karger, Basel; 1979. [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlands J. The dynamics of cough in health and in chronic bronchitis. Thorax. 1967;22:88–96. doi: 10.1136/thx.22.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith DE, Butler JP, Sneddon SL, Brain JD. Handbook of Physiology. The Respiratory System, V. III. Mechanics of Breathing, Part I. Bethesda: American Physiological Society; 1986. Cough; pp. 315–336. [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia. 1992;30:479–488. doi: 10.1038/sc.1992.102. [DOI] [PubMed] [Google Scholar]
- Loveridge BM, Dubo HI. Breathing pattern in chronic quadriplegia. Arch. Phys. Med. Rehabil. 1990;71:495–499. [PubMed] [Google Scholar]
- McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord Med. 2007;30:215–224. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J. Neurophysiol. 1987;57:1837–1853. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Mier A, Brophy C, Estenne M, Moxham J, Green M, De Troyer A. Action of abdominal muscles on rib cage in humans. J. Appl. Physiol. 1985;58:1438–1443. doi: 10.1152/jappl.1985.58.5.1438. [DOI] [PubMed] [Google Scholar]
- Miller AD. Localization of motoneurons innervating individual abdominal muscles of the cat. J. Comp. Neurol. 1987;256:600–606. doi: 10.1002/cne.902560412. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J. Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Moore BJ, Verburgt LM, King GG, Pare PD. The effect of deep inspiration on methacholine dose–response curves in normal subjects. Am. J. Respir. Crit. Care Med. 1997;156:1278–1281. doi: 10.1164/ajrccm.156.4.96-11082. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Golder FJ, Bolser DC, Hubscher C, Johnson R, Schrimsher GW, Velardo MJ. Grey matter repair in the cervical spinal cord. In: Mckerracher L, Doucet G, Rossignol S, editors. Spinal Cord Trauma: Regeneration, Neural Repair and Functional Recovery. Elsevier. 2002. pp. 49–70. [DOI] [PubMed] [Google Scholar]
- Routal RV, Pal GP. Location of the phrenic nucleus in the human spinal cord. J. Anat. 1999;195(Pt 4):617–621. doi: 10.1046/j.1469-7580.1999.19540617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Bishop BP, Hyatt RE. Discharge of abdominal muscle alpha and gamma motoneurons during expiratory loading in cats. Exp.Neurol. 1987;97:179–192. doi: 10.1016/0014-4886(87)90292-5. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J. Physiol. 2007;579:765–782. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir. Physiol. Neurobiol. 2009;166:129–141. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J. Physiol. 1993;461:647–665. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Midha M, McKenzie N. Medical complications of spinal cord disease. Neurol. Clin. 1991;9:779–795. [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp. Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Chen CY, Bechtold AG, Tabor JM, Bric JM, Pinkerton KE, Joad JP, Bonham AC. Extended secondhand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs. Eur. J. Neurosci. 2008;28:771–781. doi: 10.1111/j.1460-9568.2008.06378.x. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm. Pharmacol. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J. Appl. Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm. Pharmacol. Ther. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Siebens AA, Kirby NA, Poulos DA. Cough following transection of spinal cord at C-6. Arch. Phys. Med. Rehabil. 1964;45:1–8. [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J. Appl. Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J. Clin. Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Mead J, Banzett RB, Loring SH, Kosch PC. Regional differences in abdominal muscle activity during various maneuvers in humans. J. Appl. Physiol. 1981;51:1471–1476. doi: 10.1152/jappl.1981.51.6.1471. [DOI] [PubMed] [Google Scholar]
- Tomori Z, Widdicombe JG. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J. Physiol. 1969;200:25–49. doi: 10.1113/jphysiol.1969.sp008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E, Daniels R, Deal EC, Jr, Haxhiu MA. Role of costal and crural diaphragm and parasternal intercostals during coughing in cats. J. Appl. Physiol. 1989;66:135–141. doi: 10.1152/jappl.1989.66.1.135. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Haxhiu MA, Cherniack NS, Arnold JS. Role of triangularis sterni during coughing and sneezing in dogs. J. Appl. Physiol. 1988a;65:2440–2445. doi: 10.1152/jappl.1988.65.6.2440. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Prabhakar NR, Cherniack NS, Haxhiu MA, Dick TE. Inhibition of expiratory muscle EMG and motor unit activity during augmented breaths in cats. Respir. Physiol. 1988b;72:303–314. doi: 10.1016/0034-5687(88)90089-8. [DOI] [PubMed] [Google Scholar]