Abstract
Background
We previously found that activation of the glial cell line-derived neurotrophic factor (GDNF) pathway in the ventral tegmental area (VTA) reduces ethanol-drinking behaviors. In this study, we set out to assess the contribution of endogenous GDNF or its receptor GFRα1 to the regulation of ethanol-related behaviors.
Methods
GDNF and GFRα1 heterozygote mice (HET) and their wild-type littermate controls (WT) were used for the studies. Ethanol-induced hyperlocomotion, sensitization, and conditioned place preference (CPP), as well as ethanol consumption before and after a period of abstinence were evaluated. Blood ethanol concentration (BEC) was also measured.
Results
We observed no differences between the GDNF HET and WT mice in the level of locomotor activity or in sensitization to ethanol-induced hyperlocomotion after systemic injection of a nonhypnotic dose of ethanol and in BEC. However, GDNF and GFRα1 mice exhibited increased place preference to ethanol as compared with their WT littermates. The levels of voluntary ethanol or quinine consumption were similar in the GDNF HET and WT mice, however, a small but significant increase in saccharin intake was observed in the GDNF HET mice. No changes were detected in voluntary ethanol, saccharin or quinine consumption of GFRα1 HET mice as compared with their WT littermates. Interestingly, however, both the GDNF and GFRα1 HET mice consumed much larger quantities of ethanol after a period of abstinence from ethanol as compared with their WT littermates. Furthermore, the increase in ethanol consumption after abstinence was found to be specific for ethanol as similar levels of saccharin intake were measured in the GDNF and GFRα1 HET and WT mice after abstinence.
Conclusions
Our results suggest that endogenous GDNF negatively regulates the rewarding effect of ethanol and ethanol-drinking behaviors after a period of abstinence.
Keywords: GDNF, Ethanol, Reward, Consumption, Abstinence
GDNF is a secreted protein that was initially identified in a glial-derived cell line (Lin et al., 1993) and has since been shown to be highly expressed in neurons (Barroso-Chinea et al., 2005; Pochon et al., 1997). GDNF is expressed throughout the central nervous system during development (Choi-Lundberg and Bohn, 1995; Nosrat et al., 1996; Schaar et al., 1993; Stromberg et al., 1993), and high levels of GDNF mRNA are still present in adult spinal cord and specific brain areas, including the striatum (dorsal striatum and nucleus accumbens), thalamus, cortex and hippocampus (Barroso-Chinea et al., 2005; Choi-Lundberg and Bohn, 1995; Golden et al., 1998, 1999; Nosrat et al., 1996; Pochon et al., 1997; Springer et al., 1994; Trupp et al., 1997). GDNF plays an important role in promoting the survival, regeneration and maintenance of the mature phenotype of distinct central and peripheral neuronal populations, such as midbrain dopaminergic neurons (Beck et al., 1995; Granholm et al., 2000; Kowsky et al., 2007; Lin et al., 1993; Pascual et al., 2008; Tomac et al., 1995a). GDNF also promotes neurite outgrowth (Kowsky et al., 2007; Toledo-Aral et al., 2003), and contributes to some cognitive functions (Gerlai et al., 2001). GDNF acts through a multicomponent receptor system, which includes the GDNF family receptor alpha-1 (GFRα1) and the tyrosine kinase receptor Ret. Formation of the GDNF-GFRα1 signaling complex leads to the autophosphorylation of Ret and the subsequent activation of downstream signaling cascades, such as the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase and phospholipase Cγ/protein kinase C pathway (Airaksinen and Saarma, 2002; Sariola and Saarma, 2003). GFRα1 and Ret are expressed in several brain regions in the developing and adult brain and are particularly abundant in the mesencephalic dopaminergic areas projecting to the striatum, namely the substantia nigra pars compacta and the ventral tegmental area (VTA) (Burazin and Gundlach, 1999; Glazner et al., 1998; Golden et al., 1998, 1999; Matsuo et al., 2000; Trupp et al., 1997). Interestingly, GDNF is retrogradely transported to dopamine neurons of the mesencephalic system in the adult brain (Ai et al., 2003; Barroso-Chinea et al., 2005; Kordower et al., 2000; Lapchak et al., 1997; Tomac et al., 1995b), supporting an important role of the endogenous growth factor in the maintenance and functionality of these neurons.
In recent years, a role for GDNF as a negative regulator of biochemical and behavioral adaptations to drugs of abuse has been reported (Carnicella and Ron, 2009c). Specifically, Messer et al. showed that infusion of GDNF into the VTA reversed biochemical adaptations to long-term exposure to cocaine and morphine, and blocked cocaine-induced CPP (Messer et al., 2000). Transplantation of cells engineered to over-express GDNF or delivery of nanoparticles conjugated to GDNF into the striatum was shown to reduce acquisition of rat cocaine self-administration (Green-Sadan et al., 2003, 2005), and increasing GDNF expression in the brain reduced the sensitivity of mice to methamphetamine (Niwa et al., 2007b).
We found that systemic administration of the naturally occurring alkaloid, Ibogaine, and the ergotamine derivative, FDA approved drug, cabergoline, to mice and rats increases the expression of GDNF in the VTA (Carnicella et al., 2009a; He et al., 2005). We further showed that treatment of dopaminergic-like SH-SY5Y cells with Ibogaine or cabergoline results in the activation of the Ret/MAPK cascade via newly synthesized GDNF (Carnicella et al., 2009a; He et al., 2005). Importantly, we found that Ibogaine and cabergoline, via up-regulation of the GDNF pathway in the VTA, reduces rat operant self-administration of ethanol (Carnicella et al., 2009a; He et al., 2005). We also presented data to suggest that the long-lasting reduction of ethanol self-administration by Ibogaine may be mediated by the activation of an autoregulatory feedback loop in which GDNF up-regulates its own expression (He and Ron, 2006). Furthermore, we reported that direct infusion of GDNF into the VTA of rats results in a specific, rapid and sustained reduction of ethanol self-administration (Carnicella and Ron, 2009c; Carnicella et al., 2008, 2009b). We further showed that the actions of GDNF to attenuate the motivation to consume ethanol are mediated via the activation of the MAPK pathway (Carnicella et al., 2008). Finally, we found that GDNF inhibits the chronic ethanol-mediated increase in tyrosine hydroxylase immunoreactivity in the SH-SY5Y cells (He and Ron, 2008), suggesting that GDNF may also act to reduce cellular biochemical adaptations upon prolonged exposure to ethanol.
In line with these data, various studies have suggested a role for endogenous GDNF in mechanisms that underlie sensitivity of rodents to drugs of abuse. This possibility stems from studies using GDNF heterozygote knockout mice (GDNF HET) (Granholm et al., 1997; Pichel et al., 1996), as homozygote knockout mice die at birth from renal abnormalities (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996). GDNF HET mice have lower (35 to 70%) GDNF protein levels in the striatum compared with wild-type (WT) mice (Airavaara et al., 2004; Griffin et al., 2006; Niwa et al., 2007b; Yan et al., 2007), and show greater sensitivity to some effects of drugs of abuse. For example, GDNF HET mice exhibit greater place preference to morphine (Niwa et al., 2007a; but see Airavaara et al., 2007), cocaine (Messer et al., 2000), and methamphetamine (Niwa et al., 2007b), and are more vulnerable to psychomotor sensitization induced by morphine (Airavaara et al., 2007) and cocaine (Messer et al., 2000; but see Airavaara et al., 2004). In addition, administration of morphine, but not cocaine, induces a greater increase in striatal dopamine levels in GDNF HET mice than in WT mice (Airavaara et al., 2004, 2006, 2007). Furthermore, motivation to self-administer and to seek methamphetamine is potentiated in GDNF HET mice (Yan et al., 2007). Together, these studies suggest an important role for the endogenous GDNF system in the regulation of behavioral phenotypes associated with exposure to drugs of abuse. In this study, we set out to test for the interaction between endogenous GDNF and ethanol’s actions by determining the sensitivity of GDNF and GFRα1 HET mice and their WT littermates to ethanol.
MATERIALS AND METHODS
Mice
Experimental protocols were approved by the Ernest Gallo Research Center Institutional Animal Care and Use Committee and met National Institutes of Health guidelines. GDNF HET and GFRα1 HET mice were generous gifts from Drs. Barry Hoffer and Andreas Tomac (NIDA). The generation of both transgenic mouse lines is described in Pichel and colleagues (1996) and Tomac and colleagues (2000), respectively. GDNF HET (DBH/CD1 background), GFRα1 HET (C57/CD1 background), and their respective WT littermates were bred in-house by mating pairs of heterozygous transgenic mice. The genotype of the mice were determined by reverse transcription-polymerase chain reaction (RT-PCR) analysis of products derived from tail DNA. Only male mice were used for the studies and the age of the subjects ranged from 8 to 10 weeks at the start of each experiment. The generations used for the studies were F9 to F12 and F6 to F10 for the GDNF and GFRα1 mice, respectively. After weaning, mice were randomly (with respect to the genotype) housed in groups of 5 in Plexiglas cages. Food and water were available ad libitum. Animals were kept in conditions of constant temperature (23°C), humidity (50%) and light–dark cycle (lights on from 7:00 am to 7:00 pm).
Basal Locomotor Activity
Mice were placed in 43 cm × 43 cm locomotor activity chambers (Med Associates) and the distance traveled by the animals was recorded for 1 hour.
Acute Hyperlocomotion Induced by Ethanol
Mice were first habituated to handling and injection procedures with intraperitoneal (i.p.) injections of saline once a day for 4 days. On the test day, mice were placed in locomotor chambers for a 30-minute period of habituation. Mice were then injected i.p. with ethanol (1.5 or 2 g/kg, 20% v/v in saline) or saline, and locomotor activity was measured for 20 minutes.
Sensitization
Mice were habituated to the handling and the injection procedure as described previously. Mice were injected i.p. every other day for 13 days with a saline injection the first day and 1.5 g/kg ethanol injection (20% v/v solution, i.p.) on days 3, 5, 7, 9, 11, and 13 (for a total of 6 ethanol injections). On days 1, 3, 7, and 13, locomotor activity was assessed as described above.
Ethanol-Induced Conditioned Place Preference
The place conditioning boxes (Columbus) consist of 2 distinct compartments that differ in color and floor texture. On day 1, mice were allowed to freely explore the apparatus for 15 minutes in order to habituate them and obtain baseline measurements (preconditioning session). Animals that spent more than 65% of the time in either one of the compartments during the pre-conditioning baseline session were excluded from the study. This allowed us to use an unbiased design in which the 2 compartments were equally preferred before conditioning as indicated by the group average (unbiased apparatus) and to pseudorandomly assigned the compartment paired with ethanol (unbiased assignment procedure) (Cunningham et al., 2003; Tzschentke, 2007). The next day, the conditioning training started with one conditioning trial per day during 6 days (days 2 to 7). Mice were injected i.p. with saline and confined immediately to one of the compartment during 5 minutes (unpaired compartment). The next day, mice were injected with saline (saline group) or ethanol (1.8 g/kg, with a 20% v/v solution; ethanol group) and were confined to the other compartment (drug-paired compartment). This schedule was repeated twice (i.e., 3 ethanol conditioning sessions). On day 8, animals were allowed to explore the entire apparatus during 15 minutes (postconditioning session); preference was scored by dividing the time spent in the drug-paired compartment to the time spent in the unpaired compartment during this session (preference ratio; Hernandez-Rabaza et al., 2008). The dose of ethanol and the number of conditioning sessions were chosen in order to induce a moderate CPP in WT mice in order to avoid a possible ceiling effect in the HET mice.
Blood Ethanol Concentration Measurements
Trunk blood was collected in heparinized capillary tubes from GDNF and GFRα1 HET and WT mice 5, 30, or 120 minutes after an i.p. injection of 1.8 g/kg of ethanol. Serum was extracted with 3.4% trichloroacetic acid followed by a 5-minute centrifugation at 420 g, and assayed for ethanol content using the NAD-NADH enzyme spectrophotometric method (Weiss et al., 1993; Zapata et al., 2006). BECs were determined by using a standard calibration curve.
Limited Access 2-Bottle Choice Paradigm
The limited access 2-bottle choice procedure was adapted from Rhodes and colleagues (2005). Mice were housed individually on a reverse dark cycle (lights off from 10:00 am to 10:00 pm) and were allowed continuous access to tap water. After 1 week of acclimatization, mice were given access to 2 bottles: one containing ethanol, saccharin, or quinine in tap water and the other containing tap water alone. Two-bottle drinking sessions were limited to 4 hours, 5 days a week, and began 1 hour after the start of the dark cycle (i.e., from 11:00 am to 3:00 pm). During the course of the exposure period, the concentrations of ethanol (2.5, 5, 10, and 20%, v/ v), saccharin (0.03 and 0.06%, w/ v), or quinine (0.015 and 0.03 mM) were increased gradually, with 5 days of access (Monday to Friday) at each concentration. Water and ethanol, saccharin or quinine intake levels were recorded after each session. The intake values recorded on Monday were highly variable as new concentrations of ethanol, saccharin, or quinine were introduced on this day. Therefore, only the values from Tuesday to Friday were averaged for each solution and concentration. A separate ANOVA found no genotype-specific effects on intake on Mondays. The placement (left or right) of each solution was alternated daily to control for side preference. A bottle containing water in a cage without mice was used to evaluate the spillage due to the experimental manipulations during the test sessions. The spillage was always ≤0.1 ml (<4% of total fluid intake during 4 hours).
Ethanol and Saccharin Consumption After a Period of Abstinence
The 2-bottle choice drinking procedures for ethanol and saccharin intake were conducted as described above and an average of the 4 sessions of 20% ethanol or 0.06% saccharin consumption was recorded (pre-abstinence value). Mice were then deprived of either substance for 1 week, and intake of 20% ethanol or 0.06% saccharin were measured after one 4-hour 2-bottle drinking session, and compared with the pre-abstinence values. The length of the abstinence period was determined in a pilot study in order to elicit only a weak deprivation effect in the WT mice and therefore ensure the detection of any specific increase of consumption in the HET mice.
Statistical Analysis
Experiments were conducted in either between-subjects or mixed within-subjects designs and analyzed by 2-way ANOVA. Significant main effects or interactions were further investigated using the Student–Newman–Keuls test or the method of contrasts (t-test was used taking into consideration orthogonality to avoid multiple comparisons; Keppel, 1991). The levels of intake were corrected for body weight (g / kg or ml / kg) and preference was calculated as the percentage of ethanol, saccharin, or quinine solution consumed relative to total fluid consumed.
RESULTS
Haplodeficiency of the GDNF Gene Does Not Affect Locomotor Activity in Response to Ethanol
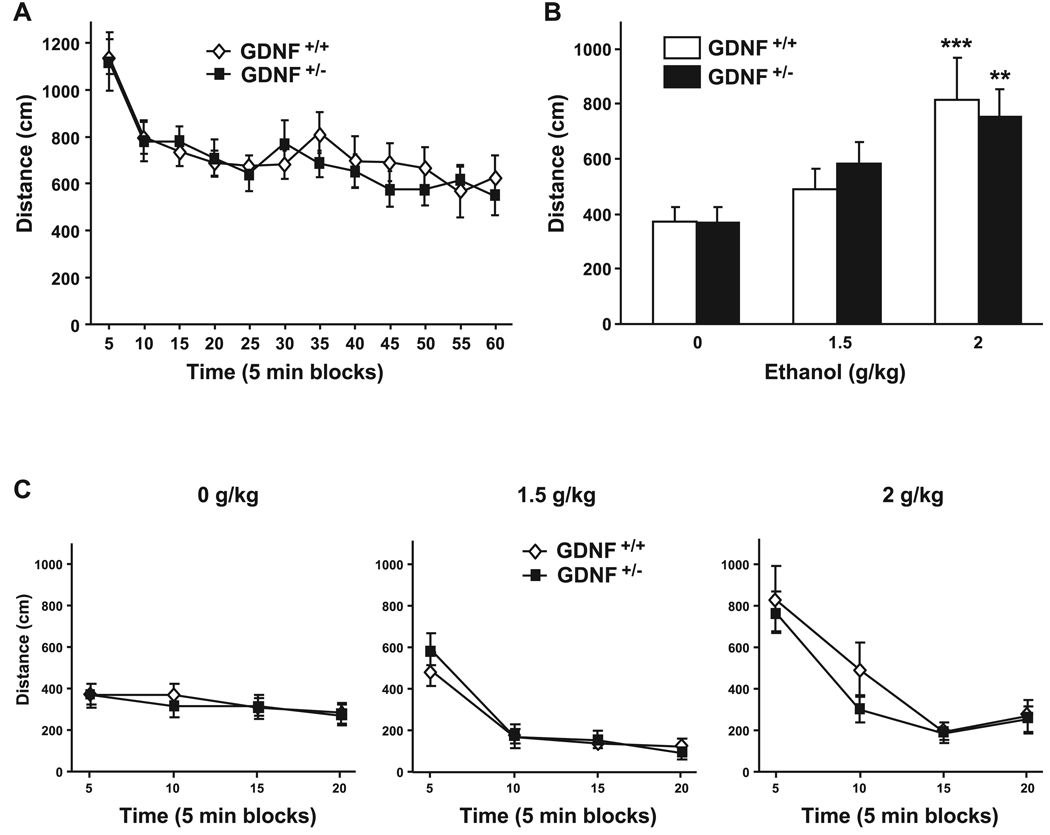
First, we monitored the basal locomotor activity in the GDNF HET and WT mice by measuring the distance traveled during a 1-hour session in an open field. As shown in Fig. 1A, we observed no differences between the genotypes [main effect of time: F(11, 197) = 28.31, p < 0.001; but no effect of genotype: F(1, 197) = 0.05, p = 0.83; and no time × genotype interaction: F(11, 193) = 1.022, p = 0.43], suggesting that the GDNF HET mice do not have any gross locomotor deficiencies. Next, we tested the sensitivity of the mice to the rapid increase in locomotion induced by systemic administration of nonintoxicating doses of ethanol (1.5 and 2 g/ kg). We observed no difference in acute ethanol-mediated hyperlocomotion between the GDNF HET and WT mice (Fig. 1B and 2C) [main effect of treatment: F(2, 49) = 13.36, p < 0.001; but no effect of genotype: F(1, 49) = 0.02, p = 0.90; and no interaction between both factors: F(2, 49) = 0.41, p = 0.67].
Fig. 1.
GDNF HET and WT mice exhibit similar locomotor responses to an acute injection of ethanol. (A) Basal locomotor activity of GDNF HET and WT mice. n = 10–11 per group. (B and C), Locomotor activity following i.p. injections of ethanol (1.5 or 2 g /kg) or saline. n = 8–14 per group. (B), Total distance traveled during the first 5 minutes following ethanol or saline injection. **p < 0.01, ***p < 0.001 compared with saline. (C) Locomotor activity in blocks of 5 minutes for ethanol and saline. Data are presented as mean ± SEM.
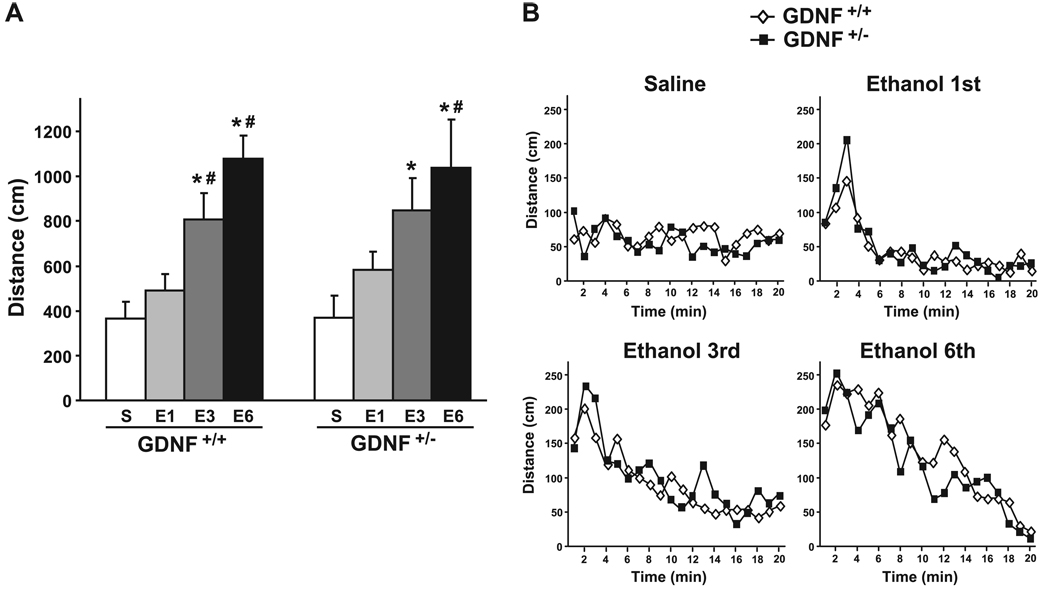
Fig. 2.
GDNF HET and WT mice exhibit similar locomotor sensitization to ethanol. (A) Locomotor activity measured as mean ± SEM of the total distance traveled during 5 minutes after a saline, a first (E1), a third (E3), or a sixth (E6) systemic injection of ethanol (1.5 g / kg). *p < 0.05 compared with saline, #p < 0.05 compared with the first injection of ethanol. (B) Distance traveled by blocks of 1 minute for each ethanol and saline injection. n = 7–8 per group.
We also determined whether sensitization to the increase in locomotion induced by ethanol is different in the 2 genotypes. The hyperlocomotion induced by the administration of a low dose of ethanol (1.5 g/ kg) was significantly increased in both GDNF HET and WT mice after multiple ethanol injections [Fig. 2A, main effect of treatment: F(3, 39) = 21.72, p < 0.001], with an increase both in amplitude and duration of the hyperlocomotion (Fig. 2B). However, repeated administration of ethanol resulted in a similar degree of ethanol sensitization in the GDNF HET and WT mice [Fig. 2A and 2B; no effect of genotype: F(1, 39) = 0.04, p = 0.85; and no interaction: F(3, 39) = 0.19, p = 0.90].
Haplodeficiency of the GDNF Gene or its Receptor (GFRα1) Results in Increased Place Preference to Ethanol
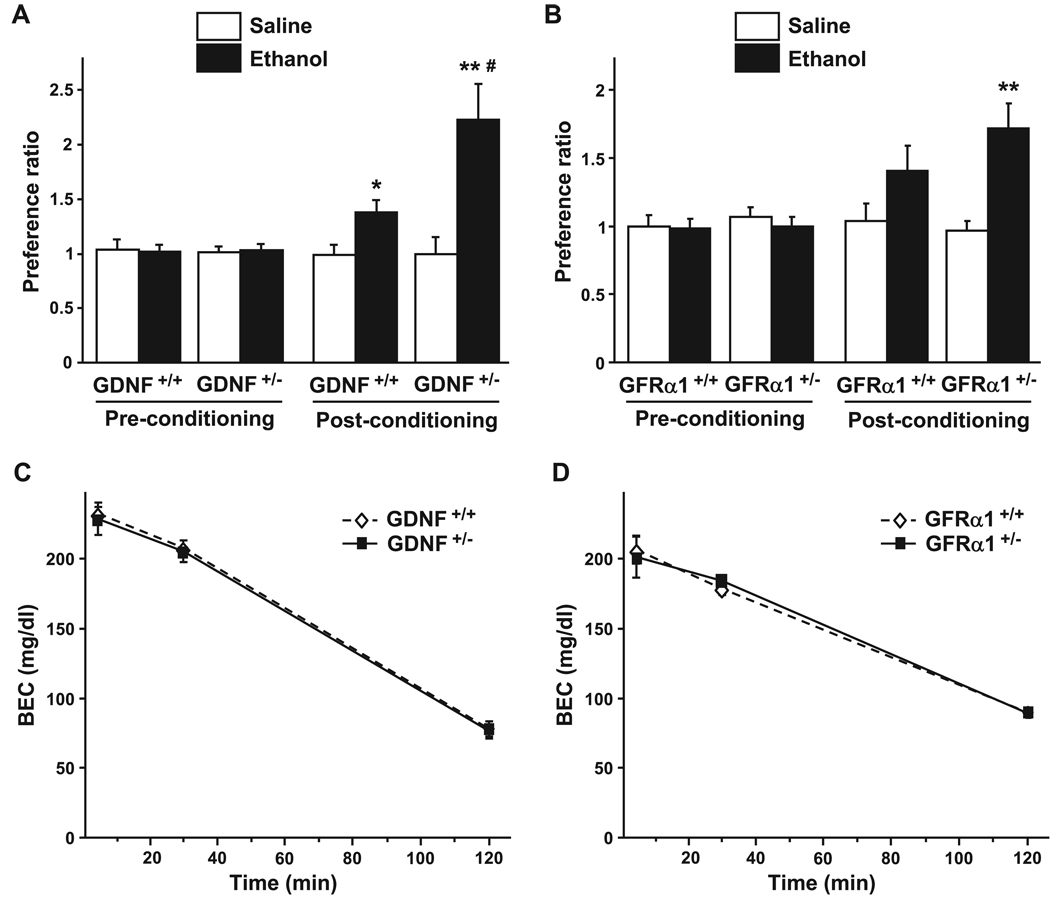
Next, we assessed the sensitivity of the GDNF HET and WT mice to the rewarding effect of a low dose of ethanol (1.8 g / kg) by using a CPP procedure. As shown in Fig. 3A, GDNF HET mice exhibited greater preference to the ethanol-paired compartment than their WT littermates [a 2-way ANOVA conducted on the postconditioning values found a main effect of conditioning: F(1, 48) = 15.63, p < 0.001; a main effect of genotype: F(1, 48) = 4.35, p < 0.05; and a significant interaction between these 2 factors: F(1, 48) = 4.23, p < 0.05].
Fig. 3.
GDNF and GFRα1 HET mice exhibit greater place preference to ethanol than their WT littermates, however BEC is unaltered. (A and B) Mice were injected i.p. with ethanol (1.8 g / kg) or saline during conditioning. Place preference to ethanol in the GDNF (A) and GFRα1 (B) mice, expressed as the ratio ± SEM of the time spent in the drug-paired and unpaired compartment. n = 12–14 per group.*p < 0.05, **p < 0.01 compared with saline and #p < 0.05 compared with WT. (C and D) Blood ethanol concentration 5, 30, and 120 minutes after an i.p. injection of 1.8 g / kg of ethanol in GDNF (C) and GFRα1 (D) mice, presented as mean ± SEM. n = 4–5 per group.
The GDNF-mediated signaling pathway is activated upon the binding of the growth factor to its receptor, GFRα1 (Airaksinen and Saarma, 2002). We were therefore interested in determining whether similar changes in ethanol-mediated reward are observed in mice in which the level of the GDNF receptor is reduced. To do so, we compared the level of ethanol place preference of the GFRα1 HET mice and their WT littermates, and observed higher place preference to ethanol in the HET mice as compared with WT mice (Fig. 3B). A 2-way ANOVA conducted on the postconditioning values found a main effect of conditioning [F(1, 52) = 14.48, p < 0.001], but no effect of genotype [F(1, 52) = 0.65, p = 0.42] and no interaction between both factors [F(1, 52) = 1.78, p = 0.19]. As the interaction failed to reach significance, the post-hoc analysis was restricted to the effect of conditioning using the method of contrasts, avoiding multiple comparisons (Keppel, 1991). A difference was found between conditioning for saline and ethanol for the GFRα1 HET mice [T(26) = 3.61, p < 0.01], but not for the WT mice [T(26) = 0.72, p = 0.24]. In order to ensure that the phenotypic differences observed above are not due to a change in ethanol metabolism, we measured the BEC of the GDNF and GFRα1 HET and WT mice after the administration of the dose of ethanol used in the CPP experiments. As shown in Fig. 3C and 3D, BEC and ethanol clearance are similar in the GDNF and the GFRα1 HET and the WT mice [GDNF mice, main effect of time: F(2, 19) = 283.47, p < 0.001; but no effect of genotype: F(1, 19) = 0.27, p = 0.61; and no interaction: F(2, 19) = 0.01, p = 0.99; GFRα1 mice, main effect of time: F(2, 22) = 124.61, p < 0.001; but no effect of genotype: F(1, 22) = 0.00, p=0.98; and no interaction: F(2, 22) = 0.21, p = 0.81]. Taken together, these data suggest that attenuation in the level of either endogenous GDNF or its receptor increases the sensitivity of mice to the rewarding effect of ethanol.
Haplodeficiency of the GDNF Gene Does Not Significantly Affect Voluntary Ethanol Intake or Preference, but Results in Increased Level of Ethanol Consumption After a Period of Abstinence
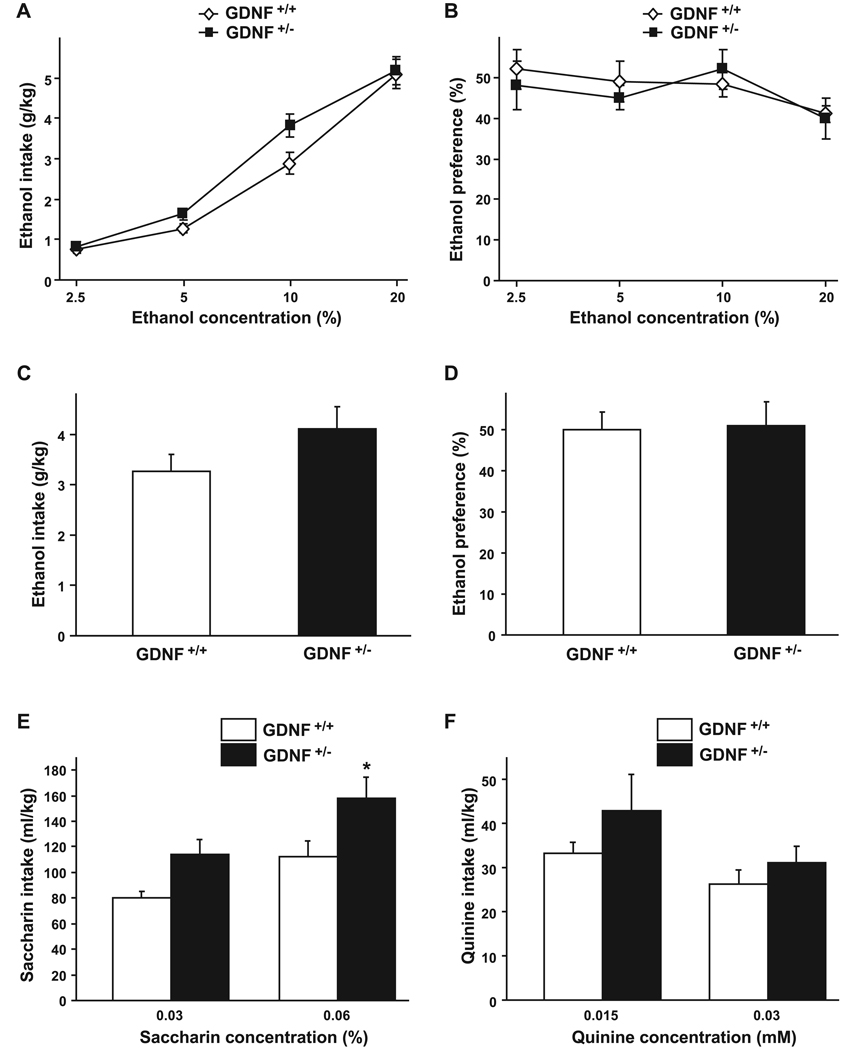
As we recently showed that exogenous application of GDNF to the VTA and the activation of the GDNF pathway reduce ethanol self-administration in rats (Carnicella et al., 2008, 2009b), we were interested in testing the contribution of endogenous GDNF to mechanisms that control ethanol-drinking behaviors by comparing voluntary ethanol consumption between the GDNF HET and WT mice in a 2-bottle choice (ethanol vs. water) limited-access paradigm (4 hours during the dark cycle; Rhodes et al., 2005). This procedure ensures high levels of ethanol intake during a short period of time. As shown in Fig. 4A, the amount of ethanol consumed was slightly greater for the GDNF HET mice than their WT littermates when mice were exposed to either a 5% or 10% ethanol solution. However, although a 2-way ANOVA revealed a significant effect of ethanol concentration [F(3, 57) = 198.81, p < 0.001], no significant effect of the genotype [F(1, 57) = 2.76, p = 0.11] and no significant interaction between both factors [F(3, 57) = 2.12, p = 0.11] were found. In addition, the GDNF HET mice did not differ from their WT littermates in the level of preference for ethanol (Fig. 4B) [main effect of ethanol concentration: F(3, 57) = 3.57, p < 0.02; but no effect of genotype: F(1, 57) = 0.11, p=0.75; and no interaction: F(3, 57) = 0.57, p = 0.64].
Fig. 4.
Ethanol consumption and preference are similar between GDNF HET and WT mice. (A and B) Voluntary ethanol intake (g / kg) and preference (percentage of ethanol solution consumed relative to total fluid consumed) plotted as a function of ethanol concentration during 4-hour limited-access sessions. n = 10–11 per group. (C and D) Voluntary ethanol intake and preference during 4-hour limited-access to a 10% ethanol solution. n = 14. E–F, Voluntary saccharin (E) or quinine (F) intake (ml / kg) plotted as a function of saccharin or quinine concentration during 4-hour limited-access sessions. n = 9–10 per group. Data are presented as mean ± SEM. *p < 0.05, compared with WT.
Ethanol intake and preference of a solution of 10% ethanol were assessed in a separate additional experiment in order to further explore a possible difference in ethanol consumption between the GDNF HET and their WT littermates. As shown in Fig. 4C, the GDNF HET mice showed a marginal, but not statistically significant, increase in ethanol consumption as compared with WT mice [T(26) = 2.15, p = 0.07], but no change in ethanol preference was observed [Fig. 4D, T (26) = 0.03, p = 0.87] as the increase in ethanol intake was associated with an increase in water consumption [38.7 ± 3.7 ml /kg and 47.3 ± 6.7 ml /kg for the GDNF WT and HET mice, respectively, T(26) = 1.85, p = 0.14]. These data suggest the haplodeficient GDNF mice do not exhibit a significant change in ethanol consummatory behavior as compared with their WT littermates.
To evaluate the taste sensitivity of GDNF HET and WT mice, we assessed the voluntary consumption of a sweet substance, saccharin, and a bitter, aversive substance, quinine. Compared with the WT mice, GDNF HET mice showed a small but significant increase in the consumption of saccharin (Fig. 4E) [main effect of saccharin concentration: F(1, 17) = 34.48, p < 0.001; main effect of genotype: F(1, 17) = 5.95, p < 0.05; no interaction between both factors: F(1, 17) = 0.82, p = 0.38]. However, consumption of quinine was similar in the 2 genotypes (Fig. 4F) [main effect of quinine concentration: F(1, 17) = 10.83, p < 0.01; but no effect of genotype: F(1, 17) = 1.53, p=0.23; and no interaction: F(1, 17) = 0.74, p = 0.40]. These results suggest that GDNF HET and WT mice may differ in their sensitivity to a sweet solution, but not to a bitter solution.
Finally, we tested whether the reduction in the level of GDNF expression alters ethanol consumption after a period of abstinence. At the end of the course of ethanol exposure, the limited 2-bottle choice drinking was discontinued for 1 week, followed by the assessment of intake of 20% ethanol and water during a single 4-hour drinking session. As shown in Fig. 5A, 1 week of abstinence resulted in increased ethanol consumption in the GDNF HET, but not in the WT mice, as compared with the level of drinking before the abstinence period. A 2-way ANOVA showed a significant effect of abstinence [F(1, 18) = 9.47, p < 0.01], but no significant effect of genotype [F(1, 18) = 2.71, p < 0.12] and no significant interaction between both factors [F(1, 18) = 3.04, p < 0.10]. In the absence of interaction, the post-hoc analysis was restricted to the effect of abstinence using the method of contrasts. A difference was found between the pre- and postperiod abstinence from ethanol intake of the GDNF HET mice [T(9) = 2.92, p < 0.01], but not of the WT mice [T(9) = 1.19, p = 0.13]. Interestingly, a 1-week period of abstinence from saccharin induced a similar increase in saccharin intake in both genotypes (Fig. 5B) [main effect of abstinence: F(1, 18) = 8.75, p < 0.01; but no effect of the genotype: F(1, 18) = 2.72, p = 0.12; and no interaction between both factors: F(1, 18) = 0.01, p = 0.93]. Taken together, these results suggest that attenuation in the level of endogenous GDNF specifically alters voluntary ethanol intake after a period of abstinence.
Fig. 5.
GDNF HET mice consume more ethanol than WT mice after a period of abstinence. (A and B) Voluntary ethanol (A) or saccharin (B) intake (g / kg) during 4-hour limited-access to a 20% ethanol or a 0.06% saccharin solution, before and after a 1-week period of abstinence. The pre-abstinence values are the average of the 4 consecutive sessions of drinking preceding the abstinence period. n = 10 per group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 compared with pre-abstinence values.
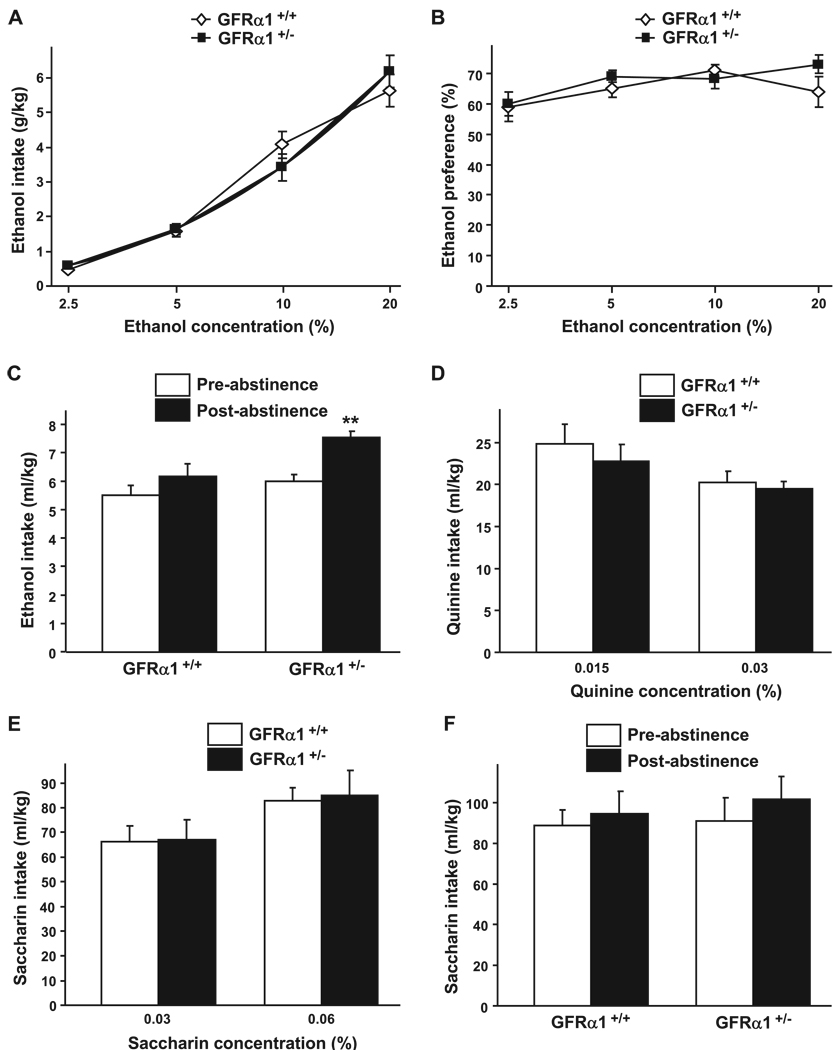
Haplodeficiency of the GFRα1 Gene Does Not Affect Voluntary Ethanol Intake or Preference, but Results in Increased Level of Ethanol Consumption After a Period of Abstinence
Next, we compared the level of ethanol intake of the GFRα1 HET mice to their WT littermates using the same limited-access 2-bottle choice paradigm described previously. As shown in Fig. 6A and 6B, the levels of ethanol consumption and preference were similar between the HET and WT mice [consumption, main effect of ethanol concentration: F(3, 39) = 174.88, p < 0.001; but no effect of genotype: F(1, 39) = 0.00, p = 0.97; and no interaction: F(3, 39) = 1.89, p = 0.15; preference, main effect of ethanol concentration: F(3, 39) = 3.52, p < 0.05; but no effect of genotype: F(1, 39) = 0.59, p = 0.46; and no interaction: F(3, 39) = 0.99, p = 0.41]. We further assessed the drinking behaviors of the GFRα1 HET and WT mice after a period of abstinence. Ethanol consumption was significantly increased after a 1-week period of abstinence as compared with the level of drinking before abstinence in the GFRα1 HET mice, but not in their WT littermates (Fig. 6C) [main effect of abstinence: F(1, 21) = 28, 98, p < 0.001, main effect of genotype: F(1, 21) = 4.33, p = 0.05, and a significant interaction between both factors: F(1, 21) = 4.52, p < 0.05].
Fig. 6.
GFRα1 HET mice consume more ethanol than WT mice after a period of abstinence. (A and B) Voluntary ethanol intake (g / kg) and preference (percentage of ethanol solution consumed relative to total fluid consumed) plotted as a function of ethanol concentration during 4-hour limited-access sessions. n = 6–9 per group. (C) Voluntary ethanol intake (g / kg) during 4-hour limited-access to 20% ethanol, before and after a 1-week period of abstinence. The pre-abstinence values are the average of the 4 consecutive sessions of drinking preceding the abstinence period. n = 10–12 per group. (D and E) Voluntary quinine (D) or saccharin (E) intake (ml / kg) plotted as a function of quinine or saccharin concentration during 4-hour limited-access sessions. n = 6–9 per group. (F) Voluntary saccharin intake (g / kg) during 4-hour limited-access to a 0.06% saccharin solution, before and after a 1-week period of abstinence. The pre-abstinence values are the average of the 4 consecutive sessions of drinking preceding the abstinence period. n = 8–10 per group. Data are presented as mean ± SEM. **p < 0.01 compared with pre-abstinence values.
Finally, we tested saccharin and quinine consumption in the GFRα1 HET and WT mice. GFRα1 HET mice showed no change in quinine or saccharin consumption compared with their WT littermates (Fig. 6D and 6E) [main effect of quinine concentration: F(1, 13) = 4.10, p < 0.05; no effect of genotype: F(1, 13) = 0.30, p = 0.59; and no interaction: F(1, 13) = 0.02, p = 0.89; main effect of saccharin concentration: F(1, 13) = 21.77, p < 0.001; no effect of genotype: F(1, 13) = 0.02, p = 0.89; and no interaction: F(1, 13) = 0.04, p = 0.85]. These results suggest that the palatability of the GFRα1 HET mice for sweet and bitter solutions is not altered. Importantly, an abstinence period did not result in changes in the level of saccharin consumption in the GFRα1 HET and WT mice (Fig. 6F) [no effect of abstinence: F(1, 16) = 2.26, p = 0.15; no effect of genotype: F(1, 16) = 0.14, p = 0.72; no interaction: F(1, 16) = 0.20, p = 0.66]. Taken together, these results suggest that reduction in the level of either GDNF or its receptor GFRα1 increases voluntary ethanol intake after a period of abstinence.
DISCUSSION
Here we report that reduction in the level of endogenous GDNF or its receptor GFRα1 results in increased sensitivity of mice to the rewarding properties of ethanol as well as in higher levels of ethanol consumption after a period of abstinence. We further show that these changes are not due to alteration in the metabolism of ethanol. Furthermore, the increase in intake after a period of abstinence was specific for ethanol, as saccharin intake was not altered in the GDNF or GFRα1 WT and HET mice.
We found that GDNF HET mice do not differ in their vulnerability to the psychomotor effects of ethanol as compared with their WT littermate controls. The study of the psychomotor effects of other drugs of abuse in GDNF HET and WT mice has lead to conflicting results. While no changes in the locomotor response of GDNF HET mice to acute injections of morphine, cocaine or methamphetamine have been reported (Airavaara et al., 2004, 2007; Yan et al., 2007), GDNF HET mice were shown to be more sensitive than WT mice to a challenge injection of morphine after repeated morphine administration (Airavaara et al., 2007), and more sensitive to repeated cocaine administration (Messer et al., 2000). However, using the same dose of cocaine and the same protocol of sensitization, Airavaara et al. observed a similar level of sensitization between the HET and the WT (Airavaara et al., 2004), and sensitization to methamphetamine was also found to be comparable in both phenotypes (Yan et al., 2007). Taken together, these data suggest that the role of endogenous GDNF in mechanisms that control the psychomotor response to ethanol and drugs of abuse merits further indepth investigation.
We observed greater place preference to ethanol in the GDNF HET mice, and to a lesser extent in the GFRα1 HET mice compared with their WT littermates. Greater place preference to morphine (Niwa et al., 2007a) and psychostimulants (Messer et al., 2000; Niwa et al., 2007b) was detected with the GDNF HET mice compared with their WT counterparts. Conversely, infusion of GDNF into the VTA during conditioning was shown to inhibit cocaine-induced CPP in rats (Messer et al., 2000). Taken together, these data strongly suggest that GDNF negatively modulates the rewarding effects of drugs of abuse, including ethanol. The results also imply that the decrease in rat ethanol self-administration upon application of GDNF in the VTA (Carnicella and Ron, 2009c; Carnicella et al., 2008, 2009b; He et al., 2005) may be due to a decrease in the rewarding / reinforcing properties of ethanol.
We found a small but significant increase in saccharin consumption in the GDNF HET mice, which is in line with previous data obtained with female GDNF HET mice in an operant sucrose self-administration paradigm (Griffin et al., 2006). However, these results are in contrast with the present results showing that GFRα1 HET mice consume similar amounts of saccharin as their WT littermates. In addition, Yan et al. reported no change in the consumption of, and motivation to consume food (another nondrug reward) in GDNF HET and WT mice (Yan et al., 2007). These discrepancies could be due to the difference in background between the GDNF and GFRα1 mice and/ or to compensatory mechanisms due to the down-regulation of the genes throughout development and adulthood.
We found that both the GDNF and GFRα1 HET mice did not significantly differ in their ethanol consumption and preference compared with their respective WT littermates before a period of abstinence, although a trend towards a higher degree of ethanol intake was observed in the GDNF (but not in GFRα1) HET mice as compared with the WT mice. We previously showed that up-regulation of the expression of endogenous GDNF in the VTA, or activation of the GDNF pathway by the exogenous application of the GDNF protein into the VTA, significantly decreased rats’ ethanol self-administration (Carnicella et al., 2008, 2009a,b; He et al., 2005). The discrepancy between the mice and rats data could be explained by the modest reduction in mRNA GDNF levels we observed in the GDNF and GFRα1 HET mice compared with the WT controls (Carnicella et al., 2009a) that could be insufficient to produce changes in ethanol consumption, and/ or to the masking of the VTA-specific actions of GDNF to reduce ethanol consumption, as we previously observed for another gene (Wang et al., 2007; Yaka et al., 2003). Finally, as in all transgenic mice in which the gene level is reduced during development, the lack of significant difference in ethanol consumption between the GDNF and GFRα1 HET and WT mice could be due to unknown compensation mechanisms that negate GDNF’s actions.
Interestingly, however, the haplodeficiency of the GDNF or GFRα1 gene is associated with an increase in ethanol consumption after a period of abstinence. The specific occurrence of this effect after abstinence and the comparable quinine consummatory behavior between the HET and the WT mice suggest that the increase in ethanol consumption is not due to a change in the palatability of ethanol. This postabstinence phenomenon is reminiscent of the observation that the BDNF HET male mice show a significant increase in ethanol consumption compared with their WT littermate controls only after a period of abstinence (McGough et al., 2004). Interestingly, we found that infusion of GDNF into the VTA was much more effective in inhibiting reacquisition of ethanol self-administration after an extinction period, than in reducing ethanol self-administration before the period of abstinence (Carnicella et al., 2008). Moreover, GDNF HET mice that self-administered the same amount of methamphetamine as the WT controls showed more seeking for the drug as compared with WT mice after several days of extinction or prolonged withdrawal periods (Yan et al., 2007). Together, these data strongly suggest an important regulatory role of the GDNF pathway during abstinence. No differences in saccharin intake after a period of abstinence were observed between the GDNF or GFRα1 HET and WT. In line with these results, Yan and colleagues found no difference in the reinstatement of food-seeking between HET and WT GDNF mice (Yan et al., 2007). Together, these data suggest that the role of GDNF during abstinence is specific to ethanol and other drugs of abuse, but is not shared with nondrug/natural rewarding substances.
Abstinence to drugs of abuse is a critical period during which drug-seeking becomes progressively stronger (Bossert et al., 2005; Grimm et al., 2001). GDNF expression in the nucleus accumbens was shown to be increased 24 hours after repeated exposure to methamphetamine and morphine (Niwa et al., 2007a,b; but see Green-Sadan et al., 2003), as well as in the VTA after repeated phencyclidine exposure (Semba et al., 2004). Interestingly, the increase in GDNF levels after repeated exposure to morphine was observed in the WT but not in the GDNF HET mice (Niwa et al., 2007a). Although it is unknown whether the GDNF level remains high during prolonged abstinence, these data and the present study suggest that GDNF plays a protective role against the neuroadaptations that occur during abstinence.
Similar levels of dopamine synthesis, storage, metabolism or reuptake have been observed in the GDNF HET and WT mice (Airavaara et al., 2004; Gerlai et al., 2001). Moreover, the GDNF HET mice do not differ from theWT mice in their number of the dopamine D1 receptor (Airavaara et al., 2004) and number / functionality of the dopamine D2 receptors (Carnicella et al., 2009a) receptors. However, GDNF was shown to be critical for the survival and outgrowth of midbrain dopaminergic neurons and other neuronal populations in the adult (Airaksinen and Saarma, 2002; Granholm et al., 2000). Thus, we cannot exclude the possibility that the increase in ethanol consumption after a period of abstinence in the GDNF and GFRα1 HET mice are a consequence of compensatory changes that result from the gene deletion (Airavaara et al., 2004) rather than an indication of the gene’s normal function. However, this is highly unlikely as the results are in line with the robust effect of GDNF to reduce ethanol self-administration after a period of extinction (Carnicella et al., 2008). The generation of conditional knockout mice (Pascual et al., 2008) or specific knock-down of the gene with small interference RNA, could be useful tools to address this question by providing postnatal and brain region-specific inactivation of GDNF. However, it should be noted that the HET mice may be an appropriate model to test for psychiatric disorders resulting from deficits in the GDNF pathway, as a complete deletion or loss of function of GDNF or its receptor leads to severe developmental abnormalities (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Tomac et al., 2000). Interestingly, reductions of 30 to 40% in the expression of GDNF were detected in blood samples of patients suffering from major depression and bipolar disorder (Otsuki et al., 2008; Takebayashi et al., 2006). As co-morbidity between alcoholism and depressive disorders has been documented (Brown et al., 1995; Davidson, 1995; Davidson and Blackburn, 1998) with a significant relationship between depression and propensity to relapse to alcohol consumption (Driessen et al., 2001; Greenfield et al., 1998), it is possible that alcohol addiction may also be associated with a decrease in the expression of GDNF.
In conclusion, our results suggest an important role for GDNF in controlling the level of ethanol reward and ethanol-drinking behaviors after a period of abstinence, and put forward the potential use of agents that up-regulate the GDNF pathway as potential drugs to prevent relapse.
ACKNOWLEDGMENTS
This work was supported by NIH-NIAAA R01 AA014366–02 (D.R., P.H.J.), and the state of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R. and P.H.J.). The authors thank Emily King and Madeline Ferwerda for technical support, and Drs. Barry Hoffer and Andreas Tomac (NIDA) for providing the transgenic mice.
REFERENCES
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse. 2006;59:321–329. doi: 10.1002/syn.20245. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Tuomainen H, Piepponen TP, Saarma M, Ahtee L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–298. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Brown SA, Inaba RK, Gillin JC, Schuckit MA, Stewart MA, Irwin MR. Alcoholism and affective disorder: clinical course of depressive symptoms. Am J Psychiatry. 1995;152:45–52. doi: 10.1176/ajp.152.1.45. [DOI] [PubMed] [Google Scholar]
- Burazin TC, Gundlach AL. Localization of GDNF/ neurturin receptor (c-ret, GFRalpha-1 and alpha-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Brain Res Mol Brain Res. 1999;73:151–171. doi: 10.1016/s0169-328x(99)00217-x. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via GDNF. Biol Psychiatry. 2009a doi: 10.1016/j.biopsych.2008.12.022. In press. DOI: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by GDNF. Alcohol. 2009b;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF - A potential target to treat addiction. Pharmacol Ther. 2009c doi: 10.1016/j.pharmthera.2008.12.001. In press. DOI: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Davidson KM. Diagnosis of depression in alcohol dependence: changes in prevalence with drinking status. Br J Psychiatry. 1995;166:199–204. doi: 10.1192/bjp.166.2.199. [DOI] [PubMed] [Google Scholar]
- Davidson KM, Blackburn IM. Co-morbid depression and drinking outcome in those with alcohol dependence. Alcohol Alcohol. 1998;33:482–487. doi: 10.1093/alcalc/33.5.482. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, Powell-Braxton L, Phillips HS. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14:1153–1163. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G, Shen L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20:3182–3190. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Srivastava N, Mott JL, Henry S, Henry M, Westphal H, Pichel JG, Shen L, Hoffer BJ. Morphological alterations in the peripheral and central nervous systems of mice lacking glial cell line-derived neurotrophic factor (GDNF): immunohistochemical studies. J Neurosci. 1997;17:1168–1178. doi: 10.1523/JNEUROSCI.17-03-01168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, Yadid G. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Boger HA, Granholm AC, Middaugh LD. Partial deletion of glial cell line-derived neurotrophic factor (GDNF) in mice: Effects on sucrose reward and striatal GDNF concentrations. Brain Res. 2006;1068:257–260. doi: 10.1016/j.brainres.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, Janak PH, Ron D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20:2420–2422. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- He DY, Ron D. GDNF reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of HSP90. J Biol Chem. 2008;283:12811–12818. doi: 10.1074/jbc.M706216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A, Barcia JA, Trejo JL, Canales JJ. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol LearnMem. 2008;90:553–559. doi: 10.1016/j.nlm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. 3rd ed. Upper, Saddla River, NJ: Prentice Hall; 1991. [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kowsky S, Poppelmeyer C, Kramer ER, Falkenburger BH, Kruse A, Klein R, Schulz JB. RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals. Proc Natl Acad Sci USA. 2007;104:20049–20054. doi: 10.1073/pnas.0706177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Jiao S, Collins F, Miller PJ. Glial cell line-derived neurotrophic factor: distribution and pharmacology in the rat following a bolus intraventricular injection. Brain Res. 1997;747:92–102. doi: 10.1016/s0006-8993(96)01265-6. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Matsuo A, Nakamura S, Akiguchi I. Immunohistochemical localization of glial cell line-derived neurotrophic factor family receptor alpha-1 in the rat brain: confirmation of expression in various neuronal systems. Brain Res. 2000;859:57–71. doi: 10.1016/s0006-8993(99)02442-7. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Shen L, Noda Y, Nabeshima T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav Brain Res. 2007a;179:167–171. doi: 10.1016/j.bbr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Shen L, Noda Y, Furukawa S, Nabeshima T. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007b;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- Otsuki K, Uchida S, Watanuki T, Wakabayashi Y, Fujimoto M, Matsubara T, Funato H, Watanabe Y. Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res. 2008;42:1145–1153. doi: 10.1016/j.jpsychires.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL / 6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Semba J, Akanuma N, Wakuta M, Tanaka N, Suhara T. Alterations in the expressions of mRNA for GDNF and its receptors in the ventral midbrain of rats exposed to subchronic phencyclidine. Brain Res Mol Brain Res. 2004;124:88–95. doi: 10.1016/j.molbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Springer JE, Mu X, Bergmann LW, Trojanowski JQ. Expression of GDNF mRNA in rat and human nervous tissue. Exp Neurol. 1994;127:167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hisaoka K, Nishida A, Tsuchioka M, Miyoshi I, Kozuru T, Hikasa S, Okamoto Y, Shinno H, Morinobu S, Yamawaki S. Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int J Neuropsychopharmacol. 2006;9:607–612. doi: 10.1017/S1461145705006085. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Mendez-Ferrer S, Pardal R, Echevarria M, Lopez-Barneo J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J Neurosci. 2003;23:141–148. doi: 10.1523/JNEUROSCI.23-01-00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac AC, Grinberg A, Huang SP, Nosrat C, Wang Y, Borlongan C, Lin SZ, Chiang YH, Olson L, Westphal H, Hoffer BJ. Glial cell line-derived neurotrophic factor receptor alpha1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience. 2000;95:1011–1023. doi: 10.1016/s0306-4522(99)00503-5. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995a;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci USA. 1995b;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL / 6J and DBA/ 2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]