Abstract
The capacity of immune complexes to augment antibody (Ab) responses is well established. The enhancing effects of immune complexes have been attributed mainly to Fc-mediated adjuvant activity, while the ability of Abs to induce antigenic alterations of specific epitopes as a result of immune complex formation have been less well studied. Previously we have shown that the interaction of anti-CD4-binding site (CD4bs) Abs with HIV-1 gp120 induces conformation changes that lead to enhanced antigenicity and immunogenicity of neutralizing epitopes in the V3 loop. The present study shows that significant increases in the antigenicity of the V3 and C1 regions of gp120 were attained for several subtype B gp120s and a subtype C gp120 upon immune complex formation with the anti-CD4bs monoclonal Ab (mAb) 654-D. Such enhancement was observed with immune complexes made with other anti-CD4bs mAbs and anti-V2 mAbs, but not with anti-C2 mAbs, indicating this activity is determined by antigen specificity of the mAb that formed the immune complex. When immune complexes of gp120LAI/654-D and gp120JRFL/654-D were tested as immunogens in mice, serum Abs to gp120 and V3 were generated at significantly higher titers than those induced by the respective uncomplexed gp120s. Notably, the anti-V3 Ab responses had distinct fine specificities; gp120JRFL/654-D stimulated more cross-reactive anti-V3 Abs than gp120LAI/654-D. Neutralizing activities against viruses with heterologous envelope were also detected in sera of mice immunized with gp120JRFL/654-D, although the neutralization breadth was still limited. Overall this study shows the potential use of gp120/Ab complexes to augment the immunogenicity of HIV-1 envelope gp120, but further improvements are needed to elicit virus-neutralizing Ab responses with higher potency and breadth.
Keywords: HIV-1, immune complex vaccine, antibody response
Introduction
Immune complexes have been tested as vaccines to augment protective immune responses against various viral and bacterial pathogens, including HIV-1 [1–3], hepatitis B surface antigen [4–6], infectious bursal disease virus [7], equine herpesvirus 1 [8], porcine parvovirus [9], and Francisella tularensis [10]. The enhancing effects of these immune complexes have been attributed mainly to the specific Fc receptor targeting. The capacity of Abs to alter the conformation and exposure of specific epitopes on antigens has not been exploited as much. A number of studies from our lab and others have demonstrated that Abs can shield specific antigenic sites [11–14] or alter the overall antigen stability to affect the antigen processing by antigen-presenting cells [15,16], resulting in modulation of T cell epitope presentation. In terms of Ab responses, immunization with immune complexes has also been shown to elicit qualitatively different Ab responses with distinct antigenic specificities from those elicited by antigens alone [17–19].
HIV-1 envelope glycoproteins gp120 and gp41 are key targets for neutralizing antibodies against the virus. However, the envelope glycoproteins expressed by HIV-1 isolates are extremely variable, and very few conserved neutralizing epitopes have been identified on gp120 and gp41 [20–22]. During natural infection with HIV-1, the vast majority of serum antibodies generated against the virus have no neutralizing activity or display highly restricted specificities effective only against selected virus strains, as the antibodies bind to antigenic sites irrelevant for virus infectivity or target the variable regions on gp120 and gp41 [23–27]. Considering the capacity of certain antibodies to better expose or stabilize selective antigenic sites on gp120, immune complexes have been evaluated as an approach to redirect Abs toward critical neutralizing epitopes on this antigen. An earlier effort to immunize animals using HIV-1 envelope glycoprotein gp120 complexed with mAb A32, which specifically induces the mAb binding to the chemokine-receptor binding site, did not enhance the production of cross-reactive neutralizing Abs against this conserved region on gp120 [3]. However, immune complexes made of gp120 and the CD4-binding site (CD4bs) mAbs were found to be potent immunogens that stimulated higher Ab titers especially to the V3 loop than the uncomplexed gp120 [1,2]. Significantly, neutralizing Abs against V3 and other undefined epitopes were induced by the immune complexes but not by gp120 alone, although the neutralizing activity was highly restricted to HIV-1 bearing the homologous gp120 strain [1].
In the present study, we examined immune complexes made of different gp120s in order to broaden the neutralizing Ab responses toward heterologous HIV-1 isolates. The anti-CD4bs975 mAb 654-D was reactive with many of the gp120s tested and the gp120/654-D complexes displayed enhanced reactivity with anti-V3 and anti-C1 mAbs. The complexes made of gp120LAI or gp120JRFL were subsequently tested to immunize BALB/c mice in the presence or absence of adjuvant. Anti-gp120 and anti-V3 Ab responses elicited in sera of mice immunized with immune complexes vs. uncomplexed gp120 were compared. Enhanced titers of Ab binding and neutralization against heterologous viruses were induced by immunization with the gp120JRFL/654-D complex in the presence of adjuvant, but the neutralization was still limited to relatively sensitive viruses. This study provides clear evidence for the superior capacity of the gp120/Ab complexes to direct Ab responses toward specific neutralizing epitopes on gp120, but more research efforts are needed to improve the immune complex design and the immunization regimen in order to attain the Ab titers and breadth required to tackle the more resistant HIV-1 isolates.
Materials and Methods
HIV-1 gp120, mAbs, and immune complexes
Recombinant soluble gp120 proteins were obtained from the following sources: Perkin Elmer at Boston, MA (LAI), Immunodiagnostics at Wolburn, MA (MN), Dr. Abraham Pinter at PHRI (BaL), Dr. Richard Wyatt at the Vaccine Research Center, NIH (YU-2), Dr. James Arthos at the NIH, NIAID (TH14-12, 93MW959, AN1, 92UG21-9), Vaccine Research and Development Branch of Division of AIDS, NIAID, NIH (JRFL), the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (93TH975). The following peptides were used: V3LAI/HXB-2 (aa 272-291), V3SF162 (aa 272–291), V3JRFL (aa 272–296), and V3MN (aa 272–296), pooled C1MN (aa 1–20, aa 23–30, aa 27–35, aa 31–50, aa 41–60, aa 61–80, aa 84–96, aa 91–110). The peptides were obtained from NIBSC Centre for AIDS Reagents (EU Programme EVA/AVIP), the NIH AIDS Research and Reference Reagent Program, or purchased commercially. Human mAbs were gifts from Drs. Susan Zolla-Pazner at New York University and James Robinson at Tulane University.
Ab binding to immune complexes
Measurement of mAb reactivity with immune complexes vs. gp120 was done as described previously [1]. Immune complexes were prepared at molar gp120/mAb ratios of ~1:2. Immune complexes or gp120 alone were serially diluted in phosphate buffered saline and used to coat ELISA plates. MAb binding was detected using biotinylated mAbs, followed by alkaline phosphatase-conjugated streptavidin and substrate.
Immunization with immune complexes
BALB/c mice (female, >6 weeks old from Jackson lab, 4–5 animals per group) were injected intraperitoneally with immune complexes, gp120 alone or gp120 + control mAb (3 µg gp120 and 9 µg mAb in 100 µl per animal). When adjuvant was used, the immunogens were mixed with 25 µg of MPL and 250 µg DDA per animal per dose (Sigma, St. Louis, MO). Animals were immunized at 3x at 2–3 weeks intervals. Blood was collected a week after the last immunization, and the sera from each group pooled. The animal studies were carried out according to the protocol approved by the VA and NYU IACUC.
Assessment of serum Ab titers in immunized mice
The levels of gp120- and V3-specific Abs in immune sera were measured by ELISA as described previously [1].
HIV-1 neutralization assays with TZM/bl target cells
Neutralizing activities of the immune sera were tested in two different neutralization assays using fully infectious HIV-1 strains and single cycle infectious pseudoviruses. HIV-1 LAI and MN were grown in mitogen-activated PBMCs, while pseudoviruses with HIV-1 envelopes of SF162, SS1196.1, 3988.25, HXB-2, and JRFL were produced by co-transfecting 293T cells with plasmids containing env (18 µg), rev (2 µg), and the env-deficient HIV-1 backbone pNL4-3.Luc.R-E– using the ProFection mammalian transfection system (Promega, Madison, WI). The plasmids bearing env genes of HIV-1 isolates SS1196.1 and 3988.25 were kindly given by Dr. D. Montefiori (Duke University). The viruses (200 TCID50) were mixed with sera at the designated dilutions for 2 hrs at 37°C, and then tested for infectivity in the TZM-bl target cells based on luciferase activity as described previously [1]. Indinavir (1 µM) was added for testing HIV-1 LAI and MN. Pseudoviruses with envelopes of YU-2 and NL4.3, on the other hand, were generated to express the enhanced green fluorescence protein reporter gene with the env-defective NL4.3 backbone NLENG1-ES-IRES [28]. These viruses were pre-treated with the immune sera as above, and their infectivity levels measured in TZM/bl cells by flow cytometry at day 2 or day 3. In each experiment, normal or pre-bleed sera were tested as negative controls. All sera were heat-inactivated at 56°C for 30 min prior to use in the assays. The neutralizing anti-V3 mAb 447-52D was also tested for comparison.
Results
Anti-CD4bs mAb 654-D binds to recombinant gp120 proteins of different HIV-1 strains and enhances Ab binding to multiple antigen sites on gp120
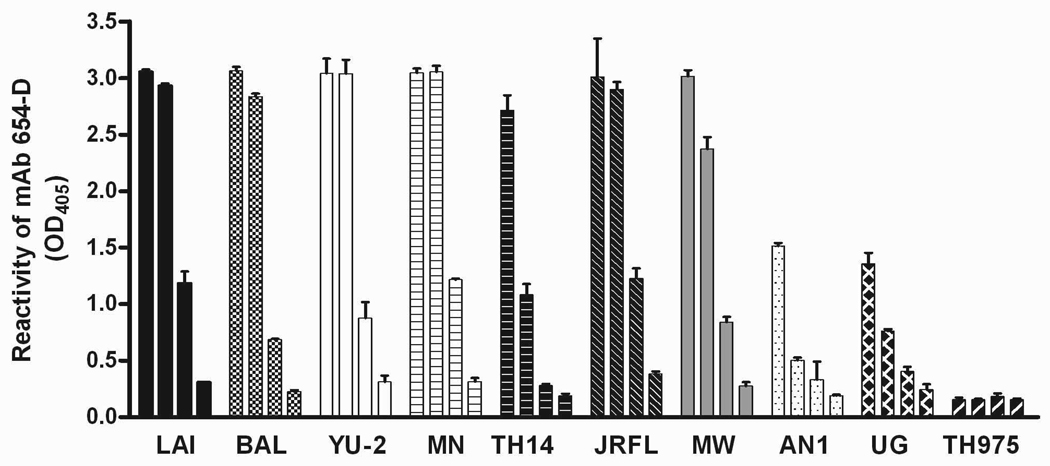
Our previous study has shown that gp120LAI complexed with different anti-CD4bs mAbs, including 654-D, displays increased antigenicity of the different regions of gp120, particularly the amino terminal C1 region and neutralizing epitopes in the V3 loop [1]. The levels of enhancement observed with the gp120/anti-CD4bs mAb complexes were markedly greater than those seen with complexes made of other anti-gp120 mAbs, such as the anti-C2 mAb. To investigate whether enhanced V3 antigenicity was also evident on immune complexes made of mAb 654-D and gp120 from different HIV-1 strains, we first tested a panel of 10 recombinant gp120 proteins for reactivity with mAb 654-D (Fig. 1) and then evaluated the binding capacity of the anti-V3 mAb 694/98-D to the complexes made of different gp120 and mAb 654-D as compared to the respective uncomplexed gp120 (Fig. 2). Similar to many cross-reactive anti-CD4bs mAbs isolated from HIV-1-infected subjects [29,30], mAb 654-D was highly reactive against six gp120 proteins from subtype B viruses (LAI, BAL, YU-2, MN, TH14-12, JRFL) and one subtype C protein (93MW959). The synthetic ancestral subtype B gp120 (AN1) and a subtype D gp120 (92UG21-9) were recognized weakly, while a subtype E gp120 (93TH975) failed to show any reactivity with the mAb.
Fig. 1.
Cross reactivity of anti-CD4bs mAb 654-D with a panel of HIV-1 gp120 proteins. Gp120 was coated on 96-microwell plates and reacted with human anti-gp120 654-D mAb (1 µg/ml). The following gp120 proteins were tested: LAI, BAL, YU-2, MN, TH14-12 (TH14), JRFL, 93MW959 (MW), ancestral subtype B (AN1), 92UG21-9 (UG), 93TH975 (TH975). Alkaline phosphatase-labeled anti-human IgG was used to detect the mAb binding. The bars in each set represent 2-fold serial dilutions of the gp120 from 1 to 0.125 µg/ml. Means and standard deviations from triplicates wells are presented in graph. OD405, optical density at 405 nm.
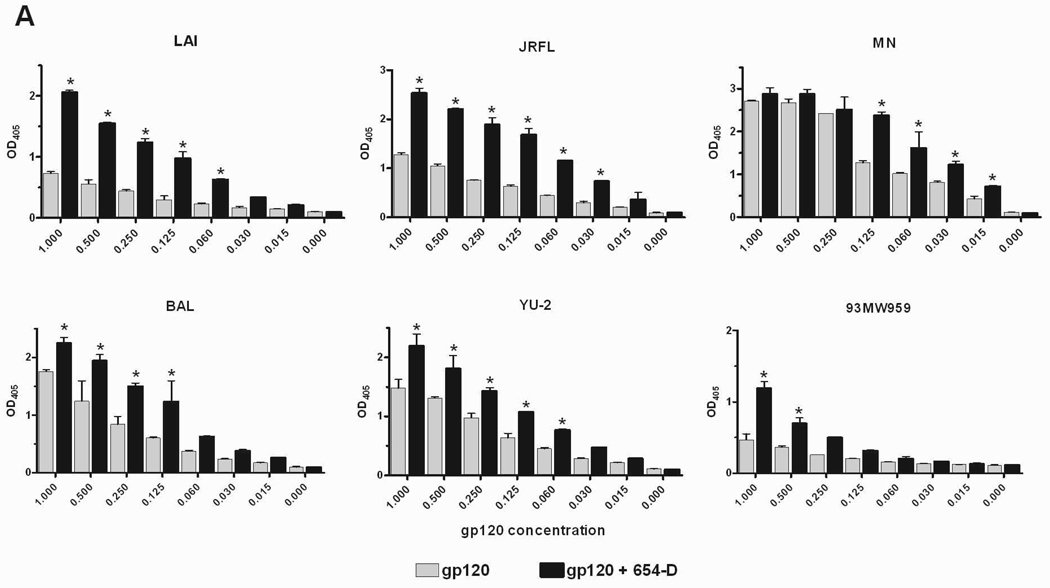
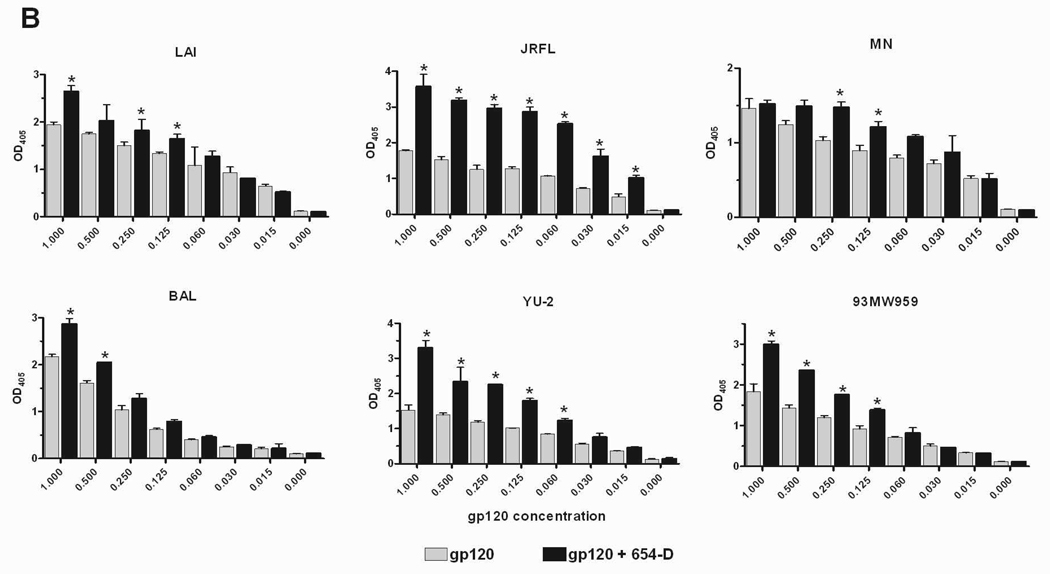
Fig. 2.
Enhanced gp120 antigenicity upon immune complex formation. (A) The binding of anti-V3 mAb to gp120/654-D complexes made of different HIV-1 gp120s. (B) The binding of anti-C1 mAb to gp120/654-D complexes made of different HIV-1 gp120s. (C) The binding of anti-V3 mAb to gp120 complexed with different anti-gp120 mAbs. The gp120/mAb complexes or gp120s alone were coated onto 96-microwell plates and reacted with biotinylated mAbs specific for V3 (694/98-D) (A and C) and C1 (EH21) (B). The binding of the biotinylated mAbs was determined using alkaline phosphatase-conjugated-streptavidin. The x-axis shows equivalent concentrations of gp120, on its own and in the gp120/mAb complexes. Means and standard deviations from duplicate wells are presented in graph. Representative results from one set of experiments are shown. * p<0.05 as compared to gp120 alone. OD405, optical density at 405 nm. Statistical analyses were performed using two-way ANOVA with Dunnett’s multiple comparision test (Graph Pad Prism).
Six of the highly reactive gp120 proteins were subsequently tested on their own or as immune complexes with mAb 654-D for reactivity with a biotinylated anti-V3 mAb 694/98-D. Each of the six gp120/654-D complexes had higher reactivity with the anti-V3 mAb relative to uncomplexed gp120 (Fig. 2A). The mAb binding curves were shifted down by as much as 16 folds for gp120JRFL and gp120LAI, with statistically significant differences of p<0.01 for the highest 5–6 concentrations tested. For the other gp120 proteins including the subtype C gp120 93MW959, 2–4 fold improvement was observed, with p<0.01 at some of the tested concentrations. The V3 loop has been shown to be shielded, at varying degrees depending on the virus strains, due to masking by N-glycans, the V1/V2 loop, and other undefined elements [31–33]. Whether these factors account for the different degrees by which the mAb 654-D is capable of exposing V3 on these monomeric gp120 proteins remain unclear. Similarly the N-terminal C1 region of gp120 was also better recognized by mAb EH21 when the gp120 proteins were bound by mAb 654-D (Fig. 2B; p<0.05), whereas Ab reactivity with C5 at the C-terminus was not altered ([1] and data not shown). Hence, the antigenicity of V3 and C1 were specifically enhanced on the various gp120/654-D complexes, most likely due to conformational changes induced by the mAb 654-D that better expose or stabilize these gp120 antigenic regions.
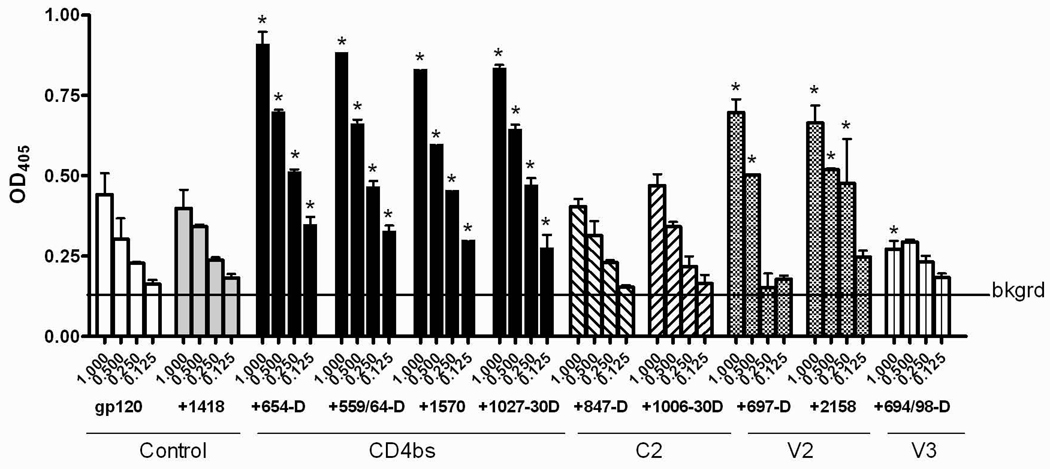
To further evaluate the specific contribution of anti-CD4bs mAb in enhancing gp120 antigenicity, we tested mAb reactivity to V3 on gp120/mAb complexes formed with other anti-gp120 mAbs (Fig. 2C). Each of these human mAbs is of IgG1 subtype. All complexes made with anti-CD4bs mAbs (654-D, 559/64-D, 1570, 1027-30D) displayed significantly higher levels of anti-V3 reactivity than uncomplexed gp120, whereas gp120 complexed with anti-C2 mAbs (1006-30D and 847-D) did not. As expected, we detected poor to no binding of the biotinylated anti-V3 mAb to gp120 already in complex with the same anti-V3 mAb, due to steric hindrance. Interestingly, enhanced V3 reactivity was also observed with gp120 complexed with anti-V2 mAbs (697-D and 2158). Hence, not all gp120/mAb complexes display enhanced V3 antigenicity and the enhancing activity is determined mainly by the antigen specificity of the mAb used to form the complex.
Immunization with the gp120/654-D complex elicits virus-neutralizing antibody responses but an additional adjuvant is required
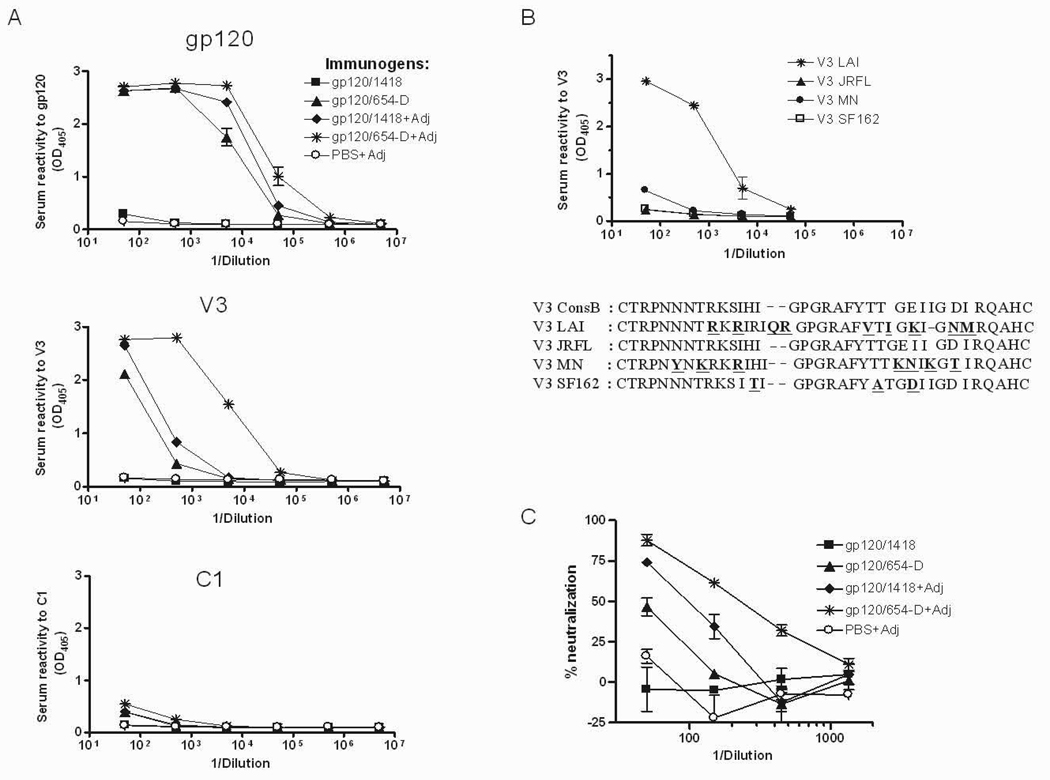
In addition to the capacity to alter epitope exposure, immune complexes have been shown to have adjuvant properties that augment the induction of the overall antibody responses to the specific antigens [6,34]. To examine whether the gp120/654-D complex could be sufficiently immunogenic to elicit gp120-specific antibodies without additional adjuvant, we immunized BALB/c mice with the gp120/654-D complex with or without MPL/DDA adjuvant. The complex was made with gp120LAI, as the gp120LAI/654-D complex has been found previously to stimulate robust anti-gp120 Ab responses when administered with an adjuvant [1]. For comparison, we also immunized mice with uncomplexed gp120LAI mixed with an irrelevant anti-parvovirus mAb 1418 in the presence or absence of adjuvant MPL/DDA. Fig. 3A (top panel) shows that the gp120/654-D complex could elicit antibodies specific for gp120 either with or without MPL/DDA (half max of 27,800 vs. 9,200, respectively), while the uncomplexed gp120 and 1418 requires adjuvant to elicit anti-gp120 responses (half max of 17,100). In the absence of MPL/DDA, the gp120 and 1418 mixture did not generate any detectable gp120-specific antibodies. The gp120/654-D complex alone was also capable of eliciting antibodies to V3, albeit at lower titers than the complex with adjuvant (half max of 120 vs. 5900; Fig. 3A middle panel). In contrast, the antibody responses to C1 were weak in all of the groups (Fig. 3A lower panel), even though both V3 and C1 regions on the gp120/654-D complex was better recognized by mAbs (Fig. 2). Hence, the anti-gp120 and anti-V3 titers induced by the gp120/654-D complex with MPL/DDA were 1–2 logs higher than those achieved following immunization with the gp120/654-D complex without the adjuvant. Consistent with our previous findings [1], when administered with MPL/DDA, the gp120/654-D complex also elicited higher levels of Ab responses to gp120 and particularly to V3 than the gp120 and 1418 mixture.
Fig. 3.
Reactivity of serum Abs from mice immunized with the gp120LAI/mAb complex with or without MPL/DDA adjuvant. (A) Serum Ab titers to the whole gp120LAI protein (top), the V3LAI peptide (middle), and the N-terminal C1 peptides (bottom). BALB/c mice were immunized with the gp120LAI/654-D complex or the mixture of gp120LAI + control mAb 1418 in the presence or absence of adjuvant MPL/DDA (Adj). For controls, a group of animals was also immunized with PBS and MPL/DDA. Immune sera were serially diluted and tested in ELISA for reactivity with recombinant gp120LAI protein, V3 peptide and C1 peptides coated directly on the microwell plates. Alkaline phosphatase-conjugated anti-mouse IgG was used as the secondary antibody. (B) Reactivity of serum Abs from mice immunized with the gp120LAI/654-D complex and MPL/DDA with different V3 peptides. To detect cross reactivity of anti-V3 Abs induced by the gp120LAI/654-D complex, V3 peptides with sequences of LAI, JRFL, MN, and SF162 strains were coated on the microwell plates and reacted with serially diluted sera from the gp120LAI/654-D + MPL/DDA-immunized animals. (C) Neutralization of HIV-1 pseudovirus expressing the homologous envelope by sera from immunized animals. Sera were serially diluted 3 fold starting from 1:50, pre-incubated with the virus, and tested for virus neutralizing activity in the single round infection assay with TZM-bl cells. Means and standard deviations were derived from duplicate wells. Data from one of two repeated experiments are shown. OD405, optical density at 405 nm.
However, the anti-V3 Abs induced by the gp120/654-D complex, in the presence or absence of MPL/DDA, were reactive mainly with the homologous V3 LAI and did not recognize other V3 peptides (Fig. 3B and data not shown). The immune sera were then tested for the capacity to neutralize pseudovirus bearing a cloned env gene (HXB-2) from HIV-1LAI. Sera from mice immunized with gp120/654-D in the presence of MPL/DDA neutralize the virus more potently than sera from mice immunized with the uncomplexed gp120/1418 plus MPL/DDA (IC50 of 225 and 100, respectively). By contrast, sera from mice immunized with gp120/654-D in the absence of adjuvant did not mediate neutralization above 50% (IC50 of <50, Fig. 3C). Immunization with the uncomplexed gp120 in the absence of adjuvant (gp120/1418) did not elicit neutralizing activity above the background level observed with the PBS control group (Fig. 3C). Similar findings were observed when the sera were tested for neutralization of the infectious HIV-1LAI strain (data not shown). These results demonstrate that the gp120/654-D complex was a potent immunogen for inducing anti-gp120 binding Abs, but an additional adjuvant was still needed to generate virus-neutralizing Abs.
Immunization with the gp120JRFL/654-D complex elicits high titers of serum Abs against the homologous and heterologous gp120, but the serum neutralizing activity remains highly restricted
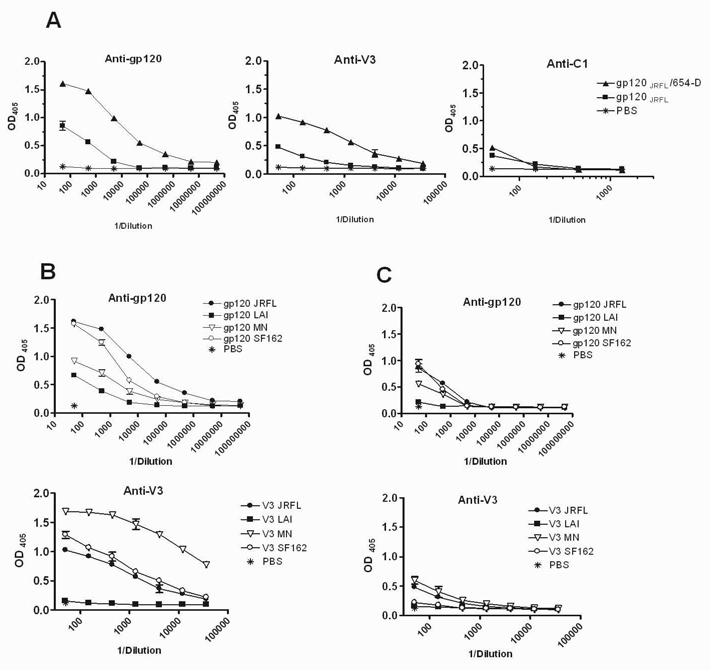
Since the gp120LAI sequence, especially in the V3 loop, deviates significantly from the consensus subtype B sequence (Fig. 3 and [1]), we sought to examine the use of a more representative subtype B gp120 antigen as an immunogen in order to generate anti-gp120 Abs that are more cross-reactive among a larger array of subtype B viruses. The V3JRFL is identical to that of the consensus subtype B (Fig. 3), and gp120JRFL sequence shows 91% similarity with the consensus B gp120. Moreover, as shown in Fig. 2A, the antigenicity of the gp120JRFL/654-D complex was much improved as compared to the uncomplexed gp120JRFL. Hence, we compared the immunogenicity of gp120JRFL alone or as a complex with mAb 654-D in the BALB/c mice. All immunogens were administered i.p. 3x with MPL/DDA. The data clearly show that consistent with the findings observed with gp120LAI/654-D, the gp120JRFL/654-D complex was a superior immunogen as compared to uncomplexed gp120JRFL in term of inducing Ab responses to the whole gp120 and to the V3 loop (Fig. 4A). Ab response to the C1 region was again unaltered, even though the C1 epitope was better recognized on the gp120JRFL/654-D complex by mAb EH21. These data indicate that enhanced in vitro antigenicity of a given epitope does not necessarily result in improvement of its immunogenicity in vivo. The anti-gp120 Ab responses induced by gp120JRFL/654-D were highest against the homologous gp120JRFL, but were reactive, in a descending order, with gp120 of SF162, MN, and LAI (Fig. 4B top). The anti-V3 Ab responses were also much more cross-reactive (Fig. 4B bottom). Interestingly, the Abs induced by gp120JRFL/654-D recognized V3MN best, while the homologous V3JRFL was recognized at lower levels, comparable to V3SF162. No reactivity to V3LAI was detected above background. This pattern is completely opposite from that observed with the gp120LAI/654-D complex, which induced Abs highly specific for V3LAI (Fig. 4B bottom vs. Fig. 3B). For comparison, the anti-gp120 and anti-V3 responses elicited by immunization with the uncomplexed gp120JRFL were much poorer regardless of the gp120 and V3 strains tested (Fig. 4C).
Fig. 4.
Induction of cross-reactive anti-gp120 Ab responses in sera of mice immunized with the gp120JRFL/654-D complex. (A) Serum Abs specific for homologous gp120 (left), V3 (middle) and C1 (right) induced in BALB/c mice immunized with gp120JRFL alone or the gp120 JRFL/654-D complex along with adjuvant MPL/DDA. For controls, animals were also immunized with PBS and MPL/DDA. (B) Cross-reactivity of anti-gp120 and anti-V3 mAbs induced by the gp120JRFL/654-D complex. (C) Cross-reactivity of anti-gp120 and anti-V3 mAbs induced by gp120JRFL alone. The whole gp120 proteins of JRFL, LAI, MN and SF162, and the C1 or V3 peptides were coated on the microwell plates and reacted with serially diluted sera. Alkaline phosphatase-conjugated anti-mouse IgG was used as the secondary antibody to detect serum IgG binding to gp120 or V3. Means and standard deviations were derived from duplicate wells. Data from one of two independent experiments are shown. OD405, optical density at 405 nm
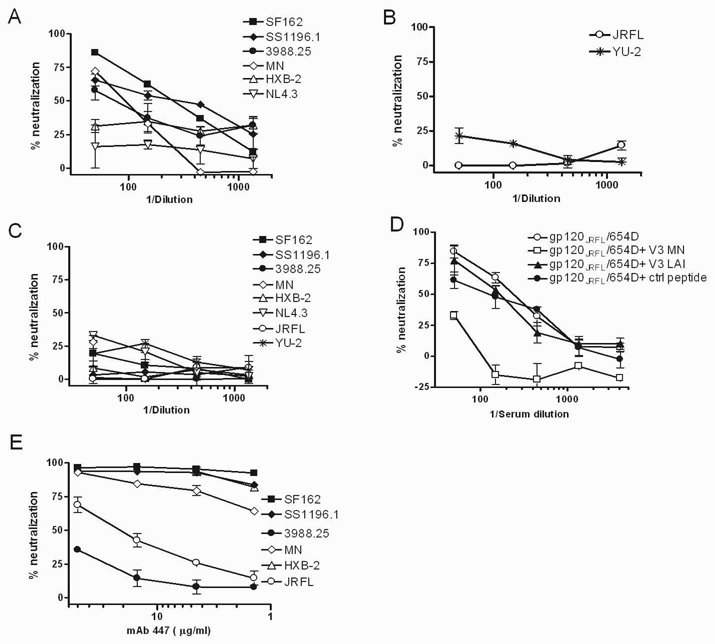
The sera from mice immunized with the gp120JRFL/654-D complex were then tested for neutralizing activities against eight viruses expressing different HIV-1 envelopes: HXB-2, NL4.3, SF162, SS1196.1, 3988.25, MN, YU-2, and JRFL (Fig. 5A–B). The first six viruses are considered to be highly sensitive to neutralization by various polyclonal and monoclonal Abs (Fig. 5A), while the last two viruses are relatively resistant (Fig. 5B) [35]. HXB-2 and NL4.3 are molecular clones whose envelopes are derived from HIV-1LAI. Sera from mice receiving PBS and MPL/DDA were also tested to determine the background neutralization level of the assays (IC50 of <50 against all eight viruses tested; Fig. 5C). Consistent with poor serum Ab binding detected in ELISA to gp120LAI and V3LAI, the sera from gp120JRFL/654-D-immunized mice had little or no neutralizing activity against HXB-2 and NL4.3. Potent neutralization was detected against SF162 (IC50 of 260), but not against the homologous JRFL, even though high levels of serum Abs binding to gp120 and V3 of these two virus strains were detected (Fig. 4B). Neutralization was also detected against SS1196.1, 3988.25, and MN (IC50 of 290, 80, and 106, respectively), but not against YU-2. Hence, the neutralizing Ab response elicited by the gp120JRFL/654-D complex was cross-reactive and effective against highly sensitive HIV-1 viruses, but they were ineffective against resistant viruses, including virus with the homologous JRFL envelope. For comparison, we tested the anti-V3 mAb 447-52D, which was able to neutralize four of the highly sensitive viruses (SF162, SS1196.1, HXB-2, and MN) at IC50 <1 µg/ml and the resistant virus JRFL at IC50 of 20 µg/ml, but not 3988.25 (IC50 >50 µg/ml).
Fig. 5.
Virus neutralization by sera from mice immunized with the gp120JRFL/654-D complex. (A–B) Sera collected after the final immunization with the gp120JRFL/654-D complex and MPL/DDA was serially diluted and tested for neutralizing activity with TZM-bl target cells against viruses expressing different HIV-1 envelope proteins in two different assays described in the Materials and Methods section. (C) Sera from control animals immunized with PBS and MPL/DDA were also tested against the same set of viruses to establish the background neutralization levels. (D) Sera from mice immunized with the gp120JRFL/654-D complex were pre-treated with serum-reactive V3MN peptide, non-reactive V3LAI peptide, or control peptide (40 µg/ml) and tested for the capacity to neutralize SF162. (E) For comparison, neutralization activity of the anti-V3 mAb 447-52D was assessed against viruses expressing envelope proteins of SF162, SS1196.1, 3988.25, MN, HXB-2 and JRFL. The averages and standard deviations are shown, and each experiment was repeated at least twice.
To determine the contribution of anti-V3 Abs in mediating the neutralizing activity observed with sera from mice immunized with the gp120JRFL/654-D complex, V3 peptides were used to block the activity of anti-V3 Abs present in the sera. Serially diluted sera were pre-treated with a fixed amount of peptide (40 µg/ml), and tested for the capacity to neutralize SF162. Pre-treatment with the serum-reactive V3MN peptide significantly reduced the neutralizing activity. In contrast, the non-reactive V3LAI peptide had no inhibitory activity, comparable to the control peptide. These data clearly demonstrate that the neutralizing activity elicited by immunization with the gp120JRFL/654-D complex was to a large extent due to anti-V3 Abs. Hence, in correlation to the enhanced V3 antigenicity observed on the gp120/654-D complex, the immune complex is also a potent immunogen for eliciting and directing Ab responses against neutralizing epitopes in the V3 loop.
Discussion
The HIV-1 envelope gp120 is a critical target for neutralizing Ab responses against HIV-1, but gp120 is poorly immunogenic, and designing immunogens that stimulate Ab responses against the conserved neutralizing epitopes on gp120 has been a formidable challenge. This study demonstrates that gp120 antigenicity and immunogenicity are significantly enhanced when gp120 is presented as an immune complex with the anti-CD4bs mAb 654-D rather than as uncomplexed gp120. Significantly, we show for the first time that the enhanced antigenicity and immunogenicity can be observed with gp120s from different HIV-1 strains, indicating that the approach is applicable to a wide array of gp120s. The enhanced Ab reactivity is directed toward the V3 region of gp120 that needs to adopt a conserved structure for interacting with the chemokine receptors CCR5 or CXCR4 and contains multiple neutralizing epitopes, some of which are broadly cross-reactive [36–38]. However, the V3 region also includes highly variable elements, and many anti-V3 mAbs target these variable sites and display narrow specificities [38]. Indeed, immunization with immune complexes made of gp120LAI with an unusual V3 sequence results in induction of serum Abs that are highly specific for V3LAI and fail to recognize any other V3 sequences (Fig. 3B and [1]). The gp120LAI/654-D complex also induces potent neutralizing activity but the neutralization is only effective against the homologous HIV-1 LAI (Fig. 3C and [1]). In the current study, we tested an immune complex made of gp120JRFL that expresses the HIV-1 subtype B consensus V3 sequence and observed that anti-V3 Abs with broader reactivity were generated, as indicated with serum IgG binding to the homologous V3JRFL and the heterologous V3 of MN and SF162 (Fig. 4B). In contrast to the gp120LAI/654-D complex, the gp120JRFL/654-D complex did not elicit Abs reactive with V3LAI, demonstrating the completely distinct specificities of anti-V3 Ab responses induced by the two complexes.
Although anti-gp120 and anti-V3 Abs were produced to higher titers and with broader reactivities upon immunization with the gp120JRFL/654-D complex, the Ab responses were effective in neutralizing only HIV-1 isolates that belong to the highly sensitive Tier 1 category (SF162, MN, SS1196.1 and 3988.25) (Fig. 5A). Neutralization was not achieved against the more resistant viruses, including the homologous JRFL (Fig. 5B). The neutralizing activity elicited by the gp120/654-D complex is mediated in large part by anti-V3 Abs, as it can be significantly reduced by pretreatment with the relevant V3 peptide (Fig. 5D and [1]). We further showed that JRFL neutralization was achievable by the anti-V3 mAb 447-52D, albeit with IC50 that was many folds higher than those for the Tier 1 viruses such as SF162 and SS1196.1 (Fig. 5E). Nevertheless, the Ab responses induced by the gp120JRFL/654-D complex did not display a mAb 447-52D-like neutralizing specificity since the immune serum neutralized 3988.25, which was relatively resistant to mAb 447-52D. These results indicate that JRFL can be neutralized by anti-V3 Abs, but such anti-V3 Abs may not be elicited by the gp120JRFL/654-D complex. Alternatively, much higher titers of anti-V3 Abs are required and such high Ab levels were not reached by the current immunization regimen used with the gp120JRFL/654-D complex. It is of interest to note that the Ab titer elicited by the gp120JRFL/654-D complex against V3MN was ~100x higher than that against the homologous V3JRFL, and neutralization was achieved against MN. While the reason for this is still unclear, the data suggest that significantly higher Ab titers against V3 can be induced, although improvements on the immune complex design are required to further boost and re-direct the Ab responses toward common epitopes on V3JRFL that represents a subtype B consensus V3 sequence.
Although the gp120/654-D complexes have been shown consistently to be superior immunogens as compared to gp120 alone, the use of anti-CD4bs mAb 654-D to form the immune complexes may not be ideal. The mAb 654-D has been selected because of its capacity to enhance the antigenicity of gp120 and especially of the V3 loop. However, the mAb 654-D blocks the binding of other mAbs to the CD4 binding site and the bridging sheet, which are two highly conserved gp120 regions required for HIV-1 interaction with CD4 and the chemokine receptor, respectively ([39,40] and unpublished data). Indeed, our previous studies show that antibodies to these two regions were not detected in sera of animals immunized with the gp120/654-D complex [1]. Moreover, the binding of mAb 654-D and several other mAbs to the CD4bs has been shown to inhibit antigen processing and MHC class II presentation of gp120 from several HIV-1 isolates, resulting in poor recognition of these gp120 antigens by CD4 T cells [15,16,41]. Consistent with these in vitro findings, our recent study further shows that immunization of mice with the gp120LAI/654-D complex resulted in lower levels of lymphoproliferation than immunization with uncomplexed gp120LAI [2]. Nevertheless, further studies are needed to determine if in vivo helper T cell responses to gp120JRFL is similarly suppressed by mAb 654-D. In future studies, mAbs with other specificities should also be evaluated in order to identify those that can augment the immunogenicity of specific neutralizing epitopes on gp120 without preventing access and induction of Abs to the binding sites for CD4 and the chemokine receptors and without negative effects on anti-gp120 helper T cell responses. Since the CD4 binding site and the chemokine receptor binding site which includes both V3 and the bridging sheet are masked to different degrees and are not fully accessible to Abs in the vast majority of primary HIV-1 isolates [31,42,43], mAbs that better expose epitopes in these three critical regions of gp120 will be good candidates. The potential candidates are anti-V2 mAbs, some of which have been shown to enhance the exposure of certain epitopes on V3 and the CD4bs [44]. In agreement with this earlier report, our study demonstrates that the binding of anti-V2 mAbs indeed enhances V3 antigenicity, without blocking the CD4bs (Fig. 2C and data not shown). The work to test immunogenicity of immune complexes made of anti-V2 mAbs is now in progress.
This study also demonstrates that the gp120/654-D complex alone is inadequate to elicit virus-neutralizing Abs when administered by parenteral route of immunization without any adjuvant (Fig. 3C). This contrasts with previous reports on immune complex vaccines against F. tularensis, infectious bursal disease virus, and hepatitis B virus that elicited protective immunity even without any additional adjuvant [4,7,10]. Notably, the immune complex vaccine against F. tularensis was administered intranasally to elicit mucosal immunity for protection against this virulent intracellular mucosal pathogen [10]. In case of the immune complex vaccine against hepatitis B virus, adjuvants could be added to skew the anti-viral immune responses from Th2-like toward Th1-like [4]. The reason for the difference is not known at the moment. One likely explanation is our use of a human mAb 654-D (IgG1) to form the immune complex, which was tested in the murine system. Human IgG1 may not bind as efficiently to mouse FcRs, as the association constant of human IgG1 Fc binding to mouse splenic macrophages was found to be ~30x lower than that to human peripheral monocytes [45]. Moreover, different binding modes were reported for human IgG1 Fc binding to mouse vs. human Fc receptors expressed on mononuclear cells [45,46]. Hence, the enhancing effects observed with the gp120/654-D complexes may be in large part only due to the Fab-mediated activity of mAb 654-D to better expose or stabilize of the neutralizing epitopes on gp120 and particularly the V3 loop. If this idea is proven to be correct, immune complexes made with human anti-gp120 mAbs are expected to be much more potent immunogens in humans, where the Fc-mediated activity would work optimally. Nevertheless, the contribution of Fab vs. Fc fragments in enhancing the immunogenicity of the gp120/mAb complexes remains unclear and needs further investigation.
In summary, the gp120/mAb complexes are superior immunogens as compared to gp120 alone. The immune complexes display enhanced antigenicity and immunogenicity of the neutralizing V3 epitopes. The use of gp120JRFL bearing the subtype B consensus V3 sequence to form the immune complex vaccine increases the cross-reactivity of Abs generated in the immune sera, but the neutralization is restricted to the highly sensitive viruses. Therefore, in order to construct more effective immune complex-based vaccines, further improvements on this vaccine platform are warranted to significantly boost the Ab titers and expand the breadth of neutralizing Abs toward the more resistant HIV-1 isolates.
Acknowledgments
The authors would like to thank Ms. Diana Virland for performing flow cytometry analyses. The work was supported by a Merit Review Award and the Research Enhancement Award Program of the U. S. Department of Veterans Affairs, the New York University Center for AIDS Research Immunology Core (AI-27742), and by NIH grant AI-48371 (C.E.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Visciano ML, Tuen M, Gorny MK, Hioe CE. In vivo alteration of humoral responses to HIV-1 envelope glycoprotein gp120 by antibodies to the CD4-binding site of gp120. Virology. 2008;372(2):409–420. doi: 10.1016/j.virol.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visciano ML, Tuen M, Chen PD, Hioe CE. Antibodies to the CD4-binding site of HIV-1 gp120 suppress gp120-specific CD4 T cell response while enhancing antibody response. Infect Agent Cancer. 2008;3:11. doi: 10.1186/1750-9378-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao HX, Alam SM, Mascola JR, et al. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoproteins. J Virol. 2004;78(10):5270–5278. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCluskie MJ, Wen YM, Di Q, Davis HL. Immunization against hepatitis B virus by mucosal administration of antigen-antibody complexes. Viral Immunol. 1998;11(4):245–252. doi: 10.1089/vim.1998.11.245. [DOI] [PubMed] [Google Scholar]
- 5.Xu DZ, Zhao K, Guo LM, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE. 2008;3(7):e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen YM. Antigen-antibody immunogenic complex: promising novel vaccines for microbial persistent infections. Expert Opin Biol Ther. 2009;9(3):285–291. doi: 10.1517/14712590802715749. [DOI] [PubMed] [Google Scholar]
- 7.Ivan J, Velhner M, Ursu K, et al. Delayed vaccine virus replication in chickens vaccinated subcutaneously with an immune complex infectious bursal disease vaccine: quantification of vaccine virus by real-time polymerase chain reaction. Can J Vet Res. 2005;69(2):135–142. [PMC free article] [PubMed] [Google Scholar]
- 8.Alber DG, Killington RA, Stokes A. Solid matrix-antibody-antigen complexes incorporating equine herpesvirus 1 glycoproteins C and D elicit anti-viral immune responses in BALB/c (H-2K(d)) and C3H (H-2K(k)) mice. Vaccine. 2000;19(7–8):895–901. doi: 10.1016/s0264-410x(00)00222-x. [DOI] [PubMed] [Google Scholar]
- 9.Roic B, Cajavec S, Ergotic N, et al. Immune complex-based vaccine for pig protection against parvovirus. J Vet Med B Infect Dis Vet Public Health. 2006;53(1):17–23. doi: 10.1111/j.1439-0450.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 10.Rawool DB, Bitsaktsis C, Li Y, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180(8):5548–5557. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corradin G, Engers HD. Inhibition of antigen-induced T-cell clone proliferation by antigen-specific antibodies. Nature. 1984;308(5959):547–548. doi: 10.1038/308547a0. [DOI] [PubMed] [Google Scholar]
- 12.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181(6):1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manca F, Fenoglio D, Kunkl A, Cambiaggi C, Sasso M, Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988;140(9):2893–2898. [PubMed] [Google Scholar]
- 14.Williams JG, Tomer KB, Hioe CE, Zolla-Pazner S, Norris PJ. The antigenic determinants on HIV p24 for CD4+ T cell inhibiting antibodies as determined by limited proteolysis, chemical modification, and mass spectrometry. J Am Soc Mass Spectrom. 2006;17(11):1560–1569. doi: 10.1016/j.jasms.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Chien PC, Jr, Cohen S, Tuen M, et al. Human immunodeficiency virus type 1 evades T-helper responses by exploiting antibodies that suppress antigen processing. J Virol. 2004;78(14):7645–7652. doi: 10.1128/JVI.78.14.7645-7652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuen M, Visciano ML, Chien PC, Jr, et al. Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation. Eur J Immunol. 2005;35(9):2541–2551. doi: 10.1002/eji.200425859. [DOI] [PubMed] [Google Scholar]
- 17.Bouige P, Iscaki S, Cosson A, Pillot J. Molecular analysis of the modulatory factors of the response to HBsAg in mice as an approach to HBV vaccine enhancement. FEMS Immunol Med Microbiol. 1996;13(1):71–79. doi: 10.1111/j.1574-695X.1996.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 18.Oli MW, Rhodin N, McArthur WP, Brady LJ. Redirecting the humoral immune response against Streptococcus mutans antigen P1 with monoclonal antibodies. Infect Immun. 2004;72(12):6951–6960. doi: 10.1128/IAI.72.12.6951-6960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isoda R, Robinette RA, Pinder TL, McArthur WP, Brady LJ. Basis of beneficial immunomodulation by monoclonal antibodies against Streptococcus mutans adhesin P1. FEMS Immunol Med Microbiol. 2007;51(1):102–111. doi: 10.1111/j.1574-695X.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 20.Zolla-Pazner S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol. 2004;4(3):199–201. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 22.DeVico AL. CD4-induced epitopes in the HIV envelope glycoprotein, gp120. Curr HIV Res. 2007;5(6):561–571. doi: 10.2174/157016207782418560. [DOI] [PubMed] [Google Scholar]
- 23.Moore PL, Crooks ET, Porter L, et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80(5):2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blish CA, Dogan OC, Derby NR, et al. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82(24):12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82(23):11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83(2):757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Svehla K, Louder MK, et al. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83(2):1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. Viral complementation allows HIV-1 replication without integration. Retrovirology. 2008;5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore JP, McCutchan FE, Poon SW, et al. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68(12):8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinter A, Honnen WJ, Racho ME, Tilley SA. A potent, neutralizing human monoclonal antibody against a unique epitope overlapping the CD4-binding site of HIV-1 gp120 that is broadly conserved across North American and African virus isolates. AIDS Res Hum Retroviruses. 1993;9(10):985–996. doi: 10.1089/aid.1993.9.985. [DOI] [PubMed] [Google Scholar]
- 31.Lusso P, Earl PL, Sironi F, et al. Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains. J Virol. 2005;79(11):6957–6968. doi: 10.1128/JVI.79.11.6957-6968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol. 2006;80(14):7127–7135. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Cleveland B, Klots I, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82(2):638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getahun A, Heyman B. How antibodies act as natural adjuvants. Immunol Lett. 2006;104(1–2):38–45. doi: 10.1016/j.imlet.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zolla-Pazner S. Improving on nature: focusing the immune response on the V3 loop. Hum Antibodies. 2005;14(3–4):69–72. [PubMed] [Google Scholar]
- 37.Cardozo T, Kimura T, Philpott S, Weiser B, Burger H, Zolla-Pazner S. Structural basis for coreceptor selectivity by the HIV type 1 V3 loop. AIDS Res Hum Retroviruses. 2007;23(3):415–426. doi: 10.1089/aid.2006.0130. [DOI] [PubMed] [Google Scholar]
- 38.Cardozo T, Swetnam J, Pinter A, et al. Worldwide distribution of HIV type 1 epitopes recognized by human anti-V3 monoclonal antibodies. AIDS Res Hum Retroviruses. 2009;25(4):441–450. doi: 10.1089/aid.2008.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karwowska S, Gorny MK, Buchbinder A, et al. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992;8(6):1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 40.Raja A, Venturi M, Kwong P, Sodroski J. CD4 binding site antibodies inhibit human immunodeficiency virus gp120 envelope glycoprotein interaction with CCR5. J Virol. 2003;77(1):713–718. doi: 10.1128/JVI.77.1.713-718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hioe CE, Tuen M, Chien PC, Jr, et al. Inhibition of human immunodeficiency virus type 1 gp120 presentation to CD4 T cells by antibodies specific for the CD4 binding domain of gp120. J Virol. 2001;75(22):10950–10957. doi: 10.1128/JVI.75.22.10950-10957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong PD, Doyle ML, Casper DJ, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 43.Labrijn AF, Poignard P, Raja A, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77(19):10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70(3):1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratcliffe A, Stanworth DR. The localization of the binding site(s) on human IgG1 for the Fc receptors on homologous monocytes and heterologous mouse macrophages. Immunology. 1983;50(1):93–100. [PMC free article] [PubMed] [Google Scholar]
- 46.Ratcliffe A, Stanworth DR. The use of synthetic gamma-chain peptides in the localization of the binding site(s) on human IgG1 for the Fc receptors of homologous monocytes and heterologous mouse macrophages. Immunol Lett. 1982;4(4):215–221. doi: 10.1016/0165-2478(82)90017-7. [DOI] [PubMed] [Google Scholar]