Abstract
Heme oxygenase-1 (HO-1) catalyzes the oxidative degradation of heme to biliverdin, carbon monoxide, and free iron in a reaction requiring the interaction of HO-1 with NADPH-cytochrome P450 reductase (CPR). HO-1 is bound to the endoplasmic reticulum by 23 C-terminal amino acids; however, a soluble HO-1 (sHO-1) lacking this membrane spanning region has been extensively studied. The goal of this project was to characterize the effect of the C-terminal hydrophobic domain on formation of the HO-1/CPR complex. Full-length HO-1 was shown to exhibit higher reaction rates than sHO-1, particularly at subsaturating CPR; indicating that the C-terminal region influences HO-1 binding to CPR. The increased activity of HO-1 was attributable to a time dependent formation of a low Km HO-1/CPR complex that was not seen with sHO1. Gel filtration analysis confirmed the formation of multiple high molecular weight complexes in the presence and absence of the synthetic lipid dilauroylphosphatidylcholine (DLPC). However, the largest complex appeared following a two hour incubation of HO-1 and CPR in DLPC, suggesting that C-terminal region was required for the high affinity HO-1/CPR complex formation and membrane incorporation. These data demonstrate that the C-terminal region of HO-1 influenced complex formation and ultimately its affinity for CPR.
The oxidative degradation of Fe-protoporphyrin IX (heme) by microsomal heme oxygenase (HO) generates biliverdin, carbon monoxide (CO), and free iron (1) in a reaction that requires seven electrons and three molecules of oxygen (2). In order to receive the necessary reducing equivalents for this oxidative cleavage, HO must associate with NADPH-cytochrome P450 reductase (CPR) (3). The cytosolic enzyme, biliverdin reductase (BVR) reduces biliverdin to bilirubin, which following glucuronidation is more readily excreted (4;5). Two definitive isoforms of heme oxygenase are responsible for maintaining heme homeostasis, which is essential due to the detrimental effects of free heme on various cell types and tissues (6). HO-1 is induced by a variety of stimuli (7;8), and provides an initial defense mechanism against subsequent inflammation (9–11) and oxidative stress (12). The cytoprotective role of HO-1 has long been attributed to biliverdin and bilirubin (13;14), but recently CO has also been described as essential to this response. Not only has CO emerged as a potent gaseous signaling molecule similar to nitric oxide (15;16), but recent reports also demonstrate its anti-inflammatory effects (11). HO-2, a constitutive isoform expressed mainly in the brain and testis, is thought to be the major source of CO in these tissues (8;17;18). There have been reports of the existence of a third HO-1 isoform; however, the function and character of this form remain a point of debate (19–22).
HO-1 exists largely in the spleen and liver (23) bound to the endoplasmic reticulum membrane via its hydrophobic, C-terminal membrane-spanning region (24;25). Recombinant rat (26) and human (27) HO-1 (sHO-1), devoid of this C-terminal membrane anchor, can be rapidly purified with a superior yield compared to earlier purification procedures (28–31). As a result, truncated rat and human HO-1 have offered valuable insight on the mechanism and structure of HO-1. Crystal structures of rat/human sHO-1 in the apo form (32;33) as well as in complex with heme (34;35), verdoheme (36), biliverdin-iron chelate (37), and biliverdin (38), have been solved. Detailed kinetic studies of sHO-1 have been reported and include rate constants for heme degradation and subsequent intermediate and product formation (2;39). In single turnover studies, reported by Liu and coworkers, biliverdin release from the sHO-1 active site is the rate limiting step in heme degradation; however, in the presence of BVR, the rate limiting step becomes the conversion of Fe2+-verdoheme to Fe3+-biliverdin (2).
Bilirubin formation is a multi-step reaction thought to require the formation of an enzyme complex between HO-1,CPR, and BVR (40–42). Site specific mutagenesis, as well as fluorescence resonance energy transfer and mass spectrometric experiments, have revealed what appear to be either shared or overlapping binding sites for CPR and BVR on sHO-1; suggesting a potential competition between the two reductases (43;44). Other recent studies demonstrated that NADP(H) increased the binding affinity of CPR for sHO-1, which allowed CPR to compete more effectively than BVR for the mutual binding site on HO-1 (45). Using soluble HO-1 and CPR, devoid of the membrane binding domain, these studies revealed that 1) a competition between CPR and BVR for a common binding site on HO-1 exists and 2) this competition can be influenced by cofactors such as NADP(H).
Recently, we reported on the generation and characterization of a full-length human HO-1 by site directed mutagenesis of arginine 254 to lysine. This conservative modification permits the retention of the C-terminal membrane spanning region and increases the stability of the resulting protein. HO-1 was shown to possess the same catalytic characteristics as the wild-type full-length protein. Interestingly, HO-1 appeared to be quite different than the sHO-1, exhibiting a significantly higher catalytic activity and more avid binding to CPR (46).
In the present study, the catalytic characteristics of HO-1 were further examined. Our results clearly demonstrate that retention of the C-terminal membrane spanning region not only promoted association with the lipid membrane, but also influenced the interaction between HO-1 and CPR leading to the formation of a complex between CPR and HO-1 having a much lower Km value when compared to soluble HO-1. Size exclusion chromatography of HO-1 and sHO-1 along with detailed kinetic analysis has established that retention of the hydrophobic membrane anchor both supports the formation of high molecular weight complexes in purified HO-1 when in solution, and the efficient incorporation of the protein into phosphatidylcholine membranes. Furthermore, these studies also clearly illustrate that the HO-1 complexes enhanced the association with CPR, permitting the formation of time-dependent, high affinity HO-1/CPR complexes. Preincubation of HO-1 with CPR in a lipid environment is required for the formation of a stable, high affinity complex between CPR and HO-1.
EXPERIMENTAL PROCEDURES
Materials
Heme, NADPH, EDTA, glycerol, dilauroylphosphatidylcholine (DLPC), and bovine serum albumin (BSA) were purchased from Sigma. Triton X-100 was obtained from Acros. The AKTA© FPLC system and the gel filtration columns used for size exclusion analysis (Superose 6 and Superdex 200) were purchased from GE Healthcare Life Sciences. All spectrophotometric analyses of HO-1 activity were performed on a SpectraMax M5 plate reader from Molecular Devices (Sunnyvale, CA).
Enzymes
Recombinant, full-length, human HO-1 containing the single site R254K mutation and truncated human HO-1 (provided by Dr. Paul Ortiz de Montellano, University of California – San Francisco), lacking the C-terminal hydrophobic tail, were expressed in E. coli and purified as previously described (26;27;46). The full-length HO-1 expression system was kindly provided by Dr. Mahin Maines (University of Rochester), and mutated at position 254 as described previously (46). Both HO-1 and sHO-1 were purified in the apo-form and quantified using the heme titration method (47). In this manuscript, HO-1 refers to the R254K mutant containing the C-terminal membrane binding domain as described previously (46), whereas sHO-1 refers to the form lacking this C-terminal region. Unless otherwise stated, the HO-1 preparations contain heme. Recombinant rat CPR (provided by Dr. Grover P. Miller, UAMS) with a single amino acid mutation of K56Q was expressed in C41 cells, purified and quantified according to previously described methods (48;49). Rat liver cytosol served as the source for partially purified biliverdin reductase (BVR) and was prepared and quantified as previously reported (4;50).
Gel Filtration Analysis of HO-1
Size exclusion chromatography was used to verify successful incorporation of HO-1 into the DLPC membranes. DLPC liposomes had an estimated molecular size of greater than 5 MDa. Samples were injected onto a Superose 6 10/300 GL (GE Healthcare Life Sciences) size exclusion column that had been pre-equilibrated in 0.05 M KPO4, pH 7.25, 0.1 M NaCl, and 0.1 mM EDTA at a flow rate of 0.3 ml/min. The elution profile of HO-1 that had not been preincubated in DLPC served as a control. sHO-1 (1–2 nmol), lacking the membrane binding region, was treated in a similar fashion as the HO-1 and injected on the size exclusion column. HO-1 and sHO-1 were reconstituted with equimolar hemin prior to any treatment in order to visualize the elution profile at 405 nm.
HO-1 (1 nmol) and CPR (1 nmol) that were preincubated for 0 and 120 minutes in DLPC were also injected onto the size exclusion column. sHO-1 incubated with CPR served as a control. To demonstrate the ability of HO-1 to draw CPR into the membrane, CPR (1 nmol) was incubated with increasing levels of HO-1 (0, 4, and 8 nmol) in DLPC for 2 hours prior to injection. Fractions were collected from the elute and the location of CPR was determined by monitoring the rate of cytochrome c reduction (51).
Preparation of reconstituted systems
HO-1 was reconstituted into DLPC liposomes according to a previously described method for P450 reconstitution (52). DLPC was prepared in a 5 mM stock solution in 50 mM potassium phosphate buffer, pH 7.25, containing 20% glycerol, 0.1 M NaCl, and 5 mM EDTA (DLPC sonication buffer) (53). The DLPC stock solution was clarified by water-bath sonication for approximately 20 minutes. CPR, HO-1 and DLPC were preincubated as a concentrated solution (in a minimal volume) for 2 hr unless otherwise stated. The molar DLPC:HO-1 was 320:1, similar to the preincubation conditions involving various cytochrome P450 enzymes (54). After preincubation, the reconstituted systems were mixed with the other assay components.
In the initial characterization experiments, the reconstituted systems (RCS) with constant HO-1 consisted of 0.1 µM HO-1, 0–0.5 µM CPR and 16 µM DLPC, which incubated for two hours at room temperature prior to the addition of the other assay components. To determine the effects of lipid on HO-1 activity, HO-1 and CPR were incubated together with and without DLPC for various time points (0, 20, 60, 120 minutes). In the CPR-dependent complex-formation experiments, RCS in which HO-1 or CPR were absent from the preincubation procedure were also made; however the omitted protein was added just prior to initiation of the reaction. During the reconstitution process, the HO-1 concentration was greater than 4 µM, and each system was brought to equivalent volume with DLPC sonication buffer to normalize CPR concentrations, where necessary.
Measurement of Heme Oxygenase activity
HO-1 catalytic activity was calculated by monitoring the rate of bilirubin formation, according to previously published methods (27;29;46). Subsequent to the designated preincubation time, partially purified rat BVR (40U/ml), heme (15 µM), and 0.1 M KPO4, pH 7.4 (0.25mg/ml BSA) were added to the reconstituted systems to a final volume of 0.1 ml. A 2.5 mM heme stock was prepared as previously prescribed (47). The levels of BVR used in this assay were saturating with either full-length HO-1 or sHO-1, based on preliminary characterization of the assay system. The reactions were preincubated for 2 minutes at 37° C and initiated by the addition of 0.5 mM NADPH. The increase at 468 nm corresponded to bilirubin formation, which remained linear for at least 5 minutes. HO-1 activity was quantified using an extinction coefficient of 43.5 mM−1 cm−1 and reported in nmoles of bilirubin hr−1 nmol−1 HO-1.
Other methods
The eluted fractions from the FPLC that were derived from incubations of the HO-1 with and without DLPC were analyzed via immunoblotting. The samples were separated by electrophoresis in 10% SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were probed with a human HO-1 primary antibody from a mouse host (U.S. Biological), blocked and treated with an anti-mouse-HRP conjugate (Sigma) as a secondary antibody and visualized by chemiluminescence (Pierce). Protein concentrations were determined using a BCA protein assay kit purchased from Pierce according to published methods (55).
RESULTS
Characterization of HO-1 using gel filtration
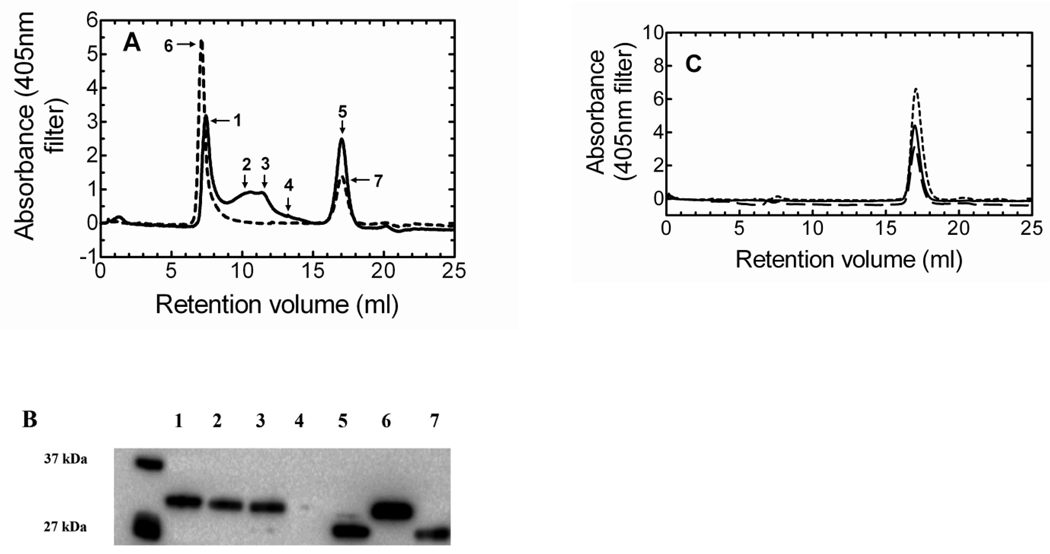
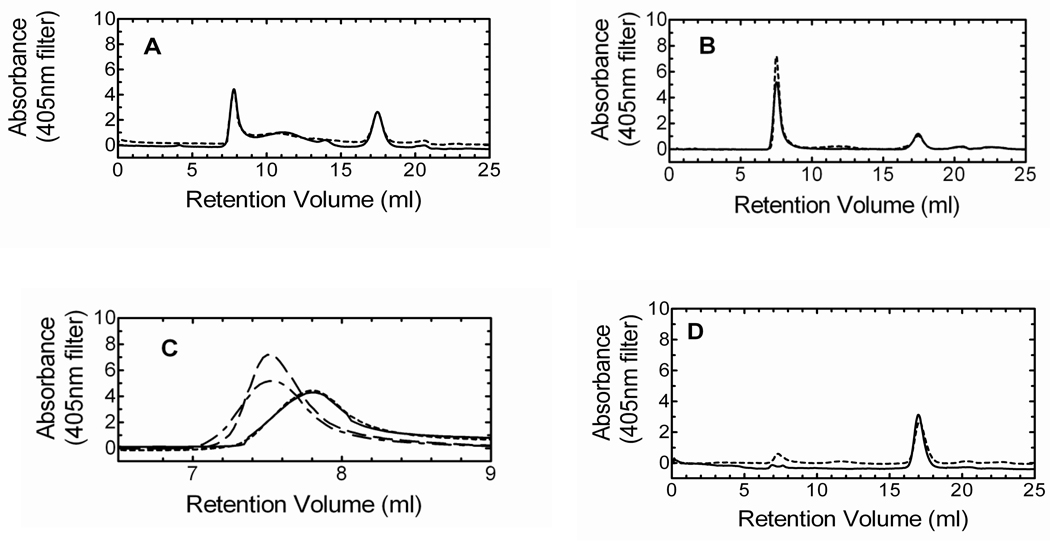
To verify reconstitution of HO-1 into DLPC liposomes, we applied the HO-1/heme complex to a Superose 6 size exclusion column. The Superose 6 column has an exclusion limit of 5 million Daltons, so a complex in excess of this molecular weight would elute in the void volume, indicating reincorporation of the protein into the lipid. In the initial experiments, we wanted to examine the elution profile of HO-1/heme in the absence of other proteins. As seen in Fig. 1A, multiple high molecular weight complexes ranging from ~1 to < 5 million Daltons existed in the HO-1 sample in an aqueous solution regardless of incubation time (2 hour incubation of HO-1 without DLPC – Supplementary Figure A). When HO-1 was preincubated with DLPC for 2 hours, the high molecular weight complexes shifted into the void volume (>5 million Daltons), forming one major peak (peak 6). A smaller peak that eluted late in both chromatograms was estimated to have a molecular weight of ~26–28kDa.
Figure 1. Gel filtration and Western blot analysis demonstrating the formation of high molecular weight HO-1 complexes.
A, The size exclusion chromatogram shows HO-1 complex formation following: no incubation without DLPC (———); and 2hr incubation with DLPC (- - - - -). B, Western blot of the peaks following gel filtration of HO-1 displayed the HO-1 in the earlier fractions (lanes 1–3) and a truncated HO-1 in the later fraction (lane 5). Following the 2hr incubation of HO-1 in DLPC only two peaks were observed. The first peak (lane 6) contained only HO-1, whereas the later fraction consisted of truncated HO-1 (lane 7). The unmarked lane served as molecular weight markers. C, sHO-1 size exclusion gel filtration: no incubation (———), sHO-1 and DLPC no incubation (— — —), and sHO-1 and DLPC 2hr incubation (- - - - -).
The movement of HO-1 into the void volume after 2 hours was corroborated by immune blot analysis of the respective peaks, as shown in Fig. 1B. The immune blots clearly indicated that, without prolonged incubation with DLPC, a high concentration of HO-1 was detected in multiple high molecular weight peaks (lanes 1–3). After the 2 hour incubation in DLPC, the void volume contained a concentrated level of HO-1 (lane 6). The small contaminant that eluted late in both chromatograms was shown to be degraded HO-1 (lanes 5 and 7). Degradation can be minimized by inclusion of protease inhibitors and delicate handling of the protein.
To determine if the C-terminal binding segment of HO-1 was essential for complex formation and lipid reconstitution into DLPC, sHO-1, which lacks the 23 amino acids comprising the C-terminal hydrophobic membrane binding domain was used. Fig. 1C illustrates the Superose 6 size exclusion chromatogram of sHO-1 and the effect of DLPC preincubation. Unlike the HO-1, there were no complexes present in the sHO-1 sample. Also, neither the addition of DLPC nor the preincubation procedure with DLPC caused a shift of the sHO-1 peak into the void volume. Using a Superdex 200 column, the single peak for all three sHO-1 chromatograms was calculated to be ~30 kDa; which is consistent with the molecular weight of monomeric sHO-1. Thus, collectively, the gel filtration results obtained for the HO-1 and sHO-1 demonstrate that the hydrophobic region of HO-1 was responsible for (1) large complex formation among several HO-1 molecules and (2) successful incorporation of HO-1 into the DLPC membranes.
Effect of C-terminal membrane spanning region on HO-1 activity
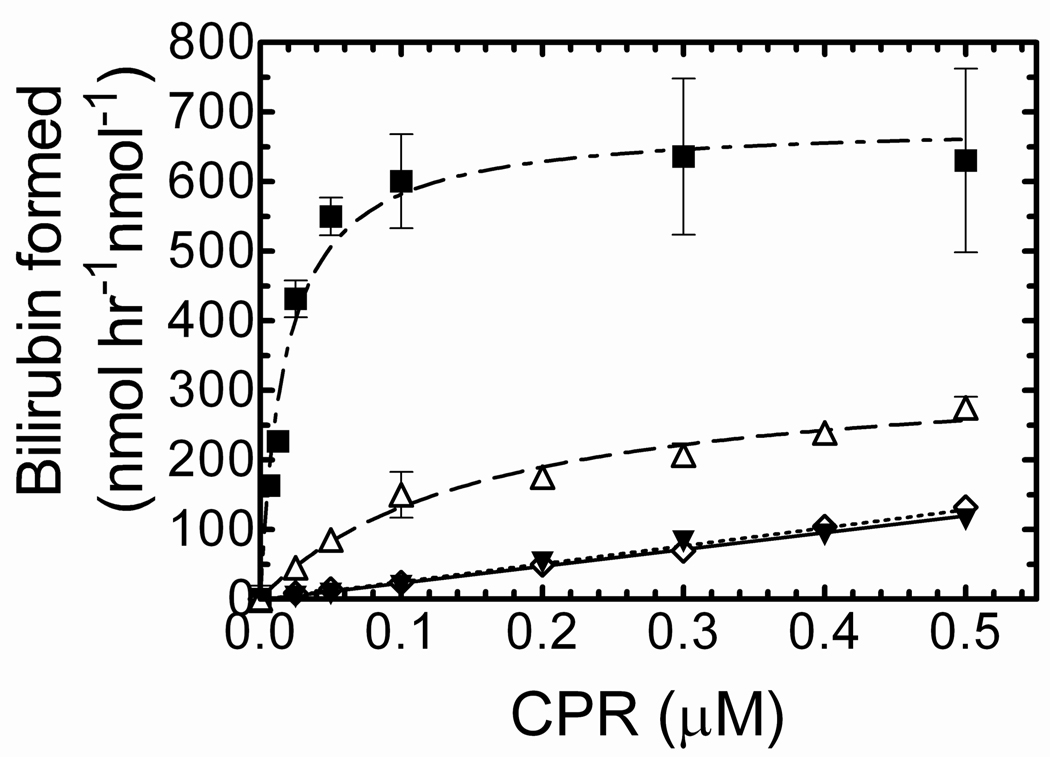
The goal of this experiment was to compare HO-1 and sHO-1 in the presence of a lipid membrane by monitoring the rate of bilirubin formation over a range of CPR concentrations (0 µM–0.5 µM). As shown in Fig. 2, HO-1 activity under “standard” assay conditions (26;29;46), which did not include preincubation of the proteins with DLPC, was increased compared to that measured for sHO-1. In agreement with our previous report (46), presence of the C-terminus of HO-1 also resulted in a lower Km for CPR. The specific activity of full-length HO-1 in the absence of lipid ranged from 200–250 nmol of bilirubin hr−1 nmol−1 HO-1, compared to 100 nmol of bilirubin hr−1 nmol−1 HO-1 for sHO-1 (46).
Figure 2. Full-length and soluble HO-1 activity as a function of CPR concentration.
The rate of bilirubin formation was monitored at 468nm for 0.1 µM purified, E. coli expressed HO-1 and sHO-1 at increasing CPR levels, under the following conditions; CPR was combined with either sHO-1 (▼), or full-length HO-1 (Δ) without DLPC. Under these conditions HO-1 activity was measured without preincubation. CPR was reconstituted with sHO-1 (◊), or full-length HO-1 (■), by preincubation in DLPC for 2 h. After preincubation for 2 h with DLPC, the reconstituted system was combined with the other components and HO-1 activity was measured. Enzyme assays are detailed in “Experimental procedures.” Values represent the mean +/− S.E. of n=3.
Interestingly, when HO-1 was preincubated with DLPC and CPR for two hours at room temperature prior to the assay, the overall activity was significantly increased; particularly at subsaturating CPR levels, where protein ratios were less than 1:4 (CPR:HO-1) (Fig 2). The specific activity levels ranged from 600–650 nmol of bilirubin hr−1 nmol−1 HO-1. However, the augmented activity displayed by reconstituted HO-1 was not observed for sHO-1. sHO-1 showed a linear response to increasing CPR levels similar to that seen with no preincubation and had some tendency to plateau at CPR:sHO-1 levels above 20:1. At these elevated CPR:sHO-1 levels, the reaction rates were comparable to those of full-length HO-1 (not shown). One explanation for these results is that retention of the membrane spanning region increased the HO-1 affinity for CPR, but also that reinsertion of the HO-1 into DLPC facilitated efficient formation of these time-dependent complexes. These results strongly suggest that the overall organization of CPR and HO-1 was quite different with full-length HO-1, but the efficiency of electron transfer was not significantly affected.
Effects of incubation time and DLPC on HO-1 activity
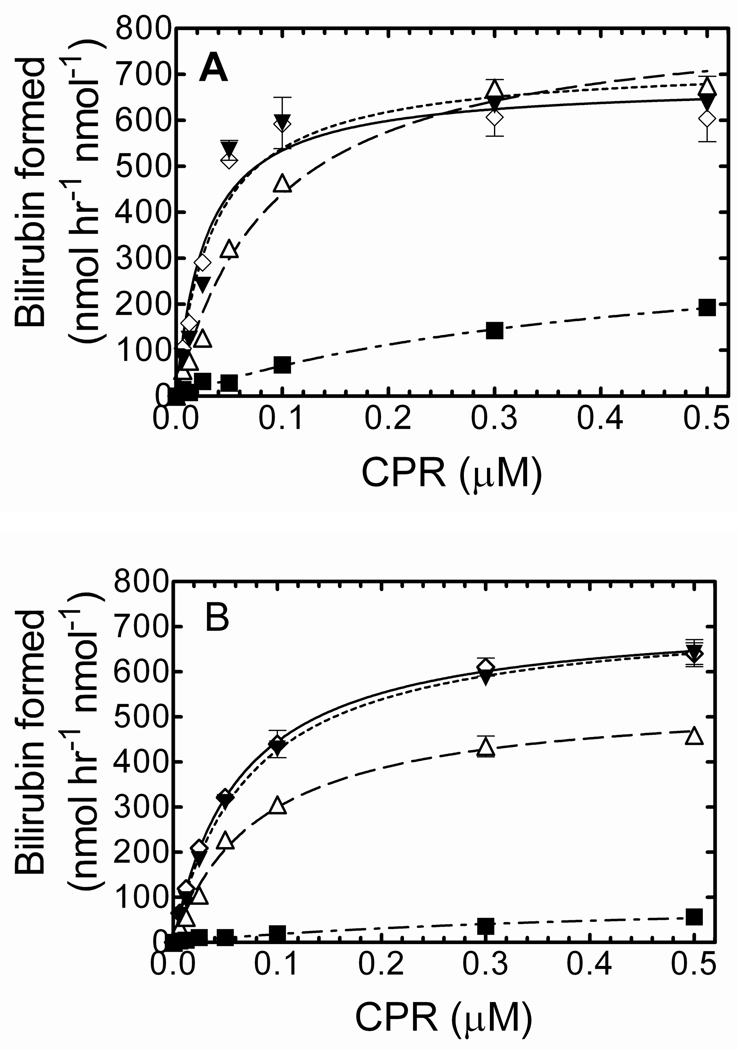
Due to the dramatic catalytic increase following the reincorporation of HO-1 in DLPC, the next experiments were designed to determine if the increase in activity was a result of the preincubation time and/or presence of DLPC. To examine the effect of incubation time on the RCS, HO-1 and CPR were preincubated in DLPC for times ranging from 0–120 min prior to the addition of the remaining assay components. As shown in Fig. 3A, longer incubation periods resulted in a higher rate of bilirubin formation, suggesting a time-dependent complex formation between HO-1 and CPR mediated by reinsertion into the lipid membrane. Interestingly, HO-1 activity also increased with incubation time in the absence of DLPC (Fig. 3B). In both experiments, HO-1 reached its maximal catalytic efficiency following long preincubation periods; however, there was no further increase in activity after preincubation times exceeding 2 hours.
Figure 3. Effect of preincubation time and presence of DLPC on HO-1-dependent rate of bilirubin formation.
HO-1 (0.1 µM) was incubated prior to the activity assay with various levels of CPR ranging from 0 – 0.5 µM. Incubation times included 0 min (— - — - — ■), 20 min (— — — Δ), 60 min (——— ▼), and 120 min (- - - - - ◊) periods in the presence (panel A) and absence (panel B) of DLPC. Assay conditions are detailed in “Experimental procedures.” Values represent the mean +/− S.E. of n=3.
Although HO-1 activity showed a time-dependent increase with and without DLPC, catalytic differences were observed when comparing plus and minus DLPC. The addition of DLPC to the RCS caused an immediate increase in bilirubin formation (Fig 3A), which suggested that DLPC augmented HO-1 activity, and possibly affinity for CPR, very rapidly. Following the 2 hour preincubation, HO-1 that was reconstituted into the DLPC displayed a much lower Km for CPR (compare Fig 3A and 3B open diamonds). The HO-1 curves fit a simple hyperbolic model; the apparent Km in the presence of DLPC (2 h preincubation) was approximately 0.017 µM compared to 0.063 µM when DLPC was omitted. These results suggest that HO-1 and CPR retain the ability to associate and form a functional complex in the absence of DLPC, but the apparent affinity of the HO-1 and CPR complex increases when the proteins are reincorporated into the lipid membrane.
Effect of CPR on time-dependent HO-1 complex formation
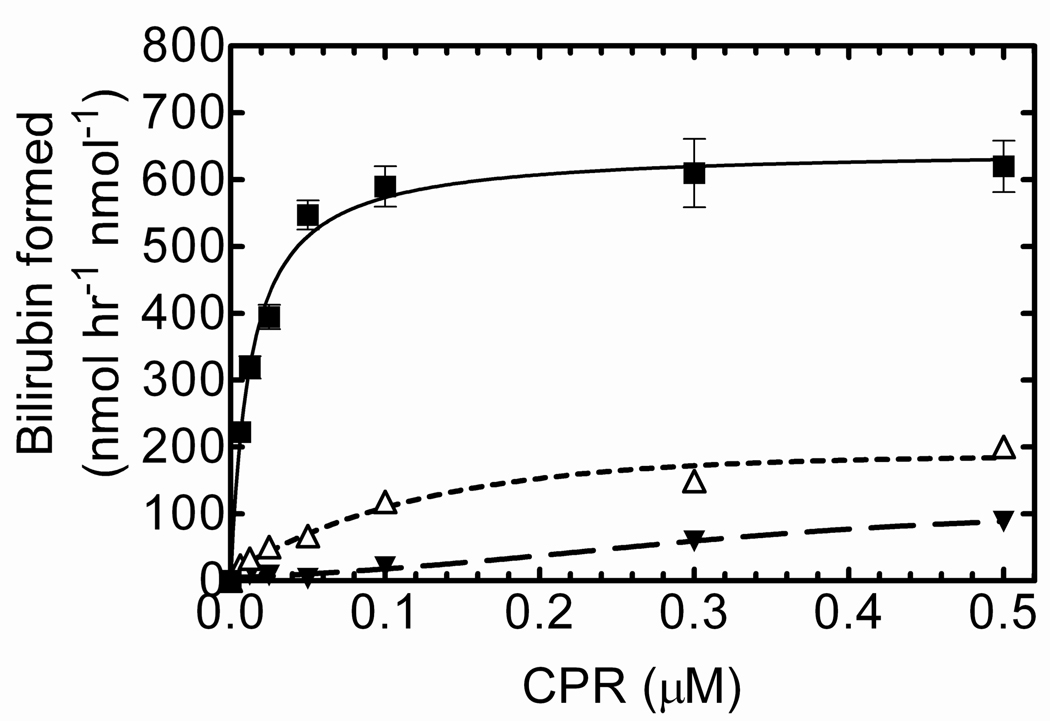
The next set of experiments were designed to determine if the formation of the high affinity complex required the preincubation of HO-1 with CPR in DLPC, or if the preincubation of HO-1 and DLPC alone would allow for complex formation. Although HO-1 existed in a high molecular weight membrane fraction following the 2 hour incubation, it was still unclear as to whether the high affinity CPR-HO-1 complex simply required the time-dependent incorporation of HO-1 with the membrane, or whether both CPR and HO-1 needed to be together during that time. As shown in Fig 4, the RCS system containing HO-1, CPR and DLPC that was preincubated for 2 hours resulted in the high affinity complex formation between HO-1 and CPR. In contrast, when CPR was omitted from the 2 hour preincubation procedure, the activity was significantly lower and did not saturate at 1:1 CPR:HO-1, suggesting that simply reincorporating HO-1 into the membrane did not result in the high affinity complex. Preincubation of CPR alone in DLPC produced a similar response, showing the formation of a lower affinity (i.e. high Km) complex. As shown here, incorporation of HO-1 into the lipid membrane alone was not able to speed the CPR-HO-1 complex formation; co-incubation with CPR was required.
Figure 4. Effect of Preincubation of HO-1 with CPR on the Rate of Bilirubin Formation.
Reconstituted systems containing CPR and DLPC (▼), HO-1 and DLPC (Δ), and HO-1, CPR, and DLPC (■) were preincubated for 2 hrs at room temperature prior to assay. Proteins not included in pre-incubation procedure were added just prior to the initiation of the reaction. The final HO-1 concentration was 0.1 µM, and bilirubin production was monitored at 468nm. Assay details are discussed in “Experimental Procedures.” Values represent the mean +/− S.E. of n=3.
Gel filtration of HO-1/CPR complexes
Kinetic data is consistent with the formation of a complex between CPR, HO-1 when preincubated for 2 h in phospholipid. To further characterize these complexes reconstituted systems were prepared and examined after preincubation for 2 h using size exclusion chromatography. When CPR and HO-1 were combined in DLPC and immediately added to the Superose column, the elution profile was the same as that for HO-1 alone (Fig. 5A); however, following a 2 hour incubation in DLPC, the HO-1 shifted into the void volume as expected. Interestingly, if CPR was included in the preincubation procedure, the complex not only eluted in the void volume, but also increased the absorbance at the peak, which is consistent with both CPR and HO-1 being incorporated into the liposomes (Fig. 5B). Our lab has shown that CPR alone does not efficiently associate with DLPC (56), suggesting that HO-1 may attract CPR into the lipid membrane in the process of complex formation. The differences among the major peaks in Figs. 5A and 5B are depicted in Fig. 5C. These data show that preincubation of HO-1 increased the size of the major HO-1 peak from a < 5 MDa complex to the void volume, which is consistent with the incorporation of HO-1 into the DLPC liposomes. Such behavior is unique to the full length form of HO-1; the soluble form leading to the elution of the 30 kDa form that is not influenced by the presence of DLPC or CPR (Fig. 5D). These data further illustrate the time-dependent formation of a high affinity complex between CPR and full-length HO-1 when reconstituted into preformed DLPC liposomes.
Figure 5. Gel filtration analysis of HO-1/CPR complex formation in reconstituted systems.
HO-1 or HO-1/CPR were reconstituted with DLPC and the effect on complex formation was examined by Superose 6 gel filtration chromatography. The HO-1/heme complex was monitored at 405 nm. A, HO-1 (1 nmol) in the absence (———) and presence (- - - - - - -) of CPR (1 nmol) were combined with DLPC and immediately applied to the gel filtration column. B, HO-1 (1 nmol) in the absence (———) and presence (- - - - - - -) of CPR (1 nmol) were combined with DLPC, preincubated at room temperature for 2 h prior to loading onto the Superose 6 column. C, The void volume regions of Panels A and B showing differences in elution volumes depending on incubation conditions: HO-1 no incubation (———), HO-1/CPR no incubation (- - - - - - - ), HO-1, 2 hr incubation (— - — - —), and HO-1/CPR 2 hr incubation (— — —). D, sHO-1 (1 nmol) was added to DLPC and incubated for 2 hr with (- - - - - - -) and without (———) CPR.
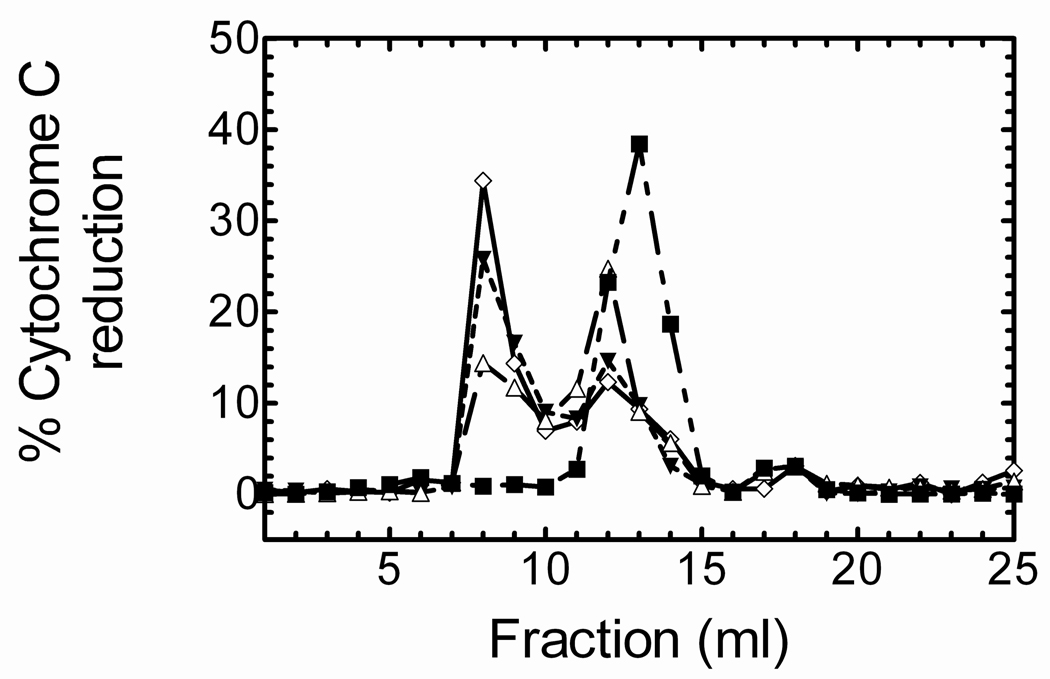
Because CPR did not absorb well at 405 nm, we used cytochrome c reduction to more precisely identify the CPR-containing fractions, and to demonstrate that HO-1 attracted CPR into the liposome. As shown in Fig. 6, CPR alone exists in a high molecular weight aggregate estimated to be ~ 650,000–700,000 Da. When CPR was preincubated in DLPC for 2 hours, about 40% was able to associate with the liposomal fraction; which is consistent with previous reports (56). When HO-1, which efficiently incorporates into the liposomes (Fig. 1), was included in the preincubation procedure at a 4:1 HO-1 to CPR ratio, ~55% of the CPR shifted into the void volume. The inclusion of increasing amounts of HO-1 to the reconstituted systems (8:1 HO-1 to CPR) only marginally increased the amount of CPR localized to the liposomal vesicle fraction (57%). These data demonstrate that preincubation of CPR with DLPC causes the incorporation of some CPR with the phospholipid, and the HO-1 increases the association of CPR with DLPC, providing further evidence for high affinity complex formation between HO-1 and CPR, particularly in phospholipid vesicles.
Figure 6. Effect of HO-1 on the incorporation of CPR in DLPC containing liposomes.
CPR (1 nmol) was injected onto a Superose 6 size exclusion column following: no pre-incubation (— -- — -- — ■), 2hr pre-incubation in DLPC (— — — Δ), 2hr pre-incubation in DLPC and 4 nmol HO-1 (- - - - - ▼), and 2hr pre-incubation in DLPC and 8 nmol HO-1 (——— ◊). Fractions were collected and assayed for the presence of CPR by measuring the rate of cytochrome c reduction.
DISCUSSION
HO-1 is one of many proteins associated with the endoplasmic reticulum that requires an interaction with CPR to receive the necessary reducing equivalents for catalysis, degrading its heme cofactor into biliverdin, CO, and Fe2+. The mechanism and structure of HO-1 has been extensively studied using a soluble rat or human HO-1 lacking the C-terminal membrane anchor. Mutagenesis studies involving multiple residues on sHO-1 have provided important insight on the location and functional significance of binding sites on sHO-1 for constituents such as heme, NADPH, CPR, and BVR (43–45;57;58). Although these structural findings offer valuable information on HO-1, not much is known regarding how this enzyme behaves in the lipid membrane with other resident proteins. Previously, our lab isolated and purified a recombinant, human HO-1, which binds to CPR with a much higher affinity than sHO-1, using commonly reported assay conditions that were performed in the absence of a lipid membrane (46). The overall goal of the present study was to reincorporate HO-1 into a lipid membrane and determine if the phospholipid influenced the catalytic characteristics of HO-1 by incorporating into preformed DLPC liposomes.
Although HO-1 associates with membrane lipid very rapidly following solubilization (59), we wanted to verify that HO-1 was incorporated into the DLPC-containing liposomes, using size exclusion chromatography. The HO-1 that was incorporated into DLPC eluted as a single peak in the void volume indicating a high molecular weight complex in excess of 5 million Daltons. Interestingly, HO-1 without the membrane also eluted as high molecular weight complexes that were likely the result of aggregation of multiple HO-1 molecules due to the presence of the C-terminal hydrophobic region; however, there was a difference in the size of the complex when DLPC was present (Fig. 5C). Because sHO-1 lacks this membrane spanning region, no large hydrophobic complexes were present, nor was sHO-1 able to associate with the DLPC membrane. When HO-1 was pre-treated with Triton X-100 prior to the gel filtration procedure, the high molecular weight complexes disappeared, with concomitant formation of smaller complexes (data not shown).
Having verified that HO-1 successfully incorporated into liposomes, the catalytic behavior of HO-1 in the DLPC membrane was examined. Membrane-bound HO-1 activity was monitored using the traditional coupled assay to monitor bilirubin formation (42). When reconstituted HO-1 was compared to HO-1 and sHO-1 that was not preincubated, it was clear that the preincubation procedure had a significant influence on the Km for CPR as well as the maximal activity. Incubation time, DLPC, and presence of CPR during preincubation all contributed to the increased affinity of CPR for HO-1.
Phospholipids have been shown to influence the activity and conformation of other CPR-dependent enzymes, including multiple P450 enzymes (60;61). Lipid reconstitution is not required for HO-1 activity, but presence of the lipid binding domain alone increases the affinity for CPR (46), and as a consequence has a significant influence on the catalytic behavior of this enzyme. Taken together with the subtle increase in the apparent molecular weight of the HO-1 complex to > 5 MDa after preincubation with phospholipid, it is reasonable to conclude that HO-1 reconstitutes into the membrane in a time-dependent manner and undergoes a conformational change that allows for more avid CPR binding. Although a similar trend was seen in the absence of lipid, comparison of the time courses for reconstitution of HO-1 activity illustrated that the DLPC allowed for the formation of a more avid complex. Thus, optimal complex formation required a 2 h preincubation and the presence of DLPC, which significantly affected the catalytic behavior of this enzyme.
DLPC vesicles containing HO-1 alone could not be combined with separate CPR-containing DLPC vesicles to produce the significant stimulation seen when the two proteins were preincubated together in DLPC (data not shown). In a report describing the binding sites of CPR and BVR on HO-1, Wang and Ortiz de Montellano (43) speculate that reinsertion of the HO-1 and CPR may effectively concentrate the proteins, altering the ability of BVR to compete for the binding site shared by CPR on HO-1. Clearly, our results demonstrate that the membrane environment significantly affects the ability of CPR to interact with HO-1 and supports a potential alteration in the relative ability of BVR to compete with CPR for the HO-1 binding site. These studies are currently in progress.
In conclusion, the results illustrate the importance of the C-terminal binding region of heme oxygenase-1, not only for its membrane binding characteristics, but also for its catalytic behavior. The presence of the C-terminal region leads to the incorporation of HO-1 into the liposomal membrane and to the efficient co-localization of reductase into the membrane. Additionally, catalytic characterization shows that preincubation of both CPR and HO-1 leads to the formation of a high affinity complex, and that the affinity of this complex is increased when incorporated into preformed DLPC vesicles. Additional studies are required to further characterize full-length HO-1 function in the membrane.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr. Mahin Maines and Dr. Paul Ortiz de Montellano for supplying the full-length and truncated hHO-1 expression plasmids, respectively. The rat CPR-K56Q expression plasmid was kindly provided by Dr. Grover P. Miller.
These studies were supported by a US Public Health Service Research Grant from the National Institute of Environmental Health Sciences ES004344 (WLB)
ABBREVIATIONS USED
- HO
Heme oxygenase
- HO-1
full-length, recombinant HO-1 containing R254K mutation
- sHO-1
30-kDa soluble, human HO-1
- CPR
NADPH-cytochrome P450 reductase containing the K56Q mutation
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- CO
carbon monoxide
- DLPC
dilauroylphosphatidylcholine
- EDTA
ethylenediaminetetraacetic acid
- BSA
bovine serum albumin
- RCS
reconstituted systems
- ER
endoplasmic reticulum
- BCA
Bicinchoninic acid
- BVR
biliverdin reductase
Footnotes
SUPPORTING INFORMATION AVAILABLE
The data in Figure 1 of this paper shows a comparison of the characteristics of HO-1 when using standard conditions, which are used throughout the literature (no DLPC and no preincubation), to our conditions where the full-length protein was incorporated into DLPC. Supplemental Figure 1 shows that preincubation of HO-1 for 2 h at room temperature in the absence of the phospholipid DLPC does not affect the size distribution of the HO-1 aggregates as measured by gel-filtration. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 2.Liu Y, Ortiz de Montellano PR. Reaction intermediates and single turnover rate constants for the oxidation of heme by human heme oxygenase-1. J. Biol. Chem. 2000;275:5297–5307. doi: 10.1074/jbc.275.8.5297. [DOI] [PubMed] [Google Scholar]
- 3.Schacter BA, Nelson EB, Marver HS, Masters BS. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol. Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 4.Tenhunen R, Ross ME, Marver HS, Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochem. 1970;9:298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SC, Ziurys JC, Gollan JL. Subcellular distribution and regulation of hepatic bilirubin UDP-glucuronyltransferase. J. Biol. Chem. 1984;259:4527–4533. [PubMed] [Google Scholar]
- 6.Alam J. Heme oxygenase-1: past, present, and future. Antioxid. Redox. Signal. 2002;4:559–562. doi: 10.1089/15230860260220049. [DOI] [PubMed] [Google Scholar]
- 7.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 8.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 9.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 10.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 11.Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 12.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 15.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 16.Stevens CF, Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature. 1993;364:147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 17.Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J. Biol. Chem. 1986;261:11131–11137. [PubMed] [Google Scholar]
- 19.McCoubrey WK, Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–59. doi: 10.1016/s0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang H, Pin S, Li X, Dore S. Regulation of heme oxygenase expression by cyclopentenone prostaglandins. Exp. Biol. Med. (Maywood. ) 2003;228:499–505. doi: 10.1177/15353702-0322805-13. [DOI] [PubMed] [Google Scholar]
- 23.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 24.Yoshida T, Sato M. Posttranslational and direct integration of heme oxygenase into microsomes. Biochem. Biophys. Res. Commun. 1989;163:1086–1092. doi: 10.1016/0006-291x(89)92332-2. [DOI] [PubMed] [Google Scholar]
- 25.Shibahara S, Muller R, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 1985;82:7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilks A, Ortiz de Montellano PR. Rat liver heme oxygenase. High level expression of a truncated soluble form and nature of the meso-hydroxylating species. J. Biol. Chem. 1993;268:22357–22362. [PubMed] [Google Scholar]
- 27.Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochem. 1995;34:4421–4427. doi: 10.1021/bi00013a034. [DOI] [PubMed] [Google Scholar]
- 28.Kutty RK, Maines MD. Oxidation of heme c derivatives by purified heme oxygenase. Evidence for the presence of one molecular species of heme oxygenase in the rat liver. J. Biol. Chem. 1982;257:9944–9952. [PubMed] [Google Scholar]
- 29.Yoshida T, Kikuchi G. Purification and properties of heme oxygenase from pig spleen microsomes. J. Biol. Chem. 1978;253:4224–4229. [PubMed] [Google Scholar]
- 30.Yoshinaga T, Sassa S, Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J. Biol. Chem. 1982;257:7778–7785. [PubMed] [Google Scholar]
- 31.Yoshida T, Kikuchi G. Purification and properties of heme oxygenase from rat liver microsomes. J. Biol. Chem. 1979;254:4487–4491. [PubMed] [Google Scholar]
- 32.Sugishima M, Sakamoto H, Kakuta Y, Omata Y, Hayashi S, Noguchi M, Fukuyama K. Crystal structure of rat apo-heme oxygenase-1 (HO-1): mechanism of heme binding in HO-1 inferred from structural comparison of the apo and heme complex forms. Biochem. 2002;41:7293–7300. doi: 10.1021/bi025662a. [DOI] [PubMed] [Google Scholar]
- 33.Lad L, Schuller DJ, Shimizu H, Friedman J, Li H, Ortiz de Montellano PR, Poulos TL. Comparison of the heme-free and -bound crystal structures of human heme oxygenase-1. J. Biol. Chem. 2003;278:7834–7843. doi: 10.1074/jbc.M211450200. [DOI] [PubMed] [Google Scholar]
- 34.Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1. Nat. Struct. Biol. 1999;6:860–867. doi: 10.1038/12319. [DOI] [PubMed] [Google Scholar]
- 35.Sugishima M, Omata Y, Kakuta Y, Sakamoto H, Noguchi M, Fukuyama K. Crystal structure of rat heme oxygenase-1 in complex with heme. FEBS Lett. 2000;471:61–66. doi: 10.1016/s0014-5793(00)01353-3. [DOI] [PubMed] [Google Scholar]
- 36.Lad L, Ortiz de Montellano PR, Poulos TL. Crystal structures of ferrous and ferrous-NO forms of verdoheme in a complex with human heme oxygenase-1: catalytic implications for heme cleavage. J. Inorg. Biochem. 2004;98:1686–1695. doi: 10.1016/j.jinorgbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Sugishima M, Sakamoto H, Higashimoto Y, Noguchi M, Fukuyama K. Crystal structure of rat heme oxygenase-1 in complex with biliverdin-iron chelate. Conformational change of the distal helix during the heme cleavage reaction. J. Biol. Chem. 2003;278:32352–32358. doi: 10.1074/jbc.M303682200. [DOI] [PubMed] [Google Scholar]
- 38.Lad L, Friedman J, Li H, Bhaskar B, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1 in a complex with biliverdin. Biochem. 2004;43:3793–3801. doi: 10.1021/bi035451l. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Moenne-Loccoz P, Loehr TM, Ortiz de Montellano PR. Heme oxygenase-1, intermediates in verdoheme formation and the requirement for reduction equivalents. J. Biol. Chem. 1997;272:6909–6917. doi: 10.1074/jbc.272.11.6909. [DOI] [PubMed] [Google Scholar]
- 40.Maines MD, Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc. Natl. Acad. Sci. U. S. A. 1974;71:4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshinaga T, Sassa S, Kappas A. The occurrence of molecular interactions among NADPH-cytochrome c reductase, heme oxygenase, and biliverdin reductase in heme degradation. J. Biol. Chem. 1982;257:7786–7793. [PubMed] [Google Scholar]
- 42.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Ortiz de Montellano PRO. The binding sites on human heme oxygenase-1 for cytochrome P450 reductase and biliverdin reductase. J. Biol. Chem. 2003;278:20069–20076. doi: 10.1074/jbc.M300989200. [DOI] [PubMed] [Google Scholar]
- 44.Higashimoto Y, Sugishima M, Sato H, Sakamoto H, Fukuyama K, Palmer G, Noguchi M. Mass spectrometric identification of lysine residues of heme oxygenase-1 that are involved in its interaction with NADPH-cytochrome P450 reductase. Biochem. Biophys. Res. Commun. 2008;367:852–858. doi: 10.1016/j.bbrc.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higashimoto Y, Sakamoto H, Hayashi S, Sugishima M, Fukuyama K, Palmer G, Noguchi M. Involvement of NADPH in the interaction between heme oxygenase-1 and cytochrome P450 reductase. J. Biol. Chem. 2005;280:729–737. doi: 10.1074/jbc.M406203200. [DOI] [PubMed] [Google Scholar]
- 46.Huber WJ, III, Backes WL. Expression and characterization of full-length human heme oxygenase-1: the presence of intact membrane-binding region leads to increased binding affinity for NADPH cytochrome P450 reductase. Biochem. 2007;46:12212–12219. doi: 10.1021/bi701496z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber WJ, III, Backes WL. Quantitation of heme oxygenase 1: heme titration increases yield of purified protein. Anal. Biochem. 2008;373:167–169. doi: 10.1016/j.ab.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasukochi Y, Masters BS. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J. Biol. Chem. 1976;251:5337–5344. [PubMed] [Google Scholar]
- 49.Shen AL, Porter TD, Wilson TE, Kasper CB. Structural analysis of the FMN binding domain of NADPH-cytochrome P-450 oxidoreductase by site-directed mutagenesis. J Biol. Chem. 1989;264:7584–7589. [PubMed] [Google Scholar]
- 50.Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J. Biol. Chem. 1981;256:3956–3962. [PubMed] [Google Scholar]
- 51.Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J. Biol. Chem. 1962;237:2652–2660. [PubMed] [Google Scholar]
- 52.Causey KM, Eyer CS, Backes WL. Dual role of phospholipid in the reconstitution of cytochrome P-450 LM2-dependent activities. Mol. Pharmacol. 1990;38:134–142. [PubMed] [Google Scholar]
- 53.Huang C-H. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochem. 1969;8:344–351. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- 54.Cawley GF, Zhang S, Kelley RW, Backes WL. Evidence supporting the interaction of CYP2B4 and CYP1A2 in microsomal preparations. Drug Metab Dispos. 2001;29:1529–1534. [PubMed] [Google Scholar]
- 55.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 56.Reed JR, Kelley RW, Backes WL. An evaluation of methods for the reconstitution of cytochromes P450 and NADPH P450 reductase into lipid vesicles. Drug Metab Dispos. 2006;34:660–666. doi: 10.1124/dmd.105.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun J, Wilks A, Ortiz de Montellano PR, Loehr TM. Resonance Raman and EPR spectroscopic studies on heme-heme oxygenase complexes. Biochem. 1993;32:14151–14157. doi: 10.1021/bi00214a012. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S, Wang J, Rousseau DL, Ishikawa K, Yoshida T, Host JR, Ikeda-Saito M. Heme-heme oxygenase complex. Structure of the catalytic site and its implication for oxygen activation. J. Biol. Chem. 1994;269:1010–1014. [PubMed] [Google Scholar]
- 59.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Escherichia coli as a catalytically active, full-length form that binds to bacterial membranes. Eur. J. Biochem. 1991;202:161–165. doi: 10.1111/j.1432-1033.1991.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 60.Yun CH, Song M, Kim H. Conformational Change of Cytochrome P450 1A2 Induced by Phospholipids and Detergents. J. Biol. Chem. 1997;272:19725–19730. doi: 10.1074/jbc.272.32.19725. [DOI] [PubMed] [Google Scholar]
- 61.Yun CH, Ahn T, Guengerich FP. Conformational change and activation of cytochrome P450 2B1 induced by salt and phospholipid. Arch. Biochem. Biophys. 1998;356:229–238. doi: 10.1006/abbi.1998.0759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.