Abstract
This study was to explore a potential role of epithelium-derived cytokines in Th17 differentiation. Th17 induction was evaluated by murine CD4+ T cells treated with different combinations of 5 inducing cytokines, or conditioned media of human corneal epithelial cells (HCECs) exposed to a variety of stimuli. Th17 differentiation was determined by measuring Th17 associated molecules, IL-17A, IL-17F, IL-22, CCL-20 and STAT3 at mRNAand protein levels, and numbers of IL-17-producing T cells by real-time PCR, and cytokine immunobead and ELISPOT assays, respectively. IL-23 was the strongest inducer for expanding Th17 cells in the presence of TGF-β1+IL-6; and IL-1β was the strongest Th17 amplifier in the presence of TGF-β1+IL-6+IL-23. These inducing cytokines were found to be significantly stimulated in HCECs challenged by hyperosmotic media (450 mOsM), microbial components (polyI:C, flagellin, R837 and other TLR ligands) and TNF-α. Interestingly, when incubated with conditioned media of HCECs irritated by polyI:C or TNF-α, CD4+ T cells displayed increased mRNA levels of IL-17A, IL-17F, IL-22, CCL-20 and STAT3, increased IL-17 protein in the supernatant, and increased numbers of IL-17-producing T cells (Th17 cells). These findings demonstrate for the first time that Th17 differentiation can be promoted by cytokines produced by corneal epithelium that are exposed to hyperosmotic, microbial and inflammatory stimuli.
Keywords: Th17, epithelial cell, CD4+ T cell
INTRODUCTION
Epithelia are the first line of defense. A long-recognized property of epithelial cells is their physical barrier function against potentially harmful substances and microbial pathogens. Increasing new evidence reveals a critical role of epithelial cells as both initiators and regulators in innate and adaptive immune responses (Kato and Schleimer, 2007;Schleimer et al, 2007). Recently, Th17 has been identified as a new T helper cell subset. Th17 cells produce IL-17, a pro-inflammatory cytokine, presenting an intriguing new bridge between adaptive and innate immunity. Produced by T cells, mediators of adaptive immunity, IL-17 promotes expansion and recruitment of innate immune cells such as neutrophils, and also cooperates with TLR ligands, IL-1β and TNFα to enhance inflammatory reactions (Liang et al, 2006;Weaver et al, 2007;Yu and Gaffen, 2008;Ouyang et al, 2008).
CD4+ T helper (Th) cells now include threedifferent types based on their cytokine signatures: interferon-γ (IFN-γ)-secreting Th1, interleukins (IL)-4, -5, and -13-secreting Th2 (Mosmann and Coffman, 1989), and IL-17-producing Th17 cells (Dong, 2006). Th17 cells appear to be key effector T cells in a variety of human inflammatory and autoimmune diseases as well as experimental animal models (Komiyama et al, 2006;Hwang and Kim, 2005). IL-17A (also known as IL-17) and IL-17F are the founding members of the IL-17 cytokine family. The genes encoding IL-17Aand IL-17F are localized in the same chromosomal region in mice and in humans. But IL-17F has significantly weaker activity than IL-17A (Chang and Dong, 2007;Williams, 2006). It has been recently reported that Th17 cells also produce IL-22 (Liang et al, 2006;Zheng et al, 2007) and CC-chemokine attractant ligand 20 (CCL20) (Hirota et al, 2007) in mice and humans. Therefore, distinct from Th1 and Th2 cells, Th17 cells produce a unique and expanding array of pro-inflammatory products.
The differentiation of Th17 cells from naive CD4+ T cells is regulated by cytokines. Transforming growth factor-β (TGF-β) and IL-6, broadly expressed by many cell types in the body, including dendritic and epithelial cells, are dominant in the initiation of Th17 cell differentiation (Bettelli et al, 2006;Zhou et al, 2007;Mangan et al, 2006;Veldhoen et al, 2006); IL-23, IL-1β and IL-21, which are products of activated dendritic cells, macrophages, activated T cell or inflamed epithelial cells, possibly expand and maintain the differentiated Th17 cells in the presence of IL-6 and TGF-β1 (Hunter, 2005;Manel et al, 2008;Nurieva et al, 2007;Veldhoen et al, 2006;Zhou et al, 2007;Korn et al, 2007). Furthermore, signal transducer and activator of transcription 3 (STAT3) has been found to mediate the initiation of Th17 cell differentiation by these inducing cytokines (Yang et al, 2007).
Th17 has also been found to play an important role in the ocular surface disease. IL-17 actively influences early herpes virus-induced corneal inflammation (Molesworth-Kenyon et al, 2008); and increased expression of Th17 associated cytokines has been found in the ocular surface tissues of human dry eye patients and in an experimental murine dry eye model (de Paiva et al, 2009). It is recognized that mucosal epithelia, including ocular surface epithelia, are sites enriched with Th17 inducing cytokines, including TGF-β1, IL-6, IL-23, and IL-1β (Aujla et al, 2007;Holtta et al, 2008;Ma et al, 2009;de Paiva et al, 2009) However, it is not clear whether certain mucosal epithelia indeed promote Th17 differentiation vis a vis their production of these inducing cytokines. The present study was to investigate the potential role in the induction of Th17 differentiation by cytokines derived from human corneal epithelial cells exposed to hyperosmotic stress, microbial components and inflammatory stimuli, in three in vitro culture models.
MATERIAL AND METHODS
Materials and reagents
Cell culture dishes, plates, centrifuge tubes and other plastic ware were purchased from Becton Dickinson (Lincoln Park, NJ), Dulbecco modified Eagle medium (DMEM), RPMI medium, amphotericin B, and gentamicin were from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). The TLR ligands, Pam3CSK4, peptidoglycan from Staphylococcus aureus (PGN-SA), flagellin from S. typhimurium, synthetic diacylated lipoprotein (FSL-1), imiquimod (R837), single-stranded GU-rich oligonuleotide complexed with LyoVec (ssRNA40/LyoVec), and Type C CpG oligonucleotide (ODN 2395) were purchased from InvivoGen (San Diego, CA). Polyinosinic-polycytidylic acid (polyI:C) and LPS from Escherichia coli were from Sigma-Aldrich (St. Louis, MO). Human recombinant cytokines, TNF-α, TGF- β1, IL-6, IL-23, IL-21 and IL-1β, anti-mouse IL-17 capture antibody, biotinylated goat anti-mouse IL-17 detection antibody and Streptavidin-HRP were from R&D Systems (Minneapolis, MN). Rat anti-mouse CD4-conjugated magneticmicro beads were from MACS system (Miltenyi Biotec). RNeasy Micro Kit from Qiagen (Valencia, CA), Ready-To-Go-Primer First-Strand Beads from GE Health Care, Inc. (Piscataway, NJ), TaqMan™ gene expression assays and real-time PCR master mix from Applied Biosystems (Foster City, CA). Luminex immunobead assay from Upstate-Millipore (Lake Placid, NY).
Human corneal epithelial culture models for inducing Th17 inducing cytokines
Fresh human corneoscleral tissues (<72 hours after death) not suitable for clinical use, from donors aged 19 to 58 years, were obtained from the Lions Eye Bank of Texas (Houston,TX). Human corneal epithelial cells (HCECs) were cultured in 6-well plates with explants from corneal limbal rims in a supplemented hormonal epidermal medium containing 5%FBS (SHEM, 312mOsm) using our previous methods (Solomon et al, 2000;Li et al, 2001;Kim et al, 2004). Corneal epithelial cell growth was carefully monitored, and only the epithelial cultures without visible fibroblast contamination were used for this study. Confluent corneal epithelial cultures were switched to complete RPMI 1640 (RPMI 1640 supplemented with 10% FBS,2mM L-glutamine, 50μg/ml gentamicin, 1.25μg/ml amphotericin B and 50μm 2-ME) and treated for 4–48 hours with (1) hyperosmotic media (450 mOsM), which was achieved by adding 70 mM sodium chloride (NaCl) as previous described (Li et al, 2006); (2) a series of extracted or synthetic microbial components in different concentrations, representing 9 ligands for TLRs 1–9; (3) pro-inflammatory cytokine TNF-α (10ng/ml). Each experiment was repeated at least three times. The cells treated for 4 hours were lysed for total RNA extraction and evaluation of mRNA expression. The conditioned media in the cultures treated for 48 hour were collected and centrifuged in 14000rpm for 10 minutes at 4°C to remove stray cells and debris. The supernatants were then stored at −80°C for Luminex immunobeadassay and further study.
Isolation of murine CD4+ T cells
Animal research was approved by the Center for Comparative Medicine at Baylor College ofMedicine, and it conformed tothe standards in the ARVO Statement for the Use of Animals inOphthalmic and Vision Research.
Murine CD4+ T cells were isolated from the spleens and cervical lymph nodes of 6–8 week-old C57BL/6 mice as described previously (Lutz et al, 1999). In brief, the spleens and cervical lymph nodes of mice were surgically excised, crushed between two sterile frosted glass slides and made into a single cell suspension. Red blood cells in the suspension were lysed with ACT (ammonium chloride) and the suspension was centrifuged at 1000 rpm for 5 minutes, filtered and resuspended with rat anti-mouse CD4-conjugated magneticmicrobeads diluted with cold 0.5% BSA in PBS (1:10 dilution, 90μL of buffer and 10μL of beads for every 1 × 107 cells). After incubation for 15 minutes at 4°C and washing, the cell were resuspended in 500uL buffer per 1 × 108 cells, and loaded onto a micro-column. Positive cells attached to the column were removed with a plunger and the CD4+ T cells were cultured in complete RPMI 1640. The CD4-enriched cell suspensions contained >80% CD4+ T cells as determined by flow cytometry.
In vitro Th17 differentiation of murine CD4+ T cells by various cytokines or HCEC supernatants
The freshly isolated murine CD4+ T cells (5 × 106/ml/well) were cultured in 24-well plates in complete RPMI 1640 (Control) and treated for 4–7 days with (1) various combinations of Th17 inducing cytokines that included TGF-β (10 ng/ml), IL-6 (50 ng/ml), IL-23 (25 ng/ml) and IL-1β (10 ng/ml), (2) conditioned media from unstressed or polyI:C or TNF-α stimulated HCECs. After 4 days, CD4+ T cells were lysed for total RNA extraction and evaluation mRNA expression, and the supernatants were collected for Luminex immunobeadprotein assay. After 7 days, CD4+ T cells were harvested and the number of IL-17-producing cells was evaluated by ELISPOT bioassay.
Total RNA extraction, reverse transcription and quantitative real-time PCR
Total RNA from the HCECs and CD4+ T cells (in 4 independent experiments) was extracted using a Qiagen RNeasy® Micro Kit according to the manufacturer’s instructions, quantified by a NanoDrop® ND-1000 Spectrophotometer, and stored at −80°C. First-strand cDNA was synthesized with random hexamers by Ready-To-Go You-Prime First-strand Beads as previously described (Luo et al, 2004;Yoon et al, 2007).
Real-time PCR was performed with specific TaqMan® Primes and PCR master mix in Mx3005P QPCRSystem (Stratagene). TaqMan® Gene Expression Assay IDs for murine GAPDH, IL-17A, IL-17F, IL-22, CCL20 and STAT3, and human GAPDH, TGF-β1, IL-6, IL-23 and IL-1β, were Mm99999915, Mm00439619, Mm00521423, Mm00444241, Mm00444228, Mm00456961, Hs99999905_m1, Hs00174131_m1, Hs00171257_m1, Hs00372324_m1, and Hs00174097_m1, respectively. The results were analyzed by the comparative threshold cycle (Ct) method and normalized by GAPDH (Yoon et al, 2007;de Paiva et al, 2006).
Luminex immunobead cytokine assay
The levels of total TGF-β1 were measured using Luminex bead assay following acid activation. The levels of IL-6, IL-1β and IL-17 were measured using a multiplex immunobead assay (Millipore-Upstate, Lake Placid, NY). Total protein concentration in 2 day supernatants of HCECs and 4 day supernatants of CD4+ T cells were measured by a Micro BCA protein assay kit. For TGF-β1 assay, 1N HCl was added to lysates for 1 hour, in a shaker, at room temperature, followed by addition of 1N NaOH prior to detection. The samples were added to wells containing 10 μl of the appropriate cytokine bead mixture that included mouse monoclonal antibodies specific for TGF-β1, IL-6, IL-1β and IL-17. Serial dilutions of cytokines were added to wells in the same plate as the supernatant samples to generate a standard curve. The plate was incubated overnight at 4°C to capture the cytokines by the antibody conjugated fluorescent beads. After 3 washes with assay buffer, 25 μl of biotinylated secondary cytokine antibody mixture was applied for 1.5 hours in the dark at room temperature. The reactions were detected with streptavidin-phycoerythrin with a Luminex 100 IS 2.3 system (Austin, TX). Assays were performed on samples from 3 separate experiments and the results were averaged.
ELISPOT assay for IL-17-producing cells
Replicate 50uL cell suspensions containing 1.0×106 CD4+ T cells isolated (as described above) were added to 96-well PVDF plates, precoated with anti-mouse IL-17 or capture antibody. Wells containing either cells or positive controls (3ng/well of recombinant mouse IL-17A) or media alone (negative control) were incubated at 37°C with CO2 for 24 hours in RPMI media. After washing, the plate was incubated overnight at 4°C, with biotinylated goat anti-mouse IL-17 detection antibody, followed by incubation with Streptavidin-HRP, for 2 hours. Red color development was achieved by incubating NovaRed peroxidase substrate (Vector) for 15 minutes. The PVDF membrane was dried and the individual wells were punched out from the plate. The positive, red spots were counted under a dissecting microscope (SMZ 1500, Melville, NY). Replicate wells were averaged from 3 individual experiments. Results are presented as number of spots/1 ×106 cells.
Statistical analysis
Student’s t-test was used to compare difference between two groups. One-way ANOVA test was used to make comparisons among three or more groups, and the Dunnett’s test was further used to compare each treated group with the control group. Statistical significance was retained for P values <0.05.
RESULTS
Induction of Th17 differentiation from CD4+ T cells in vitro by inducing cytokines
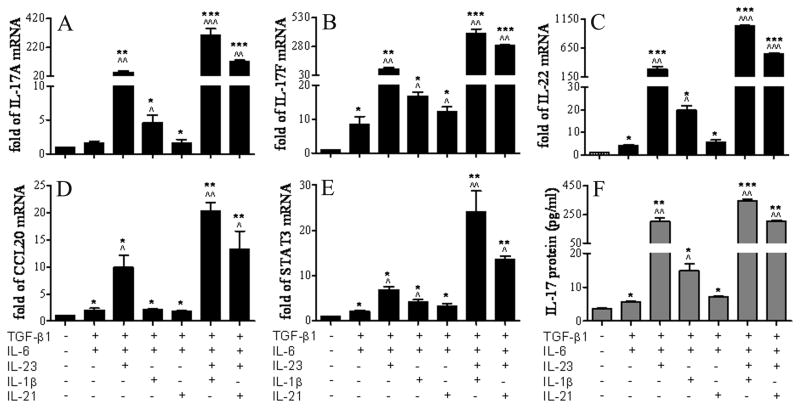
To evaluate the differential effects of currently identified cytokines in promoting differentiation of IL-17 secreting Th17 cells from freshly isolated CD4+ T cells from C57BL/6 mice, CD4+ T cells were cultured in vitro for 4 days with TGF-β1 and IL-6 (Th17 initiation cytokines) in the presence or absence of different combination of IL-23, IL-1β and IL-21 (Th17 expansion cytokines). The profile of Th17 cell expressed cytokines (IL-17A, IL-17F, IL-22 and CCL-20) and transcription regulator STAT3 at the mRNA levels were evaluated by RT and real time PCR, and IL-17 protein level was measured by Luminex immunobead assay.
As shown in Figure 1A-E and Table 1, when treated with TGF-β1 and IL-6 together (TGF-β1+IL-6), the CD4+ T cells expressed slightly to moderately higher mRNA levels of IL-17A, IL-17F, IL-22, CCL-20 and STAT3 than control, all reaching statistical significance (N=4, P<0.05) except for IL-17A. When IL-23 was added (TGF-β1+IL-6+IL-23), the CD4+ T cells expressed dramatically higher mRNA levels of IL-17A (46.52±16.73 fold), IL-17F (85.31±35.61), IL-22 (286.2±81.69), CCL-20 (9.93±3.86) and STAT3 (6.86±1.00) than control (all n=4, P<0.01) and the TGF-β1+IL-6 group (STAT3 P<0.05, all others P<0.01, n=4). Addition of IL-1β (TGF-β1+IL-6+IL-1β) also significantly increased mRNA levels of these cytokines, significantly higher than control and TGF-β1+IL-6 groups (n=4, P<0.05, respectively), but not to the level observed with the TGF-β1+IL-6+IL-23 group. In contrast, addition of IL-21 (TGF-β1+IL-6+IL-21) only increased the level of IL-17F mRNA, but not others, significantly higher than the TGF-β1+IL-6 group. It appears IL-23 is the strongest inducer for expanding Th17 cells in the presence of TGF-β1+IL-6. We further use this 3-cytokine model (TGF-β1+IL-6+IL-23) to evaluate the effects of IL-1β and IL-21 on Th17 differentiation. Interestingly, addition of IL-1β to this 3-cytokine model (TGF-β1+IL-6+IL-23+IL-1β), promoted the highest levels of Th17 differentiation from CD4+ T cells, evidenced by the highest mRNA of these Th17 cytokines, CCL-20 and regulator STAT3, among which the IL-17A mRNAlevel reached to 302.4±82.7 fold over the control, that is a 6.5-fold increase compared to the 3-cytokine model (TGF-β1+IL-6+IL-23). IL-21 also significantly induced a Th17 cytokine profile in the 4-cytokine system (TGF-β1+IL-6+IL-23+IL-21), but the induced mRNA levels were weaker than IL-1β in this 4-cytokine system (P < 0.05, respectively). The levels of IL-17 (Figure 1F and Table 1) measured in the CD4+ T cell supernatants were highly consistent with the IL-17 mRNA levels in the 2-, 3-, and 4-cytokine systems tested for their Th17 inducing capacity in vitro. TGF-β1+IL-6+IL-23, was the strongest stimulator of IL-17 protein (199.9±46.9 pg/ml) in 3-cytokine systems, while TGF-β1+IL-6+IL-23+IL-1β stimulated the highest level of IL-17 protein (346.8±33.8 pg/ml) in 4-cytokine systems, representing 56 and 98-fold increases over the control, respectively. These findings indicate that IL-23 is the strongest inducer for Th17 production in the presence of initiating cytokines TGF-β1+IL-6; and IL-1β, a pro-inflammatory cytokine, is the strongest amplifier for further promoting Th17 expansion in the presence of TGF-β1+IL-6+IL-23.
Figure 1.
Induction of Th17 differentiation in CD4+ T cells by inducing cytokines. A-E. Real-time PCR data showing the relative fold of mRNA of Th17 associated cytokines (IL-17A, IL-17F, IL-22, CCL-20) and regulator STAT3 in CD4+ T cells incubated with various combinations of TGF-β1, IL-6, IL-23, IL-1β and IL-21 for 4 days. F. Luminex immunobead assay showing IL-17 concentration in the supernatant of CD4+ T cells incubated with same combinations of inducing cytokines for 4 days. Results shown are mean ± SD of 3–5 independent experiments. *, P < 0.05; **, P <0.01; ***, P < 0.001 each treated groups vs. untreated control. ^, P < 0.05; ^^, P <0.01; ^^^, P < 0.001 other treated groups vs. TGF-β1+IL-6 group.
Table 1.
Th17 differentiation of CD4+ T cells by inducing cytokines
| Cytokine mRNA (Fold) | Control | TGFβ1 +IL-6 | TGFβ1 +IL-6 +IL-23 | TGFβ1+IL-6 +IL-1β | TGFβ1 +IL-6 +IL-21 | TGFβ1+IL-6 +IL-23 +IL-1β | TGFβ1+IL-6 +IL-23 +IL-21 |
|---|---|---|---|---|---|---|---|
| IL-17A | 1 | 1.5±0.5 | 46.5±16.7 ** ^^ | 4.6±1.7 * ^ | 1.7±0.7 * | 302.4±82.7 *** ^^^ ## | 118.5±17.8 *** ^^ # |
| IL-17F | 1 | 8.4±3.4 * | 85.3±35.6 ** ^^ | 16.8±1.7 * ^ | 12.3±1.9 * ^ | 393.8±51.9 *** ^^ ## | 286.1±11.2 *** ^^ # |
| IL-22 | 1 | 4.2±0.7 * | 286.2±81.7 *** ^^ | 19.7±3.6 * ^ | 5.6±1.3 * | 1031±18.3 *** ^^^ ## | 545.7±23.2 *** ^^^ # |
| CCL20 | 1 | 2.0±0.7 * | 9.9±3.9 * ^ | 2.1±0.2 * | 1.8±0.2 * | 20.3±2.8 ** ^ # | 13.2±5.9 ** ^ |
| STAT3 | 1 | 2.1±0.4 * | 6.9±1.0 * ^ | 4.1±0.9 * ^ | 3.2±0.8 * | 24.0±9.4 **^^ ## | 13.6±0.9 ** ^ # |
| Protein (pg/ml) IL-17 | 3.6±0.6 | 5.7±0.4 * | 199.9±46.9 ** ^^ | 14.9 ±3.7 * ^ | 7.2±0.2 * | 346.8±33.8 *** ^^ # | 201.8±13.5 ** ^^ |
Results shown are mean ± SD of 3–5 independent experiments;
P < 0.05;
P <0.01;
P < 0.001 each treated groups vs. untreated control;
P < 0.05;
P <0.01;
P < 0.001 other treated groups vs. TGF-β1+IL-6 group;
P < 0.05;
P < 0.01;
P < 0.001 4 cytokines groups vs. TGF-β1+IL-6+IL-23 group.
Corneal epithelium was enriched for Th17 inducing cytokines in response to hyperosmotic, microbial and inflammatory stimuli
Corneal epithelium, as one type of mucosal epithelia, is a target of Th17 inflammatory cytokine, but is also enriched for cytokines that participate in the induction of Th17 differentiation. Using human corneal epithelial cells (HCECs) as an in vitro model, we evaluated how these Th17 inducing cytokines were stimulated by physiological inflammatory stressor, including high osmolarity media, microbial components and inflammatory cytokines.
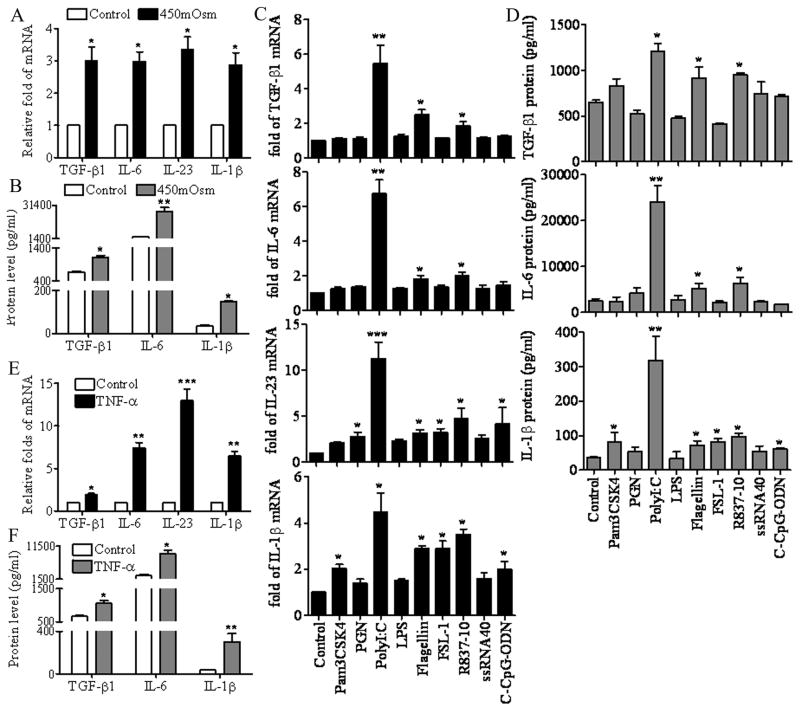
As shown in Figure 2A, the mRNA levels of TGF-β1, IL-6, IL-23 and IL-1β were significantly increased in primary cultured HCECs when switched from normal medium with an osmolarity of 312 mOsM to a 450 mOsM hyperosmotic medium by adding NaCl2 for 4 hours (all P<0.05). Luminex immunobead assay confirmed the stimulation of TGF-β1, IL-6 and IL-1β at the protein levels in culture supernatants of HCECs exposed to hyperosmotic media for 48 hours (Figure 2B, P<0.05, 0.01, 0.05, respectively).
Figure 2.
Th17 inducing cytokines in HCECs exposed to hyperosmotic media, microbial ligands and a pro-inflammatory cytokine TNF-α. A. The mRNA levels of TGF-β1, IL-6, IL-23 and IL-1β expressed by confluent primary HCECs in normal 312 mOsM and 450 mOsM hyperosmotic media for 4 hours, as evaluated by RT and real-time PCR. B. The protein concentrations of TGF-β1, IL-6 and IL-1β in the culture supernatants of HCECs receiving the same treatments for 48 hours, as measured by Luminex immunobead assay. C. The mRNA levels of TGF-β1, IL-6, IL-23 and IL-1β expressed by confluent primary HCECs exposed to 50μg/ml polyI:C or 10μg/ml of Pam3CSK4, PGN, LPS, flagellin, FSL-1, R-837, ssRNA40 or C-CpG-ODN for 4 hours as evaluated by RT and real-time PCR. D. The protein concentrations of TGF-β1, IL-6 and IL-1β in the culture supernatants of HCECs receiving the same treatments for 48 hours, as measured by Luminex immunobead assay. E. The mRNA levels of TGF-β1, IL-6, IL-23 and IL-1β expressed by confluent primary HCECs treated with TNF-α (10ng/ml) for 4 hours, as evaluated by RT and real-time PCR. F. The protein concentrations of TGF-β1, IL-6 and IL-1β in the culture supernatants of HCECs treated with TNF-α for 48 hours, as measured by Luminex immunobead assay. Results shown are mean ± SD of 3–5 independent experiments. *, P < 0.05; **, P <0.01; ***, P < 0.001 each treated groups vs. untreated control.
The effect of nine microbial ligands on induction of TGF-β1, IL-6, IL-23 and IL-1β by HCECs is shown in Figure 2C (for mRNA) and 2D (for protein). Levels of TGF-β1 and IL-6 mRNAs were significantly induced by polyI:C, flagellin and imiquimod (R837), ligands respective to TLRs, 3, 5 and 7. Interestingly, IL-23 mRNA was largely induced in HCECs challenged by a variety of viral or bacterial components, including peptidoglycan (PGN), polyI:C, flagellin, FSL-1, R837 and C-CpG-ODN, ligands respective to TLRs, 2, 3, 5, 6, 7 and 9. IL-1β expression was also greatly enhanced by Pam3CSK4 (TLR1 ligand), polyI:C, flagellin, FSL-1, R837 and C-CpG-ODN. For the most part, levels of these cytokines in supernatants of HCECs stimulated by these viral and bacterial components were parallel to mRNA levels (Fig 2D). PolyI:C, representing double-stranded viral RNA (dsRNA), was noted to be the strongest stimulator for TGF-β1 (1209±167 pg/ml), IL-6 (24100±4950 pg/ml) and IL-1β (319±99 pg/ml), inducing 1.9-, 9.3- and 8.9-fold increases over the control, respectively.
As shown in Figure 2E and 2F, TNF-α, a proinflammatory cytokine, strongly stimulated mRNA expression of TGF-β1, IL-6, IL-23 and IL-1β (P<0.05, 0.01, 0.001, 0.01, respectively), with corresponding increases of TGF-β1 (1056±110 pg/ml), IL-6 (9120±1542 pg/ml) and IL-1β (302±106 pg/ml), in HCEC supernatants of 1.5-, 3.6- or 8.6-fold over the control (all P<0.05), respectively.
Th17 cell differentiation was induced from CD4+ T cells exposed to the conditioned media of HCECs challenged by polyI:C or TNF-α
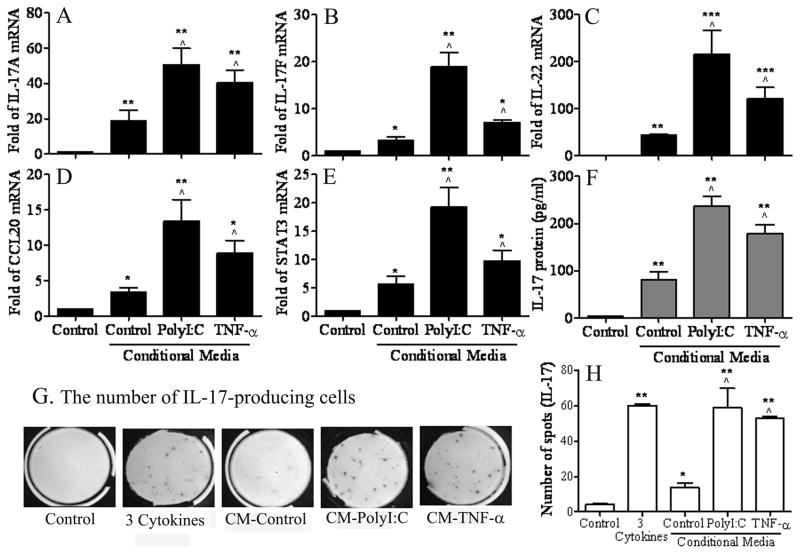
To investigate whether corneal epithelium-derived cytokines indeed promote Th17 cell differentiation from CD4+ T cells, isolated murine CD4+ T cells were cultured in complete RPMI 1640 (Control) or treated with the complete RPMI 1640 containing 50% conditioned media of HCECs without (CM-Control) or with stimulation by polyI:C (CM-PolyI:C) or TNF-α (CM-TNF-α) for 4–7 days. As shown in Figures 3A-E and Table 2, the mRNA levels of IL-17A, IL-17F, IL-22, CCL-20 and STAT3 were significantly higher in CD4+ T cells treated with 50% CM-PolyI:C or CM-TNF-α for 4 days compared with control medium (100% complete RPMI 1640) or 50% conditioned media of HCEC culture without any stressors (CM-Control, all P<0.05, n=3). The IL-17 protein levels in the supernatants of CD4+ T cells exposed to 50% CM-PolyI:C or CM-TNF-α for 4 days (Figure 3F and Table 2) were also significantly higher than the media (both P<0.01, n=3) and CM (P<0.05, n=3) controls. Furthermore, the number of IL-17-producing cells differentiated from CD4+ T cells, determined by ELISPOT bioassay (Figure 3G, 5H and Table 2), displayed the same pattern found for IL-17 cytokine mRNA and protein expression. The numbers of IL-17-producing cells stimulated by CM-PolyI:C or CM-TNF-α reached the levels similar to that seen in the 3-cytokine model (TGF-β1+IL-6+IL-23), suggesting that cytokines in the conditioned media of HCECs exposed to polyI:C or TNF-α were capable to promote Th17 cell expansion to levels induced by IL-6+TGF-β1+IL-23.
Figure 3.
Induction of Th17 differentiation in CD4+ T cells by cultured in RPMI media mixed 1:1 with conditioned media of HCECs. A-E. Real-time PCR data showing the relative fold of mRNA of Th17 associated cytokines (IL-17A, IL-17F, IL-22, CCL-20) and regulator STAT3 in CD4+ T cells incubated for 4 days with 50% of conditioned media of HCECs irritated by polyI:C (CM-PolyI:C) or TNF-α (CM-TNF-α) for 48 hrs. F. Luminex immunobead assay showing IL-17 concentration in the supernatant of CD4+ T cells receiving the same treatment for 4 days. G-H. ELISPOT bioassay showing the spots/3×105 cells/well, representing the numbers of IL-17-producing T cells, in CD4+ T cells treated with CM-PolyI:C, CM-TNF-α, or 3 cytokines (TGF-β1+IL-6+IL-23) for 7 days. Results shown are mean ± SD of 3–5 independent experiments. *, P < 0.05; **, P <0.01; ***, P < 0.001 each treated groups vs. media control. ^, P < 0.05; ^^, P <0.01; ^^^, P < 0.001 CM-PolyI:C or CM-TNF-α groups vs. CM-control group.
Table 2.
Induction of Th17 differentiation in CD4+ T cells treated with the conditioned media of HCECs.
| mRNA (Fold) | Control | CM-Control | CM-PolyI:C | CM-TNF-α |
|---|---|---|---|---|
| IL-17A | 1 | 19.0±8.1** | 50.7±15.8** ^ | 40.3±12.7** ^ |
| IL-17F | 1 | 3.3±1.1* | 18.9±4.3** ^ | 7.1±0.8* ^ |
| IL-22 | 1 | 43.5±4.7** | 214.9±89.5***^ | 120.7±43.4***^ |
| CCL20 | 1 | 3.3±1.5* | 13.4±6.2** ^ | 8.9±3.7* ^ |
| STAT3 | 1 | 5.6±2.0* | 19.3±6.0** ^ | 9.8±3.3* ^ |
| IL-17 protein (pg/ml) | 3.6±0.6 | 81.2±27.8** | 237.4±41.2** ^ | 178.5±38.5** ^ |
| IL-17-producing T cells | 4.25±0.96 | 13.7±4.5* | 59.0±15.6** ^ | 53.0±1.4** ^ |
Results shown are mean ± SD of 3–5 independent experiments;
, P < 0.05;
P <0.01;
P < 0.001 each treated groups vs. media control;
P < 0.05;
P <0.01;
P < 0.001
CM-polyI:C or CM-TNF-α groups vs. CM-control group. HCECs: human corneal epithelial cells; CM: conditioned media.
DISCUSSION
Several key cytokines have been previously identified for their ability to induce Th17 differentiation. Compelling evidence has demonstrated that TGF-β and IL-6 are dominant in the initiation of Th17 cell differentiation although IL-6 or TGF-β as a single initiator was proposed to be necessary for antigen specific Th17 differentiation (Yang et al, 2007;Veldhoen et al, 2006;Bettelli et al, 2006;Mangan et al, 2006). IL-23 was the first cytokine shown to selectively regulate IL-17 expression (Murphy et al, 2003;Zhou et al, 2007), but it might not be required for the initial differentiation of Th17 cells in vivo (Langrish et al, 2005). Recently, IL-1β was found to promote Th17 cell development and proliferation in the presence of TGF-β and IL-6 (Veldhoen et al, 2006). IL-21, produced by activated T cells and NK cells (Leonard and Spolski, 2005), may be required for full commitment of Th17 cells (Korn et al, 2007;Nurieva et al, 2007). Hence IL-23, IL-1β and IL-21 may possibly maintain and expand the differentiated Th17 cells in the presence of IL-6 and TGF-β (Bettelli et al, 2006;Veldhoen et al, 2006). In this study we evaluated the differential effects of IL-23, IL-1β and IL-21 in promoting Th17 differentiation from peripheral CD4+T cell isolated from mouse cervical lymph nodes and spleens. The results at both mRNA and protein levels showed that IL-6 and TGF-β1, only minimally induced Th17 differentiation. We evaluated the ability of 3 additional cytokines, IL-23, IL-1β and IL-21, in the presence of IL-6 and TGF-β1 to stimulate the Th17 profile. IL-23 was the strongest stimulator of the Th17 signature cytokines IL-17A and IL-17F, as well as IL-22, chemokine CCL20, and STAT3 among these 3 cytokines, while IL-1β was intermediate and IL-21 had the least effect. Interestingly, in the 4 cytokine model, IL-1β stimulated much higher levels of IL-17 family cytokines, 1.5–2 fold greater than IL-21 in the presence of TGF-β1, IL-6 and IL-23. These findings suggest that TGF-β1 and IL-6 initiate low level differentiation of Th17 cells; and their maintenance and development need other expanding factors, among which IL-23 plays a central role and IL-1β amplifies this expansion further in Th17 differentiation. In this in vitro model with murine CD4+ T cells, we used recombinant human cytokines with the rationale that we were going to compare their Th17 inducing capacity with supernatants of human corneal epithelium cells. Of note, we found no significant difference between recombinant human and mouse cytokines on inducing Th17 differentiation in murine CD4+ T cells in our preliminary experiments (data not shown).
A variety of mucosal epithelia have been found to produce Th17 inducing cytokines, including TGF-β1, IL-6, IL-23, and IL-1β (Aujla et al, 2007;Holtta et al, 2008). IL-1β is a well recognized proinflammatory cytokine produced by mucosal epithelia that increase in response to stress, infection or wounding. Our finding that IL-1β strongly promoted the Th17 differentiation in the presence of TGF-β1, IL-6 and IL-23, triggered us to investigate whether the mucosal epithelia indeed promote Th17 differentiation through their stimulated production of these inducing cytokines. In an attempt to mimic known stressors of the ocular surface, we measured production of Th17 inducing cytokines in cultured human corneal epithelial cells in response to hyperosmotic stress, microbial components and inflammatory cytokines.
The ocular surface epithelium is subjected to hyperosmotic stress in dry eye. Exposure of human epithelial cells to hyperosmotic stress has been noted to activate mitogen-activated protein kinase (MAPK) pathways and stimulate production of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-8 (Li et al, 2006). In this study, we showed that in addition to IL-1β, TGF-β1, IL-6 and IL-23 were highly induced in the corneal epithelium in response to hyperosmotic stress (Figure 2A, B).
The discovery of toll-like receptors (TLRs) represents a breakthrough in the understanding of the ability of the innate immune system to rapidly recognize pathogens. HCECs have been shown to express functional TLRs 1–7 and 9 (Kumar and Yu, 2006) and to be capable of recognizing the presence of Gram-positive bacterial components by producing pro-inflammatory cytokines to initiate an innate immune response (Bogiatzi et al, 2007). In this study, we evaluated the expression and production of Th17 inducing cytokines by HCECs in response to 9 extracted or synthetic microbial components that are ligands of TLRs 1–9, respectively. As shown in Figure 2C, D, TGF-β1, IL-6, IL-23, and IL-1β expression and production were found to be largely induced by polyI: C, flagellin, and R837, the respective ligands for TLRs3, 5 and 7, representing viral or bacterial infections. Among these TLR agonists, polyI:C was the strongest stimulator of Th17 inducing cytokines by HCECs.
Osmotic stress and microbial components also promoted production of pro-inflammatory cytokines including TNF-α (data not shown), which plays an important role in ocular surface disease (Li et al, 2006;Barton et al, 1997). Consequently, TNF-α stimulus was chosen as the third model we evaluated. Similar to the TLR agonists, we found that TGF-β1, IL-6, IL-23, and IL-1β were greatly induced by TNF-α (Figure 2E, F).
Based on our findings that among TLR ligands, PolyI:C is the potent stimulator of Th17 inducing cytokines, and that TNF-α is a representative pro-inflammatory factor, we compared the Th17 inducing capacity of conditioned media of HCECs treated with PolyI:C and TNF-α, with media from unstimulated cultures. We were unable to test hyperosmotic stress as a condition for induction of Th17 differentiation because CD4+ T cells could not survive in the high salt hyperosmotic media.
Th17 cells express a unique profile of pro-inflammatory products, comprising IL-17A, IL-17F, IL-22 and CCL20 (Chang and Dong, 2007;Chung et al, 2006;Liang et al, 2006;Williams, 2006). During T helper cell differentiation, regulatory cytokines act through selective members of the STAT family to regulate gene transcription (Afkarian et al, 2002). The differentiation of Th17 cells is initiated by STAT3, downstream of IL-6 induced signaling (Zhou et al, 2007;Yang et al, 2007). As showed in Figure 3, when HCEC conditioned media were incubated with murine CD4+ T cells in vitro, CM-PolyI:C and CM-TNF-α, the media from polyI:C and TNF-α stimulated corneal epithelia respectively, showed significantly higher stimulation of the Th17 profile including IL-17A, IL-17F, IL-22, CCL-20 and STAT3 than conditioned media and media alone controls. The induction of Th17 differentiation by CM-PolyI:C and CM- TNF-α was further confirmed by a significant increase in the number of IL-17-producing cells measured by ELISPOT. The stimulated levels of Th17 differentiation by CM-PolyI:C or CM-TNF-α groups was comparable that achieved by the 3-cytokine model (TGF-β1+IL-6+IL-23), suggesting that the Th17 cells can indeed be promoted by factors produced by corneal epithelium in response to a variety of inflammatory stimuli. It remains to be determined if the Th17 promoting activity of the corneal epithelium was due to production of known Th17 inducers or to other yet to be determined factors.
Based on our previous finding of a significant increase in the number of IL-17 producing cells in the ocular surface tissues and in cervical lymph node of mice with experimental desiccating stress (de Paiva et al, 2009), we hypothesize that Th17 inducing cytokines produced by the corneal epithelium may participate in Th17 differentiation in three ways: (1) activation of immature dendritic cells on the ocular surface; (2) direct transfer to the lymph node in lymphatic liquid; or (3) direct promotion of differentiated Th17 cells that infiltrate the ocular surface. Further study is necessary to investigate whether this promotion of Th17 differentiation by the corneal epithelium can be blocked by topical administration of neutralizing antibodies to these inducing factors on the ocular surface. In conclusion, these findings demonstrate for the first time that Th17 differentiation can be promoted, at least in part, by factors produced by stressed corneal epithelial cells.
Acknowledgments
The authors thank the Lions Eye Bank of Texas for their kindly providing human corneoscleral tissues. This work was supported by Department of Defense Congressionally Directed Medical Research Programs (CDMRP) PRMRP grant FY06 PR064719 (DQL), National Institutes of Health grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Support: DOD CDMRP PRMRP grant FY06 PR064719 (DQL), NIH grant EY11915 (SCP), Research to Prevent Blindness, Oshman Foundation, William Stamps Farish Fund.
Abbreviations
- HCECs
human corneal epithelial cells
- CM
conditioned media
- PGN
Peptidoglycan
- PolyI
C, polyinosinic-polycytidylic acid
- R837
imiquimod
Footnotes
Commercial relationship: None
CONFLICT OF INTEREST
The all authors have no financial and commercial conflicts of interest.
References
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K, Monroy DC, Nava A, Pflugfelder SC. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997;104:1868–1874. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, III, Bian F, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li D-Q, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccation stress. Mucosal Immunology. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li D-Q, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtta V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, Kolho KL, Farkkila M, Savilahti E, Vaarala O. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–1184. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells. 2005;19:180–184. [PubMed] [Google Scholar]
- Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- Li D-Q, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Li D-Q, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Ma P, Bian F, Wang Z, Zheng X, Chotikavanich S, Pflugfelder SC, Li DQ. Human Corneal Epithelium-Derived Thymic Stromal Lymphopoietin - A Potential Link between the Innate and Adaptive Immune Responses via Toll-Like Receptors and Th2 Cytokines. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Li D-Q, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am J Ophthalmol. 2000;130:688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]