Abstract
In Japanese quail, males will readily exhibit the full sequence of male-typical sexual behaviors but females never show this response even after ovariectomy and treatment with male-typical concentrations of exogenous testosterone. Testosterone aromatization plays a key-limiting role in the activation of this behavior but the higher aromatase activity in the brain of males compared to females is not sufficient to explain the behavioral sex difference. The cellular and molecular bases of this prominent sex difference in the functional consequences of testosterone have not been identified so far. We hypothesized that the differential expression of sex steroid receptors in specific brain areas could mediate this behavioral sex difference and therefore quantified by radioactive in situ hybridization histochemistry the expression of the mRNA coding for the androgen receptor (AR) and the estrogen receptors (ER) of the α and β sub-types. All three receptors were expressed in an anatomically discrete manner in various nuclei of the hypothalamus and limbic system and, at usually lower densities, in a few other brain areas. In both sexes, the intensity of the hybridization signal for all steroid receptors was highest in the medial preoptic nucleus (POM), a major site of testosterone action related to the activation of male sexual behavior. Although no sex difference in the optical density of the AR hybridization signal could be found in POM, the area covered by AR mRNA was significantly larger in males than in females, indicating a higher overall degree of AR expression in this region in males. In contrast, females tended to have significantly higher levels of AR expression than males in the lateral septum. ERα was more densely expressed in females than males throughout the medial preoptic and hypothalamic areas (including the POM and the medio-basal hypothalamus [MBH)], an area implicated in the control of female receptivity) and in the mesencephalic nucleus intercollicularis. ERβ was more densely expressed in the medio-basal hypothalamus of females but a difference in the reverse direction (males>females) was observed in the nucleus taeniae of the amygdala. These data suggest that a differential expression of steroid receptors in specific brain areas could mediate at least certain aspects of the sex differences in behavioral responses to testosterone but they do not appear to be sufficient to explain the complete lack of activation by testosterone of male-typical copulatory behavior in females.
Keywords: androgen receptor, estrogen receptor, in situ hybridization, Japanese quail, sex differences, preoptic area
1. Introduction
In many vertebrates there is a pronounced sex difference in the behavioral effectiveness of testosterone (1, 2) such that males will regularly perform more frequent or more intense sexual behaviors than females in response to a same treatment with this steroid. This sex difference in responsiveness to testosterone is particularly prominent in Japanese quail (Coturnix japonica) in which treatment of castrated males with exogenous testosterone reliably activates male-typical copulatory behaviors but the same treatment has no effect on ovariectomized females (3–5). It has been established that these behavioral differences in response to testosterone are organized in an enduring fashion by embryonic sex steroids (for review, see (6, 7)).
In many species, testosterone does not act on neural sites controlling sexual behavior directly as an androgen. Rather, the conversion of testosterone into 17β-estradiol, which is catalyzed in the preoptic area of the brain by the enzyme aromatase, is crucial for the activation of male reproductive behavior (8–10). In Japanese quail (Coturnix japonica), immunocytochemical and in situ hybridization studies have revealed that the preoptic aromatase is specifically expressed in the sexually dimorphic (larger in males than in females) medial preoptic nucleus (POM) (11–13), a structure where testosterone action is necessary and sufficient for the activation of male sexual behavior (14, 15). Given the prominent role played by preoptic aromatase in the activation of sexual behavior, it was hypothesized that the behavioral sex difference in response to testosterone could relate to a sex difference in aromatase activity. It was indeed found in several species that aromatase activity in the preoptic area is higher in sexually mature males than in sexually mature females (rat: (16); quail: (17)). However, this sex difference in enzymatic activity does not appear to be sufficient in and of itself to explain the differential responsiveness to testosterone because it is not always observed in birds treated with the same dose of testosterone (18, 19) and, most importantly, because treatment of ovariectomized females with an estrogen (which should bypass the putative enzymatic limiting step related to aromatase) is not sufficient to activate male-typical copulatory behavior while the same treatment is effective in males (20).
Other aspects of steroid action and/or the neural circuits modulated by steroids must therefore be sexually differentiated in the brain and among the most obvious candidates that could display such sex differences are the receptors for sex steroid hormones because they represent the next logical cellular step in steroid action on behavior. It has indeed been shown in a number of studies on other species that sex steroid receptor expression in the brain can be a limiting factor for the activation of sexual behavior. For example, in rats and guinea pigs, the induction by 17β-estradiol of progesterone receptors in the hypothalamus is required for the activation of female sexual receptivity (21). In rats also, it is well established that there are marked individual differences in the behavior of males when placed with a sexually receptive female. Some males will readily mount and copulate with the female while others are generally inactive (sometimes called the dud-stud phenomenon). This behavioral difference is correlated with the presence of a significantly higher concentration of nuclear estrogen receptors in the preoptic area of sexually active males (i.e., studs) as compared to inactive males (duds)(22). Similarly in songbirds such as canaries, song control nuclei such as HVC (previously High Vocal Center but now used as a proper name, see (23)) or the robust nucleus of the arcopallium, RA contain more cells accumulating testosterone or its metabolites after an injection of tritiated testosterone in males than in females. The percentage of positive cells per unit of volume is the same in these nuclei for both sexes but the volumes of the nuclei are larger in males so that in total males have more hormone-sensitive cells than females. This might contribute to explain why males sing more readily and with more complex songs than females (24). These selected examples clearly highlight the critical role played by brain steroid receptors in the control of behavior.
Limited evidence, however, is available to support the notion that sex differences in brain steroid receptors could play a key role in the control of sex differences in the hormonal activation of behavior. In rats for example, there are no overall sex differences in the general distribution of androgen or estrogen receptors in the brain but brain regions that are sexually dimorphic in size (larger in males such as the medial preoptic area or in females such as the ventromedial nucleus of the hypothalamus) have more steroid receptor expressing cells in one sex than in the other (25). A similar sex difference in the number of steroid-sensitive cells expressing androgen receptors is found in the sexually dimorphic song control nucleus HVC (24). Some sex differences in receptor expression density have been reported (males displaying in general higher androgen receptors and females higher estrogen receptors concentrations) but these differences always have a small magnitude and suffer many exceptions (see discussion). Whether these neural differences control the sex differences in reproductive behaviors has not be investigated in great detail (see also (7) for additional discussion of this topic).
We therefore decided to test the hypothesis that the differential expression of sex steroid receptors in specific brain areas of Japanese quail could mediate the sexually differentiated behavioral responses to testosterone in this species. Three types of steroid receptors that have been previously implicated in the control of sexual behavior were considered here: the androgen receptor (AR), the estrogen receptor alpha (ERα) and the second form of estrogen receptor discovered in the late nineties in rats and then in quail that was named estrogen receptor beta (ERβ)(26, 27).
Initial studies investigating by in vivo autoradiography the binding of tritiated testosterone, dihydrotestosterone and estradiol in the male and female quail brain failed to detect any sex differences with the exception of a higher binding of estradiol in the nucleus taeniae of the amygdala of males as compared to females (28). No sex difference in the number of cells immunoreactive for estrogen receptor as detected with antibody H222SPγ (that was raised against estrogen receptor α from human breast cancer cells) (29) was subsequently identified in this nucleus therefore questioning the reliability of this difference (30). The distribution of the ERα and ERβ mRNAs was also described specifically in the preoptic area of male and female quail but no quantitative analysis was carried out and no qualitative difference in the density or localization of either of the mRNAs could be detected between males and females (31). Besides these three specific studies, there are no other data available on sex differences in sex steroid receptor expression in the quail brain. The gene sequences of these receptors have now been characterized in quail or other avian species (AR: (32, 33), ERα: (34) and ERβ: (27)), which makes it possible to assess in a quantitative manner their distribution in both sexes. In the present study, we quantified by radioactive in situ hybridization the expression of the mRNA coding for the androgen receptor (AR) and estrogen receptors (ER) of the α and β sub-types in the brain of males and females to test the hypothesis that their different behavioral responses to testosterone could be mediated by differences in the expression of some of these receptors. These studies were first carried out in sexually mature gonadally intact males and females because they display a larger number of behavioral sex differences than gonadectomized testosterone-treated birds. Future work should then investigate whether any of the differences in steroid receptor expression described here are maintained when males and females experience a similar endocrine condition as a result of a treatment with exogenous testosterone and only male-typical copulatory behavior is differentially expressed.
2. Materials and Methods
2.1. Animals
This study was carried out with sexually mature gonadally intact male (n=9) and female (n=9) Japanese quail (Coturnix japonica) that were purchased from a local breeder in Belgium. Throughout their life, birds were exposed to a photoperiod simulating long days (16 light:8 h dark cycles), a condition that stimulates the activity of the hypothalamo-pituitary-gonadal axis and therefore gonadal development. Food and water were always available ad libitum. Experimental procedures were in agreement with the Belgian laws on “Protection and Welfare of Animals” and on the “Protection of experimental animals” and the International Guiding Principles for Biomedical Research involving Animals published by the Council for International Organizations of Medical Sciences. The protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Sexual behavior of all males was quantified during three 5 min tests that took place 2–3 weeks before sacrifice. In these tests the frequency of neck grabs, mount attempts and cloacal contact movements was recorded (see (35–37) for procedures and a description of the behaviors that were measured). The area of the cloacal gland, an androgen-sensitive structure (38, 39), was measured in all birds with a calliper to the nearest millimeter (area = largest length × largest width). Their body weight was recorded to the nearest gram. At the time of brain collection the mass of the testes, of the ovary and of the brain was recorded to the nearest mg.
2.2. Brain histology
Birds were killed by decapitation, brains were dissected out of the skull, frozen on dry ice and stored at −80°C until used. Frozen brains were cut on a cryostat into 30 μm coronal sections (from the level of the tractus septopallio-mesencephalicus to the third nerve). The plane of the sections was adjusted to match as closely as possible the plane of the quail brain atlas (40). Sections were mounted onto Superfrost Plus slides (Menzel-Gläser, Braunschweig, Germany) in five different series, so that one series of slides contained a section every 150 μm. One series of sections was Nissl-stained with thionin blue to provide anatomical landmarks for the interpretation of the in situ hybridization signals. In situ hybridization for aromatase, androgen receptor (AR), estrogen receptor α (ERα) and estrogen receptor β (ERβ) mRNA was carried out on adjacent series of sections. Results concerning aromatase were described in a previous report (13).
2.3. Cloning of cDNA probes
Based on sequence information available for quail, PCR was used to amplify fragments of the AR, ERα and ERβ genes from Japanese quail. mRNA was prepared from brain tissue by using the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). The synthesis of first-strand cDNA was done with SUPERSCRIPT II Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) and oligo (dT)-primer. The resulting RNA-DNA hybrids were subsequently used in PCR to generate pieces of the appropriate genes. For AR the forward primer was 5′-CTGCGGAAGTGCTACGAGGC-3′ and the reverse primer was 5′-CGAAGTTCATCAAAGAGCTTCTG-3′. For ERα the forward primer was 5′-GAAGTGGGAATGATGAAAGG-3 ′ and the reverse primer was 5 ′-CTCCATTCCTTTGTTGCTCAT-3′. For ERβ the forward primer was 5′-CCTCGAGGTTCCGAGAGC-3′ and the reverse primer was 5′-TCAGACCTGGAAATGTGAAACTT-3′. PCR was carried out for 40 cycles by using the following parameters: 94°C for 1 minute, 52°C for 45 seconds, 72°C for 1 minute. Amplified fragments were purified, blunt-ended and cloned into the Sma I site of the plasmid vector pGEM7ZF (Promega, Mannheim, Germany). Resultant clones were sequenced to verify the authenticity and fidelity of the amplification. The cloned AR sequence is 646 bp in length and matches nucleotides 119-764 of the previously cloned AR sequence of Japanese quail (GenBank no. AB 188828). The cloned ERα sequence is 829 bp in length and matches nucleotides 722-1551 of the previously cloned sequence of Japanese quail (GenBank no. AF 442965). The cloned ERβ sequence is 409 bp in length and matches nucleotides 1011-1419 of the previously cloned ERβ sequence of Japanese quail (GenBank no. AF 045149).
2.4. In situ hybridization
The expression of AR, ERα and ERβ in brain sections was detected with antisense RNA probes labeled with 35S-CTP. Labeling of the probes with 35S-CTP (1250 Ci/mmol; Perkin Elmer, Rodgau, Germany) was performed using the Riboprobe System (Promega). Our in situ hybridization procedure followed a previously published protocol (41) with modifications as previously described in detail (42). For signal detection, sections were exposed to autoradiographic film (Kodak Biomax MR, Rochester, NY, USA) for different lengths (AR, 5.5 weeks; ERα, 11 weeks; ERβ, 9.5 weeks). Male and female brain were run through the entire procedure at the same time and sections from both sexes were placed on each autoradiographic film to avoid any possible effect of small differences in procedures on the observed sex differences. Control sections from two different birds were also labeled by the exact same procedure with the three sense probes corresponding to the in situ probes described above. Autoradiograms from these sections exhibited no discernable signal for the three probes. These control data will therefore not be discussed in more detail below.
2.5. Data analysis
Images from autoradiograms were trans-illuminated with a ChromaPro 45 light source and acquired with a CCD digital camera connected to a Macintosh computer running the image analysis software Image J 1.36b (NIH, USA; see http://rsb.info.nih.gov/ij/). Before acquisition the system was calibrated by using a calibrated optical density step tablet (Kodak photographic step tablet no. 3) and a calibration curve was fitted with the Rodbard function of Image J [y= d + (a − d)/(1 + (x/c)^b)]. This calibration was applied to all images and extended beyond the darkest spot to be measured in the autoradiograms so that the signals that were measured never did reach saturation. Regions of interest in each section (defined by the presence of a denser signal density than surrounding areas) were delineated on screen with the computer mouse and their average optical density (OD) and area were calculated by built-in functions of the software. The volume of brain regions of interest was calculated by summing the area measurements on both sides of the brain and multiplying them by the interval between sections (150 μm). All volumes reported in this paper thus represent the total volume of the structure of interest on both sides of the brain. Background optical density of the film was measured in a rectangular area (2 mm2) in the same image immediately ventral to the brain section of interest. Final OD measurements were obtained by subtracting the film background OD value from the OD value of the region of interest. Before analysis, sections of all birds were realigned using the commissura anterior (CA) as a landmark. The neuroanatomical nomenclature employed in this paper is based on the quail and chicken brain atlases (40, 43) but includes the most recent modifications introduced by the Avian Nomenclature Forum (23). The definition of the medial preoptic nucleus (POM) and of the medial division of the bed nucleus of the stria terminalis (BSTM) is based on specific papers that described these nuclei in the quail brain (14, 44, 45).
2.6. Statistical analysis
Statistical analyses were carried out using Systat 12.0 (Systat Software, Point Richmond, CA, USA) or GraphPad Prism 5.0a for MacIntosh (GraphPad Software Inc., La Jolla, CA, USA). Data are presented as means ± SEM. Morphological differences between sexes were analyzed with t-tests. Repeated-Measures Analyses of Variance (ANOVAs) were used to analyze the AR, ERα and ERβ mRNA expression in the brain, with sex as independent factor and brain regions as repeated factor. They were followed when appropriate by the relevant post hoc tests (Bonferroni or pairwise comparisons between levels of within-subject factor). Differences were considered significant for P < 0.05.
More detailed analyses of the mRNA signals in regions of interest were performed along the rostro-caudal axis. These analyses used separate t-tests to compare both sexes at each rostro-caudal level because sample sizes varied at the most rostral and most caudal ends of the nuclei (expression of the mRNA did not extent through the same number of sections in all subjects), which prevented the use of Repeated Measures ANOVA and associated post-hoc tests (this would result in a major loss of statistical power). Significant results derived from these tests are listed in the text but not indicated in the figures because they should be considered as only suggested differences, not as statistically established effects given that these repeated tests are thought to violate the statistical criteria for these tests (see (46) for additional discussion on this topic). The multiple t-tests were used here as a descriptive tool to indicate the rostro-caudal levels where sex differences are most likely to occur.
3. Results
3.1. Morphological measurements
The morphological and behavioral characterization of the birds used in the present experiments has been previously reported in the paper describing aromatase expression in their brain (13) and will only be briefly summarized here. As expected based on previous studies (5), female quail were heavier than males (males: 242.5 ± 5.0 g; females: 275.6 ± 6.0 g; t16= 4.2, P= 0.001) but brain mass was similar in the two sexes (males: 0.869 ± 0.024 g; females: 0.864 ± 0.015 g; t= 0.18, P= 0.862). In agreement with their exposure to a photoperiod simulating long days, all birds were sexually mature (large testes in males: 6.30 ± 0.66 g and large ovary [7.31 ± 0.27 g] and oviduct [9.23 ± 0.62 g] in females). All females but one had an egg in the oviduct at autopsy except for one bird that had laid an egg earlier in the day. All males showed full copulatory behavior during behavioral tests with a sexually receptive female. The androgen-dependent cloacal gland was much larger in males than in females (males: 343.6 ± 17.7 mm2; females: 152.6 ± 13.8 mm2; t16= 8.5, P < 0.0001). The size of this gland is androgen-dependent and reflects in a proportional manner the circulating concentration of testosterone in the male (38, 47). Based on our previous work, these physiological conditions in sexually mature subjects correspond to circulating concentrations of testosterone equal to approximately 1.5 ng/ml and concentrations of estradiol lower than 100 pg/ml in males (48), and to concentrations in the range of 200–300 pg/ml and 1 to 3.5 ng/ml respectively for estradiol and progesterone in females (48, 49). These concentrations vary rapidly within one day, especially as a function of the stage in the ovulatory cycle of females (e.g. (49)). They were therefore not measured in the blood at the time of brain collection since they would only reflect indirectly the endocrine condition experienced by the experimental subjects.
3.2. Distribution of AR, ERα and ERβ mRNA
The three steroid receptors were expressed in a specific manner in various nuclei of the hypothalamic and limbic system (Fig. 1, 2). These areas include the lateral septum (LS), the medial preoptic nucleus (POM), the nucleus taeniae of the amygdala (TnA) and the mediobasal hypothalamus (MBH), as well as the mesencephalic nucleus intercollicularis (ICo).
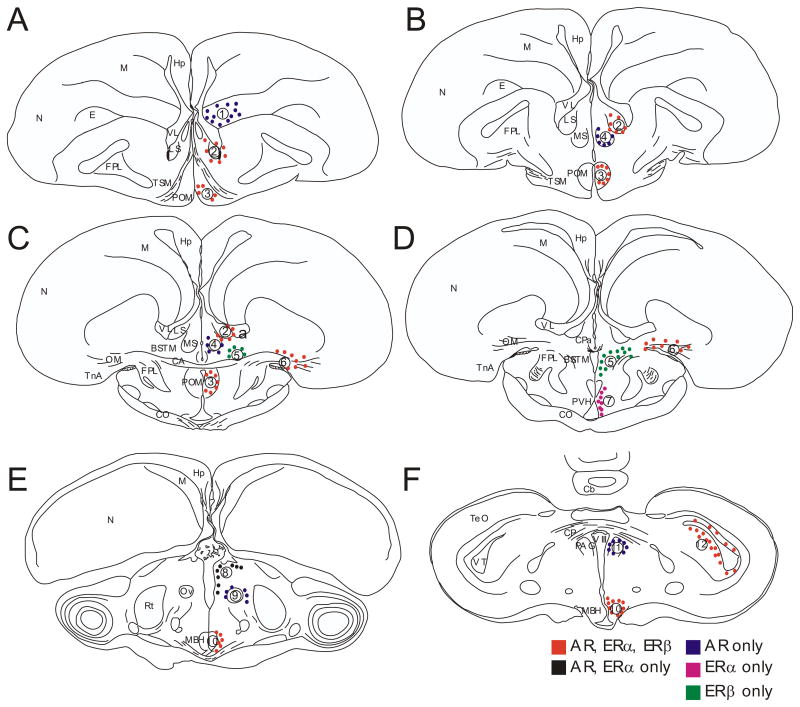
Fig. 1.
Schematic drawings of coronal sections in rostral to caudal order through the quail brain illustrating areas where expression of AR, ERα and ERβ was quantified (dots and numbers). The following clusters were distinguished: 1: nidopallium; 2: nucleus septalis lateralis (LS); 3: nucleus preopticus medialis (POM); 4: nucleus septalis medialis (MS); 5: bed nucleus of the stria terminalis, medial part (BSTM); 6: nucleus taeniae of the amygdala (TnA); 7: periventricular hypothalamus (PVH); 8: dorsal thalamus (dTh); 9: around nucleus ovoidalis (Ov); 10: mediobasal hypothalamus (MBH); 11:periaqueductal gray (PAG); 12: nucleus intercollicularis (ICo).
Abbreviations: CA, commissura anterior; Cb, cerebellum; CO, chiasma opticum; E, entopallium; FPL, fasciculus prosencephali lateralis; Hp, hippocampus; M, mesopallium; N, nidopallium; OM, tractus occipito-mesencephalicus; Ov, nucleus ovoidalis; Rt, nucleus rotundus; TeO, tectum opticum; TnA, nucleus taeniae of the amygdala; TSM, tractus septopallio-mesencephalicus; VL, ventriculus lateralis; VT, ventriculus tecti mesencephali; VIII, ventriculus tertius.
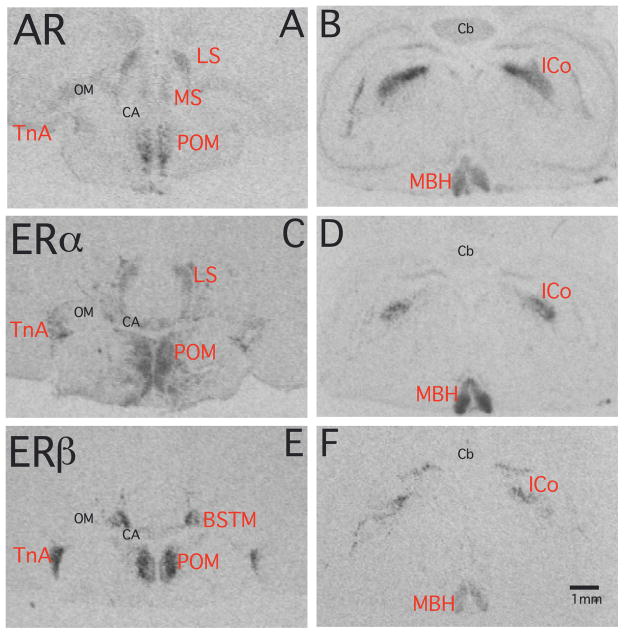
Fig. 2.
Autoradiograms of coronal sections through the quail brain illustrating the distribution of the AR, ERα and ERβ mRNA visualized by in situ hybridization. Panels A, C and E illustrate adjacent sections from a female brain at the level of the preoptic area that were stained for the mRNA of the three receptors. Panels B, D and F come from sections through another female brain at the level of the optic lobes and caudal hypothalamus. Abbreviations as in figure 1.
We found a dense expression of AR mRNA in all areas that were previously described as containing AR-ir cells (50, 51) or were labeled by in vivo autoradiography with [3H]-testosterone as a ligand (28). In addition to the above-mentioned areas, which expressed all three receptors, we also detected AR mRNA expression in the rostral nidopallium (N), the medial septum (MS), the dorsal thalamus (dTh), the periaqueductal gray (PAG) and surrounding nucleus ovoidalis (Ov). Areas with the strongest AR expression were the POM, LS and ICo. One additional area expressing AR was identified in the present study, namely the area surrounding nucleus ovoidalis, which had not been described before. The quantitative analyses presented below focused on a total number of 10 androgen mRNA-positive areas.
We identified 5 different clusters of ERα mRNA expressing cells, which are the LS, the dorsal thalamus (dTh), the TnA, the nucleus intercollicularis (ICo), and a broad periventricular area extending from the POM to the caudal part of the MBH in tuberal hypothalamus. Since this cluster does not correspond to any specific hypothalamic nucleus and actually overlaps with several of them, we simply call it the periventricular hypothalamus (PVH) in the rest of this presentation. The area expressing ERα only that is located in the middle of this cluster along the rostro-caudal axis (between POM and MHB) is specifically labeled PVH in figure 1 but quantitative measures presented later in this paper refer to the entire rostro-caudal extent of this cluster. This area showed the densest ERα hybridization signal of all positive clusters. We did not divide it into separate structures since the mRNA expression appears as a continuum throughout the rostro-caudal axis.
The distribution of ERα mRNA found in this study matches the findings of an earlier immunnocytochemical study (30) and of a more recent paper based on in situ hybridization (31). This latter study, however, did not consider the more caudal parts of the brain and therefore does not mention the dense signal we found in the dTh and ICo.
ERβ mRNA, was present in the 5 distinct cell groups expressing all three types of steroid receptors, namely the LS, POM, TnA, MBH and ICo. It was additionally present in high densities in the medial part of the bed nucleus striae terminalis (BSTM). Except for LS and ICo, the hybridization signals were very strong in these areas. A similar distribution pattern of ERβ mRNA was reported previously (27, 31). ERβ mRNA was in all brain regions where it occurs co-expressed by both AR and ERα, except in the BSTM where it was the only steroid receptor under study that was present. The anatomical distribution of this labeling in BSTM closely matched with the previously reported distribution of aromatase mRNA (13). Positive signal first appeared at the level of the anterior commissure (CA) in a position slightly dorsal to this commissure and more caudally, merged with the POM to form a V-shaped structure (Fig. 1D, 2E). A close comparison of the distribution of the ERα and ERβ signals throughout the rostro-caudal extent of TnA revealed that the latter is mainly expressed in the ventral part whereas ERα can be found predominantly in the dorsal part of the nucleus (Fig. 2C, E). The two subtypes of ER are thus expressed in slightly different portions of the avian amygdala.
3.3. Sex differences in AR expression
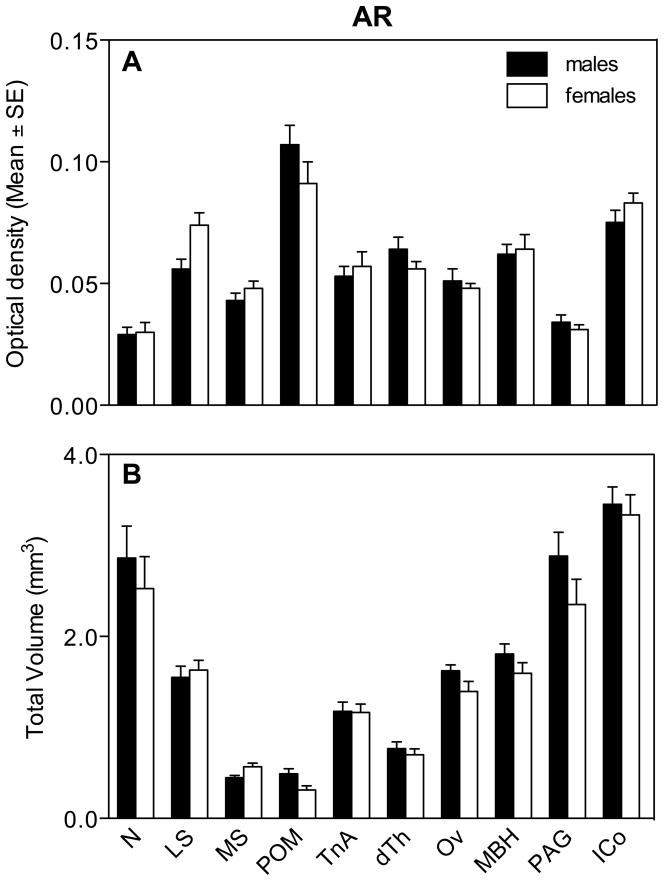
Two-way repeated measures ANOVA were used to analyze the different aspects of the AR hybridization signal (optical density [OD] and volume) simultaneously in the 10 selected clusters of positive cells. The analysis of the average optical density of the AR hybridization signal in all nuclei revealed no overall effect of sex (F1,16= 0.05, P= 0.826) but a significant effect of the brain region (F9,144= 46.25, P= 0.0001) and a significant interaction between these two factors (F9,144= 2.31, P= 0.019; Fig. 3A). Post hoc tests (pairwise comparisons between levels of within-subject factor in Systat 12.0) analyzing the anatomical distribution showed that the density of AR expression in both sexes was higher (highest concentration of the corresponding mRNA) in the POM and ICo compared to all other regions. Analysis of the interaction by the Bonferroni post-hoc test failed to identify any significant difference between males and females. In LS there was a tendency for females to have a denser AR expression as compared to males but this trends was not significant in the Bonferroni test, even if isolated t tests suggested the existence of such a difference.
Fig. 3.
Average optical density of the AR hybridization signal (A) and total volume covered by the signal (B) for different brain regions expressing androgen receptor in male and female quail.
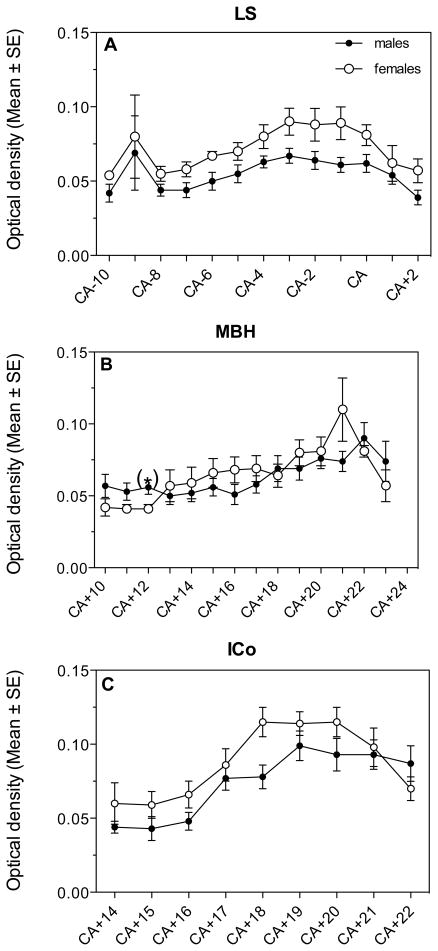
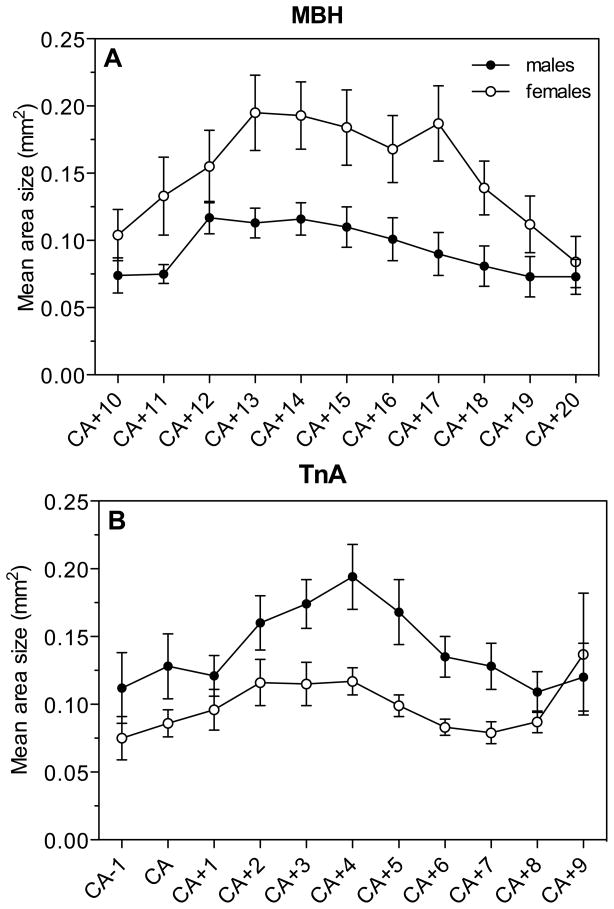
In LS, POM, MBH and ICo, the areas with the highest expression, we also analyzed changes in the density of the AR hybridization signal along the rostro-caudal axis. Significant changes in optical density were observed throughout the extent of this axis in three of the four nuclei (LS, MBH and ICo; see figure 4). Repeated measures ANOVA of these changes were however not carried out since, as explained above, the numbers of sections was different in different birds (length of nucleus variable between individuals resulting in missing sections at both ends for some subjects) so that sample size was not stable throughout the nucleus. Analysis by t-tests of the sex differences at specific levels indicated that in LS, females had a denser AR expression in the medial and caudal part of the nucleus (CA-6: t= 2.64, df= 15, P= 0.018; CA-3: t= 2.19, df= 16, P= 0.044; CA-1: t= 2.35, df= 16, P= 0.032; Fig. 4A) but not at the most rostral levels (P>0.05). No sex difference in AR mRNA density was found throughout POM. In the MBH, males had a significantly higher AR expression in the rostral part of the nucleus (CA+12: t= 2.79, df= 16, P= 0.013; Fig. 4B). In ICo, females had a higher AR expression in the central part of the nucleus compared to males (CA+18: t= 3.06, df= 15, P= 0.008; Fig. 4C).
Fig. 4.
Optical density of the hybridization signal reflecting AR expression in the LS (A), MBH (B) and ICo (C) of male and female quail as detected along the rostro-caudal axis of the nuclei.
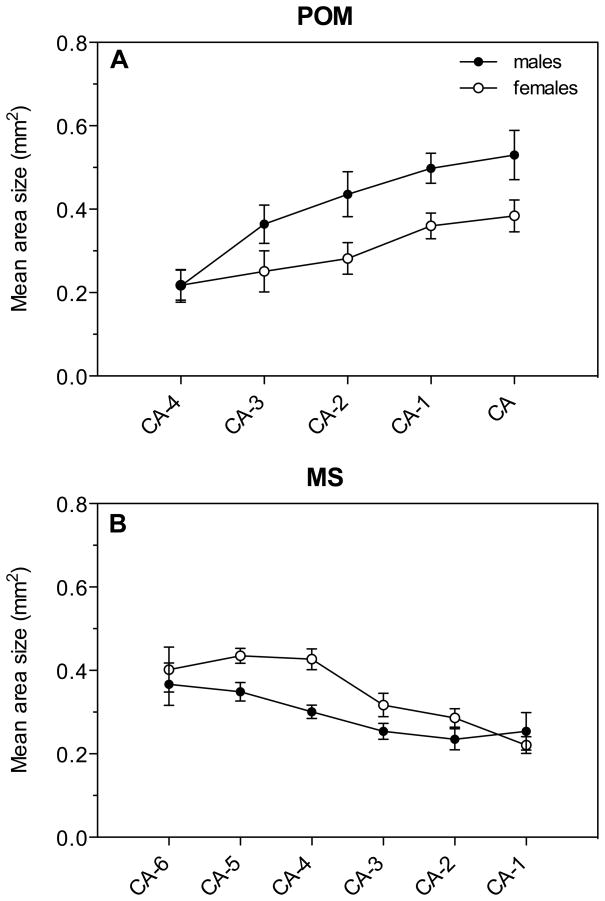
We also measured at each level through the 10 different regions the area that expressed AR more densely than the surrounding tissue and integrated these measures to compute the total volume of the brain regions expressing AR. The general ANOVA of these volumes across 10 brain regions revealed no overall effect of sex (F1,16= 2.48, P= 0.135), a significant effect of region (F9,144= 73.33, P= 0.0001) and no significant interaction (F9,144= 0.70, P= 0.712; Fig. 3B). Numerical sex differences in the volume of some brain regions were present but were in the overall ANOVA masked by the presence of many regions of equal volume in males and females. For descriptive purposes, these volumes in males and females were thus compared by t tests and these tests suggested the presence of sex differences in MS (females>males) and POM (males>females) volumes as defined by the dense AR mRNA expression (Figure 3B). These differences should, however, be confirmed in future studies since the statistical tests performed here violate the basic conclusions of the general ANOVA (post hoc t-tests performed in the absence of a significant interaction in the general ANOVA).
We analyzed along the rostro-caudal axis the change of surface in the above-mentioned four nuclei and additionally in MS, since this nucleus showed a sex difference in volume, and detected important variations. The larger volume of POM in males compared to females was mainly derived from surface differences in the central part of the nucleus (CA-2: t= 2.34, df= 16, P= 0.032, CA-1: t= 2.93, df= 14, P= 0.011; Fig. 5A). The larger volume of MS in females compared to males derived from a strong sex difference in the rostral part of the nucleus (CA-5: t= 3.08, df= 16, P= 0.007; CA-4: t= 4.18, df= 16, P= 0.001; Fig. 5B).
Figure 5.
Areas showing dense AR expression along the rostro-caudal axis of the POM (A) and MS (B).
3.3. Sex differences in ERα expression
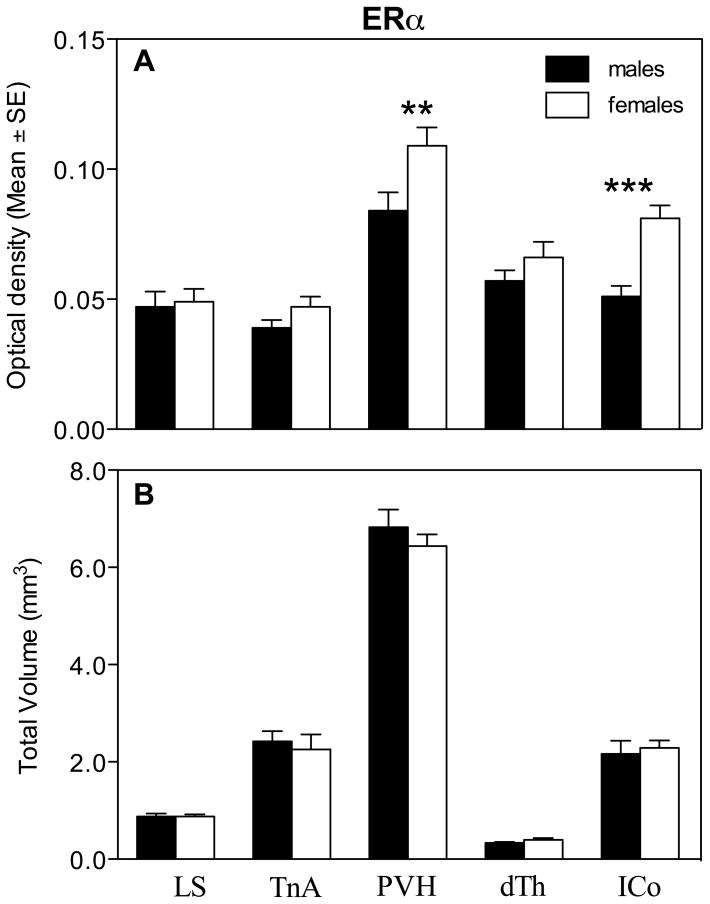
Two–way ANOVAs were similarly used to analyze the different aspects of the ERα hybridization signal simultaneously in the 5 selected clusters of positive cells. The analysis of the average optical density of the ERα hybridization signal in all nuclei revealed an overall effect of sex (F1,16= 9.36, P= 0.007), a significant effect of the brain region (F4,64= 41.97, P= 0.0001) and a significant interaction between these two factors (F4,64= 3.60, P= 0.010; Fig. 6). Post hoc Bonferroni tests identified a significant sex difference in the PVH and ICo with females having a denser ERα expression compared to males. Furthermore, ERα expression in both sexes was higher in the PVH than in all other regions (pairwise comparisons between levels of within-subject factor).
Fig. 6.
Average optical density of the ERα hybridization signal (A) and total volume covered by the signal (B) for different brain regions expressing ERα in male and female quail. Symbols above the bars indicate the results of the posthoc tests comparing both groups for different brain regions. ** P < 0.01, *** P < 0.001.
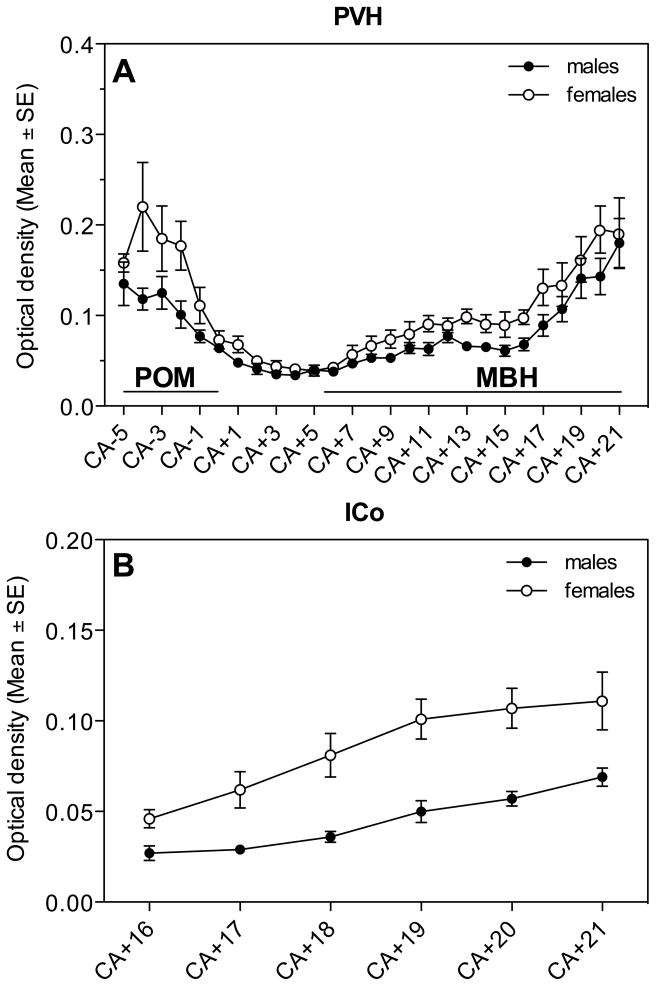
In PVH and ICo we also analyzed changes in the density of the ERα hybridization signal along the rostro-caudal axis (Figure 7). Significant changes in optical density were observed throughout the extent of this axis in both nuclei. Analysis by t-tests of the sex differences at specific levels indicated that in PVH, females had a denser ERα expression in the rostral and caudal part of the area (CA-4: t= 2.32, df= 12, P= 0.038, CA-2: t= 2.49, df= 16, P= 0.024; CA+11: t= 2.48, df= 16, P= 0.025, CA+13: t= 3.48, df= 16, P= 0.003, CA+14: t= 2.47, df= 16, P= 0.025, CA+16: t= 2.70, df= 16, P= 0.016; Fig. 7A). In the ICo, females had significantly higher ERα expression throughout the nucleus (CA+16: t= 2.72, df= 12, P= 0.019, CA+17: t= 3.29, df= 16, P= 0.005, CA+18: t= 3.64, df= 16, P= 0.002, CA+19: t= 4.27, df= 16, P= 0.001, CA+20: t= 3.92, df= 15, P= 0.001, CA+21: t= 2.66, df= 11, P= 0.022; Fig. 7B).
Fig. 7.
ERα expression along the rostro-caudal axis throughout the PVH (A) and ICo (B) of male and female quail.
We also measured at each rostro-caudal level through the 5 different regions the area that expressed ERα and integrated these measures to compute the total volume of the brain regions. The general ANOVA of these volumes across 5 brain regions revealed no overall effect of sex (F1,16=0.21, P= 0.656), a significant effect of region (F4,64= 301.02, P= 0.0001) and no significant interaction (F4,64= 0.52, P= 0.729; Fig. 6B).
3.3. Sex differences in ERβ expression
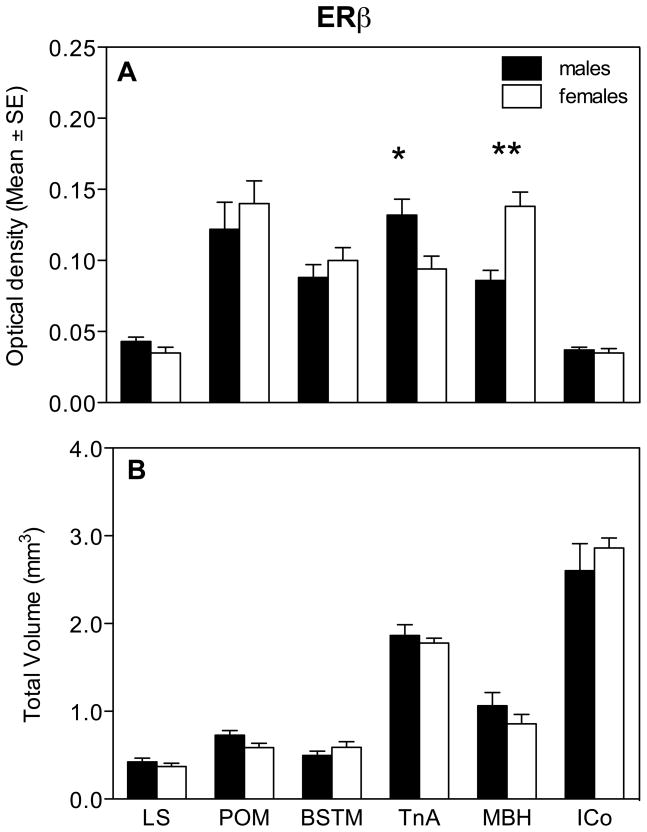
Analysis of the optical density of the ERβ hybridization signal by two-way ANOVAs in the 6 selected positive clusters revealed no overall effect of sex (F1,16= 0.31, P= 0.584) but a significant effect of the brain region (F5,80= 72.26, P= 0.0001) and a significant interaction between these two factors (F5,80= 9.96, P= 0.0001; Fig. 8A). Analysis of this interaction with the Bonferroni post-hoc test identified significant sex differences in TnA and MBH with males having a denser ERβ expression in TnA and with females having a denser expression in MBH.
Fig. 8.
Average optical density of the ERβ hybridization signal (A) and total volume covered by the signal (B) for different brain regions expressing ERβ in male and female quail. Symbols above the bars indicate the results of the posthoc tests comparing both groups for different brain regions. * P < 0.05, ** P < 0.01.
In MBH and TnA we also analyzed changes in the density of the ERβ hybridization signal along the rostro-caudal axis (Fig. 9). Significant changes in optical density were observed throughout the extent of most of this axis in both nuclei. Analysis by t-tests of the sex differences at specific levels indicated that in MBH, females had a denser ERβ expression in the medial part of the nucleus (CA+13: t= 2.70, df= 16, P= 0.016; CA+14: t= 2.73, df= 16, P= 0.015; CA+15: t= 2.33, df= 16, P= 0.033; CA+16: t= 2.21, df= 16, P= 0.042, CA+17: t= 2.96, df= 16, P= 0.009, CA+18: t= 2.32, df= 16, P= 0.034, Fig. 9A). In the TnA, males had significantly higher ERβ expression in the medial rostro-caudal part of the nucleus (CA+3: t= 2.39, df= 16, P= 0.030, CA+4: t= 2.90, df= 16, P= 0.010, CA+5: t= 2.77, df= 16, P= 0.014, CA+6: t= 3.16, df= 16, P= 0.006, CA+7: t= 2.63, df= 16, P= 0.018; Fig. 9B).
Fig. 9.
ERβ expression along the rostro-caudal axis throughout the MBH (A) and TnA (B) of male and female quail.
We also measured at each level through these 6 different regions the area that expressed ERβ at high density and integrated these measures to compute the total volume of the brain regions expressing ERβ. The general ANOVA of these volumes across 6 brain regions revealed no overall effect of sex (F1,16= 0.046, P= 0.833), a significant effect of region (F5,80= 154.40, P= 0.0001) and no significant interaction (F5,80= 1.35, P= 0.254; Fig. 8B).
3.4. Expression pattern of AR, ERα and ERβ in POM
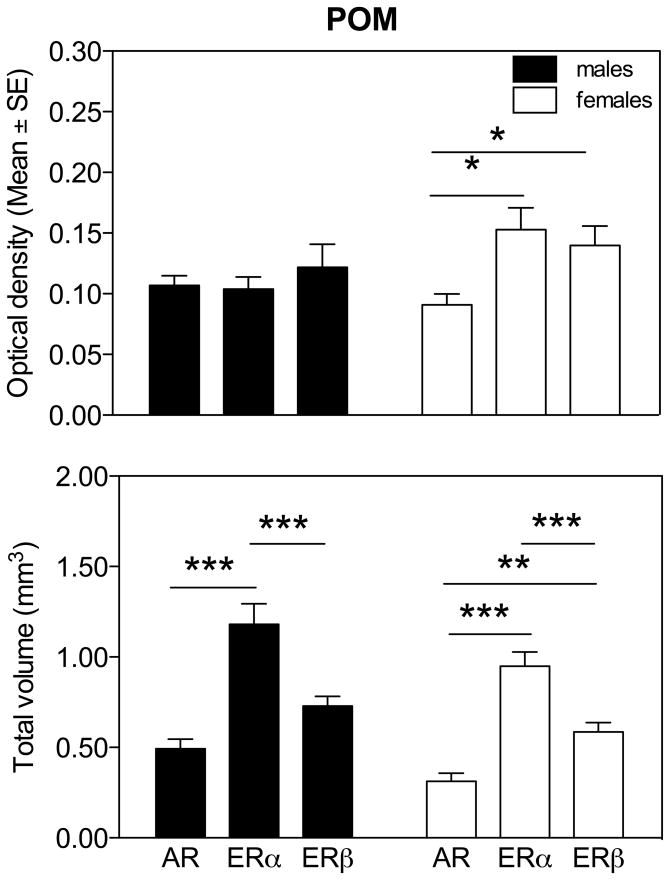
In the POM, which is of special interest since this nucleus plays a key role in the activation by sex steroids of male copulatory behavior, we compared the hybridization signals of the three probes separately for males and females (Fig. 10). The analysis revealed significant differences in the optical density among females (F2,16 = 6.11, P = 0.011) but not males (F2,16 = 0.53, P = 0.60). In females only, post-hoc Tukey multiple comparison tests indicated that the optical density of the ERα and ERβ signals was significantly higher than for AR (Fig. 10A). No conclusion should be drawn from these data concerning the relative concentrations of the three receptors in one sex because autoradiograms for different receptors had been exposed to film for different durations (AR, 5.5 weeks; ERα, 11 weeks; ERβ, 9.5 weeks) and specific activities probably differed between probes. Optical densities for different receptors thus cannot be directly compared. The present data only demonstrate the presence of a sex difference in the ratio of the different sex steroid receptors mRNAs, with females expressing relatively more estrogen receptors than males in comparison to the androgen receptor.
Fig. 10.
Comparison of optical density (A) and total volume covered by the AR, ERα and ERβ hybridization signal (B) in POM separately for male and female quail. Symbols above the bars indicate the results of the posthoc tests comparing the three receptor types within each sex. * P < 0.05, ** P < 0.01, *** P < 0.001.
In contrast, the volumes defined by the dense expression of the three steroid receptors showed the same patterns of differences in both sexes. These volumes were significantly different depending on the type of signal used to define them (females: F2,16 = 44.71, P = 0.0001; males: F2,16 = 25.20, P= 0.0001) and post-hoc Tukey multiple comparison tests showed that the POM volume was systematically larger, in both males and females, for ERα than for ERβ and AR. In addition, in males the volume defined by a dense expression of ERβ was larger than the volume defined by AR, a difference that was not found in females although a trend in the same direction was present.
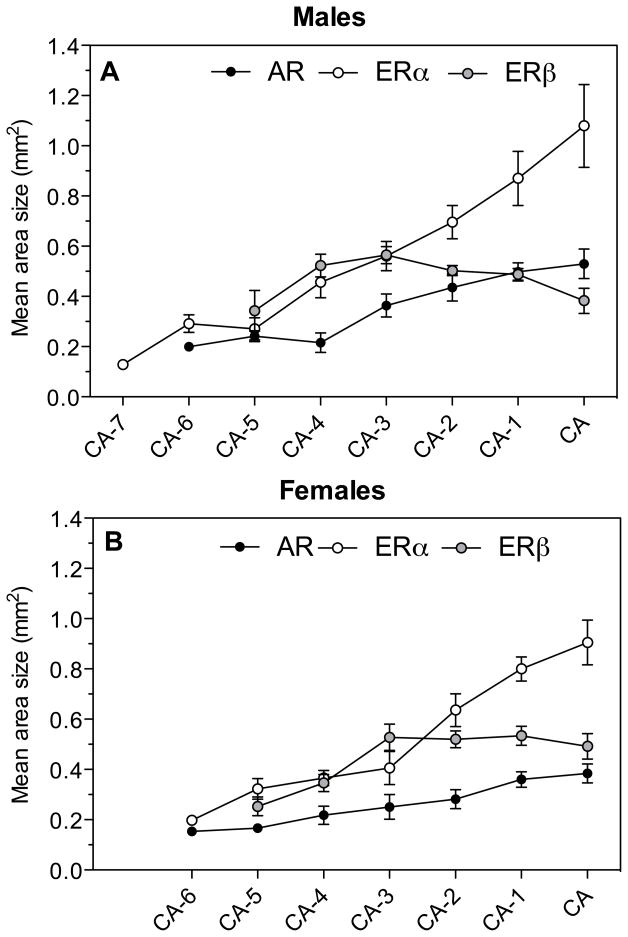
The origin of these differences in volume can be identified in figure 11 that displays for each rostro-caudal level the area expressing a high density of each receptor type. At the most caudal levels of the POM (at the level of the CA or just rostral to this fiber tract) the area showing a dense ERα expression was, in both sexes, much larger than for the other two receptor types. This is visible in the autoradiograms of sections at the level of CA shown in figure 2 in which one can clearly observe that the dense ERα mRNA signal extends ventrally outside of the POM as defined by ERβ. The dense ERβ expression was shown previously to label POM specifically (27) in the same manner as the dense group of aromatase-immunoractive cells that also outlines the boundaries of this nucleus (11, 52). Accordingly the volumes defined here by ERβ were very similar to those identified by the aromatase in situ hybridization signal observed in a previously published study based on alternate sections originating from the same brains (13).
Fig. 11.
Comparison of the AR, ERα and ERβ expression along the rostro-caudal axis of the POM in male and female quail. The figure shows at each level the area expressing levels of receptor mRNA that are denser than in the surrounding tissue. The area covered by dense ERβ mRNA specifically labels the POM throughout its rostro-caudal extent. AR covers a smaller area that corresponds to the medial part of POM and conversely the dense ERα label extends outside of POM.
Conversely, the area covered by a dense AR mRNA signal was consistently smaller at each rostro-caudal level than the area corresponding to ERβ. The origin of this difference is also visible in Figure 2 where one can see that the dense AR mRNA signal only highlights the medial part of the POM whereas ERβ covers the full medio-lateral extent of the nucleus with the densest expression of this receptor being actually located in the lateral parts of the nucleus.
4. Discussion
The present study identified in the brain of male and female Japanese quail anatomically discrete patterns of expression for the three main sex steroid receptors, namely AR, ERα and ERβ. These overall patterns of expression were qualitatively similar in both sexes but differed from one receptor to the other. Quantitative analyses revealed in addition the presence of a number of significant sex differences in the expression levels of these receptors (affecting the density of the signal and/or the volume over which the corresponding mRNA is densely expressed) in several brain areas. In particular, the optical density of the ERα hybridization signal was found to be higher in the periventricular hypothalamus (PVH), including the medial preoptic nucleus (POM) and medial part of the basal hypothalamus (MBH), and in the ICo of females as compared to males. In the MBH, the ERβ also showed a higher expression in females than in males. Furthermore, in the LS, females tended to display a higher expression level of AR mRNA compared to males. The only male-biased sex difference in the hybridization signal that we found concerns the nucleus taeniae of the amygdala (TnA), where males displayed a denser expression of ERβ than females. A detailed analysis of the POM also showed that the total volume expressing AR mRNA in this brain area is larger in males than in females. Comparison of the three receptors separately for both sexes finally identified a denser expression of both estrogen receptors compared to AR in the female but not in the male POM.
Neuroanatomical distribution of steroid receptor mRNAs
The distribution of AR, ERα and ERβ mRNA observed here in the brain of male and female quail generally matches the distribution of these genes or of the corresponding proteins previously observed using either in situ hybridization or immunocytochemistry (27, 30, 31, 51, 53).
The AR mRNA distribution observed here also matches very closely the results of an earlier autoradiographic study that used [H3]-testosterone as a ligand (28). Since testosterone can be metabolized into an estrogen by the enzyme aromatase, this autoradiographic study did not necessarily discriminate with certainty between androgen and estrogen binding sites. However, we observed here that most sites expressing AR also express at least one form of ER while few areas express one or the other subtype of ER only in the absence of AR. The correspondence between results of in vivo autoradiography and in situ hybridization is therefore not surprising.
A more recent immunocytochemical study, which identified the AR protein with the polyclonal antibody PG-21, also provided results in general agreement with the present in situ hybridization work (51). Nearly all areas showing dense populations of cells containing androgen receptor-immunoreactive material in their nucleus were identified here as expressing the AR mRNA. However, a number of brain regions in which androgen receptor-immunoreactive material was identified could not be shown here to express AR mRNA. These include small brain nuclei that contained only a limited number of androgen receptor-immunoreactive cells that potentially were not detected here due to differences in sensitivity between immunocytochemistry and in situ hybridization (e.g., the supraoptic nucleus (SOv), the nucleus suprachiasmaticus pars medialis, the nucleus anterior medialis-hypothalami (AM) and a part of the striatum labeled at that time paleostriatum ventrale (PVT) based on the atlas of the chicken brain of (43)) but also a number of brain regions where androgen receptor immunoreactivity was seen partly or entirely in the cytoplasm of the positive cells such as the PVT mentioned above, the area ventralis of Tsai (AVT) and the substantia nigra (SN). This cytoplasmic immunocytochemical signal was not entirely removed by a preadsorbtion of the antibody with the peptide used for immunization suggesting that this signal might not be specific. The absence of the corresponding mRNA in these brain areas certainly supports this interpretation (51). Quite interestingly, in these three studies based on completely independent technical approaches (binding of tritiated testosterone, detection of the androgen receptor protein or of the corresponding mRNA), the largest population of AR expressing cells was found in the mesencephalic ICo. This nucleus also represents, in the present study, the region with the largest volume of dense AR mRNA in both sexes. A broad area of the rostral nidopallium was also found to express AR in the present study but the density of the signal was relatively weak in this brain area probably explaining why AR had not been detected there in previous studies.
The distribution of both estrogen receptor subtypes in the quail brain was also anatomically discrete. The areas showing the densest expression comprise the preoptic-hypothalamic region, including in particular the POM and MBH, as well as the BSTM (ERβ only). Similar distribution patterns were found in a previous in situ hybridization study focusing on the same brain regions (31). The only mismatch concerns BSTM, where these authors describe a few ERα-expressing cells, whereas we could not detect such a signal. In a recent study investigating estrogen receptor expression in quail embryos, no ERα expression could be found in BSTM during late embryonic development while the expression of ERβ in that region was already intense (53). These minor discrepancies presumably relate to differences in sensitivity between the techniques and probes used in these studies.
Areas expressing steroid receptors and brain nuclei as defined by classical histological stains
It is important to note that brain regions expressing one or multiple steroid receptors do not necessarily match exactly with brain nuclei as defined with traditional histological techniques that have been used for the definition of brain areas such as the Nissl stain. This is a fairly common finding of chemical neuroanatomical studies and the study of steroid hormone receptors is no exception. ERα was, for example, observed to be densely expressed in periventricular position throughout the full rostro-caudal extent of the preoptic area and hypothalamus. Its expression thus overlapped with many different nuclei including the POM, the antero-medial nucleus of hypothalamus, the ventromedial nucleus and the tuberal region.
In other instances, expression of a given receptor was more closely related to specific nuclei. That was the case for nuclei such as the POM, TnA or ICo that seem to be specifically labeled by a dense expression of all three receptors under study. This correspondence with “traditional” brain nuclei must, however, be considered carefully and mismatches are frequently detected when the different patterns of expression are considered in detail. For example, the ERα and AR expression in TnA did not seem to overlap with that of ERβ. In the caudal TnA specifically, ERα appeared to be expressed more dorsally than ERβ (Fig. 2). This suggests that TnA mediates its different functions by differential steroid receptor expression in discrete subdivisions of the nucleus (54–56).
ERβ labeled the POM in a manner that completely agrees with the definition of this nucleus in Nissl-stained sections or in sections stained for aromatase by immunocyctochemistry but this was not the case for AR and ERα which labeled parts of the preoptic area that are respectively smaller and larger than the POM sensu stricto. The specific (transcription) factors that control these anatomically discrete expressions of receptors and why these control mechanisms have identical or divergent neuroanatomical distributions is largely unknown and represents one very challenging question in modern neurosciences.
Quite interestingly, there was a complete similarity between the patterns of expression of aromatase and ERβ expression in the preoptic area and BSTM. Both mRNAs specifically label the POM throughout its rostro-caudal extent and label the rostral part of the BSTM that is dorsal to the anterior commissure. At the most caudal levels of the POM, i.e. at the caudal end of the anterior commissure, the signal for both receptors that is filling the POM merges with the signal present in the BSTM to form a characteristic V-shaped structure that clearly defines the caudal part of the BSTM. Previous work demonstrated that although they are expressed in the general same brain areas, aromatase and ERα are truly co-localized in only a small percentage of neurons (18 % of aromatase cells contains ERα in POM, 4% in BSTM (57)). The fact that aromatase and ERβ are co-expressed in exactly the same nuclei might be taken as a suggestion that these two neurochemical markers are colocalized in a large number of neurons in this area. This possibility should now be tested in double-label experiments.
Functional significance of the differential expression of steroid receptor mRNAs in males and females
Male and female adult quail exhibit a qualitative sex difference in their behavioral response to exogenous testosterone. While in males a treatment with testosterone induces a very active male-typical copulatory behavior, including mounts and cloacal contact movements, such a treatment has no activating effect on these behaviors in females (3, 5). This differential responsiveness to testosterone is known to be the result of an early organizational action of ovarian estrogens. The capacity to show male-typical copulatory is lost in females essentially before day 12 of egg incubation (out of a 17 day-incubation period) following exposure to their ovarian estrogens (3, 4, 58). This process can be mimicked in males by injecting them with an estrogen before day 12 of incubation (they are demasculinized and lose the capacity to show male-typical behavior) or blocked in females by injection into the egg of an aromatase inhibitor that will suppress ovarian estrogen production so that these genetic females will retain the capacity to show male-typical behavior in adulthood if injected with testosterone (59).
While the endocrine mechanisms controlling the sexual differentiation of male copulatory behaviors during ontogeny are very well understood in quail, the neural substrate responsible for the sex difference in behavioral responsiveness to testosterone remains unclear (7). It is well established that in order to activate male sexual behavior in adulthood testosterone must be aromatized into an estrogen within the POM and the resulting estrogens are, at the cellular level, largely responsible for the behavioral effects of testosterone (10). This aromatization is catalyzed by the preoptic aromatase that is specifically expressed in the POM, and the expression and activity of the enzyme is itself up-regulated by testosterone (12, 17, 47, 52, 60). There is, however, evidence that androgens and estrogens act synergistically to activate male sexual behavior and therefore, it is most likely that both, androgen and estrogen receptors are involved in behavioral activation (20, 61–65). This notion is supported by our finding that both steroid receptor types (AR and ER) are densely expressed in all major preoptic-hypothalamic regions.
It had been expected that one or several of these steps in testosterone action (testosterone aromatization, AR or ER expression) should be sexually differentiated with a male bias in order to explain the differential action of testosterone in males and females. Previous work focusing on aromatase indeed identified a higher aromatase activity in the preoptic area of males as compared to females and interestingly, this sex difference was still present when birds of both sexes were placed in similar endocrine conditions (gonadectomized and treated with the same dose of testosterone) (17); Balthazart, et al., 1990b; Schumacher and Balthazart, 1986) thus suggesting that this enzymatic sex difference could be responsible for the differential response to testosterone of males and females (males would produce larger amounts of the behaviorally active metabolite, estradiol). However, this sex difference in aromatase activity was found in subsequent studies to have a smaller magnitude than originally thought and was not entirely reproducible (18, 19), it was not accompanied by a clear-cut sex difference in aromatase expression as measured at the level of the corresponding protein (semi-quantitative assessment obtained by counting in sections stained by immunohistochemistry the number of aromatase-immunoreactive cells) (52, 60) or mRNA (quantitative in situ hybridization) (13), and most importantly, treatment of females with exogenous estrogens, a manipulation that should bypass the enzymatic bottleneck that would be potentially resulting from the lower enzymatic activity still failed to activate male-typical copulatory behavior (20). Together, these data indicate that the sex difference in aromatase activity cannot by itself explain the sex difference in behavioral effects of testosterone in quail.
The present study therefore researched whether a sex difference affecting the next logical step in steroid action, namely the binding to specific receptors could explain the behavioral sex difference. Surprisingly, only one significant male-biased difference in steroid receptor expression was detected in the present studies investigating the three sex steroid receptors that are most likely involved in the control of male copulatory behavior, i.e. AR, ERα and ERβ. In a part of the amygdala overlapping with nucleus taeniae, (TnA), males displayed a denser expression of ERβ than females. The functional significance of this sex difference remains, however, unclear for a number of reasons. First the implication of TnA and the amygdala in general in the control of copulatory behavior is not firmly established in quail at present. Thompson et al. reported that electrolytic lesions of TnA decreased several measures of appetitive sexual behavior in male quail such as crowing, approaches of the female and rhythmic contractions of the cloacal gland muscles (54). In contrast, Absil et al. identified no effects of lesions of the TnA on similar measures of appetitive sexual behavior but found that measures of copulatory behavior sensu stricto (mount attempts and cloacal contact movements) were enhanced by the lesions (56). These discrepancies are presumably explained by different aspects of the procedures employed in the two studies and by the slightly different locations of the lesions within the amygdala (see (56) for detailed discussion).
Together, these studies and another set of experiments in ring doves (55) indicate that, in some way, TnA is implicated in the control of aspects of male sexual behavior but they do not help in providing a functional interpretation for the role of the denser expression of ERβ in the TnA of males as compared to females. Aromatase is indeed very poorly or not at all expressed in this brain area (13, 66, 67) so that the origin of the estrogens that could potentially activate these receptors more in males than in females remains unclear (males have lower circulating concentrations of estradiol than females; (48)). A few aromatase-immunoreactive cells were, however, detected in or near TnA (11) and it cannot be excluded that these cells or their distal processes release some estrogens in the vicinity of these ERβ. The magnitude of this release must however be very small since it was barely detectable by aromatase activity assays in microdissected nuclei (66).
Quite interestingly, however, this sex difference in ERβ expression within TnA provides an explanation of why the autoradiographic study of Watson & Adkins-Regan (28) had identified a sex difference in tritiated estradiol binding in this nucleus (males>females) while a subsequent immunocytochemical experiment failed to report a sex difference in the number of estrogen receptor immunoreactive cells (30). This later experiment was performed at a time when only one estrogen receptor was known. In 1996, a second form of ER was cloned in rats and later in several mammalian and avian species including the Japanese quail. This new receptor was called ERβ to distinguish it from the previously known ER now renamed ERα (26, 27, 68). It turns out that the antibody used in the 1989 immunocytochemical study (H222SPγ) specifically recognizes ERα, which is not expressed in a sexually dimorphic manner in TnA according to the present hybridization study. It therefore seems probable that the sex difference in tritated estradiol binding that had been identified by Watson and Adkins-Regan (28) reflects the differential expression of ERβ in TnA.
The POM is the major site responsible for the activation by testosterone of copulatory behavior in male quail. The volume of this nucleus is significantly larger in males than in females when its boundaries are delineated in Nissl-stained sections (44, 69) or in sections stained by immunocytochemistry for aromatase (52). Interestingly the medial part of the POM that displays a dense expression of AR tended in the present study to be larger in males than in females although this trend did not reach statistical significance (p=0.109; Fig 3). When successive rostro-caudal levels of this area were compared by exploratory t tests, a larger area expressing dense AR mRNA was identified in males than in females at the two levels that are just rostral to the anterior commissure, that is the levels that are precisely supposed to play the most prominent role in the activation of male sexual behavior (15, 70, 71). This more widely distributed expression of AR could contribute to explain the sex difference in behavioral responsiveness to testosterone.
All other sex differences in the density of steroid receptor expression were in contrast favoring females as compared to males. A trend towards a female-biased difference in the density of AR expression was found in the LS. Other differences concerned the density of ERα expression throughout the PVH (including POM and MBH) and in ICo, and the density of ERβ in the MBH. The functional significance of these differences in estrogen receptor expression is difficult to assess. It is known that estrogens activate sexual receptivity in female quail (72–74) but the specific brain sites concerned by this activation have never been investigated in this species. Studies in mammals (75, 76) and in one avian species, the ring dove (77) point however to the ventromedial hypothalamus as a key site of estrogen action for the activation of sexual receptivity. It is therefore likely that the same location is also concerned in quail. The higher expression of ERα throughout the PVH and in particular in the rostral part of the medio-basal hypothalamus (Fig 7; levels CA+11 to CA+15) and of ERβ in a slightly more caudal region (Fig. 9; levels CA+13 to CA+18) is therefore presumably implicated in the activation of female sexual behavior. The significance of a denser expression of ERα in the POM or ICo is however not obviously linked to a specific behavioral or physiological function.
It must also be noted that the activity of aromatase, the estrogens-producing enzyme, is in most brain areas significantly higher in adult males than in females (17, 19, 47) while aromatase activity appears to be absent in ICo (66). The two types of ER that are expressed in higher density in several areas of the female brain are thus presumably activated by estrogens coming from the periphery rather than by locally produced steroids. As mentioned above, females have indeed higher concentrations of estradiol in their blood than males (48).
Regulation of steroid receptors expression in males and females
The observation that sex steroid receptors are to some extent expressed differentially in males and females in specific brain areas also raises the obvious question of the mechanisms controlling these anatomically discrete sex differences. Such differences could be under direct genetic control (78) or result from organizational (irreversible effects induced during early life) or activational (reversible effects of the adult hormonal environment) actions of sex steroids. The denser expression of ERα and ERβ in several brain areas of females could for example reflect the higher concentration of estradiol in the female plasma even if a down-regulation of the receptor’s transcription by estradiol is more likely to occur in most brain areas (for review see (21, 79)).
Some interaction with the local brain environment is, however, needed to explain why the higher expression of estrogen receptors in females is not found in all brain areas that express these receptors and even more intriguingly why ERβ is expressed at higher levels in the TnA of males as compared to females. Identification of the mechanisms implicated in these controls will require the analysis of the expression of these receptors in subjects that will have been gonadectomized and treated with sex steroids (testosterone or estradiol) to experimentally determine what are the effects of these steroids on the expression of the receptors mRNA. Additionally, factors determining the tissue specificity in expression of the receptors will have to be determined which will require detailed molecular studies of the transcription factors that are implicated. These studies should simultaneously shed important light on the mechanisms controlling reproductive behaviors and the sex differences affecting these behaviors.
Sex steroid receptors and the behavioral sex differences
As discussed in detail in a previous section, it is difficult to relate in direct causal manner the somewhat limited degree to which there are sex differences in steroid receptor expression in the quail brain to the very prominent behavioral dimorphism affecting this species. This difficulty is certainly not specific to quail and has been encountered on many occasions in other behavioral neuroendocrine studies. A large number of neuroendocrine and neurochemical differences between males and females have been and continue to be discovered (see for example (80) for a recent compilation) but, for two main types of reasons, none of them seems able to explain the sex differences in adult reproductive behaviors that are frequently observed.
First, it has to be recognized that the magnitude of the neurochemical brain differences that have been reported are usually fairly small (from a few to 20–30%; e.g., see (81)) whereas sex differences in behavior are often more pronounced and sometimes qualitative (female quail will never show cloacal contact movements in response to testosterone (3), male rats will almost never display lordosis, the posture of sexual receptivity, even after a sequential treatment with estradiol and progesterone that is fully effective in females (1)). It is therefore conceptually difficult to understand why a small quantitative brain difference could cause a qualitative difference in behavior. It was thought originally that neurochemical differences of a large magnitude should be present in limited brain regions specifically related to behavior but had been diluted during measurements in larger volumes of non-differentiated tissue. Studies focusing on smaller brain areas should thus have been more informative. However even in brain nuclei where specific areas have been microdissected by approaches such as the “Palkovits punch technique” (82) no major difference in steroid receptor concentration has been identified in most cases. One could still argue that such differences are present in specific neurons within these areas and that these neurons are the key to behavior control (See (83) for a detailed discussion of this idea) but this proposition is difficult to evaluate and has not been extensively tested.
A second critical problem relates to the fact that many behavioral differences between males and females can be observed in physiological conditions in adult sexually mature subjects and remain present when animals are gonadectomized and submitted to standard endocrine replacement therapies (e.g. treatment of ovariectomized females with testosterone or of castrated males with estrogens and progesterone). In contrast, most of the neurochemical sex differences that have been reported so far in adult animals disappears when the endocrine condition of males and females are standardized by gonadectomy associated or not with hormone replacement therapy. In other words, the behavioral sex differences result from organizational effects of steroids during ontogeny whereas the differences in steroid receptors would rather be due to differences in the adult endocrine milieu, i.e., to activational effects of steroids (see however (81) for a difference in estrogen receptor concentration in the preoptic area and hypothalamus favoring females that results from early developmental effects of sex steroids). In general, these neurochemical differences can thus not be held responsible to the differential response to exogenous steroids (see (7) for additional discussion). Songbirds displaying an extreme sexual brain dimorphism such as the zebra finch (Taeniopygia guttata) could, however represent a potential exception here. In this species several song control nuclei such as HVC and MAN (the magnocellular nucleus of the anterior nidopallium) are several fold larger in males than in females (84) and since these nuclei are outlined by a dense expression of androgen receptors, this corresponds to an expression of androgen receptors in a much larger number of cells in males (85). These neural sex differences are maintained in adult birds placed in similar endocrine conditions and could therefore contribute to explain the sex differences in behavior (female zebra finches do not sing even if treated with testosterone) (59, 86).
For most species, other aspects of brain structure and function should contribute to behavioral sex differences. Differences in expression of steroid receptor co-activators and co-repressors have been considered and may play a significant role but they do not appears sufficient by themselves to explain sex differences observed in adulthood even if they contribute to their development during ontogeny (87–91). Other steps in steroid action especially in the cascade of intracellular responses and transcriptional events that are initiated by steroids certainly deserve attention in this context but the number of possibilities is large and the question therefore difficult to tackle.
One alternative promising avenue that has been considered relates to the presence of some sort of differential brain connectivity between males and females that could explains their different response to the same treatment with exogenous steroids. Major sex differences in the connections between specific nuclei have been identified in a number of species. In rats for example, the projection from the principal nucleus of the bed nucleus of the stria terminalis to the periventricular hypothalamus is significantly more robust in males than in females (92, 93). In zebra finches, the projection from HVC to another song control nucleus, RA is very prominent in males and nearly completely absent in females (94). This difference develops during ontogeny under the influence of steroids (95) and cannot be suppressed in adulthood by placing subjects of both sexes in similar endocrine conditions (e.g. by treating females with testosterone). Hodological sex differences of this nature are thus excellent candidates as critical factors that could control the differential responses of adult males and females to sex steroids. In quail, it has similarly been shown that the aromatase-immunoreactive cells of the POM send much denser projections to the mesencephalic periaqueductal gray in males than in females (96). We do not know whether this hodological sex difference in quail is affected by steroids in adulthood but this is unlikely to be the case because it is hard to imagine how growing axons could find their way in an adult brain to a target located several millimeters away. Given the key role played by the POM and the aromatase present in this nucleus in the control of male sexual behavior, this difference in connectivity is certainly a strong candidate to be a factor involved in the control of the qualitative sex dimorphism in male-typical copulatory behavior affecting this species. Studies are currently in progress to test this idea.
In general, it could be argued that clear connections between sex differences in brain steroid receptors and in behavior were not identified previously because brain receptors had not been studied in sufficient detail or they had been investigated in species that do not displaying prominent sex differences in the hormonal activation of behavior. The present study analyzed in great detail (measures of expression density, of volumes of dense expression and of the rostro-caudal extension of these signals in specific nuclei) both androgen and the two estrogen receptors in a species, the Japanese quail, that displays a qualitative sex difference in response to testosterone (i.e., females will never show male-typical copulatory behavior even after treatment with doses of testosterone that are much higher than the active doses in males). Even in this case though, we still ended up detecting only sex differences in receptors of a relatively small magnitude that do not seem capable of explaining the sex differences in the hormonal activation of behavior. It is therefore likely that, in general, sex differences in brain steroid receptors do not play a key role in the control of such behavioral dimorphisms.
Conclusions
In summary, the present study identified in quail a number of discrete sex differences in the expression of sex steroid receptors. This differential expression of steroid receptors in specific brain areas could be implicated in the control of some aspects of reproductive behavior but they do not appear to be sufficient to explain the complete lack of activation by testosterone of male-typical copulatory behavior in females. Other aspects of brain neurochemistry or connectivity should probably be invoked to explain these behavioral sex differences.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NIH/MH50388) to GFB and JB and the Belgian FRFC (2.4537.09) to JB. We thank the late Reinhold Metzdorf for help in cloning the quail fragments.
References
- 1.Goy RW, McEwen BS. Sexual differentiation of the brain. Cambridge, MA: The MIT Press; 1980. [Google Scholar]
- 2.Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA. Sex differences in the brain. From Genes to behavior. Oxford: Oxford University Press; 2008. [Google Scholar]
- 3.Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol. 1975;89:61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- 4.Adkins EK. Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of the quail brain. Neuroendocrinol. 1979;29:178–185. doi: 10.1159/000122920. [DOI] [PubMed] [Google Scholar]
- 5.Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 6.Adkins-Regan E. Nonmammalian psychosexual differentiation. In: Adler NT, Goy R, Pfaff D, editors. Handbook of Neurobiology. Vol. 7. New York: Plenum Press; 1985. pp. 43–76. [Google Scholar]
- 7.Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- 8.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 9.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 10.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–70. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 12.Aste N, Panzica GC, Viglietti-Panzica C, Harada N, Balthazart J. Distribution and effects of testosterone on aromatase mRNA in the quail forebrain: A non-radioactive in situ hybridization study. J Chem Neuroanat. 1998;14:103–115. doi: 10.1016/s0891-0618(97)10023-0. [DOI] [PubMed] [Google Scholar]
- 13.Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007;33:75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–78. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 17.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J. Correlation between the sexually dimorphic aromatase of the preoptic area and sexual behavior in quail: effects of neonatal manipulatons of the hormonal milieu. Arch Int Physiol Bioch. 1989;97:465–481. doi: 10.3109/13813458909075078. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- 21.Blaustein JD. Feminine reproductive behavior and physiology in rodents: Integration of homonal, behavioral, and environmental influences. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. San Diego, CA: Academic Press; 2009. pp. 67–107. [Google Scholar]
- 22.Clark AS, Davis LA, Roy EJ. A possible physiological basis for the dud-stud phenomenon. Horm Behav. 1985;19:227–230. doi: 10.1016/0018-506x(85)90022-4. [DOI] [PubMed] [Google Scholar]
- 23.Reiner AD, Perkel J, Bruce L, Butler A, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, CS, EDJ Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenowitz EA, Arnold AP. Hormone accumulation in song regions of the canary brain. J Neurobiol. 1992;23:871–80. doi: 10.1002/neu.480230708. [DOI] [PubMed] [Google Scholar]
- 25.Pak TR, Handa RJ. Steroid hormone receptors and sex differences in behavior. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex differences in the brain from genes to behavior. New York: Oxford University Press; 2008. pp. 109–138. [Google Scholar]
- 26.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foidart A, Lakaye B, Grisar T, Ball GF, Balthazart J. Estrogen receptor-beta in quail: Cloning, tissue expression and neuroanatomical distribution. J Neurobiol. 1999;40:327–342. [PubMed] [Google Scholar]
- 28.Watson JT, Adkins-Regan E. Neuroanatomical localization of sex steroid-concentrating cells in the Japanese quail (Coturnix japonica): Autoradiography with [3H]-testosterone, [3H]-estradiol, and [3H]-dihydrotestosterone. Neuroendocrinol. 1989;49:51–64. doi: 10.1159/000125091. [DOI] [PubMed] [Google Scholar]
- 29.Greene GL, Sobel NB, King WJ, Jensen EV. Immunochemical studies of estrogens receptors. J Steroid Biochem. 1984;20:51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- 30.Balthazart J, Gahr M, Surlemont C. Distribution of estrogen receptors in the brain of the Japanese quail: an immunocytochemical study. Brain Res. 1989;501:205–214. doi: 10.1016/0006-8993(89)90638-0. [DOI] [PubMed] [Google Scholar]
- 31.Halldin K, Axelsson J, Holmgren C, Brunström B. Localization of estrogen receptor -alpha and -beta mRNA in brain areas controlling sexual behavior in Japanese quail. J Neurobiol. 2006;66:148–154. doi: 10.1002/neu.20199. [DOI] [PubMed] [Google Scholar]
- 32.Nastiuk KL, Clayton DF. Seasonal and tissue-specific regulation of canary androgen receptor messenger ribonucleic acid. Endocrinology. 1994;134:640–649. doi: 10.1210/endo.134.2.8299561. [DOI] [PubMed] [Google Scholar]
- 33.Perlman WR, Arnold AP. Expression of estrogen receptor and aromatase mRNAs in embryonic and posthatch zebra finch brain. J Neurobiol. 2003;55:204–219. doi: 10.1002/neu.10190. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: Cloning and mRNA expression. J Steroid Biochem Mol Biol. 1996;59:135–145. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- 35.Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- 36.Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher M, Balthazart J. The postnatal demasculinization of sexual behavior in the Japanese quail. Horm Behav. 1984;18:298–312. doi: 10.1016/0018-506x(84)90018-7. [DOI] [PubMed] [Google Scholar]
- 38.Sachs BD. Photoperiodic control of reproductive behavior and physiology of the male Japanese quail (Coturnix coturnix japonica) Horm Behav. 1969;1:7–24. [Google Scholar]
- 39.Delville Y, Hendrick JC, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol Behav. 1984;33:817–823. doi: 10.1016/0031-9384(84)90053-2. [DOI] [PubMed] [Google Scholar]
- 40.Baylé JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail. J Physiol (Paris) 1974;68:219–241. [PubMed] [Google Scholar]
- 41.Whitfield HJ, Jr, Brady LS, Smith MA, Mamalaki E, Fox RJ, Herkenham M. Optimization of cRNA probe in situ hybridization methodology for localization of glucocorticoid receptor mRNA in rat brain: a detailed protocol. Cell Mol Neurobiol. 1990;10:145–57. doi: 10.1007/BF00733641. [DOI] [PubMed] [Google Scholar]
- 42.Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 43.Kuenzel WJ, Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus) Baltimore: The Johns Hopkins University Press; 1988. [Google Scholar]
- 44.Panzica GC, Viglietti-Panzica C, Calcagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic preoptic medial nucleus in quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- 45.Aste N, Balthazart J, Absil P, Grossmann R, Mülhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. [PubMed] [Google Scholar]
- 46.Sinclair JD. Multiple t-tests are appropriate in science. TIPS. 1988;9:12–13. doi: 10.1016/0165-6147(88)90234-9. [DOI] [PubMed] [Google Scholar]
- 47.Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physio lBehav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 48.Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica) General Endocrinol (Life SciAdv) 1987;5:31–36. [Google Scholar]
- 49.Delville Y, Sulon J, Balthazart J. Diurnal variations of sexual receptivity in the the female Japanese quail. Horm Behav. 1986;20:13–33. doi: 10.1016/0018-506x(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 50.Balthazart J, Foidart A, Wilson EM, Ball GF. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J Comp Neurol. 1992;317:407–420. doi: 10.1002/cne.903170407. [DOI] [PubMed] [Google Scholar]
- 51.Balthazart J, Foidart A, Houbart M, Prins GS, Ball GF. Distribution of androgen receptor-immunoreactive cells in the quail forebrain and their relationship with aromatase immunoreactivity. J Neurobiol. 1998;35:323–340. [PubMed] [Google Scholar]
- 52.Balthazart J, Tlemçani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 53.Axelsson J, Mattsson A, Brunstrom B, Halldin K. Expression of estrogen receptor-alpha and -beta mRNA in the brain of Japanese quail embryos. Dev Neurobiol. 2007;67:1742–50. doi: 10.1002/dneu.20544. [DOI] [PubMed] [Google Scholar]
- 54.Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- 55.Cheng MF, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53:243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- 56.Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav Evol. 2002;60:13–35. doi: 10.1159/000064119. [DOI] [PubMed] [Google Scholar]
- 57.Balthazart J, Foidart A, Surlemont C, Harada N. Neuroanatomical specificity in the co-localization of aromatase and estrogen receptors. J Neurobiol. 1991;22:143–157. doi: 10.1002/neu.480220205. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher M, Hendrick JC, Balthazart J. Sexual differentiation in quail: critical period and hormonal specificity. Horm Behav. 1989;23:130–149. doi: 10.1016/0018-506x(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 59.Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press, Elsevier; 2009. pp. 1746–1787. [Google Scholar]
- 60.Foidart A, De Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. Physiol Behav. 1994;55:453–464. doi: 10.1016/0031-9384(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 61.Adkins EK, Pniewski EE. Control of reproductive behavior by sex steroids in male quail. J Comp Physiol Psychol. 1978;92:1169–1178. [Google Scholar]
- 62.Adkins-Regan E. Sex steroids and the differentiation and activation of avian reproductive behaviour. In: Balthazart J, Gilles R, editors. Hormones and behaviour in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 219–228. [Google Scholar]
- 63.Balthazart J, Schumacher M, Malacarne G. Interaction of androgens and estrogens in the control of sexual behavior in male Japanese quail. Physiol Behav. 1985;35:157–166. doi: 10.1016/0031-9384(85)90330-0. [DOI] [PubMed] [Google Scholar]
- 64.Alexandre C, Balthazart J. Effects of metabolism inhibitors, antiestrogens and antiandrogens on the androgen and estrogen induced sexual behavior in Japanese quail. Physio lBehav. 1986;38:581–591. doi: 10.1016/0031-9384(86)90429-4. [DOI] [PubMed] [Google Scholar]
- 65.Harada N, Abe-Dohmae S, Loeffen R, Foidart A, Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. doi: 10.1016/0006-8993(93)90825-8. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- 67.Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- 68.Lakaye B, Foidart A, Grisar T, Balthazart J. Partial cloning and distribution of estrogen receptor beta in the avian brain. Neuroreport. 1998;9:2743–2748. doi: 10.1097/00001756-199808240-00011. [DOI] [PubMed] [Google Scholar]
- 69.Panzica GC, Viglietti-Panzica C, Sanchez F, Sante P, Balthazart J. Effects of testosterone on a selected neuronal population within the preoptic sexually dimorphic nucleus of the Japanese quail. J Comp Neurol. 1991;303:443–456. doi: 10.1002/cne.903030310. [DOI] [PubMed] [Google Scholar]
- 70.Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci. 2006;23:1869–87. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 72.Noble R. The effects of estrogen and progesterone on copulation in female quail (Coturnix coturnix japonica) housed in continuous dark. Horm Behav. 1972;3:199–204. doi: 10.1016/0018-506x(72)90032-3. [DOI] [PubMed] [Google Scholar]
- 73.Noble R. Hormonal control of receptivity in female quail (Coturnix coturnix japonica) Horm Behav. 1973;4:61–72. [Google Scholar]
- 74.Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese quail. Horm Behav. 1987;21:288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 75.Barfield RJ, Rubin BS, Glaser JH, Davis PG. Sites of action of ovarian hormones in the regulation of oestrous responsiveness in rats. In: Balthazart J, Pröve E, Gilles R, editors. Hormones and behaviour in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 2–17. [Google Scholar]
- 76.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: Correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 77.Gibson MJ, Cheng MF. Neural mediation of estrogen-dependent courtship behavior in female ring doves. J Comp Physiol Psychol. 1979;93:855–867. [Google Scholar]
- 78.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blaustein JD, Mani SK. Feminine sexual behavior from neuroendocrine and molecular neurobiological perspectives. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology (J D Blaustein Editor) New York: Springer; 2007. pp. 97–149. [Google Scholar]
- 80.Ellis L, Hershberger S, Field E, Wersinger S, Pelis S, Geary D, Palmer C, Hoyenga K, Hetsroni A, Karadi K. Sex differences: summarizing more than a century of scientific research. New York: Psychology Press; 2008. [Google Scholar]
- 81.Kühnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Horm Behav. 1994;28:483–491. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- 82.Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- 83.Blaustein JD, Olster DH. Gonadal steroid hormone receptors and social behaviors. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology, vol 3. 3. Berlin: Springer Verlag; 1989. pp. 31–104. [Google Scholar]
- 84.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–3. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 85.Arnold AP, Saltiel A. Sexual difference in pattern of hormone accumulation in the brain of a song bird. Science. 1979;205:702–705. doi: 10.1126/science.205.4407.702. [DOI] [PubMed] [Google Scholar]
- 86.Arnold AP. Effects of androgens on volumes of sexually dimorphic brain regions in the zebra finch. Brain Res. 1980;185:441–444. doi: 10.1016/0006-8993(80)91083-5. [DOI] [PubMed] [Google Scholar]
- 87.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid receptor coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- 90.Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator 1 in the Japanese quail brain: effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;140:1381–94. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–42. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460:542–62. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- 93.Simerly RB. Wired for reproduction: organisation and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 94.Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch. Nature. 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- 95.Holloway CC, Clayton DE. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- 96.Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. Journal of Comparative Neurology. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]