Abstract
In this review, evidence is presented to support the hypothesis that mechanosensory transduction occurs in tubes and sacs and can initiate visceral pain. Experimental evidence for this mechanism in urinary bladder, ureter, gut, lung, uterus, tooth-pulp and tongue is reviewed. Potential therapeutic strategies are considered for the treatment of visceral pain in such conditions as renal colic, interstitial cystitis and inflammatory bowel disease by agents that interfere with mechanosensory transduction in the organs considered, including P2X3 and P2X2/3 receptor antagonists that are orally bioavailable and stable in vivo and agents that inhibit or enhance ATP release and breakdown.
Introduction
Visceral pain is one of the most common forms of pain associated with pathological conditions like renal colic, dyspepsia, inflammatory bowel disease (IBD), angina, dysmenorrhoea and interstitial cystitis. While it is generally accepted that IBD is associated with pain (see [1,2]) there are reports that in some patients with IBD, there is hyposensitivity. P2X3 (homomultimer) and P2X2/3 (heteromultimer) receptors were cloned and shown to be largely located on small nociceptive sensory neurons in the dorsal root ganglia (DRG) in 1995 [3,4]. A schematic showing the initiation of nociception by ATP on primary afferent fibres in the periphery and purinergic relay pathways in the spinal cord are shown in Figure 1.
Figure 1.
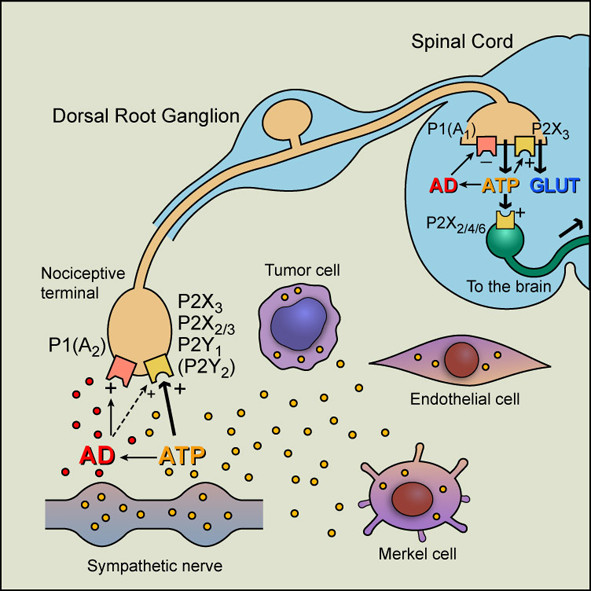
Hypothetical schematic of the roles of purine nucleotides and nucleosides in pain pathways. At sensory nerve terminals in the periphery, P2X3 and P2X2/3 receptors have been identified as the principal P2X purinoceptors present, although recent studies have also shown expression of P2Y1 and possibly P2Y2 receptors on a subpopulation of P2X3 receptor-immunopositive fibers. Other known P2X purinoceptor subtypes (1--7) are also expressed at low levels in dorsal root ganglia. Although less potent than ATP, adenosine (AD) also appears to act on sensory terminals, probably directly via P1(A2) purinoceptors; however, it also acts synergistically (broken black line) to potentiate P2X2/3 receptor activation, which also may be true for 5-hydroxytryptamine, capsaicin, and protons. At synapses in sensory pathways in the CNS, ATP appears to act postsynaptically via P2X2, P2X4 and/or P2X6 purinoceptor subtypes, perhaps as heteromultimers, and after breakdown to adenosine, it acts as a prejunctional inhibitor of transmission via P1(A2) purinoceptors. P2X3 receptors on the central projections of primary afferent neurons in lamina II of the dorsal horn mediate facilitation of glutamate and probably also ATP release. Sources of ATP acting on P2X3 and P2X2/3 receptors on sensory terminals include sympathetic nerves as well as endothelial, Merkel, and tumor cells. Yellow dots, molecules of ATP; red dots, molecules of adenosine. (Reproduced from [114] and modified from [105], used with permission from the American Physiological Society.)
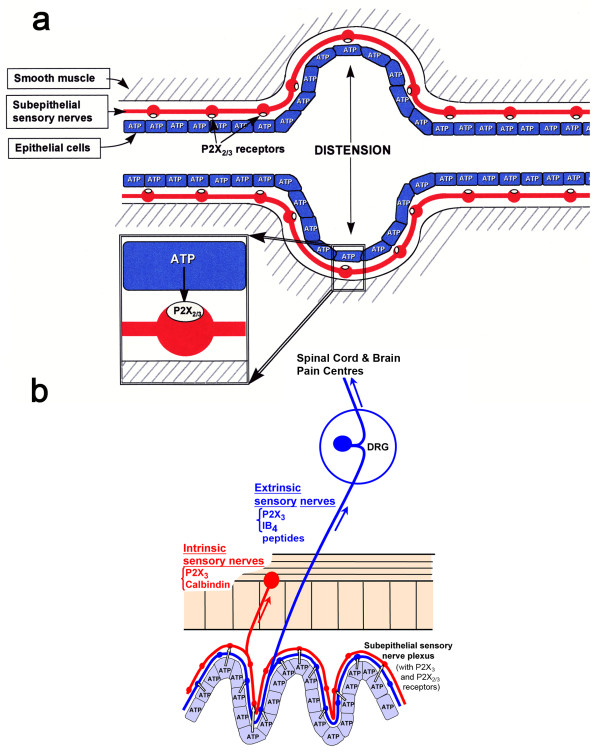
A hypothesis was proposed that purinergic mechanosensory transduction occurred in visceral tubes and sacs, including ureter, bladder and gut, where ATP released from epithelial cells during distension acted on P2X3 homomeric and P2X2/3 heteromeric receptors on subepithelial sensory nerves initiating impulses in sensory pathways to pain centres in the central nervous system (CNS) [5] (Figure 2a). Evidence supporting this hypothesis in various organs is reviewed below.
Figure 2.
A. Schematic representation of hypothesis for purinergic mechanosensory transduction in tubes (e.g., ureter, vagina, salivary and bile ducts, gut) and sacs (e.g., urinary and gall bladders, lung). It is proposed that distension leads to release of ATP from epithelium lining the tube or sac, which then acts on P2X3 and/or P2X2/3 receptors on subepithelial sensory nerves to convey sensory/nociceptive information to the CNS. (Reproduced from [5], with permission from Blackwell.) B. schematic of a novel hypothesis about purinergic mechanosensory transduction in the gut. It is proposed that ATP released from mucosal epithelial cells during moderate distension acts preferentially on P2X3 and/or P2X2/3 receptors on low-threshold subepithelial intrinsic sensory nerve fibers (labelled with calbindin) to modulate peristaltic reflexes. ATP released during extreme (colic) distension also acts on P2X3 and/or P2X2/3 receptors on high-threshold extrinsic sensory nerve fibers [labelled with isolectin B4 (IB4) or are peptidergic] that send messages via the dorsal root ganglia (DRG) to pain centres in the CNS. (Reproduced and modified from [115], published by John Wiley and Sons, Inc.)
Urinary bladder
Early evidence for ATP release from rabbit urinary bladder epithelial cells by hydrostatic pressure changes was presented by Ferguson et al. [6], who speculated about this being the basis of a sensory mechanism. Prolonged exposure to a desensitizing concentration of α,β-methylene ATP (α,β-meATP) significantly reduced the activity of mechanosensitive pelvic nerve afferents in an in vitro model of rat urinary bladder [7]. Later, it was shown that mice lacking the P2X3 receptor exhibited reduced inflammatory pain and marked urinary bladder hyporeflexia with reduced voiding frequency and increased voiding volume, suggesting that P2X3 receptors are involved in mechanosensory transduction underlying both inflammatory pain and physiological voiding reflexes [8]. Subsequently, using P2X2 knockout mice and P2X2/P2X3 double knockout mice, a role for the P2X2 subtype was shown to be involved in mediating the sensory effect of ATP [9]. In a systematic study of purinergic mechanosensory transduction in the mouse urinary bladder, ATP was shown to be released from urothelial cells during distension, and activity initiated in pelvic sensory nerves was mimicked by ATP and α,β-meATP and attenuated by P2X3 antagonists as well as in P2X3 knockout mice; P2X3 receptors were localized on suburothelial sensory nerve fibres [10]. It appears that the bladder sensory DRG neurons, projecting via pelvic nerves, express predominantly P2X2/3 heteromultimer receptors [11].
Sensory information from the urinary bladder is conveyed by both lumbar splanchnic (LSN) and sacral pelvic (PN) nerves to the spinal cord. A study comparing the mechanosensitive properties of single afferent fibres in these two pathways showed that both low and high threshold stretch-sensitive afferents were present in both pathways [12]. Single unit analysis of sensory fibres in the mouse urinary bladder revealed both low- and high-threshold fibres sensitive to ATP contributing to physiological (non-nociceptive) and nociceptive mechanosensory transduction, respectively [13]. It was also shown that purinergic agonists increase the excitability of afferent fibres to distension. The roles of ATP released from urothelial cells and suburothelial myofibroblasts on various bladder functions have been considered at length in several reviews [14,15], and evidence presented that urothelial-released ATP alters afferent nerve excitability [16]. Amiloride, a blocker of epithelial Na+ channels, has been shown to suppress ATP release from cultured urothelial cells by a hypotonic (mechanical) stimulus [17] or by stretch of intact bladder [18]. Raising the intracellular Ca2+ concentration inhibits stimulation-evoked ATP release from urothelial cells [19].
ATP given intravesically stimulates the micturition reflex in awake freely moving rats, probably by stimulating suburothelial C-fibres, although other mediators are likely to be involved [20]. Studies of resiniferatoxin desensitization of capsaicin-sensitive afferents on detrusor overactivity induced by intravesicle ATP in conscious rats supported the view that increased extracellular ATP has a role in mechanosensory transduction and that ATP-induced facilitation of the micturition reflex is mediated, at least partly, by nerves other than capsaicin-sensitive afferents [8,21]. ATP has also been shown to induce a dose-dependent hypereflexia in conscious and anesthetized mice, largely via capsaicin-sensitive C-fibres; these effects were dose-dependently inhibited by pyridoxalphosphate-6-azonphenyl-2',4'-disulfonic acid (PPADS) and 2',3'-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) [22] (Figure 2a). P2X1 and P2X3 receptors play a fundamental role in the micturition reflex in female urethane-anesthetized rats; P2X3 receptor blockade by phenol red raised the pressure and volume thresholds for the reflex, while P2X1 receptor blockade diminished motor activity associated with voiding [23]. In TRPV1 receptor knock-out mice, release of ATP is significantly depressed [24] and afferent sensitivity to distension is attenuated, especially those effects mediated by low threshold fibres related to the micturition reflex, rather than the high threshold nociceptive fibres [25].
Four functionally distinct populations of bladder sensory neurons were identified with electrophysiological recordings when guinea-pig bladder was subjected to a range of mechanical stimuli (stretch, von Frey hair stroking and focal compression of receptive fields) and chemical stimuli (α,β-methylene ATP and capsaicin) [26]. Four different major classes of extrinsic sensory C fibres have been identified in the guinea-pig bladder: one mediates muscle mechanoresponses and was unaffected by removal of the urothelium; another was activated by stretch and α,β-meATP and was reduced by urothelial removal; the third were stretch insensitive, but could be activated by mucosal stroking with von Frey hairs or α,β-meATP and reduced by urothelium removal; while the fourth class were stretch insensitive, but could be weakly activated by mucosal stroking, but not by α,β-meATP [26].
Despite the compelling evidence in support of purinergic mechanosensory transduction from several independent laboratories (including stimulation by α,β-meATP of 2 of the 4 sensory afferents classes described by Zagorodnyuk et al. [26]), a recent paper from this group claims that urothelial release of ATP and stimulation of sensory fibres is not involved in mechanosensory transduction in the bladder, but that benzamil-sensitive stretch-activated ion channels are more likely to be involved [27]. Further experiments will hopefully resolve this issue.
In rats with detrusor overactivity induced by bladder outlet obstruction, there is an increase in expression of muscarinic receptors and an increase, but to a smaller extent, of P2X3 receptor immunostaining [28]. Cyclophosphamide-induced bladder inflammation (a model for interstitial cystitis), sensitizes and enhances P2X3 and P2X2/3 receptor function in rat bladder sensory neurons [29]. Botulinum toxin A, which has antinociceptive effects in treating interstitial cystitis, inhibits distension-mediated urothelial release of ATP in conditions of bladder inflammation [30] as well as ATP release as a cotransmitter with acetylcholine from parasympathetic nerves [31].
In summary, there is now strong evidence from several different laboratories that ATP is released from urothelial cells during distension of the bladder wall. The ATP then activates sensory nerve endings beneath the urothelium, via P2X3 and P2X2/3 receptors, that leads, via low threshold fibres, to modulation of the voiding reflex and via high threshold fibres to reach pain centres in the CNS.
Ureter
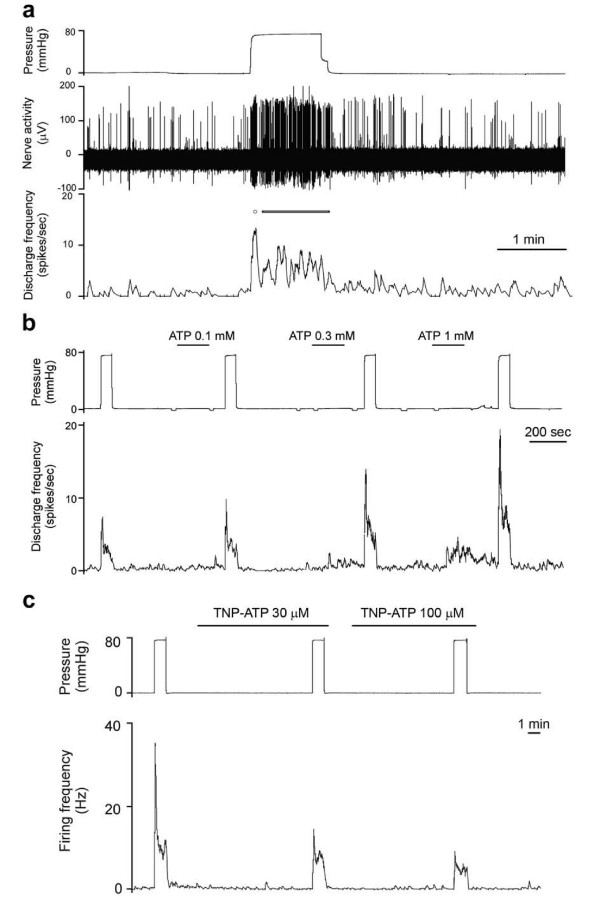
The uroteric colic that is induced by the passage of a kidney stone causes severe pain. Immunostaining of P2X3 receptors in sensory nerves in the subepithelial region was reported [32]. Multifibre recordings of ureter afferent nerves were made using a guinea pig preparation perfused in vitro [33]. Distension of the guinea-pig ureter increased spike discharge in sensory neurons, which was mimicked by ATP and reduced by ATP antagonists [33] (Figure 3a). The afferent responses consisted of both fast and slow components. The P2 receptor antagonists TNP-ATP and PPADS reduced distension-induced afferent activity (Figure 3b) and blocked the rapid and reduced the slower response to ATP, while the remaining responses were blocked by the selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine. The ecto-ATPase inhibitor (ARL-67156) produced an increase in base-line and distension-induced sensory discharge.
Figure 3.
A. Spontaneous and distension-induced activity in ureter afferent fibres. Multifibre afferent responses to rapid distension. Note that background afferent activity occurs in bursts and that ureter distension results in an initial burst of discharge (circle) followed by a phase of maintained activity (bar). B. ATP can sensitise ureter afferent fibres. An example representative of distension-induced afferent activity before and following intraluminal application of increasing concentrations of ATP. c. TNP-ATP inhibits distension-induced afferent activity. A multifibre recording to show distension-induced afferent activity in control and in the presence of TNP-ATP. (Reproduced from [33], with permission of Elsevier.)
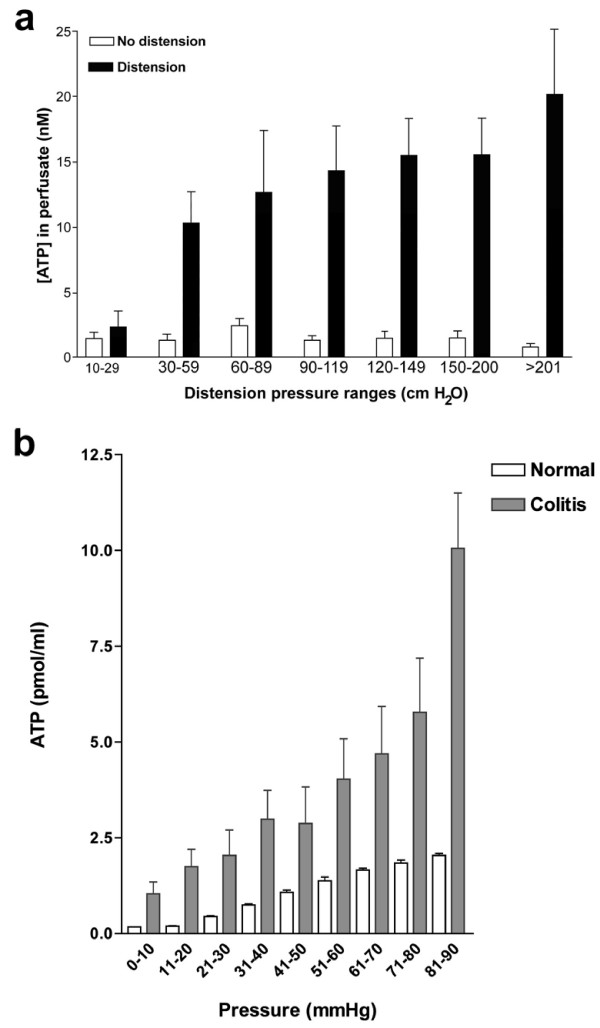
Knight et al. [34] found that distending the perfused guinea-pig ureter at pressures from 20-700 cm H2O caused a pressure-dependent release of ATP from urothelial cells, approximately 10 times the basal release levels. The ATP release was abolished by removal of the urothelium and scanning electronmicroscopy confirmed an intact urothelium after distension. ATP was not released due to activation of stretch-activated channels since gadolinium failed to affect ATP release, nor did glibenclamide, known to inhibit ATP-binding cassette proteins. However, both monensin and brefeldin A, which interfere with vesicular formation and trafficking, inhibited distension-evoked ATP release, which was Ca2+-dependent, indicating that ATP release from ureter urothelium might be largely mediated by vesicular exocytosis. In a recent study in our laboratory, experiments have been carried out to show that ATP is released from the human ureter upon distension (Figure 4a) and that human ureteric suburothelial sensory nerves express P2X3 receptors [35].
Figure 4.
A. ATP concentration ([ATP]) in perfusate immediately before and after distension of the human ureter, grouped in pressure ranges. The mean [ATP] after distension is significantly greater than before distension in each pressure range P < 0.01; n = 7, error bars represent s.e.m. (Reproduced from [35], with permission from Springer.) B. ATP concentration in luminal fluid samples from normal and inflamed rat colorectum during distension. Values are means ± SE. (Reproduced from [67] and used with permission from the American Physiological Society.)
The release of ATP only occurred above a threshold of 25-30 com H2O. This is similar to the uroteric pressure threshold for pain measured by Risholm [36]. In a recent review of the physiology and pharmacology of the human ureter, it was suggested that purinergic receptors might be target analgesics for the treatment of ureteral colicky pain and that an additional advantage might be facilitating spontaneous ureteral stone passage [37].
Gut
A hypothesis was proposed suggesting that purinergic mechanosensory transduction in the gut initiated both physiological reflex modulation of peristalsis via intrinsic sensory fibres and nociception via extrinsic sensory fibres [38,39] (Figure 2b). Evidence in support of this hypothesis was obtained from a rat pelvic sensory nerve-colorectal preparation [40]. Distension of the colorectum led to pressure-dependent increase in release of ATP from mucosal epithelial cells (Figure 4b) and also evoked pelvic nerve excitation. This excitation was mimicked by application of ATP and α,β-meATP and attenuated by the selective P2X3 and P2X2/3 antagonist TNP-ATP and by PPADS. The sensory discharge was potentiated by ARL-67156, an ATPase inhibitor. Single fibres analysis showed that high-threshold fibres were particularly affected by α,β-meATP. In addition to release of ATP from mucosal epithelial cells in the rat gut in response to distension (see [40]), ATP has also been shown to be released from human intestinal epithelial cells in response to osmotic swelling [41,42]. The interactions of ATP with other mediators that activate pelvic afferent fibres in the rat colorectum, including capsaicin, 5-hydroxytryptamine (5-HT), bradykinin, prostaglandins and substance P (SP), have been described [43,44]. In addition, TRPV1 channels are activated and sensitised by ATP that is released during distension [45,46], especially in pathological states such as colitis [47-49]. Carvacral, an agonist for TRPV3 channels, caused increased ATP release from colonic epithelial cells [50] and TRPV4 channels have also been shown to mediate stretch-release of ATP from urothelial cells [51]. LSN and PN nerves convey different mechanosensory information from the colon to the spinal cord. Forty percent of LSN afferents responded to α,β-meATP compared with only 7% of PN afferents [52].
Purinergic mechanosensory transduction has been described in other regions of the gastrointestinal tract. For instance, α,β-meATP was shown to stimulate mechanosensitive mucosal and tension receptors in mouse stomach and oesophagus leading to activity in vagal afferent nerves [53]. The sensitizing effects of P2X3 receptor agonists on mechanosensory function are induced in oesophagitis [54]. Vagal nodose (placode-derived) nociceptive fibres in guinea-pig oesophagus are exclusively C-fibres sensitive to P2X3 receptor agonists and rarely express SP, while jugular (neural crest-derived) nociceptive fibres include both A- and C-fibres and are insensitive to P2X3 agonists and mostly express SP [55]. Adenosine has been claimed to activate a subset of nociceptive vagal sensory nerves in guinea-pig oesophagus [56]. Visceral hypersensitivity may play a role in the pathogenesis of functional chest pain claimed to be of oesophageal origin. Theophylline ameliorated chest pain in 7 out of 8 patients in a clinical trial, perhaps by reducing adenosine-mediated nociception [57]. Purinergic mechanosensory transduction has also been implicated in reflex control of intestinal secretion, whereby ATP released from mucosal epithelial cells acts on P2Y1 receptors on enterochromaffin cells to release 5-HT (and ATP, which is stored and co-released with 5-HT from enterochromaffin cells [58]), which leads to regulation of secretion either directly or via intrinsic reflex activity [59].
Subepithelial fibroblasts in intestinal villi are highly sensitive to mechanical stimulation and release ATP during touch or stretch and probably act as mechanosensors [60]. The ATP released activates P2Y1 receptors on surrounding cells, which leads to intercellular propagation of Ca2+ waves and contractions in networks of subepithelial fibroblasts and a signal to sensory nerve terminals in the villi [61]. Intrinsic enteric sensory nerves express P2X3 and P2X2/3 receptors [62-66]. In P2X2 or P2X3 knock-out mice, intraluminal pressure-induced peristalsis is inhibited [65,66].
ATP release and P2X3 and P2X2/3 receptor-mediated nociceptive sensory nerve responses were enhanced in a model of colitis consisting of administration to adult rats of an intrarectal enema of 30% trinitro benzene sulfonic acid in ethanol at a dose of 80 mg/kg body weight [67]. An increase in the number of DRG neurons supplying the colorectum expressing P2X3 receptors was also claimed and there was also a substantial increase in release of ATP with distension (Figure 4b). The excitability of visceral afferent nerves is enhanced following injury or ischemia and during inflammation, for example, in irritable bowel syndrome (IBS) [68]. Under these conditions, substances are released from various sources that often act synergistically to cause sensitization of afferent nerves to mechanical or chemical stimuli. Receptors to these substances (including ATP) represent potential targets for drug treatment aimed at attenuating the inappropriate visceral sensation and subsequent reflex activities that underlie abnormal bowel function and visceral pain (see [69,70]). Chronic functional visceral hyperalgesia induced in a rat model for IBS, induced by colonic injection of 0.5% acetic acid, is associated with potentiation of ATP-evoked responses and an enhanced expression of P2X3 receptors in colon-specific sensory neurons [71]. In addition, activation of spinal A1 receptors with adenosine, following breakdown of ATP, has been shown to modulate visceral hyperalgesia [72].
Non-erosive reflux disease shows the classic symptoms of gastro-oesophageal reflux, but in the absence of oesophageal mucosal injury. Visceral hypersensitivity plays an important role in the pathology of this disease [73]. ATP has been found to sensitise vagal afferents to mechanical stimuli in the ferret oesophagus [54] and the protein expression of P2X3 receptors is increased in nodose and DRG with chronic oesophageal acid exposure in a rat model [74].
Lung
In the lung, pulmonary neuroepithelial bodies (NEBs) and more recently subepithelial receptor-like endings associated with smooth muscle (SMARs) [75] have been shown to serve as sensory organs in the lung, and P2X3and P2X2/3 receptors are expressed on a subpopulation of vagal sensory fibres that supply NEBs and SMARs with their origin in the nodose ganglia. Quinacrine staining of NEBs indicates the presence of high concentrations of ATP in their secretory vesicles, and it has been suggested that ATP is released in response to both mechanical stimulation during high-pressure ventilation and during hypoxia [76]. NEBs are oxygen sensors especially in early development, before the carotid system has matured [77]. In a study of bronchopulmonary afferent nerve activity of a mouse isolated perfused nerve-lung preparation, it was found that C fibres could be subdivided into two groups: fibres that conduct action potentials at < 0.7 ms-1 and are responsive to capsaicin, bradykinin and ATP; and fibres that conduct action potentials on an average of 0.9 ms-1 and respond vigorously to ATP, but not to capsaicin or bradykinin [78]. Both the TRPV1 receptor and P2X receptors mediate the sensory transduction of pulmonary reactive oxygen species, especially H2O2 and OH, by capsaicin-sensitive vagal lung afferent fibres [79].
Vagal C-fibres innervating the pulmonary system are derived from cell bodies situated in two distinct vagal sensory ganglia: the jugular (superior) ganglion neurons project fibres to the extrapulmonary airways (larynx, trachea, bronchus) and the lung parenchymal tissue, while the nodose (inferior) neurons innervate primarily structures within the lungs. Nerve terminals in the lungs from both jugular and nodose ganglia responded to capsaicin and bradykinin, but only the nodose C-fibres responded to α,β-meATP. Vagal afferent purinergic signaling may be involved in the hyperactivity associated with asthma and chronic obstructive pulmonary disease [80]. Th1 and Th2 cytokines reciprocally regulate P2X7 receptor function, suggesting a role for P2X7 receptors in pulmonary diseases, particularly lung hypersensitivity associated with chronic inflammatory responses [81].
Uterus
It has been hypothesised that tissue stress or damage in the uterine cervix during late pregnancy and parturition leads to ATP release and sensory signalling via P2X receptors [82]. In support of this proposal, these authors have shown P2X3 receptor immunoreactivity in axons in the cervix, in small and medium sized neurons in L6/S1 DRG and in lamina II of the L6/S1 spinal cord segments and increases in P2X3 receptor expression between pregnancy day 10 and parturition (day 22/23) in the rat cervix, although not in DRG or spinal cord.
Tooth pulp
P2X3 and P2X2/3 receptors on sensory afferents in tooth pulp appear to mediate nociception [83-86], perhaps from ATP released by mechanical distension or inflammation of odontoblasts. Mustard oil application to the tooth pulp in anaesthetised rats produced long-lasting central sensitisation, reflected by increases in neuronal mechanoreceptive field size; TNP-ATP reversibly attenuated the mustard oil sensitisation for more than 15 minutes [87].
Tongue
P2X3 receptors are abundantly present on sensory nerve terminals in the tongue [88] and ATP and α,β-meATP have been shown to excite trigeminal lingual nerve terminals in an in vitro preparation of intra-arterially perfused rat mimicking nociceptive responses to noxious mechanical stimulation and high temperature [89]. A purinergic mechanosensory transduction mechanism for the initiation of pain was considered. Taste sensations appear to be mediated both by P2Y1 receptor-activated impulses in sensory fibres in the chorda tympani [90] and by P2X2 and P2X3 and, perhaps, P2X2/3 receptors [91].
Potential Therapeutic Strategies
The search is on for selective P2X3 and P2X2/3 receptor antagonists that are orally bioavailable and do not degrade in vivo for the treatment of pain (see [92-96]). Table 1 summarises the drugs widely available. Suramin, PPADS and Reactive blue 2 have been used as non-selective antagonists at P2X3 and P2X2/3 receptors on nociceptive sensory nerve endings. PPADS has the advantage that it associates and dissociates approximately 100 to 10,000 times more slowly than other known antagonists [97]. The trinitrophenyl-substituted nucleotide, TNP-ATP, is a very potent antagonist at both P2X3 and P2X2/3 receptors. A-317491 (synthesised by Abbott Laboratories) and compound RO3 (synthesised by Roche Palo Alto) are both effective P2X3 and P2X2/3 antagonists, the latter being orally bioavailable and stable in vivo. Antagonism of P2X1 and P2X3 receptors by phenol red has been reported and tetramethylpyrazine, a traditional Chinese medicine, used as an analgesic for dysmenorrhoea, was claimed to block P2X3 receptor signalling [98].
Table 1.
P2X3 and P2X2/3 receptor antagonists
| Antagonist | P2X3 | P2X2/3 |
|---|---|---|
| Suramin and analogues NF449, NF110 | √ | √ |
| PPADS and derivatives MRS2159 & MRS2257 | √√ | √ |
| Reactive blue 2 and derivatives TNP-ATP | √ | √ |
| A-317491 (selective) | √√√ | √√√ |
| Phenol red | √√√ | √√√ |
| Tetramethylpyrazine | √√ | - |
| RO4 (orally bioavailable, stable in vivo) | √√ | ? |
| Ip5I | √√ | √√ |
| βγcarboxymethylene ATP | √√ | - |
| βγchlorophosphnomethylene ATP | ? | √√ |
| ? | √√ |
Antisense oligonucleotides have been used to down-regulate the P2X3 receptor, and in models of neuropathic (partial sciatic nerve ligation) and inflammatory (complete Freund's adjuvant pain, inhibition of the development of mechanical hyperalgesia as well as significant reversal of established hyperalgesia, were observed within 2 days of treatment [99-101]. P2X3 antisense oligonucleotides or antagonists appear to be less effective for treating discogenic (lumbar intervertebral disc) than cutaneous tissue pain [102]. Combined antisense and RNA interference-mediated treatment for specific inhibition of the recombinant rat P2X3 receptor appears to be promising for pain therapy [103]. P2X3 double-stranded short interfering RNA relieves chronic neuropathic pain and opens up new avenues for therapeutic pain strategies in man [104].
While P2X3 and P2X2/3 receptors, expressed in sensory neurons, were the predominant P2 receptor subtypes first recognised to be involved in the initiation of nociception (see [105,106]), it has become apparent more recently that P2Y receptors are also present [107] and that these are involved in modulation of pain transmission [108]. P2Y receptors appear to potentiate pain induced by chemical or physical stimuli via capsaicin sensitive TRPV1 channels and it has been proposed that the functional interaction between P2Y2 receptors and TRPV1 channels in nociceptors could underlie ATP-induced inflammatory pain [45]. P2Y1 receptor-mediated responses also enhance the sensitivity of TRPV1-mediated responses to capsaicin, protons and temperature in a protein kinase C-dependent manner [109]. ATP-induced hyperalgesia was abolished in mice lacking TRPV1 receptors.
It has been claimed that opioids inhibit purinergic nociception in rat sensory neurons and fibres via a G protein-dependent mechanism [110]. Cannabinoids act as inhibitory modulators of nociceptive responses produced by P2X2/3 receptors [111].
There are no publications to date describing clinical evaluations of P2 receptor antagonists and related purinergic compounds for the relief of pain, although clinical trials for some compounds are in progress (see [93,94]). Other therapeutic approaches to pain are being considered, including the development of agents that control the expression of receptors and those that enhance ATP breakdown. Further, while it is now clear that many different cell types release ATP physiologically in response to mechanical distortion, hypoxia, and various agents, we still await clear understanding of the mechanisms that underlie ATP transport. Hopefully, when this becomes clearer, agents will be developed that will be able to inhibit ATP release, another useful way forward as a therapeutic strategy.
Conclusion
Compelling evidence has been presented for the role of purinergic mechanosensory transduction where ATP, released from epithelial cells lining the bladder, ureter and gut during distension, acts on P2X3 and/or P2X2/3 receptors on subepithelial sensory nerve terminals to relay nociceptive messages via sensory ganglia and spinal cord to pain centres in the CNS.
Antagonists to P2X3 and P2X2/3 receptors are being explored to treat visceral pain and the possibilities for development of agents that inhibit ATP transport from epithelial cells or enhance ATP breakdown after its release are discussed.
Competing interests
The author declares that he has no competing interests.
Acknowledgements
The author thanks Dr Gillian E. Knight for her excellent editorial assistance.
References
- Bueno L. Gastrointestinal pharmacology: irritable bowel syndrome. Curr Opin Pharmacol. 2005;5:583–588. doi: 10.1016/j.coph.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kraneveld AD, Rijnierse A, Nijkamp FP, Garssen J. Neuro-immune interactions in inflammatory bowel disease and irritable bowel syndrome: future therapeutic targets. Eur J Pharmacol. 2008;585:361–374. doi: 10.1016/j.ejphar.2008.02.095. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes - a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam S, Eardley I, Morrison JFB. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Hamilton SG, Cain GR, Knight G, Ruan H-Z, Ping Y, Nunn P, Bei M, McMahon SB, Burnstock G, Ford APDW. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford APDW, Burnstock G. P2X3 knockout mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Banning AS, Cockayne DA, Ford APDW, Burnstock G, McMahon SB. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/S0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Spyer M, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri M. The physiological function of the urothelium - more than a simple barrier. Urol Int. 2006;76:289–295. doi: 10.1159/000092049. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int. 2006;97:1327–1331. doi: 10.1111/j.1464-410X.2006.06200.x. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Du S, Araki I, Mikami Y, Zakoji H, Beppu M, Yoshiyama M, Takeda M. Amiloride-sensitive ion channels in urinary bladder epithelium involved in mechanosensory transduction by modulating stretch-evoked adenosine triphosphate release. Urology. 2007;69:590–595. doi: 10.1016/j.urology.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Kagase A, Murakawa Y, Momota Y, Kawatani M. Extracellular Ca2+ regulates the stimulus-elicited ATP release from urothelium. Auton Neurosci. 2009;150:94–99. doi: 10.1016/j.autneu.2009.05.253. [DOI] [PubMed] [Google Scholar]
- Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Hu ST, Gever J, Nunn PA, Ford AP, Zhu Q-M. Cystometric studies with ATP, PPADS and TNP-ATP in conscious and anaesthetised C57BL/6 mice. J Urol. 2004;171:461–462. doi: 10.1097/01.ju.0000107964.61300.f6. [DOI] [Google Scholar]
- King BF, Knowles I, Burnstock G, Ramage A. Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetised rats. Br J Pharmacol. 2004;142:519–530. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ, Gregory SJ. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ, Spencer NJ, Gregory S. Mechanotransduction and chemosensitivity of two major classes of bladder afferents with endings in the vicinity to the urothelium. J Physiol. 2009;587:3523–3538. doi: 10.1113/jphysiol.2009.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Yoo JS, Park EY, Hong SH, Seo SI, Hwang TK. Muscarinic and purinergic receptor expression in the urothelium of rats with detrusor overactivity induced by bladder outlet obstruction. BJU Int. 2008;101:371–375. doi: 10.1111/j.1464-410X.2007.07251.x. [DOI] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Mackenzie I, Burnstock G, Dolly JO. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982;7:997–1006. doi: 10.1016/0306-4522(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. doi: 10.1016/S0022-5347(05)67618-5. [DOI] [PubMed] [Google Scholar]
- Rong W, Burnstock G. Activation of ureter nociceptors by exogenous and endogenous ATP in guinea pig. Neuropharmacology. 2004;47:1093–1101. doi: 10.1016/j.neuropharm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Calvert RC, Thompson CS, Burnstock G. ATP release from the human ureter on distension and P2X3 receptor expression on suburothelial sensory nerves. Purinergic Signalling. 2008;4:377–381. doi: 10.1007/s11302-008-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risholm L. Studies on renal colic and its treatment by posterior splanchnic block. Acta Chir Scand. 1954;184(Suppl):5–64. [PubMed] [Google Scholar]
- Canda AE, Turna B, Cinar GM, Nazli O. Physiology and pharmacology of the human ureter: basis for current and future treatments. Urol Int. 2007;78:289–298. doi: 10.1159/000100830. [DOI] [PubMed] [Google Scholar]
- Burnstock G. In: Handbook of Experimental Pharmacology. Purinergic and Pyrimidinergic Signalling II - Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Function. Abbracchio MP, Williams M, editor. 151/II. Berlin: Springer-Verlag; 2001. Purinergic signalling in gut; pp. 141–238. [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/S0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Dezaki K, Tsumura T, Maeno E, Okada Y. Receptor-mediated facilitation of cell volume regulation by swelling-induced ATP release in human epithelial cells. Jpn J Physiol. 2000;50:235–241. doi: 10.2170/jjphysiol.50.235. [DOI] [PubMed] [Google Scholar]
- Wijk T van der, Tomassen SF, Houtsmuller AB, De Jonge HR, Tilly BC. Increased vesicle recycling in response to osmotic cell swelling. Cause and consequence of hypotonicity-provoked ATP release. J Biol Chem. 2003;278:40020–40025. doi: 10.1074/jbc.M307603200. [DOI] [PubMed] [Google Scholar]
- Barthó L, Lénárd LJ, Lázár Z, Maggi CA. Connections between P2 purinoceptors and capsaicin-sensitive afferents in the intestine and other tissues. Eur J Pharmacol. 1999;375:203–210. doi: 10.1016/S0014-2999(99)00253-8. [DOI] [PubMed] [Google Scholar]
- Wynn G, Burnstock G. Adenosine 5'-triphosphate and it's relationship with other mediators that activate pelvic afferent neurons in the rat colorectum. Purinergic Signalling. 2006;2:517–526. doi: 10.1007/s11302-005-5305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60:171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol Cell Physiol. 2007;292:C1768–C1774. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- De Schepper HU, De Winter BY, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Man JG. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol. 2008;586:5247–5258. doi: 10.1113/jphysiol.2008.159731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Yamada T, Ugawa S, Ishida Y, Shimada S. TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem Biophys Res Commun. 2009;383:130–134. doi: 10.1016/j.bbrc.2009.03.143. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Blackshaw LA. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J Physiol. 2000;523:403–411. doi: 10.1111/j.1469-7793.2000.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831–842. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru F, Kollarik M. Adenosine activates a subset of nociceptive vagal sensory nerves in oesophagus [abstract] Gastroenterology. 2006;130:A252. [Google Scholar]
- Rao SS, Mudipalli RS, Mujica V, Utech CL, Zhao X, Conklin JL. An open-label trial of theophylline for functional chest pain. Dig Dis Sci. 2002;47:2763–2768. doi: 10.1023/A:1021017524660. [DOI] [PubMed] [Google Scholar]
- Winkler H, Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Wunderlich J, Christofi FL. "The force be with you": ATP in gut mechanosensory transduction. News Physiol Sci. 2003;18:43–49. doi: 10.1152/nips.01411.2002. [DOI] [PubMed] [Google Scholar]
- Furuya K, Sokabe M, Furuya S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: cell-shape-dependent ATP release and P2Y1 signaling. J Cell Sci. 2005;118:3289–3304. doi: 10.1242/jcs.02453. [DOI] [PubMed] [Google Scholar]
- Furuya S, Furuya K. Subepithelial fibroblasts in intestinal villi: roles in intercellular communication. Int Rev Cytol. 2007;264:165–223. doi: 10.1016/S0074-7696(07)64004-2. full_text. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/S1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol. 2003;551:309–322. doi: 10.1113/jphysiol.2003.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol. 2003;552:809–821. doi: 10.1113/jphysiol.2003.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn G, Bei M, Ruan H-Z, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. in press . [DOI] [PMC free article] [PubMed]
- Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: Potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- Holzer P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin Ther Targets. 2004;8:107–123. doi: 10.1517/14728222.8.2.107. [DOI] [PubMed] [Google Scholar]
- Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Straub H, Wenk M, Pogatzki-Zahn EM. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiology. 2007;107:797–806. doi: 10.1097/01.anes.0000286982.36342.3f. [DOI] [PubMed] [Google Scholar]
- Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57:674–683. doi: 10.1136/gut.2007.127886. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Shaker R, Sengupta JN. TRPV1 and P2X3 expression in vagal and spinal pathways following acid-induced esophagitis in rats [abstract] Gastroenterology. 2006;130:A133. [Google Scholar]
- Brouns I, De Proost I, Pintelon I, Timmermans JP, Adriaensen D. Sensory receptors in the airways: neurochemical coding of smooth muscle-associated airway receptors and pulmonary neuroepithelial body innervation. Auton Neurosci. 2006;126-127:307–319. doi: 10.1016/j.autneu.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Rich PB, Douillet CD, Mahler SA, Husain SA, Boucher RC. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J Trauma. 2003;55:290–297. doi: 10.1097/01.TA.0000078882.11919.AF. [DOI] [PubMed] [Google Scholar]
- Brouns I, Van Genechten J, Burnstock G, Timmermans J-P, Adriaensen D. Ontogenesis of P2X3 receptor-expressing nerve fibres in the rat lung, with special reference to neuroepithelial bodies. Biomedical Research. 2003;14:80–86. [Google Scholar]
- Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol. 2005;565:563–578. doi: 10.1113/jphysiol.2005.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaensen D, Timmermans JP. Purinergic signalling in the lung: important in asthma and COPD? Curr Opin Pharmacol. 2004;4:207–214. doi: 10.1016/j.coph.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lemaire I, Leduc N. Purinergic P2X7 receptor function in lung alveolar macrophages: pharmacologic characterisation and bidirectional regulation by Th1 and Th2 cytokines. Drug Dev Res. 2004;59:118–127. doi: 10.1002/ddr.10209. [DOI] [Google Scholar]
- Papka RE, Hafemeister J, Storey-Workley M. P2X receptors in the rat uterine cervix, lumbosacral dorsal root ganglia, and spinal cord during pregnancy. Cell Tissue Res. 2005;321:35–44. doi: 10.1007/s00441-005-1114-8. [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Alavi AM, Dubyak GR, Burnstock G. Immunohistochemical evidence for ATP receptors in human dental pulp. J Dental Res. 2001;80:476–483. doi: 10.1177/00220345010800021501. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gu J. Expression of adenosine triphosphate P2X3 receptors in rat molar pulp and trigeminal ganglia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:622–626. doi: 10.1067/moe.2002.128973. [DOI] [PubMed] [Google Scholar]
- Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–250. [PubMed] [Google Scholar]
- Hu B, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. P2X receptors in trigeminal subnucleus caudalis modulate central sensitization in trigeminal subnucleus oralis. J Neurophysiol. 2002;88:1614–1624. doi: 10.1152/jn.2002.88.4.1614. [DOI] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- Rong W, Burnstock G, Spyer KM. P2X purinoceptor-mediated excitation of trigeminal lingual nerve terminals in an in vitro intra-arterially perfused rat tongue preparation. J Physiol. 2000;524:891–902. doi: 10.1111/j.1469-7793.2000.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Toyono T, Seta Y, Ogura T, Toyoshima K. Expression of P2Y1 receptors in rat taste buds. Histochem Cell Biol. 2004;121:419–426. doi: 10.1007/s00418-004-0647-3. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Therap. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Gever J, Cockayne DA, Dillon MP, Burnstock G, Ford APDW. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Gever JR, Rothschild S, Henningsen R, Martin R, Hackos D, Panicker S, Milla ME, Oglesby I, Dillon MP, Burnstock G, Ford APDW. RO-4, a novel, potent orally bioavailable P2X3/P2X2/3 antagonist. Br J Pharmacol. 2009. in press . [DOI] [PMC free article] [PubMed]
- Carter DS, Alam M, Cai H, Dillon MP, Ford AP, Gever JR, Jahangir A, Lin C, Moore AG, Wagner PJ, Zhai Y. Identification and SAR of novel diaminopyrimidines. Part 1: The discovery of RO-4, a dual P2X3/P2X2/3 antagonist for the treatment of pain. Bioorg Med Chem Lett. 2009;19:1628–1631. doi: 10.1016/j.bmcl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Jahangir A, Alam M, Carter DS, Dillon MP, Bois DJ, Ford AP, Gever JR, Lin C, Wagner PJ, Zhai Y, Zira J. Identification and SAR of novel diaminopyrimidines. Part 2: The discovery of RO-51, a potent and selective, dual P2X3/P2X2/3 antagonist for the treatment of pain. Bioorg Med Chem Lett. 2009;19:1632–1635. doi: 10.1016/j.bmcl.2009.01.097. [DOI] [PubMed] [Google Scholar]
- Spelta V, Jiang LH, Surprenant A, North RA. Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol. 2002;135:1524–1530. doi: 10.1038/sj.bjp.0704591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SD, Gao Y, Xu CS, Xu BH, Mu SN. Effect of tetramethylpyrazine on acute nociception mediated by signaling of P2X receptor activation in rat. Brain Res. 2004;995:247–252. doi: 10.1016/j.brainres.2003.09.070. [DOI] [PubMed] [Google Scholar]
- Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Mikusa J, Bianchi B, McDonald H, Cartmell J, Faltynek C, Jarvis MF. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/S0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L. The pain of antisense: in vivo application of antisense oligonucleotides for functional genomics in pain and analgesia. Adv Drug Deliv Rev. 2003;55:1081–1112. doi: 10.1016/S0169-409X(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, Ino H, Ozawa T, Douya H, Chiba T, Moriya H. P2X3-immunoreactive primary sensory neurons innervating lumbar intervertebral disc in rats. Brain Res. 2003;989:214–220. doi: 10.1016/S0006-8993(03)03365-1. [DOI] [PubMed] [Google Scholar]
- Hemmings-Mieszczak M, Dorn G, Natt FJ, Hall J, Wishart WL. Independent combinatorial effect of antisense oligonucleotides and RNAi-mediated specific inhibition of the recombinant rat P2X3 receptor. Nucleic Acids Res. 2003;31:2117–2126. doi: 10.1093/nar/gkg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/S0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- Ruan H-Z, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochemistry and Cell Biology. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Borvendeg SJ, Schroder W, Franke H, Wirkner K, Norenberg W, Furst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov I, Yudin Y, Mamenko N, Prudnikov I, Tamarova Z, Krishtal O. Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. Neuropharmacology. 2005;48:639–647. doi: 10.1016/j.neuropharm.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Krishtal O, Lozovaya N, Fedorenko A, Savelyev I, Chizhmakov I. The agonists for nociceptors are ubiquitous, but the modulators are specific: P2X receptors in the sensory neurons are modulated by cannabinoids. Pflugers Arch. 2006;453:353–360. doi: 10.1007/s00424-006-0094-1. [DOI] [PubMed] [Google Scholar]
- Hausmann R, Rettinger J, Gerevich Z, Meis S, Kassack MU, Illes P, Lambrecht G, Schmalzing G. The suramin analog 4,4',4",4"'-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol. 2006;69:2058–2067. doi: 10.1124/mol.106.022665. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Brennan TJ, Subieta A, van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-31 a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 7491;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Expanding field of purinergic signaling. Drug Dev Res. 2001;52:1–10. doi: 10.1002/ddr.1093. [DOI] [Google Scholar]