Summary
In the absence of thymic emigration, the peripheral T cell pool is maintained by division of mature lymphocytes. We have examined the molecular interactions required for peripheral CD8+ T cell expansion in lymphopenic mice without conventional antigenic stimulation. Expansion of CD8+ T cells in lymphopenic hosts was found to be peptide specific. An antagonist peptide known to serve as a ligand for positive selection of these T cells promoted expansion; however, a control peptide that binds the same class I molecule did not. Surprisingly, the cells undergoing proliferation in lymphopenic hosts did not mature to cytotoxic effectors and displayed a partially activated surface phenotype. These data suggest that division of T cells in the periphery of lymphopenic hosts requires specific recognition of self-peptide/MHC complexes, similar to the signal for thymocyte maturation.
Introduction
During thymocyte maturation, T cells are selected for their ability to interact productively with self-MHC and their inability to recognize self with high affinity (Jameson et al., 1995). Low-affinity interactions between the TCR and self-peptide/MHC complexes deliver a signal to developing thymocytes that promotes both survival and maturation (Allen, 1994; Alam et al., 1996, 1999). The result of this process is a naive T cell population that is self-MHC restricted and tolerant. T cells leave the thymus and populate the secondary lymphoid organs, joining the peripheral T cell pool that consists of both naive and memory T cells.
Even in the absence of thymic output, peripheral homeostatic mechanisms maintain the pools of both naive and memory T cell subsets (Sprent et al., 1991; von Boehmer and Hafen, 1993). Without overt antigen exposure, both naive and memory T cells divide in the periphery. Memory cells divide rapidly, and naive T cells divide less frequently while maintaining a naive phenotype (Tough and Sprent, 1994). Peripheral T cells have a great potential for expansion and can divide many times in empty lymphoid compartments in the absence of cognate antigen (Bell et al., 1987, 1989; Rocha et al., 1989; Pereira and Rocha, 1991; Sprent et al., 1991; McDonagh and Bell, 1995; Bruno et al., 1996).
The survival of mature T cells in the periphery clearly involves a MHC-dependent signal for both naive CD8+ and CD4+ T cells. Recently it has been shown that naive CD8+ T cells rapidly disappear in MHC class I–deficient hosts (Tanchot et al., 1997a; Nesic and Vukmanovic, 1998; Freitas and Rocha, 1999). While CD4+ T cells are able to survive for a longer time in the absence of MHC class II expression, they also demonstrate a dependence on MHC class II for prolonged survival (Takeda et al., 1996; Brocker, 1997; Kirberg et al., 1997; Rooke et al., 1997). This MHC dependence is specific, as both CD8+ and CD4+ T cells require the restricting MHC allele for survival. These data suggest that ongoing TCR ligation is required for survival of naive T cells in the periphery. The peptide specificity of this interaction is of much interest.
Many studies have described the ability of mature T cells to divide spontaneously in the periphery of lymphopenic hosts. It has been shown that following transfer into SCID or nude mice, T cells divide slowly, resulting in the expansion of the peripheral lymphocyte pool (Bell et al., 1987, 1989; Rocha et al., 1989; Pereira and Rocha, 1991; Sprent et al., 1991; McDonagh and Bell, 1995; Bruno et al., 1996). Similarly, transfer of T cells into sublethally irradiated or RAG-deficient hosts results in proliferation of the transferred cells (Oehen and Brduscha-Riem, 1999). Furthermore, estimates of thymic output do not fully account for either the level of T cell turnover in the periphery or for all of the T lymphocytes that contribute to peripheral reconstitution following severe lymphocyte depletion (Scollay et al., 1980; Bell et al., 1987, 1989; Rocha et al., 1989; Bell and Sparshott, 1997). The mechanism that drives the expansion of peripheral T cells is not well understood. It has been speculated that expansion is due to encounter with antigen or growth factors; however, recent evidence also suggests that this division may be independent of cognate antigen yet involve interactions with self-peptide/MHC (Bruno et al., 1996; Mackall et al., 1996; Bell and Sparshott, 1997; Ernst et al., 1999 [this issue]; Oehen and Brduscha-Riem, 1999; Viret et al., 1999).
In this study, we directly address the specificity of the TCR interaction with self-MHC that is required for cell division in a depleted lymphoid compartment. We transferred mature CD8+ OT-1 TCR T cells, which recognize an ovalbumin-derived peptide in the context of H2-Kb (Hogquist et al., 1994), into mice expressing wild-type levels of MHC class I or into TAP-deficient mice expressing low levels of MHC class I. To assess the role of specific peptides in this process, we utilized as hosts transgenic mice expressing single H2-Kb binding peptides with different affinities for the OT-1 TCR that are independent of TAP° function for presentation. Our results imply that peripheral T cell expansion is dependent upon peptide-specific, low-affinity interactions similar to those required for positive selection in the thymus.
Results
Expansion of Peripheral CD8+ T Cells in Lymphopenic Hosts Is Impaired in TAP° Hosts
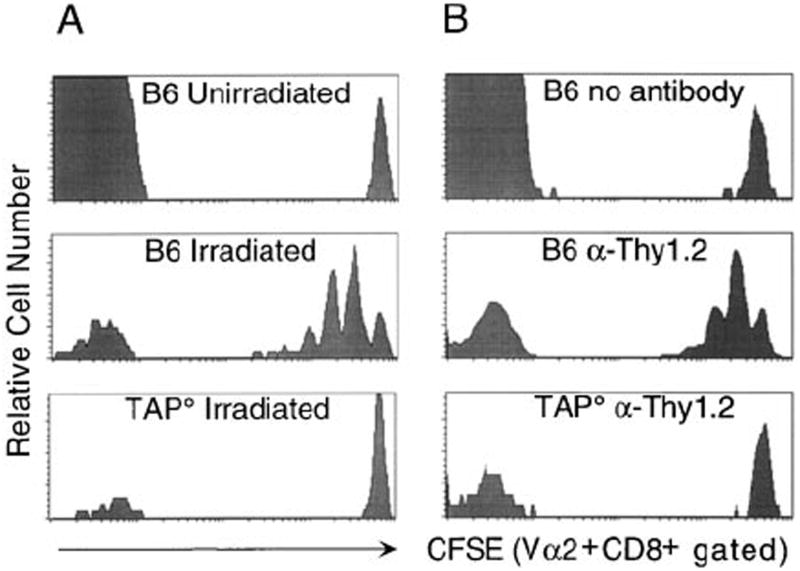
Upon transfer into irradiated or lymphopenic syngeneic hosts, both CD4+ and CD8+ T cells divide and persist (Bell et al., 1987, 1989; Rocha, 1989; Pereira and Rocha, 1991; Sprent et al., 1991; McDonagh and Bell, 1995; Bruno et al., 1996). To assess the MHC requirements for CD8+ T cell proliferation, we used OT-1 TCR (Vα2+Vβ5+) transgenic CD8+ T cells, which were labeled with the cytosolic fluorescent dye CFSE. Labeling with CFSE allows the identification of donor cells and the quantitation of donor cell proliferation by decreasing CFSE fluorescence with each division. Labeled cells were transferred into control MHC class I–positive hosts or MHC class I–low hosts (TAP°), which were irradiated (Figure 1A) or T cell depleted by treatment with anti-Thy1.2 antibody (Figure 1B). When transferred into untreated B6 hosts, the OT-1 CD8+ T cells did not measurably divide, as indicated by a single CFSE peak of Vα2+CD8+ cells. Cells transferred into irradiated or T cell–depleted B6 hosts proliferated, with the majority of transferred cells having divided at least once 5 days following transfer (Figure 1). The proliferation was not due to a side effect of irradiation, as similar results were obtained in T cell–depleted hosts (Figure 1B). Interestingly, no significant division was observed following transfer into irradiated or T cell–depleted TAP° hosts, showing that division of CD8+ T cells in lymphopenic hosts is impaired in the absence of TAP° function. We hypothesized that the inability of OT-1 CD8+ T cells to proliferate in irradiated TAP° hosts could be due to the decreased levels of surface MHC class I or, alternatively, the limited repertoire of self-peptides bound to MHC class I on TAP-deficient cells. As MHC class I appeared to be crucial in this proliferation, we became interested in the specificity of MHC recognition in this process.
Figure 1. Proliferation of Donor Cells in Lymphopenic Hosts Is MHC Class I Dependent.
CD8+ T cells were enriched from pooled spleen and lymph node of OT-1 or OT-1 Thy1.1+ mice, labeled with CFSE, and 2 × 106 CD8+Vα2+ cells were injected per recipient. Splenocytes from hosts were analyzed 5 days after transfer for detection of CFSE+ donor OT-1 cells by staining with anti-CD8 and anti-Vα2.
(A) CFSE expression is shown for OT-1 CD8+Vα2+ cells transferred into unirradiated B6, irradiated B6, or irradiated TAP° hosts. Similar results were observed with OT-1 RAG° donor cells and on days 3 and 6 post transfer in irradiated hosts.
(B) CFSE expression is shown for OT-1 Thy1.1+ CD8+Vα2+ cells transferred into untreated B6, Thy1.2-depleted B6, or Thy1.2-depleted TAP° hosts.
An Antagonist Ligand Restores Proliferation in TAP° Hosts
To determine the peptide specificity of the TCR-MHC class I–mediated interaction that promoted the expansion of OT-1 CD8+ T cells, we utilized transgenic mice expressing different MHC class I–binding peptides. The transgenic peptides were expressed as fusion proteins with an amino-terminal endoplasmic reticulum insertion signal sequence (ss). This strategy has previously been shown to allow TAP-independent loading and presentation of MHC class I–binding peptides (Bacik et al., 1994). Three lines of transgenic mice were generated, each expressing a peptide that interacts with the OT-1 TCR with different affinity when bound to H2-Kb (Alam et al., 1996, 1999). The ss-OVAp line expresses an agonist peptide that is the cognate ligand for the OT-1 TCR (Hogquist et al., 1994). The ss-R4 line expresses an antagonist peptide for the OT-1 TCR that is a low-affinity ligand which antagonizes cytolytic activity of OT-1 CTL and promotes positive selection of the OT-1 TCR in fetal thymic organ culture (Jameson et al., 1993; Hogquist et al., 1994). The ss-VSV line expresses a control H2-Kb binding peptide derived from vesicular stomatitis virus that binds Kb similarly to OVAp and R4 but does not measurably interact with the OT-1 TCR (Fremont et al., 1995; Alam et al., 1996).
Transgenic founders were bred onto the TAP° background. Expression of the ss-peptide transgenes in TAP-deficient mice did not result in detectable upregulation of surface levels of Kb molecules compared to TAP° littermates (Figure 2A). Furthermore, cells from ss-peptide transgenic mice did not stain with the OVAp-Kb complex specific monoclonal antibody, which cross reacts with R4-Kb (data not shown) (Porgador et al., 1997). To test for presentation of the ss-peptides by Kb on the surface of transgenic cells, splenocytes from ss-R4 and ss-VSV transgenic mice were used to stimulate proliferation of either R4 or VSV peptide-specific CTL. Both R4- and VSV-specific CTL lines proliferated in reponse ss-R4 and ss-VSV transgenic cells, respectively, but not to transgene negative controls, showing that the transgenes are expressed and that the peptides are presented by Kb in these mice (Figure 2B). These data indicate that the ss-R4 and ss-VSV transgenes are presenting the transgenic peptides on the cell surface, though at low levels. These transgenic peptides are presumably presented along with the low levels of self-peptides bound to the MHC class I found on TAP° cells.
Figure 2. Expression of ss-Peptide Transgenes.
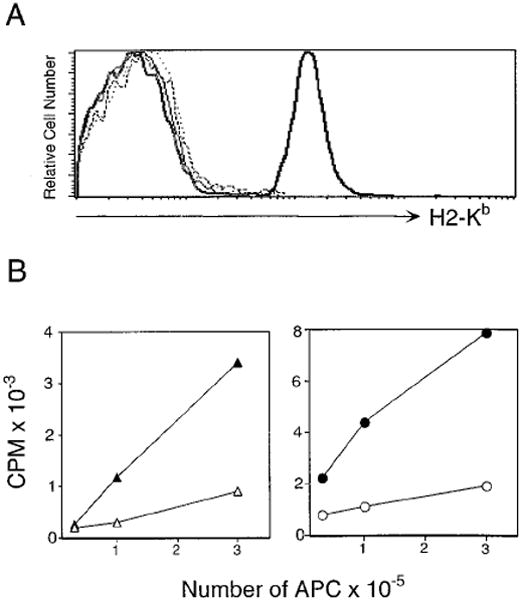
(A) Expression of H-2Kb is not upregulated in TAP° mice expressing ss-peptide transgenes. Expression levels of the MHC class I molecule Kb were compared on splenocytes from ss-OVApTAP° (thin black line), ss-R4TAP° (dashed line), ss-VSVTAP° (dotted line), TAP° (bold gray line), and B6 (bold black line) mice.
(B) ss-peptide transgenic APC stimulate proliferation of peptide-specific CTL. Filled symbols represent ss-peptide transgene positive and open symbols represent control transgene negative APC. R4-specific CTL were incubated with ss-R4TAP° (filled triangles) and control TAP° (open triangles) splenocytes (left panel). VSV-specific CTL were incubated with ss-VSV (filled circles) and control B6 (open circles) splenocytes (right panel). Peptide-specific CTL (5 × 104) were cultured with increasing numbers of irradiated APC for 72 hr and were pulsed with [3H]thymidine for 18 hr.
We next used ss-peptide-TAP° mice as recipients for CFSE-labeled OT-1 CD8+ T cells to determine if interactions between the OT-1 TCR and specific peptide/MHC complexes could restore the proliferation of donor cells in irradiated TAP° hosts. OT-1 CD8+ T cells were transferred into irradiated ss-VSVTAP°, ss-R4TAP°, ss-OVAp TAP°, or TAP° hosts (Figure 3). No division was observed among Vα2+CD8+ donor cells in the ss-VSV TAP° or TAP° hosts. No proliferation was observed in a second ss-VSV transgenic line (data not shown). As expected, extensive proliferation was observed when OT-1 CD8+ T cells were transferred into mice expressing the cognate antigen (ss-OVAp), as indicated by a total loss of CFSE label and the fact that greater than 70% of the splenocytes were Vα2+CD8+ (data not shown). Surprisingly, significant division was observed by cells transferred into ss-R4TAP° hosts of two different transgenic founder lines (Figure 3). The proliferation observed by cells transferred into ss-R4TAP° hosts was similar to that observed in B6 hosts (Figure 1), with much less division than antigen-stimulated cells (Figure 3). No differences were observed between OT-1 RAG+ (Figure 3A) and OT-1 RAG° (Figure 3B) CD8+ donor cells. Thus, proliferation of OT-1 T cells in TAP° hosts can be restored by the addition of a single Kb-binding antagonist peptide without upregulating class I levels (Figure 2). Significantly, this proliferation was mediated by a TCR interaction that is peptide specific; no signal is provided by VSV, a control peptide that binds and stabilizes Kb similarly to R4 but does not interact with the OT-1 TCR, or in TAP° mice, which display a similar low level of self-peptides/MHC class I complexes. R4, an antagonist of the OT-1 TCR, can deliver a signal for in vivo proliferation, suggesting that weak, low-affinity interactions promote division in lymphopenic hosts in the absence of conventional antigenic stimulation.
Figure 3. Proliferation of OT-1 CD8+ T Cells in Irradiated TAP° Hosts Is Restored by Expression of an Antagonist Peptide.
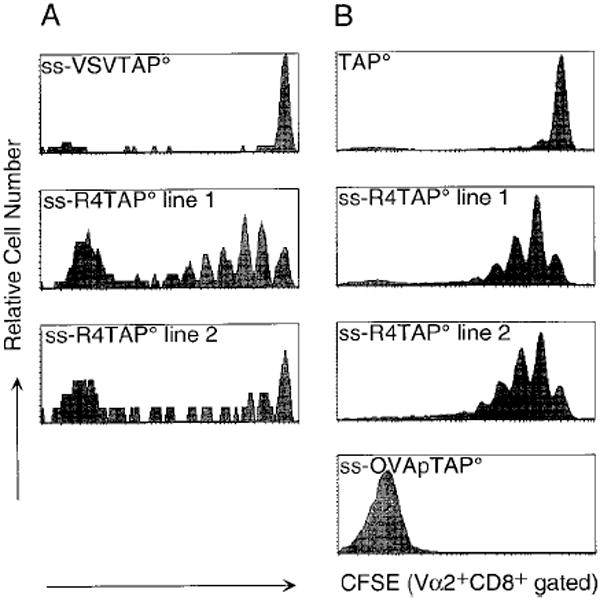
Donor cells were pooled spleen and lymph node from OT-1 (A) or OT-1 RAG° mice (B). CD8+Vα2+ CFSE-labeled cells (2 × 106) were injected per indicated host. CFSE expression is shown for CD8+Vα2+ cells from spleen on day 5 (A) or day 6 (B) post transfer. Data are representative of at least five experiments.
R4 Is Not an Agonist for Proliferation or Lysis by OT-1 CD8+ T Cells In Vitro
We next wanted to determine if the ability of R4 to induce proliferation of OT-1 CD8+ T cells in irradiated hosts is due to previously undescribed agonist properties of this peptide. R4, a single amino acid variant of the cognate ligand OVAp, has previously been shown to be a pure antagonist of OT-1 CTL activity with no agonist properties (Jameson et al., 1993; Barnden et al., 1994). R4 also efficiently promotes positive selection of OT-1 thymocytes in fetal thymic organ culture without inducing any deletion of developing thymocytes (Hogquist et al., 1994). To examine further the capacity of the R4 peptide to interact with the OT-1 TCR, we tested the ability of high concentrations of the R4 peptide to stimulate proliferation or lysis by OT-1 T cells (Figures 4A and 4B). Freshly isolated OT-1 splenocytes proliferated in response to concentrations of OVAp as low as 10 pM. No proliferation was observed to the R4 peptide at concentrations as high as 10 μM (Figure 4A). Similarly, OT-1 CTL lysed targets coated with 1 pM concentrations of OVAp but did not lyse targets at any concentration of R4 (Figure 4B).
Figure 4. Exogenous R4 and ss-R4 Transgene-Expressing Cells Do Not Stimulate Proliferation or CTL Targeting by OT-1 CD8+ T cells.
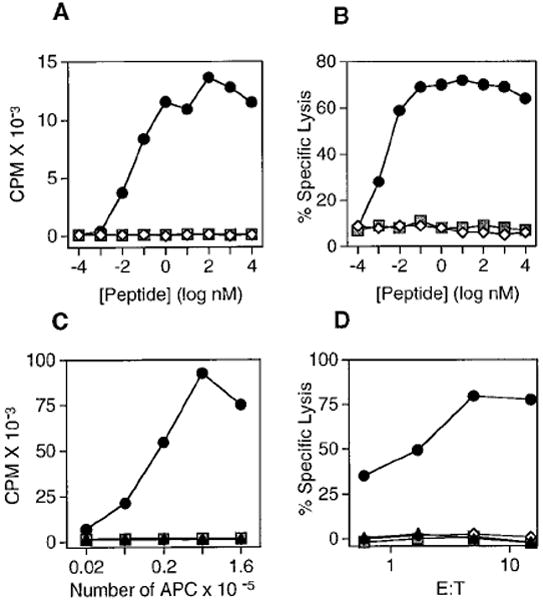
(A) OT-1 splenocytes were cultured with OVAp (circles), R4 (diamonds), or VSV (squares) peptides and irradiated B6 splenocytes and were pulsed with [3H]thymidine for 6 hr after 36 hr of culture.
(B) OT-1 CTL were used at 10:1 effector to target ratio with OVAp (circles), R4 (diamonds), or VSV (squares) peptide coated and EL4 target cells.
(C) OT-1 splenocytes were cultured with increasing numbers of irradiated splenocytes from the following mice: ss-OVATAP° (circles), B6 (triangles), ss-R4TAP° line 1 (open diamonds), ss-R4TAP° line 2 (filled diamonds), and ss-VSVTAP° (squares). The assay was pulsed with [3H]thymidine for 18 hr after 36 hr of culture.
(D) OT-1 CTL were used at the indicated effector to target ratio with 51Cr-labeled Con A blasts as targets made from the following mice: ss-OVATAP° (circles), TAP° (triangles), ss-R4TAP° line 1 (open diamonds), and ss-VSVTAP° (squares).
To assure that the ss-R4 transgenic peptide was not different from the exogenously added synthetic peptide and therefore able to mediate antigenic stimuli of OT-1 T cells, irradiated splenocytes from the different ss-peptide transgenic mice were used as stimulators in a proliferation assay (Figure 4C). OT-1 splenocytes proliferated strongly to ss-OVApTAP° antigen-presenting cells (APC) but did not proliferate to either ss-R4TAP° or ss-VSVTAP° APC, suggesting that irradiation did not induce the transgenic APC to be antigenic. Also, we found that when Con A blasts generated from the ss-peptide mice were used as targets for OT-1 CTL, only ss-OVAp blasts were lysed (Figure 4D). Hence, the cells expressing the transgenic ss-R4 peptide did not stimulate OT-1 cells in vitro. Furthermore, OT-1 cells did not proliferate when transferred into unirradiated ss-R4TAP+ hosts, and we saw no evidence of immune-mediated tissue damage in ss-R4 hosts compared to ss-OVAp hosts, which had obvious symptoms of GVH disease, following OT-1 transfer (data not shown). These findings indicate that ss-R4 is not acting as a conventional antigen in vivo.
OT-1 Cells Proliferating in Irradiated Syngeneic Hosts Are Phenotypically Distinct from Those that Have Encountered Cognate Antigen
Next, we characterized the activation state of the OT-1 cells that were proliferating in irradiated B6 and ss-R4TAP° hosts. OT-1 CD8+ T cells were phenotyped for expression of activation markers on day 6 after transfer into different hosts (Figure 5). Cells transferred into irradiated TAP° hosts did not divide, while cells transferred into ss-OVApTAP° hosts went through more than eight divisions, losing all of their CFSE label (Figure 5A). OT-1 cells transferred into irradiated B6 or ss-R4TAP° hosts proliferated at a similar rate, with most of the cells having divided 2–5 times in 6 days (more slowly than the cells that had been transferred into the host expressing ss-OVAp).
Figure 5. Surface Phenotype of CD8+ T Cells Proliferating in Various Hosts.
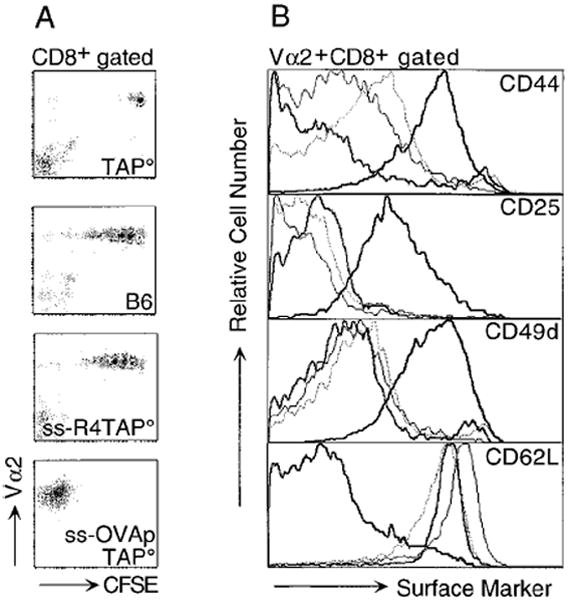
CD8+Vα2+ cells (2 × 106) from OT-1 RAG° mice were CFSE labeled, injected into irradiated hosts, and splenocytes were analyzed 6 days post transfer.
(A) This panel shows Vα2 versus CFSE levels for CD8+ cells transferred into irradiated TAP°, B6, ss-R4TAP°, and ss-OVApTAP° hosts.
(B) This panel compares levels of activation markers on the transferred OT-1 CD8+ T cells. Donor cells were gated on CD8+Vα2+ from TAP° (bold gray line), B6 (thin black line), ss-R4TAP° (dotted line), and ss-OVApTAP° (bold black line). Similar results were obtained 4 and 7 days post transfer.
Prior to transfer, OT-1 CD8+ T cells were 92% CD44lo and 96% CD62Lhi and were negative for CD25 and CD49d, typical of naive lymphocytes (data not shown). The OT-1 T cells that had proliferated in the ss-OVAp mice upregulated expression of CD44, while the nondividing cells in the TAP° hosts remained CD44lo (Figure 5B). OT-1 cells transferred into the B6 or ss-R4TAP° mice expressed CD44 at levels intermediate to donor cells in TAP° and ss-OVAp recipients. However, the OT-1 T cells proliferating in B6 and ss-R4TAP° maintained a naive phenotype for the other activation markers analyzed. OT-1 cells, which had encountered an antigenic ligand in the ss-OVAp mice, upregulated expression of CD25 and CD49d and downregulated CD62L in a manner characteristic of fully activated effector cells. The cells proliferating in the B6 or ss-R4TAP° hosts remained low for expression of CD25 and CD49d and high for CD62L as did the nondividing cells in the TAP° host, a phenotype typical of naive cells. Similar results were seen at days 4 and 7 following transfer (data not shown). Thus, the cells that undergo proliferation upon transfer into irradiated recipients expressing only self or antagonist ligands have a phenotype distinct from both naive and effector cells, suggesting a state of partial activation.
OT-1 CD8+ T Cells Proliferating in Irradiated, Syngeneic Hosts Are Not Ex Vivo Effectors
We next evaluated the functional status of OT-1 CD8+ T cells that had been transferred into irradiated recipients. Donor OT-1 CD8+ T cells were used as effectors in a CTL assay 4 days after transfer into irradiated B6, ss-R4TAP°, or ss-OVApTAP° hosts. The OT-1 cells proliferated extensively in ss-OVAp hosts, as seen by a low level of CFSE+ cells among the Vα2+CD8+ population, and had undergone several rounds of division in the irradiated B6 and ss-R4TAP° hosts (data not shown). While OT-1 cells recovered from ss-OVAp hosts lysed peptide-pulsed targets efficiently, cells recovered from the B6 and ss-R4TAP° hosts showed no CTL activity (Figure 6). Dilution of the cells recovered from ss-OVAp hosts with fresh B6 splenocytes (to normalize for total numbers of cells) did not significantly inhibit lysis of peptide-pulsed targets, showing that the failure to detect lysis by the cells recovered from B6 and ss-R4TAP° hosts was not due to the lower percentage of OT-1 cells in that population (Figure 6, filled circles). Therefore, OT-1 cells proliferating in irradiated hosts are not ex vivo CTL effectors.
Figure 6. OT-1 Cells Proliferating in Irradiated B6 and ss-R4TAP° Mice Do Not Become Cytotoxic Effector Cells.
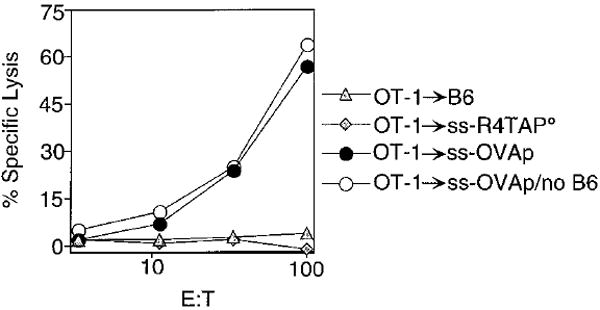
CD8+Vα2+ cells (2 × 106) from OT-1 RAG° mice were CFSE labeled and injected into irradiated B6 (triangles), ss-R4TAP° (diamonds), or ss-OVApTAP° (circles) hosts. Spleen cells were harvested on day 4 and assayed for lysis of OVAp-coated EL4 targets. Flow cytometry revealed that transferred OT-1 Vα2+CD8+ cells represented 15%, 6%, and 75% of splenocytes recovered from B6, ss-R4TAP°, or ss-OVApTAP° hosts, respectively (data not shown). The E:T was based on the number of CD8+Vα2+ cells used as effectors. The wells were normalized for total cell number by diluting cells recovered from B6 (triangles) and ss-OVApTAP° hosts (filled circles) with fresh B6 splenocytes to 6% CD8+Vα2+ cells, or cells recovered from ss-OVApTAP° hosts were used undiluted (open circles). For all effectors at all E:T ratios, lysis of EL4 targets in the absence of peptide was less than 3% (data not shown).
Discussion
In this study we show that proliferation of mature CD8+ T cells in lymphopenic hosts depends on the specific interaction of the TCR with MHC class I. The ligand that drives this proliferation in the absence of cognate antigen has low affinity for the TCR and has been previously characterized as an antagonist peptide for the T cells (Jameson et al., 1993; Alam et al., 1996, 1999). In previous in vitro studies, we have shown that the R4 variant of the OVA agonist peptide can antagonize the response of effector OT-1 cells but has no ability to stimulate effector functions (Jameson et al, 1993; Barnden et al., 1994). In fetal thymic organ culture, the R4 peptide restores positive selection of OT-1 double-positive thymocytes in MHC class I–deficient cultures (Hogquist et al., 1994). The binding of the OT-1 TCR to the OVAp/Kb agonist ligand has a Kd of 7.1 μM while binding to the R4/Kb antagonist with a Kd of 53.6 μM, approximately 7-fold lower than the cognate ligand (Alam et al., 1996). Thus, we demonstrate here that peptide/MHC complexes, which are of low affinity for the TCR and are not conventional antigens, can nevertheless stimulate proliferation of mature T cells in vivo when lymphocyte numbers are decreased.
The signal for proliferation in irradiated B6 hosts is delivered by self-peptide/MHC complexes. We propose that a ligand similar to R4, in terms of its affinity for the OT-1 TCR, exists in wild-type B6 mice, and that this ligand (or ligands) is responsible for the positive selection of OT-1 thymocytes and for the peripheral expansion of mature OT-1 T cells in a T cell depleted environment. We show that such proliferation is absent in TAP° hosts, which have low levels of self-peptide/MHC complexes (Figures 1 and 3). The trigger for proliferation is restored by expression of an antagonist peptide, R4, which is presented by MHC class I in a TAP-independent manner (Figure 3). In ss-R4TAP° mice, R4 is expressed at very low levels, as indicated by the fact that the expression of MHC class I molecules is not upregulated by the presence of the transgenic peptide (Figure 2A). A control peptide that binds the same class I molecule but has no detectable interaction with the OT-1 TCR does not support proliferation of transferred cells, showing the specificity of this interaction (Figure 3A).
It is surprising that we observe proliferation of T cells in response to an antagonist peptide, as antagonist ligands have been identified by their ability to inhibit cytolytic activity and proliferation in vitro (Evavold et al., 1993; Jameson and Bevan, 1995). Recently, these findings were confirmed in vivo when proliferation of transgenic cells to cognate antigen was inhibited by the expression of a TCR antagonist peptide in a full lymphocyte compartment (Basu et al., 1998). Many antagonist ligands have been shown to induce partial activation, such as cytokine release or killing without proliferation, and are also known to mediate positive selection of thymocytes, suggesting that they mediate a range of functions by T cells (Evavold et al., 1993; Jameson and Bevan, 1995). Nevertheless, our data clearly show that efficient proliferation of mature CD8+ T cells in lymphopenic hosts requires specific, low-affinity peptide ligands. We observe division of a very small number of cells in TAP° and ss-VSVTAP° hosts (Figures 1, 3, and 5). These cells may interact with the low levels of MHC class I on TAP° cells sufficiently to drive proliferation. Alternatively, the presence of these few dividing cells may suggest that an additional, MHC-independent mechanism exists that supports proliferation of lymphocytes when T cell numbers are reduced. It is important to note that this proliferation is not a radiation-specific phenomenon, since it also occurs in mice that have been depleted of peripheral T cells by injection of anti-Thy1 antibody (Figure 1).
Interestingly, the signal that mediates positive selection and allows developing thymocytes to survive and escape programmed cell death is thought to be provided by self-peptide/MHC complexes that serve as antagonists for effector cells (Hogquist et al., 1994; Jameson et al., 1995). These ligands are thought to provide partial signals via the TCR (Sloan-Lancaster et al., 1994; Sousa et al., 1996; Jameson and Bevan, 1995). Previously, it was thought that self-peptide/MHC ligands are not significantly perceived by mature T cells in the periphery. However, experiments published recently by several groups have shown an important role for MHC in the maintenance of both CD4+ and CD8+ T cells. In the absence of the restricting MHC allele, CD4+ and CD8+ T cells are unable to survive for prolonged periods of time (Brocker, 1997; Kirberg et al., 1997; Rooke et al., 1997; Takeda et al., 1996; Tanchot et al., 1997a; Nesic and Vukmanovic, 1998; Viret et al., 1999). New data presented here for CD8+ T cells and by Viret et al. and Ernst et al. for CD4+ T cells show an important role for specific peptide/MHC complexes in supporting proliferation of T cells when lymphocyte numbers are reduced (Ernst et al., 1999; Viret et al., 1999). Together, these data highlight a more central role for low-affinity interactions between the TCR of peripheral T cells and self-peptide/MHC in regulating homeostasis of peripheral T cell populations.
We show that OT-1 cells recovered from irradiated B6 and ss-R4TAP° hosts have no ex vivo CTL activity, while cells transferred into antigen-expressing mice become potent CTL (Figure 6). This finding appears to contradict a recent report by Oehen and coworkers which suggests that CD8+ T cells, specific for lymphocytic choriomeningitis virus (LCMV), proliferating in lymphopenic hosts can differentiate into effector cells. Their conclusion is based on the finding that these cells are at an advantage in eliminating LCMV when compared to naive T cells (Oehen and Brduscha-Riem, 1999). However, no ex vivo CTL activity was shown for these cells in this study. We suggest that due to the partially activated phenotype of cells undergoing division in lymphopenic hosts, a faster or more efficient response can be elicited in their system, but that the cells are not effectors in the absence of conventional antigen. We also show that proliferating cells display a partially activated surface phenotype, suggesting that they are not mature effectors. The cells upregulate CD44 levels as do antigen-stimulated cells, but they retain a naive phenotype for CD25, CD49d, and CD62L (Figure 5). This observation is of interest, as CD44 has been widely used as a marker for memory cells. Thus, we show proliferation of naive cells without the acquisition of a true effector or memory phenotype.
How a T cell measures the affinity between its TCR and a given ligand and then translates that measurement into a cellular readout (i.e., proliferation, differentiation) is unknown. However, the fact that T cells proliferate significantly in response to low-affinity/self ligands when T cell numbers are reduced demonstrates that this process can be altered by global homeostatic signals. We can envision at least three different mechanisms by which this could work. (1) Competition for niches or space would be reduced when T cell numbers are depleted, allowing greater accessibility to specific peptide/MHC ligands or growth factors that promote division. However, we think it is unlikely that a decrease in direct competition for peptide/MHC complexes would explain the ability of T cells to proliferate in response to self-peptide/MHC ligands for at least two reasons. First, it has been shown that CD4 and CD8 compartments can compensate for each other (for instance, in MHC class II–deficient mice, CD8 T cells compensate for the total number of T cells), suggesting that homeostasis of the T cell compartment is based on total lymphocyte number (Tanchot et al., 1997b). Second, proliferation of a small number of CD4 T cells in a lymphopenic host can be inhibited by cotransfer of a large number of CD8 T cells and vice versa (Ernst et al., 1999). Otherwise, T cells could compete for non-MHC derived stimulatory signals such as growth factors that would promote their ability to receive a stimulus from self-peptide/MHC, allowing proliferation. (2) In a second scenario, T cells may “sense” the levels of lymphocytes and regulate the lymphocyte pool. This could work directly through T cell–T cell interactions; cell contact would inhibit proliferation or decrease sensitivity to TCR signals. Alternatively, T cell number could regulate a second cell type such as APC, which would influence the sensitivity of T cells to TCR/MHC interactions. (3) Finally, T cell loss could be detected by another cell type that would in turn cause the upregulation of a ligand or factor that could promote T cell division upon TCR/MHC engagement with low-affinity ligands. While the homeostatic mechanisms that control peripheral T cell proliferation are not understood, data presented here show that TCR/MHC interactions are essential for this process to occur when the T cell pool is depleted. Similar mechanisms may regulate the low levels of division normally observed by naive T cells within a full lymphocyte compartment.
Experimental Procedures
Mice
All mice were maintained in a specific pathogen-free facility. OT-1 TCR transgenic mice, which express transgenes encoding a Vα2, Vβ5 TCR specific for an octomeric peptide derived from ovalbumin (OVAp) presented by H2-Kb (Hogquist et al., 1994), were bred to C57BL/6 (B6), RAG° (129 × B6), or B6.PL Thy1a/Cy (Thy1.1+) mice. Mice expressing transgenes encoding H2-Kb binding peptides preceded by an endoplasmic reticulum signal sequence (ss) to allow for TAP-independent presentation of the transgene-encoded peptide (ss-peptide) were generated as follows. An expression cassette including a 4 kb fragment of the MHC class I promoter and a multiple cloning site followed by 0.8 kb of SV40 intron and polyadenylation sequences was provided by Dr. Dimitris Kioussis (Jat et al., 1991). H2-Kb-binding peptides were expressed with the endoplasmic reticulum insertion signal sequence derived from mouse β2-microglobulin at the amino termini. Constructs were generated to encode one of three H2-Kb-binding peptides, OVAp, R4, and VSV. TAP-deficient RMA-S cells transfected with these constructs served as targets for peptide-specific CTL, indicating appropriate expression and processing of the Kb-binding peptides (data not shown). Vector sequences were removed by digestion with ClaI and NotI, and the constructs were injected into (H-2b × H-2d)F2 fertilized mouse eggs. Multiple founders for each construct were bred to TAP° mice (129 × B6).
Host mice were irradiated (650 cGy) 2 days prior to adoptive transfer or were given 75 μL of anti-Thy1.2 (30H12) ascites for 3 consecutive days starting 10 days prior to adoptive transfer. Donor OT-1 CD8+ T cells were pooled from spleen and lymph node and were of RAG° or Thy1.1 origin where indicated. OT-1 CD8+ RAG+ and OT-1 CD8+ Thy1.1+ donor cells were enriched by purification over nylon wool columns followed by depletion of HSA+ cells with J11D antibody plus complement lysis. Transferred cells were >70% Vα2+CD8+ for OT-1 RAG° and OT-1 RAG+, and OT-1 Thy1.1+ donor populations. Donor cells were labeled with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) prior to transfer (Lyons and Parish, 1994). Cells were washed twice in PBS 0.1% BSA, resuspended to 107 cells/ml in PBS 0.1% BSA with 10 μM CFSE, and incubated for 10 min at 37°C. Cells were washed twice with cold RPMI-1640/10% FCS followed by two washes in PBS. CFSE-labeled Vα2+CD8+ T cells (2 × 106) were transferred in 200 μL of PBS by tail vein injection into various host mice.
Antibodies and Peptides
The H2-Kb-binding peptides OVAp (SIINFEKL), R4 (SIIRFEKL), and VSV (RGYVYQGL) were synthesized by Research Genetics or with an Applied Biosystems Synergy peptide synthesizer. Peptides were quantitated by a BCA assay (Pierce) and tested for Kb binding in a RMA-S stabilization assay (Schumacher et al., 1990). The following antibodies were used for flow cytometry (Pharmingen): CD8 (53–6.7), Vα2 (B20.1), CD62L (MEL-14), CD49d (R1–2), CD25 (7D4), and CD44 (IM7). PerCP-conjugated streptavidin was used to detect biotinylated antibodies (Becton Dickinson). J11D cell supernatants were used for complement depletion of HSA+ cells (low toxicity complement, Cedarlane). 30H12 ascites was used for in vivo depletion of Thy.1.2+ host T cells. The anti-OVAp/Kb complex–specific antibody 25-D1.16 was used to stain ss-peptide transgenic splenocytes (Porgador et al., 1997). All flow cytometry was conducted on a FACS Calibur and analyzed with Cellquest software (Becton Dickinson).
CTL Lysis Assay
A CTL line was established from OT-1 splenocytes by stimulation with irradiated B6 splenocytes pulsed with 1 μM OVAp. CTL were used as effectors on days 4–7 following stimulation. Con A blasts were made by incubation of splenocytes with 2.5 μg/ml of Con A for 3 days. Targets were prepared by incubation with [51Cr]sodium chromate for 1 hr at 37°C with peptide at 5 μM where indicated. Targets were washed three times and placed in wells of round-bottom plates at 104 cells per well. Effectors and test peptides at indicated concentrations were added to each well. Plates were incubated at 37°C for 4 hr, and supernatants from each well were counted and percent specific lysis was calculated as follows: [(experimental CPM-spontaneous CPM) / (maximum CPM-spontaneous CPM)] × 100.
Proliferation Assays
R4 and VSV peptide-specific CTL lines were used as responders to detect presentation of the ss-peptides on splenocytes from transgenic mice. Anti-R4 or -VSV CTL lines were generated from peptide/IFA-immunized B6 mice and used as responders in a proliferation assay 7–9 days after stimulation with peptide-pulsed, irradiated B6 splenocytes. Splenocytes from ss-VSV or ss-R4TAP° transgenic mice were irradiated (1100 cGy) and used as stimulators at indicated numbers with 5 × 104 CTL. To detect OT-1 T cell proliferation, splenocytes from OT-1 TCR transgenic mice were added at 2 × 105 cells per well in round-bottom 96-well culture plates in RPMI-1640 10% FCS. Irradiated B6 splenocytes (5 × 105) (1100 cGy) were used as antigen presenting cells (APC) with the indicated concentrations of peptide. Alternatively, irradiated splenocytes from ss-peptide transgenic mice were added as stimulators as indicated. Plates were incubated at 37°C for 36 hr and pulsed for the indicated amount of time with 1 μCi of [3H]thymidine.
Acknowledgments
We thank Greg Barton, Pam Fink, and Jessica Hamerman for helpful discussions and critical review of the manuscript. Also, we acknowledge the excellent work of Deb Wilson for care of the animals and Katherine Forbush for generating transgenic founders. This work was supported by the following grants: National Institutes of Health AI29802 and P32CA5373209 and the Howard Hughes Medical Institute.
References
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:558–559. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NRJ, Travers PJ. Qualitiative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennik JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptide is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- Barnden MJ, Heath WR, Rodda S, Carbone FR. Peptide antagonists that promote positive selection are inefficient at T cell activation and thymocyte deletion. Eur J Immunol. 1994;24:2452–2456. doi: 10.1002/eji.1830241029. [DOI] [PubMed] [Google Scholar]
- Basu D, Williams CB, Allen PM. In vivo antagonism of a T cell response by an endogenously expressed ligand. Proc Natl Acad Sci USA. 1998;95:14332–14336. doi: 10.1073/pnas.95.24.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EB, Sparshott SM. The peripheral T-cell pool: regulation by non-antigen induced proliferation? Semin Immunol. 1997;9:347–353. doi: 10.1006/smim.1997.0092. [DOI] [PubMed] [Google Scholar]
- Bell EB, Sparshott SM, Drayson MT, Ford WL. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987;139:1379–1384. [PubMed] [Google Scholar]
- Bell EB, Sparshott SM, Drayson MT, Hunt SV. The origin of T cells in permanently reconstituted old athymic nude rats. Analysis using chromosome or allotype markers. Immunology. 1989;68:547–556. [PMC free article] [PubMed] [Google Scholar]
- Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno L, von Boehmer H, Kirberg J. Cell division in the compartment of naive and memory T lymphocytes. Eur J Immunol. 1996;26:3179–3184. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- Ernst B, Lee D-S, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Evavold BD, Sloan-Lancaster J, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;12:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Peripheral T cell survival. Curr Opin Immunol. 1999;11:152–156. doi: 10.1016/s0952-7915(99)80026-0. [DOI] [PubMed] [Google Scholar]
- Fremont DH, Stura EA, Matsumura M, Peterson PA, Wilson IA. Crystal structure of an H-2Kb-ovalbumin peptide complex reveals the interplay of primary and secondary anchor positions in the major histocompatibility complex binding groove. Proc Natl Acad Sci. 1995;92:2479–2483. doi: 10.1073/pnas.92.7.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci. 1991;12:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- McDonagh M, Bell EB. The survival and turnover of mature and immature CD8 T cells. Immunology. 1995;84:514–520. [PMC free article] [PubMed] [Google Scholar]
- Nesic D, Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pereira P, Rocha B. Post-thymic in vivo expansion of mature αβ T cells. Int Immunol. 1991;3:1077–1080. doi: 10.1093/intimm/3.11.1077. [DOI] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennick JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- Rooke R, Waltzinger C, Benoist C, Mathis D. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Heemels NT, Neefjes JJ, Kast WM, Melief CJ, Ploegh HL. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990;62:563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Sousa CR, Levine EH, Germain RN. Partial signaling by CD8+ T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Schaefer M, Hurd M, Surh CD, Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991;174:717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Rodewald H-R, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997a;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Rosado MM, Agenes F, Freitas AA, Rocha B. Lymphocyte homeostasis. Semin Immunol. 1997b;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret C, Wong SF, Janeway CA. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Hafen K. The life span of naive α/β T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]