Abstract
Under most circumstances, hepatitis B virus (HBV) is noncytopathic. However, hepatocellular regeneration that accompanies each bout of hepatitis appears to be associated with increased integration of HBV DNA fragments expressing the virus encoded hepatitis B x antigen (HBxAg). Intrahepatic HBxAg staining correlates with the intensity and progression of chronic liver disease (CLD), and additional work has shown that HBxAg blocks immune mediated killing by Fas and by tumor necrosis factor alpha (TNFα). This is not only associated with the blockage of caspase activities by HBxAg, but also by the constitutive stimulation of hepatoprotective pathways, such as nuclear factor kappa B (NF-κB), phosphoinositol 3-kinase (PI3K), and beta-catenin (β-catenin). HBxAg also appears to promote fibrogenesis, by stimulating the production of fibronectin. HBxAg also stimulates the production and activity of transforming growth factor beta 1 (TGFβ1) by several mechanisms, thereby promoting the profibrogenic and tumorigenic properties of this important cytokine. In addition, HBxAg appears to remodel the extracellular matrix (ECM) by altering the expression of several matrix metalloproteinases (MMPs), which may promote tumor metastasis. Hence, HBxAg appears to promote chronic infection by preventing immune mediated apoptosis of infected hepatocytes, by promoting the establishment and persistence of fibrosis and cirrhosis preceding the development of HCC, and by promoting the remodeling of EMC during tumor progression.
Keywords: hepatitis B virus, hepatitis B x antigen, chronic liver disease, transforming growth factor beta 1, cytokines
1. Introduction
Chronic hepatitis B virus (HBV) infection is associated with the development of chronic hepatitis, cirrhosis (end stage liver disease) and hepatocellular carcinoma (HCC) [1,2]. Although there is convincing evidence that the pathogenesis of chronic hepatitis B is immune mediated [3], and that under most conditions, HBV is not cytopathic [4], there is also evidence that sustained high levels of virus replication is associated with an elevated risk for the development of HCC [5,6]. High levels of virus replication is associated with sustained high levels of virus gene expression in the liver, which promotes recurrent inflammation and progressive chronic liver disease (CLD), the latter of which is a major risk factor for HCC. While stellate cell activation in CLD is central to the development of fibrosis and cirrhosis [7,8], and there is no firm evidence yet that HBV infects stellate cells, an important question is whether virus gene expression/replication in chronically infected hepatocytes contributes importantly to the pathogenesis of chronic infection, including the development and progression of fibrosis into cirrhosis. This question is important because cirrhosis appears to be a preneoplastic lesion that leaves patients at high risk for HCC. This idea is supported by observations showing the apparent development of HCC nodules within cirrhotic nodules in the liver [9,10], suggesting that cirrhosis may contribute importantly to tumor development. Given that both the HBV carrier state (i.e., persistent hepatitis B surface antigen [HBsAg] in the blood) and progressive CLD (i.e., the development of cirrhosis) are the most important risk factors for the development of HCC among chronically infected patients [1,2], and that the relative risk of HCC appearance in such patients is between 100 and 200 compared to uninfected patients without liver disease, raises the question as to whether development of cirrhosis results entirely from stellate cell activation or whether the virus also contributes to the lesions in the liver that develop during chronic infection. This mini-review is intended to address the putative role of HBV encoded x antigen (HBxAg) in immune mediated CLD.
2. Hepatitis B x antigen (HBxAg)
2a. HBxAg stimulates virus gene expression and replication
HBV encodes a small regulatory protein, the hepatitis B x antigen (HBxAg), that promotes virus gene expression and replication by trans-activating the virus promoters and enhancer/promoter complexes [11,12], which is thought to contribute importantly to the establishment and/or maintenance of the chronic carrier state. The carrier state is characterized by the persistence of small, spherical and variably long filamentous HBsAg particles lacking virus DNA. Some HBV carriers also demonstrate high levels of virus replication in the liver accompanied by correspondingly high titers of virus particles and virus DNA in the blood [13]. The idea that HBxAg trans-activates HBV implies that sustained high levels of intrahepatic virus gene expression provides targets for immunological recognition and long term effector function against virus infected cells. In many patients, sustained high levels of virus gene expression and replication may trigger immunological tolerance in a condition known as the asymptomatic carrier state [13], but in patients who develop a variety of antiviral immune responses, sustained virus gene expression and replication may trigger a progressive CLD in which fibrosis and cirrhosis develop. The strong and direct correlation between intrahepatic HBxAg expression and CLD [14] implies that HBxAg may contribute to this process by one or more mechanisms. For example, the inflammatory cytokines, such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), which are elevated in the serum of chronic carriers, are not only transcriptional targets of HBxAg, but also promote the expression of HBxAg in infected cells. TNFα promotes HBxAg expression by stimulating the production of free radicals, which in turn, stimulates HBxAg trans-activation function [15,16], while IL-6 stimulates HBxAg expression at the HBV enhancer 1/HBx promoter complex [17]. Since HBxAg stimulates cell growth, the HBxAg associated elevation of IL-6 may favor regeneration of cells expressing HBxAg, thereby promoting accumulation of HBxAg positive cells in the liver following successive bouts of liver cell destruction and regeneration. Moreover, the close correlation between HBxAg staining and inflammatory cell infiltrates in the liver [14] may be associated with the oxidative environment (consisting of free-radical generation) created by inflammatory cells adjacent to virus infected hepatocytes. Although CLD is an attempt by the host immune system to eliminate virus infected cells, the oxidative environment meant to kill infected cells stimulates HBxAg, which in turn stimulates hepatoprotective responses that promote chronic infection. HBxAg stimulation of the cell cycle through up-regulated expression of genes that promote cell growth, down-regulated expression of negative growth regulatory genes and tumor suppressors [18,19], combined with its ability to inhibit apoptosis, are also features characteristic of tumor cells. Together with the in vivo findings that several strains of HBx transgenic mice develop HCC [20,21], these findings provide the basis to elucidate the roles of HBxAg in the pathogenesis of CLD and HCC, which is important to identify appropriate prognostic biomarkers and therapeutic targets.
2b. X antigen and the chronic carrier state
The importance of X antigen expression to the establishment of the chronic carrier state (from which CLD then develops) was demonstrated in woodchucks that are naturally susceptible to the HBV related woodchuck hepatitis virus (WHV). Experimental infection of newborn woodchucks with a wild type clone of WHV resulted in a very high frequency of chronic infections and CLD, with virtually all animals developing HCC within 2–3 years of infection [22]. In contrast, when newborns were infected with WHV having a point mutation that prevented the expression of the X protein, the mutant genome was unable to establish chronic infection and associated CLD when introduced into newborns [23,24]. Under these circumstances, host immunity would have cleared the virus long before enough virus replication occurred in the liver to establish chronic infections. Hence, in the absence of X antigen, there probably was little or no stimulation of WHV gene expression and replication, and no liver disease, resulting in transient, subclinical infections.
2c. Integration and function of the HBx gene in chronic infections
An important characteristic of chronic HBV infection is that integration of the HBV genome into multiple sites within the host DNA readily occurs [25,26], especially during the liver regeneration that accompanies bouts of hepatitis, where the single stranded regions of HBV DNA (which most often spans the X and surface antigen genes) integrate within replication forks of host DNA. Sequence analysis of host-virus junctions in chronically infected liver and HCC have shown that most viral DNA integrates within the direct repeat (DR) sequences located at each end of the viral genome (Fig. 1) [27], although integration events characterized by variable deletions in the 3’ end of the X gene have also been described [28]. Integration with respect to host sequences appears to be more random and within many chromosomes [25,29], although multiple integration events have been found in or around the telomerase gene, h-tert [30], near or within selected oncogenes or protooncogenes [31], and within or adjacent to fragile sites or cancer susceptibility regions [32]. Integrated viral sequences often over-express full-length or 3’-truncated HBx mRNAs [33,34] (Fig. 1) that encode HBxAg polypeptides differing in trans-activation properties, with several truncated mutants being more active than the full-length molecule. Full length HBxAg transforms liver cells in culture [35,36] and gives rise to HCC in HBx transgenic mice where high levels of HBxAg expression is maintained [20,21]. Interestingly, in these transgenic mice, HBxAg expression is associated first with altered foci, then with adenomas, and finally with HCC, suggesting HBxAg associated hepatocarcinogenesis consists of multiple steps. HBxAg is selectively over-expressed in roughly 70% of chronically infected patients with HCC, and among these patients, HBxAg is present in more than 95% of their peritumor liver samples [37,38]. Staining was observed predominantly in the cytoplasm, although scattered nuclear staining was also seen in hepatocytes within cirrhotic nodules. In this context, HBxAg may alter the patterns of host gene expression that contribute importantly to the pathogenesis of chronic infection and development of tumors by two different mechanisms. As noted above, HBxAg is a trans-activating protein, but instead of trans-activating virus gene expression and replication, over-expression of HBxAg (even without virus replication going on in the same cell) alters host gene expression by constitutively activating cytoplasmic signal transduction pathways (e.g., NF-κB, src, ras, AP-1, AP-2, PI3K/AKT, JAK/STAT, Smad, and Wnt), and by binding to nuclear transcription factors (e.g., CREB, ATF-2, Oct-1, TBP, and basal transcription factors), which together contribute to increased cell growth and survival [39,40]. Importantly, integrated DNA fragments containing the HBx gene produced functional HBxAg (in trans-activation assays) [41,42] and transformed nontumorigenic liver cells into those that grew in soft agar and formed tumors in nude mice [36]. These results show that HBxAg is capable of transforming immortalized liver cells, suggesting it may act early in the transformation process. In this context, the strongest and most widespread intrahepatic HBxAg staining was observed among HBV carriers with cirrhosis [37,38], suggesting that HBxAg mediated changes in gene expression within cirrhotic nodules may be central to the development of HCC. However, HBxAg also stimulates the growth of the human adenoma cell line, HepG2, in serum free medium and in soft agar, accelerates HepG2 tumor formation in nude mice, and has been shown to promote metastasis [43,44], suggesting that HBxAg also contributes importantly to later steps in multi-step hepatocarcinogenesis. As presented below, the production of HBxAg from integrated templates appears to alter the response of infected hepatocytes to cytotoxic cytokines and activated T cells, and also promotes a profibrogenic environment and disease progression.
Fig. 1.
Model of the HBV DNA genome as it exists in the virus particle. Note that the ends of the genome, at direct repeats 1 and 2 (DR1 and DR2), respectively, are both within the X region. Integration events into host DNA often occur in or around the DR1 sequences of HBV so that the X gene is the most frequent integrated portion of HBV DNA.
3. HBxAg promotes fibrogenesis by blocking immune mediated apoptosis
Direct killing of virus infected hepatocytes by CTLs, and the recruitment of antigen nonspecific cells to the sites of intrahepatic inflammation, trigger hepatocellular injury and destruction. This process is amplified by the secretion of both pro- and anti-inflammatory cytokines during CLD that promote the development of fibrosis and cirrhosis [45]. In this context, an important question is whether HBxAg contributes to the pathogenesis of CLD by altering the response of hepatocytes to CTL and/or cytokine signaling and by altering the production of antiviral cytokines in infected hepatocytes. Interestingly, the direct relationship between HBxAg staining and the intensity of inflammation in CLD [14,37,38], suggests that HBxAg alters cytokine production and/or the response of the cell to CTL and cytokine signaling, thereby mediating survival and growth of infected cells in the presence of ongoing immune responses. If so, then the altered responses of infected cells to CTL and cytokine killing, as well as the altered production of cytokines by virus infected cells, may be among the major mechanisms whereby HBV establishes and maintains chronic infection. As outlined below, resistance to immune mediated apoptosis not only promotes the survival of infected hepatocytes, but also promotes the persistence and progression of CLD as well as the development of fibrosis.
3a. Partial protection of hepatocytes against Fas mediated apoptosis
Hepatic inflammation consists of lymphocytic infiltrations consisting of CD4+ and CD8+ T cells [46]. The latter have been shown to lyse HBV infected target cells [47] through activation of the perforin/granzyme and Fas/FasL pathways [48] in natural infections [49] and in transgenic mice [50]. Dis-regulation in proliferation vs. apoptosis during CLD is thought to distinguish normal regeneration following injury from a liver that eventually gives rise to cirrhosis and HCC [51], and it is believed that this is mediated, at least in part, by the persistent expression of HBxAg.
The centrality of the Fas/FasL system to hepatocellular death is highlighted by the observations that anti-Fas injected mice quickly die from fulminant hepatitis [52] and that primary mouse hepatocytes are exquisitely sensitive to anti-Fas treatment [53]. In CLD, activated cytotoxic T lymphocytes (CTLs) have elevated expression of FasL [54], which triggers Fas signaling and apoptosis in Fas (receptor) bearing hepatocytes. In addition, strong Fas and FasL expression are often observed in hepatocytes around areas of intense inflammation [47] and adjacent to HCC nodules [55]. Interestingly, the distribution of HBxAg is similar [14,37,38], suggesting that HBxAg may modulate Fas expression and signaling. In fact, HBxAg up-regulates expression of FasL in liver and in hepatoma cell lines [56], suggesting that HBxAg [+] cells may kill incoming CTLs and thereby “escape” immune elimination [57], while neighboring hepatocytes would remain more sensitive to killing, which would favor the survival of HBxAg [+] hepatocytes. The net outcome of this process is that the infection is not resolved in the presence of persistent and recurring immune responses, which is an environment in which stellate cells are also activated and progressive fibrotic lesions develop.
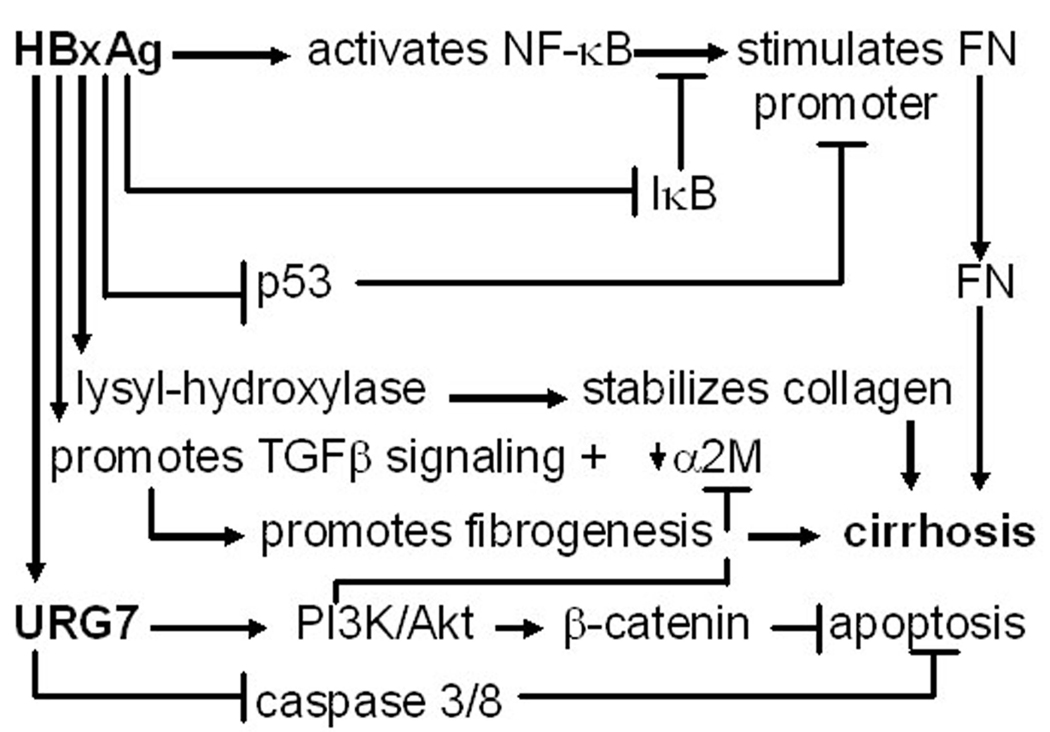
On the molecular level, when activated T or natural killer cells expressing FasL bind to hepatocytes expressing Fas receptor (or simply Fas), the receptor trimerizes, and binds to a Fas-associating protein with death domain (FADD) [58] and to procaspase 8 (or FLICE) [59]. Procaspase 8 is cleaved to yield active caspase 8, while the latter cleaves procaspase 3 (and other procaspases), which then cleave cytoplasmic and nuclear substates, resulting in apoptosis (Fig. 2) [60]. While this process does not involve mitochrondria, which also plays an important role in apoptosis, the cleavage of anti-apoptotic Bid to a proapoptotic, truncated tBid by caspase 8 [61], permits tBid to translocate to mitochrondria and trigger the release of cytochrome c, leading to the activation of caspase 9, which then activates caspase 3, and finally apoptosis (Fig. 2) [62]. Interestingly, the expression of Bid is lower in HCC than in surrounding nontumor liver [63], and a significant decrease in Bid is also observed in liver cells transfected with HBxAg [64], implying that HBxAg may block the apoptotic signal from the extrinsic (Fas) to intrinsic (mitochondrial) cell death signaling pathways [62]. Recent data has shown that the HBxAg activation of the proinflammatory NF-κB results in the up-regulation of a novel cellular gene, referred to as up-regulated gene 7 (URG7) that blocks Fas mediated killing [65] by inhibiting caspase 8 activity (Fig. 2) [66]. Since previous work showed that HBxAg blocked caspase 3 activity [67], it is possible that this effect is mediated by URG7. In addition to blocking apoptosis, URG7 also appears to override apoptosis by activating PI3K/Akt signaling [66], which had also previously been identified as one of the functions of HBxAg [68]. Interestingly, PI3K signaling results in the constitutive activation of β-catenin, which has been demonstrated to be anti-apoptotic in both HBxAg expressing and in URG7 over-expressing cells [66]. This partial protection from direct CTL activity is likely to promote prolonged activation of inflammatory and stellate cells. On the background of liver cell injury and destruction, this environment promotes the development of fibrosis and cirrhosis after repeated liver injury.
Fig. 2.
Some of the mechanisms whereby HBxAg confers resistance to immune mediated apoptosis. The pathways shown highlight TNFα Fas, β-catenin and TGFβ1 signaling.
3b. Partial protection of hepatocytes against tumor necrosis factor alpha (TNFα) mediated apoptosis
TNFα is also of central importance to the pathogenesis of HBV associated CLD. Hepatic expression of TNFα and TNFR1 are strongly up-regulated in chronic hepatitis B and correlate with the severity of liver disease [69]. Hepatocytes, Kupffer cells, other inflammatory cells, and peripheral blood mononuclear cells produce TNFα, and elevated serum levels are common among carriers [69,70]. HBV stimulates TNFα production [71], and further work has shown that HBxAg trans-activates TNFα expression in cell culture [72]. Interestingly, the ability of HBxAg to stimulate NF-κB appears to depend upon TNF signaling, since NF-κB activation by HBxAg occurred in TNF treated primary hepatocytes from TNFR1 [+/+] but not from TNFR1 [−/−] mice [73]. Recent data has also shown that HBxAg confers resistance of infected liver cells to TNFα by blocking caspase 3 and 8 activation (which are also shared with the Fas apoptotic pathway), as well as by stimulating anti-apoptotic PI3K signaling [66] (Fig. 2). Activation of PI3K/Akt may result in the inactivation of the proapoptotic BAD, and activation of the anti-apoptotic Bcl-xL, while constitutive activation of PI3K/Akt also inactivates glycogen synthase kinase 3 beta (GSK3β), resulting in the accumulation of β-catenin, which is both anti-apoptotic and protumorigenic. The ability of HBxAg to attenuate both Fas and TNFα killing results in ongoing liver damage. It is proposed that this occurs without the resolution of virus infection, favoring the development of fibrosis and cirrhosis in place of normal hepatocellular regeneration.
In addition to HBxAg, there is limited data to suggest that pregenomic HBV RNA is spliced to form a novel small protein, the hepatitis B spliced protein (HBSP) that corresponds to a fusion of the N-terminal part of the polymerase and a new ORF created by the splice event [74]. HBSP was detected in infected livers, and anti-HBSP was present in serum samples from many of the same patients [74]. Functional analysis showed that HBSP had no effect upon virus transcription or replication, although it was expressed along with markers of virus replication [74,75]. Anti-HBSP was also associated with markers of virus replication in serum [75]. Neither HBSP nor anti-HBSP correlated with liver necroinflammatory activity, but they were significantly associated with severe liver fibrosis and with elevated levels of TNFα in the serum of chronically infected patients [75]. The finding that ectopic expression of HBSP triggers apoptosis [74] may provide a stimulus for stellate and Kupffer cell activation if this occurs in vivo. Alternatively, or in addition, HBSP may contribute to fibrosis by modulating the signaling pathways of one or more cytotoxic cytokines, such as TNFα and/or TGFβ1 (see below). It is not known, however, whether these or other pathways are actually altered by HBSP.
4. HBxAg alters the composition of extracellular matrix (ECM)
Fibronectin (FN) is an important component of the extracellular matrix (ECM) that is up-regulated in the plasma of patients with CLD and down-regulated in patients who successfully respond to anti-viral treatments and resolve liver disease [76]. There is also evidence that FN in the liver sinusoids binds to HBV particles, possibly promoting the uptake of HBV by the liver [77]. Interestingly, FN is likely to be a target for HBxAg in vivo, since there is extensive co-staining between HBxAg protein and FN mRNA in chronically infected livers [78]. In addition, HBxAg stimulates the FN promoter in cell culture by activation of NF-κB and by binding to and inactivating wild type p53, which would otherwise suppress FN expression (Fig. 3) [78]. These findings suggest that HBxAg up-regulate FN expression at the transcriptional level. Despite up-regulation of FN, HBxAg expressing cells show a modest (50%) decrease in adherence to FN [79], which is associated with depressed expression of the FN receptor, α5β1 integrin. There was also an observed decrease in the levels of collagen/laminin receptor α1 subunit in HBxAg positive compared to negative cells [79], suggesting that HBxAg promotes the detachment of infected cells from the ECM. This detachment was associated with increased cell migration, indicating that changes in the ECM-cell relationship probably also contributed to metastasis. Given that activated ras and src signaling depress α5β1 expression [80], that HBxAg stimulates ras and src signaling [81], and that HBxAg disrupts adherens junctions in a src dependent manner [82], it is likely that the activation of these signaling pathways by HBxAg contribute importantly to decreased integrin expression, decreased cell adhesion, and an increased propensity for cell migration and metastasis.
Fig. 3.
Summary of various pathways whereby HBxAg may contribute importantly to the development of fibrosis and cirrhosis during CLD.
The accumulation and remodeling of ECM is a process central to the development of fibrosis and cirrhosis. In this context, HBxAg has been shown to up-regulate the expression of the enzyme, lysyl hydroxylase 3 (LH3), in liver cells. HBxAg shows extensive costaining with LH3 in cirrhotic nodules (unpublished data), suggesting that LH3 may contribute to fibrogenesis. LH3 contains three distinct catalytic activities: lysyl hydroxylase (LH), galactosyltransferase (GT), and glucosyltransferase (GGT). These domains mediate the chemical cross-linking of several collagen and collagen-like molecules [83], resulting in the stabilization of the ECM during chronic infection. In this process, hydroxylysine residues are substrates for the addition of galactose and glucose. Although no disease has been linked to abnormalities in LH3 production, LH3 knockout mice show disrupted formation of basement membranes during embryogenesis, resulting in embryonic lethality [83]. This suggests that the over-expression of LH3 during chronic HBV infection may lead to the development and persistence of basement membranes characteristic of fibrosis, which separate the intimate relationship between hepatocytes and the bloodstream observed in normal livers. Although LH3 is associated with the endoplasmic reticulum, where it may begin its modification of collagen, it has also been found in the extracellular space and in serum [84], implying that LH3 serum levels may be elevated in the blood prior to the development of HCC. In natural infection, the function of up-regulated LH3 is unknown, but it is possible that it is a mechanism whereby HBV adds sugar residues to surface antigen polypeptides during glycoprotein synthesis.
4a. HBxAg alters transforming growth factor beta 1 (TGFβ1) signaling
Transforming growth factor beta 1 (TGFβ1) is an important mediator of fibrosis and apoptosis in carriers with CLD [85,86]. In fact, there is a direct correlation between serum TGFβ1 levels, elevated aminotransferases, and fibrosis scored in liver biopsy specimens [87]. HBxAg blocks TGFβ1 mediated growth inhibition and apoptosis, in part, through the up-regulation of PI3K [68,88]. The observations that TGFβ1 expression is up-regulated by HBxAg in cell culture, in HBx transgenic mice [89], and in HCC tissue, suggest that HBxAg may confer resistance to TGFβ1 mediated growth inhibition, while uninfected cells remain sensitive, thereby favoring survival of virus infected hepatocytes. The findings that HBxAg binds the transcription factor, Egr-1, and that there is an Egr-1 binding site in the TGFβ1 promoter, further suggest that HBxAg participates in the transcriptional up-regulation of TGFβ1 expression early in infection [89], which is supported by clinical data [85,86] and is consistent with the idea that TGFβ1 plays an early, central role in the development of cirrhosis.
In addition to transcriptional trans-activation, recent work has revealed another mechanism leading to increased TGFβ1 activity. Levels of TGFβ1 activity in the supernatants of HBxAg [+] cells were significantly higher than HBxAg [−] cells, and this inversely correlated with the levels of alpha 2-macroglobulin (α2M) [90], which binds to and neutralizes TGFβ1. Suppression of α2M was inhibited by introduction of IκBα into HBxAg [+] cells, suggesting that the suppression of α2M was NF-κB dependent [90]. HBxAg may suppress α2M gene expression by either activation of NF-κB, which in turn blocks the activation of the α2M gene by STAT3, and/or by the HBxAg activation of PI3K, which then blocks α2M expression.
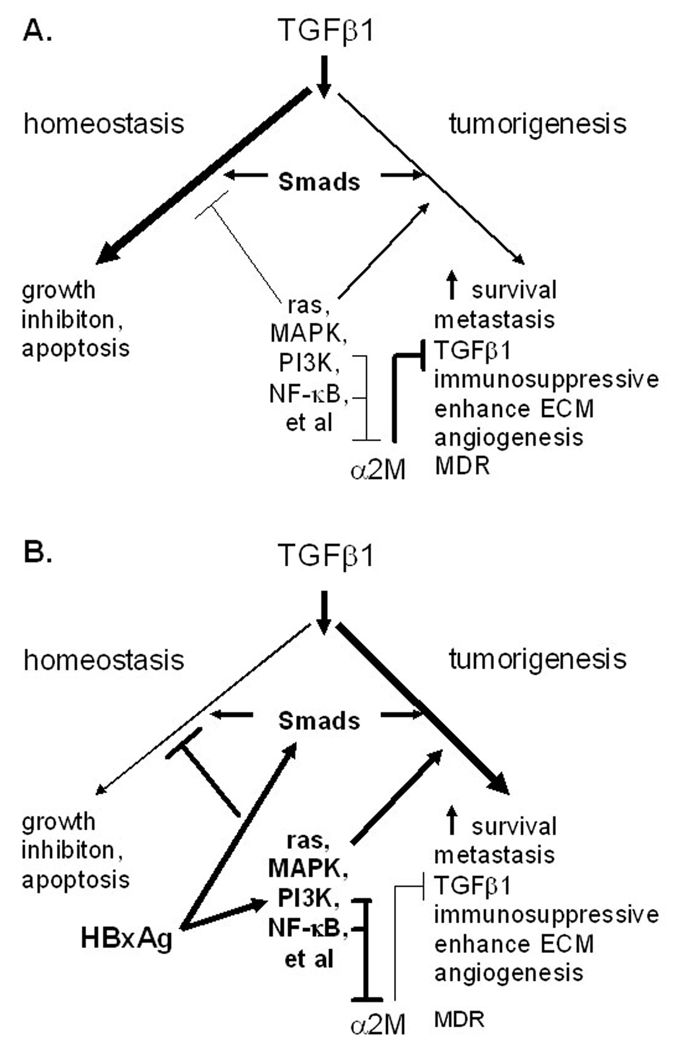
As indicated above, the findings that the HBxAg activation of NF-κB results in the up-regulated expression of FN and URG7, and down-regulation of α2M, suggest that constitutive NF-κB activation by HBxAg in chronic HBV infection promotes fibrogenesis. The activation of additional signaling pathways by HBxAg also shifts the activity of TGFβ1 from that of maintaining homeostasis by mediating growth inhibition and apoptosis as needed in the uninfected liver (Fig. 4A) to that which promotes fibrogenesis and tumorigenesis in the infected liver (Fig. 4B). Hence, HBxAg potentially promotes the development of fibrosis and cirrhosis by a number of mechanisms (Fig. 3).
Fig. 4.
Proposed changes in TGFβ1 signaling by HBxAg. Normally, TGFβ1 signaling maintains homeostatsis by acting as a negative growth regulator in the liver, but during chronic viral infection, HBxAg overrides this negative growth regulation by activating additional signaling pathways that block negative growth regulation and stimulate hepatocellular growth and survival, which may be early steps in hepatocarcinogenesis (modified from [122]).
TGFβ1 is also known to be immunosuppressive, and studies have shown that it inhibits IFNγ and anti-HBc production as well as proliferation of peripheral blood mononuclear cells (PBMC) derived from chronically infected patients, and blocks CTL activity [91]. These results suggest that HBxAg, through elevated TGFβ1 expression, is likely to be immunosuppressive, thereby promoting the development and/or persistence of the chronic carrier state.
4b. HBxAg and matrix metalloproteinases (MMPs)
Fibrosis is often the consequence of CLD independent of etiology. Under physiological conditions, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMP) promote both the synthesis and degradation of the ECM. In the context of CLD, this homeostasis is disrupted.
MMPs consist of at least 19 family members, which include collagenases, gelatinases, and stromelysins, that are distinguished by their substrate specificity. Individual MMPs are active at different times during CLD, and the active members also differ as to whether CLD is due to hepatitis B, hepatitis C, or is nonviral in origin (e.g., associated with nonalcoholic steatohepatitis or NASH) [92]. The net effect of MMP activities is tissue remodeling, which leads to the development of fibrosis and then cirrhosis prior to HCC, and then promotes angiogenesis and metastasis after the development of HCC.
Microarray analysis of HBxAg positive compared to HBxAg negative human hepatoma cells showed a significant increase in MMP-3 expression (5–7 fold), a modest increase in MMP1 (2–4 fold), and depressed integrin signaling (2–4 fold) [86]. HBxAg trans-activation function was required for elevated MMP-3 expression and enhanced cell migration in vitro. These findings are consistent with those showing that HBxAg disrupts adherens junctions and decreases cellular adhersion to ECM [79,82]. Independent results, using other cell lines, showed that HBxAg up-regulated MMP-1 and MMP-9 [94,95]. Interestingly, MMP-1 and MMP-9 are extracellular matrix-degrading MMPs that most likely promote angiogenesis, invasion and metastasis. In this context, HBV infection of hepatocytes and HepG2 cells also up-regulated MMP-9 expression, promoted MMP-9 activation and increased cell migration [96,97]. MMP-9 activation was associated with depressed expression of TIMP-1 and -3 only in HBV infected hepatoma cell lines [97], suggesting that HBV infection altered the MMP-9/TIMP-1 and MMP-9/TIMP3 ratios, thereby promoting fibrosis. Further work showed that HBxAg activation of ERK and PI3K/AKT was associated with elevated MMP-9 expression and with metastasis of liver cancer cells [95]. In addition, the fact that ERK and PI3K/AKT activate NF-κB and AP-1 signaling, and that the MMP-9 promoter has both NF-κB and AP-1 binding sites [95], suggests that HBxAg transcriptionally targets the MMP-9 gene during chronic infection. On the other hand, serum MMP-1 concentration was directly associated with the histological degree of periportal and intralobular necrosis, with portal inflammation, and with liver fibrosis [87,98], while serum levels of MMP-2 are also elevated in patients with fibrosis and liver cirrhosis [98]. HBxAg up-regulated expression of MMP-2 and of membrane type 1 matrix metalloproteinase (MT-MMP) was also associated with increased cell migration and with metastasis [43]. Independent work has shown that HBxAg up-regulates MMP-1, which in turn, activates MMP-2 [94], suggesting that HBxAg alters the expression of multiple MMPs in the development of fibrosis, cirrhosis and cancer.
Proinflammatory cytokines, such as TNFα, IFNγ, and interleukin-18 (IL-18) often up-regulate the synthesis of one of more MMPs in a cell type specific manner [45,99]. At the onset of fibrogenesis, the basement membrane-like matrices making up the ECM in the space of Disse are proteolytically degraded by MMPs. The latter are produced by stellate cells (which live in the space of DIsse) which convert normal ECM into the fibrillar ECM characteristic of fibrosis [99]. Interestingly, MMPs are expressed prior to the onset of stellate cell activation in liver fibrogenesis, suggesting that they may be made by other cell types as well, but there is evidence that MMPs are also transiently elevated during the early stages of liver regeneration, suggesting that in both contexts, elevated MMPs contribute importantly to ECM remodeling in wound healing. These observations suggest that the MMP mediated degradation of the ECM in the space of Disse triggers the activation of hepatic stellate cells [99]. If so, then the up-regulation of profibrogenic cytokines (e.g., TGFβ1) and/or MMPs by HBxAg may contribute early on as part of the trigger for the activation of stellate cells. After the initial elevations in MMPs, TIMPs are produced, which inhibit MMP activities, thereby permitting the accumulation of ECM and the development of fibrosis. The finding that elevated TIMP-1 is associated with liver fibrosis [100], and that successful antiviral treatment with lamivudine is associated with improved liver histology (decrease in fibrosis) and decreased serum levels of TIMP-1 [87], suggests that fibrosis may be reversible with sustained antiviral treatment [101]. Fibrosis may also be reversible by promoting stellate cell apoptosis, elevating MMP expression, and/or blocking TIMP expression/activity, although these approaches remain to be proven.
5. HBxAg alterations in cytokine signaling
The evidence outlined above suggest that HBxAg promotes the development of fibrosis by partially altering the outcome of Fas, TNFα and TGFβ1 signaling as well as by up-regulating TGFβ1 production in virus infected hepatocytes [89]. TNFα, which is transcriptionally up-regulated by HBxAg [72], has been shown to delay Fas mediated apoptosis by partially suppressing caspase 3 activation [102]. HBxAg also blocks caspase 3 activity, which is common to both Fas and TNFα mediated apoptosis (Fig. 2). TNFα, in turn, up-regulates interleukin-6 (IL-6) production in the liver. IL-6, which is also transcriptionally trans-activated by HBxAg [103], induces acute phase proteins and promotes liver cell regeneration during and following a bout of hepatitis. IL-6 also blocks TGFβ1 mediated apoptosis by activation of PI3K/AKT and JAK/STAT signaling pathways [104]. Activation of these same pathways by HBxAg [39,40] appear to override TGFβ1 induced hepatocellular growth arrest and apoptosis. HBxAg also appears to induce interleukin-8 (IL-8), which is a chemotactic factor for neutrophils and T cells early in the inflammatory process [105]. Interestingly, neutrophils produce high levels of MMP-8 (neutrophil collagenase) and MMP-9 (gelatinase B), which cleave and thereby modulate the function of various chemokines and ECM proteins [106]. HBxAg also induces the proinflammatory cytokine, interleukin-18 (IL-18) [107]. Given that IL-18 [107] and HBxAg [56] up-regulate FasL, this would provide a mechanism whereby HBxAg triggers the injury and lysis of CTLs, thereby protecting infected hepatocytes from Fas killing. The finding that IL-18 also stimulates many other proinflammatory cytokines, such as IL-8, IL1β, and TNFα [108], as well as natural killer (NK) and T cells [109,110], suggests that the up-regulation of IL-18 by HBxAg may be one of the major reasons underlying the close correlation between HBxAg expression and the severity of CLD. Evidence in support of this comes from HBx transgenic mice. In addition to being HBx and IL-18 positive in the liver, these mice develop hepatic necrosis, steatosis, and chronic hepatitis prior to the appearance of HCC [107,111]. In these mice, IL-18 staining correlated with regions of chronic hepatitis [111], which is consistent with epidemiologic observations showing that progressive CLD is a major risk factor for the development of HCC [1,2], and that HCC is a tumor associated with chronic inflammation. In human carriers, the latter serves to trigger stellate cell activation and fibrogenesis.
Fibroblast growth factors (FGF) consist of a family of cytokines, some of which have mitogenic, angiogenic and/or fibrogenic activities [112]. Up-regulated expression of FGFs is associated with the development of HCC in FGF transgenic mice [113,114], in c-myc/TGFα transgenic mice, and in HBxAg transgenic mice [115], suggesting that the latter may promote the over expression of FGF and/or FGF inducible genes. On the other hand, the profibrogenic cytokine, platelet derived growth factor (PDGF), appears to be a target for HBV integration [26]. In fact, there is evidence that PDGF expression is elevated in the blood of patients with fibrosis [116] while elevated intrahepatic PDGF expression correlates with inflammatory activity, fibrosis stage and the grade of histological findings [117]. Independent work has recently shown that PDGF-C transgenic mice develop activated stellate cells, fibrosis, adenomas and then HCC [118]. Elevated expression of additional profibrotic proteins, such as TGFβ1, PDGF receptors α and β, as well as TIMP-1 and TIMP-2, were also detected in preneoplastic liver. In addition to fibrosis, these changes in gene expression were associated with steatosis and liver cell dysplasia [118], strongly suggesting that PDGF plays a central role in the development of fibrosis and its progression to HCC. Although PDGF expression is elevated in HBV carriers with CLD, it is not clear whether this is triggered by the virus.
Although there is no evidence that HBV infects stellate cells, or that stellate cells express HBxAg, there is work to suggest that the conditioned medium from HBxAg expressing hepatocytes stimulated stellate cells to make collagen 1, connective tissue growth factor, alpha smooth muscle actin, MMP-2, and TGFβ. Conditioned media from HBxAg expressing cells also stimulated stellate cell proliferation [119], suggesting that HBxAg, through paracrine mechanisms, may activate stellate cells. The finding that anti-TGFβ1 blocked these paracrine effects [119] suggested that the HBxAg up-regulated expression of TGFβ1 contributed centrally to stellate cell activation. Independent observations also showed that conditioned media from HBxAg expressing liver cells stimulated the expression of angiopoietin-2 (Ang2) in hepatocytes and stellate cells [120]. Ang2 is known to be associated with both angiogenesis and inflammation at the sites of over-expression, again suggesting a role for HBxAg in stellate cell activation. These observations also strengthen the association between HBxAg expression, CLD and the development of fibrosis.
6. Conclusions
There is considerable evidence that HBV is noncytopathic, as shown by the persistently high levels of virus replication observed in asymptomatic HBV carriers. In addition, patients with immunodeficiencies often have high levels of virus but little or no liver pathology, while HBV persistently replicating to high levels in liver cell cultures demonstrate no cytopathic effects. In contrast, among chronically infected patients who develop antiviral immune responses, HBxAg appears to contribute to the development and/or progression of CLD and fibrogenesis. This is based upon clinical observations showing a correlation between HBxAg expression and markers of CLD and fibrosis. Although it is not yet clear whether these correlations represent cause and effect, cell culture studies suggest several mechanisms that are consistent with the clinical observations. For example, the ability of HBxAg to partially inhibit immune mediated apoptosis may promote the persistence of chronic inflammatory liver disease, which contributes importantly to hepatic stellate cell activation. Activated stellate cells, in turn, secrete inflammatory chemokines and express cell adhesion molecules that modulate the activation of inflammatory cells [119,121]. This cycle, whereby inflammatory and stellate cells activate each other, appears to further contribute to the observed changes in the composition of the ECM that is associated with the development and progression of fibrosis. In addition, the findings that HBxAg alters expression of several MMPs and TIMPs, may not only contribute to tissue remodeling during fibrogenesis, but also to the release of latent growth factors within the ECM that promote hepatocellular migration and promote tumor formation. Although interesting, it is not clear whether this occurs in vivo, since most of the supporting data was gathered from in vitro experiments with cell lines. However, the finding that HBxAg up-regulates the expression and activity of TGFβ1 in vitro and in vivo, suggests that it is also likely to contribute importantly to the development and progression of fibrosis. The inhibition of α2M expression by HBxAg not only impacts upon TGFβ1 activity from hepatocytes, but also upon the TGFβ1 made from other cell types during the inflammatory process. The observations that HBxAg shifts TGFβ1 signaling from that which promotes homeostasis (involving growth inhibition and apoptosis) to tumorigenesis (involving immunosuppression, enhanced ECM production, and angiogenesis) (Fig. 4) underscore some of the putative (but unproven) roles of HBxAg to the development of fibrosis and the close relationship between progressive CLD and the appearance of HCC observed in early epidemiological studies [1,2]. Given that TGFβ1 strongly up-regulates production and deposition of major ECM components, it is possible that intrahepatic HBxAg expression, in the context of CLD, is both profibrogenic, and later on, promotes the development of HCC. This suggests that in additional to nucleoside and nucleotide analogs, that strongly reduce virus replication and improve liver histology, HBxAg may be a novel and important therapeutic target among chronically patients with progressive CLD (fibrosis → cirrhosis) with little or no evidence of virus replication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and HBV. A prospective study of 22,707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 2.Beasley RP, Hwang LY. Epidemiology of hepatocellular carcinoma. In: Vyas GN, Dienstag JL, Hoofnagle JH, editors. Viral Hepatitis and Liver Disease. New York: Grune and Stratton, Inc; 1984. pp. 209–224. [Google Scholar]
- 3.Chisari FV, Ferrari C. Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 1995;17:261–281. doi: 10.1007/BF00196169. [DOI] [PubMed] [Google Scholar]
- 4.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antiviral Ther. 2006;11:669–679. [PubMed] [Google Scholar]
- 6.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN. REVEAL-HBV Study Group, Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert Rev. Mol. Med. 2003;5:1–23. doi: 10.1017/S1462399403005684. [DOI] [PubMed] [Google Scholar]
- 8.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch. Pathol. Lab. Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 9.Sadek AG, Mitchell DG, Siegelman ES, Outwater EK, Matteucci T, Hann HW. Early hepatocellular carcinoma that develops within macroregenerative nodules: growth rate depicted at serial MR imaging. Radiology. 1995;195:753–756. doi: 10.1148/radiology.195.3.7754006. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DG, Rubin R, Siegelman ES, Burk DL, Jr, Rifkin MD. Hepatocellular carcinoma within siderotic regenerative nodules: appearance as a nodule within a nodule on MR images. Radiology. 1991;178:101–103. doi: 10.1148/radiology.178.1.1845784. [DOI] [PubMed] [Google Scholar]
- 11.Keasler VV, Hodgson AJ, Madden CR, Slagle BL. Enhancement of hepatitis B virus replication by the regulatory X protein in vitro and in vivo. J. Virol. 2007;81:2656–2662. doi: 10.1128/JVI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97:977–983. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoofnagle JH, Shafritz DA, Popper H. Chronic type B hepatitis and the "healthy" HBsAg carrier state. Hepatology. 1987;7:758–763. doi: 10.1002/hep.1840070424. [DOI] [PubMed] [Google Scholar]
- 14.Jin YM, Yun C, Park C, Wang HJ, Cho H. Expression of hepatitis B virus X protein is closely correlated with the high periportal inflammatory activity of liver diseases. J. Viral Hepat. 2001;8:322–330. doi: 10.1046/j.1365-2893.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim WH, Hong F, Jaruga B, Zhang SS, Fan SJ, Liang TJ, Gao B. Hepatitis B virus X protein sensitizes primary mouse hepatocytes to ethanol- and TNF-alpha-induce apoptosis by a caspase-3-dependent mechanism. Cell. Mol. Immunol. 2005;2:40–48. [PubMed] [Google Scholar]
- 16.Meyer M, Caselmann WH, Schluter V, Schrenk R, Hofschneider PH, Baeuerle PA. Hepatitis B virus transactivator MHBxt: activation of NF-κB, selective inhibition by antioxidants and integral membrane localization. EMBO J. 1992;11:2991–3001. doi: 10.1002/j.1460-2075.1992.tb05369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno H, Kaneko S, Kobayashi K, Murakami S. Human hepatitis B virus enhancer 1 is responsive to human interleukin-6. J. Med. Virol. 1997;52:413–418. doi: 10.1002/(sici)1096-9071(199708)52:4<413::aid-jmv12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Feitelson MA, Reis HMGPV, Tufan LS, Liu J, Zhu M, Pan J, Lian Z. Hepatitis B x antigen interference with tumor suppressor pathways in hepatocarcinognesis. In: Kobarg J, editor. The Pleiotropic Functions of the Viral protein HBx in HBV Infection and the Development of Liver Cancer. Kerala, India: Research Signpost; 2008. in press. [Google Scholar]
- 19.Feitelson MA, Reis HMGPV, Liu J, Lian Z, Pan J. Hepatitis B virus X antigen (HBxAg) and cell cycle control in chronic infection and hepatocarcinogenesis. In: Zhao R, editor. Frontiers in Bioscience. Viral Infection and Cell Cycle Control. vol. 10. 2005. pp. 1558–1572. [DOI] [PubMed] [Google Scholar]
- 20.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of HBV induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 21.Koike K, Moriya K, Iino S, Yotsuyanagi H, Endo Y, Miyamura T, Kurokawa K. High level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994;19:810–819. [PubMed] [Google Scholar]
- 22.Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc. Natl. Acad. Sci. USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HS, Kaneko S, Girones R, Anderson RW, Hornbuckle WE, Tennant BC, et al. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 1993;67:1218–1226. doi: 10.1128/jvi.67.3.1218-1226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsubara K, Tokino T. Integration of HBV DNA and its implications for hepatocarcinogenesis. Mol. Biol. Med. 1990;7:243–260. [PubMed] [Google Scholar]
- 26.Murakami Y, Saigo K, Takashima H, Minami M, Okanoue T, Brechot C, et al. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut. 2005;54:1162–1168. doi: 10.1136/gut.2004.054452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dejean A, Sonigo P, Wain-Hobson S, Tiollais P. Specific hepatitis B virus integration in hepatocellular carcinoma DNA through a viral 11-base-pair direct repeat. Proc. Natl. Acad. Sci. USA. 1984;81:5350–5354. doi: 10.1073/pnas.81.17.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poussin K, Kienes H, Sirma H, Urban S, Beaugrand M, Franco D, et al. Expression of mutated hepatitis B virus X genes in human hepatocellular carcinomas. Int. J. Cancer. 1999;80:497–505. doi: 10.1002/(sici)1097-0215(19990209)80:4<497::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen JY, Harrison TJ, Lee CS, Chen DS, Zuckerman AJ. Detection of hepatitis B virus DNA in hepatocellular carcinoma: Analysis by hybridization with subgenomic DNA fragments. Hepatology. 1988;8:518–523. doi: 10.1002/hep.1840080315. [DOI] [PubMed] [Google Scholar]
- 30.Paterlini-Brechot P, Saigo K, Murakami Y, Chami M, Gozuacik D, Mugnier C, et al. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene. 2003;22:3911–3916. doi: 10.1038/sj.onc.1206492. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Chenivesse X, Henglein B, Brechot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 32.Feitelson MA, Lee JM. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007;252:157–170. doi: 10.1016/j.canlet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Diamantis ID, McGandy CE, Chen TJ, Liaw YF, Gudat F, Bianchi L. Hepatitis B X-gene expression in hepatocellar carcinoma. J. Hepatol. 1992;15:400–403. doi: 10.1016/0168-8278(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 34.Paterlini P, Poussin K, Kew M, Franco D, Brechot C. Selective accumulation of the X transcript of HBV in patients negative for HBsAg with HCC. Hepatology. 1995;21:313–321. [PubMed] [Google Scholar]
- 35.Hohne M, Schaefer S, Seifer M, Feitelson MA, Paul D, Gerlich WG. Malignant transformation of immortalized hepatocytes by HBV DNA. EMBO J. 1990;9:1137–1145. doi: 10.1002/j.1460-2075.1990.tb08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifer M, Hohne M, Schaefer S, Gerlich WH. In vitro tumorigenicity of hepatitis B virus DNA and HBx protein. J. Hepatol. 1991;13 Suppl 4:S61–S65. doi: 10.1016/0168-8278(91)90026-8. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, London WT, Lega L, Feitelson MA. HBxAg in liver from carrier patients with chronic hepatitis and cirrhosis. Hepatology. 1991;14:29–37. doi: 10.1002/hep.1840140106. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, London WT, Feitelson MA. HBxAg in HBV carrier patients with liver cancer. Cancer Res. 1991;51:4971–4977. [PubMed] [Google Scholar]
- 39.Feitelson MA, Duan LX. Hepatitis B virus x antigen in the pathogenesis of chronic infections and the development of hepatocellular carcinoma. Am. J. Pathol. 1997;150:1141–1157. [PMC free article] [PubMed] [Google Scholar]
- 40.Henkler F, Koshy R. Hepatitis B virus transcriptional activators: mechanisms and possible role in oncogenesis. J. Viral Hepat. 1996;3:109–121. doi: 10.1111/j.1365-2893.1996.tb00001.x. 1996. [DOI] [PubMed] [Google Scholar]
- 41.Takada S, Koike K. Trans-activation function of a 3' truncated X gene-cell fusion product from integrated HBV DNA in chronic hepatitis tissues. Proc. Natl. Acad. Sci. USA. 1990;87:5628–5632. doi: 10.1073/pnas.87.15.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wollersheim M, Debelka U, Hofschneider PH. A transactivating function encoded in the hepatitis B virus X gene is conserved in the integrated state. Oncogene. 1988;3:545–552. [PubMed] [Google Scholar]
- 43.Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by up-regulation of matrix metalloproteinases. Intl. J. Cancer. 2007;120:1208–1214. doi: 10.1002/ijc.22452. [DOI] [PubMed] [Google Scholar]
- 44.Ou DP, Tao YM, Chang ZG, Tang FQ, Yang LY. Hepatocellular carcinoma cells containing hepatitis B virus X protein have enhanced invasive potential conditionally. Digest. Liver Dis. 2006;38:262–267. doi: 10.1016/j.dld.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Tilg H, Kaser A, Moschen AR. How to modulate inflammatory cytokines in liver diseases. Liver Intl. 2006;26:1029–1039. doi: 10.1111/j.1478-3231.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 46.Takehara T, Hayashi N, Katayama K, Kasahara A, Fusamoto H, Kato M, et al. Two-dimensional flow cytometric analysis of intrahepatic lymphocyte subsets from patients with chronic hepatitis. Dig. Dis. Sci. 1991;36:87–91. doi: 10.1007/BF01300093. [DOI] [PubMed] [Google Scholar]
- 47.Mondelli M, Mieli-Vergani G, Bortolotti F, Cadrobbi P, Rortmann B, Alberti A, et al. Different mechanisms responsible for in vitro cell mediated cytotoxicity to autologous hepatocytes in children with autoimmune and HBsAg-positive chronic liver disease. J. Pediatr. 1985;106:899–906. doi: 10.1016/s0022-3476(85)80234-1. [DOI] [PubMed] [Google Scholar]
- 48.Kagi D, Vignauz F, Ledermann B, Burki K, Depraetere V, Nagata S, et al. Fas and perforin pathways as major mechanisms of T cell-mediated chytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 49.Hodgson PD, Grant MD, Michalak TI. Perforin and Fas/Fas ligand-mediated cytotoxicity in acute and chronic woodchuck viral hepatitis. Clin. Exp. Immunol. 1999;118:63–70. doi: 10.1046/j.1365-2249.1999.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamoto Y, Guidotti LG, Pasquetto V, Schreiber RD, Chisari FV. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J. Immunol. 1999;158:5692–5697. [PubMed] [Google Scholar]
- 51.Park YN, Chae KJ, Kim YB, Park C, Theise N. Apoptosis and proliferation in hepatocarcinogenesis related to cirrhosis. Cancer. 2001;92:2733–2736. doi: 10.1002/1097-0142(20011201)92:11<2733::aid-cncr10126>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effects of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 53.Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, et al. Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp. Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 54.Luo KX, Zhu YF, Zhang LX, He HT, Wang XS, Zhang L. In situ investigation of Fas/FasL expression in chronic hepatitis B infection and related liver diseases. J. Viral Hepat. 1997;4:303–307. doi: 10.1046/j.1365-2893.1997.00053.x. [DOI] [PubMed] [Google Scholar]
- 55.Roskams T, Libbrecht L, van Dame B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoral cells? J. Pathol. 2000;191:150–153. doi: 10.1002/(SICI)1096-9896(200006)191:2<150::AID-PATH612>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 56.Shin EC, Shin JS, Park JH, Kim SJ. Expression of fas ligand in human hepatoma cell lines: role of hepatitis B virus X (HBX) in induction of Fas ligand. Int. J. Cancer. 1999;82:587–591. doi: 10.1002/(sici)1097-0215(19990812)82:4<587::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi N, Mita E. Involvement of Fas system-mediated apoptosis in pathogenesis of viral hepatitis. J. Viral Hepat. 1999;6:357–365. doi: 10.1046/j.1365-2893.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- 58.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 59.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenk A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED 3-like protease, is recruited to the CD95 (Fas/APO-1) death inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 60.Gupta S. Molecular steps of death receptor and mitochrondrial pathways of apoptosis. Life Sci. 2001;69:2957–2964. doi: 10.1016/s0024-3205(01)01404-7. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 62.Roy S, Nicholson DW. Cross-talk in cell death signaling. J. Exp. Med. 2000;192:F21–F25. [PMC free article] [PubMed] [Google Scholar]
- 63.Chen GG, Lai PBS, Chak ECW, Xu H, Lee KM, Lau WY. Immunohistochemical analysis of proapoptotic Bid levels in chronic hepatitis, hepatocellular carcinoma and liver metastases. Cancer Lett. 2001;172:75–82. doi: 10.1016/s0304-3835(01)00630-9. [DOI] [PubMed] [Google Scholar]
- 64.Chen GG, Lai PBS, Chan PKS, Chak ECW, Yip JHY, Ho RLK, et al. Decreased expression of Bid in human hepatocellular carcinoma is related to hepatitis B virus X protein. Eur. J. Cancer. 2001;37:1695–1702. doi: 10.1016/s0959-8049(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 65.Pan J, Duan LX, Sun BS, Feitelson MA. Hepatitis B virus X protein decreases the anti-Fas induced apoptosis in human liver cells by inducing NF-κB. J. Gen. Virol. 2001;82(Part 1):171–182. doi: 10.1099/0022-1317-82-1-171. [DOI] [PubMed] [Google Scholar]
- 66.Pan J, Lian Z, Wallett S, Feitelson MA. The hepatitis B x antigen effector, URG7, blocks tumor necrosis factor alpha mediated apoptosis by activation of phosphoinositol 3-kinase and β-catenin. J. Gen. Virol. 2007;88:3275–3285. doi: 10.1099/vir.0.83214-0. [DOI] [PubMed] [Google Scholar]
- 67.Gottlob K, Fulco M, Levrero M, Greessmann A. The hepatitis B virus HBx protein inhibits caspase 3 activity. J. Biol. Chem. 1998;273:33347–33353. doi: 10.1074/jbc.273.50.33347. [DOI] [PubMed] [Google Scholar]
- 68.Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein inhibits transforming growth factor-beta -induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 2000;275:25858–25864. doi: 10.1074/jbc.M003578200. [DOI] [PubMed] [Google Scholar]
- 69.Fang JW, Shen WW, Meager A, Lau JY. Activation of the tumor necrosis factor alpha system in the liver in chronic hepatitis B virus infection. Am. J. Gastroenterol. 1996;91:748–753. [PubMed] [Google Scholar]
- 70.Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L, Moreno-Otero R, Alonso JL, Yague E, et al. Induction of tumor necrosis factor α production by human hepatocytes in chronic viral hepatitis. J. Exp. Med. 1994;179:841–848. doi: 10.1084/jem.179.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guang LX. Hepatitis B virus infection in monocytes and their functional changes in patients with chronic hepatitis B. Chin. Med. J. 1992;72:651–654. [PubMed] [Google Scholar]
- 72.Lara-Pezzi E, Majano PL, Gomez-Gonzalo M, Garcia-Monzon C, Moreno-Otero R, Levrero M, et al. The hepatitis B virus X protein up-regulates tumor necrosis factor α gene expression in hepatocytes. Hepatology. 1998;28:1013–1021. doi: 10.1002/hep.510280416. [DOI] [PubMed] [Google Scholar]
- 73.Kim WH, Hong F, Jaruga B, Hu Z, Fan S, Liang TJ, et al. Additive activation of hepatic NF-κB by ethanol and HBx or HCV core protein: involvement of TNF-αreceptor 1-independent and –dependent mechanisms. FASEB J. 2001;15:2551–2553. doi: 10.1096/fj.01-0217. [DOI] [PubMed] [Google Scholar]
- 74.Soussan P, Garreau F, Zylberberg A, Ferray C, Brechot C, Kremsdorf D. In vivo expression of a new hepatitis B virus protein encoded by a spliced RNA. J. Clin. Invest. 2000;105:55–60. doi: 10.1172/JCI8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soussan P, Tuveri R, Nalpas B, Garreau F, Zavala F, Masson A, et al. The expression of hepatitis B spliced protein (HBSP) encoded by a spliced hepatitis B virus RNA is associated with viral replication and liver fibrosis. J. Hepatol. 2003;38:343–348. doi: 10.1016/s0168-8278(02)00422-1. [DOI] [PubMed] [Google Scholar]
- 76.Kandemir O, Polat G, Sahin E, Bagdatoglu O, Camdeviren H, Kaya A. Fibronectin levels in chronic viral hepatitis and response of this protein to interferon therapy. Hepato-Gastroenterol. 2004;51:811–814. [PubMed] [Google Scholar]
- 77.Budkowska A, Bedossa P, Groh F, Louise A, Pillot J. Fibronectin of human liver sinusoids binds hepatitis B virus: identification by an anti-idiotypic antibody bearing the internal image of the pre-S2 domain. J. Virol. 1995;69:840–848. doi: 10.1128/jvi.69.2.840-848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Norton PA, Reis MGPV, Feitelson MA. Activation of fibronection gene expression by hepatitis B virus X antigen. J. Viral Hepat. 2004;11:332–341. doi: 10.1111/j.1365-2893.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 79.Lara-Pezzi E, Majano PL, Yanez-Mo M, Gomez-Gonzalo M, Carretero M, Moreno-Otero R, et al. Effect of the hepatitis B virus HBx protein on integrin-mediated adhesion to and migration on extracellular matrix. J. Hepatol. 2001;34:409–415. doi: 10.1016/s0168-8278(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 80.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol. Biol. Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein NP, Schneider RJ. Activation of src family kinases by hepatitis B virus HBx protein and coupled signaling to ras. Mol. Cell Biol. 1997;17:6427–6436. doi: 10.1128/mcb.17.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lara-Pezzi E, Roche S, Andrisani OM, Sanchez-Madrid F, Lopez-Cabrera M. The hepatitis B virus protein induces adherens junction disruption in a src-dependent manner. Oncogene. 2001;20:3323–3331. doi: 10.1038/sj.onc.1204451. [DOI] [PubMed] [Google Scholar]
- 83.Myullyla R, Wang C, Keikkinen J, Juffer A, Lampela O, Risteli M, et al. Expanding the lysyl hydroxylase toolbox: New insights into the localization and activities of lysyl hydroxylase 3 (LH3) J. Cell. Physiol. 2007;212:323–329. doi: 10.1002/jcp.21036. [DOI] [PubMed] [Google Scholar]
- 84.Salo AM, Wang C, Sipila L, Sormunen R, Vapola M, Kervinen P, et al. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J. Cell. Physiol. 2006;207:644–653. doi: 10.1002/jcp.20596. [DOI] [PubMed] [Google Scholar]
- 85.Castilla A, Prieto J, Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alpha therapy. N. Engl. J. Med. 1991;324:933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 86.Liu F, Li B, Nan Y. The effect of serum TGFβ1 of patients with chronic hepatitis B in liver fibrosis. Chin. J. Hepatol. 1999;7:196–198. [PubMed] [Google Scholar]
- 87.Flisiak R, Al-Kadasi H, Jaroszewicz J, Prokopowicz D, Flisiak I. Effect of lamivudine treatment on plasma levels of transforming growth factor beta1, tissue inhibitor of metalloproteinases-1 and metalloproteinase-1 in patients with chronic hepatitis B. World J. Gastroenterol. 2004;10:2661–2665. doi: 10.3748/wjg.v10.i18.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oshikawa O, Tamjura S, Kawata S, Ito N, Tsushima H, Kiso S, et al. The effect of hepatitis B virus X gene expression on response to growth inhibition by transforming growth factor beta 1. Biochem. Biophys. Res. Commun. 1996;222:770–773. doi: 10.1006/bbrc.1996.0819. [DOI] [PubMed] [Google Scholar]
- 89.Yoo YD, Ueda H, Park K, Flanders KC, Lee YI, Jay G, et al. Regulation of transforming growth factor-beta 1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV pathogenesis. J. Clin. Invest. 1996;97:388–395. doi: 10.1172/JCI118427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan J, Clayton MM, Feitelson MA. HBxAg promotes transforming growth factor β1 (TGFβ1) activity by up-regulation of TGFβ1 and down-regulation of alpha 2- macroglobulin. J. Gen. Virol. 2004;85:275–282. doi: 10.1099/vir.0.19650-0. [DOI] [PubMed] [Google Scholar]
- 91.Kakumu S, Ito Y, Takayanagi M, Yoshioka K, Wakita T, Ishikawa T, et al. Effect of recombinant human transforming growth factor beta 1 on immune responses in patients with chronic hepatitis B. Liver. 1993;13:62–68. doi: 10.1111/j.1600-0676.1993.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 92.Ljumovic D, Diamantis I, Alegakis AK, Kouroumalis EA. Differential expression of matrix metalloproteinases in viral and non-viral chronic liver diseases. Clin. Chim. Acta. 2004;349:203–211. doi: 10.1016/j.cccn.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 93.Yu FL, Liu HJ, Lee JW, Liao MH, Shih WL. Hepatitis B virus X protein promotes cell migration by inducing matrix metalloproteinase-3. J. Hepatol. 2005;42:520–527. doi: 10.1016/j.jhep.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 94.Lara-Pezzi E, Gome-Gaviro MV, Galvez BG, Mira E, Iniguez MA, Fresno M, et al. The hepatitis B virus X protein promotes tumor cells invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J. Clin. Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase 9 gene expression through activation of ERKs and PI3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123–1125. doi: 10.1096/fj.03-1429fje. [DOI] [PubMed] [Google Scholar]
- 96.Chung TW, Moon SK, Lee YC, Kim JG, Ko JH, Kim CH. Enhanced expression of matrix metalloproteinase-9 by hepatitis B virus infection in liver cells. Arch. Biochem. Biophys. 2002;408:147–154. doi: 10.1016/s0003-9861(02)00522-2. [DOI] [PubMed] [Google Scholar]
- 97.Kim JR, Kim CH. Association of high activity of matrix metalloproteinase-9 to low levels of tissue inhibitors of metalloproteinase-1 and -3 in human hepatitis B –viral hepatoma cells. Int. J. Biochem. Cell Biol. 2004;36:2293–2306. doi: 10.1016/j.biocel.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 98.Yin SS, Li XM, Wang BE, Wang TL, Jia JD, Qian LX. The relationship of serum metalloproteinase with the severity of liver fibrosis and inflammation. Chin. J. Hepatol. 2004;12:666–668. [PubMed] [Google Scholar]
- 99.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J. Gastroenterol. Hepatol. 2006;21:S88–S91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flisiak R, Maxwell P, Prokopowicz D, Timms PM, Panasiuk A. Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta-1: possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepato-Gastroenterol. 2002;49:1369–1372. [PubMed] [Google Scholar]
- 101.Farrell GC, Teoh NC. Management of chronic hepatitis B virus infection: a new era of disease control. Int. Med. J. 2006;36:100–113. doi: 10.1111/j.1445-5994.2006.01027.x. [DOI] [PubMed] [Google Scholar]
- 102.Takehara T, Hayashi N, Mita E, Kanto T, Tatsumi T, Sasaki Y, et al. Delayed Fas-mediated hepatocytes apoptosis during liver regeneration in mice: hepatoprotective role of TNF alpha. Hepatology. 1998;27:1643–1651. doi: 10.1002/hep.510270625. [DOI] [PubMed] [Google Scholar]
- 103.Lee Y, Park US, Choi I, Yoon SK, Park YM, Lee YI. Human interleukin 6 gene is activated by hepatitis B virus X protein in human hepatoma cells. Clin. Cancer Res. 1998;4:1711–1717. [PubMed] [Google Scholar]
- 104.Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189–205. doi: 10.1016/s1359-6101(00)00034-4. [DOI] [PubMed] [Google Scholar]
- 105.Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, et al. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J. Biol. Chem. 1991;266:13759–13763. [PubMed] [Google Scholar]
- 106.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee MO, Choi YH, Shin EC, Kang HJ, Kim YM, Jeong SY, et al. Hepatitis B virus X protein induced expression of interleukin 18 (IL-18): a potential mechanism for liver injury caused by hepatitis B virus (HBV) infection. J. Hepatol. 2002;37:380–386. doi: 10.1016/s0168-8278(02)00181-2. [DOI] [PubMed] [Google Scholar]
- 108.Puren AJ, Fantuzzi O, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, et al. IFN-gamma-inducing factor up-regulates Fas ligands mediated cytotoxic activity of murine natural killer cell clones. J. Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 110.Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell. Immunol. 1996;173:230–235. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- 111.Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X protein. J. Hepatol. 1999;31:123–132. doi: 10.1016/s0168-8278(99)80172-x. [DOI] [PubMed] [Google Scholar]
- 112.Huang X, Yu C, Jin C, Kobayashi M, Bowles CA, Wang F, et al. Ectopic activity of fibroblast growth factor receptor 1 in hepatocytes accelerates hepatocarcinogenesis by driving proliferation and vascular endothelial growth factor-induced angiogenesis. Cancer Res. 2006;66:1481–1490. doi: 10.1158/0008-5472.CAN-05-2412. [DOI] [PubMed] [Google Scholar]
- 113.Qiu WH, Zhou BS, Chu PG, Chen WG, Chung C, Shih J, et al. Over-expression of fibroblast growth factor receptor 3 in human hepatocellular carcinoma. World J. Gastroenterol. 2005;11:5266–5272. doi: 10.3748/wjg.v11.i34.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, et al. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am. J. Pathol. 2000;156:1253–1261. doi: 10.1016/S0002-9440(10)64996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang BB, Cai WM, Weng HL, Hu ZR, Lu J, Zheng M, et al. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J. Gastroenterol. 2003;9:2490–2496. doi: 10.3748/wjg.v9.i11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lou SM, Li YM, Wang KM, Cai WM, Weng HL. Expression of platelet-derived growth factor-BB in liver tissues of patients with chronic hepatitis B. World J. Gastroenterol. 2004;10:385–588. doi: 10.3748/wjg.v10.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin-Vilchez S, Sanz-Cameno P, Rodriguez-Munoz Y, Majano PL, Molina-Jimenez F, Lopez-Cabrera M, Moreno-Otero R, Lara-Pezzi E. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology. 2008;47:1872–1883. doi: 10.1002/hep.22265. [DOI] [PubMed] [Google Scholar]
- 120.Sanz-Cameno P, Martin-Vilchez S, Lara-Pezzi E, Borque MJ, Salmeron J, Munoz de Rueda P, Solis JA, Lopez-Cabrera M, Moreno-Otero R. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: role of HBV x protein. Am. J. Pathol. 2006;169:1215–1222. doi: 10.2353/ajpath.2006.051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gutierrez-Reyes G, Gutierrez-Ruiz MC, Kershenobich D. Liver fibrosis and chronic viral hepatitis. Arch. Med. Res. 2007;38:644–651. doi: 10.1016/j.arcmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 122.Akhurst RJ. TGF-beta antagonists: why suppress a tumor suppressor? J. Clin. Invest. 2002;109:1533–1536. doi: 10.1172/JCI15970. [DOI] [PMC free article] [PubMed] [Google Scholar]