Abstract
Using a partially purified bovine brain extract, our lab identified three novel endogenous acyl amino acids in mammalian tissues. The presence of numerous amino acids in the body and their ability to form amides with several saturated and unsaturated fatty acids indicated the potential existence of a large number of heretofore unidentified acyl amino acids. Reports of several additional acyl amino acids that activate G-protein coupled receptors (e.g., N-arachidonoyl glycine, N-arachidonoyl serine) and transient receptor potential channels (e.g., N-arachidonoyl dopamine, N-acyl taurines) suggested that some or many novel acyl amino acids could serve as signaling molecules. Here, we used a targeted lipidomics approach including specific enrichment steps, nano-LC/MS/MS, high-throughput screening of the datasets with a potent search algorithm based on fragment ion analysis, and quantification using the multiple reaction monitoring mode in Analyst software to measure the biological levels of acyl amino acids in rat brain. We successfully identified 50 novel endogenous acyl amino acids present at 0.2 to 69 pmol g−1 wet rat brain.
Keywords: nervous system, lipid, mass spectrometry
Conjugates of fatty acids and amino acids, lipoamino acids, have been identified in biological systems for at least 50 years (1), mostly from bacteria and marine organisms. Significant biological activities have been demonstrated by many lipoamino acids, such as N-(3-hydroxydecanoyl)-L-serine (2), acyl homoserine lactone (3), mololipids (4), and lipstatin (5). Simple lipoamino acids, called acyl amino acids, contain one fatty acid and one amino acid linked by an amide bond. A family of lipids similar to acyl amino acids, containing only one amide bond connecting a fatty acid with dopamine, amine, or ethanolamine, has been identified in mammalian tissues. These have gained prominence with the identification of the endocannabinoid N-arachidonoyl ethanolamide (6). Other similar bioactive lipids include the putative sleep-inducing lipid oleamide (7), the anti-inflammatory peroxisome proliferator-activated receptor (PPAR)α activator N-palmitoyl ethanolamide (3, 4), and the pro-nociceptive transient receptor potential vanilloid type 1 (TRPV1) channel activators N-arachidonoyl dopamine and N-oleoyl dopamine (8–10).
Earlier we reported the presence of N-arachidonoyl glycine (NAGly) and N-arachidonoyl gamma aminobutyric acid (GABA) in rats (11), which produced anti-inflammatory and anti-nociceptive effects (11–14). Recently NAGly has been identified as an endogenous ligand for the orphan G-protein coupled receptor GPR18 (15). More recent reports of acyl amino acids with biological effects in mammalian tissues include N-arachidonoyl serine, a vasodilator (16); a series of N-acyl taurines (17) including N-arachidonoyl taurine, a TRPV1, and TRPV4 receptor activator (18); and N-palmitoyl glycine, which inhibits the heat-evoked response of nociceptive neurons in the rat dorsal horn (19). NAGly, N-arachidonoyl GABA, N-arachidonoyl serine, and N-arachidonoyl taurine also bind to GPR72 at submicromolar concentrations (20). A host of psychopharmacological effects have also been reported for several synthetic acyl amino acids (21–25) although their existence as endogenous molecules is unknown. Collectively, these findings underscore the realization that the brain and other tissues produce several acyl amino acids and highlight the fact that some of them also exhibit significant biological activities.
The observation that NAGly could be formed by an enzymatically-mediated condensation of arachidonic acid and glycine (11) led us to speculate that a large number of acyl amino acids are formed in mammalian tissues (11), a hypothesis that has received support from the identification of several additional acyl amino acids (26). We have already developed a targeted lipidomic strategy that led to identification and quantification of previously identified acyl amino acids with high efficiency and sensitivity using nano-HPLC/MS/MS and information dependent acquisition (IDA) mode from Analyst QS software, automated computer analysis of MS and MS/MS data, and database searching against mass spectra of synthesized analogs (27). Herein, we report identification of 50 novel acyl amino acids from extracts of rat brains or bovine spinal cords using a targeted lipidomics method. The results demonstrate the occurrence of a large family of acyl amino acids in mammals.
MATERIALS AND METHODS
Materials
Chloroform, tetrahydrofuran, triethylamine, dichloromethane, 2-butyl alcohol, HPLC grade methanol, and acetonitrile were purchased from VWR International, Plainview, NY. Mass spectrometry/HPLC grade acetic acid, formic acid, ammonium acetate, amino acid ethyl ester hydrochlorides, amino acid methyl ester hydrochlorides, and LiOH were purchased from Sigma-Aldrich (St. Louis, MO). HPLC water was purified to a quality of ≥18.0 MΩ cm (e.g., Milli-Q system, Millipore, Billerica, MA). All fatty acids were purchased from Nu-Chek Prep (Elysian, MN). NAGly-d8 was purchased from Cayman Chemical, Ann Arbor, MI.
Tissue samples
Bovine spinal cords were obtained from the local slaughter house. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in polycarbonate cages (four per cage) with corncob bedding and stainless steel wire tops under standard conditions (22 ± 1°C, 55% relative humidity, 12 h light/dark cycle). Rats were provided with Teclad 7001 Rat Chow (Harlan, Madison, WI) and water ad libitum. Rats (160–190 g) were randomly selected for experiments. All protocols were approved by the Indiana University Institutional Animal Care and Use Committee.
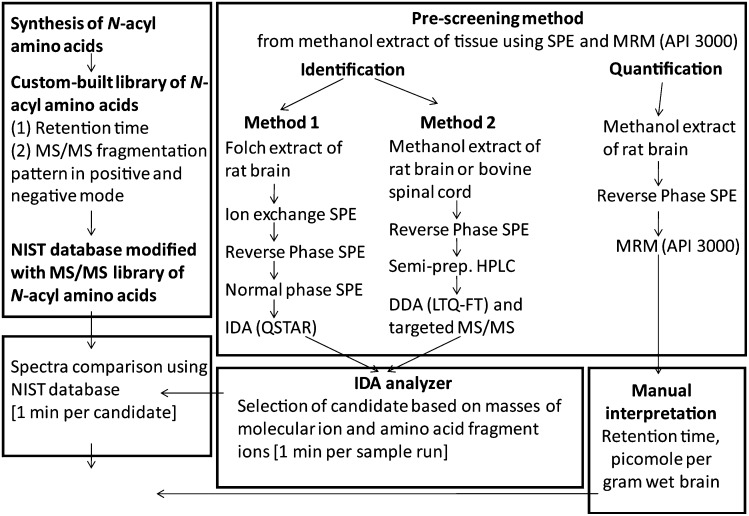
Work flow of the targeted lipidomics approach
Synthesis.
Synthesis of N-arachidonoyl GABA and L-serine was first described in 1995 using iso-butylchloroformate and N-methylmorpholine to couple arachidonic acid anhydride with GABA and serine (28). In our experiment, the N-acyl amino acid ethyl esters were synthesized by coupling the amino acid ethyl ester hydrochloride with the corresponding fatty acid followed by hydrolysis of the ethyl esters with LiOH to generate the acids, as reported previously (19). The reactions gave yields of 80%. Congeners of L-phenylalanine, L-methionine, l-tryptophan, GABA, L-glutamic acid, L-threonine, L-alanine, L-tyrosine, L-valine, L-proline, L-leucine, L-isoleucine, and L-serine, a general structure shown in Scheme 1, were prepared starting with either the methyl or ethyl ester of the amide and using Hunig's base during the coupling step. In order to circumvent racemization, hydrolysis of N-acyl serine and threonine methyl esters was carried out with methanol/water/triethylamine (29). N-acyl serines were also prepared according to the literature procedure (30). N-arachidonoyl L-glutamines were prepared by adding the sodium salt of glutamine to the mixed anhydride formed after mixing ethyl chloroformate with a solution of the fatty acid, as described previously for the synthesis of volicitin (31). Additional details to the synthesis, purification procedure, and characterization of N-acyl amino acids using NMR, TLC, and melting point were the same as reported previously (19).
Scheme 1.
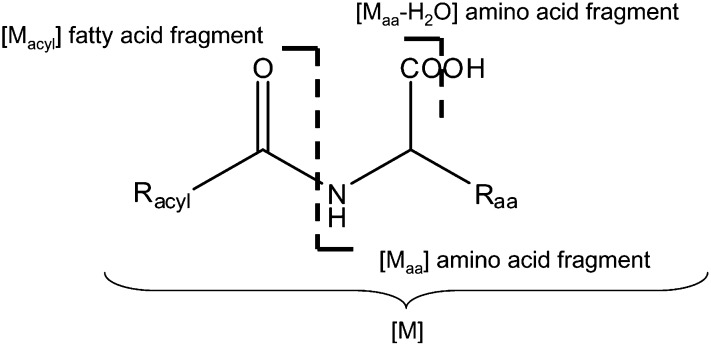
General chemical structure of the N-acyl amino acids synthesized. The most prevalent fragments are [Maa+H]+ and [Maa-H2O+H]+ in positive mode and [Maa-H]− and [Maa-H2O-H]− in negative mode, all represented by [Maa] and [Maa-H2O]. Racyl denotes the type of acyl chains (i.e., those in arachidonic acid, palmitic acid, oleic acid, stearic acid, and docosahexaenic acid). Raa denotes the type of amino acids.
Prescreening method.
Methanol (20 vols, g ml−1) was added to extract a whole rat brain (Scheme 2) at 4°C. After addition of HPLC-grade water (2.3 vols), the supernatant was loaded onto preconditioned C18 Bond-Elute solid phase extraction (SPE) columns (Varian, Harbor City, CA). Water and 50% methanol/water (2 ml) were added to wash the columns and the target lipids were eluted with 2 ml methanol. The samples (10 µL) were injected into an ESI-triple quadrupole mass spectrometer (API3000, Applied Biosystems/MDS Sciex, Foster City, CA), coupled with a Zorbax eclipse XDB 2.1 × 50 mm C18 column, a pair of Shimadzu 10ADVP pumps, and a Shimadzu SIL-10A autosampler (Columbia, MD). With a two solvent system (A: 20% methanol/water with 1 mM ammonium acetate, B: 100% methanol with 1 mM ammonium acetate) and a flow rate of 0.2 ml min−1, the gradient program was as follows: 0–1 min, 5% B; 1–3 min, 5–100% B; 3–6 min, 100% B, 6–7 min, 100–5% B. The MS acquisition methods were multiple reaction monitoring (MRM) methods containing the predicted molecular ions of target acyl amino acids and the theoretical amino acid fragment ions because all the synthetic standards were not made while the prescreening study was conducted. Other parameters of MRM methods were optimized using infusion of NAGly.
Scheme 2.
Targeted lipidomics strategy for identification and quantification of acyl amino acids.
Identification methods.
Method 1
We followed the purification procedure reported previously (11, 27). In brief, six fresh male rat brains (Scheme 2) were obtained by decapitation and extracted using a modified folch extract method at 4°C. The organic phase was purified using diethylaminopropyl, C18, and normal phase column silica (SI) Bond-Elut SPE columns. The samples were dried and reconstituted in 100 μl methanol/water (1:1) prior to nano-LC/MS/MS analysis using a quadrupole time-of-flight LC/MS/MS mass spectrometer, QSTARTM Pulsar (QSTAR, Applied Biosystems-MDS Sciex) and an ESI nanospray source (New Objective, Woburn, MA). The ion spray voltage was +2000 V for positive mode and −2000 V for negative ion mode detection. All the parameters for the QSTAR were optimized using infusion of N-oleoyl GABA. The QSTAR was coupled with a capillary C18 column and a gradient nano-HPLC pump (Micro-Tech Scientific Inc., Vista, CA) at a flow rate of 250 nL min−1 and mass spectra were obtained by using targeted MS/MS or IDA mode with the QSTAR (27). The capillary C18 columns (∼9 cm × 75 μm, New Objective, Woburn, MA, self-pack with integrated tips) were packed on a home-built device with a C18 phase (Magic C18, 3 µm 100 Å, Microm Bioresources, Inc., Auburn, CA) using helium pressure. With a two solvent system (solvent A, 2% acetonitrile/water with 0.1% formic acid and solvent B, 98% acetonitrile/water with 0.1% formic acid), the gradient program was as follows: 0–30 min, 20% B (loading time); 30–45 min, 20–100% B; 45–75 min, 100% B; 75–85 min, 100–20%B. In a typical IDA run, the three most intense singly charged ions were selected in the full scan (1 s, 300–600 m/z) for following MS/MS scans (3 s). Then, the previously selected target ions were excluded for 30 s. In addition, targeted MS/MS scans were used to aid identification of species detected by the prescreening study but not detected in the initial IDA scans.
Method 2
Method 2 was developed to aid identification of low abundance species not successfully identified with Method 1. It utilized additional purification prior to analysis with MS analysis. Six grams of bovine spinal cords or three fresh rat brains (Scheme 2) were extracted in methanol (20 vols) at 4°C. HPLC-grade water (2.3 vols) was added to the supernatant, and the solutions were loaded onto preconditioned C18 Bond-Elut 500 mg SPE columns (Varian, Harbor City, CA). The cartridges were washed in sequence with water and 50% methanol/water (2 ml) and eluted with methanol (2 ml). The methanol fractions were further purified by a semi-preparative HPLC system including Hewlett Packard 1100 binary pumps and a semi-preparative HPLC column (Zorbax Eclipse XDB-C18, 9.4 × 250 mm, 5 μM, Agilent, Palo Alto, CA) protected by a guard column (9.4 × 12.5 mm, 5 μM). The HPLC system used two solvent systems (A: 2% methanol/water, B: methanol) with a flow rate of 4 ml min−1. The gradient program was as follows: 0 min, 20% B; 0–20 min, 20–100% B; 20–60 min, 100% B, 60–65 min, 100–20% B. All the fractions were dried, reconstituted in 100 μl methanol/water (1:1), and examined using MRM analysis with transition ion pairs listed in Table 1 using the API3000. Fractions with the desired MRM peaks were analyzed with the QSTAR and a hybrid linear ion trap Fourier transform ion cyclotron resonance mass spectrometer (LTQ-FT, Thermo-Fisher Scientific, San Jose, CA) coupled with a nanoflow LC system (UltiMate 3000 LC Systems, Dionex, Sunnyvale, CA) for MS/MS analysis. The reverse-phase capillary column, the solvent system, and the gradient used in the LTQ-FT are the same as the ones used in Method 1. The LTQ-FT was operated in data dependent acquisition (DDA) positive mode and set up to perform one full MS scan with the FT analyzer and five MS/MS scans with the ion trap. Mass range and the exclusion time used in DDA LTQ-FT were set to be identical for IDA in the QSTAR.
TABLE 1.
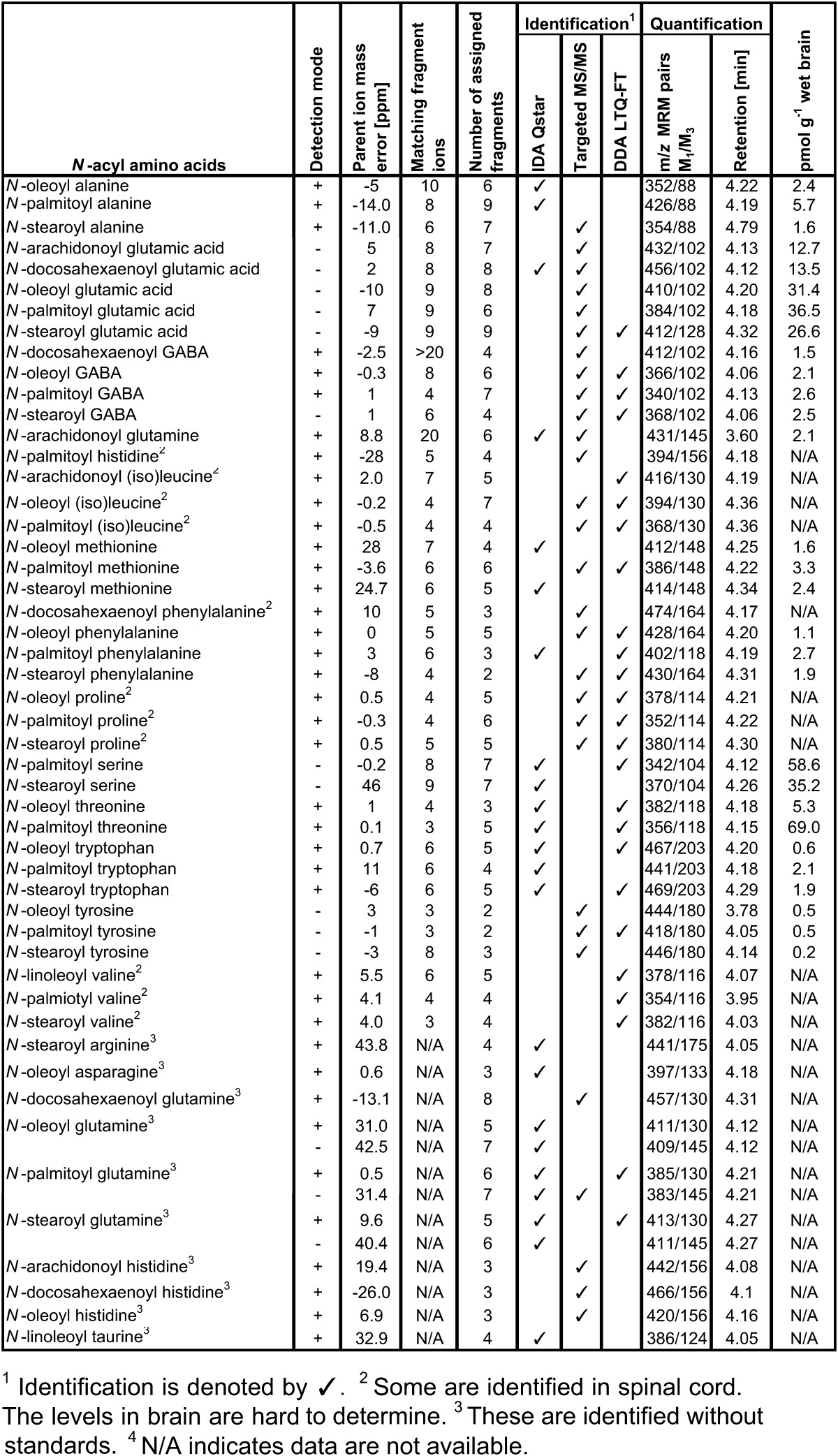
Summary of novel acyl amino acids identified using targeted lipidomics approach
Quantification method.
The instrumentation, settings, and purification method were identical to the prescreening method except that MRM conditions were optimized using the quantitative optimization module of Analyst software and infusion of synthetic standards into the API 3000 (27). The transition ions used under negative or positive modes are listed in Table 1. HPLC retention times of acyl amino acids were compared with those obtained with synthetic standards. Exact retention times to aid identification were obtained in positive or negative modes and listed in Table 1. NAGly-d8 (10 pmol g−1) was spiked into the rat brain extract as an internal standard.
Data analysis.
Data from IDA and DDA experiments were analyzed using the IDA Analyzer as described before (27) to select candidate spectra that contained the mass of a theoretical acyl amino acid and one of the putative fragment ions, [Maa] or [Maa-H2O] (Scheme 1). Candidate spectra were interpreted using the National Institute of Standards and Technology (NIST) database (Gaithersburg, MD) equipped with the library of reference MS/MS spectra. To build this library, all 111 acyl amino acids synthesized were flow injected individually at a rate of 5–20 µL min−1 using the QSTAR or LTQ-FT. MS/MS spectra were acquired in the positive and negative ion modes at five different collision energies (20, 25, 30, 35, 40 V). All the MS/MS data were averaged and exported to the NIST database to form the library.
The compounds that we identified using synthetic standards and the QSTAR met the following criteria: <50 ppm error for the molecular ion mass, <200 ppm error for the mass of the amino acid fragment ion, a matching fragmentation pattern, and a minimum number of two monitored ions, the precursor ion and one fragment ion that could be assigned to structures based upon the predicted fragmentation of the candidate molecule. Data obtained using the LTQ-FT provided high-accuracy molecular weight determination of the precursor ions whereas the MS/MS spectra were generated in the ion trap with unit mass resolution. The compounds identified in the positive or negative ion modes without the use of standards met the following minimum criteria: <45 ppm error for the theoretical mass of the molecular ion, <200 ppm error for the theoretical mass of the amino acid fragment, and a minimum number of three monitored ions that could be assigned to structures of the candidate molecule.
RESULTS AND DISCUSSION
Prescreening method
Our confidence in the presence of acyl amino acids came from the prescreening study. Rat brain methanolic extracts were purified using C18 SPE columns and analyzed by MRM methods using an API3000. We selected the predicted molecular ions and the amino acid fragment ions [Maa] or [Maa-H2O] (Scheme 1) as the transition ions for MRM analysis due to their high ion intensities and structural significance. As reported before (27), there were MRM signals corresponding to numerous unknown acyl amino acids in the extract of the rat brains. After prescreening for all acyl amino acids (combination of arachidonic acid, palmitic acid, oleic acid, stearic acid, and docosahexaenic acid and 24 amino acids), we found signals for most of them. We decided to focus our attention on the species with high intensity signals, which were about 70 acyl amino acids.
Identification method
Identification was based on matching the HPLC retention times and the fragmentation patterns of the constituents in tissues with the synthetic standards. To obtain information for our targeted acyl amino acids, we divided the identification into several steps: enriching the target lipids, using nano-LC/MS/MS to produce MS/MS spectra with high sensitivity, and analyzing spectra with all possible acyl amino acids using software. After several years’ research on NAGly and other acyl amino acids, our laboratory developed a specific purification method based on the chemical structures of acyl amino acids by combining anion exchange, reverse phase, and normal phase SPE to enrich acyl amino acids (11, 27). Without these purification steps, it is impossible to dissolve rat brain extracts in such a small volume (100 µl) with methanol/water mixtures. Our nano-HPLC/MS/MS system has demonstrated the ability to provide good quality MS/MS spectra with 150 femtomoles of acyl amino acids on column (11). IDA and DDA modes, commonly used in proteomics, were also used in our workflow and enabled us to identify several compounds in one run due to the computer-aided selection of ions for MS/MS. Therefore, we can obtain MS/MS spectra with high sensitivity and high efficiency after we combined IDA or DDA with nano-LC. For the third step, we designed a computer program known as IDA Analyzer to analyze more than one thousand spectra generated in IDA experiments with the more than one hundred possible acyl amino acids. The algorithm of this program was to search the predicted parent ions and the most abundant and characteristic fragments, the amino acid fragment ions [Maa] or [Maa-H2O]. The candidate spectra were imported to the NIST database to be compared with the MS/MS library. In another study, we used this workflow to identify 11 previously identified acyl amino acids with high efficiency and sensitivity (27).
In an attempt to maximize the number of identified novel low-abundance lipids, we employed two different MS instruments, the QSTAR and LTQ-FT. LTQ-FT has high-mass accuracy for the molecular ions but a low-mass cutoff and unit mass resolution for the fragment ions collected in the ion trap. As summarized in Table 1, 15 compounds identified in the LTQ-FT have mass errors of less than 1 ppm, 5 with errors between 1 and 5 ppm, and 5 with errors of 5 and 10 ppm. This accuracy provides greater confidence in our assigned structures. Because of the low-mass cutoff, the MS/MS spectra obtained using the LTQ-FT often lack the most abundant low-mass fragment ions such as the amino acid moiety that can play a vital role for the assignment of structures, e.g., m/z 88 for acyl alanines. We addressed this limitation by employing the QSTAR, which has good mass accuracy and the ability to capture ions down to m/z 50. Following our workflow and using both the QSTAR and LTQ-FT enabled us to identify 39 out of 50 compounds. We used targeted MS/MS scans on the QSTAR for additional 11 lipids.
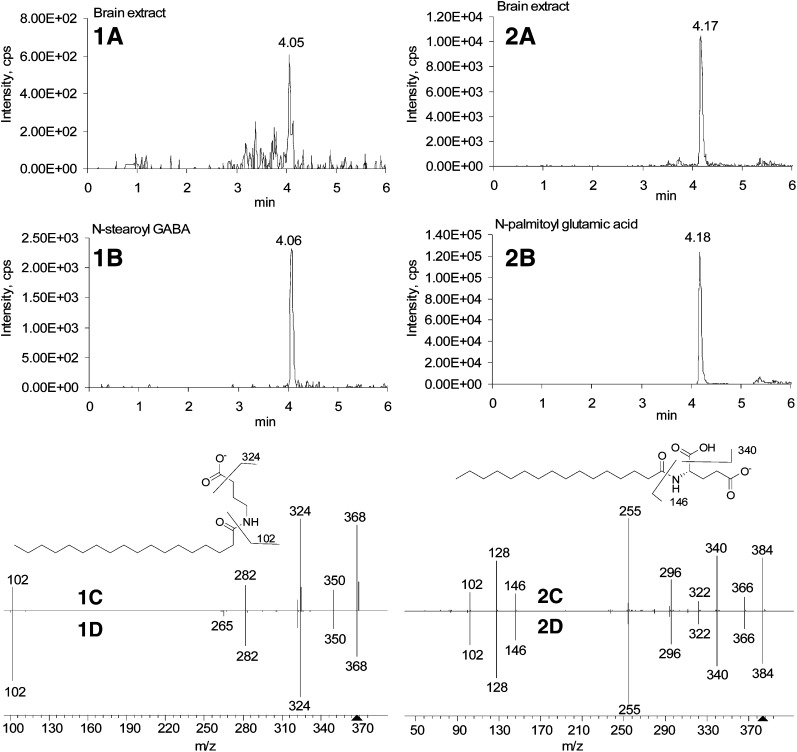
Acyl amino acids, except 10 without synthetic standards, identified in the tissues had the matching retention times and fragmentation patterns with the synthetic standards. Two typical examples, N-stearoyl GABA and N-palmitoyl glutamic acid, are provided in Fig. 1. Both exhibited almost identical MS/MS spectra for the endogenous lipids and the synthetic standards. Although the presence of these compounds in mammalian tissues was not known until now, they were reported to exhibit a variety of biological effects. N-stearoyl GABA antagonized catalepsy induced by haloperidol at low doses, potentiated sedation induced by apomorphine, and caused antimobility and hypothermia (25, 32–36). N-palmitoyl GABA, N-palmitoyl glutamic acid, and N-stearoyl glutamic acid exhibit similar psychopharmacological effects (25, 32–36). They also have neuroprotective or neurodegenerative effects (25, 32–36). Among the rest of acyl amino acids identified here, N-stearoyl proline, N-stearoyl phenylalanine, N-stearoyl tryptophan, N-stearoyl arginine, and N-stearoyl tyrosine showed antimicrobial activity against bacteria and yeast (37).
Fig. 1.
Chromatogram and MS/MS spectra of N-stearoyl GABA (1a–d) and N-palmitoyl glutamic acid (2a–d) as standards and in rat brain extract. MRM chromatograms of constituents in rat brain extract (1a and 2a) and of synthetic standards (1b and 2b) using quantification method. MS/MS spectra of constituents in rat brain extract (1c) and synthetic standard (1d) in negative mode. MS/MS spectra of constituents in rat brain extract (2c) and synthetic standard (2d). Important peaks are listed in supplementary data.
Method 1
Using Method 1, 14 acyl amino acids were found in a positive IDA experiment of the 55% methanol/chloroform SI SPE fraction of the DEA fraction of 0.5% ammonium acetate, which was the highest number of novel compounds in one single run using Method 1. Analysis of the total of six IDA experiments in positive and negative modes for all three SI SPE fractions (25%, 55%, and 85% methanol/chloroform SI SPE fractions) identified 20 compounds (Table 1).
Analysis of all DEA fractions using MRM scans in the API 3000 showed that N-acyl glutamic acids are present only in the DEA fraction of 5% ammonium acetate/methanol. This fraction was collected, further purified (C18 SPE), and analyzed by targeted MS/MS using the QSTAR. We identified the N-acyl glutamic acids N-arachidonoyl, N-palmitoyl, N-stearoyl, N-oleoyl, and N-docosahexaenoyl glutamic acid (Table 1).
In the negative mode, all glutamic acid series, such as N-palmitoyl glutamic acid in Fig. 1, fragmented to palmitic anion [RCOO]− (m/z 255) with high intensity. The majority of acyl amino acids only generated anions such as [RCONH]− with extremely low intensity, as shown for N-stearoyl tyrosine (supplementary data). The anions [RCOO]− generated from glutamic acid series are due to the cyclization reaction of glutamic acid side chain. Similar phenomenon occurred to the dipeptide of leucine and aspartic acid in which aspartic acid is on the C- terminal position (37). The mechanism is shown in the supplementary data.
Method 2
Using Method 1 we were able to identify 25 acyl amino acids, which is less than half of the total number of acyl amino acids with high intensity in our prescreening result. For the identification of additional target lipids, the purification procedure was modified (Method 2; ) to further reduce the sample complexity. Three rat brains generated 20 fractions after SPE and semi-preparative HPLC, compared with only three fractions where most acyl amino acids existed using Method 1. All fractions were then subjected to MRM analysis using the API3000. Fractions that indicated the presence of acyl amino acids were subjected to targeted MS/MS scans in the QSTAR and the LTQ-FT. Fourteen novel acyl amino acids were identified in addition to those identified using Method 1 (Table 1). To test our theory of the existence of acyl amino acids in other nervous tissues, Method 2 was applied to bovine spinal cord, which led to the identification of 11 acyl amino acids (Table 1).
By this method, we also identified several fatty acid conjugates of leucine or isoleucine. The N-acyl leucine and N-acyl isoleucine pairs had the same retention times, identical m/z of the parent ions, and indistinguishable fragmentation patterns. As a result, the compounds identified in our experiments (Table 1) could be either or both series of compounds. N-acy leucine and isoleucines (myristic, palmitic, palmitoleic, oleic acid) were found in Deleya marine (bacteria) (38) and N-arachidonoyl isoleucine was reported to inhibit the hydrolysis of anandamide by fatty acid amide hydrolase at nmol concentrations (39).
Quantification method
The API3000 LC/MS/MS system with a flow rate of 0.2 ml min−1 was used to provide information on the retention times and the endogenous concentrations. Samples after a simple C18 SPE clean up were analyzed by LC/MS/MS API3000 system, which is the same as the prescreening method. The retention times from this study were compared with the synthetic standards, as shown in Fig. 1. All identified acyl amino acids, except the ones without corresponding synthetic standards, have the same retention times as the synthetic standards.
To quantify the concentrations of our target lipids in rat brain extracts, we used NAGly-d8 as an internal standard to correct for matrix effects because it was the only deuterated acyl amino acid commercially available. The recovery rate was 85% and this was used as the recovery rate for all acyl amino acids. Although we synthesized 111 N-acyl amino acid standards, their high cost precluded synthesis of stable labeled analogs. Our experiments were designed to minimize the postmortem delay. Typically, only two minutes elapsed between decapitation of the rat and the start of centrifugation in chloroform/methanol. Compared with microwave irradiation, ischemia induced by decapitation was shown to increase the levels of anandamide (40) and arachidonoyl-CoA by 4-fold and to decrease the level of docosahexaenoyl-CoA by 2-fold (41) in rat brains within two to five minutes of decapitation. All the other acyl-CoAs (41) and amino acids had changes of less than 40% (42). Based on the structural similarity of anandamide and possible roles played by acyl-CoAs in biosynthesis of N-acyl amino acids (explained in the section on biosynthesis and metabolism), decapitation in our method probably caused changes of 2-fold or less within 5 min except that arachidonoyl amino acids could increase 4-fold.
Using this quantification method, we determined rat brain concentrations for 29 of 50 novel acyl amino acids. For some lipids with very low abundance in the rat brain, such as the valine and proline series, bovine spinal cord tissue was used to obtain identification information so the biological levels in the rat brains were not determined. For lipids identified from tissues without corresponding synthetic standards such as most of glutamine and histidine series, information on biological levels could not be obtained. The amount of the compounds varied widely (Table 1), some occurring at low levels (e.g., N-stearoyl tyrosine, 0.2 pmol g−1) and others at much higher levels (e.g., N-palmitoyl threonine, 69 pmol g−1). Among the compounds with the same fatty acid group, the serine and glutamic acid series are the most abundant and the species with the tyrosine moiety are less abundant. The high concentrations of N-acyl taurine (100–300 pmol/g) (27) and glutamic acid (12–16 pmol/g) may reflect the abundance of taurine and glutamic acid in brains. To confirm this claim, further study of distributions of acyl amino acids in different tissues will need to be done. The high concentrations of N-acyl serines (35–58 pmol/g) might be due to abundance of N-acyl phosphatidyl serines (0.1% porcine brain lipids) (43), (explained in the section on biosynthesis and metabolism). Among the compounds with the same amino acid group, those with palmitoyl, stearoyl, and oleoyl groups are more abundant than those with arachidonoyl and docosahexaenoyl groups, which matches the abundance of these fatty acids either as free acids or as phospholipids (44, 45), especially in phosphatidylcholines.
Biosynthesis and metabolism
These observations suggested that acyl amino acids may be generated by conjugation of fatty acids and amino acids in biological systems or may be metabolites of the corresponding phospholipids. N-acetylglutamate synthase, required for the urea cycle in animals and arginine biosynthesis in plants, catalyses the production of N-acetylglutamate from acetyl-CoA and glutamate. Palmitoylcarnitine with an O-linked ester bond is generated from N-palmitoyl-CoA and carnitine by carnitine palmitoyl transferase I in the outer membrane of mitochondria. It is possible that an enzyme with functions similar to N-acetyl glutamate and carnitine palmitoyl transferase I can catalyze the formation of N-acyl amino acids. This theory is supported by reports that an enzyme isolated from rat liver promoted the condensation of both palmitic and oleic acids with phenylalanine (46) and that cytochrome c has shown in vitro to catalyze the formation of N-acyl glycines and N-arachidonoyl dopamine, serotonin, serine, and GABA (47, 48) in the presence of acyl-CoAs, H2O2, and amino acids at pH 7.4.
Analogous to the observation that N-acyl phosphatidyl ethanolamine can serve as the precursor of N-acyl ethanolamines such as anandamide (49, 50), N-acyl serines could be products of N-acyl phosphatidyl serines, endogenous compounds in brain and other cells (43), either by the hydrolysis reaction with phospholipase D or deacylation with phospholipase A and then hydrolysis with phosphodiesterases. Other biosynthetic routes may exist following on the example of anandamide being oxidized to N-arachidonoyl glycine (51). Degradation of N-acyl amino acids could be carried out by fatty acid amide hydrolase based on its ability to hydrolyze anandamide (52).
Conclusions
Using a targeted lipidomics approach adapted from proteomics, we identified and quantified 50 novel endogenous acyl amino acids with high sensitivity. Our results are the first comprehensive survey of these compounds in mammalian tissues and support our initial hypothesis that there exists a large family of endogenous acyl amino acids that was based upon observations of the presence of a few endogenous acyl amino acids. These results stress the utility and power of an MS-based targeted lipidomics approach as several low-abundance acyl amino acids were readily identified despite the presence of other more abundant lipids. The strategies developed here should hasten the process of identification and biological characterization of these and many other novel low-abundance signaling lipids in the future.
Supplementary Material
Acknowledgments
The authors are grateful for Dr. Kenneth D. Roth for critical reading of the manuscript.
Footnotes
Abbreviations:
- DDA
- data dependent acquisition
- GABA
- gamma aminobutyric acid
- IDA
- information dependent acquisition
- MRM
- multiple reaction monitoring
- NAGly
- N-arachidonoyl glycine
- NIST
- National Institute of Standards and Technology
- PPAR
- peroxisome proliferator-activated receptor
- SI
- silica
- SPE
- solid phase extraction
- TRPV
- transient receptor potential vanilloid
This work was supported by grants from the National Institutes of Health/National Institute on Drug Abuse (DA16825, DA018224, DA17969), the Linda and Jack Gill Center for Biomolecular Science, Indiana University, a Faculty Research Support grant from Indiana University, Bloomington, and the MetaCyt Grant to Indiana University from the Lilly Foundation Inc., Indianapolis, IN. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health/National Institute on Drug Abuse.
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Cartwright N. J. 1955. Serratamic acid, a derivative of L-serine produced by organisms of the serratia group. Biochem. J. 60: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartwright N. J. 1957. The structure of serratamic acid. Biochem. J. 67: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA. 91: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross S. A., Weete J. D., Schinazi R. F., Wirtz S. S., Tharnish P., Scheuer P. J., Hamann M. T. 2000. Mololipids, a new series of anti-HIV bromotyramine-derived compounds from a sponge of the order Verongida. J. Nat. Prod. 63: 501–503. [DOI] [PubMed] [Google Scholar]
- 5.Weibel E. K., Hadvary P., Hochuli E., Kupfer E., Lengsfeld H. 1987. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J. Antibiot.(Tokyo) 40: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 6.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 7.Cravatt B. F., Prospero-Garcia O., Siuzdak G., Gilula N. B., Henriksen S. J., Boger D. L., Lerner R. A. 1995. Chemical characterization of a family of brain lipids that induce sleep. Science. 268: 1506–1509. [DOI] [PubMed] [Google Scholar]
- 8.Huang S. M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T. J., Krey J. F., Chu C. J., et al. 2002. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA. 99: 8400–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu C. J., Huang S. M., De Petrocellis L., Bisogno T., Ewing S. A., Miller J. D., Zipkin R. E., Daddario N., Appending G., Di Marzo V., et al. 2003. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 278: 13633–13639. [DOI] [PubMed] [Google Scholar]
- 10.Huang S. M., Walker J. M. 2006. Enhancement of spontaneous and heat-evoked activity in spinal nociceptive neurons by the endovanilloid/endocannabinoid N-arachidonoyldopamine (NADA). J. Neurophysiol. 95: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 11.Huang S. M., Bisogno T., Petros T. J., Chang S. Y., Zavitsanos P. A., Zipkin R. E., Sivakumar R., Coop A., Maeda D. Y., De Petrocellis L., et al. 2001. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 276: 42639–42644. [DOI] [PubMed] [Google Scholar]
- 12.Vuong L. A. Q., Mitchell V. A., Vaughan C. W. 2008. Actions of N-arachidonyl-glycine in a rat neuropathic pain model. Neuropharmacology. 54: 189–193. [DOI] [PubMed] [Google Scholar]
- 13.Burstein S. H. 1999. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol. Ther. 82: 87–96. [DOI] [PubMed] [Google Scholar]
- 14.Burstein S. H., Adams J. K., Bradshaw H. B., Fraioli C., Rossetti R. G., Salmonsen R. A., Shaw J. W., Walker J. M., Zipkin R. E., Zurier R. B. 2007. Potential anti-inflammatory actions of the elmiric (lipoamino) acids. Bioorg. Med. Chem. 15: 3345–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. 2006. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 347: 827–832. [DOI] [PubMed] [Google Scholar]
- 16.Milman G., Maor Y., Abu-Lafi S., Horowitz M., Gallily R., Batkai S., Mo F. M., Offertaler L., Pacher P., Kunos G., et al. 2006. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. USA. 103: 2428–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saghatelian A., Trauger S. A., Want E. J., Hawkins E. G., Siuzdak G., Cravatt B. F. 2004. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 43: 14332–14339. [DOI] [PubMed] [Google Scholar]
- 18.Saghatelian A., McKinney M. K., Bandell M., Patapoutian A., Cravatt B. F. 2006. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 45: 9007–9015. [DOI] [PubMed] [Google Scholar]
- 19.Rimmerman N., Bradshaw H. B., Hughes H. V., Chen J. S-C., Hu S. S-J., McHugh D., Vefring E., Jahnsen J. A., Thompson E. L., Masuda K., et al. 2008. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol. Pharmacol. 74: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy M-O., Hannedouche S. 2007. Ligand for G-protein coupled receptor GPR72 and uses thereof. Canadian Patent Application. 1–99. [Google Scholar]
- 21.Vamvakides A., Kolocouris N. 1989. Synthesis and pharmacological study of the diethyl N-palmitoyl glutamate. Ann. Pharm. Fr. 47: 205–212. [PubMed] [Google Scholar]
- 22.Kolocouris N., Foscolos G., Papadopoulou-Daifoti Z., Vamvakides A. 1985. Synthesis and pharmacology of some new GABA-ergics. Ann. Pharm. Fr. 43: 389–396. [PubMed] [Google Scholar]
- 23.Vamvakides A. 1987. Effect of glycine stearamide on the rectal temperature of rats treated by reserpine. Ann. Pharm. Fr. 44: 411–416. [PubMed] [Google Scholar]
- 24.Vamvakides A., Kolokouris N. 1986. Synergistic effect of GABA and glycine in antagonizing pentetrazole-induced convulsions in rats: effect of glycine valproamide. Ann. Pharm. Fr. 44: 501–508. [PubMed] [Google Scholar]
- 25.Vamvakides A. 1984. Catalepsy in rats after haloperidol and some new GABA-ergics. Agressologie. 25: 1011–1016. [PubMed] [Google Scholar]
- 26.Bradshaw H. B., Rimmerman N., Hu S. S-J., Burstein S., Walker J. M. 2008. Novel endogenous N-acyl glycines: identification and characterization. Vitam. Horm. 81: 191–205. [DOI] [PubMed] [Google Scholar]
- 27.Tan B., Yu Y. W., Monn M. F., Hughes H. V., O'Dell D. K., Walker J. M. 2009. Targeted lipidomics approach for endogenous N-acyl amino acids in rat brain tissue. J. Chromatagr. B. 877: 2890–2894. [DOI] [PubMed] [Google Scholar]
- 28.Priller J., Briley E. M., Mansouri J., Devane W. A., Mackie K., Felder C. C. 1995. Mead ethanolamine, a novel eicosamoid, is an agonist for the cetral (CB1) and peripheral (CB2) cannabinoid receptors. Mol. Pharmacol. 48: 288–292. [PubMed] [Google Scholar]
- 29.Takeda T., Sawaki M., Ogihara Y., Shibata S. 1985. Synthesis of N-glycopeptides and a neoglycoprotein. Carbohydr. Res. 139: 133–140. [DOI] [PubMed] [Google Scholar]
- 30.Sheskin T., Hanus L., Slager J., Vogel Z., Mechoulam R. 1997. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 40: 659–667. [DOI] [PubMed] [Google Scholar]
- 31.Spiteller D., Boland W. 2003. N-(17-Acyloxy-acyl)-glutamines: novel surfactants from oral secretions of lepidopteran larvae. J. Org. Chem. 68: 8743–8749. [DOI] [PubMed] [Google Scholar]
- 32.Vamvakides A. 1987. Effect of three new GABAergic drugs on reserpine-induced catalepsy and apomorphine-induced sedation in rats. Agressologie. 28: 535–539. [PubMed] [Google Scholar]
- 33.Vamvakides A. 1990. Effect of GABA, glycine or glutamic acid derivatives in the forced swimming test in mice. Ann. Pharm. Fr. 48: 154–159. [PubMed] [Google Scholar]
- 34.Vamvakides A. 1990. Working hypothesis on the effect of GABAergic, glycinergic or glutamatergic drugs in the treatment of Parkinson's disease. Ann. Pharm. Fr. 48: 70–80. [PubMed] [Google Scholar]
- 35.Vamvakides A., Kassianides A., Papadopoulou-Daifoti Z. 1985. Effect of some new GABAergics on rat motor and oral apomorphine-induced stereotypic behavior. J. Pharmacol. (Paris.) 16: 403–411. [PubMed] [Google Scholar]
- 36.Vamvakides A. 1989. Effect of glutamic acid palmitamide or linoleamide on convulsions induced by pentetrazole. Agressologie. 30: 469–471. [PubMed] [Google Scholar]
- 37.Sivasamy A., Krishnaveni M., Rao P. G. 2001. Preparation, characterization, and surface and biological properties of N-stearoyl amino acid. J. Am. Oil Chem. Soc. 78: 897–902. [Google Scholar]
- 38.Yagi H., Corzo G., Nakahara T. 1997. N-acyl amino acid biosynthesis in marine bacterium, Deleya marina. Biochim. Biophys. Acta. 1336: 28–32. [DOI] [PubMed] [Google Scholar]
- 39.Grazia Cascio M., Minassi A., Ligresti A., Appendino G., Burstein S., Di Marzo V. 2004. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 314: 192–196. [DOI] [PubMed] [Google Scholar]
- 40.Bazinet R. P., Lee H-J., Felder C. C., Porter A. C., Rapoport S. I., Rosenberger T. A. 2005. Rapid high-energy microwave fixation is required to determine the anadamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem. Res. 30: 597–601. [DOI] [PubMed] [Google Scholar]
- 41.Deutsch J., Rapoport S. I., Purdon D. A. 1997. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem. Res. 22: 759–765. [DOI] [PubMed] [Google Scholar]
- 42.Miller J. M., Jope R. S., Ferraro T. N., Hare T. A. 1990. Brain amino acid concentrations in rats killed by decapitation and microwave irradiation. J. Neurosci. Methods. 31: 187–192. [DOI] [PubMed] [Google Scholar]
- 43.Guan Z., Li S., Smith D. C., Shaw W. A., Raetz C. R. 2007. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry. 46: 14500–14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sastry P. S. 1985. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res. 24: 69–176. [DOI] [PubMed] [Google Scholar]
- 45.Soderberg M., Edlund C., Kristensson K., Daliner G. 1991. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 26: 421–425. [DOI] [PubMed] [Google Scholar]
- 46.Fukui T., Axelrod B. 1961. Enzymatic formation of lipo-amino acids by rat liver preparations and the nature of the product. J. Biol. Chem. 236: 811–816. [PubMed] [Google Scholar]
- 47.McCue J. M., Driscoll W. J., Mueller G. P. 2008. Cytochrome c catalyzes the in vitro synthesis of arachidonoyl glycine. Biochem. Biophys. Res. Commun. 365: 322–327. [DOI] [PubMed] [Google Scholar]
- 48.Mueller G. P., Driscoll W. J. 2007. In vitro synthesis of oleoylglycine by cytochrome c points to a novel pathway for the production of lipid signaling molecules. J. Biol. Chem. 282: 22364–22369. [DOI] [PubMed] [Google Scholar]
- 49.Simon G. M., Cravatt B. F. 2006. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for α/β hydrolase 4 in this pathway. J. Biol. Chem. 281: 26465–26472. [DOI] [PubMed] [Google Scholar]
- 50.Schmid P. C., Reddy P. V., Natarajan V., Schmid H. H. 1983. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J. Biol. Chem. 258: 9302–9306. [PubMed] [Google Scholar]
- 51.Aneetha H., O'Dell D. K., Tan B., Walker J. M., Hurley T. D. 2009. Alcohol dehydrogenase-catalyzed in vitro oxidation of anandamide to N-arachidonoyl glycine, a lipid mediator: synthesis of N-acyl glycinals. Bioorg. Med. Chem. Lett. 19: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giang D. K., Cravatt B. F. 1997. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. USA. 94: 2238–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.