Abstract
We performed detailed biophysical studies of transfer of long-chain fatty acids (FAs) from methyl-β-CD (MBCD) to model membranes (egg-PC vesicles) and cells and the extraction of FA from membranes by MBCD. We used i) fluorescein phosphatidylethanolamine to detect transfer of FA anions arriving in the outer membrane leaflet; ii) entrapped pH dyes to measure pH changes after FA diffusion (flip-flop) across the lipid bilayer; and iii) soluble fluorescent-labeled FA binding protein to measure the concentration of unbound FA in water. FA dissociated from MBCD, bound to the membrane, and underwent flip-flop within milliseconds. In the presence of vesicles, MBCD maintained the aqueous concentration of unbound FA at low levels comparable to those measured with albumin. In studies with cells, addition of oleic acid (OA) complexed with MBCD yielded rapid (seconds) dose-dependent OA transport into 3T3-L1 preadipocytes and HepG2 cells. MBCD extracted OA from cells and model membranes rapidly at concentrations exceeding those required for OA delivery but much lower than concentrations commonly used for extracting cholesterol. Compared with albumin, MBCD can transfer its entire FA load and is less likely to extract cell nutrients and to introduce impurities.
Keywords: flip-flop, desorption, unbound fatty acid, albumin, cholesterol
The very low aqueous solubility of long-chain fatty acids (FAs) is one of the major limitations in studies of FA transport in vitro and in vivo. A common misconception is that FA can form micelles at physiological pH because the pka values for monomeric FA are typically 4.8. Instead, they form insoluble structures similar to phospholipid bilayers, in which ∼50% of the FA is ionized (1). The aqueous solubilities of the monomeric forms of the common 16- and 18-carbon dietary FA are <10 µM (2).
A frequently used approach for adding FA at higher concentration is to complex the FA with albumin, which allows preparation of FA that is solubilized at millimolar concentrations. Although albumin is a physiologically relevant carrier of FA, careful consideration must be given to the facts that albumin can deliver impurities to cells and can extract FA and other nutrients from cells, which could alter their metabolic state (3). In addition, there is continuing disagreement about the rates of FA dissociation from albumin and whether this affects biophysical measurements of FA transport (4, 5). Furthermore, the binding properties of albumin are complex: different FA binding sites have different relative affinities, and the kinetics of desorption are dependent on acyl chain length (6). The high affinity of albumin for FA may result in very little delivery to membranes (7).
A promising new vehicle for solubilization of FA is the family of cyclodextrins (CDs), which are cyclic oligosaccharides formed by bacterial degradation of starch. These molecules typically contain six (α), seven (β), or eight (γ) glucose residues linked by (1→ 4) glycosidic bonds. They have a polar surface and a hydrophobic cylindrical cavity that can bind and solubilize a wide variety of hydrophobic molecules, such as cholesterol and FA, while remaining soluble in water (8). The number of CD molecules required to solubilize one FA molecule increases with an increase in the hydrocarbon chain length of FA (9), although the exact stoichiometry is difficult to determine.
In pharmaceutical applications, CD is widely used to solubilize hydrophobic drugs and enhance drug absorption in the gastrointestinal tract (8). It is used as a lipid-binding agent in the culture media of bacteria and animal cells (10, 11) and as a substitute for albumin for intravenous infusion of FA (12). In biochemical and physiological studies, CD is used primarily to manipulate levels of cholesterol in cell membranes, often focusing on the research of lipid rafts (13, 14). Despite these uses of CD to deliver lipids to membranes, there is a dearth of biophysical data to explain how CD interacts with lipids. For example, because the kinetics of dissociation of FA from CD are not known, the incubation time of the CD:FA complex with cells is chosen arbitrarily (15, 16).
We hypothesize that using CD to deliver FA to membranes and cells could overcome some of the technical difficulties with other delivery vehicles, especially albumin. Very rapid delivery of FA by CD would avert the current uncertainty about how fast FAs dissociate from albumin and how this affects measured transport rates. Furthermore, a low concentration of unbound FA in the aqueous phase would be maintained.
In this study, we tested the usefulness of methyl-β-CD (MBCD) as a FA donor to model membranes and cells with a focus on the common dietary FA, myristic (MA; C14:0), palmitic (PA; C16:0), stearic (SA; C18:0), and oleic acids (OA; C18:1). We employed different fluorescence assays developed in our laboratory for monitoring the binding and transbilayer diffusion of FA (17–19). The transmembrane diffusion of FA (flip-flop) typically is measured directly using a pH-sensitive fluorophore, such as pyranine or 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxy-fluorescein (BCECF). The FA adsorption step can be measured using the soluble fluorescent-labeled FA binding protein [acrylodan-labeled intestinal fatty acid binding protein (ADIFAB)] in the external medium (18). A newer assay measures adsorption of FA by the fluorescence of FPE, a phosphatidylethanolamine phospholipid molecule covalently linked to fluorescein in the headgroup region (17, 19). FPE added in the external buffer inserts into the outer leaflet of membranes and detects binding of ionized FA to the lipid bilayer. Furthermore, when FA is complexed with a donor, all of the above assays also include the kinetics of desorption of FA from the donor.
Our major findings were i) MBCD maintains a low concentration of unbound FA throughout the delivery of FA to cell membranes and lipid membranes, and ii) FA dissociates from MBCD very rapidly compared with albumin. These results confirm our previous estimates of flip-flop in the low millisecond range. Our results show also that the MBCD concentration required to solubilize and deliver FA to cells is low (<0.1 mM) but that higher concentrations used to extract cholesterol from the plasma membrane of cells (millimolar) will likely extract FA and other amphipathic nutrients from cells.
EXPERIMENTAL PROCEDURES
Materials
3T3-L1 and HepG2 cell lines were purchased from American Type Culture Collection (Manassas, VA). Cell culture supplies were purchased from Fisher Scientific (Agawam, MA) or Gibco Life Technology (Long Island, NY). Cellstripper™ nonenzymatic cell dissociation solution was purchased from Mediathec (Manassas, VA). Pyranine (8-hydroxypyrene-1.3.6-trisulfonic acid), BCECF-AM (acetoxymethyl ester), and fluorescein phosphatidylethanolamine (FPE) were purchased from Molecular Probes (Eugene, OR). ADIFAB was from FFA Sciences (San Diego, CA). Egg phosphatidylcholine was purchased from Avanti Polar Lipids (Alabaster, AL). MBCD, oleic acid-methyl-β-cyclodextrin complex (oleic acid-water soluble), BSA, all fatty acids (MA, PA, SA, and OA) and all buffer materials were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of FA solutions, FA:MBCD, and FA:BSA complexes
Stock solutions of MA (C14:0), PA (C16:0), SA (C18:0), and OA (C18:1) (10 mM) were prepared in ethanol or DMSO or by dissolving FA in 0.1 mM KOH to make the K+ salt. FA:MBCD complexes were prepared in two ways: i) purchased FA:MBCD compound (Sigma-Aldrich) was dissolved in water at 10 mM final concentration in FA (only OA, molar ratio ∼1:6 OA:MBCD); ii) aliquots of a MBCD solution in water were added to a microcentrifuge tube containing pure FA followed by incubation at 70°C for 1 h and sonication for 5 min. The molar ratios of FA:MBCD were 1 MA:10 MBCD, 1 PA:12 MBCD, and 1 SA:22 MBCD. All stock solutions of FA:MBCD were clear at room temperature. A stock solution of MBCD alone was made in water at 100 mM. BSA complexed with OA was prepared as described before (20) at OA:BSA molar ratios of 4:1 and 8:1. For stopped-flow experiments, appropriate volumes of OA, MBCD, and OA:MBCD stocks were dissolved in 50 or 20 mM HEPES-KOH (pH 7.4).
Preparation of cultured cells
The 3T3-L1 preadipocytes and HepG2 cells were cultured in high-glucose DMEM supplemented with 10% FBS, 100 μg/ml streptomycin, and 100 units/ml penicillin. BCECF was incorporated into cells by incubating cells for 20 min with 2 µM BCECF-AM at 37°C followed by three washes in PBS buffer. Cells were resuspended in albumin-free Krebs-MOPS buffer (118 mM NaCl, 5 mM KCl, 1.1 mM KH2PO4, 20 mM MOPS, 2.5 mM CaCl2, and 5.1 mM glucose, pH 7.4) and then treated with nonenzimatic cell dissociation solution for 5 min.
Preparation of lipid vesicles
Small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) of egg-PC were prepared by sonication and extrusion, respectively, as described previously (21). Briefly, the pH probe pyranine (0.05–0.5 mM) was trapped inside the vesicles by placing it in the buffer when the lipids were hydrating. The surface potential probe, FPE, resuspended in 95% ethanol (2 mg/ml), was added to vesicles in suspension (1 mol% FPE relative to egg-PC) and incubated for 1 h in the dark at room temperature to label the outer bilayer leaflet. For fluorescence experiments with pyranine or FPE, the buffer used was 20 or 50 mM HEPES-KOH (pH 7.40). In the presence of ADIFAB, the buffer used was 20 mM HEPES, 150 mM NaCl, 5 mM KCl, and 1 mM Na2HPO4 (pH 7.40). In stopped-flow experiments, in which FA are delivered as a complex with SUV, an aliquot of FA in ethanol solution was added to a suspension of donor SUV.
pH calibration with nigericin
The relationship between internal pH and pyranine fluorescence was calibrated by permeabilizing SUV to H+ with 1 µM nigericin. The external pH was adjusted with aliquots of KOH and H2SO4, and the pH was measured with a mini-pH electrode.
Fluorescence measurements
Online fluorescence measurements were performed with a Spex FluoroMax-2 fluorometer (Yvon Jobin, NJ). The sampling time was set to 1.0 s (the mixing time of the cuvette). Rapid kinetics were measured with a High Tech stopped-flow apparatus (TgK Scientific, UK) attached to the fluorometer (dead time of 10 ms). The ratiometric fluorescence of BCECF was measured using excitation at 439 and 505 nm (R = F439/F505) and emission at 535 nm (sampling time, 2.0 s; band-pass 3–3 nm). Pyranine was measured with excitation at 455 nm and emission at 509 nm (3–3 nm band-pass minimum). FPE was measured with excitation at 490 nm and emission at 520 nm (5–10 nm band-pass). The ratiometric fluorescence of ADIFAB was measured with excitation at 390 nm and emission at 505 and 432 nm (R = F505/F432) (sampling time, 2.0 s; band-pass 3–5 nm). Experiments with vesicles and cells were carried out at room temperature and 37°C, respectively.
Measurement of aqueous OA concentration
The fluorescent FA indicator ADIFAB was dissolved in buffer (0.2 µM) and used for measurements with vesicles. The concentration of unbound aqueous OA ([OA]a) was calculated from the following equation: [OA]a = Kd · 19.5 · (R × R0)/(11.5 − R), where Kd is the dissociation constant (Kd = 0.23 μM for OA at 25°C), and R and R0 are the fluorescence ratios of ADIFAB in the presence and absence of FA, respectively. The value of R0 was either used as provided by FFA Sciences or measured before adding FA to the suspension of vesicles.
Data analysis
Analysis of fluorescence data was performed to determine the t1/2 for FA transfer. The observed rate constant of the fluorescence change was obtained by fitting the fluorescence trace to a first-order exponential decay function: F(t) = F(∞) + F(0)exp(−tkobs), where t is time, F(0) is the initial fluorescence intensity, and F(∞) is the fluorescence at t = ∞. The rate constant is related to the half-time of fluorescence change (t1/2) by the following equation: t1/2 = ln2/kobs.
RESULTS
Delivery of FA to lipid vesicles by MBCD
Our initial experiments aimed to find conditions under which MBCD would deliver FA to lipid vesicles (SUV and LUV) and to determine the kinetics of the partitioning. For these aims we used i) the surface potential probe FPE, which was inserted in the outer leaflet of the membrane to measure the adsorption of FA that dissociated from MBCD, and ii) the pH probe pyranine, which measures the release of H+ to the intravesicular compartment that results from the transbilayer movement and achievement of ionization equilibrium of FA at the inner leaflet of the lipid membrane. Therefore, pyranine measures the combined steps of adsorption and flip-flop.
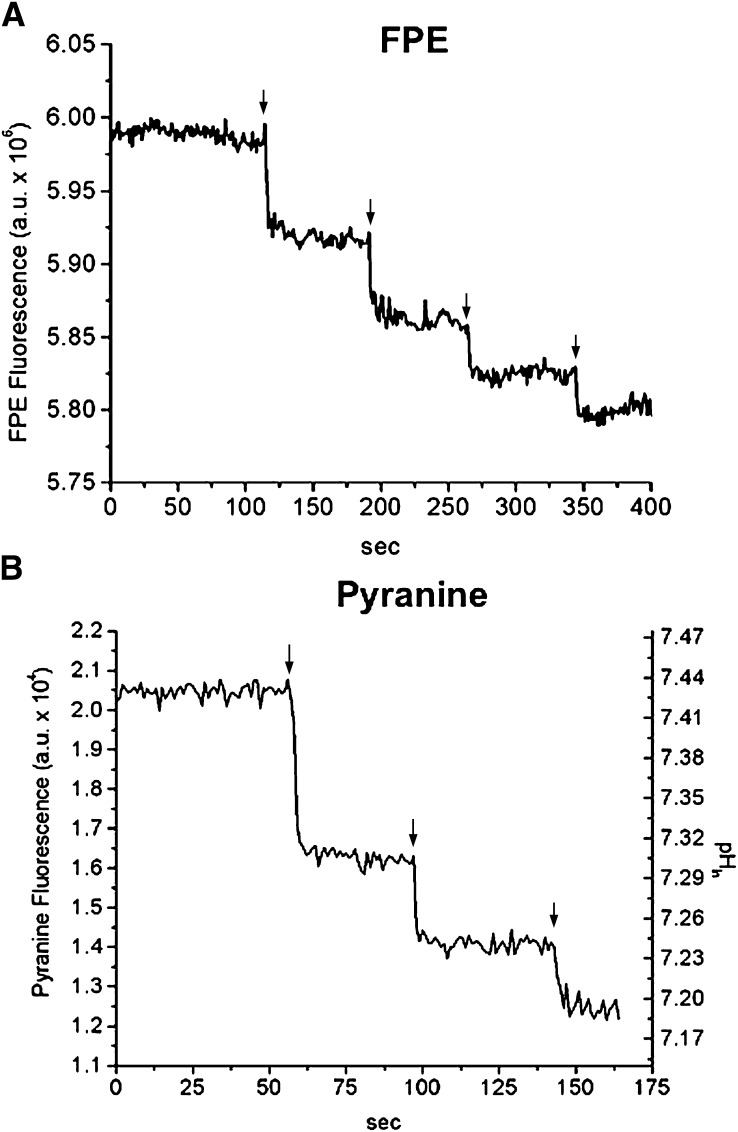
As shown in Fig. 1, sequential additions of 1OA:6MBCD complexes (1.2 μM OA:7.2 μM MBCD) to SUV (100 μM egg-PC) labeled with FPE (Fig. 2A) or containing entrapped pyranine (Fig. 2B) resulted in a rapid decrease in the fluorescence of both probes. These results indicate that OA rapidly dissociated from MBCD, bound to the membrane, and underwent flip-flop within 1–2 s, the time resolution of the online fluorescence measurements.
Fig. 1.
Delivery of OA by MBCD to lipid vesicles (SUV) measured by FPE and pyranine (online fluorescence). Multiple doses of 1.2 µM OA complexed with 7.2 µM MBCD (as indicated by the arrows) were added to a suspension of SUV either containing 0.2 mM entrapped pyranine (A) or labeled with 1 mol% FPE (B). The pyranine fluorescence values from A were converted into pH values using a pH calibration curve obtained with nigericin. SUV was used at a concentration of 100 μM egg-PC in 20 mM HEPES buffer, pH 7.4. One representative experiment is shown in each panel.
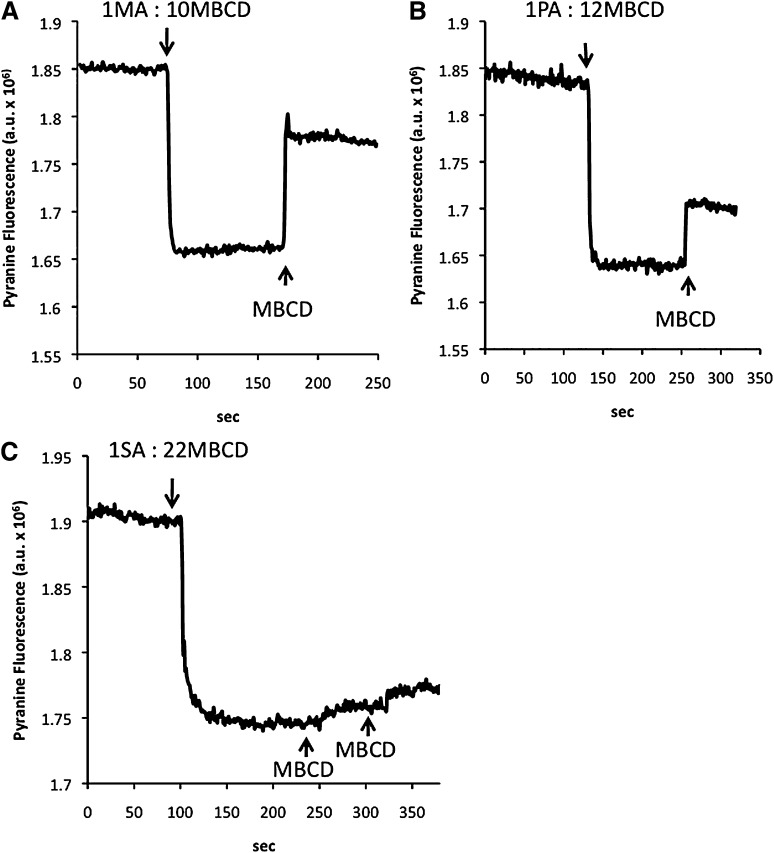
Fig. 2.
Delivery and extraction of MA (A), PA (B), and SA (C) by MBCD to/from lipid vesicles (LUV) measured by pyranine (online fluorescence). Single doses of 4 µM fatty acids complexed with MBCD (as indicated by the arrows; molar ratio FA:MBCD shown) were added to a suspension of LUV containing 0.2 mM entrapped pyranine. MBCD (667 µM) was added to the same LUV suspensions in order to extract the fatty acids delivered to the membrane. LUV was used at a concentration of 400 μM egg-PC in 50 mM HEPES buffer, pH 7.4. One representative experiment is shown in each panel.
To complement the above data with OA, we measured the delivery of other FA by MBCD to LUV containing entrapped pyranine. As shown in Fig. 2, the addition of 1MA:10MBCD (Fig. 2A), 1PA:12MBCD (Fig. 2B), and 1SA:22MBCD (Fig. 2C) complexes to LUV (400 μM egg-PC) also produced a fast reduction in pyranine fluorescence, similar to that observed with FA added in the unbound form (22). Note that higher ratios of MBCD to FA were required to solubilize longer-chain FA. Subsequent addition of an excess of MBCD (FA-free) produced a fast increase in pyranine fluorescence that can be attributed to extraction of the added FA. MBCD was not able to extract the delivered SA when added at the concentration used for extraction of MA, PA, and OA. Furthermore, MBCD did not extract SA after its addition in the unbound form (data not shown). These results indicate a much higher affinity of SA for the lipid membrane relative to MBCB.
As observed before by our group with the addition of unbound FA or FA:albumin complexes (19, 22), subsequent additions of FA:MBCD resulted in progressively smaller changes in pHin (Fig. 1A). These results are explained by the interfacial ionization of FA and flip-flop: as pHin decreases, the ionization of FA at the inner leaflet is suppressed and the net delivery of H+ per dose of added FA is reduced.
Partitioning of OA between phospholipid membranes and MBCD
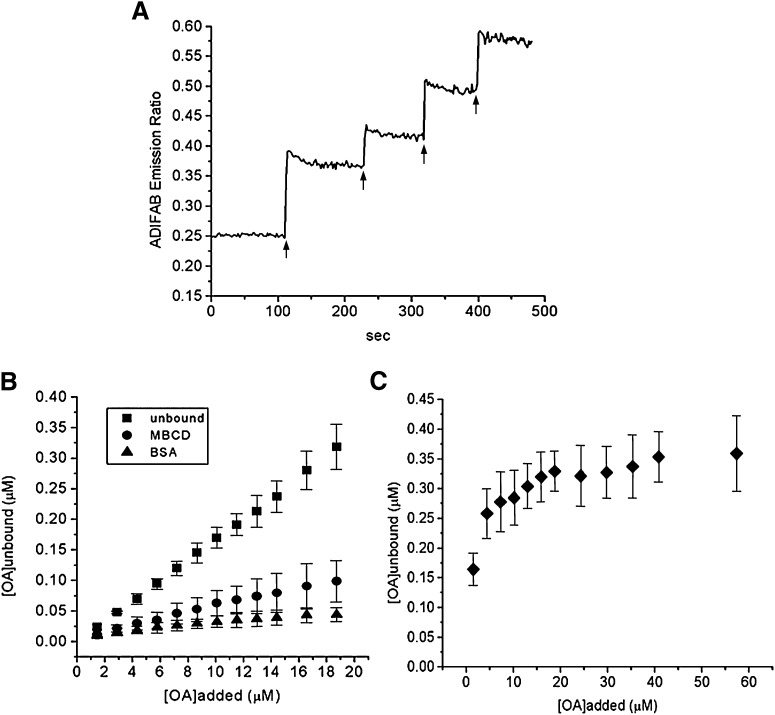
An independent assay used the fluorescent probe ADIFAB in the external buffer to monitor the concentration of unbound OA and to assess the delivery of OA to vesicles by MBCD. As shown in Fig. 3A, the fluorescence of ADIFAB increased rapidly upon addition of 1OA:6MBCD complexes to a suspension of SUV. Each plateau in Fig. 3A reflects the concentration of unbound OA in water and in equilibrium with the membrane, MBCD, and the ADIFAB probe. This result, together with results obtained with FPE and pyranine, indicates rapid dissociation of OA from MBCD; moreover, it does not depend on previous information or assumptions about flip-flop.
Fig. 3.
Partitioning of OA between lipid vesicles (SUV) and MBCD. Multiple doses of 3 μM OA complexed with 18 μM MBCD (as indicated by the arrows) were added to a suspension of SUV (100 µM egg-PC, without any fluorescent probe) with ADIFAB in the external buffer (A). The concentration of unbound OA ([OA]a) in the external buffer in equilibrium with the SUVs was calculated from the fluorescence ratio of ADIFAB obtained after each addition of OA added either unbound, from a stock solution in DMSO or ethanol (squares), complexed to MBCD (circles), or complexed to BSA (molar ratio 8OA:1BSA; triangles) (B). The concentration of [OA]a in equilibrium with MBCD in the absence of vesicles is also shown (C). Data in A are a representative experiment of three to four independent experiments. Data in B and C are means ± standard deviation of three to four independent experiments.
As also shown in Fig. 3A, subsequent addition of 1OA:6MBCD produced smaller incremental changes in the ADIFAB fluorescence. This does not reflect true saturation of binding to the membrane but the partition of FA between donor (MBCD) and acceptor (SUV).
From the ADIFAB fluorescence ratio measured in Fig. 3A, we calculated the concentration of unbound OA ([OA]a) in the external buffer after each addition of 1OA:6MBCD complex. For comparison with these results, we also measured the concentration of unbound OA produced by the delivery of either 1) OA not complexed with any carrier (added in the unbound form from a stock solution of FA in ethanol or DMSO); or 2) OA complexed with BSA (OA:BSA molar ratios of 4:1 and 8:1). Because albumin contains three high-affinity FA binding sites, we used OA:BSA complexes at molar ratios higher than 3FA:1BSA to enhance delivery of FA by albumin (20, 22).
As shown in Fig. 3B in the presence of MBCD, the concentrations of unbound OA in equilibrium with vesicles were much lower than those obtained with OA delivered free of any carrier. In the presence of a fixed SUV concentration of 100 μM, the concentration of unbound OA was between 0.01 and 0.1 μM over the 1OA:6MBCD concentration range investigated, far below the solubility limit for OA (6 μM) (2).
For lower concentrations of total added OA, the reduction in the concentration of unbound OA by MBCD was comparable to levels obtained in the presence of BSA when initial mole ratios of OA:BSA were either 8:1 or 4:1 (data not shown). For instance, at a concentration of 6 μM of total OA, the concentration of unbound OA measured in the presence of MBCD and BSA was 0.035 ± 0.013 and 0.024 ± 0.01 μM, respectively. In contrast, in the absence of a delivery system, the concentration of unbound OA in equilibrium with vesicles produced by 6 μM of total OA was 0.094 ± 0.0085 μM. However, as the concentration of total OA increased together with the concentration of the FA carrier (i.e., constant FA:carrier mole ratio), BSA was more effective in buffering the concentration of unbound OA than MBCD. This result reflects the higher affinity of BSA for FA than for MBCD.
In the presence of 200 μM SUV, the concentration of unbound OA produced by the delivery of 6 μM OA complexed to MBCD was 0.017 ± 0.002 μM, which is half of that measured in the presence of 100 μM SUV. Under these conditions, the concentration of unbound FA measured in the absence of any carrier (OA added unbound) was 0.06 ± 0.015 μM.
The partition coefficient of OA added in the unbound form (Kp) was quantified from the linear relationship between the membrane-bound OA ([OA]m), calculated after every addition of OA to the vesicle suspension and [OA]a: Kp = [OA]m/[OA]a = {([OA]t – [OA]a)/[OA]a}/ Vm/Va), where [OA]m is the FA concentration in the membrane based on the volume of the membrane (Vm); [OA]t and [OA]a are the total and unbound OA concentrations, respectively, which are based on the volume of water phase (Va). For a phospholipid bilayer, Vm/Va has been calculated as 10−3/mmol/l phospholipid (23).
The Kp calculated when uncomplexed OA was added to the vesicle suspension was 0.5 × 106, in agreement with the Kp for protein-free vesicles previously reported by our group (7).
In parallel experiments, ADIFAB was used to measure the concentration of unbound OA in equilibrium with MBCD in the absence of vesicles (Fig. 3C). These results confirmed that MBCD efficiently maintained the concentration of unbound OA in water of 0.3 ± 0.05 µM over a wide range of concentrations.
Kinetics of OA transfer between MBCD and lipid membranes: stopped-flow experiments
The preceding online fluorescence assays with FPE and pyranine showed rapid equilibration of binding of OA and saturated long-chain FA to model membranes when MBCD was a donor. However, the changes in fluorescence were completed within the mixing time of the online experiments (1–2 s). To improve on the time resolution and accurately measure the kinetics of FA transfer between MBCD and lipid membranes, we used stopped-flow fluorimetry to achieve a time resolution of milliseconds.
Stopped-flow fluorescence assays were carried out with OA and with lipid vesicles (SUVs and LUVs) containing entrapped pyranine or labeled with the surface potential probe FPE. To minimize possible artifacts from osmotic changes, we prepared both vesicles and 1OA:6MBCD solutions in HEPES buffer. We defined two populations of egg-PC vesicles: FA donor vesicles containing OA and FA acceptor vesicles with no added OA. The fluorescence probes were placed in either donor or acceptor vesicles depending on the goal of the experiment.
Kinetics of OA transfer from MBCD to phoshpolipid membranes.
To measure the kinetics of OA transfer (or delivery) from MBCD to lipid membranes, 1OA:6MBCD complexes were mixed in a stopped-flow apparatus with acceptor vesicles containing either FPE (Fig. 4) or pyranine (Fig. 5).
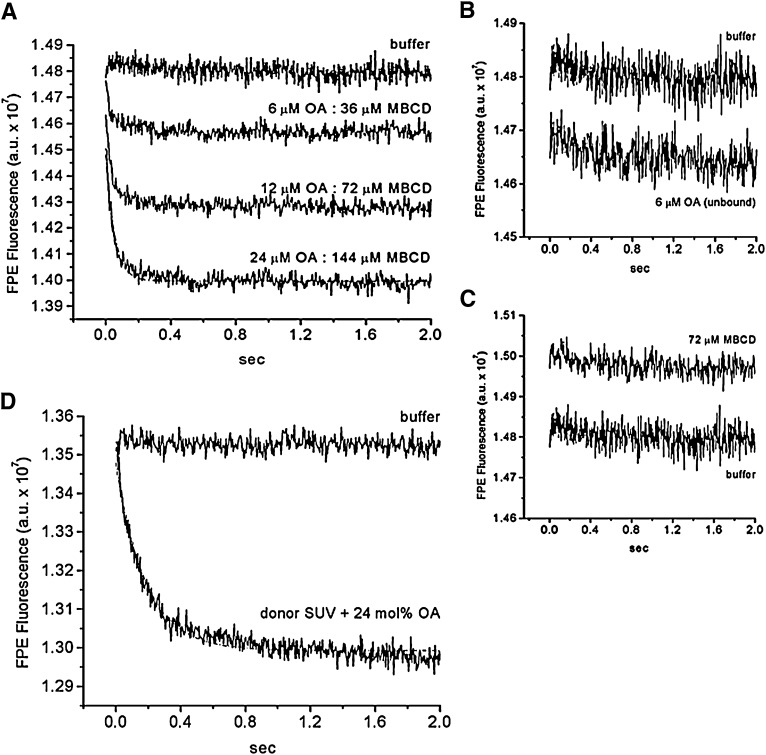
Fig. 4.
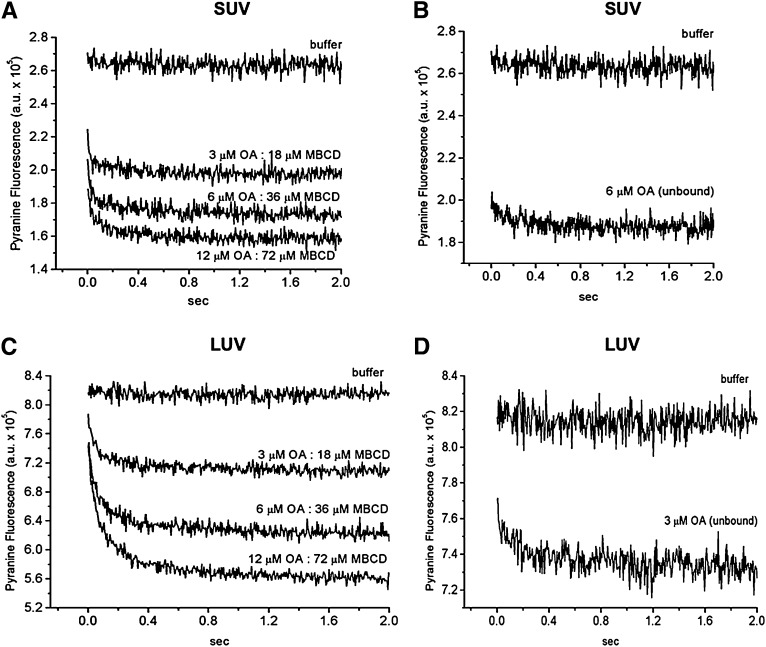
The kinetics of OA transfer from MBCD to lipid vesicles (SUV) measured with FPE. A suspension of SUV labeled with FPE (100 μM egg-PC + 1 mol% FPE) was rapidly mixed with increasing amounts of OA:MBCD complexes at a fixed molar ratio of 1OA:6MBCD (A). The final concentration of OA:MBCD complexes were 6 μM OA:36 μM MBCD, 12 μM OA:72 μM MBCD, and 24 μM OA:144 μM MBCD. The curve with 24 µM OA:144 µM MBCD was fitted to one phase exponential decay with a t1/2 of ∼30 ms (dashed line). As a control, the same SUV preparation was mixed with 6 μM unbound OA (B) and with MBCD not complexed with OA (empty MBCD; 72 μM CD) (C). A different SUV preparation also labeled with FPE (100 µM egg-PC + 1mol% FPE) was mixed with FA donor SUV (300 µM egg-PC) containing 24 mol% OA (D). The curve shown in D was fitted to one phase exponential decay with t1/2 of 128 ms (dashed line). SUV mixed with buffer served as a mixing control. Each fluorescence trace is the average of 5 to 10 measurements and represents one experiment.
Fig. 5.
The kinetics of OA transfer from MBCD to lipid vesicles (SUV and LUV) measured with pyranine. A suspension of lipid vesicles containing entrapped pyranine (100 μM egg-PC) was rapidly mixed with increasing amounts of OA:MBCD complex at a fixed molar ratio of 1 OA:6 MBCD (A, SUV; C, LUV). The final concentrations of OA:MBCD complexes were 3 μM OA:18 μM MBCD, 6 μM OA:36 μM MBCD, and 12 μM OA:72 μM MBCD. As controls, the same lipid vesicle preparations were mixed with unbound OA (3 μM, B; 6 μM, D). Lipid vesicles were also mixed with only buffer as mixing control. Each fluorescence trace is the average of 5 to 10 measurements and represents one experiment.
As shown in Fig. 4A, the mixing of 1OA:6MBCD complexes with FPE-labeled SUV produced a very fast dose-dependent decrease in FPE fluorescence with a t1/2 of 30 ms. The maximal fluorescence change was dose dependent but not the kinetic rate constants. The rate of change in FPE fluorescence corresponds to the kinetics of OA dissociation from MBCD because, as reported recently by our group (19) and confirmed here, the change in FPE fluorescence produced by OA delivered without carrier, a protocol that reports only adsorption of OA to the lipid membrane is faster (Fig. 4B; t1/2 < 10 ms). As control, mixing SUV with MBCD that was not complexed with OA (empty MBCD) did not significantly affect FPE fluorescence (Fig. 4C).
To compare the delivery of OA by MBCD with other delivery systems, such as lipid vesicles under the same conditions, we measured the transfer of OA from donor vesicles to acceptor vesicles labeled with FPE. As shown in Fig. 4D, donor vesicles delivered OA at a slower rate than MBCD. The t1/2 of OA transfer from vesicle to vesicle was 128 ms, which is within the range of vesicle-to-vesicle transfer rates recently reported by our group (t1/2 = 100 ms) (20).
For comparison with the FPE experiments, we conducted parallel experiments with pyranine. As shown in Fig. 5, mixing 1OA:6MBCD complexes with either SUV or LUV (100 µM egg-PC) both containing entrapped pyranine produced a very fast dose-dependent reduction in pyranine fluorescence. As observed for FPE, the maximal fluorescence changes were dose dependent but not the kinetic rate constants. We did not perform a curve-fitting analysis for the data with SUV because the drop in pyranine fluorescence from the initial value (SUV mixed with buffer) was almost complete before the first time point was measured. Instead, we estimated an upper limit for the t1/2 of 50 ms, which falls within the same range reported by FPE (Fig. 4; t1/2 = 30 ms). Therefore, we conclude that both probes present in the SUV are measuring the same kinetic step of OA dissociation from MBCD. For the data with LUV, the mixing of 1OA:6MBCD with LUV produced a reduction in pyranine fluorescence with a longer estimated upper limit (t1/2 of 100 ms). The slower rate probably reflects the slightly slower flip-flop of FA in LUV compared with SUV, as in Fig. 5B and D and as reported before (21). MBCD alone did not affect the pyranine fluorescence significantly in both SUVs and LUVs (data not shown).
In the stopped-flow experiments, the concentration of unbound OA in water after transfer reached equilibrium was estimated using the data obtained with ADIFAB. The mixing of 3, 6, and 12 μM OA complexed to MBCD with 100 μM SUV (Figs. 4A and 5A) resulted in a concentration of unbound OA of 0.02, 0.35, and 0.7 μM, respectively. In contrast, the concentration obtained with the mixing of 6 μM of OA not complexed with any carrier (Figs. 4B and 5B) was approximately 1 μM.
The kinetics of MBCD-mediated efflux of OA from phospholipid membranes.
To supplement these OA transfer experiments, the reversibility of exchange of OA between MBCD and vesicles was examined by measuring the transfer of OA from FA donor SUV to empty MBCD (not complexed to FA) using the fluorescence probes FPE and pyranine in the donor SUV. Changes in FPE fluorescence reflect OA desorption from the outer leaflet of the SUV and binding to MBCD, whereas pyranine kinetics reflect flip-flop in addition to these two kinetic steps.
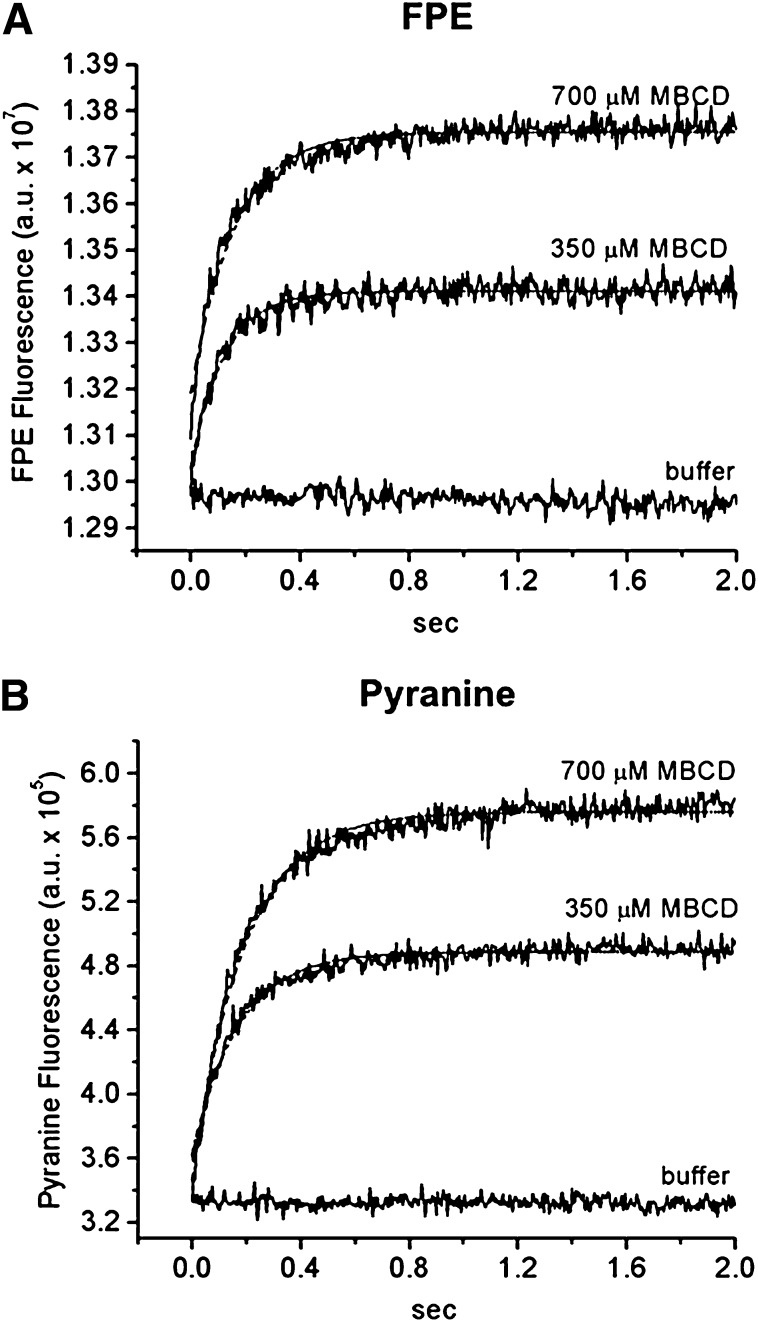
As shown in Fig. 6, when donor SUV (100 µM egg-PC) containing 12 mol% OA and labeled with FPE (Fig. 6A) or containing entrapped pyranine (Fig. 6B) were mixed with MBCD in buffer (350 or 700 µM MBCD), a rapid increase in the fluorescence of both probes was observed. The maximal fluorescence change but not the kinetic rate constant was dose dependent. Moreover, there was no significant difference between the rates of OA extraction detected by FPE (t1/2 of 84 and 100 ms for 350 and 700 μM MBCD, respectively) and by pyranine (t1/2 of 118 and 132 ms for 350 and 700 μM MBCD, respectively). Therefore, the kinetic step of flip-flop did not slow down the FA transfer from vesicles to MBCD. The rates of OA transfer from vesicle to MBCD were similar to those measured in experiments of OA transfer from vesicle to vesicle (t1/2 = 100 ms) (20), which further suggests that the measured rates are limited by the OA desorption from the donor SUV into the aqueous phase in both cases.
Fig. 6.
The kinetics of OA transfer from lipid vesicle (SUV) to MBCD measured with FPE and pyranine. FA donor SUVs (100 μM egg-PC + 12 mol% OA) either labeled with 1 mol% FPE (A) or containing 0.1 mM entrapped pyranine (B) were mixed rapidly with increasing amounts of empty MBCD (350 or 700 μM MBCD). The fluorescence traces from both FPE and pyranine were well fitted by a single exponential function (dashed line). The t1/2 values of FPE fluorescence after addition of MBCD to SUV labeled with FPE were 84 ms for 350 μM MBCD and 100 ms for 700 μM MBCD. The t1/2 values of pyranine were 118 ms for 350 μM MBCD and 132 ms for 700 μM MBCD. Donor SUVs were mixed with only buffer as a mixing control. Each fluorescence trace is the average of 5 to 10 measurements and represents one experiment.
We conclude that the kinetics reported by these two probes corresponded to the desorption of OA from the donor SUV and that OA binding to MBCD was relatively rapid. Note that the concentration of MBCD needed to extract OA from the lipid membrane was much higher than the concentration of MBCD used in the delivery of OA and the concentration of FA donor vesicles. These observations confirm the preferential partitioning of OA into the lipid membrane than into the MBCD cavity.
Delivery of oleic acid by MBCD to cells
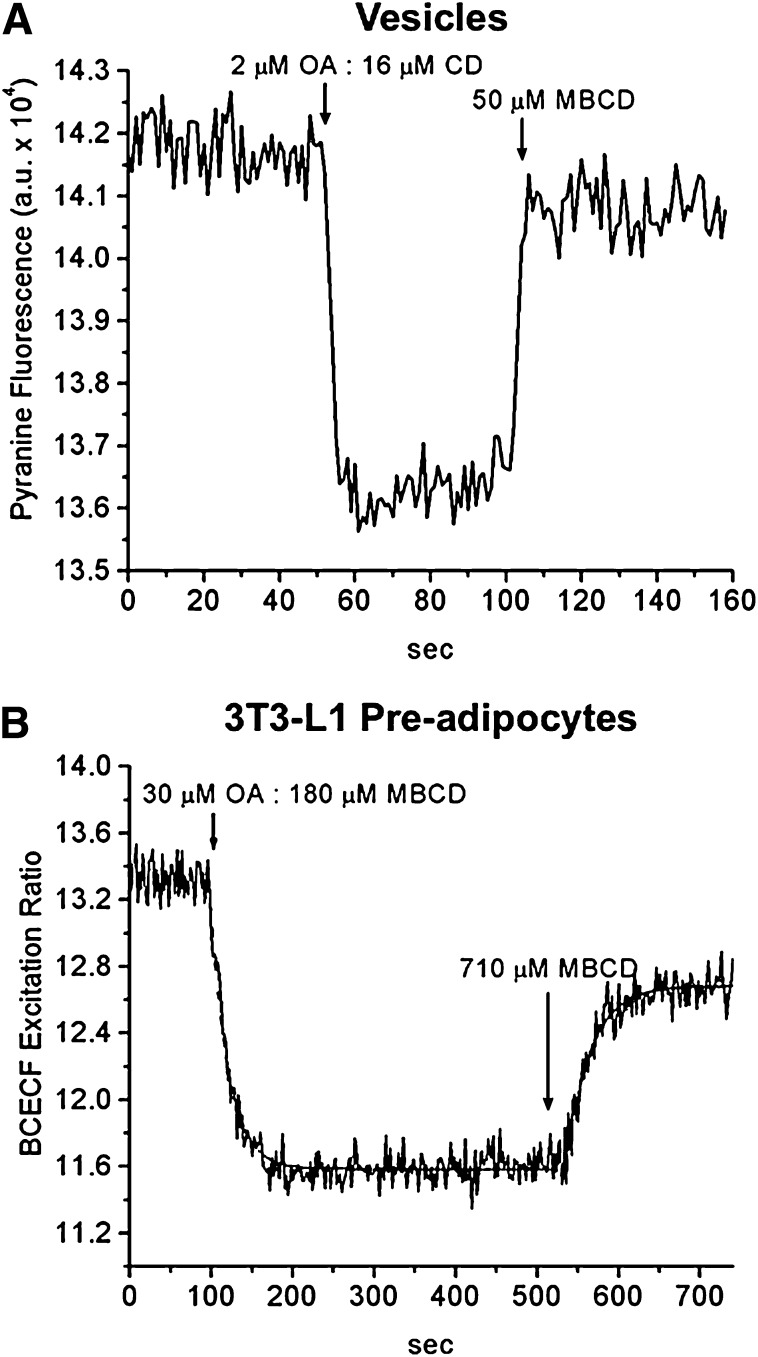
Because the long-term goal of our studies of FA transport in membrane models is to apply our methods to cells, we tested the ability of MBCD to deliver OA to different types of cells, one with active metabolism and the other with very slow utilization of OA. First, for comparison with cell experiments, we illustrate a standard protocol in model membranes. As shown in Fig. 7, following the addition of 1OA:6MBCD complexes to the external buffer, a rapid drop (t1/2 < 2s) in the fluorescence corresponding to an internal acidification was observed in protein-free LUVs containing entrapped pyranine (Fig. 7A). A slightly slower drop with a t1/2 of 15 s was observed when the complexes were added to cultured 3T3-L1 preadipocytes loaded with BCECF (Fig. 7B). Subsequent addition of excess MBCD (3- to 4-fold) before recovery of the fluorescence resulted in a rapid rise in fluorescence as predicted for extraction of FA from the membrane. These results mirror our published results with addition of uncomplexed OA followed by BSA in vesicles (23) and in adipocytes (24). Compared with BSA, a much higher ratio of MBCD to FA is required to extract FA from lipid vesicles and cells, a reflection of the lower affinity of MBCD for FA.
Fig. 7.
Delivery and extraction of OA to/from lipid vesicles (LUV) and cells by MBCD. Fluorescence changes in LUVs (50 µM egg-PC) containing entrapped pyranine (A) and in 3T3L-1 preadipocytes with BCECF (B) upon addition of 1OA:6MBCD complex. The concentration of OA complexed to MBCD is shown. The dashed line is the single exponential fit (two separate fittings). The t1/2 values for OA delivery and extraction by MBCD in cells are 15 and 20 s, respectively. One representative experiment is shown in each panel.
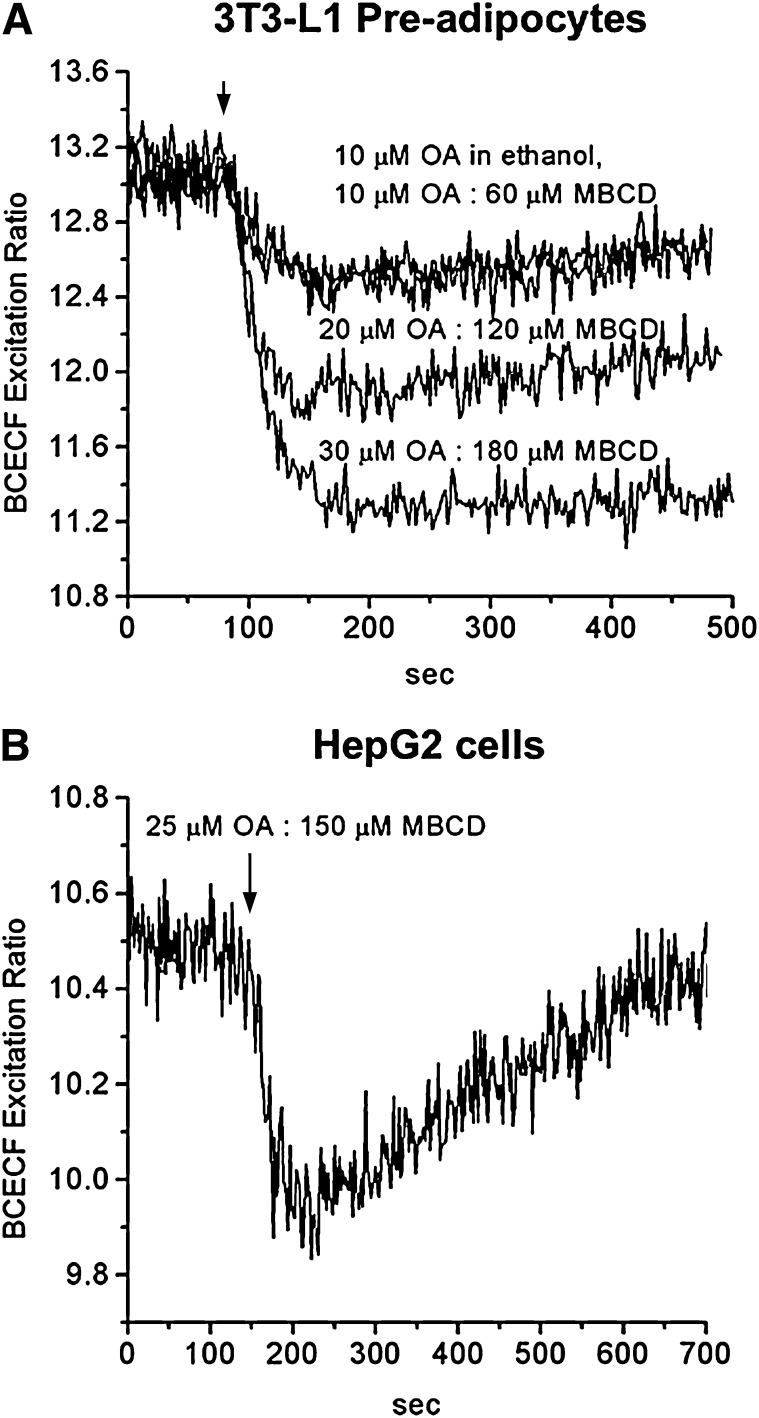
As shown in Fig. 8A, 3T3-L1 preadipocytes showed dose-dependent pH drops that were complete within 60 s and were indistinguishable from those for OA added in ethanol. The magnitude of the fluorescence drop obtained with 10 µM OA complexed with 60 µM MBCD was the same as with 10 µM OA in ethanol (unbound OA), suggesting that all of the OA partitioned from MBCD to the cell membrane. The same results were observed with 20 µM OA:120 µM MBCD compared with 20 µM OA in ethanol and for 30 µM OA:180 µM MBCD compared with 30 µM OA in ethanol (data not shown). These experiments established experimental conditions for delivery of OA by MBCD that result in complete transfer of OA from MBCD. It is important to note that conditions can be modified to results in partial transfer of OA (partitioning) if this protocol is desired.
Fig. 8.
Delivery of OA to cells by MBCD. Fluorescence changes in 3T3L-1 preadipocytes containing entrapped BCECF upon addition of 10 µM OA:60 µM MBCD, 20 µM OA:120 µM MBCD, and 30 µM OA:180 µM MBCD. Uncomplexed OA, added from an OA stock solution in ethanol, was used as a control (upper trace). The data with 30 µM OA in MBCD were fitted to a single exponential decay (t1/2 of 20s, dashed line) (A). Fluorescence changes in HepG-2 cells containing entrapped BCECF upon addition of 25 µM OA:150 µM MBCD. The decrease in BCECF fluorescence was complete within 45 s after addition of 1OA:6MBCD (B). One representative experiment is shown in each panel.
Finally, we tested the delivery of OA by MBCD to hepatocytes (cultured HepG2 cells). These cells metabolize FA faster than 3T3-L1 preadipocytes, which is detected as recovery in the BCECF fluorescence (25). As shown in Fig. 8B, addition of 1OA:6MBCD resulted in a rapid fluorescence drop that was completed within 45 s, in agreement with our previous study with OA delivered in ethanol to HepG2 cells (25). Additionally, MBCD did not affect the BCECF fluorescence recovery in HepG2 cells.
DISCUSSION
Although various CDs have been used in cell studies, especially for extraction and delivery of cholesterol, few details of the kinetics and partitioning have been reported. We took advantage of the ionization properties of FA and our novel fluorescence assays developed to study the FA transport in membranes (17–19), to monitor the movement of FA without separation of donor and acceptor. We show that MBCD has ideal properties for delivering FA. MBCD rapidly releases FA to the acceptor membrane, and the FA so delivered binds instantly to outer leaflet of the membrane and undergo fast flip-flop to equilibrate in the membrane leaflets. However, unlike our previous protocol with micromolar concentrations of unbound FA, the instantaneous concentration of unbound FA with MBCD is always much lower. Our major findings are summarized below.
Conditions for preparation of MBCD complexes with FA
Formation of FA:CD complexes is considered a true molecular dispersion and not micellization as achieved by detergents (26). Hydrogen bonds between the FA carboxyl group and the hydroxyl groups of CD and hydrophobic interactions between the FA acyl chain and CD occur during complex formation (27). The pKa of the carboxyl group of the FA is not changed in the presence of CD (28). The number of CD molecules required to solubilize one FA molecule increases with an increase in the hydrocarbon chain length of FA; one MBCD can accommodate about 5 to 6 CH2 units of FA (26). Accordingly, one molecule of stearic acid (C18:0) would require at least three molecules of MBCD, which implies that in our experiments with SA:MBCD of 1:22, either some empty MBCD was present or, possibly, additional MBCD capped the ends of the complex. For the saturated FA with 14 to 18 carbons, we found that higher ratios of MBCD to FA were required to produce a clear solution at room temperature and that the MBCD/FA increased with increasing FA chain length, as noted before (26).
MBCD maintains a low concentration of unbound OA in buffer
Physiological concentrations of unbound FA in the extracellular compartment are in the very low nanomolar range (7) because FA binds to cell membranes, lipoproteins, and albumin in the blood and tissue compartments. As the concentration of unbound FA in equilibrium with MBCD and lipid membranes has not been reported, we showed (Fig. 3B) that MBCD maintained a low concentration of unbound FA (OA) in the range of FA:MBCD total concentration suitable for delivery of FA to membranes while delivering most of its bound FA.
Our comparison of different methods of presenting OA to membranes showed that the concentration of aqueous unbound OA, measured after equilibration was established between the donor and the lipid membrane, depends on the vehicle used to deliver the OA (unbound, complexed to MBCD or to albumin). At lower concentrations of total OA, the reduction in the concentration of unbound OA by MBCD was comparable to very low nanomolar levels obtained in the presence of albumin (Fig. 3B). For higher concentrations of total OA, unbound OA was 2 to 3 times lower in the presence of albumin than in the presence of MBCD because of the multiple binding sites and higher affinity of albumin. MBCD could be advantageous as a delivery vehicle for studying FA transport and uptake because partitioning favors FA binding to membranes, and the concentration delivered approximates the concentration of FA in the MBCD complex. When albumin is used as a donor, the concentration delivered initially to cells is much lower than the total amount of FA in the complex (7) and cannot be determined unless the FA concentration in the cell is measured independently, an assay that rarely is performed.
MBCD delivers FA to phospholipid membranes in milliseconds
Another important consideration for effective delivery of FA to membranes is the kinetics of transfer. Previous cell studies using CD as a donor have not been able to measure the rate of dissociation of FA from CD because they typically used incubation times of hours (15, 16). Here, for the first time, we measured the kinetics of FA delivery by MBCD using our well-defined fluorescence assays with FPE and pyranine.
We conclude that the small decrease in the rate of binding to the vesicle outer leaflet (SUV and LUV) in the presence of MBCD compared with the rate with unbound FA represents the kinetics of dissociation of OA from the MBCD complex. These kinetics are faster than the kinetics of FA dissociation for albumin (hundreds of milliseconds) (20).
An important conclusion of this study is that delivery of uncomplexed OA in the concentrations that we used does not produce an artifactual faster rate of FA flip-flop as suggested by another investigator (29). They confirm the same conclusion recently drawn from transfer studies using vesicles and albumin as FA donors (19).
MBCD extracts OA rapidly from phospholipid membranes
Our direct measurement of the desorption of OA from the outer leaflet of the membrane bilayer by FPE showed definitively that the rate limiting step for transfer of OA from vesicle to MBCD is desorption from the membrane. In our experimental design with empty MBCD, the concentration to partition OA out of the vesicles was much higher (∼3 times) than that used for delivery, another reflection of the weak affinity of OA for MBCD. Furthermore, a higher ratio of MBCD to lipid vesicles also had to be used (MBCD in excess) to extract all of OA from the lipid membrane. Thus, when designing experiments with MBCD; it is imperative to consider that FA could be extracted from rather than delivered to the cell membrane, depending on the ratio of MBCD to lipid membrane or to cell.
CD has been used widely in raft membrane studies to enhance desorption of cholesterol from membranes (30, 31). In contrast to our results for FA desorption, CD enhances the rate of cholesterol desorption, [t1/2 of hours without MBCD (32)], probably by reducing the free energy of hydration of cholesterol (33, 34). The concentration of MBCD used in our cell studies is much lower than the concentrations required to extract cholesterol from cells. It is unlikely that MBCD extracted phospholipids from the cells or vesicles in our experiments because our MBCD concentrations never exceeded 0.2 mM for delivery or 0.7 mM in the extraction experiments. CD at a concentration of 10 mM extracted <0.2% of phospholipids present in a lipid membrane, but considerable extraction of phospholipids with perturbation of the membrane structure has been observed at CD concentrations of 30–50 mM (35, 36). However, our study does reveal an important pitfall in the use of MBCD to extract cholesterol and/or phospholipids in cells: MBCD at these higher concentrations also will extract FA, and possibly other cellular constituents, and other single-chain amphiphiles, such as lysophospholipids and acyl-CoA, thus altering the nutritional status of the cells and the plasma membrane structure.
MBCD delivers FA to cells rapidly and does not interfere with intracellular FA metabolism
Our cell studies were designed to investigate the utility of MBCD to deliver OA to cells with a slow rate of metabolism of exogenously added FA (3T3L1 preadipocyte cells; Figs. 7B and 8A) and cells with fast metabolism (HepG2 cells; Fig. 8B). We demonstrated in both cell types that adsorption and transbilayer movement of OA delivered by MBCD are complete within seconds and that all of the OA that had been complexed with MBCD partitioned from MBCD into the cell membrane. We also showed that MBCD did not affect the recovery of BCECF fluorescence in HepG2 cells, which closely follows FA metabolism (mainly FA esterification) (25, 37). Therefore, MBCD can deliver FA to cells without altering the plasma membrane structure and FA metabolism.
We conclude that the concentration of unbound FA in equilibrium with donors and acceptors does not affect kinetic measurements of membrane transport in cell experiments, which has been a frequent reservation about the use of unbound FA as a source of exogenous FA. MBCD appears to be an ideal vehicle for delivery of long-chain FA to both model membranes and cells. Used according to our protocols, it averts several complications and artifacts that might be introduced by albumin. However, the higher concentrations of CD commonly used to extract cholesterol from cells are likely to extract FA and other single-chain amphiphiles. Rather than affect only the plasma membrane lipid content, addition of MBCD could result in alterations in cell metabolism and plasma membrane structure independent of changes resulting from lower levels of cholesterol.
Footnotes
Abbreviations:
- ADIFAB
- acrylodan-labeled intestinal fatty acid binding protein
- BCECF
- 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxy-fluorescein
- CD
- cyclodextrin
- FPE
- fluorescein phosphatidylethanolamine
- Kp
- partition coefficient
- LUV
- large unilamellar vesicle
- MA
- myristic acid
- MBCD
- methyl-β-cyclodextrin
- OA
- oleic acid
- [OA]a
- concentration of unbound oleic acid
- [OA]m
- concentration of oleic acid in the membrane
- [OA]t
- concentration of total oleic acid
- PA
- palmitic acid
- SA
- stearic acid
- SUV
- small unilamellar vesicle
- Vm
- volume of the membrane
- Va
- volume of water phase
This work was partially funded by the National Institutes of Health (Grant 5 PO1-0216335). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Cistola D. P., Hamilton J. A., Jackson D., Small D. M. 1988. Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 27: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 2.Richieri G. V., Ogata R. T., Kleinfeld A. M. 1992. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J. Biol. Chem. 267: 23495–23501. [PubMed] [Google Scholar]
- 3.Hamilton J. A., Guo W., Kamp F. 2002. Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? Mol. Cell. Biochem. 239: 17–23. [PubMed] [Google Scholar]
- 4.Zakim D. 2000. Thermodynamics of fatty acid transfer. J. Membr. Biol. 176: 101–109. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton J. A. 1999. Transport of fatty acids across membranes by the diffusion mechanism. Prostaglandins Leukot. Essent. Fatty Acids. 60: 291–297. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton J. A. 2004. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog. Lipid Res. 43: 177–199. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton J. A., Kamp F. 1999. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 48: 2255–2269. [DOI] [PubMed] [Google Scholar]
- 8.Brewster M. E., Loftsson T. 2007. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59: 645–666. [DOI] [PubMed] [Google Scholar]
- 9.Duchene D., Bochot A., Yu S., Pepin C., Seiller M. 2003. Cyclodextrins and emulsions. Int. J. Pharm. 266: 85–90. [DOI] [PubMed] [Google Scholar]
- 10.Gorfien S., Paul B., Walowitz J., Keem R., Biddle W., Jayme D. 2000. Growth of NS0 cells in protein-free, chemically defined medium. Biotechnol. Prog. 16: 682–687. [DOI] [PubMed] [Google Scholar]
- 11.Vicanova J., Weerheim A. M., Kempenaar J. A., Ponec M. 1999. Incorporation of linoleic acid by cultured human keratinocytes. Arch. Dermatol. Res. 291: 405–412. [DOI] [PubMed] [Google Scholar]
- 12.Wray-Cahen D., Caperna T., Steele N. 2001. Methyl-beta-cyclodextrin: an alternative carrier for intravenous infusion of palmitate during tracer studies in swine (Sus scrofa domestica). Comp. Biochem. Physiol. A Mol. Integ.r Physiol. 130: 55–65. [DOI] [PubMed] [Google Scholar]
- 13.Covey S. D., Brunet R. H., Gandhi S. G., Mcfarlane N., Boreham D. R., Gerber G. E., Trigatti B. L. 2007. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem. Biophys. Res. Commun. 355: 67–71. [DOI] [PubMed] [Google Scholar]
- 14.Frank P. G., Marcel Y. L., Connelly M. A., Lublin D. M., Franklin V., Williams D. L., Lisanti M. P. 2002. Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. Biochemistry. 41: 11931–11940. [DOI] [PubMed] [Google Scholar]
- 15.Jia Z., Moulson C. L., Pei Z., Miner J. H., Watkins P. A. 2007. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J. Biol. Chem. 282: 20573–20583. [DOI] [PubMed] [Google Scholar]
- 16.Sanders R. J., Ofman R., Duran M., Kemp S., Wanders R. J. 2006. Omega-oxidation of very long-chain fatty acids in human liver microsomes. Implications for X-linked adrenoleukodystrophy. J. Biol. Chem. 281: 13180–13187. [DOI] [PubMed] [Google Scholar]
- 17.Brunaldi K., Simard J., Kamp F., Rewal C., Asawakarn T., O'Shea P., Hamilton J. 2007. Fluorescence assays for measuring fatty acid binding and transport through membranes. Methods Mol. Biol. 400: 237–255. [DOI] [PubMed] [Google Scholar]
- 18.Simard J. R., Kamp F., Hamilton J. A. 2007. Acrylodan-labeled intestinal fatty acid-binding protein to measure concentrations of unbound fatty acids. Methods Mol. Biol. 400: 27–43. [DOI] [PubMed] [Google Scholar]
- 19.Simard J. R., Kamp F., Hamilton J. A. 2008. Measuring the adsorption of Fatty acids to phospholipid vesicles by multiple fluorescence probes. Biophys. J. 94: 4493–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simard J. R., Pillai B. K., Hamilton J. A. 2008. Fatty acid flip-flop in a model membrane is faster than desorption into the aqueous phase. Biochemistry. 47: 9081–9089. [DOI] [PubMed] [Google Scholar]
- 21.Kamp F., Zakim D., Zhang F., Noy N., Hamilton J. 1995. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 34: 11928–11937. [DOI] [PubMed] [Google Scholar]
- 22.Kamp F., Hamilton J., Kamp F., Westerhoff H., Hamilton J. 1993. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 32: 11074–11086. [DOI] [PubMed] [Google Scholar]
- 23.Kamp F., Hamilton J. 1992. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc. Natl. Acad. Sci. USA. 89: 11367–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Civelek V. N., Hamilton J. A., Tornheim K., Kelly K. L., Corkey B. E. 1996. Intracellular pH in adipocytes: effects of free fatty acid diffusion across the plasma membrane, lipolytic agonists, and insulin. Proc. Natl. Acad. Sci. USA. 93: 10139–10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W., Huang N., Cai J., Xie W., Hamilton J. 2006. Fatty acid transport and metabolism in HepG2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G528–G534. [DOI] [PubMed] [Google Scholar]
- 26.Szente L., Szejtli J., Szemán J., Kató L. 1993. Fatty acid- cyclodextrin complexes: properties and applications. J. Incl. Phenom. Macrocycl. Chem. 16: 339–354. [Google Scholar]
- 27.Lopez-Nicolas J. M., Bru R., Sanchez-Ferrer A., Garcia-Carmona F. 1995. Use of ‘soluble lipids’ for biochemical processes: linoleic acid-cyclodextrin inclusion complexes in aqueous solutions. Biochem. J. 308: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jyothirmayi N., Ramadoss C., Divakar S. 1991. Nuclear magnetic resonance studies of cyclodextrin complexes of linoleic acid and arachidonic acid. J. Agric. Food Chem. 39: 2123–2127. [Google Scholar]
- 29.Cupp D., Kampf J., Kleinfeld A. 2004. Fatty acid-albumin complexes and the determination of the transport of long chain free fatty acids across membranes. Biochemistry. 43: 4473–4481. [DOI] [PubMed] [Google Scholar]
- 30.Gaus K., Gratton E., Kable E., Jones A., Gelissen I., Kritharides L., Jessup W. 2003. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl. Acad. Sci. USA. 100: 15554–15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehehalt R., Sparla R., Kulaksiz H., Herrmann T., Füllekrug J., Stremmel W. 2008. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biol. 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton J. A. 2003. Fast flip-flop of cholesterol and fatty acids in membranes: implications for membrane transport proteins. Curr. Opin. Lipidol. 14: 263–271. [DOI] [PubMed] [Google Scholar]
- 33.Niu S. L., Litman B. J. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83: 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steck T. L., Ye J., Lange Y. 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys. J. 83: 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piel G., Piette M., Barillaro V., Castagne D., Evrard B., Delattre L. 2007. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 338: 35–42. [DOI] [PubMed] [Google Scholar]
- 36.John K., Kubelt J., Müller P., Wüstner D., Herrmann A. 2002. Rapid transbilayer movement of the fluorescent sterol dehydroergosterol in lipid membranes. Biophys. J. 83: 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamp F., Guo W., Souto R., Pilch P., Corkey B., Hamilton J. 2003. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J. Biol. Chem. 278: 7988–7995. [DOI] [PubMed] [Google Scholar]